Abstract
Alterations of T-cell receptor signaling by human immunodeficiency virus type 1 (HIV-1) Nef involve its association with a highly active subpopulation of p21-activated kinase 2 (PAK2) within a dynamic signalosome assembled in detergent-insoluble membrane microdomains. Nef-PAK2 complexes contain the GTPases Rac and Cdc42 as well as a factor providing guanine nucleotide exchange factor (GEF) activity for Rac/Cdc42. However, the identity of this GEF has remained controversial. Previous studies suggested the association of Nef with at least three independent GEFs, Vav, DOCK2/ELMO1, and βPix. Here we used a broad panel of approaches to address which of these GEFs is involved in the functional interaction of Nef with PAK2 activity. Biochemical fractionation and confocal microscopy revealed that Nef recruits Vav1, but not DOCK2/ELMO1 or βPix, to membrane microdomains. Transient RNAi knockdown, analysis of cell lines defective for expression of Vav1 or DOCK2 as well as use of a βPix binding-deficient PAK2 variant confirmed a role for Vav1 but not DOCK2 or βPix in Nef's association with PAK2 activity. Nef-mediated microdomain recruitment of Vav1 occurred independently of the Src homology 3 domain binding PxxP motif, which is known to connect Nef to many cellular signaling processes. Instead, a recently described protein interaction surface surrounding Nef residue F195 was identified as critical for Nef-mediated raft recruitment of Vav1. These results identify Vav1 as a relevant component of the Nef-PAK2 signalosome and provide a molecular basis for the role of F195 in formation of a catalytically active Nef-PAK2 complex.
The Nef protein of human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) is a multifunctional factor that is critical for high virus titers in vivo. Consequently, disease progression in individuals infected with nef-deficient viruses is very slow or absent (16, 30, 32). These effects are thought to mirror several independent activities of Nef that prevent immune recognition of virally infected cells and boost HIV replication (18, 36, 57, 72). In order to achieve such an optimized spread in the infected host, Nef manipulates a variety of transport and signal transduction processes in cells infected by HIV type 1 (HIV-1). Modulation of cellular transport pathways by Nef affects the surface presentation of a large number of cell surface receptors, such as CD4, major histocompatibility complex class I and II molecules, and chemokine receptors, to prevent superinfection and to facilitate immune evasion of productively infected cells (8, 15, 22, 45, 65, 70). Equally widespread are Nef effects on host cell signaling, including various alterations of the T-cell receptor (TCR) cascade in T lymphocytes (6, 7, 40, 43, 44, 59, 61, 64, 66, 68). Since resting T lymphocytes are largely resistant to productive HIV infection, activation of target T lymphocytes is a prerequisite for efficient spread of HIV-1 in vivo (reviewed in reference 69). At the same time, high activation levels induced by potent extracellular stimulation drive cells into activation-induced cell death. According to an emerging view, Nef acts as an intracellular inducer of TCR-triggered distal signaling events in the absence of exogenous stimulation, while signaling via TCR from outside of the cell is tuned down by Nef (21, 27, 62, 71; see reference 17 for a review). By balancing these mechanisms, Nef may thus ensure both target cell permissiveness and survival for optimal virus production.
Nef alters host cell processes by interacting with components of cellular machines. Previous studies have identified a number of protein interaction surfaces in Nef that are vital for specific activities of the viral protein (3, 4, 23). Since an increasing number of host cell factors that interact with each of these Nef interfaces have been reported, the functional relevance of individual ligands have remained largely elusive. Regarding its signal transduction properties, Nef is known to associate with a number of cellular kinases, including members of the protein kinase C, Src tyrosine, and p21-activated kinase (PAK) kinase families (reviewed in reference 56). In particular, the association of Nef with PAK has been studied in more detail, demonstrating that PAK2 is the preferred PAK isoform that associates with Nef (5, 19, 54). The interaction of Nef with PAK2 activity is conserved for many Nef proteins derived from HIV-1, HIV-2, and SIV strains (50, 51, 60) and is readily detectable in HIV-1-infected T lymphocytes as well as HIV-1 provirus transgenic mice (59, 75). Many Nef alleles also augment overall cellular PAK2 activity, but PAK2 activation is less well conserved and dispensable for the association of Nef with PAK2 activity (51). Some studies using Nef mutants that fail to associate with PAK2 activity but are also defective in other Nef functions concluded that the Nef-PAK2 association may contribute to the elevated pathogenic potential of Nef-positive viruses in vivo (31, 61, 75). These reports are at odds with a similar study in which viruses encoding a Nef mutant with disruption of an interaction motif critical for Nef's association with PAK2 activity caused acute disease (35). If relevant for the pathogenic potential of lentiviruses, the underlying mechanism of the Nef-PAK2 association is not well understood but might involve established consequences such as upregulation of HIV transcription, remodeling of the actin cytoskeleton, prevention of apoptosis, and enhancement of virion infectivity (11, 19, 20, 27, 40, 76, 77). Thus, as for most protein interactions of Nef, the exact role and relevance of the Nef-PAK2 complex for virus propagation in the infected host remain to be defined.
The Nef-PAK2 association occurs in the context of a larger signalosome of approximately 1 MDa in size (19). Assembly of this complex occurs at cellular membranes, where it selectively segregates in detergent-resistant microdomains of the plasma membrane, and disruption of these microdomains potently blocks Nef's association with PAK2 activity (34, 51, 53). The association of Nef with the kinase is therefore mediated selectively by a small subpopulation that associates with these microdomains (24, 34, 51). While it has been demonstrated that Nef recruits PAK2 to membrane microdomains similarly to the physiological PAK2 activators Rac1 and CDC42 (34, 51, 53), further analysis of the composition of the Nef-PAK2 complex and its regulation has been hampered by its low stability. Although Nef-associated PAK2 activity can readily be demonstrated experimentally, Western blot detection of PAK2 protein in Nef immunoprecipitates has been challenging (2, 40, 48, 51, 59, 61). This may reflect the specific association of Nef with a highly active PAK2 subpopulation whose activity triggers rapid disassembly of the complex (51, 55).
More detailed information is available on the determinants in Nef that govern assembly of the Nef-PAK2 signalosome. Consistent with its membrane microdomain localization, Nef's N-terminal myristioylation, a prerequisite for its association with membranes, is essential for the functional interaction with PAK2 (60). Earlier studies also identified a di-arginine motif as a critical determinant. Mutation of this motif, however, has pleiotropic effects, including decreased protein stability, and it has thus been questioned whether this interface is actively involved in Nef-PAK2 complex formation (23, 49, 60). Recently, a novel protein interaction surface in Nef surrounding the key residue F195 was identified as essential for the association of Nef with active PAK2 (1, 2, 49). One study suggested that this interface facilitates the recruitment of PAK2 into the complex; the molecular basis for this role of F195, however, has not been addressed in more detail (2). Finally, Nef contains a highly conserved, proline-rich Src homology 3 domain binding (PxxPxR) motif that represents another key determinant for assembly of an enzymatically active Nef-PAK2 complex (31, 42, 76). Since functional Nef-PAK2 association depends on the activity of the upstream small GTPases Rac1 and Cdc42 (40, 48), it has been assumed that an SH3-mediated interaction by Nef directly or indirectly recruits a guanine nucleotide exchange factor (GEF) to ensure PAK2 activity within the complex. The identity of this GEF, however, has remained a matter of debate. With Vav1, βPix/Cool, and DOCK2/ELMO1, at least three GEFs containing an SH3 domain and providing GEF activity toward Cdc42 and/or Rac were reported to associate with Nef (9, 20, 28, 67). Vav1 was previously suggested as a critical component for the functional association of Nef and PAK2; this was based, however, exclusively on overexpression of a dominant-negative variant (20). Conflicting data exist on the role of βPix in the Nef-PAK2 signalosome (9, 55, 75), and DOCK2/ELMO1 has not been analyzed with respect to Nef's association with active PAK2 (28). To address which of these GEFs play functional roles in the Nef-PAK2 complex, their recruitment into membrane microdomains by Nef and the effects of reduced GEF expression levels upon specific RNA interference (RNAi) or genetic knockout on the efficiency of the Nef-PAK2 association were used in this study as independent criteria. Our results demonstrate the involvement of Vav1 in the association of Nef with PAK2 activity and unexpectedly reveal that this GEF is recruited into the complex via the F195 surface rather than the PxxPxR motif.
MATERIALS AND METHODS
Cells, reagents, and plasmids.
Jurkat Tag (JTag), JVav, and BEα16-3 cells were cultivated in RPMI 1640 supplemented with 10% fetal calf serum, 1% l-glutamine, and 1% penicillin-streptomycin (all from Invitrogen). JVav and BEα16-3 cells are Jurkat derivatives and T-cell hybridoma cells that lack Vav1 or DOCK2 expression (12, 58), respectively, and were kindly provided by Daniel Billadeau and Yoshinori Fukui. Expression constructs for Vav1.myc, green fluorescent protein (GFP), various Nef.GFP proteins, PAK2, and βPix binding-deficient Pak2 proteins were described elsewhere (13, 20, 25, 27, 34). Nef from HIV-1 SF2 was used throughout. Constructs for expression of Nef.GFP carrying the F195A or F195I mutation were generated by site-directed mutagenesis (QuikChange site-directed mutagenesis kit; Stratagene), and the Nef-coding sequences were verified by sequencing. Expression plasmids for βPix.myc, Flag.DOCK2, and ELMO1.His were kindly provided by Ivan Dikic, Shinya Tanaka, and Yoshinori Fukui, respectively (47, 58, 63).
The following antibodies were used: polyclonal rabbit anti-ELMO1, polyclonal rabbit anti-Vav2, and monoclonal mouse anti-myc (clone 9E10) (all obtained from Santa Cruz Biotechnology); monoclonal mouse anti-flag (clone M2), monoclonal mouse anti-GFP (clone GFP20), and polyclonal mouse anti-cholera toxin (anti-CTx) (all from Sigma-Aldrich); polyclonal rabbit anti-PAK1/2/3 and polyclonal rabbit anti-Vav (both from Cell Signaling Technology); polyclonal rabbit anti-βPIX and monoclonal mouse anti-His6 (clone BMG-His-1) (Roche); polyclonal rabbit anti-linker of activated T cells (LAT) (Upstate Biotechnology); and monoclonal mouse anti-transferrin receptor (anti-Tfr) (clone H68.4) (Zymed Laboratories, Inc.). Secondary fluorescent antibodies and Alexa Fluor 555-conjugated CTx subunit B were obtained from Molecular Probes, and protease inhibitor cocktail was purchased from Sigma. Polyclonal rabbit serum against GFP was kindly provided by Hans-Georg Kräusslich, and polyclonal sheep serum against Nef was a kind gift from Mark Harris (14).
Western blotting.
For Western blot analysis, samples were boiled in sodium dodecyl sulfate (SDS) sample buffer, separated by 10% SDS-polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane. Protein detection was performed following incubation with appropriate first and secondary antibodies using the Super Signal Pico detection kit (Pierce) according to the manufacturer's instructions.
IVKA.
Nef-associated PAK2 activity was analyzed in an in vitro kinase reaction following immunoprecipitation of Nef.GFP essentially as described previously (24, 34). Cells were transfected with the respective plasmids and RNAi oligonucleotides via electroporation and incubated for 24 h (BEα16-3 and JVav cells), 48 h (PAK1 RNAi), or 72 h (all other RNAi constructs). For this, 15 to 50 μg DNA and 500 pmol of RNAi specific for the targeted sequences, or unspecific as a control, were added to 1 × 107 cells in 4-mm cuvettes, and electroporation took place at 250 V (JTAg and JVav) or 230 V (BEα16-3), 950 μF, in a GenePulser Xcell (Bio-Rad). Sequences targeted by RNAi were 5′ UCU GUA UAC ACA CGG UCU GTT 3′ for PAK1, 5′ AGA AGG AAC UGA UCA UUA ATT 3′ for PAK2, 5′ CGU CGA GGU CAA GCA CAU UTT 3′ for Vav1, 5′ AGU CCG GUC CAU AGU CAA CTT 3′ for Vav2, 5′ ACC ACU GUC UGC AAU AAU ATT 3′ for ELMO1, 5′ GGA ACG ACA UCU ACA UUA CTT 3′ for DOCK2, 5′ GGA UGA AGU UCA AGA AUU ATT 3′ for βPix, and 5′ AGG UAG UGU AAU CGC CUU GTT 3′ as an unspecific control (all from MWG-Biotech). Cells were lysed in KEB (137 mM NaCl, 50 mM Tris HCl [pH 8], 2 mM EDTA, 0.5% Nonidet P-40, Na3VO4, and protease inhibitors) and subjected to immunoprecipitation using rabbit anti-GFP serum as described (27). After extensive washing in KEB, beads were resuspended in 50 μl KAB (50 mM HEPES [pH 8], 150 mM NaCl, 5 mM EDTA, 10 mM MgCl2, 0.02% Triton X-100). Treatment with 10 μCi [γ-32P]ATP (5 min, room temperature [RT]) allowed the detection of PAK2 autophosphorylation. Following extensive washing in KEB, in vitro kinase assay (IVKA) reaction products were separated by SDS-polyacrylamide gel electrophoresis and blotted on a nitrocellulose membrane. Radioactive signals were visualized and quantified with a PhosphorImager (Bio-Rad). Immunoisolated proteins were detected by Western analysis using anti-GFP antibodies and quantified using the QuantityOne software (Bio-Rad). Radioactive signals were normalized against the amount of isolated Nef in the Western blot, and signals of wild-type (wt) Nef.GFP were arbitrarily set to 100%. Statistical significance was determined by Student's t test.
Isolation of DRMs.
Detergent-resistant membranes (DRMs) were isolated by flotation experiments as described previously (34). In brief, JTAg cells were transfected via electroporation as described for the IVKA and incubated for 48 h. A total of 4 × 107 cells were transfected for each sample. Cells were lysed in 400 μl TXNE buffer (1% Triton X-100, 50 mM Tris HCl [pH 7.4], 150 mM NaCl, 5 mM EDTA) for 20 min on ice, homogenized with a Dounce homogenizer, and loaded on an Optiprep (Life Technologies) gradient (lysates were adjusted to 40% Optiprep and overlaid with 2.5 ml 28% Optiprep and 600 μl TXNE), followed by ultracentrifuged (35 000 rpm, 3 h, 4°C). Eight fractions of 500 μl each were collected from the top. For further analysis, fraction 2, which represented the DRM fraction, and fraction 8, which was indicative of the soluble fraction, were used in a Western blot. The quality of the flotation was addressed by using Tfr (excluded from DRMs) and LAT (incorporated in DRMs).
CTx clustering.
The generation of CTx-positive clusters (CTx clusters) was performed as described before (34). A total of 1 × 107 JTAg cells were transfected via electroporation and incubated with 25 μg of Alexa 555-conjugated CTx/ml in 0.1% bovine serum albumin-phosphate-buffered saline for 30 min at 4°C, followed by addition of anti-CTx antibody (1:200; 30 min at 4°C and 10 min at 37°C). Cells were bound to poly-l-lysine-coated coverslips (5 min, RT), fixed with 3% paraformaldehyde (10 min, RT), and extracted with 0.1% Triton X-100 (1 min, RT). Cells were stained using the respective anti-tag antibodies (1:50 anti-His and 1:500 anti-flag and anti-myc for 1 h), followed by Alexa 660-conjugated secondary antibody (1:2,000 for 30 min at RT), and were mounted in Histomount (Linaris). Samples were analyzed in a confocal laser scanning microscope (LSM 510; Zeiss) using a 100× oil immersion objective lens. Images were processed with Adobe Photoshop.
RESULTS
Nef-mediated recruitment of Vav1 to membrane microdomains.
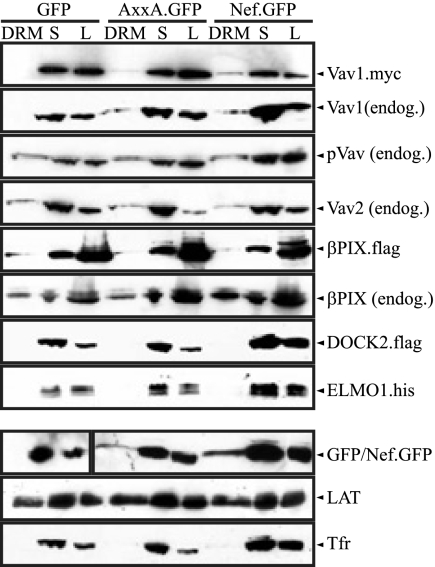
The aim of this study was to evaluate the relative involvement of three cellular GEFs, Vav, βPix, and DOCK2/ELMO1, which have been reported to interact functionally or physically with the Nef/PAK2 complex. Since the Nef-PAK2 association is confined exclusively to the detergent-insoluble fraction in microdomain flotation experiments and microdomain association of Nef correlates with its ability to associate with PAK2 (24, 34, 51), we used the presence in such microdomains as the first criterion to define a GEF relevant for the Nef-PAK2 complex. In order to allow parallel biochemical and microscopic analyses, Nef.GFP fusion protein from the HIV-1 strain SF2 was used. This fusion protein has previously been shown to be functionally equivalent to native Nef (24, 27, 34). Using previously validated experimental procedures (24, 34), microdomain association was first assessed by flotation analysis of DRMs after Triton X-100-based lysis at 4°C of transiently transfected JTag T lymphocytes. Effects of Nef on GEF segregation were assessed by comparing cells expressing Nef.GFP, a GFP control, or a Nef.GFP mutant protein that fails to associate with active PAK2 due to disruption of its SH3 domain-interacting motif (AxxA.GFP). In addition to expression plasmids for GFP/Nef.GFP, either these cells were cotransfected with expression constructs for epitope-tagged GEFs or endogenous GEFs were analyzed. Following microdomain flotation, equal volumes of DRM and soluble fractions as well as total cell lysate were analyzed by Western blotting (Fig. 1). Exclusion of Tfr from the DRM fraction and the nearly equal distribution of LAT between the DRM and soluble fractions served as quality controls for the DRM isolation procedure.
FIG. 1.
Analysis of Nef-mediated DRM recruitment upon transient expression of cellular GEFs. Membrane microdomain flotation analysis from JTag T lymphocytes transiently expressing different Nef.GFP fusion proteins or GFP is shown. Cell lysates (1% Triton X-100) were separated by Optiprep gradient ultracentrifugation, and eight fractions were collected from the top (fraction 1) to the bottom (fraction 8) of the gradient. The DRM (fraction 2) and the soluble fraction (S) (fraction 8) were analyzed together with the unfractionated cell lysate (L) by Western blotting for the distribution of GFP/Nef.GFP and the GEF. TfR and LAT were analyzed as markers for S and DRM fractions, respectively. For DRM and S, 4% of each fraction was loaded; L corresponds to 1% of the total lysate. When indicated, expression constructs of epitope tagged GEFs were cotransfected with GFP/Nef.GFP. The results presented are representative of at least four independent experiments.
As expected, soluble GFP was excluded from DRMs while Nef.GFP was detected in small but significant amounts in DRMs. In comparison, the AxxA.GFP mutant was expressed at somewhat reduced levels, as previously noted (13). While these lower levels of expression resulted in relatively small absolute amounts of DRM association, the distributions between the DRM and soluble fractions were approximately similar for Nef.GFP and AxxA.GFP. Coexpressed Vav1 was undetectable in DRMs in GFP-expressing control cells. In contrast, detectable amounts of the GEF were present in DRMs upon coexpression of Nef.GFP, suggesting recruitment of Vav1 by Nef. Consistent results were obtained when endogenous Vav1 was analyzed. Surprisingly, Vav1 was also enriched in DRMs in the presence of AxxA.GFP. Indeed, when taking the reduced relative expression levels and specific DRM localization of this Nef mutant into account, DRM recruitment by AxxA.GFP occurred essentially with efficiencies comparable to those by Nef.GFP. In contrast, the analogous analysis for βPix, Vav2, and tyrosine-phosphorylated, active Vav (pVav) revealed the presence of a minor fraction of these proteins in DRMs already in the presence of GFP. This residual DRM association remained unaltered by coexpression of Nef.GFP or its AxxA mutant. The distribution of DOCK2/ELMO1 was analyzed upon coexpression of both units of the bipartite GEF and revealed its virtual absence from DRMs irrespective of whether GFP or Nef.GFP was coexpressed. Together, these results were compatible with potential roles of Vav1 and/or βPix in the Nef-PAK2 complex but did not support an involvement of DOCK2/ELMO1.
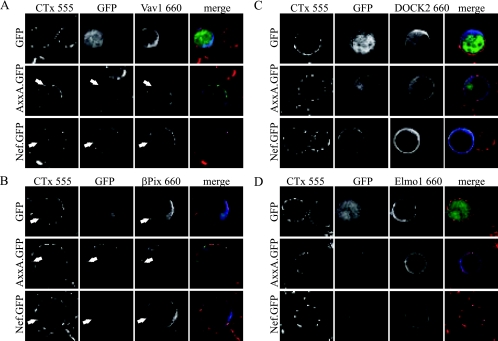
Vav1 is targeted to plasma membrane microdomains by Nef.
To verify the above results by an independent experimental approach, plasma membrane microdomains were visualized in parallel by confocal microscopy via clustering of fluorescently labeled CTx (24, 34) (Fig. 2). While Vav1 was found diffusely distributed in the cytoplasm of GFP-expressing JTag T lymphocytes (Fig. 2A), the presence of Nef.GFP and AxxA.GFP led to a marked redistribution of Vav1 to the plasma membrane. Both Nef.GFP variants localized to punctuate structures at the plasma membrane as well as intracellular perinuclear membranes that were detectable in only some z-sections of the cells and likely represent endosomal compartments (27, 41, 71). The Nef-positive plasma membrane punctae partially colocalized with CTx clusters, identifying them as membrane microdomains. In typically two to three of these clusters per optical section, Nef.GFP and Vav1 were found to colocalize. Thus, Nef triggers plasma membrane recruitment of Vav1 and leads to some incorporation of the GEF in plasma membrane microdomains. Coexpression of βPix did not have appreciable effects on the localization of Nef.GFP (Fig. 2B). Likewise, this GEF was found in the cytoplasm as well as at the plasma membrane, where it sometimes accumulated at CTx-positive clusters. This localization remained unaltered in the presence of Nef, and both proteins were occasionally found to colocalize in membrane microdomains, albeit less frequently than Nef and Vav1. Finally, DOCK2 (Fig. 2C) and ELMO1 (Fig. 2D) were found diffusely in the cytoplasm without marked plasma membrane enrichment or microdomain localization irrespective of coexpression of GFP or Nef.GFP. This independent analysis therefore confirmed a possible role of Vav1 and βPix in signaling events initiated by Nef from membrane microdomains.
FIG. 2.
Analysis of Nef-mediated recruitment of cellular GEFs into plasma membrane microdomains. Aliquots of the transfected JTag T lymphocytes analyzed in Fig. 1 were subjected to raft clustering by incubation with CTx conjugated with Alexa 555 fluorescent dye and subsequently cross-linked with anti-CTx antibody. The GEFs were stained with anti-tag antibodies and detected with Alexa 660 coupled to the appropriate secondary antibodies. Cells were analyzed by confocal microscopy, and single representative sections are presented. The merge panels depict the overlay of all three fluorescence channels, with GFP/Nef.GFP in green, CTx in red, and the respective GEF in blue. Arrows indicate colocalization of Nef with a GEF in plasma membrane microdomains. The results presented are representative of at least three independent experiments.
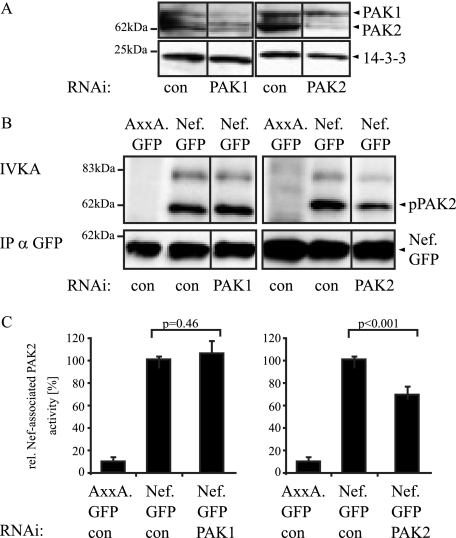
Nef-associated kinase activity following PAK knockdown.
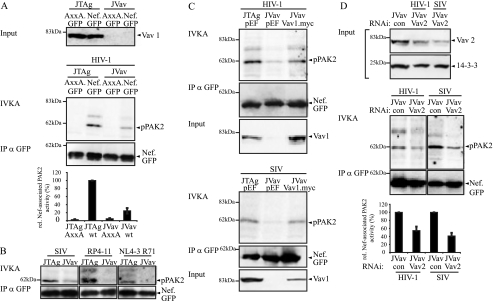
We next sought to address the role of the three GEFs more directly using RNAi-mediated reduction of protein levels. To this end, we first estimated the range of inhibition of PAK enzymatic activity that can be achieved by RNAi targeted against the kinase itself. Furthermore, while PAK2 has been identified as the preferred PAK isoform associated with Nef, some reports also suggested the involvement of PAK1 (19, 46). We therefore compared the effects of RNAi against these two kinases on Nef-associated PAK activity. As shown in Fig. 3A, RNAi oligonucleotides directed against target sequences in PAK1 or PAK2, respectively, effectively and specifically reduced the protein levels of the respective PAKs (knockdown efficiency as quantified by Western blotting: PAK1, 88% ± 15%; PAK2, 94% ± 10%). Following immunoprecipitation of various Nef.GFP fusion proteins and subsequent IVKA reaction, Nef-associated phosphorylated PAK2 was visualized by autoradiography (Fig. 3B). When an irrelevant RNAi oligonucleotide was used, Nef.GFP associated with robust PAK activity resulting in PAK autophosphorylation (62 kDa, pPAK2) as well as phosphorylation of an 80-kDa substrate. As expected, AxxA.GFP failed to associate with detectable kinase activity. The substantial reduction of PAK1 expression did not result in diminished Nef-associated PAK activity, whereas treatment with the PAK2-specific RNAi oligonucleotide caused about 35% inhibition of Nef-associated kinase activity. When the Nef-associated PAK activity from five experiments was quantified by phosphorimager analysis relative to the amounts of immunoisolated Nef.GFP, PAK2 RNAi reduced the kinase reaction to 65% ± 19% (P = 0.001), while PAK1 RNAi had no effect. Consistently, PAK2 but not PAK1 could be detected in anti-Nef immunoprecipitates by using highly sensitive luminometric detection of PAK-luciferase fusion proteins (data not shown). These results suggest that PAK1 does not contribute to Nef-associated kinase activity in our experimental system and indicate that PAK2 protein still present in the RNAi-targeted cells (approximately 6% of the levels in control cells) can be efficiently recruited by Nef to give rise to a relatively high (65% of control) residual Nef-associated kinase activity.
FIG. 3.
Effect of RNAi knockdown of PAK1 and PAK2 on Nef-associated PAK activity. (A) JTag T lymphocytes were transfected with small interfering RNA oligonucleotides specific for PAK1 or PAK2 or a nonspecific control oligonucleotide (con) and analyzed by Western blotting for PAK1 and PAK2 expression levels at 48 and 72 h posttransfection, respectively. 14-3-3 was used as a loading control. (B) Analysis of Nef-associated PAK activity following PAK1 and PAK2 knockdown. JTag T lymphocytes were transfected with small interfering RNA oligonucleotides specific for PAK1 or PAK2 or the control oligonucleotide together with an expression plasmid for Nef.GFP or the AxxA.GFP mutant, and an IVKA following anti-GFP immunoprecipitation (IP) was performed at 48 (PAK1) or 72 (PAK2) hours posttransfection. Nef-associated PAK activity is revealed by the phosphorylated 62-kDa band (IVKA, pPAK2). (C) Quantification of the Nef-associated PAK activity. Intensities of autophosphorylated PAK2 signals were quantified relative to the amounts of immunoisolated Nef.GFP. The relative associated PAK activity for Nef.GFP in the presence of a control oligonucleotide was arbitrarily set to 100%. Data are means ± standard deviations from at least five independent experiments. Statistical significance is indicated by the P values derived from Student's t test analysis. wt, wild type.
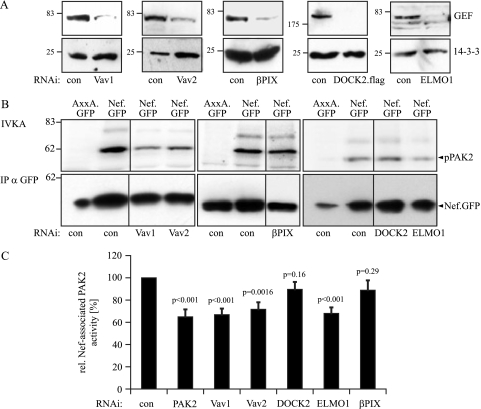
RNAi knockdown of Nef-associated GEFs.
Having determined the degree of inhibition of Nef-associated PAK2 activity that can be achieved by direct RNAi knockdown of PAK2 itself, we next tested the effect of knockdown for the GEFs previously implicated in Nef-associated protein assemblies. RNAi against Vav1, Vav2, βPix, and ELMO1 caused a marked reduction in the expression levels of the corresponding endogenous proteins (knockdown efficiency as quantified by Western blotting: Vav1, 86% ± 15%; Vav2, 76% ± 10%; DOCK2, 97% ± 5%; ELMO, 85% ± 9%; βPix, 86% ± 10%). Potent silencing of DOCK2 protein expression was confirmed using a transfected vector expressing an epitope-tagged version of DOCK2 (Fig. 4A). IVKA analysis revealed a significant reduction of Nef-associated PAK2 activity upon Vav1 RNAi treatment. The magnitude of this effect was comparable to that obtained with direct knockdown of PAK2 (reduction to 68% ± 20% for Vav1 and 65% ± 19% for PAK2). RNAi against Vav2 and ELMO1 (reduction to 71% ± 20% and 67% ± 15%, respectively) also had statistically significant effects on the Nef-PAK2 association (Fig. 4B and C; P values indicate statistical significance in comparison to reaction after treatment with RNAi control oligonucleotides). Knockdown of DOCK2 and βPix caused no significant alterations in Nef-associated PAK2 activity (reduction to 89% ± 20% and 88% ± 25%). These results further suggested a critical role for Vav1 in the Nef-PAK2 complex and argued against the involvement of DOCK2 and βPix.
FIG. 4.
Effect of RNAi knockdown of cellular GEFs on Nef-associated PAK2 activity. (A) Western blot analysis of JTag cells transfected with small interfering RNA oligonucleotides specific for the indicated GEFs or a nonspecific control oligonucleotide (con) for GEF expression levels, as in Fig. 3. Note that endogenous protein levels were analyzed, except in the case of DOCK2, where an expression plasmid for DOCK2.flag was cotransfected with the RNAi oligonucleotides. (B) Nef-associated PAK2 activity following GEF knockdown. JTag T lymphocytes transfected with GEF-specific small interfering RNA or the control oligonucleotide together with the indicated Nef.GFP expression plasmids were subjected to IVKA following anti-GFP immunoprecipitation (IP). (C) Quantification of the Nef-associated PAK2 activity following GEF knockdown. Intensities of autophosphorylated PAK2 signals were quantified relative to the amounts of immunoisolated Nef.GFP. The relative associated PAK2 activity for Nef.GFP was arbitrarily set to 100%. Data are means ± standard errors of the means from at least five independent experiments. Statistical significance is indicated by the P values derived from Student's t test analysis. wt, wild type.
Impairment of Nef-PAK2 association in Vav1-deficient T lymphocytes.
Because inhibition of Nef-associated PAK2 activity was only partial upon RNAi-mediated reduction of Vav protein levels, we next turned to the JVav cell line, a Jurkat T-lymphocyte derivative that lacks Vav1 expression due to genetic knockout (12). Consistent with the previous RNAi experiments, the Nef protein from HIV-1 SF2 associated up to fourfold less efficiently with PAK2 activity in the absence than in the presence of Vav1 (Fig. 5A, compare JVav with JTag). Although with various degree of reduction in Nef-associated PAK2 activity in the absence of Vav1, the involvement of Vav1 in Nef-PAK2 association was also conserved for SIVmac239 Nef, the T71R mutant of Nef from HIV-1 NL4-3, and the patient derived HIV-1 Nef variant RP4-11 (Fig. 5B). To verify that this reduction in Nef-associated PAK2 activity was really due to the lack of Vav1, a myc-tagged version of Vav1 was coexpressed with Nef in JVav cells (Fig. 5C). Under conditions where expression levels of overexpressed Vav1.myc were in the range of endogenous Vav1 in JTag cells, Nef-PAK2 association was markedly enhanced, reaching levels that almost matched those in parental JTag cells. Since Nef can associate with Vav2 as well as with Vav1 (20), we next tested whether the residual Nef-PAK2 association in JVav cells was due to the presence of Vav2 and performed RNAi knockdown experiments targeting Vav2 (Fig. 5D). As seen before in JTag cells, Vav2 knockdown was only partial in JVav cells; however, it reduced the residual Nef-associated PAK2 activity (to 54% ± 15% and 41% ± 20% for HIV-1 SF2 and SIVmac239 Nef, respectively). Together these results support an important role of Vav1 in the Nef-PAK2 complex and suggest that Vav2 can substitute for the Vav1 isoform.
FIG. 5.
Vav1 deficiency reduces Nef-PAK2 association in T lymphocytes. (A) IVKA for Nef-associated PAK2 activity in JTag and Vav1-deficient JVav T lymphocytes. IVKA reactions were performed for Nef from HIV-1 SF2. Vav1 expression levels are shown in the upper panels; the middle panel depicts PAK2 autophosphorylation levels and amounts of immunoisolated Nef. The graphs present relative levels of Nef-associated PAK2 activity, with values for wild-type (wt) Nef in JTag cells set to 100%. Data are means ± standard errors of the means from three independent experiments. IP, immunoprecipitation. (B) IVKA reaction and Western blot analysis for immunoisolated Nef from SIVmac239, HIV-1 RP4-11, and HIV-1 NL4-3. T71R, HIV-1 RP4-11, and SIVmac239. (C) Rescue of HIV-1 SF2 Nef-associated PAK2 activity in JVav cells by Vav1 overexpression. Shown are the IVKA reaction, amounts of immunoisolated Nef, and Vav1 expression levels. (D) Residual Nef-associated PAK2 activity in JVav cells is sensitive to RNAi against Vav2. JVav cells were treated with the indicated RNAi oligonucleotides, and knockdown efficiency was evaluated by Western blotting (upper panel). From the same lysates, an IVKA was performed (middle panel). The bottom panel presents the quantification of Nef-associated PAK2 activity, with values for wt Nef in JVav cells treated with a control oligonucleotide set to 100%. Data are means ± standard errors of the means from three independent experiments.
DOCK2 expression and βPix binding of PAK2 are dispensable for the association of Nef with PAK2 activity.
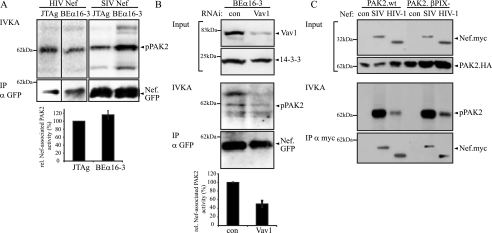
Following the genetic knockout approach, the role of DOCK2 in the association of Nef with PAK2 activity was tested using DOCK2−/− BEα16-3 T-cell hybridoma cells (58). No significant difference was observed in the PAK2 association of Nef proteins from HIV-1 and SIV between BEα16-3 and JTag cells (Fig. 6A), strongly suggesting that DOCK2 is dispensable for Nef-PAK2 association. Of note, reduction of Vav1 expression using RNAi in BEα16-3 cells caused a twofold reduction of Nef-PAK2 association (Fig. 6B). Experiments on a βPix−/− genetic background were precluded due to the lack of a βPix knockout cell line. Since βPix directly binds to PAK2 (29), we tested instead whether a PAK2 mutant carrying a mutation in the βPix binding site would be able to associate with Nef in its catalytically active form. As presented in Fig. 6C, association with PAK2 activity was equally efficient for HIV-1 as well as SIV Nef in the presence of wt and βPix binding-deficient PAK2 variants. In conjunction with the RNAi experiments, these results suggested that βPix is dispensable for the association of Nef with active PAK2. Taking together analyses on GEF membrane microdomain recruitment, GEF RNAi, and the use of GEF knockout cell lines, we conclude that among the three GEFs previously associated with Nef function, only Vav1 fulfils the criteria for a GEF with a major role in the Nef-PAK2 complex.
FIG. 6.
Nef-PAK2 association is functional in the absence of DOCK2 or the presence of βPix binding-deficient PAK2. (A) IVKA for Nef-associated PAK2 activity in JTag and DOCK2-deficient BEα16-3 cells. The IVKA reaction for Nef from HIV-1 SF2 and SIVmac239 and amounts of immunoisolated Nef are shown in the upper panels; the lower panel depicts relative levels of HIV-1 and SIV Nef-associated PAK2 activity, with values for wild-type (wt) Nef in JTag cells set to 100%. Data are means ± standard errors of the means from four independent experiments. IP, immunoprecipitation. (B) Nef-associated PAK2 activity is reduced upon Vav1 knockdown. BEα16-3 cells were treated with the indicated RNAi oligonucleotides. Knockdown efficiency was evaluated by Western blotting (upper panels). IVKA analysis and quantification of amounts of immunoisolated Nef.GFP are presented in the middle panels. The bottom panel presents the quantification of Nef-associated PAK2 activity, with values for Nef in BEα16-3 cells treated with a control oligonucleotide set to 100%. Data are means ± standard errors of the means from three independent experiments. (C) βPix binding is dispensable for functional Nef-PAK2 association. PAK2 association was analyzed for Nef from HIV-1 SF2 and SIVmac239 in the presence of wt or βPix binding-deficient PAK2. From top to bottom, the panels show expression levels of Nef and PAK2, the IVKA reaction, and the amounts of immunoisolated Nef.
Residue 195 is critical for the Nef-PAK2 association and Vav1 recruitment.
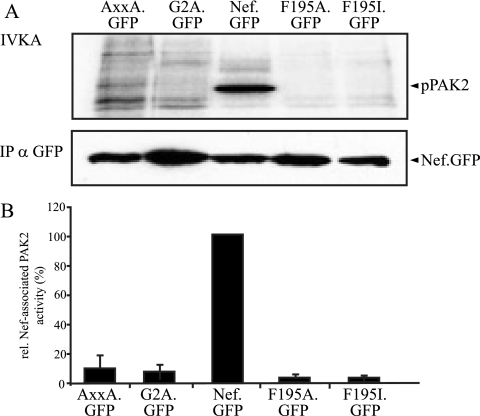
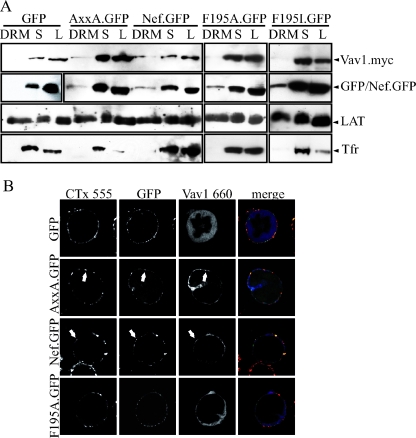
The identification of the SH3 domain-containing Vav1 protein as the relevant GEF in the Nef-PAK2 complex was at odds with the fact that its recruitment into membrane microdomains occurred independently of the highly conserved SH3 binding (PxxP) motif of Nef. Recent studies identified a novel protein interaction surface involving residue F195 in Nef that is required for the Nef-PAK2 association (2, 49). We therefore tested whether this surface is involved in the recruitment of Vav1 by Nef and created expression vectors for F195A and F195I Nef.GFP proteins. Compared to wt Nef, both mutants displayed less than 5% of Nef-associated PAK2 activity and scored lower than the AxxA.GFP or the membrane binding-deficient G2A.GFP controls (Fig. 7). DRM flotation experiments and confocal microscopy revealed that this lack of kinase association was not a consequence of exclusion of the F195 mutants from membrane microdomains (Fig. 8A) or other mislocalization in T lymphocytes (Fig. 8B). Rather, the association with DRM fractions appeared to be slightly elevated relative to Nef.GFP. In sharp contrast, both Nef variants with mutations at position F195 failed to recruit Vav1 into DRMs. Consistently, F195A.GFP (Fig. 8B) and F195I.GFP (data not shown) were defective in targeting Vav1 to the T-lymphocyte plasma membrane and did not induce colocalization of the GEF with CTx membrane microdomains. We conclude that the F195 protein interaction surface is a critical determinant for the membrane microdomain recruitment of Vav1 and thus for the association of Nef with PAK2 activity.
FIG. 7.
F195 is critical for the association of Nef.GFP with PAK2 activity. (A) IVKA analysis from JTag T lymphocytes transiently expressing the indicated Nef.GFP fusion proteins. IP, immunoprecipitation. (B) Quantification of PAK2 activity associated with the Nef.GFP variants analyzed in panel A. The relative associated PAK2 activity for Nef.GFP was arbitrarily set to 100%. Data are means ± standard deviations from at least three independent experiments. wt, wild type.
FIG. 8.
Role of F195 in microdomain recruitment of Vav by Nef. (A) Western blot analysis of DRM flotation from JTag T lymphocytes transiently expressing the indicated Nef.GFP fusion proteins or GFP together with myc-tagged Vav1 (see legend to Fig. 1 for details). (B) Raft clustering analysis of the JTag T lymphocytes analyzed in panel A. Cells were analyzed by confocal microscopy, and single representative sections are presented (see legend to Fig. 2 for details). The merge panel depicts the overlay of all three fluorescence channels, with GFP/Nef.GFP in green, CTx in red, and Vav1 in blue. Arrows indicate colocalization of Nef with Vav1 in plasma membrane microdomains. The results presented are representative of at least three independent experiments.
DISCUSSION
The composition of the Nef-PAK2 complex has remained controversial largely due to its low stability and the concomitant difficulties in detecting associated proteins directly rather than based on their activity. In a first step toward understanding the organization and regulation of this signalosome, we addressed here which of the reported Nef-associated cellular GEFs plays a role in the association of Nef with PAK2 activity. Microdomain recruitment, subcellular localization, GEF-specific RNAi, genetic knockout cells, and GEF binding-deficient PAK2 variants were applied as independent assays and tools to answer this question. Taken together, the results reveal a role for Vav1 in the association of Nef with PAK2 activity and argue that βPix and DOCK2 are dispensable for this Nef function. Surprisingly however, a recently described protein interface surrounding F195 rather than the SH3 binding PxxPxR motif in Nef mediates the recruitment of Vav1 into the signalosome.
Since each individual experimental strategy used here to dissect the role of cellular GEFs in functional Nef-PAK2 association had its specific limitations, it is important to consider the combined interpretation of the independently obtained results. The isolation of DRMs demonstrated Nef-mediated recruitment of Vav1 but not DOCK2/ELMO1, while βPix was found to be DRM resident with no detectable alteration upon Nef expression. Due to possible artifacts during detergent treatment, these results were compared with microdomain cluster colocalization. Besides providing overall confirmation of the biochemical analyses, this approach also allowed us to appreciate the specific recruitment of Vav1 by Nef to the plasma membrane on a single-cell level, which was overall more pronounced than the microdomain targeting judged by biochemical criteria. The lack of detection of DOCK2/ELMO1 in DRMs or CTx clusters in Nef-expressing cells argued against a functional role of this bipartite GEF for the Nef-PAK2 association; however, the catalytic relevance of undetectable protein amounts could not be excluded. However, since the recruitment of other catalytic components of the Nef-PAK2 complex, such as PAK2, Rac1, and Cdc42, can readily be demonstrated (34), a critical involvement of DOCK2/ELMO1 seemed unlikely. The microdomain analysis also did not yield information on the functional relevance of membrane microdomain-associated GEFs and could thus not differentiate between the roles of Vav and βPix. GEF-specific RNAi was therefore used in further analyses. Knockdown of PAK1 and PAK2 established the dynamic range that could be expected for this approach: despite reduction of PAK2 expression to less than 10% of the levels in control cells, only an approximately 30% decrease in Nef-associated PAK activity was observed. Similar difficulties in functional knockdowns of cellular enzymes, including PAK2, have been encountered by other investigators (37-39, 73) and possibly reflect cells' ability to maintain a relatively constant pool of active enzymes even upon significant reduction of overall enzyme abundance. Alternatively, and not mutually exclusive, these results could also indicate the presence of a PAK2 phosphorylating activity in the complex other than just PAK2 itself. These findings are in agreement with the finding that Nef specifically associates with a small but highly active PAK2 subpopulation (51, 55). Providing specificity to these results, similarly efficient knockdown of PAK1 expression did not affect Nef-associated PAK activity. In a previous study we had concluded that PAK1 can serve as a Nef-associated kinase based on the inhibitory effect of an interfering PAK1 fragment (19). This fragment was, however, subsequently shown to also interfere with the activity of PAK2. Use of specific antibodies and sensitivity to caspase cleavage then identified PAK2 as the Nef-associated kinase (5, 54). The RNAi data presented here confirm that PAK2 is the relevant Nef-associated PAK isoform in JTag T lymphocytes by an independent experimental approach. Within the limits of this incomplete inhibition of Nef-associated PAK2 activity, efficient knockdown of βPix and DOCK2 had no significant effect on Nef's association with PAK2 activity. Consistently, binding of βPix to PAK2 was fully dispensable for efficient association of Nef with PAK2 activity. More directly, BEα16-3 cells allowed us to verify the involvement of DOCK2 in a cellular environment of genetic DOCK2 deficiency. The results clearly demonstrated that DOCK2 expression is dispensable for the association of Nef with PAK2 activity. Thus, βPix and DOCK2 could be excluded as constituting limiting factors in the Nef-PAK2 complex. In contrast, Vav1, and to a lesser extent Vav2, were identified as relevant for functional Nef-PAK2 interactions, with the effects of Vav1 knockdown being as pronounced as knockdown of the kinase itself. Analysis of T lymphocytes devoid of Vav1 expression confirmed a critical role of Vav1 in Nef's association with PAK2 activity. Importantly, Nef-PAK2 association was rescued in these cells by reintroduction of Vav1, and residual Nef-PAK2 association was sensitive to expression levels of Vav2. Together with the microdomain analysis, these results demonstrate a functional role for Vav1 and Vav2 but not DOCK2 or βPix in the Nef-PAK2 complex.
DOCK2/ELMO1 associate with Nef in a PxxP-dependent manner and have been implicated in the inhibition of T-lymphocyte chemotaxis by the viral protein (28). This effect likely depends on the exchange activity of this GEF for Rac1, which is exerted only in the context of a direct interaction of both subunits of this bipartite GEF (10, 58). Since DOCK2 was found to be entirely dispensable for the association of Nef with active PAK2, this GEF appears not be involved in this function of Nef. In line with these findings, Janardhan and colleagues did not detect PAK2 or Vav1 proteins in their Nef-associated DOCK2-ELMO1-Rac complex (28). Together, these results suggest that the association of Nef with PAK2 and with DOCK2/ELMO1 occurs in the context of two independent protein complexes. Thus, Nef, via its PxxPxR motif, assembles multiple protein complexes in T lymphocytes that differ in abundance, composition, and possibly subcellular localization to modulate select effector functions. In contrast to DOCK2, however, knockdown of ELMO1 partially affected the functional Nef-PAK2 association, suggesting that ELMO1 can affect Nef-PAK2 independently of DOCK2. As ELMO1 was undetectable in Nef-positive microdomains, we favor the idea that these effects are indirect consequences of reduced ELMO1 expression levels, possibly reflecting the regulation of Rac signaling pathways by ELMO1 in conjunction with DOCK family members other than DOCK2 (26).
Regarding the role of βPix in the Nef-PAK2 complex, it was previously reported that this GEF is physically present in the complex (75) and serves as a substrate of Nef-associated active PAK2 (9). On the other hand, we show here that the βPix-PAK2 interaction is dispensable for Nef's ability to associate with active PAK2. Together these results suggest that βPix, possibly facilitated by its constitutive microdomain association and recruited via its interaction with PAK2, associates with the Nef-PAK2 complex as a peripheral complex component which does not contribute to PAK2 activity levels in this context. Rather, βPix might act as a downstream effector of Nef-PAK2 signalosomes. In line with such a model, βPix was recently shown to associate with Nef in membrane microdomains to regulate the activity of the ubiquitin ligase c-Cbl (67).
Vav1 is the only GEF tested here that was recruited to membrane microdomains by Nef and whose expression levels directly correlated with the efficiency of the association of Nef with PAK2 activity. These results thus identify endogenously expressed Vav1 as a critical component for the function of the Nef-PAK2 complex, thereby confirming and extending previous reports on the interaction of Nef with this particular GEF (20, 52, 74). Although not experimentally addressed here, this role most likely involves its GEF activity toward Rac1 and Cdc42, which, in their GTP bound state, ensure activity of PAK2 in the complex prior to its disassembly. Nef was previously shown to interact directly with the C-terminal SH3 domain of Vav1 in vitro via its PxxPxR motif, and the association of Nef and Vav1 in cells was also shown to depend on these interaction surfaces (20, 52, 74). These results suggested that Vav1 might be recruited into the Nef-PAK2 complex via this interaction. Unexpectedly, however, we find here that this recruitment occurs via the F195 patch rather than the Nef SH3 binding motif. In addition to Vav1, the association of PAK2 with Nef also depends on the F195 interaction surface (2). These data do not allow us to identify the direct interaction partner of this Nef interface, which could represent either one of the two proteins or a yet-unidentified adapter protein. The results presented also raise the question of which SH3 protein interacts with Nef's PxxPxR motif to facilitate functional Nef-PAK2 interactions. While the C-terminal SH3 domain of Vav1 might indeed interact with this interface within the complex, our data argue that this interaction would not significantly contribute to the stability of the Nef/Vav1/PAK2-containing signalosome. In this scenario, the PxxP-SH3 interaction might ensure correct positioning of the complex and/or Vav activity and thus provide activation instead of recruitment of critical complex components. Affinity screening of a library containing the complete collection of human SH3 domains did not reveal any SH3 domains expressed in T cells that would show distinct affinity for Nef (29), thus indirectly supporting the idea that the critical function of the Nef PxxP motif in Nef/Vav1/PAK2 assembly might be mediated via such a low-affinity SH3 interaction. Alternatively, the activity of the complex would also be facilitated by the association with yet-to-be-identified SH3 domain-containing factors that may well lack GEF activity.
The association of Nef with PAK2 activity was among the first protein interactions described for this viral pathogenicity factor (59) and represents one of the most conserved features of the different HIV and SIV Nef variants (33). However, due to the low stability of the Nef-PAK2 complex and the involvement of protein interaction surfaces required for complex assembly in several other independent Nef activities, the physiological role and the molecular principles of this complex have remained largely elusive. Based on the new insights into the Nef-PAK2 complex organization presented here, future studies can now focus on determining the full composition of the Nef-PAK2 complex and on the specificity of its downstream effector functions.
Acknowledgments
We thank Daniel Billadeau, Xose Bustelo, Margherita Doria, Ivan Dikic, Mark Harris, Shinya Tanaka, and Yoshinori Fukui for the kind gifts of reagents and Matthias Geyer for insightful discussion.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 638, project A11) as well as a group leader fellowship from the C.H.S. Stiftung to O.T.F. and by grants from the Academy of Finland, Medical Research Council of Helsinki University Hospital, and the Sigrid Juselius Foundation to K.S. O.T.F. is a member of the Cluster of Excellence “Cellular Networks” supported by the Deutsche Forschungsgemeinschaft.
Footnotes
Published ahead of print on 19 December 2007.
REFERENCES
- 1.Agopian, K., B. L. Wei, J. V. Garcia, and D. Gabuzda. 2007. CD4 and MHC-I downregulation are conserved in primary HIV-1 Nef alleles from brain and lymphoid tissues, but Pak2 activation is highly variable. Virology 358119-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agopian, K., B. L. Wei, J. V. Garcia, and D. Gabuzda. 2006. A hydrophobic binding surface on the human immunodeficiency virus type 1 Nef core is critical for association with p21-activated kinase 2. J. Virol. 803050-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arold, S. T., and A. S. Baur. 2001. Dynamic Nef and Nef dynamics: how structure could explain the complex activities of this small HIV protein. Trends Biochem. Sci. 26356-363. [DOI] [PubMed] [Google Scholar]
- 4.Arora, V. K., B. L. Fredericksen, and J. V. Garcia. 2002. Nef: agent of cell subversion. Microbes Infect. 4189-199. [DOI] [PubMed] [Google Scholar]
- 5.Arora, V. K., R. P. Molina, J. L. Foster, J. L. Blakemore, J. Chernoff, B. L. Fredericksen, and J. V. Garcia. 2000. Lentivirus Nef specifically activates Pak2. J. Virol. 7411081-11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baur, A. S., G. Sass, B. Laffert, D. Willbold, C. Cheng-Mayer, and B. M. Peterlin. 1997. The N-terminus of Nef from HIV-1/SIV associates with a protein complex containing Lck and a serine kinase. Immunity 6283-291. [DOI] [PubMed] [Google Scholar]
- 7.Baur, A. S., E. T. Sawai, P. Dazin, W. J. Fantl, C. Cheng-Mayer, and B. M. Peterlin. 1994. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity 1373-384. [DOI] [PubMed] [Google Scholar]
- 8.Benson, R. E., A. Sanfridson, J. S. Ottinger, C. Doyle, and B. R. Cullen. 1993. Downregulation of cell-surface CD4 expression by simian immunodeficiency virus Nef prevents viral super infection. J. Exp. Med. 1771561-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, A., X. Wang, E. Sawai, and C. Cheng-Mayer. 1999. Activation of the PAK-related kinase by human immunodeficiency virus type 1 Nef in primary human peripheral blood lymphocytes and macrophages leads to phosphorylation of a PIX-p95 complex. J. Virol. 739899-9907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brugnera, E., L. Haney, C. Grimsley, M. Lu, S. F. Walk, A. C. Tosello-Trampont, I. G. Macara, H. Madhani, G. R. Fink, and K. S. Ravichandran. 2002. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat. Cell Biol. 4574-582. [DOI] [PubMed] [Google Scholar]
- 11.Campbell, E. M., R. Nunez, and T. J. Hope. 2004. Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. J. Virol. 785745-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao, Y., E. M. Janssen, A. W. Duncan, A. Altman, D. D. Billadeau, and R. T. Abraham. 2002. Pleiotropic defects in TCR signaling in a Vav-1-null Jurkat T-cell line. EMBO J. 214809-4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casartelli, N., G. Giolo, F. Neri, C. Haller, M. Potesta, P. Rossi, O. T. Fackler, and M. Doria. 2006. The Pro78 residue regulates the capacity of the human immunodeficiency virus type 1 Nef protein to inhibit recycling of major histocompatibility complex class I molecules in an SH3-independent manner. J. Gen. Virol. 872291-2296. [DOI] [PubMed] [Google Scholar]
- 14.Coates, K., S. J. Cooke, D. A. Mann, and M. P. Harris. 1997. Protein kinase C-mediated phosphorylation of HIV-I nef in human cell lines. J. Biol. Chem. 27212289-12294. [DOI] [PubMed] [Google Scholar]
- 15.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391397-401. [DOI] [PubMed] [Google Scholar]
- 16.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, et al. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270988-991. [DOI] [PubMed] [Google Scholar]
- 17.Fackler, O. T., A. Alcover, and O. Schwartz. 2007. Modulation of the immunological synapse: a key to HIV-1 pathogenesis? Nat. Rev. Immunol. 7310-317. [DOI] [PubMed] [Google Scholar]
- 18.Fackler, O. T., and A. S. Baur. 2002. Live and let die: Nef functions beyond HIV replication. Immunity 16493-497. [DOI] [PubMed] [Google Scholar]
- 19.Fackler, O. T., X. Lu, J. A. Frost, M. Geyer, B. Jiang, W. Luo, A. Abo, A. S. Alberts, and B. M. Peterlin. 2000. p21-activated kinase 1 plays a critical role in cellular activation by Nef. Mol. Cell. Biol. 202619-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fackler, O. T., W. Luo, M. Geyer, A. S. Alberts, and B. M. Peterlin. 1999. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol. Cell 3729-739. [DOI] [PubMed] [Google Scholar]
- 21.Fenard, D., W. Yonemoto, C. de Noronha, M. Cavrois, S. A. Williams, and W. C. Greene. 2005. Nef is physically recruited into the immunological synapse and potentiates T cell activation early after TCR engagement. J. Immunol. 1756050-6057. [DOI] [PubMed] [Google Scholar]
- 22.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350508-511. [DOI] [PubMed] [Google Scholar]
- 23.Geyer, M., O. T. Fackler, and B. M. Peterlin. 2001. Structure-function relationships in HIV-1 Nef. EMBO Rep. 2580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giese, S. I., I. Woerz, S. Homann, N. Tibroni, M. Geyer, and O. T. Fackler. 2006. Specific and distinct determinants mediate membrane binding and lipid raft incorporation of HIV-1(SF2) Nef. Virology 355175-191. [DOI] [PubMed] [Google Scholar]
- 25.Giolo, G., F. Neri, N. Casartelli, M. Potesta, F. Belleudi, M. R. Torrisi, and M. Doria. 2007. Internalization and intracellular retention of CD4 are two separate functions of the human immunodeficiency virus type 1 Nef protein. J. Gen. Virol. 883133-3138. [DOI] [PubMed] [Google Scholar]
- 26.Grimsley, C. M., J. M. Kinchen, A. C. Tosello-Trampont, E. Brugnera, L. B. Haney, M. Lu, Q. Chen, D. Klingele, M. O. Hengartner, and K. S. Ravichandran. 2004. Dock180 and ELMO1 proteins cooperate to promote evolutionarily conserved Rac-dependent cell migration. J. Biol. Chem. 2796087-6097. [DOI] [PubMed] [Google Scholar]
- 27.Haller, C., S. Rauch, N. Michel, S. Hannemann, M. J. Lehmann, O. T. Keppler, and O. T. Fackler. 2006. The HIV-1 pathogenicity factor Nef interferes with maturation of stimulatory T-lymphocyte contacts by modulation of N-Wasp activity. J. Biol. Chem. 28119618-19630. [DOI] [PubMed] [Google Scholar]
- 28.Janardhan, A., T. Swigut, B. Hill, M. P. Myers, and J. Skowronski. 2004. HIV-1 Nef binds the DOCK2-ELMO1 complex to activate Rac and inhibit lymphocyte chemotaxis. PLoS Biol. 2E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karkkainen, S., M. Hiipakka, J. H. Wang, I. Kleino, M. Vaha-Jaakkola, G. H. Renkema, M. Liss, R. Wagner, and K. Saksela. 2006. Identification of preferred protein interactions by phage-display of the human Src homology-3 proteome. EMBO Rep. 7186-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65651-662. [DOI] [PubMed] [Google Scholar]
- 31.Khan, I. H., E. T. Sawai, E. Antonio, C. J. Weber, C. P. Mandell, P. Montbriand, and P. A. Luciw. 1998. Role of the SH3-ligand domain of simian immunodeficiency virus Nef in interaction with Nef-associated kinase and simian AIDS in rhesus macaques. J. Virol. 725820-5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332228-232. [DOI] [PubMed] [Google Scholar]
- 33.Kirchhoff, F., M. Schindler, N. Bailer, G. H. Renkema, K. Saksela, V. Knoop, M. C. Muller-Trutwin, M. L. Santiago, F. Bibollet-Ruche, M. T. Dittmar, J. L. Heeney, B. H. Hahn, and J. Munch. 2004. Nef proteins from simian immunodeficiency virus-infected chimpanzees interact with p21-activated kinase 2 and modulate cell surface expression of various human receptors. J. Virol. 786864-6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krautkramer, E., S. I. Giese, J. E. Gasteier, W. Muranyi, and O. T. Fackler. 2004. Human immunodeficiency virus type 1 Nef activates p21-activated kinase via recruitment into lipid rafts. J. Virol. 784085-4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang, S. M., A. J. Iafrate, C. Stahl-Hennig, E. M. Kuhn, T. Nisslein, F. J. Kaup, M. Haupt, G. Hunsmann, J. Skowronski, and F. Kirchhoff. 1997. Association of simian immunodeficiency virus Nef with cellular serine/threonine kinases is dispensable for the development of AIDS in rhesus macaques. Nat. Med. 3860-865. [DOI] [PubMed] [Google Scholar]
- 36.Lindwasser, O. W., R. Chaudhuri, and J. S. Bonifacino. 2007. Mechanisms of CD4 downregulation by the Nef and Vpu proteins of primate immunodeficiency viruses. Curr. Mol. Med. 7171-184. [DOI] [PubMed] [Google Scholar]
- 37.Ling, J., S. J. Morley, and J. A. Traugh. 2005. Inhibition of cap-dependent translation via phosphorylation of eIF4G by protein kinase Pak2. EMBO J. 244094-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llano, M., D. T. Saenz, A. Meehan, P. Wongthida, M. Peretz, W. H. Walker, W. Teo, and E. M. Poeschla. 2006. An essential role for LEDGF/p75 in HIV integration. Science 314461-464. [DOI] [PubMed] [Google Scholar]
- 39.Llano, M., M. Vanegas, O. Fregoso, D. Saenz, S. Chung, M. Peretz, and E. M. Poeschla. 2004. LEDGF/p75 determines cellular trafficking of diverse lentiviral but not murine oncoretroviral integrase proteins and is a component of functional lentiviral preintegration complexes. J. Virol. 789524-9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu, X., X. Wu, A. Plemenitas, H. Yu, E. T. Sawai, A. Abo, and B. M. Peterlin. 1996. CDC42 and Rac1 are implicated in the activation of the Nef-associated kinase and replication of HIV-1. Curr. Biol. 61677-1684. [DOI] [PubMed] [Google Scholar]
- 41.Madrid, R., K. Janvier, D. Hitchin, J. Day, S. Coleman, C. Noviello, J. Bouchet, A. Benmerah, J. Guatelli, and S. Benichou. 2005. Nef-induced alteration of the early/recycling endosomal compartment correlates with enhancement of HIV-1 infectivity. J. Biol. Chem. 2805032-5044. [DOI] [PubMed] [Google Scholar]
- 42.Manninen, A., M. Hiipakka, M. Vihinen, W. Lu, B. J. Mayer, and K. Saksela. 1998. SH3-domain binding function of HIV-1 Nef is required for association with a PAK-related kinase. Virology 250273-282. [DOI] [PubMed] [Google Scholar]
- 43.Manninen, A., G. H. Renkema, and K. Saksela. 2000. Synergistic activation of NFAT by HIV-1 nef and the Ras/MAPK pathway. J. Biol. Chem. 27516513-16517. [DOI] [PubMed] [Google Scholar]
- 44.Manninen, A., and K. Saksela. 2002. HIV-1 Nef interacts with inositol trisphosphate receptor to activate calcium signaling in T cells. J. Exp. Med. 1951023-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michel, N., I. Allespach, S. Venzke, O. T. Fackler, and O. T. Keppler. 2005. The Nef protein of human immunodeficiency virus establishes superinfection immunity by a dual strategy to downregulate cell-surface CCR5 and CD4. Curr. Biol. 15714-723. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen, D. G., K. C. Wolff, H. Yin, J. S. Caldwell, and K. L. Kuhen. 2006. “UnPAKing” human immunodeficiency virus (HIV) replication: using small interfering RNA screening to identify novel cofactors and elucidate the role of group I PAKs in HIV infection. J. Virol. 80130-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishihara, H., M. Maeda, A. Oda, M. Tsuda, H. Sawa, K. Nagashima, and S. Tanaka. 2002. DOCK2 associates with CrkL and regulates Rac1 in human leukemia cell lines. Blood 1003968-3974. [DOI] [PubMed] [Google Scholar]
- 48.Nunn, M. F., and J. W. Marsh. 1996. Human immunodeficiency virus type 1 Nef associates with a member of the p21-activated kinase family. J. Virol. 706157-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Neill, E., L. S. Kuo, J. F. Krisko, D. R. Tomchick, J. V. Garcia, and J. L. Foster. 2006. Dynamic evolution of the human immunodeficiency virus type 1 pathogenic factor, Nef. J. Virol. 801311-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Priceputu, E., Z. Hanna, C. Hu, M. C. Simard, P. Vincent, S. Wildum, M. Schindler, F. Kirchhoff, and P. Jolicoeur. 2007. Primary human immunodeficiency virus type 1 nef alleles show major differences in pathogenicity in transgenic mice. J. Virol. 814677-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pulkkinen, K., G. H. Renkema, F. Kirchhoff, and K. Saksela. 2004. Nef associates with p21-activated kinase 2 in a p21-GTPase-dependent dynamic activation complex within lipid rafts. J. Virol. 7812773-12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quaranta, M. G., B. Mattioli, F. Spadaro, E. Straface, L. Giordani, C. Ramoni, W. Malorni, and M. Viora. 2003. HIV-1 Nef triggers Vav-mediated signaling pathway leading to functional and morphological differentiation of dendritic cells. FASEB J. 172025-2036. [DOI] [PubMed] [Google Scholar]
- 53.Raney, A., L. S. Kuo, L. L. Baugh, J. L. Foster, and J. V. Garcia. 2005. Reconstitution and molecular analysis of an active human immunodeficiency virus type 1 Nef/p21-activated kinase 2 complex. J. Virol. 7912732-12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Renkema, G. H., A. Manninen, D. A. Mann, M. Harris, and K. Saksela. 1999. Identification of the Nef-associated kinase as p21-activated kinase 2. Curr. Biol. 91407-1410. [DOI] [PubMed] [Google Scholar]
- 55.Renkema, G. H., A. Manninen, and K. Saksela. 2001. Human immunodeficiency virus type 1 Nef selectively associates with a catalytically active subpopulation of p21-activated kinase 2 (PAK2) independently of PAK2 binding to Nck or beta-PIX. J. Virol. 752154-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Renkema, G. H., and K. Saksela. 2000. Interactions of HIV-1 NEF with cellular signal transducing proteins. Front. Biosci. 5D268-D283. [DOI] [PubMed] [Google Scholar]
- 57.Roeth, J. F., and K. L. Collins. 2006. Human immunodeficiency virus type 1 Nef: adapting to intracellular trafficking pathways. Microbiol. Mol. Biol. Rev. 70548-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanui, T., A. Inayoshi, M. Noda, E. Iwata, J. V. Stein, T. Sasazuki, and Y. Fukui. 2003. DOCK2 regulates Rac activation and cytoskeletal reorganization through interaction with ELMO1. Blood 1022948-2950. [DOI] [PubMed] [Google Scholar]
- 59.Sawai, E. T., A. Baur, H. Struble, B. M. Peterlin, J. A. Levy, and C. Cheng-Mayer. 1994. Human immunodeficiency virus type 1 Nef associates with a cellular serine kinase in T lymphocytes. Proc. Natl. Acad. Sci. USA 911539-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sawai, E. T., A. S. Baur, B. M. Peterlin, J. A. Levy, and C. Cheng-Mayer. 1995. A conserved domain and membrane targeting of Nef from HIV and SIV are required for association with a cellular serine kinase activity. J. Biol. Chem. 27015307-15314. [DOI] [PubMed] [Google Scholar]
- 61.Sawai, E. T., I. H. Khan, P. M. Montbriand, B. M. Peterlin, C. Cheng-Mayer, and P. A. Luciw. 1996. Activation of PAK by HIV and SIV Nef: importance for AIDS in rhesus macaques. Curr. Biol. 61519-1527. [DOI] [PubMed] [Google Scholar]
- 62.Schindler, M., J. Munch, O. Kutsch, H. Li, M. L. Santiago, F. Bibollet-Ruche, M. C. Muller-Trutwin, F. J. Novembre, M. Peeters, V. Courgnaud, E. Bailes, P. Roques, D. L. Sodora, G. Silvestri, P. M. Sharp, B. H. Hahn, and F. Kirchhoff. 2006. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell 1251055-1067. [DOI] [PubMed] [Google Scholar]
- 63.Schmidt, M. H., K. Husnjak, I. Szymkiewicz, K. Haglund, and I. Dikic. 2006. Cbl escapes Cdc42-mediated inhibition by downregulation of the adaptor molecule betaPix. Oncogene 253071-3078. [DOI] [PubMed] [Google Scholar]
- 64.Schrager, J. A., and J. W. Marsh. 1999. HIV-1 Nef increases T cell activation in a stimulus-dependent manner. Proc. Natl. Acad. Sci. USA 968167-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2338-342. [DOI] [PubMed] [Google Scholar]
- 66.Simmons, A., V. Aluvihare, and A. McMichael. 2001. Nef triggers a transcriptional program in T cells imitating single-signal T cell activation and inducing HIV virulence mediators. Immunity 14763-777. [DOI] [PubMed] [Google Scholar]
- 67.Simmons, A., B. Gangadharan, A. Hodges, K. Sharrocks, S. Prabhakar, A. Garcia, R. Dwek, N. Zitzmann, and A. McMichael. 2005. Nef-mediated lipid raft exclusion of UbcH7 inhibits Cbl activity in T cells to positively regulate signaling. Immunity 23621-634. [DOI] [PubMed] [Google Scholar]
- 68.Skowronski, J., D. Parks, and R. Mariani. 1993. Altered T cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 12703-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stevenson, M. 2003. HIV-1 pathogenesis. Nat. Med. 9853-860. [DOI] [PubMed] [Google Scholar]
- 70.Stumptner-Cuvelette, P., S. Morchoisne, M. Dugast, S. Le Gall, G. Raposo, O. Schwartz, and P. Benaroch. 2001. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. USA 9812144-12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thoulouze, M. I., N. Sol-Foulon, F. Blanchet, A. Dautry-Varsat, O. Schwartz, and A. Alcover. 2006. Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity 24547-561. [DOI] [PubMed] [Google Scholar]
- 72.Tolstrup, M., L. Ostergaard, A. L. Laursen, S. F. Pedersen, and M. Duch. 2004. HIV/SIV escape from immune surveillance: focus on Nef. Curr. HIV Res. 2141-151. [DOI] [PubMed] [Google Scholar]
- 73.Vandekerckhove, L., F. Christ, B. Van Maele, J. De Rijck, R. Gijsbers, C. Van den Haute, M. Witvrouw, and Z. Debyser. 2006. Transient and stable knockdown of the integrase cofactor LEDGF/p75 reveals its role in the replication cycle of human immunodeficiency virus. J. Virol. 801886-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vilhardt, F., O. Plastre, M. Sawada, K. Suzuki, M. Wiznerowicz, E. Kiyokawa, D. Trono, and K. H. Krause. 2002. The HIV-1 Nef protein and phagocyte NADPH oxidase activation. J. Biol. Chem. 27742136-42143. [DOI] [PubMed] [Google Scholar]
- 75.Vincent, P., E. Priceputu, D. Kay, K. Saksela, P. Jolicoeur, and Z. Hanna. 2006. Activation of PAK2 and its association with NEF are conserved in murine cells, but are not sufficient to induce an AIDS-like disease in CD4C/HIV transgenic mice. J. Biol. Chem. 2816940-6954. [DOI] [PubMed] [Google Scholar]
- 76.Wiskerchen, M., and C. Cheng-Mayer. 1996. HIV-1 Nef association with cellular serine kinase correlates with enhanced virion infectivity and efficient proviral DNA synthesis. Virology 224292-301. [DOI] [PubMed] [Google Scholar]
- 77.Wolf, D., V. Witte, B. Laffert, K. Blume, E. Stromer, S. Trapp, P. d'Aloja, A. Schurmann, and A. S. Baur. 2001. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat. Med. 71217-1224. [DOI] [PubMed] [Google Scholar]