Plants have evolved various strategies to defend themselves against herbivores and pathogens. Although some of these strategies are constitutive, i.e. present at all times, others are induced only in response to herbivore feeding or pathogen infection. The induction of direct and indirect plant defenses in response to herbivory and other biotic stresses is well established (Karban and Baldwin, 1997), and a wide array of studies have documented such induced defenses (Schultz and Baldwin, 1982; Haruta et al., 2001; van Dam et al., 2004; Miranda et al., 2007). Induced defenses should be adaptive when: (1) defenses are costly to implement, (2) there is spatial or temporal variability in the distribution of herbivores or pathogens so that plants do not always experience attack, and (3) there are tradeoffs between defenses against different enemies (e.g. defenses against herbivores and pathogens or against different herbivore species) so that defense against one increases susceptibility to another. Although induced defenses allow plants to avoid the costs of implementing defenses in the absence of enemies, plants may suffer considerable damage during the time required to mount defenses once attack occurs. To compensate for this vulnerability, some plants appear to prime specific defenses in response to environmental cues that reliably indicate an increased probability of attack before they actually experience an herbivore or pathogen.
In everyday language, to prime means to prepare or make ready. In plant defense, priming is a physiological process by which a plant prepares to more quickly or aggressively respond to future biotic or abiotic stress (Fig. 1). The condition of readiness achieved by priming has been termed the “primed state” (Conrath et al., 2006). Priming may be initiated in response to an environmental cue that reliably indicates an increased probability of encountering a biotic stress, but a primed state may also persist as a residual effect following an initial exposure to the stress. For example, the classic pathogen-induced hypersensitive response is often induced with greater efficiency in plants that have previously experienced pathogen attack (Kuc, 1987), and similar effects may occur in plant-herbivore interactions (Karban and Baldwin, 1997). In the context of long-lived plants such as trees, a primed state may persist across multiple growing seasons, a phenomenon commonly referred to in the ecological literature as “delayed induced resistance” (Haukioja et al., 1985; Zvereva et al., 1997). Because priming initiates a state of readiness that does not confer resistance per se but rather allows for accelerated induced resistance once an attack occurs, one presumed benefit of priming is that it does not impose the costs associated with full implementation of an induced defense response.
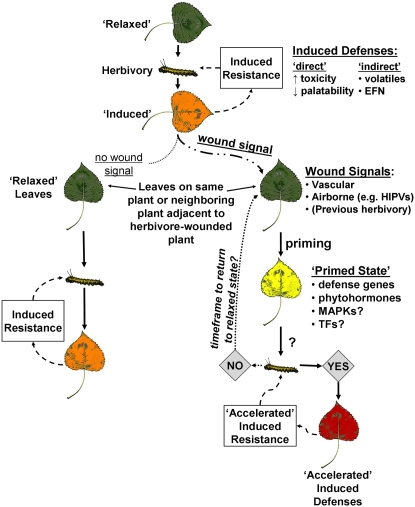
Figure 1.
A conceptual model of defense priming in plant-herbivore interactions. The classic model of induced resistance is highlighted at the top of the flow chart, where a relaxed leaf is induced by herbivore feeding. Induced defenses include a suite of chemical changes that are plant and situation specific and may include direct defenses by synthesizing chemicals that are toxic or unpalatable to the herbivore. Induced defenses may also include indirect defenses such as the production of volatile compounds or EFN, both of which can attract natural enemies of the herbivores. Some of the chemical changes to the wounded leaf may act as wound signals to undamaged regions within the plant or to adjacent plants. The wound signals include internal signals such as JA or external signals such as volatiles. The recognition of these signals may initiate priming, which evidently comprises changes at the molecular level and leads to a so-called primed state in undamaged leaves. Leaves in a primed state are then able, by mechanisms that are poorly understood, to respond more quickly or vigorously to herbivore attack should such an attack occur. But, primed leaves theoretically pay fewer costs relative to a fully induced defense in the event that they do not actually experience herbivory. TFs, Transcription factors. See text for citations.
To date, priming has most often been considered in the context of plant-pathogen interactions (for review, see Conrath et al., 2002, 2006), but plants can also be primed by signals associated with herbivore feeding. This review focuses specifically on priming responses to herbivores to highlight current knowledge and directions for future research on defense priming in plant-herbivore interactions.
SIGNALING CUES FOR PRIMING ANTIHERBIVORE DEFENSES
Any environmental cue that provides a reliable indication of the presence of herbivores can conceivably serve as a signal to induce priming. Essentially all studies on antiherbivore priming to date have focused on plant-derived cues associated with herbivore feeding on neighboring plants or on other parts of the same plant. In the former case, the most obvious cues available are herbivore-induced plant volatiles (HIPVs), a subset of volatile organic compounds (VOCs) that are emitted in response to herbivory; in the latter, signals may be transmitted either internally through the vasculature or externally via HIPVs (Fig. 1).
In the case of internal wound signals within a plant, information is most likely carried by signaling molecules transported through phloem and xylem (Malone and Alarcon, 1995; van Bel 2003) from the site of localized attack to systemic undamaged regions. Systemic regions can be either primed or induced to express defense responses depending apparently on the cost of the defense and the intensity of the signal (Rhodes et al., 1999), though it is not clear whether the priming and induced defense responses share the same systemic signal pathways. Either way, systemic signals can effectively transmit a wound signal from the site of local attack to potentially vulnerable systemic regions. Plant architecture plays a key role in such signaling (Davis et al., 1991; Orians, 2005) and can cause nonuniform distribution of systemic wound signals (Orians et al., 2000). Though a number of compounds may play a role in internal systemic signaling (for review, see Erb et al., 2008), the most likely candidate appears to be jasmonic acid (JA) and its conjugated forms (Howe, 2004; Thines et al., 2007). The majority of studies of priming in plant-pathogen interactions have focused on within-plant priming via systemic signaling (Conrath et al., 2006).
HIPVs provide a second route for signal transmission. Although HIPVs are also known to mediate a diverse array of interactions between plants and insects (Turlings et al., 1990; De Moraes et al., 1998; Hoballah and Turlings, 2001; De Moraes et al., 2001), the role of volatiles in signaling between plants has been one of the most fascinating and controversial topics in modern ecology (Baldwin and Schultz, 1983; Fowler and Lawton, 1985; Farmer and Ryan, 1990; Shonle and Bergelson, 1995; Karban and Baldwin, 1997; Agrawal, 2000; Bruin and Dicke, 2001; Baldwin et al., 2002, 2006; Karban et al., 2003; Dudareva et al., 2006). One recurring point of controversy has been the distance over which HIPV signals can be received by plants (Baldwin et al., 2002; Karban et al., 2003; Kessler et al., 2006). Recent work has shown that vascular constraints on systemic induction can be overcome with HIPVs (Karban et al., 2006; Heil and Silva Bueno, 2007; Frost et al., 2007), as hypothesized by both Farmer (2001) and Orians (2005). Within-plant signaling via HIPVs is consistent with short transmission distances, but also provides a tangible benefit of HIPV emissions to the emitting plant for the costs associated with those emissions. Thus, although between-plant signaling may remain controversial, the potential role of HIPVs in systemic wound signaling within plants has renewed interest in the physiological mechanisms by which plants respond to HIPVs. In terms of plant-herbivore interactions, the majority of studies investigating priming have considered the effects of HIPVs or other VOCs as possible signals.
CURRENT KNOWLEDGE OF PRIMING IN PLANT-HERBIVORE INTERACTIONS
The few published studies on defense priming in the context of plant-herbivore interactions indicate that priming occurs across a diverse group of plant taxa, including wild species and cultivated varieties. Engelberth et al. (2004) published the first study to explicitly address antiherbivore defense priming, showing that at least three green-leaf volatiles (GLVs)—(Z)-3-hexenal (z3HAL), (Z)-3-hexen-1-ol (z3HOL), and (Z)-3-hexenyl acetate (z3HAC)—prime defense responses in maize (Zea mays). GLV-treated maize plants subsequently induced higher JA concentrations and produced more HIPVs in response to the application of caterpillar regurgitant combined with mechanical wounding than did control plants. In a follow-up study, Engelberth et al. (2007) showed that the same GLVs up-regulated at least three genes in the octadecanoid pathway responsible for JA production. Further support for priming in maize was provided by Ton et al. (2007), who showed that undamaged maize plants exposed to volatiles from Spodoptera littoralis-wounded conspecifics up-regulated defense gene expression and were primed for HIPV emissions. They further showed that S. littoralis fed on the primed maize had lower relative growth rates (RGR), and that the parasitic wasp (Cotesia marginiventris) was preferentially attracted to the abundant HIPVs released by primed plants. Thus, priming can possibly influence herbivore dynamics by mediating a combination of direct defenses and indirect defenses via tritrophic interactions.
Although the studies above highlight priming of volatile production or stress signaling, priming can also affect direct and other indirect defenses. For example, Arabidopsis (Arabidopsis thaliana) plants exposed to z3HOL showed an increase in aliphatic glucosinolates up to 24 h sooner than did control plants when challenged with beet armyworm (Spodoptera exigua; H. Appel, personal communication). In addition, a study using lima bean (Phaseolus lunatus) and a species of herbivorous spider mite (Tetranychus urticae) has shown priming of extrafloral nectar (EFN) production in plants with previous air contact with other mite-infested plants (Choh and Takabayashi, 2006a). The EFN secretions were further shown to be attractive to predatory mites (Phytoseiulus persimilis; Choh et al., 2006), indicating that priming may influence tritrophic interactions in this system. EFN production has been described in almost 1,000 species from 93 plant families and is an important form of indirect plant defense (Pemberton, 1998; Rudgers and Gardener, 2004).
Priming also occurs in woody plants. Frost et al. (2007) showed that leaves of hybrid poplar saplings (Populus deltoides x nigra) can be primed both by exposure to HIPVs and through internal wound signaling following gypsy moth larvae (Lymantria dispar) feeding. A follow-up study (C.J. Frost, unpublished data) show that z3HAC primes the expression of genes in the octadecanoid signaling pathway, the production of α-linolenic acid and JA, the expression of genes involved in direct defense, and terpenoid volatile production in hybrid poplar leaves. In addition, recent work with blueberry shrubs (Vaccinium corymbosum) indicates that feeding by gypsy moth larvae on branches previously exposed to VOCs from adjacent damaged branches was reduced by 70% compared with controls, and HIPV emissions on undamaged branches were primed by exposure to HIPVs from damaged branches (C. Rodriguez, personal communication).
Many plant species are biologically capable of sensing and responding to HIPVs and other signals by priming future defenses. However, in an ecological context, the adaptive significance of priming is best demonstrated by field studies. An intriguing set of field experiments has explored priming of EFN production in wild lima bean plants (Heil and Kost, 2006; Kost and Heil, 2006; Heil and Silva Bueno, 2007). Both naturally produced HIPVs (Heil and Silva Bueno, 2007) and a synthetic HIPV blend (Heil and Kost, 2006) primed EFN production in wild lima bean plants. Kost and Heil (2006) further identified z3HAC as a specific VOC capable of priming EFN production. Although it is possible that HIPVs also attract parasitoids in the lima bean system, as has been demonstrated in other systems (Turlings et al., 1990; De Moraes et al., 1998), physical protection by predatory ants attracted to EFN is an effective defense strategy for wild Phaseolus and significantly reduces herbivore damage (Fig. 1 in Heil and Silva Bueno, 2007).
In another field study, Kessler et al. (2006) found that wild tobacco plants (Nicotiana attenuata) with air contact to mechanically clipped sagebrush (Artemisia tridentata), as well as the specific VOCs E-2-hexenal and methacrolein, were primed for accelerated proteinase inhibitor (PI) activity when subsequently exposed to experimental feeding by Manduca sexta. Despite being a field experiment, this study was not conducted under completely natural conditions as clipped sagebrush leaves were artificially placed around the bases of the tobacco plants. The same sagebrush/wild tobacco system was the field model for early work on plant-plant communication (Karban et al., 2000), and Kessler et al. (2006) suggested that priming mediated by VOC signals could account for apparently conflicting field results between research groups when considering between-plant signaling in this system.
A number of early studies did not use the term priming but nonetheless describe effects that reasonably fall within that category. Airborne GLVs have long been known to induce defense genes in Arabidopsis (Bate and Rothstein, 1998), and terpenoids have similar effects in lima bean (Arimura et al., 2000). Induction of defense genes can also occur systemically via internal wound signals. For example, Davis et al. (1991) showed expression of a wound-induced gene in undamaged systemic leaves following localized mechanical wounding.
Plants may respond to signals associated with the presence of herbivores in complex ways that include a mix of priming and induced defenses. Because different classes of defenses likely have different allocation costs, it is possible that fitness benefits could be derived from inducing less costly metabolites while priming more costly ones in response to a wound signal. The induction of HIPVs (Turlings et al., 1990) or EFN (Kost and Heil, 2006) to attract natural enemies of the herbivore may impose minor costs relative to the implementation of direct defenses because the costs of defense is shared with a predator or parasitoid, and might therefore be more likely to occur in response to a signal that precedes herbivore attack. For example, maize plants respond to z3HOL exposure with increased transcript accumulation of genes involved in production of both GLVs and PI, though only GLV production is actually increased (Farag et al., 2005). Although these authors did not discuss their results in the context of priming, a reasonable interpretation of their findings is that volatile production was induced by z3HOL, whereas PI defenses were primed. This would make sense because PI production entails high fitness costs (Zavala et al., 2004). In an earlier study by the same group (Farag and Paré, 2002), the application of GLVs to tomato plants triggered the release of volatile terpenoids, though direct defenses were not measured and priming effects cannot be inferred. In both studies, the amounts of VOCs released were approximately 13% to 40% of those released in response to herbivore feeding. If induction of volatile compounds in response to herbivory is a cry for help, the induction of volatiles in response to a VOC signal may be more of a whisper, which appears to be correspondingly less attractive to predators (Choh and Takabayashi, 2006b). It is not clear whether such a moderated volatile release is adaptive in its own right or is merely a by-product of priming for a full volatile response to actual herbivory.
The interactive effects of different VOCs may also be important for priming or induced defenses. For example, emissions of maize VOCs induced by exposure to z3HOL were 2.5-fold higher when the maize plants were simultaneously exposed to ethylene, whereas ethylene alone did not induce maize volatiles (Ruther and Kleier, 2005). Thus, more work will be needed to decipher the role of specific GLVs, terpenoids, other volatile compounds, and their interactive effects on plant signaling and on priming specifically. In addition, future studies that explore direct and indirect defenses simultaneously will be particularly important to determine how plants balance the costs of producing or priming various defensive metabolites against the risk of herbivory.
MECHANISMS OF DEFENSE PRIMING
The studies reviewed above and others from the plant-pathogen literature suggest that priming is based on the up-regulation of defense-related genes or other metabolites that may initiate biochemical signal transduction leading to a primed state. As with plant-pathogen interactions (Conrath et al., 2006), the mechanisms responsible for defense priming against herbivores are not known (Dudareva et al., 2006), and little is known about the primed state.
One key issue to be explored is how the signals that induce priming are received by plants. A single receptor model of recognition—such as has been found in defense responses to herbivore elicitors (Truitt et al., 2004)—is possible but may be impractical. Instead, the diverse class of receptor-like kinases (Hardie, 1999; Nishiguchi et al., 2002; Takabatake et al., 2006) may be necessary to decipher the complex mix of possible HIPV compounds that might be signals. Indeed, there is no reason to assume that the mechanism by which lima bean plants respond to terpene volatiles (Arimura et al., 2000) is the same mechanism by which poplar (C.J. Frost, unpublished data), maize (Engelberth et al., 2004), or lima bean (Kost and Heil, 2006) plants perceive and respond to GLVs.
Another key issue for exploration is the process by which priming occurs once a signal has been recognized. Just as there is no reason to assume that the mechanisms of signal reception are consistent across possible signals, there is no reason to assume that the primed state is a static condition. Up-regulation of genes involved in defense or signaling pathways clearly appears to be a component of priming (van Hulten et al., 2006; Engelberth et al., 2007; Ton et al., 2007), though a clear pattern of gene activation or repression associated with priming has yet to emerge. It is also possible that exposure to VOCs or other perceived signals induces changes in processes or metabolites upstream of defense gene expression. For example, changes may occur in cellular electrical potential or Ca2+ flux across cell membranes, as has been shown for physical damage (Maffei et al., 2007). Although such ephemeral signals by themselves are unlikely to maintain a primed state, the recent work in Arabidopsis linking γ-amino butyric acid with responsiveness to E-2-hexenal provides an intriguing signaling cascade mechanism because wound-induced accumulation of γ-amino butyric acid is regulated by the activity of a Ca2+/calmodulin-dependent Glu decarboxylase (Mirabella et al., 2008).
In the context of pathogen attack, it has also been suggested that a longer term sensitization could result from the accumulation in primed cells of “inactivated” signaling proteins upstream of gene expression that could be “hyperactivated” following a secondary elicitation and thereby amplify signal transduction (Beckers and Conrath, 2007). Candidate signaling proteins include the diverse class of mitogen-activated protein kinases (MAPKs), which have previously been shown to play important roles mediating postherbivore signaling cascades (Seo et al., 1995; Kandoth et al., 2007). Recent work in Arabidopsis shows that priming by the salicylic acid analog benzo(1,2,3) thiadiazole-7-carbothioic acid S-methyl ester results from accumulation of inactive MAPK3 (Beckers and Conrath, 2007). MAPK3 activity is then induced in response to biotic stress (pathogen infection), thereby enhancing defensive gene expression and the induction of antifungal metabolites. Whether MAPK3 is a general pre-stress marker or works in concert with other markers remains to be seen. Indeed, the MAPK family includes a diversity of MAPKs, MAPKKs, and MAPKKKs (Ichimura et al., 2002), at least some of which are herbivore inducible (Kandoth et al., 2007; Wu et al., 2007). This suggests that more molecular markers regulating primed states may yet be identified.
Seo et al. (1999) termed the MAPK that is responsive to wounding a “wound-induced protein kinase”, the expression of which could be detected within 1 min of wounding. It will be interesting to see whether volatile-induced protein kinases can be identified that may provide insights into the signal transduction mechanisms for the early recognition of HIPV emissions as well as identify regulators of, and phophorylation targets for, putative volatile-induced protein kinases (Caspersen et al., 2007).
COSTS OF DEFENSE PRIMING
Priming is often induced by environmental cues and thus should fit, alongside induced defense responses, within the larger theoretical framework of optimal defense theory (ODT; Stamp, 2003). The basic hypothesis of ODT is that “organisms evolve and allocate defenses in a way that maximizes individual inclusive fitness” (Rhoades, 1979). The key prediction of ODT relevant to this discussion is that opportunity, production, and/or allocation costs associated with induced defenses should exert a strong selective pressure for induced defenses to be low or absent when herbivores are absent but increased when herbivores are present. This prediction assumes that: (1) genetic variation exists in the production of secondary metabolites on which selection can operate (Zangerl and Berenbaum, 1990; Duquette et al., 2005); (2) herbivores exert a selective pressure on the expression of these defensive metabolites (Simms and Rausher, 1989; Mauricio and Rausher, 1997); (3) defenses confer resistance against herbivores (Haukioja and Hanhimaki, 1985; Roda and Baldwin, 2003); and (4) there are significant fitness costs associated with the production of defensive secondary metabolites (Gershenzon, 1994; Baldwin, 1998; Redman et al., 2001; Zavala et al., 2004).
Conceptually, priming should be subject to the same predictions as other induced responses associated with plant defense. Thus, priming and the primed state presumably have costs that make constitutive expression disadvantageous. However, we may predict that costs should be lower for priming than for the induction of defensive metabolites because ostensibly fewer resources have been dedicated to defense in the primed state. For example, Arabidopsis plants chemically primed with β-amino butyric acid, a salicylic acid analog, show moderately reduced growth and no effect on seed production compared to controls, whereas constitutively induced plants had significant reduction in seed set (van Hulten et al., 2006). Thus, the benefits of priming-mediated resistance may outweigh the costs in environments where pathogen infection is likely, though such an hypothesis requires support under ecological conditions. Currently, there are no published studies exploring the costs of priming in the context of plant-herbivore interactions. However, the demonstration that defense priming against herbivores exists under field conditions (Heil and Silva Bueno, 2007) implies that the benefits of priming outweigh potential costs.
A second prediction from ODT is that induced defenses should be relaxed once an herbivore stops feeding (Stamp, 2003). In the context of priming, the primed state should also attenuate over time if the wound signal discontinues and there is no subsequent herbivory (Fig. 1). Presumably, maintenance costs will determine the duration of the primed state because opportunity and allocation costs of priming have already been paid. Such maintenance costs include the possibility that costly defensive metabolites or proteins may be inadvertently produced by primed defense pathways when such defenses are not needed. There are currently no published studies that have explored whether plants primed by VOCs or other herbivore (or pathogen) signals relax or what the temporal dynamics of such relaxation would be. However, if priming is less costly to maintain than are induced defenses, the relaxation times associated with priming could be longer than those expected for induced defenses. This prediction, if supported, may help explain the ecological phenomenon of delayed inducible resistance—in which defoliation in one year influences herbivore performance in subsequent years (Neuvonen et al., 1987; Ruohomaki et al., 1992; Zvereva et al., 1997). Stamp (2003) observed that delayed inducible resistance may not fit ODT because the relaxation time occurs over multiple growing seasons. If delayed inducible resistance is a form of priming, the time frame for relaxation could be longer if herbivory in one growing season is a reliable predictor of herbivory in subsequent growing seasons and maintenance costs are low. Under such conditions, maintaining a state of readiness between growing seasons may be advantageous and therefore adaptive (Haukioja and Neuvonen, 1985).
If resource allocation to priming is indeed lower than to induced resistance, then priming should be a general characteristic of induced responses against a biotic stress. However, the adaptive benefit of priming should only be realized when a signal is a reliable indicator of experiencing a stress. Reliability is ultimately a measure of probability, which can be difficult or impossible to determine (Karban and Baldwin, 1997). Fortunately, we should be able to ask the plants which compounds are the most reliable signals. The volatile signals that are reliable cues will likely vary among plant species based on the natural history of the plant and its associated herbivore community; any specific compound could be more or less reliable in different ecological contexts. Although this may reveal context dependency, a focus on the natural history should also reveal areas of commonality between systems that provides the selective pressure to develop induced priming.
CONCLUSION
Our objective in this review was to provide an overview of defense priming in the context of plant-herbivore interactions, suggest future directions for research into the physiological mechanisms responsible for priming, and situate defense priming with a larger framework of ecological theory. Priming induced by the detection of a signal that indicates increased probability of experiencing a biotic stress prepares a plant for subsequent exposure to that stress. The priming responses documented to date appear limited to the expression of defense-related genes or other physiological machinery necessary for an accelerated response to the actual stress. Priming differs from induced defense, which confers resistance against the stress through synthesis of costly defensive compounds. As such, the costs associated with priming should be less than the costs of induced defenses. Based on the limited studies that have explicitly tested for priming and those that otherwise demonstrate priming, priming appears to occur across a diverse range of plant species. Thus, defense priming mediated by either internal or external wound signals may be a common and ecologically important phenomenon in plant-herbivore interactions. Future work on priming in plant-herbivore interactions should focus on understanding the ecological circumstances under which priming is favored and elucidate the physiology of the early steps involved in signal recognition and priming that results in the so-called primed state.
Acknowledgments
We thank Gregg Howe and Georg Jander for the invitation to contribute this review to the focus issue. Two anonymous reviewers provided helpful comments. We further thank R. Goergen for thoughtful discussion and comments on previous versions of the manuscript. C.J.F. is grateful to J.K. Cooke and G.H. Cooke for logistical support.
This work was supported by the U.S. Department of Agriculture (USDA-NRI no. 2007–35302–18087 to C.J.F.), the National Science Foundation (NSF CAREER no.0643966 to C.M.D.M.), the Penn State Center for Chemical Ecology, and the Schatz Center for Tree Molecular Genetics. This work was also supported by the David and Lucile Packard Foundation and the DuPont young investigator grant (to C.M.D.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Christopher J. Frost (cfrost@psu.edu).
References
- Agrawal AA (2000) Communication between plants: this time it's real. Trends Ecol Evol 15 446. [DOI] [PubMed] [Google Scholar]
- Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J (2000) Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406 512–515 [DOI] [PubMed] [Google Scholar]
- Baldwin IT (1998) Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc Natl Acad Sci USA 95 8113–8118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA (2006) Volatile signaling in plant-plant interactions: “talking trees” in the genomics era. Science 311 812–815 [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Kessler A, Halitschke R (2002) Volatile signaling in plant-plant-herbivore interactions: what is real? Curr Opin Plant Biol 5 351–354 [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Schultz JC (1983) Rapid changes in tree leaf chemistry induced by damage: evidence for communication between plants. Science 221 277–279 [DOI] [PubMed] [Google Scholar]
- Bate NJ, Rothstein SJ (1998) C-6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J 16 561–569 [DOI] [PubMed] [Google Scholar]
- Beckers GJ, Conrath U (2007) Priming for stress resistance: from the lab to the field. Curr Opin Plant Biol 10 425–431 [DOI] [PubMed] [Google Scholar]
- Bruin J, Dicke M (2001) Chemical information transfer between wounded and unwounded plants: backing up the future. Biochem Syst Ecol 29 1103–1113 [Google Scholar]
- Caspersen MB, Qiu JL, Zhang X, Andreasson E, Naested H, Mundy J, Svensson B (2007) Phosphorylation sites of Arabidopsis MAP kinase substrate 1 (MKS 1). Biochim Biophys Acta 1774 1156–1163 [DOI] [PubMed] [Google Scholar]
- Choh Y, Kugimiya S, Takabayashi J (2006) Induced production of extrafloral nectar in intact lima bean plants in response to volatiles from spider mite-infested conspecific plants as a possible indirect defense against spider mites. Oecologia 147 455–460 [DOI] [PubMed] [Google Scholar]
- Choh Y, Takabayashi J (2006. a) Herbivore-induced extrafloral nectar production in lima bean plants enhanced by previous exposure to volatiles from infested conspecifics. J Chem Ecol 32 2073–2077 [DOI] [PubMed] [Google Scholar]
- Choh Y, Takabayashi J (2006. b) Intact lima bean plants exposed to herbivore-induced plant volatiles attract predatory mites and spider mites at different levels according to plant parts. Appl Entomol Zool 41 537–543 [Google Scholar]
- Conrath U, Beckers GJM, Flors V, Garcia-Agustin P, Jakab G, Mauch F, Newman MA, Pieterse CM, Poinssot B, Pozo MJ, et al. (2006) Priming: getting ready for battle. Mol Plant Microbe Interact 19 1062–1071 [DOI] [PubMed] [Google Scholar]
- Conrath U, Pieterse CMJ, Mauch-Mani B (2002) Priming in plant-pathogen interactions. Trends Plant Sci 7 210–216 [DOI] [PubMed] [Google Scholar]
- Davis JM, Gordon MP, Smit BA (1991) Assimilate movement dictates remote sites of wound-induced gene expression in poplar leaves. Proc Natl Acad Sci USA 88 2393–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393 570–573 [Google Scholar]
- De Moraes CM, Mescher MC, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410 577–580 [DOI] [PubMed] [Google Scholar]
- Dudareva N, Negre F, Nagegowda DA, Orlova I (2006) Plant volatiles: recent advances and future perspectives. CRC Crit Rev Plant Sci 25 417–440 [Google Scholar]
- Duquette SL, Altwegg R, Anholt BR (2005) Factors affecting the expression of inducible defences in Euplotes: genotype, predator density and experience. Funct Ecol 19 648–655 [Google Scholar]
- Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH (2004) Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci USA 101 1781–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberth J, Seidl-Adams I, Schultz JC, Tumlinson JH (2007) Insect elicitors and exposure to green leafy volatiles differentially upregulate major octadecanoids and transcripts of 12-oxophytodienoic acid reductases in Zea mays. Mol Plant Microbe Interact 20 707–716 [DOI] [PubMed] [Google Scholar]
- Erb M, Ton J, Degenhardt J, Turlings TCJ (2008) Interactions between arthropod-induced aboveground and belowground defenses in plants. Plant Physiol 146 867–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag MA, Fokar M, Zhang HA, Allen RD, Paré PW (2005) (Z)-3-Hexenol induces defense genes and downstream metabolites in maize. Planta 220 900–909 [DOI] [PubMed] [Google Scholar]
- Farag MA, Paré PW (2002) C-6-green leaf volatiles trigger local and systemic VOC emissions in tomato. Phytochemistry 61 545–554 [DOI] [PubMed] [Google Scholar]
- Farmer EE (2001) Surface-to-air signals. Nature 411 854–856 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA (1990) Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci USA 87 7713–7716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SV, Lawton JH (1985) Rapidly induced defenses and talking trees—the devils advocate position. Am Nat 126 181–195 [Google Scholar]
- Frost CJ, Appel HM, Carlson JE, De Moraes CM, Mescher MC, Schultz JC (2007) Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol Lett 10 490–498 [DOI] [PubMed] [Google Scholar]
- Gershenzon J (1994) Metabolic costs of terpenoid accumulation in higher plants. J Chem Ecol 20 1281–1328 [DOI] [PubMed] [Google Scholar]
- Hardie DG (1999) Plant protein serine threonine kinases: classification and functions. Annu Rev Plant Physiol Plant Mol Biol 50 97–131 [DOI] [PubMed] [Google Scholar]
- Haruta M, Major IT, Christopher ME, Patton JJ, Constabel CP (2001) A Kunitz trypsin inhibitor gene family from trembling aspen (Populus tremuloides Michx.): cloning, functional expression, and induction by wounding and herbivory. Plant Mol Biol 46 347–359 [DOI] [PubMed] [Google Scholar]
- Haukioja E, Hanhimaki S (1985) Rapid wound-induced resistance in white birch (Betula pubescens) foliage to the geometrid Epirrita autumnata: a comparison of trees and moths within and outside the outbreak range of the moth. Oecologia 65 223–228 [DOI] [PubMed] [Google Scholar]
- Haukioja E, Neuvonen S (1985) Induced long-term resistance of birch foliage against defoliators—defensive or incidental. Ecology 66 1303–1308 [Google Scholar]
- Haukioja E, Soumela J, Neuvonen S (1985) Long-term inducible resistance in birch foliage: triggering cues and efficacy on a defoliator. Oecologia 65 363–369 [DOI] [PubMed] [Google Scholar]
- Heil M, Kost C (2006) Priming of indirect defences. Ecol Lett 9 813–817 [DOI] [PubMed] [Google Scholar]
- Heil M, Silva Bueno JC (2007) Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA 104 5467–5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoballah MEF, Turlings TCJ (2001) Experimental evidence that plants under caterpillar attack may benefit from attracting parasitoids. Evol Ecol Res 3 553–565 [Google Scholar]
- Howe GA (2004) Jasmonates as signals in the wound response. J Plant Growth Regul 23 223–237 [Google Scholar]
- Ichimura K, Shinozaki K, Tena G, Sheen J, Henry Y, Champion A, Kreis M, Zhang SQ, Hirt H, Wilson C, et al (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7 301–308 [DOI] [PubMed]
- Kandoth PK, Ranf S, Pancholi SS, Jayanty S, Walla MD, Miller W, Howe GA, Lincoln DE, Stratmann JW (2007) Tomato MAPKs LeMPK1, LeMPK2, and LeMPK3 function in the systemin-mediated defense response against herbivorous insects. Proc Natl Acad Sci USA 104 12205–12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karban R, Baldwin IT (1997) Induced Responses to Herbivory. University of Chicago Press, Chicago
- Karban R, Baldwin IT, Baxter KJ, Laue G, Felton GW (2000) Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 125 66–71 [DOI] [PubMed] [Google Scholar]
- Karban R, Maron J, Felton GW, Ervin G, Eichenseer H (2003) Herbivore damage to sagebrush induces resistance in wild tobacco: evidence for eavesdropping between plants. Oikos 100 325–332 [Google Scholar]
- Karban R, Shiojiri K, Huntzinger M, Mccall AC (2006) Damage-induced resistance in sagebrush: volatiles are key to intra- and interplant communication. Ecology 87 922–930 [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Diezel C, Baldwin IT (2006) Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 148 280–292 [DOI] [PubMed] [Google Scholar]
- Kost C, Heil M (2006) Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. J Ecol 94 619–628 [Google Scholar]
- Kuc J (1987) Translocated signals for plant immunization. Ann N Y Acad Sci 494 221–223 [Google Scholar]
- Maffei ME, Mithofer A, Boland W (2007) Before gene expression: early events in plant-insect interaction. Trends Plant Sci 12 310–316 [DOI] [PubMed] [Google Scholar]
- Malone M, Alarcon JJ (1995) Only xylem-borne factors can account for systemic wound signaling in the tomato plant. Planta 196 740–746 [Google Scholar]
- Mauricio R, Rausher MD (1997) Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense. Evolution Int J Org Evolution 51 1435–1444 [DOI] [PubMed] [Google Scholar]
- Mirabella R, Rauwerda H, Struys EA, Jakobs C, Triantaphylides C, Haring MA, Schuurink RC (2008) The Arabidopsis her1 mutant implicates GABA in E-2-hexenal responsiveness. Plant J 53 197–213 [DOI] [PubMed] [Google Scholar]
- Miranda M, Ralph SG, Mellway R, White R, Heath MC, Bohlmann J, Constabel CP (2007) The transcriptional response of hybrid poplar (Populus trichocarpa x P. deltoides) to infection by Melampsora medusae leaf rust involves induction of flavonoid pathway genes leading to the accumulation of proanthocyanidins. Mol Plant Microbe Interact 20 816–831 [DOI] [PubMed] [Google Scholar]
- Neuvonen S, Haukioja E, Molarius A (1987) Delayed inducible resistance against a leaf-chewing insect in 4 deciduous tree species. Oecologia 74 363–369 [DOI] [PubMed] [Google Scholar]
- Nishiguchi M, Yoshida K, Sumizono T, Tazaki K (2002) A receptor-like protein kinase with a lectin-like domain from lombardy poplar: gene expression in response to wounding and characterization of phosphorylation activity. Mol Genet Genomics 267 506–514 [DOI] [PubMed] [Google Scholar]
- Orians C (2005) Herbivores, vascular pathways, and systemic induction: facts and artifacts. J Chem Ecol 31 2231–2242 [DOI] [PubMed] [Google Scholar]
- Orians CM, Pomerleau J, Ricco R (2000) Vascular architecture generates fine scale variation in systemic induction of proteinase inhibitors in tomato. J Chem Ecol 26 471–485 [Google Scholar]
- Pemberton RW (1998) The occurrence and abundance of plants with extrafloral nectaries, the basis for antiherbivore defensive mutualisms, along a latitudinal gradient in east Asia. J Biogeogr 25 661–668 [Google Scholar]
- Redman AM, Cipollini DF, Schultz JC (2001) Fitness costs of jasmonic acid-induced defense in tomato, Lycopersicon esculentum. Oecologia 126 380–385 [DOI] [PubMed] [Google Scholar]
- Rhoades DF (1979) Evolution of plant chemical defense against herbivores. In GA Rosenthal, DH Janzen, eds, Herbivores: Their Interaction with Secondary Plant Metabolites. Academic Press, New York, pp 1–55
- Rhodes JD, Thain JF, Wildon DC (1999) Evidence for physically distinct systemic signalling pathways in the wounded tomato plant. Ann Bot (Lond) 84 109–116 [Google Scholar]
- Roda AL, Baldwin IT (2003) Molecular technology reveals how the induced direct defenses of plants work. Basic Appl Ecol 4 15–26 [Google Scholar]
- Rudgers JA, Gardener MC (2004) Extrafloral nectar as a resource mediating multispecies interactions. Ecology 85 1495–1502 [Google Scholar]
- Ruohomaki K, Hanhimaki S, Haukioja E, Isoiivari L, Neuvonen S, Niemala P, Suomela J (1992) Variability in the efficacy of delayed inducible resistance in mountain birch. Entomol Exp Appl 62 107–115
- Ruther J, Kleier S (2005) Plant-plant signaling: ethylene synergizes volatile emission in Zea mays induced by exposure to (Z)-3-hexen-1-ol. J Chem Ecol 31 2217–2222 [DOI] [PubMed] [Google Scholar]
- Schultz JC, Baldwin IT (1982) Oak leaf quality declines in response to defoliation by gypsy moth larvae. Science 217 149–151 [DOI] [PubMed] [Google Scholar]
- Seo S, Okamoto N, Seto H, Ishizuka K, Sano H, Ohashi Y (1995) Tobacco map kinase—a possible mediator in wound signal-transduction pathways. Science 270 1988–1992 [DOI] [PubMed] [Google Scholar]
- Seo S, Sano H, Ohashi Y (1999) Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell 11 289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonle I, Bergelson J (1995) Interplant communication revisited. Ecology 76 2660–2663 [Google Scholar]
- Simms EL, Rausher MD (1989) The evolution of resistance to herbivory in Ipomoea purpurea. 2. Natural-selection by insects and costs of resistance. Evolution Int J Org Evolution 43 573–585 [DOI] [PubMed] [Google Scholar]
- Stamp N (2003) Out of the quagmire of plant defense hypotheses. Q Rev Biol 78 23–55 [DOI] [PubMed] [Google Scholar]
- Takabatake R, Seo S, Ito N, Gotoh Y, Mitsuhara I, Ohashi Y (2006) Involvement of wound-induced receptor-like protein kinase in wound signal transduction in tobacco plants. Plant J 47 249–257 [DOI] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu GH, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCFCO11 complex during jasmonate signalling. Nature 448 661–662 [DOI] [PubMed] [Google Scholar]
- Ton J, D'Alessandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TC (2007) Priming by airborne signals boosts direct and indirect resistance in maize. Plant J 49 16–26 [DOI] [PubMed] [Google Scholar]
- Truitt CL, Wei HX, Pare PW (2004) A plasma membrane protein from Zea mays binds with the herbivore elicitor volicitin. Plant Cell 16 523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlings TCJ, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250 1251–1253 [DOI] [PubMed] [Google Scholar]
- van Bel AJE (2003) Transport phloem: low profile, high impact. Plant Physiol 131 1509–1510 [PMC free article] [PubMed] [Google Scholar]
- van Dam NM, Witjes L, Svatos A (2004) Interactions between aboveground and belowground induction of glucosinolates in two wild Brassica species. New Phytol 161 801–810 [DOI] [PubMed] [Google Scholar]
- van Hulten M, Pelser M, van Loon LC, Pieterse CMJ, Ton J (2006) Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci USA 103 5602–5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JQ, Hettenhausen C, Meldau S, Baldwin IT (2007) Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell 19 1096–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangerl AR, Berenbaum MR (1990) Furanocoumarin induction in wild parsnip—genetics and populational variation. Ecology 71 1933–1940 [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Baldwin IT (2004) Constitutive and inducible trypsin proteinase inhibitor production incurs large fitness costs in Nicotiana attenuata. Proc Natl Acad Sci USA 101 1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvereva EL, Kozlov MV, Niemela P, Haukioja E (1997) Delayed induced resistance and increase in leaf fluctuating asymmetry as responses of Salix borealis to insect herbivory. Oecologia 109 368–373 [DOI] [PubMed] [Google Scholar]