Phytophages breach the integrity of plant tissues to recover nutrients from foliage, seeds, pollen, nectar, roots, or shoots. While many herbivores cause extensive damage, phloem-feeding insects, such as aphids and whiteflies, cause modest to barely perceptible damage, respectively. Phloem-feeding insects provide additional challenges to plants as they deplete photosynthates, vector viruses, and introduce chemical and/or protein effectors that alter plant defense signaling, infestation symptoms, and plant development (Kaloshian and Walling, 2005). When these attributes are combined with broad host ranges, breeding strategies that promote invasiveness, highly evolved feeding strategies, the ability to adapt to a wide range of plant habitats, and the emergence of insecticide-resistant strains, it is not surprising that phloem-feeding insects cause heavy losses in agriculture and horticulture (Goggin, 2007).
With the tools of cell and molecular biology, genetics, genomics, electrophysiology, and biochemistry, investigators are providing novel insights into the complexity and dynamics of plant-herbivore interactions. Many of the reviews in this issue describe the initial events in perception, as well as the defense signals and biochemical reprogramming that influence direct (antibiotic and antixenotic) and indirect (interactions with natural enemies) defenses to tissue-damaging herbivores. This review will highlight intricacies of plant-/phloem-feeding insect interactions, with a primary focus on whiteflies and comparisons to aphids.
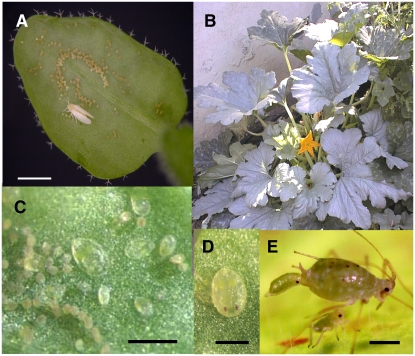
Although whiteflies and aphids are members of the Hemipteran suborder Sternorrhyncha, their life cycles, endosymbiont populations, and feeding activities are distinct (Baumann, 2005; Kaloshian and Walling, 2005). These insects use highly modified mouthparts (stylets) to navigate the cuticle, epidermis, and mesophyll and establish feeding sites in phloem sieve elements (SEs). Aphid adults and nymphs are mobile and utilize several feeding sites during their lifetime. In contrast, once the whitefly nymph establishes a feeding site on a minor vein of the phloem, nymphs (first–fourth instars) feed at this site almost continuously for 21 to 30 d (Fig. 1). The immobility of nymphs, longer life cycle, and prolonged nymph feeding are features that distinguish the whitefly-plant and aphid-plant interactions.
Figure 1.
Hemipteran development and SLWF-plant interactions. A, SLWF adult with arcs of eggs deposited on the abaxial side of an Arabidopsis ecotype Columbia leaf. Scale bar = 1 mm. B, Silverleaf symptoms on squash caused by a severe infestation in Riverside, CA. C, SLWF eggs and first, second, third, and early fourth instar nymphs on Arabidopsis leaves after 23 d of infestation. Scale bar = 0.7 mm. D, SLWF red-eye nymphs (late fourth instar) on Arabidopsis leaves after 28 d of SLWF infestation. Scale bar = 0.5 mm. E, Bluegreen aphid female giving birth to a nymph (John Klinger, University of Arizona). Scale bar = 1 mm.
Aphids and whiteflies take advantage of their adept feeding strategies and avoid or deter many plant defenses. These insects disguise themselves and deceive their hosts and natural enemies by using their stylets to deliver salivary chemicals and/or proteins into the plant to influence wound healing, defense-signaling pathways, and volatile emissions. Similar deceptive strategies are routinely employed by phytopathogenic microbes to avoid recognition and combat plant defenses (da Cunha et al., 2007). Pathogens introduce effectors into plant cells manipulating many biochemical and cellular processes to enhance phytopathogen success on host plants. In plant-biotroph interactions, effectors influence three stages of interaction (pre-entry, entry, and colonization). These interaction stages will form the framework for discussing adaptations and evasive strategies employed by phloem-feeding insects.
PRE-ENTRY STRATEGY 1: WATCH WHERE YOU STEP
The plant selection mechanisms used by phloem-feeding insects vary. Whiteflies use color, while aphids use both visual and olfactory cues to direct flight responses to host plants (Gerling, 1990; Powell et al., 2006). Upon landing, adults evaluate the tactile and chemical cues of the plant surface to determine the suitability of a plant as shelter or as a feeding and/or oviposition host. For insects that have sessile instars, like whiteflies, the plant chosen for egg deposition is a crucial maternal decision. On a good host, the next generation will thrive; on a poor host, insect populations will decline.
While on the leaf surface, insects are exposed to chemicals that are imbedded in the hydrophobic cuticular waxes, including nonvolatile secondary metabolites, as well as volatile and semivolatile compounds (i.e. monoterpenes and glucosinolate-derived volatiles), which serve to attract or repel insects (Müller and Riederer, 2005). Leaf trichomes contribute to this complex environment by exuding secondary metabolites and proteins that have antibiotic or antixenotic effects (Wagner et al., 2004). Glandular trichome exudates deter whitefly settling and entrap whiteflies, providing one of the most effective whitefly-resistance mechanisms known to date. Surprisingly, whiteflies prefer plants with nonglandular trichomes over glabrous plants and preferentially oviposit near trichome bases (Neal and Bentz, 1999). These trichomes provide shelter for the sessile nymphs, and trichome exudates deter natural enemies. Whiteflies are not perturbed by these exudates because whiteflies shed particles of wax from their coat to form a physical barrier to trichome exudates.
Interestingly, trichomes also induce a beneficial polyphenism in whiteflies (Guershon and Gerling, 2006). On glabrous leaves, all whitefly nymph cases are flat. On leaves with trichomes, most of the fourth-instar cases are decorated with dorsal waxy projections (setae). Setea are induced by the tactile experiences (collisions with trichomes, exuvia, and eggs) of the mobile whitefly crawler. Setose nymphs are smaller, develop more rapidly, and provide less time for enemies to identify their prey. Furthermore, predators prefer non-setate nymphs.
PRE-ENTRY STRATEGY 2: TAKE A FREE SAMPLE PRIOR TO CHOOSING A MAIN DISH
Whiteflies and aphids use tactile and gustatory cues to determine the value of a plant as a feeding and oviposition host (Gerling, 1990; Powell et al., 2006). During initial encounters with a plant, these insects often use their stylets to tap on and make shallow probes of the leaf surface. Combined with secretion of small amounts of watery saliva to dissolve surface chemicals and imbibition of the liquids at the surface (Miles, 1999), whiteflies and aphids can determine physical features and “taste” the chemical defenses of the phylloplane. These behaviors detect differences in the carbohydrate content of cell walls, epicuticular waxes, and presence or absence of secondary metabolites to determine nonhost or host status (Müller and Riederer, 2005). If the plant is unacceptable, the winged adults depart in search of a more suitable site or host. Therefore, like tissue-damaging caterpillars, the ability to move within and between plants is important for avoiding defenses in initial hemipteran-plant interactions (Paschold et al., 2007).
ENTRY STRATEGY 1: DODGE DEFENSES
The damage caused by cell punctures and the nature of salivary effectors will determine the defense-signaling pathways that are activated and metabolites and proteins that accumulate in the infested plant. In addition, the stylet path determines the constitutive and induced defenses an herbivore will encounter. To limit damage to epidermal cells and contact with extracellular defenses, hemiptera deposit beads of rapidly gelling saliva to form a flange at the leaf surface (to limit stylet slippage) and a sheath that insulates the stylets from apoplastic defenses, respectively (Miles, 1999). In addition, the sheath's polyphenol oxidases may polymerize apoplastic phenolics (an induced defense) to prevent damage to plant cells. Finally, the sheath provides a track along which stylets move. This limits cellular damage as evidenced by the tracks of whitefly nymph stylets after larval molts and Astegopteyx minuta's opportunistic use of dislodged aphid sheaths to guide its own stylet to an SE (Foster, 1996; Freeman et al., 2001).
Aphid and whitefly stylet sheath paths are multi-branched, showing that stylets take tortuous routes to the phloem (Freeman et al., 2001; Tjallingii, 2006). During this journey, aphids puncture and “taste” virtually all mesophyll cells on their path to a major vein of the phloem. This appears to orient the stylet's progression toward an SE. Plant cell damage can be moderate to extensive depending on the mechanics and vigor of aphid stylet probing and the effectors introduced by the salivas. Therefore, it is not surprising that wound-signaling pathways are transiently activated by aphids (Martinez de Ilarduya et al., 2003). In contrast, whiteflies rarely puncture mesophyll cells and, thereby, avoid activation of wound responses and contact with the potent defenses that are stored within vacuoles and apoplasts of these cells (Walling, 2000; Kempema et al., 2007). Like aphids, whiteflies secrete saliva to allow appraisal of the chemical composition of the apoplast (Lei et al., 1998); these gustatory cues may provide directionality to stylet movement and provide up-to-date information about host suitability for feeding and oviposition.
ENTRY STRATEGY 2: PLUG THE HOLES
When a stylet pierces a phloem SE, the plasma membrane lesion must be rapidly sealed to prevent leakage of phloem sap into the apoplast (Will and van Bel, 2006). Plants repair SE wounds by depositing callose and proteins, and hemiptera modify these responses to enhance their own success. Gelling saliva cements the stylet sheath to the SE, rapidly sealing the puncture site. Apoplastic callose deposits also reinforce this repair (Kempema et al., 2007; Saheed et al., 2007). In the cytoplasm, proteins aggregate at an SE lesion to occlude the wound. In the Fabaceae, a unique protein complex (forisome), which changes conformation in response to wound-induced alterations in free Ca2+ or redox state, is used to seal wounds (Will and van Bel, 2006).
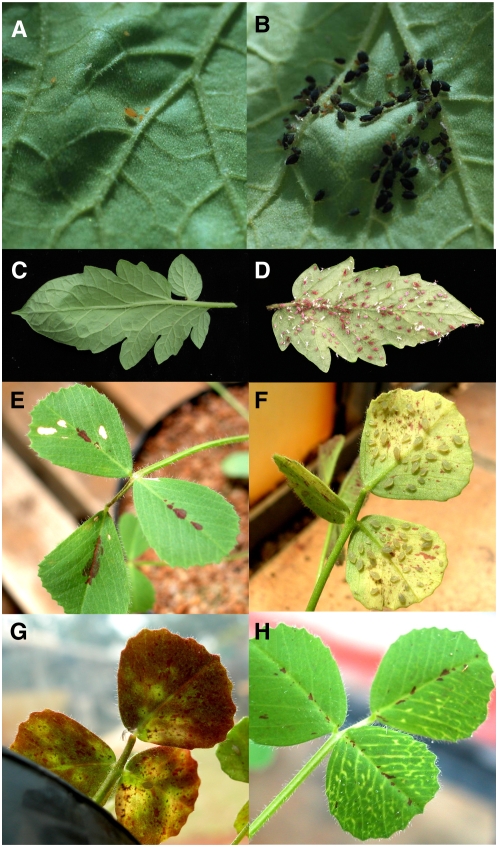
The closure of stylet-induced lesions, while essential for maintaining SE turgor, can inadvertently block an insect's food canal. In fact, the melon (Cucumis melo) Vat gene appears to enhance SE wound healing and, thereby, confers resistance to aphids (Martin et al., 2003; Fig. 2). Therefore, it is not surprising that hemiptera antagonize the cytosolic wound-healing events (Will et al., 2007). By sealing the SE lesion with sheath saliva, aphids thwart the influx of apoplastic Ca2+ and prevent the coagulation of proteins. In addition, aphid watery saliva contains Ca2+-binding proteins and prevents the Ca2+-dependent structural changes to forisomes in vitro and presumably antagonizes protein depositions that could block the aphid's food canal in planta. In addition, watery saliva peroxidases may maintain SE redox conditions to prevent feeding site occlusion (Miles, 1999). Aphids clearly antagonize the innate plant wound responses to make the plant a more suitable host. It is presumed that the salivas of other phloem-feeders have similar roles.
Figure 2.
Infestations and symptoms on aphid-resistant and -susceptible melons, tomatoes, and Medicago. Cotton aphid (Aphis gossypii)-melon interactions: A and B, Cotton aphid infestation of the resistant melon ‘AR5’ line (Vat gene; A) and susceptible melon ‘PMR5’ line (B; G. Thompson, Oklahoma State University). Notice lack of overt symptoms in the incompatible and compatible interactions. Potato aphid-tomato interactions: C and D, Potato aphid infestation of the resistant tomato variety ‘Motelle’ (Mi-1.2 gene; C) and susceptible ‘Moneymaker’ (mi-1.2; D; Isgouhi Kaloshian, UC Riverside). Aphid-M. truncatula interactions: E, Bluegreen aphid infestation of the susceptible ‘A17’ line causes necrosis. F, Spotted alfalfa aphid-infested ‘Borung’ (spotted alfalfa aphid-susceptible line; ttk AKR) displaying local chlorosis. G, Spotted alfalfa aphid infestation of the resistant ‘Mogul’ line (TTR AKR) displaying local purple haze. H, Spotted alfalfa aphid infestation of the susceptible ‘A20’ line displaying systemic vein chlorosis. All Medicago-aphid interaction photos were provided by John Klinger (University of Arizona).
COLONIZATION STRATEGY 1: DECEIVE YOUR HOST AND SUPPRESS EFFECTIVE DEFENSES
Most insects are deterred by the chemical complexity of a plant's phylloplane. However, some insects tolerate these constitutive defenses and use a plant as a host (a compatible interaction). During compatible interactions, plants perceive the amount of tissue damage, the quality and quantity of salivary signals (effectors), and the magnitude of electrical and/or hydraulic signals caused by hemipteran attack (Walling, 2000). After integration of this suite of signals, plants deploy signal transduction pathways to regulate large cohorts of genes to provide the “best” defense response to its intruder. Many induced genes appear to address the changes in physiological status imposed by hemipteran feeding, and defense-response genes are activated or suppressed (Thompson and Goggin, 2006). Almost without exception, pathogenesis-response (PR) gene RNAs, proteins, and/or activities are elevated after phloem-feeding insect attack (Walling, 2000).
Salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) control many of the cellular and biochemical responses to pathogens and pests. These signaling pathways cross talk and may act antagonistically or synergistically (Koornneef and Pieterse, 2008). The SA, JA, and ET networks also liaise with other known (i.e. auxin, abscisic acid, and brassinosteroids) and novel defense-signaling networks to provide the innate immunity to pests/pathogens (Robert-Seilaniantz et al., 2007). The integration of defense networks may minimize expression of costly and ineffective defenses that divert carbon and nitrogen resources from plant growth and reproduction. However, similar to pathogens, insects have leveraged the molecular communication between the signaling networks to enhance their success on host plants.
The Bemisia tabaci biotype B (silverleaf whitefly [SLWF])-Arabidopsis (Arabidopsis thaliana) interaction is a clear example of an insect manipulating plant signaling to suppress effective defenses, increase plant susceptibility, and enhance insect performance (Kempema et al., 2007; Zarate et al., 2007). In response to SLWF nymph feeding, SA-regulated RNAs increase locally and systemically, while JA- and ET-regulated RNAs are unchanged or decline. The correlation of defense gene RNA levels and SLWF performance on JA and SA defense mutants show that JA-regulated defenses are important in deterring SLWF nymph development. Furthermore, when the npr1 mutant, which impairs SA-regulated defenses and uncouples SA-JA cross talk, is treated with methyl jasmonate (MeJA), SLWF nymph development is severely delayed, demonstrating that JA controls defenses that actively thwart whitefly development.
SLWFs deceive Arabidopsis plants and prevent the activation of the JA-regulated defenses that actively deter nymph development. SLWFs may merely evade JA-regulated defenses due to the absence of tissue damage once a feeding site is established. Alternatively, nymph saliva may contain effectors that directly antagonize JA-regulated defenses. There is precedent for an insect effector (Glc oxidase) to suppress effective direct (nicotine production) and indirect (volatile biosynthesis) defenses (Musser et al., 2002; Bede et al., 2006). It is also possible that a nymph effector could act indirectly by increasing SA levels and leveraging SA-JA cross talk mechanisms to inhibit the expression of JA-regulated defenses. This is supported by the fact that SLWFs increase SA levels locally and systemically during infestation of Arabidopsis (S.I. Zarate, D.A. Navarre, and L.L. Walling, unpublished results).
Similar evasive strategies appear to be active during the aphid interactions with Arabidopsis, sorghum (Sorghum bicolor), and Medicago truncatula. Behavioral studies on aphid-preinfested plants indicate that aphid feeding reduces the defenses that deter aphids (Prado and Tjallingii, 2007). In addition, SA-regulated RNAs increase and JA-regulated RNAs are reduced or increase modestly in aphid-infested leaves (Moran and Thompson, 2001; Ellis et al., 2002; Zhu-Salzman et al., 2004; De Vos et al., 2005; Gao et al., 2007). Unlike whiteflies, only modest systemic responses occur, and changes in SA or JA levels are not detected after Myzus persicae infestation of Arabidopsis (De Vos et al., 2005). Aphid performance on a large number of Arabidopsis mutants has been reported (Thompson and Goggin, 2006; de Vos et al., 2007). While the impact of SA and JA defense mutants on aphid population growth has varied, JA-regulated defenses appear to be important in deterring aphid population expansion in Arabidopsis. In addition, MeJA treatment of Arabidopsis, sorghum, and Medicago plants retards aphid population expansion (Ellis et al., 2002; Zhu-Salzman et al., 2004; Gao et al., 2007). Collectively, these data indicate that aphids, like whiteflies, express “decoy” defenses and suppress or avoid the JA-regulated defenses that antagonize insect performance (Thompson and Goggin, 2006).
The identities of most of the JA-regulated resistance traits suppressed during whitefly and aphid infestations are unknown. However, one of these traits appears to be synthesis of glucosinolates, which is complexly regulated by SA, JA, and ET (Mewis et al., 2006). Upon tissue damage, glucosinolates and their hydrolyzing enzymes (myrosinases) mix to release highly toxic products (de Vos et al., 2007). Phloem feeders avoid cellular damage and, therefore, do not generate myrosinase-hydrolysis products, do not activate glucosinolate biosynthesis/catabolism genes, and reduce total glucosinolate levels (Mewis et al., 2006; Kempema et al., 2007; Kim and Jander, 2007). A reduction in glucosinolate levels creates a more insect-friendly environment for generalists, which are repelled by glucosinolates. However, reduced glucosinolates may be a disadvantage for specialist aphids that are attracted to and utilize these compounds for their own defense (Bridges et al., 2002). While aphids appear to effectively maneuver around most glucosinolates, one indolic glucosinolate, 4M13M, is synthesized at elevated levels after aphid infestation and is a potent aphid deterrent (Kim and Jander, 2007), indicating that some effective constitutive defenses can be up-regulated in response to hemipteran feeding.
It is not clear if all hemipterans suppress a subset of plant defenses to enhance their success. These conclusions can be made only when defense mutants are used in performance assays and RNAs for genes for each defense-signaling network are monitored. This is a challenge in most plants, even Arabidopsis, where networks are complex and novel defense-signaling networks are being revealed (Robert-Seilaniantz et al., 2007). In crops, the roles of the JA-, SA-, and ET-signaling networks are not completely understood and appear to vary (Karban and Chen, 2007). Therefore, it is not surprising to find exceptions to the decoy hypothesis. For example, both SA and JA appear important in tomato's (Solanum lycopersicum) innate immunity to the potato aphid (Macrosiphum euphorbiae) and controlling antibiotic and antixenotic traits, respectively (Li et al., 2006; Bhattarai et al., 2007b).
COLONIZATION STRATEGY 2: TOLERATE DEFENSES THAT ARE EFFECTIVE AGAINST OTHER HEMIPTERA
Whiteflies and aphids increase PAD4 and SAG RNA levels (Pegadaraju et al., 2005; Kempema et al., 2007). The PAD4 lipase promotes senescence, increases in SAG RNAs, and SA accumulation. PAD4 controls a phloem-mediated resistance to aphids that is independent of EDS1. Surprisingly, PAD4 does not influence SLWF nymph development. SLWFs may lack effectors to activate or possess effectors to deter the PAD4 phloem-based resistance. Alternatively, SLWFs may tolerate the PAD4-regulated antibiosis using chemical detoxification and/or sequestration mechanisms, which are effective strategies to counter plant defenses.
While defenses effective against aphids and whiteflies vary, it is difficult to assess the degree of overlap and specificity in the molecular responses to different phloem feeders (De Vos et al., 2005; Kempema et al., 2007). This is largely due to the wide variety of experimental parameters that influence these assessments, including natal plants for rearing colonies, infestation levels, duration of infestation, duration of feeding, and variation in microarray experimental design and statistical analysis. However, specificity in plant molecular, biochemical, and physiological responses to insects is observed frequently in volatile production and tritrophic interactions (Arimura et al., 2005) and is also supported by whitefly-crop interactions. Squash (Curcubita pepo) plants can discriminate between two closely related whitefly biotypes (SLWF and B. tabaci biotype A) as evidenced by the induction of different sets of genes and developmental disorders (van de Ven et al., 2000; Fig. 1).
COLONIZATION STRATEGY 3: EVEN WHEN YOUR HOST RECOGNIZES YOU, DON'T GIVE UP
During incompatible interactions, a plant with a resistance (R) gene rapidly recognizes an avirulent insect and the infestation is curtailed (Kaloshian and Walling, 2005). While little is known about the genes encoding effectors in avirulent insects, R genes effective against insects have been genetically characterized and mapped and genome walking strategies are being employed to identify these loci. To date, only one R gene (Mi-1.2) that confers resistance to insects is characterized at the molecular level. Mi-1.2 plants are resistant to the potato aphid, two whitefly biotypes (SLWF and biotype Q), a psyllid, and three nematode species, and the mechanisms of resistance appear distinct (Kaloshian and Walling, 2005; Casteel et al., 2006, and refs. therein). For example, Mi-1.2 resistance to nematodes displays a hypersensitive response (cell death), which does not occur in the resistance response to aphids (Fig. 2). Potato aphid resistance is antibiotic and phloem based, while resistance to phloem-feeding psyllids is antixenotic. Finally, Mi-1.2-mediated resistance to whiteflies is expressed in the epidermis or mesophyll and deters whitefly settling. If a whitefly establishes a feeding site, it can develop unimpaired on Mi-1.2 plants. The biochemical basis for the Mi-1.2 resistance to four animal taxa and the pest effectors in these incompatible interactions are not presently known.
The defense-signaling mechanisms that control plant-mediated aphid resistance have parallels to incompatible responses in plant-pathogen interactions. For example, aphids cause more rapid increases in SA levels and/or PR gene RNAs in resistant than in susceptible plants (Forslund et al., 2000; Mohase and van der Westhuizen, 2002; Martinez de Ilarduya et al., 2003). In addition, Mi-1.2-mediated resistance to aphids is dependent on HSP90, SGT1, and SA (Bhattarai et al., 2007a). However, this contrasts with the M. truncatula AKR gene that imparts resistance to the bluegreen aphid (Acyrthosiphon kondoi) by leveraging JA-regulated defenses (Gao et al., 2007). Bluegreen aphid and spotted alfalfa aphid (Therioaphis trifolii) interactions with Medicago are complex. Distinctive phenotypes are observed in both compatible and incompatible interactions (Fig. 2).
It is presumed that insect saliva contains the signals (avirulence effectors) that trigger the incompatible interaction using mechanisms proposed in the guard hypothesis (Kaloshian and Walling, 2005). Based on the diversity of avirulence effectors in microbes and the known salivary effectors from herbivores that influence volatile production, insect effectors may be a chemical, protein, or peptide (Arimura et al., 2005; da Cunha et al., 2007; Schmelz et al., 2007). While an insect avirulence effector has not yet been identified, significant strides in identifying avirulence genes from diptera and effectors important in compatible phloem-feeding insect interactions are being made. For example, a salivary protein extract from Russian wheat aphids (Diuraphis noxia) can mimic the symptoms of infested susceptible plants (Lapitan et al., 2007).
COLONIZATION STRATEGY 4: AVOID ELICITING VOLATILE RELEASE
Immediately after herbivore attack or egg deposition, plants release stored volatiles and initiate synthesis of new volatiles for emission from the infested and distal uninfested leaves (Arimura et al., 2005). Herbivore-induced plant volatiles (HIPVs) are used by tissue-damaging and phloem-feeding herbivores and their natural enemies to discriminate between uninfested and infested host plants, providing a potent indirect defense. Volatiles also act directly to decrease fecundity, enhance or deter feeding, and provide information about herbivore density. Volatiles can also boost direct defenses by activating defense signal transduction pathways. In addition, similar to the induced systemic resistance seen with phytopathogens, HIPVs can prime plants for enhanced defense responses upon subsequent challenge (Turlings and Ton, 2006; Frost et al., 2008).
Like caterpillars, hemipteran saliva can stimulate volatile production (Williams et al., 2005), and the volatile blends emitted by phloem-feeding insects have chemical compositions similar to those of tissue-damaging herbivores. HIPVs contain dozens of chemicals, including C6 volatiles, cis-jasmone, MeJA, methyl salicylate, indoles, and terpenoids. While some phloem feeders synthesize new volatile compounds (Birkett et al., 2003), others merely change the proportions of the chemicals in the volatile blend (Lu et al., 2006) to impart specificity. Unlike tissue-damaging herbivores, the quantities of volatiles emitted in response to phloem feeders are low and at times undetectable (Du et al., 1998; Turlings et al., 1998; Rodriguez-Saona et al., 2003). This is likely due to the minimal damage inflicted or introduction of salivary effectors that deter volatile synthesis. Avoiding the emission of HIPVs could be beneficial; smaller volatile emissions could result in fewer direct and indirect defenses.
SUMMARY AND FUTURE PROSPECTIVES
While the molecular, biochemical, and physiological changes that dictate the outcome of plant-insect interactions are beginning to be revealed, it is clear our knowledge remains in a relatively primitive state. To date, it is not clear if all herbivores manipulate one or multiple host responses for their success or if evasive tactics are employed only by a distinctive subset of phytophages. Phloem-feeding whiteflies and aphids clearly employ a wide variety of tactics to avoid or suppress effective defenses.
The importance of hemipteran salivary proteins in stimulating or suppressing plant wound and defense responses is now clear and with one exception (i.e. Ca2+-binding proteins of aphid watery saliva), the biochemical nature of effectors from phloem-feeding insects are unknown. Therefore, a renewed emphasis on biochemical characterization of insect salivas is emerging and will benefit from the increased sensitivity of current metabolomic and proteomics technologies and is likely to utilize novel transgenic bioassays to assess effector activity. These biochemical strategies will be enhanced by the growing genomics resources (genome sequences, EST collections, and microarrays) for hemipterans and their endosymbionts (Leshkowitz et al., 2006). The recent development of RNA interference technologies for aphids and whiteflies that allow down-regulation of insect genes will enable the identification of the effectors that enhance hemipteran success (Ghanim et al., 2007). These integrative approaches are also likely to allow the identification of the components of hemipteran saliva that stimulate developmental disorders, infestation symptoms, and elicit incompatible plant-insect interactions (Figs. 1 and 2). Ultimately, the identity of the factors that bind to these effectors in planta will be revealed.
Complementary studies to explore the complexity of plant defense-signaling networks are crucial. Today, we have snapshots of gene expression profiles after hemipteran attack. Even in Arabidopsis, where genomics resources are abundant and expression profiles are available for numerous plant-pathogen interactions, we have an incomplete knowledge of these defense networks. While oscillations of JA- and SA-regulated pathways are apparent, the integrative hubs and interconnections to other signaling pathways during plant-insect interactions have yet to be identified. Systems biology approaches to amalgamate our knowledge of pathogen and pest signaling will be critical to understand the similarities and distinctions with pathogenic biotrophs and insects. In addition, the identity of novel attacker-specific defense pathways will need to be revealed and integrated with well-characterized defense signal transduction pathways. The impact of chemical genomics screens with reporter gene bioassays should become increasingly important tools. These assays can be used to identify the molecules that induce or perturb novel insect-regulated signaling pathways and should provide clues about the chemical nature of the novel plant signal molecules and/or the insect effectors or elicitors that elude biochemical characterization.
Finally, enhanced genomics resources for crop plants will be critical tools for understanding if the principles emerging from studies in model plants (Arabidopsis, Medicago) pertain to crops. High-quality and comprehensive microarrays are emerging for a variety of crops and should reveal the dynamics of defense signaling in hemipteran interactions with both monocots and dicots. Complementation of these genomics resources with genetic approaches (i.e. use of mutants, viral-induced gene silencing, RNA interference strategies, and ectopic expression of defense gene products) will reveal the signaling networks and the specific factors that dictate innate immunity and gene-for-gene-mediated defense to phloem-feeding insects. These data, when coordinated with assays to assess insect performance and interactions at the third and fourth trophic levels, should allow for the development of cogent strategies to enhance plant-based resistance to hemiptera.
Acknowledgments
I thank Fran Holzer (UC Riverside), Dr. Mien van de Ven (UC Riverside), and Dr. David Carter (UC Riverside) for images of whiteflies and whitefly-infested plants, and Drs. Isgouhi Kaloshian (UC Riverside), Gary Thompson (Oklahoma State University), and John Klingler (University of Arizona) for aphid-plant interaction photos. I thank helpful and creative suggestions of two anonymous reviewers and members of the Walling lab for helpful discussions.
This work was supported in part by the California Agricultural Experiment Station, by the U.S. Department of Agriculture (USDA)-Southwest Consortium Grant for Genetics and Water Resources, and by the USDA-NRI Cooperative State Research, Education, and Extension Service (award no. 99–35301–8077 to L.L.W.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Linda L. Walling (linda.walling@ucr.edu).
References
- Arimura G, Kost C, Boland W (2005) Herbivore-induced, indirect plant defences. Biochim Biophys Acta 1734 91–111 [DOI] [PubMed] [Google Scholar]
- Baumann P (2005) Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol 59 155–189 [DOI] [PubMed] [Google Scholar]
- Bede JC, Musser RO, Felton GW, Korth KL (2006) Caterpillar herbivory and salivary enzymes decrease transcript levels of Medicago truncatula genes encoding early enzymes in terpenoid biosynthesis. Plant Mol Biol 60 519–531 [DOI] [PubMed] [Google Scholar]
- Bhattarai KK, Li Q, Liu Y, Dinesh-Kumar SP, Kaloshian I (2007. a) The Mi-1-mediated pest resistance requires Hsp90 and Sgt1. Plant Physiol 144 312–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai KK, Xie QG, Pourshalimi D, Younglove T, Kaloshian I (2007. b) Coi1-dependent signaling pathway is not required for Mi-1-mediated potato aphid resistance. Mol Plant Microbe Interact 20 276–282 [DOI] [PubMed] [Google Scholar]
- Birkett MA, Chamberlain K, Guerrieri E, Pickett JA, Wadhams LJ, Yasuda T (2003) Volatiles from whitefly-infested plants elicit a host-locating response in the parasitoid, Encarsia formosa. J Chem Ecol 29 1589–1600 [DOI] [PubMed] [Google Scholar]
- Bridges M, Jones AME, Bones AM, Hodgson C, Cole R, Bartlet E, Wallsgrove R, Karapapa VK, Watts N, Rossiter JT (2002) Spatial organization of the glucosinolate-myrosinase system in brassica specialist aphids is similar to that of the host plant. Proc R Soc Lond B Biol Sci 269 187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteel CL, Walling LL, Paine TD (2006) Behavior and biology of the tomato psyllid, Bactericerca cockerelli, in response to the Mi-1.2 gene. Entomol Exp Appl 121 67–72 [Google Scholar]
- da Cunha L, Sreerekha MV, Mackey D (2007) Defense suppression by virulence effectors of bacterial phytopathogens. Curr Opin Plant Biol 10 349–357 [DOI] [PubMed] [Google Scholar]
- de Vos M, Kim JH, Jander G (2007) Biochemistry and molecular biology of Arabidopsis-aphid interactions. Bioessays 29 871–883 [DOI] [PubMed] [Google Scholar]
- De Vos M, Van Oosten VR, Van Poecke RMP, Van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Metraux JP, Van Loon LC, Dicke M, et al (2005) Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact 18 923–937 [DOI] [PubMed] [Google Scholar]
- Du YJ, Poppy GM, Powell W, Pickett JA, Wadhams LJ, Woodcock CM (1998) Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J Chem Ecol 24 1355–1368 [Google Scholar]
- Ellis C, Karafyllidis I, Turner JG (2002) Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol Plant Microbe Interact 15 1025–1030 [DOI] [PubMed] [Google Scholar]
- Forslund K, Pettersson J, Bryngelsson T, Jonsson L (2000) Aphid infestation induces PR proteins differently in barley susceptible or resistant to the birdcherry-oat aphid (Rhopalosiphum padi). Physiol Plant 110 496–502 [Google Scholar]
- Foster WA (1996) Duelling aphids: Intraspecific fighting in Astegopteryx minuta (Homoptera:Hormaphididae). Anim Behav 51 645–655 [Google Scholar]
- Freeman TP, Buckner JS, Nelson DR, Chu C-c, Henneberry TJ (2001) Stylet penetration by Bemisia argentifolii (Homoptera: Aleyrodidae) into host leaf tissue. Ann Entomol Soc Am 94 761–768 [Google Scholar]
- Frost CJ, Mescher MC, Carlson JE, De Moraes CM (2008) Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol 146: 818–824 [DOI] [PMC free article] [PubMed]
- Gao LL, Anderson JP, Klingler JP, Nair RM, Edwards OR, Singh KB (2007) Involvement of the octadecanoid pathway in bluegreen aphid resistance in Medicago truncatula. Mol Plant Microbe Interact 20 82–93 [DOI] [PubMed] [Google Scholar]
- Gerling D (1990) Whiteflies: Their Bionomics, Pest Status and Management. Intercept Ltd., Andover, UK
- Ghanim M, Kontsedalov S, Czosnek H (2007) Tissue-specific gene silencing by RNA interference in the whitefly Bemisia tabaci (Gennadius). Insect Biochem Mol Biol 37 732–738 [DOI] [PubMed] [Google Scholar]
- Goggin FL (2007) Plant-aphid interactions: molecular and ecological perspectives. Curr Opin Plant Biol 10 399–408 [DOI] [PubMed] [Google Scholar]
- Guershon M, Gerling D (2006) Effects of plant and prey characteristics on the predatory behavior of Delphastus catalinae. Entomol Exp Appl 121 15–21 [Google Scholar]
- Kaloshian I, Walling LL (2005) Hemipterans as plant pathogens. Annu Rev Plant Biol 43 491–521 [DOI] [PubMed] [Google Scholar]
- Karban R, Chen Y (2007) Induced resistance in rice against insects. Bull Entomol Res 97 327–335 [DOI] [PubMed] [Google Scholar]
- Kempema LA, Cui X, Holzer FM, Walling LL (2007) Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs. Similarities and distinctions in responses to aphids. Plant Physiol 143 849–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Jander G (2007) Myzus persicae (green peach aphid) feeding on Arabidopsis induces the formation of a deterrent indole glucosinolate. Plant J 49 1008–1019 [DOI] [PubMed] [Google Scholar]
- Koornneef A, Pieterse CMJ (2008) Cross talk in defense signaling. Plant Physiol 146: 839–844 [DOI] [PMC free article] [PubMed]
- Lapitan NLV, Li YC, Peng JH, Botha AM (2007) Fractionated extracts of Russian wheat aphid eliciting defense responses in wheat. J Econ Entomol 100 990–999 [DOI] [PubMed] [Google Scholar]
- Lei H, Xu R, Tjallingii WF, Van Lenteren JC (1998) Electrical penetration graphs of greenhouse whitefly, Trialeurodes vaporariorum (Westwood). Acta Entomol Sin 41 113–123 [Google Scholar]
- Leshkowitz D, Gazit S, Reuveni E, Ghanim M, Czosnek H, McKenzie C, Shatters RL, Brown JK (2006) Whitefly (Bemisia tabaci) genome project: analysis of sequenced clones from egg, instar, and adult (viruliferous and non-viruliferous) cDNA libraries. BMC Genomics 7 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Xie QG, Smith-Becker J, Navarre DA, Kaloshian I (2006) Mi-1-mediated aphid resistance involves salicylic acid and mitogen-activated protein kinase signaling cascades. Mol Plant Microbe Interact 19 655–664 [DOI] [PubMed] [Google Scholar]
- Lu YJ, Wang X, Lou YG, Cheng JA (2006) Role of ethylene signaling in the production of rice volatiles induced by the rice brown planthopper Nilaparvata lugens. Chin Sci Bull 51 2457–2465 [Google Scholar]
- Martin B, Rahbe Y, Fereres A (2003) Blockage of stylet tips as the mechanism of resistance to virus transmission by Aphis gossypii in melon lines bearing the Vat gene. Ann Appl Biol 142 245–250 [Google Scholar]
- Martinez de Ilarduya O, Xie QG, Kaloshian I (2003) Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions. Mol Plant Microbe Interact 16 699–708 [DOI] [PubMed] [Google Scholar]
- Mewis I, Tokuhisa JG, Schultz JC, Appel HM, Ulrichs C, Gershenzon J (2006) Gene expression and glucosinolate accumulation in Arabidopsis thaliana in response to generalist and specialist herbivores of different feeding guilds and the role of defense signaling pathways. Phytochemistry 67 2450–2462 [DOI] [PubMed] [Google Scholar]
- Miles PW (1999) Aphid saliva. Biol Rev 74 41–85 [Google Scholar]
- Mohase L, van der Westhuizen AJ (2002) Salicylic acid is involved in resistance responses in the Russian wheat aphid-wheat interaction. J Plant Physiol 159 585–590 [DOI] [PubMed] [Google Scholar]
- Moran PJ, Thompson GA (2001) Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol 125 1074–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C, Riederer M (2005) Plant surface properties in chemical ecology. J Chem Ecol 31 2621–2651 [DOI] [PubMed] [Google Scholar]
- Musser RO, Hum-Musser SM, Eichenseer H, Peiffer M, Ervin G, Murphy JB, Felton GW (2002) Herbivory: caterpillar saliva beats plant defences: a new weapon emerges in the evolutionary arms race between plants and herbivores. Nature 416 599–600 [DOI] [PubMed] [Google Scholar]
- Neal JW, Bentz JA (1999) Evidence for the stage inducing phenotypic plasticity in pupae of the polyphagous whiteflies Trialeurodes vaporariorum and Bemisia argentifolii (Homoptera: Aleyrodidae) and the raison d'etre. Ann Entomol Soc Am 92 774–787 [Google Scholar]
- Paschold A, Halitschke R, Baldwin IT (2007) Co(i)-ordinating defenses: NaCOI1 mediates herbivore- induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant J 51 79–91 [DOI] [PubMed] [Google Scholar]
- Pegadaraju V, Knepper C, Reese J, Shah J (2005) Premature leaf senescence modulated by the Arabidopsis PHYTOALEXIN DEFICIENT4 gene is associated with defense against the phloem-feeding green peach aphid. Plant Physiol 139 1927–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell G, Tosh CR, Hardie J (2006) Host plant selection by aphids: behavioral, evolutionary, and applied perspectives. Annu Rev Entomol 51 309–330 [DOI] [PubMed] [Google Scholar]
- Prado E, Tjallingii WF (2007) Behavioral evidence for local reduction of aphid-induced resistance. J Insect Sci 7 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Seilaniantz A, Navarro L, Bari R, Jones JDG (2007) Pathological hormone imbalances. Curr Opin Plant Biol 10 372–379 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Saona C, Crafts-Brandner SJ, Canas LA (2003) Volatile emissions triggered by multiple herbivore damage: beet armyworm and whitefly feeding on cotton plants. J Chem Ecol 29 2539–2550 [DOI] [PubMed] [Google Scholar]
- Saheed SA, Larsson KAE, Delp G, Botha CEJ, Jonsson LMV, Bradley G (2007) Wound callose synthesis in response to Russian wheat aphid and bird cherry-oat aphid feeding on barley cv Clipper. S Afr J Bot 73 310 [Google Scholar]
- Schmelz EA, LeClere S, Carroll MJ, Alborn HT, Teal PEA (2007) Cowpea chloroplastic ATP synthase is the source of multiple plant defense elicitors during insect herbivory. Plant Physiol 144 793–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GA, Goggin FL (2006) Transcriptomics and functional genomics of plant defence induction by phloem-feeding insects. J Exp Bot 57 755–766 [DOI] [PubMed] [Google Scholar]
- Tjallingii WF (2006) Salivary secretions by aphids interacting with proteins of phloem wound responses. J Exp Bot 57 739–745 [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Bernasconi M, Bertossa R, Bigler F, Caloz G, Dorn S (1998) The induction of volatile emissions in maize by three herbivore species with different feeding habits: possible consequences for their natural enemies. Biol Control 11 122–129 [Google Scholar]
- Turlings TCJ, Ton J (2006) Exploiting scents of distress: the prospect of manipulating herbivore-induced plant odours to enhance the control of agricultural pests. Curr Opin Plant Biol 9 421–427 [DOI] [PubMed] [Google Scholar]
- van de Ven WTG, LeVesque CS, Perring TM, Walling LL (2000) Local and systemic changes in squash gene expression in response to silverleaf whitefly feeding. Plant Cell 12 1409–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GJ, Wang E, Shepherd RW (2004) New approaches for studying and exploiting an old protuberance, the plant trichome. Ann Bot (Lond) 93 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19 195–216 [DOI] [PubMed] [Google Scholar]
- Will T, Tjallingii WF, Thonnessen A, van Bel AJE (2007) Molecular sabotage of plant defense by aphid saliva. Proc Natl Acad Sci USA 104 10536–10541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will T, van Bel AJE (2006) Physical and chemical interactions between aphids and plants. J Exp Bot 57 729–737 [DOI] [PubMed] [Google Scholar]
- Williams L, Rodriguez-Saona C, Pare PW, Crafts-Brandner SJ (2005) The piercing-sucking herbivores Lygus hesperus and Nezara viridula induce volatile emissions in plants. Arch Insect Biochem Physiol 58 84–96 [DOI] [PubMed] [Google Scholar]
- Zarate SI, Kempema LA, Walling LL (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu-Salzman K, Salzman RA, Ahn JE, Koiwa H (2004) Transcriptional regulation of sorghum defense determinants against a phloem-feeding aphid. Plant Physiol 134 420–431 [DOI] [PMC free article] [PubMed] [Google Scholar]