Abstract
Binding to target cell receptors is a critical step in the virus life cycle. Coxsackievirus A24 variant (CVA24v) has pandemic potential and is a major cause of acute hemorrhagic conjunctivitis, but its cellular receptor has hitherto been unknown. Here we show that CVA24v fails to bind to and infect CHO cells defective in sialic acid expression. Binding of CVA24v to and infection of corneal epithelial cells are efficiently inhibited by treating cells with a sialic acid-cleaving enzyme or sialic acid-binding lectins and by treatment of the virus with soluble, multivalent sialic acid. Protease treatment of cells efficiently inhibited virus binding, suggesting that the receptor is a sialylated glycoprotein. Like enterovirus type 70 and influenza A virus, CVA24v can cause pandemics. Remarkably, all three viruses use the same receptor. Since several unrelated viruses with tropism for the eye use this receptor, sialic acid-based antiviral drugs that prevent virus entry may be useful for topical treatment of such infections.
Two members of the family Picornaviridae, coxsackievirus A24 variant (CVA24v) and enterovirus type 70 (EV70), are responsible for the majority of cases of acute hemorrhagic conjunctivitis (AHC). AHC is characterized by conjunctivitis, keratitis, foreign body sensation, and pain, but respiratory symptoms and severe neurological symptoms similar to those caused by poliovirus (acute flaccid paralysis) have also been reported (7, 44, 64, 67). AHC was first reported in Ghana in 1969 (10), and since then numerous epidemics and three pandemics have occurred: in 1969 to 1971 (36), 1980 to 1981 (18, 27), and in 2002 to 2004. It has been estimated that there have been about 100 million cases of AHC since identification of the causative agents (1). With a few exceptions, most outbreaks during the past 15 to 20 years, including the 2002 to 2004 pandemic, have been caused by CVA24v. The last pandemic started in the eastern hemisphere in 2002, probably in South Korea, where more than one million people were reported to be affected over a three-month period (41). It continued with an outbreak in Malaysia the same year (18). In 2003, outbreaks of AHC were reported from South Korea (46), India (19), Nepal (25), Tunisia (60), and Congo (29), and in the western hemisphere from Nicaragua, Honduras, Guatemala, El Salvador, and Caribbean countries (45), French Guiana and the West Indies (15), Puerto Rico (2), and Brazil (38). In 2004, more outbreaks hit both the western (58) and eastern (29) hemispheres.
To date, cellular receptors used by coxsackie A viruses have been described only for CVA9 and CVA21, but neither of these viruses is associated with AHC. CVA9 uses αVβ3 integrins, αVβ6 integrins, and/or glucose-regulated protein 78 as cellular receptors (48, 59, 62), whereas CVA13, -15, -18, and -20 use intercellular adhesion molecule 1 (ICAM-1) (39) and CVA21 uses CD55 and/or ICAM-1 (53).
EV70, the other member of the Picornaviridae family that causes AHC has been found to use either CD55 (26) or sialic acid (1) as a cellular receptor, and the choice of receptor may depend on the cell line under investigation. Early on members of the Picornaviridae family were shown to compete with members of the Adenoviridae family for receptors (32). The coxsackie-adenovirus receptor (CAR) was the first cellular receptor shown to be used by both virus families (9). Subsequently, sialic acid has been shown to be used by both EV70 and specific adenoviruses that cause epidemic keratoconjunctivitis (5, 6). Other receptors shared by these two unrelated virus families are heparan sulfate, integrins, and members of the regulators of complement activation protein family (49, 69). In this study we set out to investigate whether CVA24v can make use of any of the receptors used by members of the Picornaviridae or Adenoviridae. We identified sialic acid as a cellular receptor for CVA24v on human ocular cells, and we conclude that usage of sialic acid is a common feature of at least three different viruses with pandemic potential: CVA24v, EV70, and influenza A viruses. Another common feature of these viruses is their primary sites of replication in humans: the eyes and airways. Usage of sialic acid has previously been suggested to explain, at least in part, the tropism of these viruses (42). Here we provide results that support this suggestion.
MATERIALS AND METHODS
Cells and viruses.
CHO-MOCK, CHO-CAR, CHO-CD55, CHO-CD46, CHO-ICAM-1, Lec2, Pro-5, psgB-618, HCE (human corneal epithelial), NHC (normal human conjunctiva), A549 (alveolar), Hep2 (larynx), HeLa (cervix), and GMK (green monkey kidney) cells were grown as described in the references listed in Table 1, except for GMK cells, which were grown in Dulbecco's modified essential medium (DMEM; Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal calf serum (FCS; Sigma-Aldrich), HEPES at pH 7.4 (EuroClone, Milan, Italy), and penicillin-streptomycin (PEST; Gibco, Carlsbad, CA). The CVA24v strain used in this work (110390) originates from the outbreak of AHC in Malaysia in 2002 to 2003 and was isolated in Hep2 cells (18). New stocks were propagated in NHC cells. 35S-labeled CVA24v virions were propagated as follows. NHC cells were incubated with freshly prepared CVA24v stocks in serum-free medium containing DMEM, HEPES, and PEST. After one hour, unadsorbed virions were removed by washing with phosphate-buffered saline (PBS; Medicago AB, Uppsala, Sweden), and cells were starved of methionine and cysteine in Met/Cys-free medium (Sigma-Aldrich) with 1% FCS (Sigma-Aldrich) for three hours and then incubated with Met/Cys-free medium supplemented with 35S-Met/Cys mixture (NEG-772 Easytag express protein-labeling mix; Perkin-Elmer, Wellesley, MA). Thirty hours after infection, Triton X-100 (Sigma-Aldrich) was added to a final concentration of 0.5%. After 15 min of centrifugation at 3,000 × g, sodium dodecyl sulfate (VWR, Leicestershire, United Kingdom) was mixed with the supernatant to a final concentration of 0.5%. The mixture was laid onto a 30% sucrose solution and centrifuged for 3 h at 113,000 × g at 4°C. The pellets were dissolved in 4 ml of 10 mM Tris-HCl, pH 7.5, and sonicated for 20 s. The mixture was loaded onto a discontinuous gradient of 1.2 and 1.4 g/ml CsCl and centrifuged at 107,000 × g for 17 h at 4°C. The virion band was harvested and desalted on a NAP-10 column (Amersham Biosciences, Uppsala, Sweden) and stored in Tris-buffered saline with 10% glycerol at −80°C until use. Unlabeled virions were propagated essentially as described above except for the 35S-Met/Cys-labeling step, which was omitted.
TABLE 1.
Cell lines used in this study and expression of candidate receptors
| Cell line | Candidate receptor expressiona
|
||||||
|---|---|---|---|---|---|---|---|
| SA | GAG | hCAR | hCD55 | hCD46 | hICAM-1 | Reference | |
| Pro-5 | + | + | − | − | − | − | 55 |
| CHO-CAR | + | + | + | − | − | − | 9 |
| CHO-MOCK | + | + | − | − | − | − | 9 |
| CHO-CD55 | + | + | − | + | − | − | 33 |
| CHO-CD46 | + | + | − | − | + | − | 31 |
| CHO-ICAM-1 | + | + | − | − | − | + | 20 |
| pgsB-618 | + | − | − | − | − | − | 16 |
| Lec2 | − | + | − | − | − | − | 13 |
| HCE | + | + | + | + | + | + | 4 |
| NHC | + | ND | + | + | + | + | 14 |
| A549 | + | ND | + | + | + | +/− | 30 |
| Hep2 | + | ND | + | + | + | − | 37 |
| HeLa | + | ND | + | + | + | +/− | 51 |
| GMK | + | ND | + | + | + | +/− | 21 |
Abbreviations: SA, sialic acid; GAG, glycosaminoglycan; hCAR, human coxsackie-adenovirus receptor; hICAM-1, human intracellular adhesion molecule 1; and ND, not determined.
Binding and binding inhibition assays.
The binding assay was performed essentially as described previously (5). Briefly, adherent cells were detached with PBS containing 0.05% EDTA (PBS-EDTA; Merck, Darmstadt, Germany) and recovered in growth medium for one hour at 37°C. After washing, 2 × 105 cells/sample were incubated with 5,000 35S-labeled CVA24v virions (physical particles) per cell in 100 μl binding buffer (BB) consisting of DMEM, PEST, HEPES (pH 7.4), and 1% bovine serum albumin (Roche AB, Stockholm, Sweden) at 4°C with gentle agitation. One hour later, unbound virions were removed by washing, and the cell-associated radioactivity was measured with a Wallac 1409 scintillation counter (Perkin-Elmer). The experiments were varied in that (i) cells were pretreated with Vibrio cholerae neuraminidase (10 mU/100 μl BB; Sigma Aldrich) and washed, prior to incubation with virions, (ii) cells were preincubated with Sambucus nigra lectin (SNA), Maackia amurensis II lectin (MAA II), or wheat germ agglutinin lectin (WGA) (all from Vector Laboratories, Burlingame, CA) at 20 μg/100 μl in BB prior to incubation with virions, or (iii) virions were preincubated with 13-valent sialic acid-human serum albumin (SA-HSA) (24) in BB prior to incubation with cells. Each experiment was performed at least twice with duplicate samples in each experiment.
Infection and infection inhibition assays.
The infection assay was performed essentially as described previously (5). Briefly, 2 × 105 adherent cells were incubated with various numbers of unlabeled CVA24v virions (physical particles) at 4°C, which allows binding to cells but not internalization. One hour later, unbound virions were removed by washing, and the cells were incubated at 37°C. Sixteen to eighteen hours later, the cells were fixed in 99.5% methanol and stained through incubation with (i) mouse monoclonal antibodies against enterovirus VP1 (DakoCytomation, Glostrup, Denmark) diluted 1:200 in PBS, for one hour at room temperature, and (ii) fluorescein isothiocyanate (FITC)-labeled rabbit anti-mouse immunoglobulin G (DakoCytomation) diluted 1:100 in PBS, for one hour at room temperature. After each incubation, the cells were washed to remove unbound antibodies. Infected cells (i.e., antigen-positive cells) were then quantified with a fluorescence microscope (Axioskop2; Zeiss, Jena, Germany) at ×20 magnification; this was linked to a digital camera (AxioCam MRm; Zeiss) and Axiovision AC software (Zeiss). Counting of infected cells was performed using ImageJ software (downloaded from http://rsb.info.nih.gov/ij/). The experiments were varied in that cells were (i) pretreated with 10 mU V. cholerae neuraminidase in 300 μl PBS and washed, prior to incubation with virions, or (ii) pretreated with 20 μg WGA in 300 μl PBS, prior to incubation with virions. Each experiment was performed at least twice with duplicate samples in each experiment.
Flow cytometry.
NHC, HCE, A549, HeLa, and Hep2 cells were rinsed and harvested with 0.05% EDTA in PBS, counted, and recovered in DMEM containing HEPES (pH 7.4), PEST, and 2% bovine serum albumin, for one hour at 37°C. Cells were then resuspended in PFN buffer (PBS containing 1% FCS and 0.05% NaN3) (from J. T. Baker, Tamro MedLab AB, Mölndal, Sweden) and transferred to 96-well plates with 5 × 105 cells per well, in a volume of 100 μl. The cells were then incubated with PFN containing biotinylated MAA II, SNA, or WGA (1 μg/100 μl) or monoclonal antibodies to CD55 (IF7; diluted 1:200), CD46 (FITC conjugated) (E4.3; 0.5 μg/100 μl; Ancell, Bayport, NM), ICAM-1 (FITC conjugated) (6.5B5; 0.1 μg/100 μl; DakoCytomation), or CAR (RmcB; 1 μg/100 μl; Millipore, Charlottesville, VA) and incubated for one hour on ice. Thereafter, the cells were washed with PFN and (i) in the case of the lectins, incubated for one more hour in the dark with FITC-labeled streptavidin in PFN (0.5 μg/100 μl; DakoCytomation) or (ii) in the case of anti-CD55 and anti-CAR, incubated with FITC-labeled rabbit anti-mouse antibody in PFN (0.1 μg/100 μl; DakoCytomation). The cells were washed with PFN and resuspended in 200 μl PBS per sample, transferred to fluorescence-activated cell sorter tubes (Becton Dickinson, Franklin Lakes, NJ) and then analyzed using a FACScan flow cytometer (Becton Dickinson).
RESULTS
CVA24v requires sialic acid for efficient binding to CHO cells.
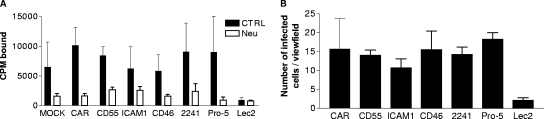
In order to investigate whether any of the previously identified picornavirus and adenovirus receptors can also be used by CVA24v, we first investigated binding of metabolically 35S-labeled CVA24v virions to CHO cell lines that expressed specific receptors in trans or were deficient in endogenous receptor expression, and then we compared these results with binding of CVA24v to ordinary CHO cells. CVA24v virions bound with similar efficiency to the reference CHO cell line Pro-5, which expresses both sialic acid and heparan sulfate, and to CHO cell lines transfected with cDNAs encoding human CAR (CHO-CAR), CD55 (CHO-CD55), CD46 (CHO-CD46), ICAM-1 (CHO-ICAM-1), and also to a CHO cell line that is deficient in synthesis of glycosaminoglycans (pgsB-618) (Fig. 1A; Table 1). However, CVA24v bound much more weakly to Lec2, a CHO cell line that is deficient in sialic acid expression. When sialic acid was removed from cell surfaces by treatment with neuraminidase, the binding of CVA24v virions to all CHO cell lines was reduced by 65 to 85%, except for Lec2 cells, for which the originally low binding was reduced further by 10% only.
FIG. 1.
CVA24v requires cell surface sialic acid for efficient binding to and infection of CHO cells. (A) CHO cells in suspension were first pretreated at 37°C for one hour with or without V. cholerae neuraminidase, incubated with 5,000 35S-labeled CVA24v virions per cell at 4°C for one hour, washed to remove unbound virions, and then analyzed in a scintillation counter for cell-associated radioactivity. CPM, counts per minute. (B) Adherent CHO cells were infected with 20,000 CVA24v virions per cell, fixed in methanol, and stained as described in Materials and Methods. CVA24v antigen-positive cells were then quantified in a fluorescence microscope. The results are presented as means ± standard deviations of duplicate samples from at least three independent experiments (MOCK, CHO-MOCK [mock with respect to CAR]; CAR, CHO-CAR; CD55, CHO-CD55; ICAM1, CHO-ICAM-1; CD46, CHO-CD46; 2241, CHO-2241; Lec2; and Pro-5 [parental cell line to Lec2]).
To test whether any of these molecules is also important for subsequent entry and production of viral proteins, we allowed CVA24v virions to infect the same range of CHO cells and then quantified the numbers of cells positive for CVA24v antigens (Fig. 1B). Similar to the binding experiment, all cells were equally infected (range: 11 to 18 infected cells per viewfield), except for Lec2, where only two infected cells per viewfield were detected. These data suggested that sialic acid allows not only virus binding but also the subsequent steps in infection.
CVA24v efficiently binds to and infects ocular cells.
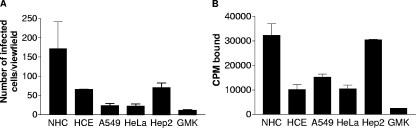
To identify human cell lines that would be suitable for further characterization of CVA24v receptors, we first compared the ability of CVA24v to bind to and infect various epithelial cell lines that have been used previously for successful isolation of CVA24v (HeLa, A549, Hep2, and GMK) and also two additional epithelial cell lines that reflect the pronounced tropism of CVA24v for the eye, NHC cells and HCE cells. Production of CVA24v antigens was most pronounced in NHC cells, followed by HCE and Hep2 cells, suggesting efficient infection of these cell lines (Fig. 2A). Fewer antigens were found in A549, HeLa, and GMK cells, suggesting less-efficient infection of these cells. The relative number of antigen-positive cells correlated reasonably well with the ability of CVA24v to bind to these cells (Fig. 2B). CVA24v bound most efficiently to NHC and Hep2 cells, with intermediate efficiency to A549, HCE, and HeLa cells, and with the least efficiency to GMK cells. In agreement with the ocular and upper respiratory tropism of CVA24v, epithelial cells derived from the conjunctiva (NHC), the cornea (HCE), and the larynx (Hep2) appeared to be most suitable for further studies.
FIG. 2.
CVA24v virions efficiently infect and bind to human ocular epithelial cells. (A) Adherent cells (2 × 105) were incubated with 5,000 CVA24v virions per cell at 4°C for one hour, washed to remove unbound virions, and incubated for 16 to 18 h at 37°C. The cells were fixed with methanol, stained as described in Materials and Methods, and quantified in a fluorescence microscope. (B) Binding experiments were carried out exactly as described for Fig. 1A, but without neuraminidase and with different cells. The results are presented as means ± standard deviations of duplicate samples from at least three independent experiments. CPM, counts per minute.
NHC cells express high amounts of previously recognized virus receptors.
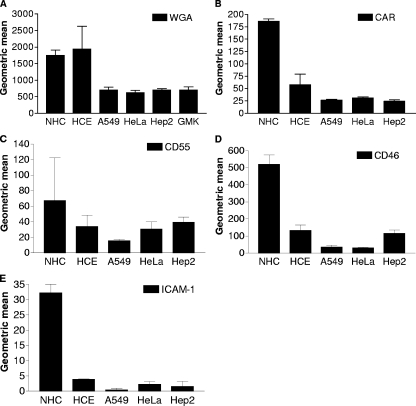
Next, we set out to determine the relative levels of expression of sialic acid, CAR, CD55, CD46, and ICAM-1 on epithelial cell surfaces using flow cytometry (Fig. 3). Sialic acid is a cellular receptor for EV70 on ocular cells (1, 40) and for adenoviruses that cause epidemic keratoconjunctivitis (5, 6). NHC and HCE cells were found to express approximately three to four times as much sialic acid as A549, HeLa, Hep2, and GMK cells, as determined by WGA staining (Fig. 3A). However, since WGA also has affinity for N-acetylglucosamine, it could not be excluded that some of the WGA binding was due to N-acetylglucosamine on cell surfaces. CAR has been suggested to be a cellular receptor for all six coxsackie B viruses (34) and for many, but not all, adenoviruses (47). CAR was expressed in relatively high amounts on NHC cells, in intermediate amounts on HCE cells, and in relatively low amounts on A549, HeLa, and Hep2 cells (Fig. 3B). CD55 and CD46 both belong to the protein family termed regulators of complement activation. CD55 is a receptor for echoviruses, coxsackieviruses, and EV70 on HeLa cells (8, 26, 52, 53), whereas CD46 is a receptor for seven out of nine species B adenoviruses (35). CD55 was found in relatively high amounts on NHC cells, in intermediate amounts on HCE, HeLa, and Hep2 cells, and in the lowest amounts on A549 cells (Fig. 3C), whereas CD46 was expressed in relatively high amounts on NHC cells, in intermediate amounts on HCE and Hep2 cells, and in relatively low amounts on A549 and HeLa cells (Fig. 3D). The only receptor known to be used by other coxsackie A viruses that was included in this study, ICAM-1, was found to be expressed in relatively high amounts on NHC cells but was hardly detectable on the other cell lines (Fig. 3E). Since species-specific monoclonal antibodies were used to detect expression of human CAR, CD55, CD46, and ICAM-1 proteins, the presence of these proteins was not investigated on GMK cells. The candidate receptor sialic acid was expressed in relatively high amounts on NHC and HCE cells. Therefore, these cell lines were included in all subsequent experiments.
FIG. 3.
Flow cytometry analysis of candidate receptors on epithelial cells. Epithelial cells (5 × 105) in suspension were incubated with biotinylated WGA to detect sialic acid, followed by incubation with streptavidin-FITC (A) or with monoclonal antibodies to human CAR (B), human CD55 (C), human CD46 (FITC conjugated) (D), or human ICAM-1 (FITC conjugated) (E), followed by FITC-conjugated anti-antibodies (against CAR or CD55), and analyzed by flow cytometry as described in Materials and Methods. Data represent the geometric means ± standard deviations of the fluorescence measured in three experiments in duplicate.
CVA24v uses sialic acid as a receptor on corneal cells.
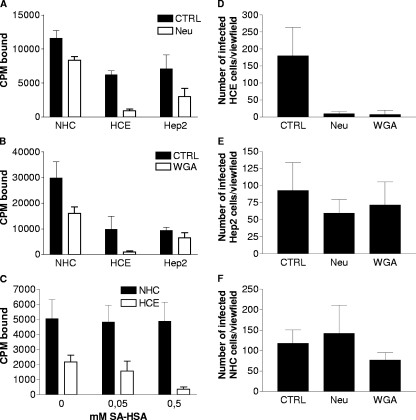
To test whether sialic acid is a receptor for attachment of CVA24v on human epithelial cells, we first treated NHC, HCE, and Hep2 cells with neuraminidase prior to binding of 35S-labeled CVA24v virions. This resulted in a modest (28%) decrease in binding to NHC cells but a strong decrease in binding to HCE cells (85%) and to Hep2 cells (58%) (Fig. 4A). Next, we preincubated cells with WGA, a lectin that binds to both sialic acid and N-acetylglucosamine. Compared to control (without lectin), this resulted in inhibition of binding to NHC cells (46%), HCE cells (93%), and Hep2 cells (30%) (Fig. 4B). Since WGA also binds to N-acetylglucosamine, this molecule was considered to be a candidate receptor for CVA24v. However, up to 100 mM N-acetylglucosamine monosaccharides did not affect CVA24v binding to either NHC or HCE cells (data not shown), suggesting that N-acetylglucosamine is not a receptor for CVA24v and that the inhibitory effect of WGA is due to blocking of sialic acid on the cell surface. In a third type of binding experiment, sialic acid monosaccharides linked to human serum albumin (SA-HSA; 13-valent with respect to sialic acid) did not inhibit binding of CVA24v to NHC cells. However, increasing concentrations of SA-HSA efficiently inhibited binding to HCE cells (Fig. 4C). Similar results were obtained when the effect on CVA24v infection was investigated. Treatment of cells with neuraminidase or incubation of cells with WGA prior to infection with CVA24v resulted in a strong reduction in infection of HCE cells, whereas little or no effect was seen on infection of NHC and Hep2 cells (Fig. 4D to F).
FIG. 4.
Cell surface sialic acid is required for efficient binding of CVA24v to and infection of human ocular cells. Binding was carried out as described for Fig. 1. (A) Cells (2 × 105) in suspension were pretreated with or without neuraminidase from V. cholerae prior to incubation of the cells with 5,000 35S-labeled CVA24v virions per cell. (B) Cells (2 × 105) in suspension were preincubated with or without WGA prior to incubation with 5,000 35S-labeled CVA24v virions per cell. (C) 35S-labeled CVA24v virions (5,000 virions/cell) were preincubated with or without 13-valent sialic acid linked to albumin (SA-HSA) prior to virion-cell incubation (2 × 105 cells/sample; concentrations of SA-HSA are in mM and with respect to sialic acid monosacharrides). (D to F) Adherent HCE (D), NHC (E), or Hep2 (F) epithelial human cell lines were pretreated with or without neuraminidase from V. cholerae or preincubated with or without WGA prior to incubation with virus (500 virions per cell) at 4°C. After one hour, the cells were washed to remove unbound virions and then incubated for 16 to 18 h at 37°C. They were fixed with methanol, stained as described in Materials and Methods, and quantified in a fluorescence microscope. The results are presented as means ± standard deviations of duplicate samples from at least three independent experiments. CPM, counts per minute.
Specific glycosidic bonds are not required for CVA24v binding to NHC and HCE cells.
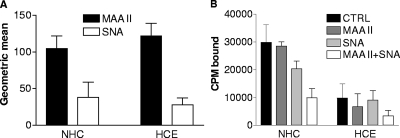
Four other viruses with pronounced ocular tropism, EV70 (40), Ad37 (5), avian influenza A virus H7N7 (23), and Newcastle disease virus (57), all bind more efficiently to sialic acid linked via α2,3 glycosidic bonds to galactose (SAα2,3Gal) than to SAα2,6Gal, suggesting that this could also be the case for CVA24v. To test this, we first set out to compare the relative expression of SAα2,3Gal and SAα2,6Gal on NHC and HCE cells. Using Maackia amurensis lectin (MAA II), which specifically binds SAα2,3Gal (22), and SNA, which specifically binds SAα2,6Gal (54), we found by flow cytometry that MAA II bound more efficiently than SNA to both NHC and HCE cells (Fig. 5), thus indicating that SAα2,3Gal mainly, but also SAα2,6Gal to some extent, is available to CVA24v on these cells. Preincubation of these cells with MAA II or SNA, or both together, prior to CVA24v binding showed that SNA is a slightly more-efficient inhibitor of CVA24v binding to NHC cells but also that neither of the lectins alone efficiently inhibited CVA24v binding to HCE cells. However, when both lectins were incubated together, there was an efficient reduction in binding of CVA24v to both NHC cells (67%) and HCE cells (66%). Taken together, these experiments support the suggestion that sialic acid is a cellular receptor for CVA24v on ocular cells. However, we could not determine whether α2,3 or α2,6 glycosidic bonds between sialic acid and galactose are preferred for CVA24v binding to these cells.
FIG. 5.
Roles of SAα2,3Gal and SAα2,6Gal in binding of CVA24v to human ocular cells. (A) Flow cytometric analysis of expression of SAα2,3Gal (MAA II binding) and SAα2,6Gal (SNA binding) on NHC and HCE cells. Cells (5 × 105) in suspension were first incubated with biotinylated lectins, followed by streptavidin-FITC, prior to flow cytometric analysis. Data represent the geometric means ± standard deviations of the fluorescence measured in three experiments in duplicate. (B) Effect of SAα2,3Gal-binding MAA II and SAα2,3Gal-binding SNA on CVA24v virion binding to NHC and HCE cells. Cells (2 × 105) in suspension were preincubated with lectins prior to incubation with 5,000 35S-labeled CVA24v virions/cell. Cell-associated radioactivity (CPM [counts per minute] bound) was determined by using a scintillation counter. The results are presented as means ± standard deviations of duplicate samples from at least three independent experiments.
The receptor used by CVA24v on NCH and HCE cells has a protein component.
Since sialic acid can be linked to the cell surface through additional saccharides attached either to a protein or to a lipid, we wanted to determine whether the CVA24v receptor is a sialylated glycoprotein or a sialylated glycolipid (i.e., a ganglioside). To test this, we treated NHC and HCE cells with three different proteases. Bromelain and ficin are cysteine proteases; bromelain is relatively nonspecific, cleaving at several different amino acid sites, while ficin cleaves after aromatic amino acids. V8 protease, on the other hand, specifically cleaves polypeptides at Glu or Asp residues. All three proteases efficiently reduced binding of CVA24v to both NHC and HCE cells, indicating that the cellular receptor used by CVA24v on these cells contains a protein component and that this component contains exposed and protease-sensitive cysteine, glutamic acid, and/or aspartic acid residues (Fig. 6). However, on NHC cells, ficin protease was the most efficient, since maximum inhibition was obtained at concentrations as low as ≤2 mU, whereas V8 protease was more efficient than bromelain and ficin on HCE cells, since maximum inhibition was already obtained at ≤2 mU. This suggests that there may be two nonidentical protein components that serve as receptors for CVA24v on corneal and conjunctival cells.
FIG. 6.
Protease treatment of ocular cells inhibits binding of CVA24v virions to NHC and HCE cells. Cells (2 × 105) in suspension were pretreated with bromelain and ficin proteases (which cleave at cysteine residues) or V8 protease (which cleaves at glutamic acid or aspartic acid residues), prior to incubation with 5,000 35S-labeled CVA24v virions/cell. Cell-associated radioactivity (CPM [counts per minute] bound) was determined by using a scintillation counter. Cell viability was assessed with the trypan blue method immediately after proteolytic digestion and no cytotoxic effects mediated by the protease treatment could be seen, compared to untreated control cells. The results are presented as means ± standard deviations of duplicate samples from at least three independent experiments.
DISCUSSION
Members of the Picornaviridae use a broad range of cellular receptors, including CAR, CD55, ICAM-1, CD155 (poliovirus receptor), sialic acid, heparan sulfate, and various integrins (49). Several of these are also used by members of the Adenoviridae. In this study, we investigated whether any of these previously recognized receptors could also be used by CVA24v. Using cells unable to express sialic acid (Lec2), sialic acid-blocking lectins, soluble sialic acid-containing molecules, and enzymes (neuraminidase) that remove cell surface sialic acid, we have demonstrated that sialic acid is a major cellular receptor for CVA24v on CHO cells and on human epithelial cells of corneal origin. On epithelial cells derived from human conjunctiva and larynx, sialic acid contributes to some extent to viral binding and infection, but alone, it is insufficient to mediate efficient binding and infection. Treatment of NHC and HCE cells with three different proteases resulted in a strong inhibition of the binding of CVA24v to ocular cells, which suggests that the cellular receptor(s) used by CVA24v on these cells contains at least one and probably two different protein components, thus excluding gangliosides as candidate receptors for CVA24v.
Replication of CVA24v takes place in palpebral and bulbar conjunctiva and also in corneal epithelial cells (63). Replication of a virus in the cornea leads to destruction of cells and is followed by punctate epithelial keratitis, which is thought to be the cause of foreign body sensation (64). This symptom is reported as being predominant in most outbreaks of AHC (∼80% of all cases) (12, 18, 25). Thus, replication in corneal cells contributes considerably to the clinical picture of AHC, so the use of sialic acid as a cellular receptor by CVA24v in the cornea is likely to affect the severity of AHC. On conjunctival cells, however, the role of sialic acid needs to be investigated further, and usage of additional receptors or coreceptors cannot be excluded.
In several cases, it has been demonstrated that viruses that bind to sialic acid show a preference for either SAα2,3Gal or SAα2,6Gal (42) and that this is largely associated with the tropism of the virus. With few exceptions, the rule of thumb has been that respiratory viruses, such as human influenza A, bind to SAα2,6Gal and ocular viruses, including EV70, bind to SAα2,3Gal (40, 42). With this in mind, we first found it somewhat surprising that CVA24v was not inhibited more efficiently by SAα2,3Ga-specific MAA II lectin. However, unlike EV70, which is rarely or never associated with respiratory disease, CVA24v is frequently associated with respiratory disease (28, 64, 66-68). Conjunctival secretions and transmission via direct contact are likely routes of transmission, but respiratory transmission may also occur (67, 68) and may even explain the rapid and extensive spread of AHC during outbreaks (67). Thus, the dual tropism of CVA24v for eyes and airways matches its capacity to bind to both SAα2,3Gal and SAα2,6Gal.
Immunity to AHC decreases considerably within as little as seven years (3). Accordingly, loss of herd immunity has been suggested to contribute to widespread transmission of AHC (2). The epidemic and pandemic potential of AHC-causing picornaviruses matches only one other viral disease: influenza (66). From the present work, we can conclude that one other feature that these viruses have in common is their usage of sialic acid as a cellular receptor. With few exceptions (50), avian influenza A viruses, including the highly pathogenic H5 and H7 subtypes, bind mainly to SAα2,3Gal, whereas human influenza A viruses bind only to SAα2,6Gal (56). Conjunctivitis seems to be a predominant symptom in zoonotic cases or outbreaks caused by H7 subtypes (17), and zoonotic cases caused by H5N1 strains have also been associated with conjunctivitis (11, 43, 61). When isolated from humans, however, H5N1 strains tend to have adapted to using both SAα2,3Gal and SAα2,6Gal (65). We conclude that usage of sialic acid as a receptor is a conserved feature among several oculo-respiratory viruses with pandemic potential. Further studies of the receptors used by these viruses may help us on the road to fully understanding the determinants of virus tropism and potentially also the development and establishment of pandemics caused by these viruses.
Acknowledgments
We thank Jeffrey M. Bergelson for the generous gift of CHO-CAR and CHO-DAF cells and antibodies to DAF (IF7), John P. Atkinson for the generous gift of CHO-CD46 cells, and Magnus Evander for the generous gift of psgB-618 cells. We also thank our colleagues Ken Dimock and Mark Pallansch for critically reading the manuscript.
This work was supported by the Swedish Research Council (grants no. 2003-6008 and 2004-6174), the Kempe Foundation, the Swedish Society for Medical Research, the Swedish Society of Medicine, the Jeansson Foundation, the Sven and Dagmar Salén Foundation, and the Petrus and Augusta Hedlund Foundation.
The authors declare that there are no conflicts of interest in this study.
Footnotes
Published ahead of print on 9 January 2008.
REFERENCES
- 1.Alexander, D. A., and K. Dimock. 2002. Sialic acid functions in enterovirus 70 binding and infection. J. Virol. 7611265-11272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso-Echanove, J., Y. García-Guadalupe, P. Rullán, M. A. Pallansch, F. Alvarado-Ramy, and B. Cauthen. 2004. Acute hemorrhagic conjunctivitis outbreak caused by coxsackievirus A24—Puerto Rico, 2003. Morb. Mortal. Wkly. Rep. 53632-634. [PubMed] [Google Scholar]
- 3.Aoki, K., and H. Sawada. 1992. Long-term observation of neutralization antibody after enterovirus 70 infection. Jpn. J. Ophthalmol. 36465-468. [PubMed] [Google Scholar]
- 4.Araki-Sasaki, K., K. Y. Ohasi, T. Sasabe, K. Hayashi, H. Watanabe, Y. Tano, and H. Handa. 1995. An SV-40-immortalized human corneal epithelial cell line and its characterization. Investig. Ophthalmol. 36614-621. [PubMed] [Google Scholar]
- 5.Arnberg, N., K. Edlund, A. H. Kidd, and G. Wadell. 2000. Adenovirus type 37 uses sialic acid as a cellular receptor. J. Virol. 7442-48. [PMC free article] [PubMed] [Google Scholar]
- 6.Arnberg, N., A. H. Kidd, K. Edlund, F. Olfat, and G. Wadell. 2000. Initial interactions of subgenus D adenoviruses with A549 cellular receptors: sialic acid versus αv integrins. J. Virol. 747691-7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahri, O., D. Rezig, B. B. Nejma-Oueslati, A. B. Yahia, J. B. Sassi, N. Hogga, A. Sadraoui, and H. Triki. 2005. Enteroviruses in Tunisia: virological surveillance over 12 years (1992-2003). J. Med. Microbiol. 5463-69. [DOI] [PubMed] [Google Scholar]
- 8.Bergelson, J. M., M. Chan, K. R. Solomon, N. F. St. John, H. Lin, and R. W. Finberg. 1994. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc. Natl. Acad. Sci. USA 916245-6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 2751320-1323. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee, S., C. O. Quarcoopome, and A. Apenteng. 1970. Unusual type of epidemic conjunctivitis in Ghana. Br. J. Ophthalmol. 54628-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi, S., and T. Tsang. 1998. An update on influenza A H5N1 in Hong Kong. Public Health Epidemiol. Bull. 71-8. [Google Scholar]
- 12.Chou, M. Y., and M. D. Malison. 1988. Outbreak of acute hemorrhagic conjunctivitis due to coxsackie A24 variant—Taiwan. Am. J. Epidemiol. 127795-800. [DOI] [PubMed] [Google Scholar]
- 13.Deutscher, S. L., N. Nuwayhid, P. Stanley, E. I. Briles, and C. B. Hirschberg. 1984. Translocation across Golgi vesicle membranes: a CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell 39295-299. [DOI] [PubMed] [Google Scholar]
- 14.Diebold, Y., M. Calonge, A. E. de Salamanca, S. Callejo, R. M. Corrales, V. Saez, K. F. Siemasko, and M. E. Stern. 2003. Characterization of a spontaneously immortalized cell line (IOBA-NHC) from normal human conjunctiva. Investig. Ophthalmol. Vis. Sci. 444263-4274. [DOI] [PubMed] [Google Scholar]
- 15.Dussart, P., G. Cartet, P. Huguet, N. Leveque, C. Hajjar, J. Morvan, J. Vanderkerckhove, K. Ferret, B. Lina, J. J. Chomel, and H. Norder. 2005. Outbreak of acute hemorrhagic conjunctivitis in French Guiana and West Indies caused by coxsackievirus A24 variant: phylogenetic analysis reveals Asian import. J. Med. Virol. 75559-565. [DOI] [PubMed] [Google Scholar]
- 16.Esko, J. D., J. L. Weinke, W. H. Taylor, G. Ekborg, L. Roden, G. Anantharamaiah, and A. Gawish. 1987. Inhibition of chondroitin and heparan sulfate biosynthesis in Chinese hamster ovary cell mutants defective in galactosyltransferase I. J. Biol. Chem. 26212189-12195. [PubMed] [Google Scholar]
- 17.Fouchier, R. A., P. M. Schneeberger, F. W. Rozendaal, J. M. Broekman, S. A. Kemink, V. Munster, T. Kuiken, G. F. Rimmelzwaan, M. Schutten, G. J. Van Doornum, G. Koch, A. Bosman, M. Koopmans, and A. D. Osterhaus. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. USA 1011356-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghazali, O., K. B. Chua, K. P. Ng, P. S. Hooi, M. A. Pallansch, M. S. Oberste, K. H. Chua, and J. W. Mak. 2003. An outbreak of acute haemorrhagic conjunctivitis in Melaka, Malaysia. Singapore Med. J. 44511-516. [PubMed] [Google Scholar]
- 19.Gopalkrishna, V., P. R. Patil, R. M. Kolhapure, H. Bilaiya, P. V. Fulmali, and R. P. Deolankar. 2007. Outbreak of acute hemorrhagic conjunctivitis in Maharashtra and Gujarat states of India, caused by coxsackie virus A-24 variant. J. Med. Virol. 79748-753. [DOI] [PubMed] [Google Scholar]
- 20.Hasler, T., G. R. Albrecht, M. R. Van Schravendijk, J. C. Aguiar, K. E. Morehead, B. L. Pasloske, C. Ma, J. W. Barnwell, B. Greenwood, and R. J. Howard. 1993. An improved microassay for Plasmodium falciparum cytoadherence using stable transformants of Chinese hamster ovary cells expressing CD36 or intercellular adhesion molecule-1. Am. J. Trop. Med. Hyg. 48332-347. [DOI] [PubMed] [Google Scholar]
- 21.Horta-Barbosa, L., and J. Warren. 1969. Comparative sensitivity of tissue cultures to rubella virus: use of guinea pig cells for virus titration. Appl. Microbiol. 18251-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imberty, A., C. Gautier, J. Lescar, S. Perez, L. Wyns, and R. Loris. 2000. An unusual carbohydrate binding site revealed by the structures of two Maackia amurensis lectins complexed with sialic acid-containing oligosaccharides. J. Biol. Chem. 27517541-17548. [DOI] [PubMed] [Google Scholar]
- 23.Ito, T., Y. Suzuki, L. Mitnaul, A. Vines, H. Kida, and Y. Kawaoka. 1997. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology 227493-499. [DOI] [PubMed] [Google Scholar]
- 24.Johansson, S. M., E. C. Nilsson, M. Elofsson, N. Ahlskog, J. Kihlberg, and N. Arnberg. 2007. Multivalent sialic acid conjugates inhibit adenovirus type 37 from binding to and infecting human corneal epithelial cells. Antivir. Res. 7392-100. [DOI] [PubMed] [Google Scholar]
- 25.Karki, D. B., C. D. Shrestha, and S. Shrestha. 2003. Acute haemorrhagic conjunctivitis: an epidemic in August/September 2003. Kathmandu Univ. Med. J. 1234-236. [PubMed] [Google Scholar]
- 26.Karnauchow, T. M., D. L. Tolson, B. A. Harrison, E. Altman, D. M. Lublin, and K. Dimock. 1996. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55). J. Virol. 705143-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kew, O. M., B. K. Nottay, M. H. Hatch, J. C. Hierholzer, and J. F. Obijeski. 1983. Oligonucleotide fingerprint analysis of enterovirus 70 isolates from the 1980 to 1981 pandemic of acute hemorrhagic conjunctivitis: evidence for a close genetic relationship among Asian and American strains. Infect. Immun. 41631-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosrirukvongs, P., R. Kanyok, S. Sitritantikorn, and C. Wasi. 1996. Acute hemorrhagic conjunctivitis outbreak in Thailand, 1992. Southeast Asian J. Trop. Med. Public Health 27244-249. [PubMed] [Google Scholar]
- 29.Leveque, N., I. L. Amine, G. Cartet, A. B. Hammani, Y. C. Khazraji, B. Lina, J. J. Muyembe, H. Norder, and J. J. Chomel. 2007. Two outbreaks of acute hemorrhagic conjunctivitis in Africa due to genotype III coxsackievirus A24 variant. Eur. J. Clin. Microbiol. Infect. Dis. 26199-202. [DOI] [PubMed] [Google Scholar]
- 30.Lieber, M., B. Smith, A. Szakal, W. Nelson-Rees, and G. Todaro. 1976. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int. J. Cancer 1762-70. [DOI] [PubMed] [Google Scholar]
- 31.Liszewski, M. K., and J. P. Atkinson. 1996. Membrane cofactor protein (MCP; CD46). Isoforms differ in protection against the classical pathway of complement. J. Immunol. 1564415-4421. [PubMed] [Google Scholar]
- 32.Lonberg-Holm, K., R. L. Crowell, and L. Philipson. 1976. Unrelated animal viruses share receptors. Nature 259679-681. [DOI] [PubMed] [Google Scholar]
- 33.Lublin, D. M., and K. E. Coyne. 1991. Phospholipid-anchored and transmembrane versions of either decay-accelerating factor or membrane cofactor protein show equal efficiency in protection from complement-mediated cell damage. J. Exp. Med. 17435-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martino, T. A., M. Petric, H. Weingartl, J. M. Bergelson, M. A. Opavsky, C. D. Richardson, J. F. Modlin, R. W. Finberg, K. C. Kain, N. Willis, C. J. Gauntt, and P. P. Liu. 2000. The coxsackie-adenovirus receptor (CAR) is used by reference strains and clinical isolates representing all six serotypes of coxsackievirus group B and by swine vesicular disease virus. Virology 27199-108. [DOI] [PubMed] [Google Scholar]
- 35.Marttila, M., D. Persson, D. Gustafsson, M. K. Liszewski, J. P. Atkinson, G. Wadell, and N. Arnberg. 2005. CD46 is a cellular receptor for all species B adenoviruses except types 3 and 7. J. Virol. 7914429-14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirkovic, R. R., R. Kono, M. Yin-Murphy, R. Sohier, N. J. Schmidt, and J. L. Melnick. 1973. Enterovirus type 70: the etiologic agent of pandemic acute haemorrhagic conjunctivitis. Bull. W. H. O. 49341-346. [PMC free article] [PubMed] [Google Scholar]
- 37.Moore, A. E., L. Sabachewsky, and H. W. Toolan. 1955. Culture characteristics of four permanent lines of human cancer cells. Cancer Res. 15598-602. [PubMed] [Google Scholar]
- 38.Moura, F. E., D. C. Ribeiro, N. Gurgel, A. C. da Silva Mendes, F. N. Tavares, C. N. Timoteo, and E. E. da Silva. 2006. Acute haemorrhagic conjunctivitis outbreak in the city of Fortaleza, northeast Brazil. Br. J. Ophthalmol. 901091-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newcombe, N. G., P. Andersson, E. S. Johansson, G. G. Au, A. M. Lindberg, R. D. Barry, and D. R. Shafren. 2003. Cellular receptor interactions of C-cluster human group A coxsackieviruses. J. Gen. Virol. 843041-3050. [DOI] [PubMed] [Google Scholar]
- 40.Nokhbeh, M. R., S. Hazra, D. A. Alexander, A. Khan, M. McAllister, E. J. Suuronen, M. Griffith, and K. Dimock. 2005. Enterovirus 70 binds to different glycoconjugates containing α2,3-linked sialic acid on different cell lines. J. Virol. 797087-7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh, M. D., S. Park, Y. Choi, H. Kim, K. Lee, W. Park, Y. Yoo, E. C. Kim, and K. Choe. 2003. Acute hemorrhagic conjunctivitis caused by coxsackievirus A24 variant, South Korea, 2002. Emerg. Infect. Dis. 91010-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olofsson, S., U. Kumlin, K. Dimock, and N. Arnberg. 2005. Avian influenza and sialic acid receptors: more than meets the eye? Lancet Infect. Dis. 5184-188. [DOI] [PubMed] [Google Scholar]
- 43.Oner, A. F., A. Bay, S. Arslan, H. Akdeniz, H. A. Sahin, Y. Cesur, S. Epcacan, N. Yilmaz, I. Deger, B. Kizilyildiz, H. Karsen, and M. Ceyhan. 2006. Avian influenza A (H5N1) infection in eastern Turkey in 2006. N. Engl. J. Med. 3552179-2185. [DOI] [PubMed] [Google Scholar]
- 44.Palacios, G., and M. S. Oberste. 2005. Enteroviruses as agents of emerging infectious diseases. J. Neurovirol. 11424-433. [DOI] [PubMed] [Google Scholar]
- 45.Pan American Health Organization. 2003. Hemorrhagic conjunctivitis in Central America and the Caribbean. EID Weekly Updates. http://www.paho.org/english/ad/dpc/cd/eid-eer-25-sep-2003.htm/.
- 46.Park, K., K. Lee, J. Lee, S. Yeo, S. Lee, D. S. Cheon, W. Choi, J. Ahn, S. Kim, and Y. Jee. 2006. Acute hemorrhagic conjunctivitis epidemic caused by coxsackievirus A24 variants in Korea during 2002-2003. J. Med. Virol. 7891-97. [DOI] [PubMed] [Google Scholar]
- 47.Roelvink, P. W., A. Lizonova, J. G. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 727909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roivainen, M., L. Piirainen, T. Hovi, I. Virtanen, T. Riikonen, J. Heino, and T. Hyypia. 1994. Entry of coxsackievirus A9 into host cells: specific interactions with alpha v beta 3 integrin, the vitronectin receptor. Virology 203357-365. [DOI] [PubMed] [Google Scholar]
- 49.Rossmann, M. G., Y. He, and R. J. Kuhn. 2002. Picornavirus-receptor interactions. Trends Microbiol. 10324-331. [DOI] [PubMed] [Google Scholar]
- 50.Saito, T., W. Lim, T. Suzuki, Y. Suzuki, H. Kida, S. I. Nishimura, and M. Tashiro. 2001.Characterization of a human H9N2 influenza virus isolated in Hong Kong. Vaccine 20125-133. [DOI] [PubMed] [Google Scholar]
- 51.Scherer, W. F., J. T. Syverton, and G. O. Gey. 1953. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J. Exp. Med. 97695-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shafren, D. R., R. C. Bates, M. V. Agrez, R. L. Herd, G. F. Burns, and R. D. Barry. 1995. Coxsackieviruses B1, B3, and B5 use decay accelerating factor as a receptor for cell attachment. J. Virol. 693873-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shafren, D. R., D. J. Dorahy, R. A. Ingham, G. F. Burns, and R. D. Barry. 1997. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J. Virol. 714736-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shibuya, N., I. J. Goldstein, W. F. Broekaert, M. Nsimba-Lubaki, B. Peeters, and W. J. Peumans. 1987. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2-6)Gal/GalNAc sequence. J. Biol. Chem. 2621596-1601. [PubMed] [Google Scholar]
- 55.Stanley, P., V. Caillibot, and L. Siminovitch. 1975. Selection and characterization of eight phenotypically distinct lines of lectin-resistant Chinese hamster ovary cell. Cell 6121-128. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki, Y. 2005. Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses. Biol. Pharm. Bull. 28399-408. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki, Y., T. Suzuki, M. Matsunaga, and M. Matsumoto. 1985. Gangliosides as paramyxovirus receptor. Structural requirement of sialo-oligosaccharides in receptors for hemagglutinating virus of Japan (Sendai virus) and Newcastle disease virus. J. Biochem. 971189-1199. [DOI] [PubMed] [Google Scholar]
- 58.Tavares, F. N., E. V. Costa, S. S. Oliveira, C. C. Nicolai, M. Baran, and E. E. da Silva. 2006. Acute hemorrhagic conjunctivitis and coxsackievirus A24v, Rio de Janeiro, Brazil, 2004. Emerg. Infect. Dis. 12495-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Triantafilou, K., D. Fradelizi, K. Wilson, and M. Triantafilou. 2002. GRP78, a coreceptor for coxsackievirus A9, interacts with major histocompatibility complex class I molecules which mediate virus internalization. J. Virol. 76633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Triki, H., D. Rezig, O. Bahri, N. Ben Ayed, A. Ben Yahia, A. Sadraoui, and S. Ayed. 2007. Molecular characterisation of a coxsackievirus A24 that caused an outbreak of acute haemorrhagic conjunctivitis, Tunisia 2003. Clin. Microbiol. Infect. 13176-182. [DOI] [PubMed] [Google Scholar]
- 61.Van Borm, S., I. Thomas, G. Hanquet, B. Lambrecht, M. Boschmans, G. Dupont, M. Decaestecker, R. Snacken, and T. van den Berg. 2005. Highly pathogenic H5N1 influenza virus in smuggled Thai eagles, Belgium. Emerg. Infect. Dis. 11702-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams, C. H., T. Kajander, T. Hyypia, T. Jackson, D. Sheppard, and G. Stanway. 2004. Integrin αvβ6 is an RGD-dependent receptor for coxsackievirus A9. J. Virol. 786967-6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wright, P. F., G. Neumann, and Y. Kawaoka. 2007. Orthomyxoviruses, p. 1691-1740. In D. M. Knipe and P. M. Howley (ed.), Fields Virology, 5th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 64.Wright, P. W., G. H. Strauss, and M. P. Langford. 1992. Acute hemorrhagic conjunctivitis. Am. Fam. Physician 45173-178. [PubMed] [Google Scholar]
- 65.Yamada, S., Y. Suzuki, T. Suzuki, M. Q. Le, C. A. Nidom, Y. Sakai-Tagawa, Y. Muramoto, M. Ito, M. Kiso, T. Horimoto, K. Shinya, T. Sawada, M. Kiso, T. Usui, T. Murata, Y. Lin, A. Hay, L. F. Haire, D. J. Stevens, R. J. Russell, S. J. Gamblin, J. J. Skehel, and Y. Kawaoka. 2006. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 444378-382. [DOI] [PubMed] [Google Scholar]
- 66.Yin-Murphy, M., Baharuddin-Ishak, M. C. Phoon, and V. T. Chow. 1986. A recent epidemic of Coxsackie virus type A24 acute haemorrhagic conjunctivitis in Singapore. Br. J. Ophthalmol. 70869-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yin-Murphy, M., K. T. Goh, M. C. Phoon, J. Yao, and Baharuddin-Ishak. 1993. A recent epidemic of acute hemorrhagic conjunctivitis. Am. J. Ophthalmol. 116212-217. [DOI] [PubMed] [Google Scholar]
- 68.Yin-Murphy, M., K. H. Lim, and Y. M. Ho. 1976. A coxsackievirus type A24 epidemic of acute conjunctivitis. Southeast Asian J. Trop. Med. Public Health 71-5. [PubMed] [Google Scholar]
- 69.Zhang, Y., and J. M. Bergelson. 2005. Adenovirus receptors. J. Virol. 7912125-12131. [DOI] [PMC free article] [PubMed] [Google Scholar]