Abstract
Regulation of carbon partitioning is essential for plant growth and development. To gain insight into genes controlling carbon allocation in leaves, we identified mutants that hyperaccumulate carbohydrates. tie-dyed2 (tdy2) is a recessive mutant of maize (Zea mays) with variegated, nonclonal, chlorotic leaf sectors containing excess starch and soluble sugars. Consistent with a defect in carbon export, we found that a by-product of functional chloroplasts, likely a sugar, induces tdy2 phenotypic expression. Based on the phenotypic similarities between tdy2 and two other maize mutants with leaf carbon accumulation defects, tdy1 and sucrose export defective1 (sxd1), we investigated whether Tdy2 functioned in the same pathway as Tdy1 or Sxd1. Cytological and genetic studies demonstrate that Tdy2 and Sxd1 function independently. However, in tdy1/+; tdy2/+ F1 plants, we observed a moderate chlorotic sectored phenotype, suggesting that the two genes are dosage sensitive and have a related function. This type of genetic interaction is referred to as second site noncomplementation and has often, though not exclusively, been found in cases where the two encoded proteins physically interact. Moreover, tdy1; tdy2 double mutants display a synergistic interaction supporting this hypothesis. Additionally, we determined that cell walls of chlorotic leaf tissues in tdy mutants contain increased cellulose; thus, tdy mutants potentially represent enhanced feedstocks for biofuels production. From our phenotypic and genetic characterizations, we propose a model whereby TDY1 and TDY2 function together in a single genetic pathway, possibly in homo- and heteromeric complexes, to promote carbon export from leaves.
All aspects of plant growth and development are dependent upon proper control of carbon allocation. For carbon to be correctly partitioned, photoassimilates fixed in source leaf tissues must be distributed through the veins to carbon-importing sink tissues (Turgeon, 1989, 2006). Maize (Zea mays) leaves have three different types of longitudinally arranged veins, small, intermediate (collectively referred to as minor), and large (lateral), which perform different roles in carbon distribution (Russell and Evert, 1985). Minor veins principally function to load fixed carbon from the photosynthetic cells (Fritz et al., 1983). These veins intergrade into the lateral veins, which primarily function in the long-distance transport of assimilates (Fritz et al., 1989). Maize is a C4 plant with two distinct photosynthetic cell types, mesophyll and bundle sheath cells, that cooperatively carry out carbon assimilation. Mesophyll cells encircle the bundle sheath cells, which surround the veins (Esau, 1977). Maize is an apoplastic phloem-loading species (Evert et al., 1978). Suc synthesized in mesophyll cells diffuses along the symplastic transport pathway through plasmodesmata into bundle sheath cells and then into vascular parenchyma cells (Russin et al., 1996). From there, Suc is exported to the apoplasm prior to loading into the phloem. Although the translocation path of fixed carbon in maize leaves was described nearly 40 years ago (Hofstra and Nelson, 1969), little is known concerning the mechanisms that regulate this process.
In most plant species, Suc is the major form of fixed carbon translocated, and it is loaded into the companion cells and/or sieve elements of the phloem by Suc transporters (SUTs; Lalonde et al., 2004; Sauer, 2007). It is also known that Suc acts as a signal to regulate SUT transcript and protein levels (Chiou and Bush, 1998; Vaughn et al., 2002; Ransom-Hodgkins et al., 2003), but the components of the signaling cascade have not been elucidated. Evidence demonstrating that SUTs are essential for phloem loading comes from mutational studies (Riesmeier et al., 1994; Kuhn et al., 1996; Burkle et al., 1998; Gottwald et al., 2000). Plants with decreased SUT function from either antisense transgenic expression or a T-DNA insertion accumulate carbohydrates in their leaves. The excess photoassimilates cause feedback down-regulation of photosynthetic gene expression and chlorosis (Sheen, 1990; Goldschmidt and Huber, 1992; Krapp and Stitt, 1995; Koch, 1996; Jeannette et al., 2000; Rolland et al., 2006). SUT mutant plants also show diminished shoot growth, delayed flowering, and decreased yield due to the reduced export of carbohydrates to the growing sink tissues.
In maize, two recessive mutants have been described with carbon-accumulating leaf phenotypes resembling SUT loss-of-function mutations. Unlike SUT mutants that have uniform chlorotic phenotypes, tie-dyed1 (tdy1) and sucrose export defective1 (sxd1) mutant leaves develop variegated chlorotic sectors that hyperaccumulate carbohydrates (Russin et al., 1996; Provencher et al., 2001; Braun et al., 2006). The formation of these nonclonal sectors cannot be explained by the clonal basis of maize leaf development (Poethig and Szymkowiak, 1995; Baker and Braun, 2007), suggesting that a mobile signal, potentially a sugar, coordinates their development. Additionally, both tdy1 and sxd1 plants display reduced growth, are slow to flower, and have decreased yield. However, despite the similarities in their phenotypes, each mutant has unique properties. Sxd1 encodes tocopherol cyclase, and sxd1 mutants lack tocopherols (Sattler et al., 2003). In sxd1 mutants, callose is deposited over the plasmodesmata of the bundle sheath-vascular parenchyma cell interface in leaf minor veins (Botha et al., 2000). This blockage prevents Suc movement along the symplastic transport pathway. The resultant sxd1 chlorotic sectors progressively develop, initiating at the leaf tip and spreading toward the base. On the other hand, tdy1 mutant leaves have normal tocopherol levels and do not exhibit ectopic callose deposits or any alterations in plasmodesmatal ultrastructure along the symplastic pathway (Ma et al., 2008). Further, tdy1 chlorotic sectors appear shortly after leaves emerge from the whorl, are stable once visible, and tend to be bounded by lateral veins (Braun et al., 2006; Baker and Braun, 2007). From genetic and cytological investigations, we previously determined that Tdy1 and Sxd1 function in separate genetic pathways and that Tdy1 likely functions within the veins to limit carbohydrate accumulation in leaves (Baker and Braun, 2007; Ma et al., 2008).
The combination of leaf variegation and carbon hyperaccumulation phenotypes seen in tdy1 and sxd1 mutants is novel. Other carbon accumulation mutants have been reported, but they affect the entire leaf and are not variegated. For example, in addition to the SUT mutants described above, mutations in starch catabolism result in the build-up of excess starch throughout the leaf blades (Zeeman et al., 1998; Critchley et al., 2001; Yu et al., 2001; Dinges et al., 2003; Lu and Sharkey, 2004; Niittyla et al., 2004). Conversely, many variegated chlorotic mutants have been described, but the chlorotic tissues do not hyperaccumulate carbohydrates (Madore, 1990; Chatterjee et al., 1996; Keddie et al., 1996; Streatfield et al., 1999; Chen et al., 2000; Sakamoto et al., 2002; see Yu et al. [2007] for a recent review of Arabidopsis [Arabidopsis thaliana] variegated mutants and mechanisms for variegation). Hence, the phenotypes of tdy1 and sxd1 appear to be unique and may offer insights into the regulation of carbon accumulation in leaves. Incorporating these phenotypes and other data into a threshold model led us to propose that TDY1 acts as an osmotic stress or sugar sensor to induce SUT function (Braun et al., 2006). This SUT induction would promote Suc export and thereby decrease carbohydrate content in leaf tissues below a threshold necessary to produce a chlorotic sector.
We have taken a genetic approach to identify additional genes regulating carbon accumulation in maize leaves. In analyzing a large collection of mutants with variegated nonclonal sectors in their leaves, we discovered several new mutants that hyperaccumulate carbohydrates within the chlorotic tissues. In this article, we report the characterization of the third maize mutant affecting leaf carbon partitioning, tdy2. tdy2 mutant plants develop stable, nonclonal, chlorotic leaf sectors with greatly elevated carbohydrate content. We determined that tdy2 chlorotic sectors are frequently bounded by lateral veins and that they are induced by a chloroplast-derived signal, likely a sugar. Similar to tdy1 and sxd1 plants, tdy2 mutants have reduced stature, delayed flowering, and decreased seed yield, presumably due to the retention of carbohydrates in leaves. However, phenotypic, cytological, and genetic analyses demonstrate that Tdy1 and Tdy2 function independently of Sxd1.
To determine if Tdy2 acts in the same genetic pathway as Tdy1, we constructed double mutant families. Intriguingly, F1 plants doubly heterozygous for tdy1 and tdy2 exhibited a moderately sectored phenotype. This type of genetic interaction whereby two recessive loci show a phenotype in the F1 generation is called second site noncomplementation (SSNC, also referred to as nonallelic noncomplementation; Badano and Katsanis, 2002; Hawley and Gilliland, 2006). SSNC has been documented in animals, yeast, and in a couple of instances in plants (Stearns and Botstein, 1988; Hays et al., 1989; Harris and Juriloff, 1998; Yook et al., 2001; Folkers et al., 2002; Kaplinsky and Freeling, 2003). In many, though not all cases, SSNC is indicative of a physical association between the proteins encoded by the two loci and suggests that TDY1 and TDY2 may function in the same genetic pathway and physically interact. Double mutant plants show a synergistic interaction of severely chlorotic leaves consistent with this hypothesis. Models for the TDY1-TDY2 interaction and function are presented.
Finally, because of the carbohydrate hyperaccumulation in tdy mutant leaves, we examined whether there was an increase in carbon deposited into the cell wall as a terminal storage site. We found that tdy mutant leaves have increased cellulose levels, suggesting that these stocks may serve as enhanced feedstocks for the production of biofuels. The utility of carbon hyperaccumulating mutants is discussed.
RESULTS
tdy2 Is a Variegated Leaf Mutant That Hyperaccumulates Carbohydrates in Chlorotic Sectors
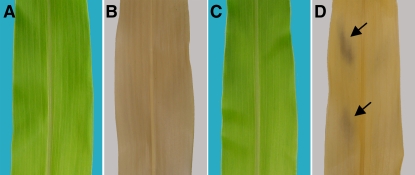
To characterize additional genes promoting carbon export from maize leaves, we screened for nonclonal sectoring mutants resembling tdy1. We identified a new mutant, tdy2, that forms chlorotic leaf sectors similar in appearance to tdy1 (Fig. 1, A–C). tdy2 plants were reciprocally crossed with inbred lines, and the F1 progeny were wild type, indicating that the mutation is not maternally inherited. The F1 plants were self-fertilized, and the F2 segregation ratio indicated that tdy2 is a recessive mutation (149 wild type:51 tdy2; χ2 = 0.03, P > 0.05). A series of crosses using B-A translocation stocks was performed to map tdy2 (Beckett, 1994). Only progeny from the chromosome 5 L cross uncovered the tdy2 mutation; however, the phenotype of the 5 L hypoploid individuals (one mutant copy of tdy2 over a deletion) was mild compared to tdy2 homozygotes (Fig. 1D). These data suggest that tdy2 is a hypomorphic mutation that still retains some function, i.e. it is not a null allele (Muller, 1932). We fine mapped tdy2 to the distal end of the long arm of chromosome 5 in bin 5.09 using molecular markers (four recombinants/214 chromosomes with IDP217), indicating that tdy2 is a distinct locus from tdy1 (mapped to chromosome 6) and sxd1 (located near the centromere of chromosome 5, bin 5.04). Similar to tdy1 but different than sxd1, tdy2 chlorotic sectors are stable once formed (Fig. 1E; Supplemental Fig. S1).
Figure 1.
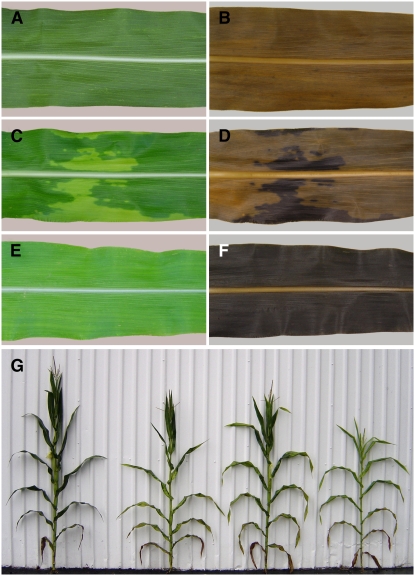
tdy2 leaves exhibit chlorotic and green regions. A, Wild-type leaves have uniform green coloration. B, tdy1 leaves display variegated chlorotic and green regions. C, tdy2 leaves display a similar chlorotic and green variegated pattern. D, Hypoploid tdy2/− leaves show a mild chlorotic phenotype. E, Mature field-grown tdy2 plant displaying chlorotic leaves.
tdy1 and sxd1 mutants hyperaccumulate carbohydrates in chlorotic leaf tissues (Russin et al., 1996; Braun et al., 2006). To determine if tdy2 mutants also contain high levels of carbohydrates, we stained leaves with iodine-potassium iodide to visualize starch. tdy2 chlorotic tissues stained much more strongly than green leaf tissues, indicating the chlorotic sectors contain higher levels of starch (Fig. 2, A–D). We quantified the amount of Suc, Glc, Fru, and starch in tissues from tdy2 chlorotic regions, tdy2 green regions, and wild-type leaves. tdy2 chlorotic regions contained approximately 3- to 8-fold higher levels of carbohydrates compared to green leaf tissues from the mutant or wild type (Fig. 2, E–H). Hence, tdy2 mutants hyperaccumulate carbohydrates in the chlorotic leaf tissues. In addition, similar to tdy1 and sxd1 mutants, tdy2 plants display a reduction in plant height, delayed time to flowering, diminished inflorescence size, and decreased kernel number, most likely due to the retention of carbohydrates in vegetative tissues (Supplemental Fig. S2).
Figure 2.
tdy2 chlorotic sectors hyperaccumulate carbohydrates. A and B, Photographs of wild-type and tdy2 leaf tissues, respectively. C and D, Photographs of cleared, starch-stained leaves in A and B showing that tdy2 chlorotic tissues hyperaccumulate starch relative to green tissues. E to H, Carbohydrate quantification in wild type (dark gray), tdy2 green tissues (light gray), and tdy2 chlorotic tissues (white). All units are milligram/gram fresh weight, and values represent averages of six samples ± se. Asterisks indicate values significantly different than wild type at P ≤ 0.01 determined using the Student's t test. E, Suc. F, Glc. G, Fru. H, Starch. [See online article for color version of this figure.]
Both tdy1 and sxd1 mutants exhibit similar levels of chlorosis associated with hyperaccumulation of carbohydrates (Ma et al., 2008). However, pigment levels in the green tissues differ between the two mutants. Green tissues in tdy1 mutants have photosynthetic pigment levels comparable to wild type, whereas sxd1 green leaf tissues show mild chlorosis and contain only approximately 70% of wild-type pigment levels (Ma et al., 2008). To characterize the photosynthetic pigment levels in tdy2 tissues, we measured their abundance. The chlorophyll a, chlorophyll b, and total carotenoid levels in tdy2 chlorotic regions were 50% to 70% of those in wild type, while the chlorophyll a/b ratio in chlorotic regions was elevated (Table I). In contrast, pigment levels in green tdy2 tissues were not statistically different from wild type. In this regard, the tdy2 phenotype is similar to tdy1 and distinct from sxd1.
Table I.
Photosynthetic pigment levels in wild-type and tdy2 leaves
Data represent means from six samples ± se.
| Leaf Tissue | Chlorophyll a | Percentage of Wild Type | Chlorophyll b | Percentage of Wild Type | Chlorophyll a/b | Total Carotenoids | Percentage of Wild Type |
|---|---|---|---|---|---|---|---|
| μg/g fresh weight | μg/g fresh weight | μg/g fresh weight | |||||
| Wild type | 1,278.6 ± 111.8 | 100 | 369.5 ± 21.5 | 100 | 3.46 | 320.9 ± 4.2 | 100 |
| tdy2 green | 1,242.2 ± 43.5 | 97.2 | 363.5 ± 19.5 | 98.4 | 3.42 | 308.9 ± 10.1 | 96.2 |
| tdy2 chlorotic | 829.9a ± 67.8 | 65.0 | 192.2a ± 17.8 | 52.0 | 4.32 | 220.5a ± 7.0 | 68.7 |
Value is significantly different from wild type at P ≤ 0.01 using the Student's t test.
Starch Accumulation Precedes Chlorosis in tdy2 Leaves
Excess accumulation of carbohydrates in leaves is known to down-regulate photosynthetic gene expression and result in chlorosis (Sheen, 1990; Goldschmidt and Huber, 1992; Krapp and Stitt, 1995; Koch, 1996; Jeannette et al., 2000; Rolland et al., 2006). To investigate whether tdy2 leaves accumulate carbohydrates prior to chlorosis, we stained emerging leaves for starch before visible sector formation. Wild-type leaves did not show any regions that differentially accumulated starch (Fig. 3). However, tdy2 leaves that visibly lacked chlorotic regions displayed leaf areas that preferentially accumulated starch. These starch-accumulating regions would presumably have developed into chlorotic tissues. Because carbohydrate accumulation precedes chlorosis, it suggests that the primary defect may be in carbon export rather than in plastid development. The discrete development of tdy2 starch-accumulating regions and chlorotic sectors parallels events in tdy1 mutant leaves (Supplemental Fig. S1; Braun et al., 2006) but contrasts with the continuous expansion of sectors seen in sxd1 leaves (Russin et al., 1996; Provencher et al., 2001).
Figure 3.
Starch accumulation precedes chlorosis in tdy2 leaves. A and C, Photographs of emerging wild-type and tdy2 leaves, respectively. B and D, Leaves in A and C were cleared, starch stained, and photographed. tdy2 leaves contain regions that preferentially accumulated starch prior to visible chlorosis (arrows), while wild type did not show any differential accumulation of starch. [See online article for color version of this figure.]
Lateral Veins Often Coincide with tdy2 Sector Boundaries
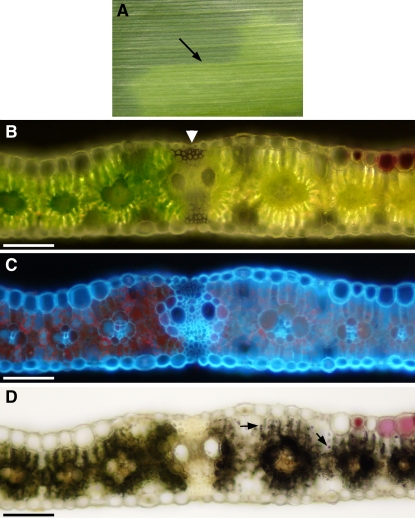
tdy2 mutant leaves often exhibited a very sharp boundary between chlorotic and green tissues (Fig. 4A; Supplemental Table S1). Cross sections through these regions determined that the sharp boundaries frequently corresponded to lateral veins. Under reflected light, it can be seen that the bundle sheath cells on either side of the lateral vein differ in their chlorophyll pigmentation (Fig. 4B). As shown by chlorophyll autofluorescence upon UV illumination, a significant reduction in chlorophyll is seen in the bundle sheath and mesophyll cells from the chlorotic tissue relative to the green tissue (Fig. 4C). Furthermore, after starch staining, it is evident that bundle sheath cells on the chlorotic side of the lateral vein hyperaccumulate starch, while those on the opposite side contain much less starch (Fig. 4D). In addition, similar to tdy1 and sxd1 chlorotic tissues, we observed that mesophyll cells in tdy2 chlorotic tissue stain strongly for starch, whereas mesophyll cells in tdy2 green tissue do not (Fig. 4D). Hence, lateral veins frequently delineate tdy2 chlorotic regions. Lateral veins also tend to be located at the sharp boundaries between tdy1 chlorotic and green tissues (Baker and Braun, 2007). However, in sxd1 mutants, it is the minor, not lateral, veins that are affected (Russin et al., 1996). As lateral veins primarily function in the long-distance transport of assimilates (Fritz et al., 1989), it suggests that the transport of Suc through this class of veins may limit expansion of a tdy2 chlorotic region.
Figure 4.
Lateral veins frequently are found at sharp boundaries between tdy2 chlorotic and green tissues. A, Photograph of tdy2 leaf segment with sharp boundary between the green and the chlorotic region (arrow). B, Leaf cross section spanning the sharp boundary viewed in reflected light shows that a lateral vein (arrowhead) separates chlorotic and green tissues. C, Same section viewed in UV light shows reduced chlorophyll autofluorescence (red color) in the chlorotic tissue compared with the green tissue. Note that bundle sheath cells on either side of the lateral vein contain differing levels of chlorophyll. D, Same section starch stained and viewed in bright-field shows that mesophyll cells in chlorotic tissue contain starch (arrows). Additionally, bundle sheath cells on the chlorotic side of the lateral vein contain more starch than bundle sheath cells on the green side of the vein. Scale bars = 100 μm.
A Chloroplast By-Product Induces tdy2 Phenotypic Expression
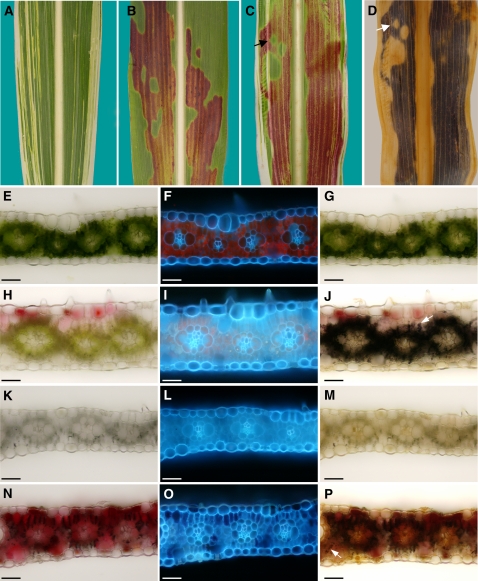
In our studies of the tdy1 mutant, we found that functional chloroplasts are required to produce a diffusible compound, potentially a sugar, that induces the tdy1 sectored phenotype (Baker and Braun, 2007). To test whether functional chloroplasts were likewise required for expression of the tdy2 chlorotic phenotype, we analyzed double mutants between tdy2 and iojap1 (ij1). ij1 is a recessive, nuclear mutation that conditions defective chloroplasts that lack ribosomes and results in albino longitudinal stripes in an otherwise largely green leaf (Fig. 5A; Walbot and Coe, 1979; Coe et al., 1988). Because it is not possible to visualize a change in chlorophyll pigmentation in white tissue, we crossed tdy2 into a genetic stock that accumulates anthocyanins exclusively in the tdy2 starch-accumulating sectors (Fig. 5B). We utilized this anthocyanin accumulation to specifically mark tdy2 phenotypic regions and detect their presence within albino tissues. In examining tdy2; ij1 double mutant leaves, we observed that albino cells expressed the tdy2 anthocyanin accumulation phenotype only if contiguous to green leaf tissue expressing a tdy2 sector (Fig. 5C). Starch staining the double mutant leaves demonstrated that starch and anthocyanin accumulation perfectly coincided and that the albino cells expressed the tdy2 starch accumulation phenotype (Fig. 5D). Histological examinations verified this finding (Fig. 5, E–P). ij1 albino tissue not expressing the tdy2 anthocyanin phenotype contained no detectable starch, consistent with previous data that ij1 albino cells lack starch (Fig. 5M; Rhoades and Carvalho, 1944). However, albino cells expressing anthocyanins in tdy2; ij1 double mutant leaves accumulated starch in the mesophyll and bundle sheath cells as in the tdy2 chlorotic regions but at a reduced level, presumably due to the heterotrophic cells metabolizing a portion of the imported sugar (Fig. 5, J and P). These data are in agreement with previous data showing that albino tissues accumulate starch if fed surplus sugar (Cox and Dickinson, 1971) and that tdy1; ij1 albino cells can accumulate starch (Baker and Braun, 2007). These data suggest that functional chloroplasts are not required to express the tdy2 phenotype, and that a mobile chloroplast by-product, possibly Suc, induces the tdy2 phenotype.
Figure 5.
A mobile chloroplast by-product induces tdy2 phenotypic expression. A, ij1 leaf with longitudinal white stripes. B, tdy2 in anthocyanin expressing genetic background with red pigments accumulated solely in chlorotic regions. C, tdy2; ij1 double mutant leaf shows anthocyanin accumulation in albino cells adjacent to chlorotic, anthocyanin-accumulating tissues (arrow). D, Cleared, starch-stained leaf from C shows starch accumulates in albino, anthocyanin-accumulating cells (arrow). E to P, Leaf cross sections of each single mutant and tdy2; ij1 double mutant viewed in bright-field (E, H, K, and N) and under UV light (F, I, L, and O). G, J, M, and P show starch-stained sections in bright-field. Arrows in J and P show starch accumulation in mesophyll cells. E to G, tdy2 green tissue. H and J, tdy2 chlorotic tissue. K to M, ij1 albino tissue. N to P, tdy2; ij1 albino tissue accumulating anthocyanin. Dark color in N corresponds to air bubbles. Scale bars = 50 μm.
Plasmodesmata along the Symplastic Pathway Appear Unobstructed in tdy2 Leaves
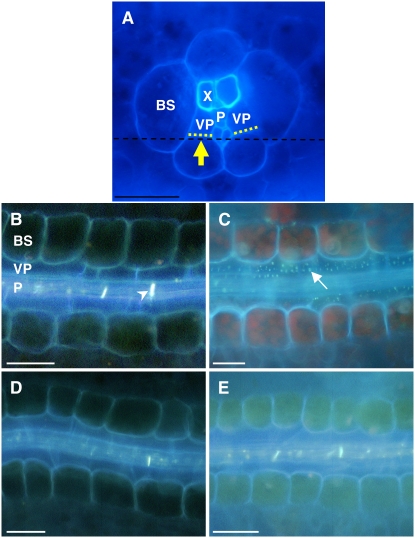
sxd1 mutants accumulate carbohydrates in their leaves due to a blockage along the Suc symplastic pathway (Russin et al., 1996; Provencher et al., 2001). Specifically, callose is deposited over the plasmodesmata between the bundle sheath and vascular parenchyma cell interface, thereby preventing Suc from being exported to the apoplast (Botha et al., 2000). tdy1 mutants do not deposit callose over this cellular interface, suggesting that they accumulate carbohydrates by a different mechanism (Ma et al., 2008). To determine whether tdy2 mutants contain callose deposits occluding the symplastic pathway as observed in sxd1 leaf minor veins, we performed aniline blue fluorescence microscopy. By gently scraping away the abaxial epidermis, mesophyll, and bundle sheath cells, we exposed the bundle sheath-vascular parenchyma cell interface and then stained the tissue with aniline blue (Fig. 6A). Aniline blue binds callose and emits blue-white light on UV excitation. As shown in paradermal sections of leaf minor veins, callose is found in wild-type tissues only in the sieve plates between sieve elements (Fig. 6B). In contrast, in sxd1 mutants, many ectopic callose deposits are detected at the bundle sheath-vascular parenchyma cell interface (Fig. 6C). No ectopic callose deposits were observed in either green or chlorotic leaf tissues from tdy2 mutants (Fig. 6, D and E). These data suggest that tdy2 mutants do not have callose deposits precluding symplastic Suc movement as found in sxd1 mutants.
Figure 6.
tdy2 leaf minor veins lack ectopic callose deposits. A, Cross section of minor vein viewed under UV light. Cells below black dotted line were removed to expose vascular interface (yellow dotted line). Arrow indicates orientation of view in B to E. BS, Bundle sheath; VP, vascular parenchyma; P, phloem; X, xylem. B to E, Paradermal sections of leaf minor veins stained with aniline blue and viewed in UV light. B, Wild type. Arrowhead indicates sieve plate. C, sxd1 mutants display ectopic callose deposits (arrow). D and E, tdy2 green and chlorotic tissues, respectively, lack ectopic callose deposits. Scale bars = 50 μm. [See online article for color version of this figure.]
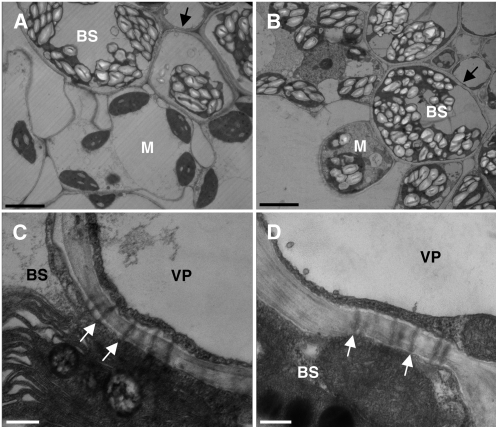
Though we did not observe callose deposits blocking the symplastic pathway in tdy2 mutants, it is possible that another plasmodesmatal structural defect could account for the carbohydrate accumulation. To test this hypothesis, we performed transmission electron microscopy (TEM) on wild-type and tdy2 leaves (Fig. 7). In wild-type leaves, starch predominantly accumulates in bundle sheath cell chloroplasts but is largely absent from mesophyll cell chloroplasts (Fig. 7A; Rhoades and Carvalho, 1944). However, in tdy2 chlorotic tissues, starch hyperaccumulates in both bundle sheath and mesophyll cell chloroplasts (Fig. 7B). Inspecting the bundle sheath-vascular parenchyma cell interface, we did not detect any structural alterations to, or deposits over, the plasmodesmata in wild-type or tdy2 leaves (Fig. 7, C and D). We similarly investigated the appearance of plasmodesmata at all cellular interfaces along the symplastic loading pathway. In every case, the tdy2 samples appeared indistinguishable from wild type (Supplemental Fig. S3). Collectively, these data suggest that tdy2 mutants do not accumulate carbohydrates due to physical blockage of the plasmodesmata as seen in sxd1 plants.
Figure 7.
tdy2 mutants have normal-appearing plasmodesmata at the BS-VP cell interface. TEM images of wild-type (A and C) and tdy2 chlorotic (B and D) tissues. BS, Bundle sheath; M, mesophyll; VP, vascular parenchyma. A, Wild-type bundle sheath chloroplasts contain abundant starch crystals, while mesophyll plastids contain little starch. B, tdy2 bundle sheath and mesophyll chloroplasts hyperaccumulate starch. Arrows in A and B indicate the BS-VP cell interface. C, Wild-type BS-VP cellular interface with plasmodesmata connecting the adjacent cytoplasms. D, tdy2 chlorotic tissue has normal appearing plasmodesmata between BS-VP cells. Arrows in C and D indicate the location of the plasma membrane in the BS cells. Scale bars in A and B = 5 μm; in C and D = 250 nm.
tdy2 and tdy1 Display SSNC and a Synergistic Interaction
In addition to the cytological investigations showing tdy2 plants accumulate carbohydrates due to a different mechanism than sxd1 mutants, we observed an additive genetic interaction in tdy2; sxd1 double mutants (data not shown). Together, these data indicate that the two genes function in distinct pathways. Similarly, we previously determined that tdy1 and sxd1 also define separate genetic pathways (Ma et al., 2008). Hence, we tested whether Tdy2 acts in the same genetic pathway as Tdy1. Most surprisingly, in the F1 generation, the doubly heterozygous tdy1/+; tdy2/+ individuals, which would be expected to display a wild-type phenotype (Fig. 8A), exhibited a tdy sectored leaf phenotype indicative of SSNC (Fig. 8C; Supplemental Fig. S4). Cases of SSNC have been reported previously in plants, for example, between genes functioning in Arabidopsis trichome morphogenesis (Folkers et al., 2002), and in maize inflorescence development (Kaplinsky and Freeling, 2003). To determine if the tdy chlorotic sectors observed in tdy1/+; tdy2/+ F1 plants accumulated carbohydrates, we starch stained the sectored leaves. Similar to tdy1 or tdy2 chlorotic sectors, the chlorotic tissues of F1 plants hyperaccumulated starch relative to wild type or green regions of F1 leaves (Fig. 8, B and D). These data indicate that Tdy1 and Tdy2 are dosage sensitive and may act in the same process.
Figure 8.
Genetic interaction between tdy1 and tdy2. A, C, and E, Leaves before staining. B, D, and F, Same leaves cleared and starch stained. A and B, Wild type. C and D, tdy1/+; tdy2/+ F1 leaf with chlorotic sectors. E and F, tdy1; tdy2 double mutant leaf displays complete chlorosis and starch hyperaccumulation throughout. G, Left to right, wild type, tdy2 single mutant, tdy1 single mutant, tdy1; tdy2 double mutant. [See online article for color version of this figure.]
To further characterize the interaction between Tdy1 and Tdy2, F2 families segregating both mutants were analyzed. Remarkably, we found that leaves of tdy1; tdy2 double mutants were virtually completely chlorotic (Fig. 8E). The reduction in chlorophyll in the double mutant leaves was comparable to that seen in the chlorotic regions of single mutants, and all of the chlorotic tissues displayed similar down-regulation of photosynthesis (Supplemental Table S2). Starch staining the double mutant chlorotic leaf blades showed that they hyperaccumulated starch throughout the tissue (Fig. 8F). By quantifying soluble sugar accumulation in the double mutant plants relative to wild type, we determined that the chlorotic tissue contained greatly elevated levels of carbohydrates (Table II). In addition, the double mutant plants had a stronger reduction in plant height compared with either single mutant or wild-type siblings (Fig. 8G). Based on the nearly identical phenotypes, the F1 SSNC effect and the synergistic genetic interaction observed in the double mutants, we conclude that Tdy1 and Tdy2 function similarly to promote carbohydrate export from leaves. Further, these data also suggest that Tdy1 and Tdy2 may act in the same genetic pathway, potentially in a complex.
Table II.
Carbohydrate quantification in wild-type versus chlorotic tdy1; tdy2 leaves
Data represent means from six samples ± se. Values for double mutant tissue were statistically different than wild type as determined by the Student's t test at P ≤ 0.001.
| Leaf Tissue | Suc | Percentage of Wild Type | Glc | Percentage of Wild Type | Fru | Percentage of Wild Type |
|---|---|---|---|---|---|---|
| mg/g fresh weight | mg/g fresh weight | mg/g fresh weight | ||||
| Wild type | 17.1 ± 3.4 | 100 | 1.8 ± 0.3 | 100 | 1.1 ± 0.3 | 100 |
| tdy1; tdy2 | 46.8 ± 1.7 | 274 | 11.3 ± 1.1 | 630 | 11.5 ± 0.8 | 1,045 |
tdy Mutants Have Increased Cellulose in Their Cell Walls
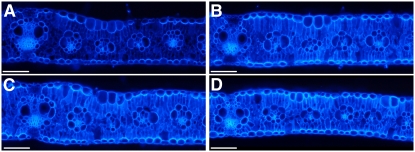
Because tdy1 and tdy2 mutants build up very high concentrations of carbohydrates within chlorotic regions of leaf tissue, we investigated whether the surplus carbon can be shunted into the cell wall as a permanent carbon storage reserve. To examine the cellulose content of the cell wall, we performed calcofluor white fluorescence microscopy. Calcofluor white binds cellulose and fluoresces upon UV light exposure. In comparing wild type to chlorotic tissues from tdy1, tdy2, or tdy1; tdy2 double mutant leaves, we determined that the single and double mutants fluoresce more strongly than wild-type siblings (Fig. 9). No differences were detected among the single and double mutants. These data suggest that the mutants have greater cellulose content in their cell walls compared with wild type. Quantifying cellulose levels in tdy2 chlorotic regions and wild-type leaves supported these findings (Supplemental Table S3). Additionally, it has been reported that carbon availability derived from starch turnover and sugar abundance positively influences lignin deposition in Arabidopsis (Rogers et al., 2005). As tdy mutants contain excess starch and soluble sugars, we analyzed whether the increased carbon pools in the chlorotic tissues were associated with increased lignin deposition by phloroglucinol staining. In contrast to the results in Arabidopsis, we did not observe changes in lignin levels in tdy chlorotic regions compared with wild type (Supplemental Fig. S5). Together, these data suggest that the tdy mutants have increased cellulose content in their leaves and that the mechanisms controlling lignin biosynthesis may differ between maize and Arabidopsis.
Figure 9.
tdy mutants deposit increased cellulose in their cell walls. A to D, Leaf cross sections stained with calcofluor white and viewed under UV light. A, Wild type. B, tdy2 chlorotic tissue. C, tdy1 chlorotic tissue. D, tdy1; tdy2 double mutant chlorotic tissue. Scale bars = 100 μm. [See online article for color version of this figure.]
DISCUSSION
In this article, we identified and characterized tdy2, a mutant that produces variegated leaves with chlorotic regions that hyperaccumulate carbohydrates. The tdy2 mutant phenotype strongly resembles the tdy1 mutant, but the new mutant maps to a different chromosomal location. This indicates that tdy2 is an independent locus from tdy1 or sxd1. Further, the chromosomal segments where the three genes reside are not syntenous, indicating that Tdy2 does not represent an ancient duplication of Tdy1 or Sxd1 (Gale and Devos, 1998). The carbohydrate accumulation within tdy2 chlorotic regions suggests that the mutation leads to a defect in Suc export from leaves. Moreover, the starch accumulation in tdy2 mesophyll cells appears similar to leaves blocked in phloem export (Russin et al., 1996; Jeannette et al., 2000). However, cytological investigations show that tdy2 mutants do not display similar physical obstruction of plasmodesmata along the Suc symplastic pathway as found in sxd1 mutants. From genetic analyses, we conclude that Tdy2 acts in the same pathway as Tdy1 and that both genes function independently from Sxd1. From our phenotypic characterization and genetic studies, we propose that Tdy2 functions with Tdy1 to promote Suc export from leaves.
tdy2 chlorotic sectors appear shortly after the leaf blade emerges from the whorl and are stable in shape and size once formed. At this time, cell differentiation is complete and the phenotype is irreversible. Interestingly, the chlorophyll a/b ratio in tdy2 chlorotic regions is greater than in wild type or green tissue from mutant leaves. This may be an acclimation response similar to that observed in leaves that develop in high light, whereby the amount of energy harvested is reduced to prevent photodamage (Lichtenthaler et al., 1981). The altered ratio may also be due to the excess carbohydrates having a greater effect on mesophyll cells than bundle sheath cells. For example, mesophyll cells contain grana stacks that are the principal site of the chlorophyll a/b-binding protein LHCII, whereas bundle sheath cells contain mostly agranal thylakoids and lack LHCII (Kirchanksi, 1975; Schuster et al., 1985). In tdy2 chlorotic tissues, the relative increase in carbohydrate accumulation and disruption of grana stacks may be much greater in mesophyll cell chloroplasts than in bundle sheath cell plastids, which normally accumulate starch. This would result in reduced LHCII levels, the greater decrease in chlorophyll b, and the increased chlorophyll a/b ratio observed in the chlorotic regions.
Down-regulation of photosynthetic gene expression and chlorosis is known to occur under conditions of high carbohydrate accumulation. Accordingly, we determined that carbohydrates accumulate in tdy2 leaf blades prior to visible chlorosis. This suggests that chlorosis is a secondary effect and that the primary defect may be in carbon export. In agreement with this idea, lateral veins frequently were found at boundaries between green and chlorotic tdy2 regions. The association of lateral veins with tdy2 sector boundaries suggests that failure to load Suc into the veins may account for the build up of carbohydrates within the photosynthetic cells and the subsequent chlorosis. This hyperaccumulation of carbohydrates in leaf tissues is correlated with decreased transport to and reduction in the size of sink tissues (Russin et al., 1996; Burkle et al., 1998; Gottwald et al., 2000; Niittyla et al., 2004) and likely accounts for the diminished inflorescence size and yield seen in tdy2 mutants. The fact that both tdy1 and tdy2 chlorotic sector boundaries predominantly occur at lateral veins is consistent with the hypothesis that they have similar functions and act within the same pathway. In addition, because albino tissues express the tdy2 phenotype only if adjacent to chlorotic regions within green tissue, it implies that a mobile chloroplast by-product, potentially a sugar, induces the phenotype. Together, these data suggest that tdy2 chlorotic regions result from a failure to load Suc into the veins rather than from a defect in chloroplast function.
Models for Tdy1-Tdy2 Interaction
Phenotypic similarities and genetic studies suggest that Tdy1 and Tdy2 function in the same pathway to promote carbohydrate export from leaves. The SSNC interaction observed in tdy1/+; tdy2/+ F1 plants indicates that these genes may encode proteins that physically interact (Stearns and Botstein, 1988; Hawley and Gilliland, 2006), although examples are known whereby two loci display SSNC and the corresponding proteins do not physically associate but still function in the same pathway (Yook et al., 2001). Further, this interaction is specific for tdy1 and tdy2 because we did not observe any phenotype in F1 progeny from either tdy1 or tdy2 crossed with sxd1. These data, along with the double mutant characterization, suggest that the Tdy1-Tdy2 pathway is distinct from the action of Sxd1. In addition, tdy1; tdy2 double mutants showed a strong enhancement of the chlorotic phenotype, indicating that the two mutations have a synergistic interaction. These data also indicate that neither single mutant completely abrogates the biological process(es) TDY1 and TDY2 functions in. Therefore, we propose a model in which TDY1 and TDY2 form homo- and heteromeric protein complexes that function to promote Suc export.
Based on dosage analysis, tdy2 is a hypomorphic allele. This suggests tdy2 is not a null mutation and potentially produces a gene product with partially reduced function. If the tdy2 mutant allele encodes a “poison” protein with reduced function, it may be able to physically compete with wild-type TDY2 protein for binding to interacting proteins, such as TDY1. Incorporating the poison TDY2 protein into a complex is hypothesized to reduce the number of functional TDY1-TDY2 and TDY2-TDY2 protein complexes. This could account for the phenotype we observe in doubly heterozygous tdy1/+; tdy2/+ F1 plants. Similarly, in the hypoploids, by decreasing the dosage of tdy2 from two to one, the amount of the poison TDY2 protein is presumably reduced. This would sequester less of the TDY1 protein into nonfunctional TDY1-TDY2 complexes, allowing more TDY1-TDY1 functional homomeric complexes to form. This would be consistent with the milder phenotype detected.
Three models have been proposed to explain the molecular basis of SSNC (Badano and Katsanis, 2002; Hawley and Gilliland, 2006). In the first case, the two proteins may function in a protein complex. Mutation in one copy of either gene does not lead to a phenotype. However, mutation of one copy of both genes prevents formation of a functional complex and leads to a phenotype. The second model is that the two genes function in a linear pathway. A hypomorphic mutation in one gene reduces the flux of product through the pathway but does not eliminate it completely. A second hypomorphic mutation in another gene in the pathway additionally reduces the flux through the pathway, effectively eliminating production of the final product and leading to a phenotype. The third model is that the two genes function in redundant parallel pathways. Again, mutation of one copy of either gene has no phenotype. However, mutation of one copy of both genes lowers flux through both pathways sufficiently to produce a phenotype.
From additional genetic studies, we have been able to rule out the second model to explain the tdy1-tdy2 SSNC. In an effort to clone Tdy1, we performed a transposon mutagenesis and identified new alleles of tdy1 with variegated leaf phenotypes indistinguishable from the reference allele. One of these alleles was found to contain a large deletion that removed the Tdy1 gene entirely and is therefore a null allele (Golubovskaya et al., 2006). The tdy1-deletion/+; tdy2/+ F1 individuals exhibited SSNC and a sectored phenotype (D. Braun, unpublished data). Thus, our genetic analysis supports either the first or third SSNC models for the tdy1-tdy2 F1 interaction but excludes the second, as the interaction we observe between tdy1 and tdy2 is not dependent on both alleles being hypomorphic mutations. Future work characterizing the molecular nature of the gene products and whether they reside in a complex will distinguish between these models.
We previously proposed that the tdy1 leaf variegation can be explained by a threshold model (Braun et al., 2006, and refs. therein). We hypothesized that Suc accumulation above the threshold induces a chlorotic sector, while a Suc concentration below the threshold produces normal-appearing green tissue. Sensitivity to the threshold must occur for only a limited developmental window prior to or up to the time that sector formation is first visible, because, after this time, the phenotype of green mutant tissue is stable and appears similar to wild type. Perhaps local variation in photoassimilate production due to different light levels or decreased rate of Suc movement from photosynthetic cells into the phloem may account for the initiation of a chlorotic sector. Upon accumulation, Suc likely diffuses into neighboring cells where the elevated concentration induces phenotypic expression. Hence, we hypothesized that Tdy1 functions as an osmotic stress or sugar sensor to regulate SUT activity to promote Suc export (Braun et al., 2006). In the absence of TDY1, improper SUT function may lead to the failure to export Suc into the vein, the overaccumulation of carbohydrates in photosynthetic cells, and ultimately to the formation of a chlorotic sector. tdy2 mutants have a variegated phenotype strongly resembling tdy1 mutants; therefore, we propose a similar threshold model to explain the tdy2 variegation. Furthermore, the dosage sensitivity we observe in F1 plants and the synergistic interaction in the double mutants suggests that the two proteins may interact. We hypothesize that TDY1 and TDY2 form multimeric protein complexes controlling Suc export, either directly, by physically interacting with a SUT, or indirectly, perhaps by functioning in a Suc-sensing signal transduction pathway that regulates SUT abundance (Chiou and Bush, 1998; Vaughn et al., 2002; Ransom-Hodgkins et al., 2003). Mutation of one copy of either Tdy1 or Tdy2 reduces the amount of functional protein complexes but does not result in a phenotype. A second mutation in the other gene reduces the amount of functional protein complexes to the point that Suc export ability may be compromised. tdy1 and tdy2 mutant phenotypes are dependent on high light conditions likely for the synthesis of sufficient photoassimilate levels (Supplemental Fig. S6; Braun et al., 2006). In high light environments where high Suc flux is expected, these conditions reveal the limited Suc export capacity under which tdy chlorotic sectors form. As Tdy1-Tdy2 function is reduced, we suggest that the regulation of SUT activity becomes severely hampered. This would cause the Suc concentration to build up, exceed the threshold, and induce a chlorotic phenotype. In tdy1; tdy2 double mutants, the above scenario leads to an almost entirely chlorotic leaf blade that accumulates excess carbohydrates throughout.
Precedence for our model for TDY1 and TDY2 interacting in protein complexes is provided by the characterization of variegated mutants in Arabidopsis. Mutations in the VARIEGATED1 (VAR1) and VAR2 loci produce plants with green and white sectored leaves. var1; var2 double mutant plants display a synergistic interaction of severely albino leaves with few green regions (Sakamoto et al., 2002; Zaltsman et al., 2005). VAR1 and VAR2 encode similar FtsH proteins that form homomeric and heteromeric protein complexes (Chen et al., 2000; Sakamoto et al., 2002, 2003; Yu et al., 2004, 2005). Further, mutations in one gene lead to a decrease in protein abundance of the other, likely due to failure to form an active protein complex and degradation of nonassembled subunits (Sakamoto et al., 2003; Yu et al., 2004, 2005). Based on the genetic and molecular interactions, a threshold model has been proposed to explain the variegation in var1 and var2 mutants (Yu et al., 2004, 2005, 2007). Hence, this paradigm may explain our observations for the tdy1 and tdy2 genetic interaction.
Another example of the parallels between the proposed role of the Tdy loci and an Arabidopsis variegated mutant is evinced by the chlorophyll a/b binding protein gene underexpressed1 (cue1) mutant (Li et al., 1995). cue1 mutants have aberrant mesophyll cell plastids leading to a pale phenotype, while the bundle sheath cell plastids appear normal and green, giving the leaf a reticulate pigmentation pattern. cue1 is defective in the chloroplast phosphoenolpyruvate/phosphate translocator (Streatfield et al., 1999). Mutations in this transporter lead to altered mesophyll cell gene expression, plastid function, and morphology, but not to the overaccumulation of carbohydrates. The cue1 phenotype has some resemblance to the tdy mutants in affecting mesophyll cell function and in the proposed roles of TDY1 and TDY2 regulating a transporter. However, there are also differences between the cue1 and tdy phenotypes in that tdy mutants show bundle sheath cell defects and carbohydrate accumulation in photosynthetic cells that are not seen in cue1 mutants.
It is worthwhile to mention that tdy1; tdy2 double mutants with severely chlorotic leaves produce tassels that shed pollen. This observation indicates that the chlorotic leaves are capable of sufficient Suc export to support development of the inflorescence sink tissue. Therefore, as reported for tdy1; sxd1 double mutant leaves (Ma et al., 2008), tdy1; tdy2 double mutant leaves do not retain sink identity but must be source leaves with reduced export capacity. Furthermore, these data also suggest that there are additional genes that control carbon export from leaves. Future work characterizing other variegated nonclonal sectoring mutants that hyperaccumulate carbohydrates may identify these genes.
tdy Mutants as Enhanced Feedstocks for Biofuels Production
tdy mutants hyperaccumulate starch and soluble sugars in chlorotic leaf sectors. In addition, we found that excess sugars are shunted to the cell wall and deposited as cellulose without an increase in lignin. These data indicate that tdy mutant leaves have elevated cellulose levels and suggest the possibility that tdy mutants may have applications in the production of biofuels. For example, the yield of sugars that could be fermented from tdy carbon hyperaccumulating chlorotic leaf tissues is expected to be increased compared with that of nonmutants. We are currently exploring this possibility. Future work combining the tdy mutants with other maize mutants with reduced lignin deposition, the brown midrib mutants (Barriere et al., 2004), will potentially create lines with greater cellulose to lignin ratios that may serve as superior stocks for biofuels production. Lastly, we note that our data showing that there is no increase in lignin deposition in tdy mutants with excess carbon pools differs from the case in Arabidopsis. Hence, this suggests that the mechanisms regulating lignin deposition may not be conserved between Arabidopsis and grasses, and that it will be necessary to characterize cell wall biosynthesis pathways in maize and other grasses for bioenergy applications utilizing these species (Bush and Leach, 2007; Lawrence and Walbot, 2007).
MATERIALS AND METHODS
Growth Conditions and Genetic Stocks
Maize (Zea mays) plants were grown in the summer at the Pennsylvania State University Rock Springs Agronomy Farm. For the sectoring developmental time course, sector boundary analysis, photosynthesis measurements, and starch staining emerging leaves experiments, plants were grown in a greenhouse supplemented with sodium vapor and mercury halide lamps at 1,400 μmol m−2 s−1 light under a 12-h day (30°C)/12-h night (20°C). Plants were grown under low light 100 μmol m−2 s−1 in a growth chamber under a 12-h day (30°C)/12-h night (20°C). The tdy2-Reference allele (hereafter tdy2) was isolated from an ethyl methylsulfonate mutagenized F2 population. tdy2 mutants were backcrossed to the B73 inbred line three or more times prior to analyses. The tdy1-Reference (tdy1), B-A translocations, anthocyanin-accumulating genetic background, ij1, and sxd1 stocks used have been described previously (Braun et al., 2006; Baker and Braun, 2007; Ma et al., 2008). For fine mapping, tdy2 introgressed five times into B73 was outcrossed to Mo17, the F1 plants self-fertilized, and F2 progeny analyzed.
Quantitative and Double Mutant Analyses
Morphometrics, photosynthetic pigments, photosynthesis, vein boundary, starch staining, and carbohydrates measurements were performed as previously described (Braun et al., 2006; Baker and Braun, 2007; Ma et al., 2008). Double mutant plants were identified in F2 families by genotyping with PCR markers tightly linked to tdy1 (umc1653) and tdy2 (IDP217). Cellulose was measured according to Updegraff (1969).
Microscopy
Free-hand leaf cross sections were examined under reflected light, UV light, bright-field, and starch stained as described (Braun et al., 2006; Baker and Braun, 2007). Aniline blue staining of callose and TEM investigations of plasmodesmata were performed as described by Ma et al. (2008). For examining cellulose abundance, free-hand leaf cross sections were stained on the slide with aqueous 0.1 mg mL−1 calcofluor white for 2 min, rinsed, and mounted (Ruzin, 1999). Samples were examined under UV illumination with a 340-380-nm excitation filter and a 435-485-nm bandpass emission filter on a Nikon Eclipse 80i fluorescent microscope with a 100-W mercury lamp. All images within a Figure were captured using identical conditions by a Nikon DXM1200F digital camera. To inspect lignin accumulation, leaf cross sections were photographed under UV light, then stained by acidified phloroglucinol according to Ruzin (1999) and photographed in bright-field.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Developmental time course for tdy2 sector formation.
Supplemental Figure S2. Morphometric analyses of wild type and tdy2 mutants.
Supplemental Figure S3. Plasmodesmata ultrastructure in wild type and tdy2 mutants.
Supplemental Figure S4. SSNC phenotypes of tdy1/+; tdy2/+ F1 leaves.
Supplemental Figure S5. Phloroglucinol-stained wild-type and mutant leaves.
Supplemental Figure S6. Wild-type and tdy2 leaves grown under low light.
Supplemental Table S1. tdy2 chlorotic sector boundary analysis.
Supplemental Table S2. Chlorophyll levels and photosynthesis rates of wild-type and mutant leaves.
Supplemental Table S3. Cellulose quantification in wild-type and tdy2 chlorotic leaves.
Supplementary Material
Acknowledgments
We thank Tony Omeis and Scott Harkcom for excellent plant care. We thank Lisa Harper for performing the mutagenesis that generated the tdy2 allele and Mike Muszynski for help fine mapping. We are grateful to Tom Slewinski for performing the photosynthesis measurements, Sarah Nilson for expert advice, and Sally Assmann for the use of the LICOR 6400. We also thank Dan Cosgrove and Nick Kaplinsky for advice on the cellulose assays. We appreciate the suggestions of two anonymous reviewers, Mike Muszynski and Surinder Chopra for critically reading the paper, and members of the Braun and McSteen labs for discussion of the data and comments on the manuscript.
This work was partially supported by the National Research Initiative of the U.S. Department of Agriculture Cooperative State Research, Education and Extension Service (grant no. 2004–35304–14948 to D.M.B.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: David M. Braun (dbraun@psu.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Badano JL, Katsanis N (2002) Beyond Mendel: an evolving view of human genetic disease transmission. Nat Rev Genet 3 779–789 [DOI] [PubMed] [Google Scholar]
- Baker RF, Braun DM (2007) tie-dyed1 functions non-cell autonomously to control carbohydrate accumulation in maize leaves. Plant Physiol 144 867–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriere Y, Ralph J, Mechin V, Guillaumie S, Grabber J, Argillier O, Chabbert B, Lapierre C (2004) Genetic and molecular basis of grass cell wall biosynthesis and degradability. II. Lessons from brown-midrib mutants. C R Biol 327 847–860 [DOI] [PubMed] [Google Scholar]
- Beckett JB (1994) Locating recessive genes to chromosome arm with B-A translocations. In M Freeling, V Walbot, eds, The Maize Handbook. Springer-Verlag, New York, pp 315–327
- Botha CEJ, Cross RHM, van Bel AJE, Peter CI (2000) Phloem loading in the sucrose-export-defective (SXD-1) mutant maize is limited by callose deposition at plasmodesmata in bundle sheath-vascular parenchyma interface. Protoplasma 214 65–72 [Google Scholar]
- Braun DM, Ma Y, Inada N, Muszynski MG, Baker RF (2006) tie-dyed1 regulates carbohydrate accumulation in maize leaves. Plant Physiol 142 1511–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkle L, Hibberd JM, Quick WP, Kuhn C, Hirner B, Frommer WB (1998) The H+-sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves. Plant Physiol 118 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DR, Leach JE (2007) Translational genomics for bioenergy production: there's room for more than one model. Plant Cell 19 2971–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Sparvoli S, Edmunds C, Garosi P, Findlay K, Martin C (1996) DAG, a gene required for chloroplast differentiation and palisade development in Antirrhinum majus. EMBO J 15 4194–4207 [PMC free article] [PubMed] [Google Scholar]
- Chen M, Choi YD, Voytas DF, Rodermel S (2000) Mutations in the Arabidopsis VAR2 locus cause leaf variegation due to the loss of a chloroplast FtsH protease. Plant J 22 303–313 [DOI] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR (1998) Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA 95 4784–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe EH, Thompson D, Walbot V (1988) Phenotypes mediated by the iojap genotype in maize. Am J Bot 75 634–644 [DOI] [PubMed] [Google Scholar]
- Cox EL, Dickinson DB (1971) Identification of maize seedling mutants lacking starch accumulation capacity. Biochem Genet 5 15–25 [DOI] [PubMed] [Google Scholar]
- Critchley JH, Zeeman SC, Takaha T, Smith AM, Smith SM (2001) A critical role for disproportionating enzyme in starch breakdown is revealed by a knock-out mutation in Arabidopsis. Plant J 26 89–100 [DOI] [PubMed] [Google Scholar]
- Dinges JR, Colleoni C, James MG, Myers AM (2003) Mutational analysis of the pullulanase-type debranching enzyme of maize indicates multiple functions in starch metabolism. Plant Cell 15 666–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K (1977) Anatomy of Seed Plants, Ed 2. John Wiley and Sons, New York
- Evert RF, Eschrich W, Heyser W (1978) Leaf structure in relation to solute transport and phloem loading in Zea mays L. Planta 138 279–294 [DOI] [PubMed] [Google Scholar]
- Folkers U, Kirik V, Schöbinger U, Falk S, Krishnakumar S, Pollock MA, Oppenheimer DG, Day I, Reddy AR, Jürgens G, et al (2002) The cell morphogenesis gene ANGUSTIFOLIA encodes a CtBP/BARS-like protein and is involved in the control of the microtubule cytoskeleton. EMBO J 21 1280–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz E, Evert RF, Heyser W (1983) Microautoradiographic studies of phloem loading and transport in the leaf of Zea mays L. Planta 159 193–206 [DOI] [PubMed] [Google Scholar]
- Fritz E, Evert RF, Nasse H (1989) Loading and transport of assimilates in different maize leaf bundles: digital image analysis of 14C microautoradiographs. Planta 178 1–9 [DOI] [PubMed] [Google Scholar]
- Gale MD, Devos KM (1998) Plant comparative genetics after 10 years. Science 282 656–659 [DOI] [PubMed] [Google Scholar]
- Goldschmidt EE, Huber SC (1992) Regulation of photosynthesis by end-product accumulation in leaves of plants storing starch, sucrose, and hexose sugars. Plant Physiol 99 1443–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovskaya IN, Hamant O, Timofejeva L, Wang CJR, Braun D, Meeley R, Cande WZ (2006) Alleles of afd1 dissect REC8 functions during meiotic prophase I. J Cell Sci 119 3306–3315 [DOI] [PubMed] [Google Scholar]
- Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR (2000) Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc Natl Acad Sci USA 97 13979–13984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M, Juriloff D (1998) Nonallelic noncomplementation models in mice: the first arch and lidgap-Gates mutations. Genome 41 789–796 [DOI] [PubMed] [Google Scholar]
- Hawley RS, Gilliland WD (2006) Sometimes the result is not the answer: the truths and the lies that come from using the complementation test. Genetics 174 5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays T, Deuring R, Robertson B, Prout M, Fuller M (1989) Interacting proteins identified by genetic interactions: a missense mutation in alpha-tubulin fails to complement alleles of the testis-specific beta-tubulin gene of Drosophila melanogaster. Mol Cell Biol 9 875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra G, Nelson C (1969) The translocation of photosynthetically assimilated 14C in corn. Can J Bot 47 1435–1442 [Google Scholar]
- Jeannette E, Reyss A, Gregory N, Gantet P, Prioul JL (2000) Carbohydrate metabolism in a heat-girdled maize source leaf. Plant Cell Environ 23 61–69 [Google Scholar]
- Kaplinsky NJ, Freeling M (2003) Combinatorial control of meristem identity in maize inflorescences. Development 130 1149–1158 [DOI] [PubMed] [Google Scholar]
- Keddie JS, Carroll B, Jones JDG, Gruissem W (1996) The DCL gene of tomato is required for chloroplast development and palisade cell morphogenesis in leaves. EMBO J 15 4208–4217 [PMC free article] [PubMed] [Google Scholar]
- Kirchanksi SJ (1975) The ultrastructural development of the dimorphic plastids of Zea mays L. Am J Bot 62 695–705 [Google Scholar]
- Koch KE (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47 509–540 [DOI] [PubMed] [Google Scholar]
- Krapp A, Stitt M (1995) An evaluation of direct and indirect mechanisms for the “sink-regulation” of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 195 313–323 [Google Scholar]
- Kuhn C, Quick WP, Schulz A, Riesmeier JW, Sonnewald U, Frommer WB (1996) Companion cell-specific inhibition of the potato sucrose transporter SUT1. Plant Cell Environ 19 1115–1123 [Google Scholar]
- Lalonde S, Wipf D, Frommer WB (2004) Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu Rev Plant Biol 55 341–372 [DOI] [PubMed] [Google Scholar]
- Lawrence CJ, Walbot V (2007) Translational genomics for bioenergy production from fuelstock grasses: maize as the model species. Plant Cell 19 2091–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HM, Culligan K, Dixon RA, Chory J (1995) CUE1: a mesophyll cell-specific positive regulator of light-controlled gene-expression in Arabidopsis. Plant Cell 7 1599–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler H, Buschmann C, Doll M, Fietz H-J, Bach T, Kozel U, Meier D, Rahmsdorf U (1981) Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth Res 2 115–141 [DOI] [PubMed] [Google Scholar]
- Lu Y, Sharkey T (2004) The role of amylomaltase in maltose metabolism in the cytosol of photosynthetic cells. Planta 218 466–473 [DOI] [PubMed] [Google Scholar]
- Ma Y, Baker R, Magallanes-Lundback M, DellaPenna D, Braun D (2008) Tie-dyed1 and Sucrose export defective1 act independently to promote carbohydrate export from maize leaves. Planta 227 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore MA (1990) Carbohydrate metabolism in photosynthetic and nonphotosynthetic tissues of variegated leaves of Coleus blumei Benth. Plant Physiol 93 617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HJ (1932) Further studies on the nature and causes of gene mutations. In DF Jones, ed, Proceedings of the 6th International Congress of Genetics. Brooklyn Botanic Gardens, Menasha, WI, pp 213–255
- Niittyla T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC (2004) A previously unknown maltose transporter essential for starch degradation in leaves. Science 303 87–89 [DOI] [PubMed] [Google Scholar]
- Poethig RS, Szymkowiak EJ (1995) Clonal analysis of leaf development in maize. Maydica 40 67–76 [Google Scholar]
- Provencher LM, Miao L, Sinha N, Lucas WJ (2001) Sucrose export defective1 encodes a novel protein implicated in chloroplast-to-nucleus signaling. Plant Cell 13 1127–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom-Hodgkins W, Vaughn M, Bush D (2003) Protein phosphorylation plays a key role in sucrose-mediated transcriptional regulation of a phloem-specific proton-sucrose symporter. Planta 217 483–489 [DOI] [PubMed] [Google Scholar]
- Rhoades M, Carvalho A (1944) The function and structure of the parenchyma sheath plastids of the maize leaf. Bull Torrey Bot Club 71 335–346 [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB (1994) Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J 13 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers LA, Dubos C, Cullis IF, Surman C, Poole M, Willment J, Mansfield SD, Campbell MM (2005) Light, the circadian clock, and sugar perception in the control of lignin biosynthesis. J Exp Bot 56 1651–1663 [DOI] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57 675–709 [DOI] [PubMed] [Google Scholar]
- Russell SH, Evert RF (1985) Leaf vasculature in Zea mays L. Planta 164 448–458 [DOI] [PubMed] [Google Scholar]
- Russin WA, Evert RF, Vanderveer PJ, Sharkey TD, Briggs SP (1996) Modification of a specific class of plasmodesmata and loss of sucrose export ability in the sucrose export defective1 maize mutant. Plant Cell 8 645–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzin S (1999) Plant Microtechnique and Microscopy. Oxford University Press, New York
- Sakamoto W, Tamura T, Hanba-Tomita Y, Murata M (2002) The VAR1 locus of Arabidopsis encodes a chloroplastic FtsH and is responsible for leaf variegation in the mutant alleles. Genes Cells 7 769–780 [DOI] [PubMed] [Google Scholar]
- Sakamoto W, Zaltsman A, Adam Z, Takahashi Y (2003) Coordinated regulation and complex formation of YELLOW VARIEGATED1 and YELLOW VARIEGATED2, chloroplastic FtsH metalloproteases involved in the repair cycle of photosystem II in Arabidopsis thylakoid membranes. Plant Cell 15 2843–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler SE, Cahoon EB, Coughlan SJ, DellaPenna D (2003) Characterization of tocopherol cyclases from higher plants and cyanobacteria. Evolutionary implications for tocopherol synthesis and function. Plant Physiol 132 2184–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer N (2007) Molecular physiology of higher plant sucrose transporters. FEBS Lett 581 2309–2317 [DOI] [PubMed] [Google Scholar]
- Schuster G, Ohad I, Martineau B, Taylor W (1985) Differentiation and development of bundle sheath and mesophyll thylakoids in maize. Thylakoid polypeptide composition, phosphorylation, and organization of photosystem II. J Biol Chem 260 11866–11873 [PubMed] [Google Scholar]
- Sheen J (1990) Metabolic repression of transcription in higher plants. Plant Cell 2 1027–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T, Botstein D (1988) Unlinked noncomplementation: isolation of new conditional-lethal mutations in each of the tubulin genes of Saccharomyces cerevisiae. Genetics 119 249–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streatfield SJ, Weber A, Kinsman EA, Hausler RE, Li J, Post-Beittenmiller D, Kaiser WM, Pyke KA, Flugge U-I, Chory J (1999) The phosphoenolpyruvate/phosphate translocator is required for phenolic metabolism, palisade cell development, and plastid-dependent nuclear gene expression. Plant Cell 11 1609–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R (1989) The sink-source transition in leaves. Annu Rev Plant Physiol Plant Mol Biol 40 119–138 [Google Scholar]
- Turgeon R (2006) Phloem loading: how leaves gain their independence. Bioscience 56 15–24 [Google Scholar]
- Updegraff DM (1969) Semimicro determination of cellulose in biological materials. Anal Biochem 32 420–424 [DOI] [PubMed] [Google Scholar]
- Vaughn MW, Harrington GN, Bush DR (2002) Sucrose-mediated transcriptional regulation of sucrose symporter activity in the phloem. Proc Natl Acad Sci USA 99 10876–10880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walbot V, Coe EH (1979) Nuclear gene iojap conditions a programmed change to ribosome-less plastids in Zea mays. Proc Natl Acad Sci USA 76 2760–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yook KJ, Proulx SR, Jorgensen EM (2001) Rules of nonallelic noncomplementation at the synapse in Caenorhabditis elegans. Genetics 158 209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Fu A, Aluru M, Park S, Xu Y, Liu H, Liu X, Foudree A, Nambogga M, Rodermel S (2007) Variegation mutants and mechanisms of chloroplast biogenesis. Plant Cell Environ 30 350–365 [DOI] [PubMed] [Google Scholar]
- Yu F, Park S, Rodermel S (2004) The Arabidopsis FtsH metalloprotease gene family: interchangeability of subunits in chloroplast oligomeric complexes. Plant J 37 864–876 [DOI] [PubMed] [Google Scholar]
- Yu F, Park S, Rodermel SR (2005) Functional redundancy of AtFtsH metalloproteases in thylakoid membrane complexes. Plant Physiol 138 1957–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Kofler H, Hausler RE, Hille D, Flugge UI, Zeeman SC, Smith AM, Kossmann J, Lloyd J, Ritte G, et al (2001) The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell 13 1907–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaltsman A, Ori N, Adam Z (2005) Two types of FtsH protease subunits are required for chloroplast biogenesis and photosystem II repair in Arabidopsis. Plant Cell 17 2782–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman SC, Northrop F, Smith AM, Rees T (1998) A starch-accumulating mutant of Arabidopsis thaliana deficient in a chloroplastic starch-hydrolysing enzyme. Plant J 15 357–365 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.