A plant's resistance to herbivore attack is thought to be principally determined by its secondary metabolism, which can be remarkably plastic and responsive to different grades and types of herbivory. Newer unbiased “omic” approaches, which characterize transcriptomic, metabolomic, and proteomic changes in herbivore-attacked plants, have laid to rest the notion that metabolism can be neatly parsed into “secondary metabolism,” which functions to meet environmental challenges, and “primary metabolism,” which supports growth. The hundreds of genes regulated during the plant-herbivore or -pathogen interaction have been analyzed with microarray studies, and almost all aspects of metabolism are represented, with a substantial fraction coming from primary metabolism (Hui et al., 2003; Both et al., 2005; Major and Constabel, 2006; Mozoruk et al., 2006; Ralph et al., 2006; Schmidt and Baldwin, 2006; Tian et al., 2006; Kant and Baldwin, 2007) Here, we consider four overlapping functional explanations for this reconfiguration:
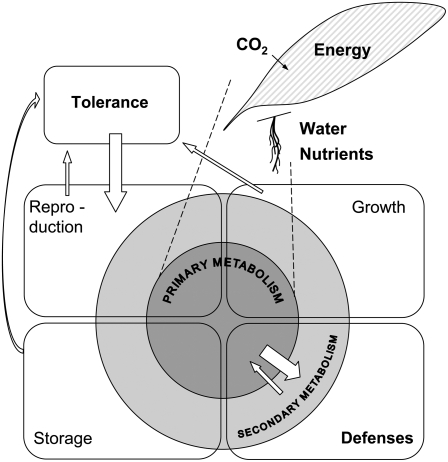
Resistance traits are costly and frequently up-regulated after attack, requiring reductions in growth, reproduction or storage, and/or increases in assimilation to meet their metabolic demands (Fig. 1). These changes in resource allocation can be either acute, driven by immediate reductions in resources, or anticipatory, occurring before resource supply limits defense activation (Smith and Stitt, 2007).
Rather than supporting defense responses, reconfiguration could support the physiological adjustments plants must make to tolerate herbivory and reduce the negative fitness consequences of herbivore attack (Fig. 1).
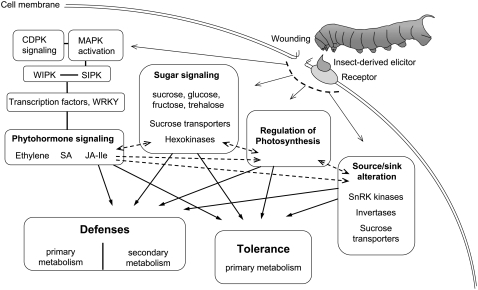
Primary metabolites could function as signals in defense pathways (Fig. 2).
Induced changes in primary metabolism could themselves be defensive (Fig. 2).
Figure 1.
Dependency of resistance traits (defenses and tolerance) and primary metabolism. Primary metabolism is fueled by energy and resources, which the plant gains from its environment. Primary metabolism involves growth, storage, and reproduction. Tolerance depends on primary metabolites and energy, both of which are taken from pools for reproduction, storage, and/or growth, and later reinvested in reproduction. Defenses from secondary metabolism are based on energy and resources from primary metabolism, which can be partially resupplied to primary metabolism. Parts of primary metabolism can function as direct defense.
Figure 2.
Resistance signaling is elicited differently from simple wounding when herbivore-specific elicitors (FACs) are introduced into wounds during caterpillar feeding. Signaling depends on primary metabolites. Herbivory induces a large reorganization of primary metabolism, including altered photosynthesis and altered sink/source relations. These changes are coordinated by a signaling network that is only partially understood. The expression of defense and tolerance traits requires changes both in primary and secondary metabolism.
We consider these four hypotheses in an overview of the literature that addresses how assimilation and the partitioning of assimilates are altered by herbivory and how primary metabolites function as signals and as defenses. In conclusion, we consider the challenges that plant biologists face in attempting to falsify these hypotheses. Compared to the falsifications of hypotheses about the defensive function of secondary metabolism, tests of the above hypotheses will seriously challenge the procedures that we use to understand resistance mechanisms and perhaps even challenge the reductionist paradigm that has proved so useful for understanding gene function in much of biology in the last century.
HERBIVORE-INDUCED CHANGES IN ASSIMILATION AND PARTITIONING OF ASSIMILATES
If the resource demands of defense production compete with those of growth and reproduction, and defenses are costly to produce (Steppuhn, 2007), then changes in the partitioning of resources among growth, storage, and reproduction would be expected (Fig. 1). Changes in how resources are partitioned according to function can be avoided if the rate of resource assimilation increases. Similar predictions hold for the activation of physiological changes that allow plants to better tolerate the negative fitness consequences of herbivory. Tolerance, which measures a plant's ability to compensate for the negative fitness effects of tissue damage, is usually described as a reaction norm of the fitness of specific genotypes at various damage levels (Strauss and Agrawal, 1999; Stowe et al., 2000). Tolerance is thought to result from the activation of dormant meristems, changes in plant architecture, resource allocation, or photosynthetic capacity. In short, the herbivory-induced activation of both defense and tolerance responses is predicted to alter resource assimilation and source-sink relationships, and the literature provides general support for these predictions.
The photosynthetic apparatus frequently responds to herbivore attack, usually with decreases in CO2 assimilation in the attacked leaf that are proportionally greater than the leaf area that is actually damaged (Zangerl et al., 2002). Otherwise, the photosynthetic response depends on the type of attacker and the age of the tissues that are measured (Welter, 1989). Defoliating herbivores can increase the photosynthetic activity of unattacked leaves, whereas stem borers and mesophyll feeders tend to decrease activity. On the other hand, transcript levels of photosynthetically related genes are commonly down-regulated (Hui et al., 2003; Ralph et al., 2006; Tang et al., 2006). It is thought that down-regulation of the photosynthetic apparatus protects it from oxidative damage (Niyogi, 2000), but decreased photosynthetic activity may also free up resources, especially nitrogen-rich compounds, making these available for use in secondary defense pathways. Decreased photosynthetic rates may be part of a global inhibition of protein synthesis, which may anticipate the need to redirect resources to defensive functions. As decreases in photosynthetic rates are more common than increases, altered photosynthesis has only rarely been correlated with tolerance (e.g. Cullen et al., 2006). Increases in photosynthetic rates could also be caused by changes in source-sink relationships resulting from the increased demand for energy and carbon (C)-based resources that the production of defensive compounds entails; separating where and for what additional C and energy are used is difficult. The activation of dormant meristems and thus new sinks has been shown to be central in tolerance in some species (Bergelson et al., 1996; Mabry and Wayne, 1997). For example, in Nicotiana attenuata, increased branching compensates for leaf damage (Schwachtje et al., 2006), and jasmonic acid (JA) signaling, which is responsible for activating several defense responses in this species, appears to suppress regrowth and contribute to apical dominance (Zavala and Baldwin, 2006).
How herbivore attack alters source-sink relationships remains unclear other than by reducing source strength when herbivores consume and damage leaves. As well as serving a variety of developmental functions, invertases are involved in the regulation of sink strength by cleaving Suc into Glc and Fru, thereby altering the osmotic gradient of Suc and leading to altered carbohydrate partitioning by turning specific tissues into metabolic sinks for carbohydrates (Roitsch and Gonzalez, 2004). Hence, invertases are often regulated after insect attack. For example, increased sink strength is elicited by JA treatment and gypsy moth feeding via the increased activity of cell wall invertases in the sink leaves of hybrid Populus deltoides × Populus nigra (Arnold and Schultz, 2002) and the wounding of leaves in Solanum lycopersicum, Solanum peruvianum, and Pisum sativum, which increases the activity of soluble (vacuolar) and cell wall invertase in damaged leaves (Zhang et al., 1996; Ohyama et al., 1998). Root wounding was shown to induce vacuolar and cell wall invertase in Beta vulgaris (Rosenkranz et al., 2001). Changes in assimilate flux after herbivore attack may occur along the transport routes, where sugar transporters are involved in Suc loading and unloading. Wounding is known to elicit a Suc transporter, AtSUC3, in sieve elements of different sink tissues (Meyer et al., 2004) and a monosaccharide transporter, STP4, in Arabidopsis (Arabidopsis thaliana; Truernit et al., 1996).
Tolerance to herbivore attack can be acquired by changing resource allocation when stored reserves are used (for example, those of root tissues). This strategy favors biennial or perennial species that normally accumulate reserves during their growing season for later growth during short-day periods (Wyka, 1999; Wise and Cummins, 2006). With nutrients stored in safe tissues, e.g. roots, plants have the possibility to regrow later in the growing season when the pressure from aboveground herbivores may have decreased. If plants are attacked by root herbivores, assimilates can be remobilized above ground. The highly tolerant Centaurea maculosa responds to root herbivory by the knapweed moth by reducing its nitrogen (N) uptake but also shifting N to aboveground tissues (Newingham et al., 2007), suggesting that N allocation can be a determinant of tolerance. This idea is supported by the finding that after its leaves were clipped, the dwarf shrub Indigofera spinosa increased its root N uptake (Coughenour et al., 1990) and that Quercus serrata accumulates higher N levels in leaves (Takashima et al., 2004). Moreover, N allocation to roots has been observed after methyl-JA treatment of Medicago sativa (Meuriot et al., 2004).
Carbon is allocated to roots in response to leaf damage or herbivory in several species, for example, after grasshopper damage to Zea mays (Holland et al., 1996) and Panicum coloratum (Dyer et al., 1991), after the defoliation of Lolium perenne (Bazot et al., 2005) and of two C4 perennial grasses (Briske et al., 1996), and after methyl-JA treatment of Populus tremuloides (Babst et al., 2005). Recently, a SnRK kinase has been found to regulate the reallocation of photoassimilates in response to herbivory, facilitating a tolerance response (Fig. 2; Schwachtje et al., 2006). SnRK kinases are involved in regulating isoprenoid, amino acid, and especially carbohydrate metabolism (Halford and Paul, 2003). The β-subunit of the kinase complex is rapidly down-regulated in the source leaves of N. attenuata after simulated attack by the tobacco hornworm, leading to 10% more photoassimilate being partitioned to roots. The same effect was seen in JA-deficient asLOX plants, which are silenced for the JA-biosynthetic enzyme lipoxygenase, making this response demonstrably independent of JA signaling. This rapid bunkering of C into root tissues is elicited when wounds are treated with fatty acid-amino acid conjugates (FACs), which are the insect-specific elicitors that activate most defense responses via the jasmonate cascade (Halitschke et al., 2003). At the end of its growing season, N. attenuata gains a measure of tolerance by reusing its additional root resources to prolong flowering, leading to increased capsule production late in the season. Recently, wild-type Arabidopsis plants overexpressing JA were observed to have reduced fitness but the same tolerance of defoliation, which is consistent with the idea that there is a JA-independent mechanism of tolerance (Cipollini, 2007).
The increased flux of C to the roots in response to herbivory would be expected to increase the rate of root growth, but in young seedlings of N. attenuata, for example, sometimes just the opposite occurs (Hummel et al., 2007). Unlike the FAC-elicited C flux, this rapid inhibition of root growth requires an intact JA-signaling cascade (G.M. Hummel, U. Schurr, I.T. Baldwin, and A. Walter, unpublished data) and may be one of the plant's anticipatory responses. Changes in growth that are anticipated in advance of resource limitations (and therefore differ from acute responses) have acquired growing importance as physiologists have shifted their focus to understanding the relationships between C balance and growth (Smith and Stitt, 2007).
By studying the growth dynamics of plants unable to synthesize starch due to a mutation in plastidial phosphoglucomutase in combination with experimental conditions in which the dark cycle was extended, researchers have discovered that plants anticipate the length of the dark period and adjust their synthesis and catabolism of starch to exactly meet energy demands during the dark period (Gibon et al., 2004; Smith and Stitt, 2007). For reasons that are not completely clear, starch accumulation at the end of the dark period is inversely correlated with growth rate (Cross et al., 2006). It will be interesting to see how these anticipatory changes in allocation and resource partitioning that are likely coordinated by a plant's circadian clock are modified when plants are elicited by insect-specific elicitors.
PRIMARY METABOLITES AS SIGNALS AND DEFENSES
A plant's resistance response to insect feeding is coordinated by different signaling pathways that depend on primary metabolites; in addition, the integration of the different signals induced by wounding and insect-specific elicitors results in a complex rearrangement of primary and secondary metabolism (Fig. 2). JA is a crucial player in defense signaling (Devoto and Turner, 2005) and requires kinases, such as WIPK and SIPK (Wu et al., 2007), and transcription factors such as WRKYs (Hui et al., 2003). After elicitation, Ile production is amplified by Thr deaminase (TD). Two hours after elicitation, the mRNA levels of N. attenuata's TD are increased by as much as 30 times (Kang et al., 2006). The Ile that is produced at the attack site is rapidly conjugated to JA, forming JA-Ile, a key activator of defense signaling (Chini et al., 2007; Thines et al., 2007). In addition to the JA-dependent signaling, several JA-independent responses to wounding and FACs have been documented (Leon et al., 1998; Rojo et al., 1999; LeBrasseur et al., 2002; Gross et al., 2004; Schwachtje et al., 2006), but knowledge about the underlying mechanisms is limited. Recently, the signaling role of sugars has received increased attention because several sugar-induced resistance genes have been found. For example, Suc, Glc, and Fru act as specific regulatory signals on the wound-inducible expression of an extensin gene (SbHRGP3) in Glycine max (Ahn et al., 1996; Ahn and Lee, 2003); additionally, a putatively defensive vegetative storage protein is Suc as well as JA induced (Berger et al., 1995). Moreover, transcripts of a hexokinase, which can function as a sugar sensor or photosynthesis repressor (Rolland et al., 2006), are induced by wounding and are sensitive to trehalose-6-P (Claeyssen and Rivoal, 2007), which itself is involved in the feedback regulation of photosynthesis and developmental transitions (Paul, 2007; Ramon and Rolland, 2007). Trehalose and SnRK protein kinases have been shown to interact (Schluepmann et al., 2004), as have sugars and lectins, which also can be induced by JA, suggesting lectins play a role in signal transduction (Chen et al., 2002; Van Damme et al., 2003; Gabius et al., 2004; Lannoo et al., 2006). Furthermore, an antagonistic interaction between Glc and ethylene, which is involved in defense signaling (von Dahl and Baldwin, 2007), has been reported (Zhou et al., 1998).
Several metabolites that play well-studied roles in primary metabolism have been found to possess defensive functions. Their dual function has been discovered because very high levels of them accumulate in plants, or because their induction patterns after herbivore attack are similar to those of defensive secondary metabolites. In the case of TD, for example, the function of the enzyme, degrading Thr, led to the hypothesis that it functioned in the insect's gut to degrade this essential amino acid. TD's regulatory domain was found to be removed by insect proteases, suppressing its negative feedback regulation by Ile (Chen et al., 2007). TD then continuously degrades Thr in the gut lumen, leading to amino acid starvation. Two TD isoforms are known in S. lycopersicum, one of which is stable in insect guts (Chen et al., 2007); in N. attenuata, in contrast, one TD serves both primary and secondary functions (Kang et al., 2006).
High levels of calcium oxalate (CaOx), a primary metabolite, accumulate in plants (up to 80% of dry mass), and in some plants CaOx synthesis is induced by herbivory (Molano-Flores, 2001; Ruiz et al., 2002). CaOx regulates bulk levels of the Ca that is involved in cell signaling and in several biochemical processes. The morphologically diverse CaOx crystals are either stored in the vacuoles of specialized cells, the crystal idioblasts, or are associated with the cell wall (Franceschi and Nakata, 2005). Crystals can be located around tissues, e.g. vascular bundles, to provide a physical barrier against chewing insects by an abrasive effect that blunts insects' mandibles (Korth et al., 2006). Moreover, CaOx is thought to act as an antinutritive defense by decreasing the efficiency with which ingested food is converted (Korth et al., 2006).
Vegetative storage proteins and lectins play dual roles in primary metabolism and resistance, and some of them are JA induced. For a detailed description of defensive proteins, see Zhu-Salzman et al. (2008). Carbohydrates can also directly function as defenses. Galactose markedly reduced larval growth of western spruce budworm when added to artificial diet in quantities of 6%, but Glc and Fru increased growth (Zou and Cates, 1994). In Acacia, mutualistic ants are attracted by nectar sugars (Heil et al., 2005). However, only after Suc is cleaved by an invertase, which is present in extrafloral nectar, are nonsymbiotic ants repelled and mutualistic ants attracted.
TESTING HYPOTHESES ABOUT DEFENSIVE FUNCTION
Whether a given secondary metabolite plays a role in herbivore protection is best determined by planting isogenic plants that both do and do not produce the metabolite into the plants' native environment where they can be confronted with native herbivore communities. With the development of transformation systems and the identification of genes that control the biosynthesis and flux into secondary metabolism, it is now possible to create these isogenic plants and to test their function. For example, nicotine-, TPI-, and JA-signaling-deficient N. attenuata plants have provided strong proof for the defensive function of individual secondary metabolites, for defensive synergies among different secondary metabolites, and for the role of JA signaling in activating metabolic changes (Kessler et al., 2004; Steppuhn et al., 2004; Zavala et al., 2004a; Steppuhn and Baldwin, 2007).
The defensive metabolites of a plant have long been thought to act synergistically; the combination of different effects is assumed to be more than their parts. A defensive synergism between nicotine and trypsin protease inhibitors (TPIs) was discovered in N. attenuata when the production of nicotine or TPI or both was silenced, and when plants were attacked by the second most common lepidopteran herbivore in this tobacco's natural habitat, Spodoptera exigua. The compensatory feeding response of this herbivore to TPI-induced amino acid starvation was inhibited by the larvae's limited ability to tolerate nicotine and the leaf area consumed was reduced when both secondary metabolites were present (Steppuhn and Baldwin, 2007). Similarly, artificial diet experiments showed that plant-derived phytic acid, a primary metabolite, reduces the detoxification of xanthotoxin, a defensive furanocoumarin, by the parsnip webworm, likely by inhibiting insect CYP450 monooxygenases (Green et al., 2001). These results demonstrate that in planta tests are essential for pinpointing defensive function because the chemical milieu in which a metabolite is expressed can profoundly influence how an herbivore responds.
These secondary-metabolite-deficient plants also provided strong support for the hypothesis that secondary metabolites, previously thought to be directed solely at agents outside the plant, play a physiological role inside the plant. Silencing a TPI gene not only increased plants' susceptibility to herbivores but also increased growth and seed production. This increase in plant fitness, apparent not only when TPI production was silenced but also when TPI production was restored in an ecotype naturally deficient in TPI production (Zavala et al., 2004b), is not likely explained by the liberation of resources that are no longer invested in TPI production. While the mechanisms remain to be worked out, it is likely that TPIs down-regulate growth through their modulation of a yet-to-be described signaling cascade.
Molecular biology made it possible to uncover the defensive functions of secondary metabolites because it was possible to silence the accumulation of a secondary metabolite with simple RNAi constructs driven by constitutive promoters without simultaneously affecting plant growth. Tests of the defensive function of primary metabolites will require subtle silencing tools that allow silencing to be both tissue specific and controlled at very precise times; the goal is to minimize the growth and developmental effects of gene silencing while determining herbivore performance and resistance under native conditions. Integrative approaches that compare the effects of gene silencing at different levels in the signaling hierarchy will be necessary to determine how resources are allocated and source-sink relations adjusted. Asking the proteome, metabolome, and transcriptome for answers using unbiased analytical tools will, we hope, identify those regulatory nodes that are altered by herbivore attack. While (ultra) high-throughput analytical and data handling platforms will be important for handling the torrent of data produced, student training that emphasizes real-world familiarity with the plant and its natural history will also be important. Students will need to be trained to use an experimental approach that inverts the normal sequence of events in the biological discovery process. Instead of proceeding step-by-step from gene, to transcript, protein, metabolite, glasshouse phenotype, and, only when the plants are fully characterized, to a field test, field tests will need to be carried out earlier in the analysis. In this way, biological intuition will again become a valued trait among plant biologists.
The discovery of the function of one of N. attenuata's RNA-direct RNA polymerases (RdR1) in mediating resistance responses to herbivore attack illustrates the procedure (Pandey and Baldwin, 2007). RdRs are essential in siRNA biogenesis, but their organismic-level function was unknown. When plants silenced in endogenous RdR1 expression were planted into native populations, they were found to be highly susceptible to attack from native herbivores, which was associated with reduced nicotine levels, which in turn are known to require JA signaling. Further analysis revealed that RdR1 was involved in coordinating the signaling of phytohormones, namely, ethylene and JA, and, after sequencing the small RNA transcriptome, a number of small RNAs that matched sequences for phytohormone biosynthetic genes were found (Pandey and Baldwin, 2008). This example demonstrates the value of field experiments in rapidly unraveling whole-organismic functions that are overlooked when plants are grown in protected glasshouse environments.
Heterotrophy was well established long before the evolution of photosynthesis. Plants have always had to cope with the ravages of consumers that want access to the resources that plants control. Their sophisticated means of defending themselves likely use all aspects of their metabolism. Their prodigious anabolic potential allows plants to throw just about everything at consumers to protect themselves. Our challenge will be to figure out what parts of metabolism are currently being maintained by natural selection as defenses. As Rick Karban predicted in his “Moving Target Hypothesis” (Karban et al., 1997), plants are very plastic and this plasticity itself may well be part of its defensive repertoire.
Although nature provides the best laboratory for testing gene function, we have trained a generation of plant biologists who are unfamiliar with field work. When unbiased transcriptional responses are used to “ask the plant” which genes are regulated in response to herbivore attack, the plant provides testable hypotheses about which genes are important in tolerance or defense. Gene annotations classify genes and specify their putative biochemical function based on sequence similarity. These annotations are extremely valuable, but they should be viewed with caution as they do not exclude other biochemical functions or functions at other levels. An example of the TD gene from N. attenuata illustrates the point. Silencing TD generated plants with stunted growth because TD is involved in Ile biosynthesis. However, other TD-silenced plants grew normally but were found to be highly susceptible to herbivores (Kang et al., 2006). Further analysis of these plants revealed a strong reduction of defense responses to herbivory, due to reduced levels of JA-Ile; defense signaling was hampered, but the effects on primary metabolism were negligible. This illustrates that the defense function of a primary gene can be studied with transformants that exhibit “mild” phenotypes and are not impaired in development.
Clearly, there will be much to be learned by “asking the plant” and using the “omic” tools for deciphering the plant's answer in the genes, proteins, and metabolites that it regulates differently when attacked by herbivores. If we are sufficiently forward thinking to ignore the gene annotations, to silence these regulated responses in ways that do not dramatically influence growth, and then to ask the community of herbivores that naturally attack plants whether the plant is more resistant to or tolerant of herbivore attack, we will undoubtedly learn much that is new about how plants survive in the real world. Although at present technical and regulatory issues impede the adoption of this procedure, the blinders that come with specialized scientific training will be as difficult to remove as the other challenges.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Ian T. Baldwin (baldwin@ice.mpg.de).
References
- Ahn JH, Choi Y, Kwon YM, Kim SG, Choi YD, Lee JS (1996) A novel extensin gene encoding a hydroxyproline-rich glycoprotein requires sucrose for its wound-inducible expression in transgenic plants. Plant Cell 8 1477–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JH, Lee JS (2003) Sugar acts as a regulatory signal on the wound-inducible expression of SbHRGP3∷GUS in transgenic plants. Plant Cell Rep 22 286–293 [DOI] [PubMed] [Google Scholar]
- Arnold TM, Schultz JC (2002) Induced sink strength as a prerequisite for induced tannin biosynthesis in developing leaves of Populus. Oecologia 130 585–593 [DOI] [PubMed] [Google Scholar]
- Babst BA, Ferrieri RA, Gray DW, Lerdau M, Schlyer DJ, Schueller M, Thorpe MR, Orians CM (2005) Jasmonic acid induces rapid changes in carbon transport and partitioning in Populus. New Phytol 167 63–72 [DOI] [PubMed] [Google Scholar]
- Bazot S, Mikola J, Nguyen C, Robin C (2005) Defoliation-induced changes in carbon allocation and root soluble carbon concentration in field-grown Lolium perenne plants: Do they affect carbon availability, microbes and animal trophic groups in soil? Funct Ecol 19 886–896 [Google Scholar]
- Bergelson J, Juenger T, Crawley MJ (1996) Regrowth following herbivory in Ipomopsis aggregata: compensation but not overcompensation. Am Nat 148 744–755 [Google Scholar]
- Berger S, Bell E, Sadka A, Mullet JE (1995) Arabidopsis thaliana Atvsp is homologous to soybean Vspa and Vspb, genes encoding vegetative storage protein acid-phosphatases, and is regulated similarly by methyl jasmonate, wounding, sugars, light and phosphate. Plant Mol Biol 27 933–942 [DOI] [PubMed] [Google Scholar]
- Both M, Csukai M, Stumpf MPH, Spanu PD (2005) Gene expression profiles of Blumeria graminis indicate dynamic changes to primary metabolism during development of an obligate biotrophic pathogen. Plant Cell 17 2107–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briske DD, Boutton TW, Wang Z (1996) Contribution of flexible allocation priorities to herbivory tolerance in C-4 perennial grasses: an evaluation with C-13 labeling. Oecologia 105 151–159 [DOI] [PubMed] [Google Scholar]
- Chen H, Gonzales-Vigil E, Wilkerson CG, Howe GA (2007) Stability of plant defense proteins in the gut of insect herbivores. Plant Physiol 143 1954–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Peumans WJ, Hause B, Bras J, Kumar M, Proost P, Barre A, Rouge P, Van Damme EJM (2002) Jasmonate methyl ester induces the synthesis of a cytoplasmic/nuclear chitooligosaccharide-binding lectin in tobacco leaves. FASEB J 16 U225–U251 [DOI] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernandez G, Adie B, Chico JM, Lorenzo O, Garcia-Casado G, Lopez-Vidriero I, Lozano FM, Ponce MR, et al (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448 666–671 [DOI] [PubMed] [Google Scholar]
- Cipollini D (2007) Consequences of the overproduction of methyl jasmonate on seed production, tolerance to defoliation and competitive effect and response of Arabidopsis thaliana. New Phytol 173 146–153 [DOI] [PubMed] [Google Scholar]
- Claeyssen E, Rivoal J (2007) Isozymes of plant hexokinase: occurrence, properties and functions. Phytochemistry 68 709–731 [DOI] [PubMed] [Google Scholar]
- Coughenour MB, Detling JK, Bamberg IE, Mugambi MM (1990) Production and nitrogen responses of the African dwarf shrub Indigofera-Spinosa to defoliation and water limitation. Oecologia 83 546–552 [DOI] [PubMed] [Google Scholar]
- Cross JM, von Korff M, Altmann T, Bartzetko L, Sulpice R, Gibon Y, Palacios N, Stitt M (2006) Variation of enzyme activities and metabolite levels in 24 Arabidopsis accessions growing in carbon-limited conditions. Plant Physiol 142 1574–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen BR, Chapman DF, Quigley PE (2006) Comparative defoliation tolerance of temperate perennial grasses. Grass and Forage Science 61 405–412 [Google Scholar]
- Devoto A, Turner JG (2005) Jasmonate-regulated Arabidopsis stress signalling network. Physiol Plant 123 161–172 [Google Scholar]
- Dyer MI, Acra MA, Wang GM, Coleman DC, Freckman DW, McNaughton SJ, Strain BR (1991) Source-sink carbon relations in 2 panicum-coloratum ecotypes in response to herbivory. Ecology 72 1472–1483 [Google Scholar]
- Franceschi VR, Nakata PA (2005) Calcium oxalate in plants: formation and function. Annu Rev Plant Biol 56 41–71 [DOI] [PubMed] [Google Scholar]
- Gabius HJ, Siebert HC, Andre S, Jimenez-Barbero J, Rudiger H (2004) Chemical biology of the sugar code. ChemBioChem 5 741–764 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Blasing O, Palacios-Rojas N, Pankovic D, Hendriks J, Fisahn J, Hohne M, Gunther MS (2004) Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J 39 847–862 [DOI] [PubMed] [Google Scholar]
- Green ES, Zangerl AR, Berenbaum MR (2001) Effects of phytic acid and xanthotoxin on growth and detoxification in caterpillars. J Chem Ecol 27 1763–1773 [DOI] [PubMed] [Google Scholar]
- Gross N, Wasternack C, Kock M (2004) Wound-induced RNaseLE expression is jasmonate and systemin independent and occurs only locally in tomato (Lycopersicon esculentum cv. Lukullus). Phytochemistry 65 1343–1350 [DOI] [PubMed] [Google Scholar]
- Halford NG, Paul MJ (2003) Carbon metabolite sensing and signalling. Plant Biotechnol J 1 381–398 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Gase K, Hui DQ, Schmidt DD, Baldwin IT (2003) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VI. Microarray analysis reveals that most herbivore-specific transcriptional changes are mediated by fatty acid-amino acid conjugates. Plant Physiol 131 1894–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, Rattke J, Boland W (2005) Postsecretory hydrolysis of nectar sucrose and specialization in ant/plant mutualism. Science 308 560–563 [DOI] [PubMed] [Google Scholar]
- Holland JN, Cheng WX, Crossley DA (1996) Herbivore-induced changes in plant carbon allocation: assessment of below-ground C fluxes using carbon-14. Oecologia 107 87–94 [DOI] [PubMed] [Google Scholar]
- Hui DQ, Iqbal J, Lehmann K, Gase K, Saluz HP, Baldwin IT (2003) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. V. Microarray analysis and further characterization of large-scale changes in herbivore-induced mRNAs. Plant Physiol 131 1877–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel GM, Naumann M, Schurr U, Walter A (2007) Root growth dynamics of Nicotiana attenuata seedlings are affected by herbivory. Plant Cell Environ 30 1326–1336 [DOI] [PubMed] [Google Scholar]
- Kang JH, Wang L, Giri A, Baldwin IT (2006) Silencing threonine deaminase and JAR4 in Nicotiana attenuata impairs jasmonic acid-isoleucine-mediated defenses against Manduca sexta. Plant Cell 18 3303–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant MR, Baldwin IT (2007) The ecogenetics and ecogenomics of plant-herbivore interactions: rapid progress on a slippery road. Curr Opin Genet Dev 17 519–524 [DOI] [PubMed] [Google Scholar]
- Karban R, Agrawal AA, Mangel M (1997) The benefits of induced defenses against herbivores. Ecology 78 1351–1355 [Google Scholar]
- Kessler A, Halitschke R, Baldwin IT (2004) Silencing the jasmonate cascade: induced plant defenses and insect populations. Science 305 665–668 [DOI] [PubMed] [Google Scholar]
- Korth KL, Doege SJ, Park SH, Goggin FL, Wang Q, Gomez SK, Liu G, Jia L, Nakata PA (2006) Medicago truncatula mutants demonstrate the role of plant calcium oxalate crystals as an effective defense against chewing insects. Plant Physiol 141 188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoo N, Peumans WJ, Van Damme EJM (2006) The presence of jasmonate-inducible lectin genes in some but not all Nicotiana species explains a marked intragenus difference in plant responses to hormone treatment. J Exp Bot 57 3145–3155 [DOI] [PubMed] [Google Scholar]
- LeBrasseur ND, MacIntosh GC, Perez-Amador MA, Saitoh M, Green PJ (2002) Local and systemic wound-induction of RNase and nuclease activities in Arabidopsis: RNS1 as a marker for a JA-independent systemic signaling pathway. Plant J 29 393–403 [DOI] [PubMed] [Google Scholar]
- Leon J, Rojo E, Titarenko E, Sanchez-Serrano JJ (1998) Jasmonic acid-dependent and -independent wound signal transduction pathways are differentially regulated by Ca2+/calmodulin in Arabidopsis thaliana. Mol Gen Genet 258 412–419 [DOI] [PubMed] [Google Scholar]
- Mabry CM, Wayne PW (1997) Defoliation of the annual herb Abutilon theophrasti: mechanisms underlying reproductive compensation. Oecologia 111 225–232 [DOI] [PubMed] [Google Scholar]
- Major IT, Constabel CP (2006) Molecular analysis of poplar defense against herbivory: comparison of wound- and insect elicitor-induced gene expression. New Phytol 172 617–635 [DOI] [PubMed] [Google Scholar]
- Meuriot F, Noquet C, Avice JC, Volenec JJ, Cunningham SM, Sors TG, Caillot S, Ourry A (2004) Methyl jasmonate alters N partitioning, N reserves accumulation and induces gene expression of a 32-kDa vegetative storage protein that possesses chitinase activity in Medicago sativa taproots. Physiol Plant 120 113–123 [DOI] [PubMed] [Google Scholar]
- Meyer S, Lauterbach C, Niedermeier M, Barth I, Sjolund RD, Sauer N (2004) Wounding enhances expression of AtSUC3, a sucrose transporter from Arabidopsis sieve elements and sink tissues. Plant Physiol 134 684–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molano-Flores B (2001) Herbivory and calcium concentrations affect calcium oxalate crystal formation in leaves of Sida (Malvaceae). Ann Bot (Lond) 88 387–391 [Google Scholar]
- Mozoruk J, Hunnicutt LE, Cave RD, Hunter WB, Bausher MG (2006) Profiling transcriptional changes in Citrus sinensis (L.) Osbeck challenged by herbivory from the xylem-feeding leafhopper Homalodisca coagulata (Say) by cDNA macroarray analysis. Plant Sci 170 1068–1080 [Google Scholar]
- Newingham BA, Callaway RM, Bassirirad H (2007) Allocating nitrogen away from a herbivore: a novel compensatory response to root herbivory. Oecologia 153 913–920 [DOI] [PubMed] [Google Scholar]
- Niyogi KK (2000) Safety valves for photosynthesis. Curr Opin Plant Biol 3 455–460 [DOI] [PubMed] [Google Scholar]
- Ohyama A, Nishimura S, Hirai M (1998) Cloning of cDNA for a cell wall-bound acid invertase from tomato (Lycopersicon esculentum) and expression of soluble and cell wall-bound invertases in plants and wounded leaves of L-esculentum and L-peruvianum. Genes Genet Syst 73 149–157 [DOI] [PubMed] [Google Scholar]
- Pandey SP, Baldwin IT (2007) RNA-directed RNA polymerase 1 (RdR1) mediates the resistance of Nicotiana attenuata to herbivore attack in nature. Plant J 50 40–53 [DOI] [PubMed] [Google Scholar]
- Pandey SP, Baldwin IT (2008) Silencing RNA-directed RNA polymerase 2 (RdR2) increases Nicotiana attenuata's susceptibility to UV in the field and in the glasshouse. Plant J (in press) [DOI] [PubMed]
- Paul M (2007) Trehalose 6-phosphate. Curr Opin Plant Biol 10 303–309 [DOI] [PubMed] [Google Scholar]
- Ralph SG, Yueh H, Friedmann M, Aeschliman D, Zeznik JA, Nelson CC, Butterfield YSN, Kirkpatrick R, Liu J, Jones SJM, et al (2006) Conifer defence against insects: microarray gene expression profiling of Sitka spruce (Picea sitchensis) induced by mechanical wounding or feeding by spruce budworms (Choristoneura occidentalis) or white pine weevils (Pissodes strobi) reveals large-scale changes of the host transcriptome. Plant Cell Environ 29 1545–1570 [DOI] [PubMed] [Google Scholar]
- Ramon M, Rolland F (2007) Plant development: introducing trehalose metabolism. Trends Plant Sci 12 185–188 [DOI] [PubMed] [Google Scholar]
- Roitsch T, Gonzalez MC (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9 606–613 [DOI] [PubMed] [Google Scholar]
- Rojo E, Leon J, Sanchez-Serrano JJ (1999) Cross-talk between wound signalling pathways determines local versus systemic gene expression in Arabidopsis thaliana. Plant J 20 135–142 [DOI] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzales E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57 675–709 [DOI] [PubMed] [Google Scholar]
- Rosenkranz H, Vogel R, Greiner S, Rausch T (2001) In wounded sugar beet (Beta vulgaris L.) tap-root, hexose accumulation correlates with the induction of a vacuolar invertase isoform. J Exp Bot 52 2381–2385 [DOI] [PubMed] [Google Scholar]
- Ruiz N, Ward D, Saltz D (2002) Calcium oxalate crystals in leaves of Pancratium sickenbergeri: constitutive or induced defence? Funct Ecol 16 99–105 [Google Scholar]
- Schluepmann H, van Dijken A, Aghdasi M, Wobbes B, Paul M, Smeekens S (2004) Trehalose mediated growth inhibition of Arabidopsis seedlings is due to trehalose-6-phosphate accumulation. Plant Physiol 135 879–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt DD, Baldwin IT (2006) Transcriptional responses of Solanum nigrum to methyl jasmonate and competition: a glasshouse and field study. Funct Ecol 20 500–508 [Google Scholar]
- Schwachtje J, Minchin PEH, Jahnke S, van Dongen JT, Schittko U, Baldwin IT (2006) SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proc Natl Acad Sci USA 103 12935–12940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Stitt M (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30 1126–1149 [DOI] [PubMed] [Google Scholar]
- Steppuhn A (2007) Costs and benefits of two direct defenses in Nicotiana attenuata: nicotine and trypsin protease inhibitors. PhD thesis. Friedrich-Schiller-University, Jena, Germany
- Steppuhn A, Baldwin IT (2007) Resistance management in a native plant: nicotine prevents herbivores from compensating for plant protease inhibitors. Ecol Lett 10 499–511 [DOI] [PubMed] [Google Scholar]
- Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT (2004) Nicotine's defensive function in nature. PLoS Biol 2 1074–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe KA, Marquis RJ, Hochwender CG, Simms EL (2000) The evolutionary ecology of tolerance to consumer damage. Annu Rev Ecol Syst 31 565–595 [Google Scholar]
- Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14 179–185 [DOI] [PubMed] [Google Scholar]
- Takashima T, Hikosaka K, Hirose T (2004) Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ 27 1047–1054 [Google Scholar]
- Tang JY, Zielinski RE, Zangerl AR, Crofts AR, Berenbaum MR, DeLucia EH (2006) The differential effects of herbivory by first and fourth instars of Trichoplusia ni (Lepidoptera : Noctuidae) on photosynthesis in Arabidopsis thaliana. J Exp Bot 57 527–536 [DOI] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu GH, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 448 661–665 [DOI] [PubMed] [Google Scholar]
- Tian ZD, Liu J, Wang BL, Xie CH (2006) Screening and expression analysis of Phytophthora infestans induced genes in potato leaves with horizontal resistance. Plant Cell Rep 25 1094–1103 [DOI] [PubMed] [Google Scholar]
- Truernit E, Schmid J, Epple P, Illig J, Sauer N (1996) The sink-specific and stress-regulated Arabidopsis STP4 gene: enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. Plant Cell 8 2169–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme EJM, Lannoo N, Fouquaert E, Peumans WJ (2003) The identification of inducible cytoplasmic/nuclear carbohydrate-binding proteins urges to develop novel concepts about the role of plant lectins. Glycoconj J 20 449–460 [DOI] [PubMed] [Google Scholar]
- von Dahl CC, Baldwin IT (2007) Deciphering the role of ethylene in plant-herbivore interactions. J Plant Growth Regul 26 201–209 [Google Scholar]
- Welter SC (1989) Arthropod impact on plant gas exchange. In EA Bernays, ed, Insect-Plant Interactions, Vol 1. CRC Press, Boca Raton, FL, pp 135–150
- Wise MJ, Cummins JJ (2006) Strategies of Solanum carolinense for regulating maternal investment in response to foliar and floral herbivory. J Ecol 94 629–636 [Google Scholar]
- Wu JQ, Hettenhausen C, Meldau S, Baldwin IT (2007) Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell 19 1096–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyka T (1999) Carbohydrate storage and use in an alpine population of the perennial herb, Oxytropis sericea. Oecologia 120 198–208 [DOI] [PubMed] [Google Scholar]
- Zangerl AR, Hamilton JG, Miller TJ, Crofts AR, Oxborough K, Berenbaum MR, de Lucia EH (2002) Impact of folivory on photosynthesis is greater than the sum of its holes. Proc Natl Acad Sci USA 99 1088–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Baldwin IT (2006) Jasmonic acid signalling and herbivore resistance traits constrain regrowth after herbivore attack in Nicotiana attenuata. Plant Physiol 134 1181–1190 [DOI] [PubMed] [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Baldwin IT (2004. a) Constitutive and inducible trypsin proteinase inhibitor production incurs large fitness costs in Nicotiana attenuata. Proc Natl Acad Sci USA 101 1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Hui DQ, Baldwin IT (2004. b) Manipulation of endogenous trypsin proteinase inhibitor production in Nicotiana attenuata demonstrates their function as antiherbivore defenses. Plant Physiol 134 1181–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Cohn NS, Mitchell JP (1996) Induction of a pea cell-wall invertase gene by wounding and its localized expression in phloem. Plant Physiol 112 1111–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Jang JC, Jones TL, Sheen J (1998) Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA 95 10294–10299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu-Salzman K, Luthe DS, Felton GW (2008) Arthropod-inducible proteins: broad spectrum defenses against multiple herbivores. Plant Physiol 146 852–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JP, Cates RG (1994) Role of Douglas-fir (Pseudotsuga-menziesii) carbohydrates in resistance to budworm (Choristoneura-occidentalis). J Chem Ecol 20 395–405 [DOI] [PubMed] [Google Scholar]