Abstract
Continuous mechanical damage initiates the rhythmic emission of volatiles in lima bean (Phaseolus lunatus) leaves; the emission resembles that induced by herbivore damage. The effect of diurnal versus nocturnal damage on the initiation of plant defense responses was investigated using MecWorm, a robotic device designed to reproduce tissue damage caused by herbivore attack. Lima bean leaves that were damaged by MecWorm during the photophase emitted maximal levels of β-ocimene and (Z)-3-hexenyl acetate in the late photophase. Leaves damaged during the dark phase responded with the nocturnal emission of (Z)-3-hexenyl acetate, but with only low amounts of β-ocimene; this emission was followed by an emission burst directly after the onset of light. In the presence of 13CO2, this light-dependent synthesis of β-ocimene resulted in incorporation of 75% to 85% of 13C, demonstrating that biosynthesis of β-ocimene is almost exclusively fueled by the photosynthetic fixation of CO2 along the plastidial 2-C-methyl-d-erythritol 4-P pathway. Jasmonic acid (JA) accumulated locally in direct response to the damage and led to immediate up-regulation of the P. lunatus β-ocimene synthase gene (PlOS) independent of the phase, that is, light or dark. Nocturnal damage caused significantly higher concentrations of JA (approximately 2–3 times) along with enhanced expression levels of PlOS. Transgenic Arabidopsis thaliana transformed with PlOS promoter∷β-glucuronidase fusion constructs confirmed expression of the enzyme at the wounded sites. In summary, damage-dependent JA levels directly control the expression level of PlOS, regardless of light or dark conditions, and photosynthesis is the major source for the early precursors of the 2-C-methyl-d-erythritol 4-P pathway.
Herbivore-induced plant volatiles (HIPVs) attract herbivore natural enemies and, hence, benefit plants indirectly. Like floral volatiles (Dudareva et al., 2003, 2005), HIPVs display diurnal or nocturnal rhythms that may be coordinated with the habits of the parasitoids that are attracted to them (Loughrin et al., 1994; Turlings et al., 1995; Arimura et al., 2004a, 2005). Whereas floral scents or light-dependent isoprene emissions are constitutively produced according to circadian rhythms (Dudareva et al., 2005; Loivamäki et al., 2007; Roeder et al., 2007), the release of HIPVs is a phenotypically plastic response of plants to herbivory that is influenced by insect performance and environmental factors (e.g. photoperiod and temperature). Herbivorous insects likewise depend on plant volatiles for information about their food plants and the surrounding environment so that they can modulate their activity accordingly. For example, caterpillars of Mythimna separata behaved as if they were in the dark when exposed during the day to volatiles emitted from host plants (infested or uninfested) in the dark (Shiojiri et al., 2006). Likewise, regardless of the amount of light available, caterpillars behaved as if they were in the light when exposed to volatiles emitted from plants during the photophase (Shiojiri et al., 2006). Moreover, volatile compounds released at night from lepidopteran-infested tobacco (Nicotiana tabacum) plants were repellent to conspecific female moths (Heliothis virescens; De Moraes et al., 2001). These examples clearly demonstrate that plants are able to generate temporally different volatile blends that address different organisms at different times of the day in order to optimize defense. Whereas many elements of damage recognition, signal transduction, de novo biosynthesis, and metabolic regulation involved in HIPV biosynthesis are known (Arimura et al., 2005), only little is known about the mechanisms of the temporal variations underlying the synthesis and release of HIPVs. In particular, the question of whether HIPV emissions are controlled by circadian clocks or by environmental factors, such as the insect's activity level or the amount of light, remain to be addressed.
Here, we report on the results of diurnal and nocturnal leaf damage using the previously developed MecWorm device to mimic herbivore-caused tissue damage in a completely reproducible and quantifiable manner. The continuous mechanical damage effected by the robotic device was able to induce in lima bean (Phaseolus lunatus) leaves the emission of volatiles whose profiles perfectly matched those of the HIPVs induced by feeding larvae of Spodoptera littoralis (Mithöfer et al., 2005) The volatile spectrum comprised monoterpenes, sesquiterpenes, and homoterpenes along with fatty acid-derived volatiles, such as (Z)-3-hexenyl acetate (Hex-Ac). The major compounds from the group of terpenoids were (E)-β-ocimene (69%), linalool (4%), and (E)-4,8-dimethyl-1,3,7-nonatriene (11%). Because this computer-controlled mechanical induction process (1) does not depend on undefined components of insect salivary secretions (Paré and Tumlinson, 1999), (2) can be fully reproduced at any time of the day, and (3) can be adjusted to predefined damage levels, this approach is ideally suited for a comparative study of the effects of diurnal or nocturnal damage on the emission of HIPVs.
Using this fully reproducible mode of mechanical damage, we present evidence that temporally different instances of leaf damage to the same plant during either the light phase (day) or the dark phase (night) play a decisive role in the control of HIPV emission. We demonstrate that continuous mechanical damage during the day or night is associated with an enhanced level of jasmonic acid (JA). This JA production is closely followed by up-regulation of the transcript level of the gene coding for the (E)-β-ocimene synthase. During the photophase, damage by MecWorm leads to the instantaneous emission of (E)-β-ocimene. In the dark, however, a very weak nocturnal emission is followed by a rather brief emission burst after the onset of the photophase. Studies with 13CO2 demonstrate that the early steps of the terpenoid pathway along the 2-C-methyl-d-erythritol 4-P (MEP) pathway appear to be responsible for the delayed response. We propose a schematic view of the elements of signaling and metabolic regulation required for the herbivore-induced β-ocimene emission and the light-independent Hex-Ac.
RESULTS
Functional Characterization of the β-Ocimene Synthase Gene in Lima Bean
The monoterpene (E)-β-ocimene, emitted from lima bean leaves after damage by the feeding larvae of S. littoralis, was one of the major volatiles (64%–69% of the total volatiles; Arimura et al., 2007). To explore the regulatory mechanisms for herbivore-induced β-ocimene emission, we first isolated the full-length clone of the cDNA of the β-ocimene synthase from lima bean (P. lunatus ocimene synthase [PlOS]; GenBank accession no. EU194553). To functionally characterize PlOS, the recombinant protein was expressed in Escherichia coli. Assays with geranyl diphosphate as a substrate produced two monoterpene hydrocarbons, namely, (E)-β-ocimene (98%) and its (Z)-isomer (2%; Fig. 1). This ratio corresponds to the composition of β-ocimene isomers emitted from the lima bean plants after 1 d of damage by the feeding larvae of S. littoralis (E:Z = 97:3). Neither farnesyl diphosphate nor geranylgeranyl diphosphate was converted by the recombinant protein.
Figure 1.
Gas chromatographic separation of the β-ocimene isomers formed by the recombinant PlOS enzyme with GDP as substrate.
Rhythmic Emission of Herbivore-Induced Volatiles β-Ocimene and Hex-Ac
Volatiles in the headspace of induced plants were analyzed with zNose, which combines a high time resolution (10 min) with the ability to run unattended over prolonged periods (Kunert et al., 2002). Figure 2A demonstrates the time course of the emission of the two selected HIPVs, Hex-Ac and β-ocimene. Emission was low on the first day after damage by feeding larvae of S. littoralis; strong diurnal emissions of both compounds followed on the second day (14/10 light-dark [LD] cycle). This was accompanied by up-regulation of the PlOS transcript. Maximal transcript levels of PlOS were observed during the photophase (7 am–9 pm) of a LD cycle. To test whether herbivore-induced terpenes are regulated according to circadian rhythms, we introduced an additional dark (AD) period for 4 h during the second and third photophases and investigated its effect on the PlOS transcript level and β-ocimene emission (Fig. 2B). Although β-ocimene was permanently emitted during the full photoperiod of Figure 2A, during the AD period (Fig. 2B) volatile emissions were reduced within 40 to 60 min following the onset of darkness. This clearly demonstrates that the herbivore-induced emission of β-ocimene is strictly linked to light and, unlike floral scents (Dudareva et al., 2005; Roeder et al., 2007), is not under the control of the circadian clock. Most important, the transcript level of the PlOS gene was affected by the AD period and dropped to very low levels at the end of AD, whereas transcript levels of PlOS remained high in the light period without AD (P < 0.05; ANOVA; see time points 29 and 53 h; P < 0.05). Note that the feeding performance of S. littoralis did not change even after the onset of the AD period.
Figure 2.
Emission of the volatiles β-ocimene and Hex-Ac and transcript levels of PlOS in damaged lima bean leaves. Damage conditions: larvae of S. littoralis under a 14:10 LD cycle (A) and under a LD cycle set with 4 h of additional darkness (AD) during the second (25–29 h) and third (49–53 h) photoperiods (B). Time of day is shown across the top x axis, and total elapsed time is shown on the bottom x axis. Headspace analyses were performed in triplicate. Emission is expressed as ng min−1 g−1 FW. Data regarding to the transcript levels represent the mean ± se (n = 4–5).
Effects of Diurnal and Nocturnal Damage on Volatile Emissions
To determine temporal relation between leaf damage and emission of HIPVs, we used the recently developed MecWorm (Mithöfer et al., 2005); with MecWorm, we could evaluate differences between induction processes during the light and dark periods. Continuous mechanical damage for 6 h during the photophase induced the rhythmic emission of volatiles. β-Ocimene and Hex-Ac (Fig. 3A) were chosen to represent the groups of terpenoids and fatty acid-derived compounds and monitored over several days. In contrast to the typical delay observed after insects fed (Fig. 2A) with MecWorm, volatiles were emitted almost instantaneously. Moreover, both compounds displayed different emission maxima: Emission of Hex-Ac closely followed the damage period, whereas emission of β-ocimene increased further after the damage had ended. Emission remained high until to the onset of the dark period followed by a sharp decrease of β-ocimene production. Emission profiles of both compounds were entirely different when MecWorm (6 h) was applied during the dark phase. Whereas Hex-Ac emission closely followed the damage period (albeit at a lower level than during the photophase), the nocturnal emission of β-ocimene started only sluggishly and was followed by a very strong emission burst at the beginning of the photophase (Fig. 3B). Unlike diurnal damage, which resulted in strong β-ocimene emissions over the full light period, nocturnally induced β-ocimene production ceased after the initial burst and rapidly dropped to the basal level at the onset of the dark phase. In addition to the rather low emission of Hex-Ac during the night, nocturnal damage resulted in additional, albeit weak, emissions of Hex-Ac during the photophase. Interestingly, the total amount of β-ocimene emitted after nocturnal damage was higher than the amount released after the damage that occurred in the light.
Figure 3.
Emissions of β-ocimene and Hex-Ac (A and B) and levels of JA and PlOS transcripts (C and D) in MecWorm-treated leaves. Damage program: 6 h during either the photophase (A and C) or the dark phase (B and D) according to a 14:10 LD cycle. Damage periods started 2 h after the onset of the light or dark phase with punching every 5 s for 6 h (9 am–3 pm during the photophase and from 11 pm–5 am during the night). Headspace analyses were performed in triplicate. Emission is expressed as ng min−1 g−1 FW. JA and transcript levels represent the mean ± se (n = 3–4).
Evidence for de Novo Biosynthesis of β-Ocimene during the Early Morning Burst
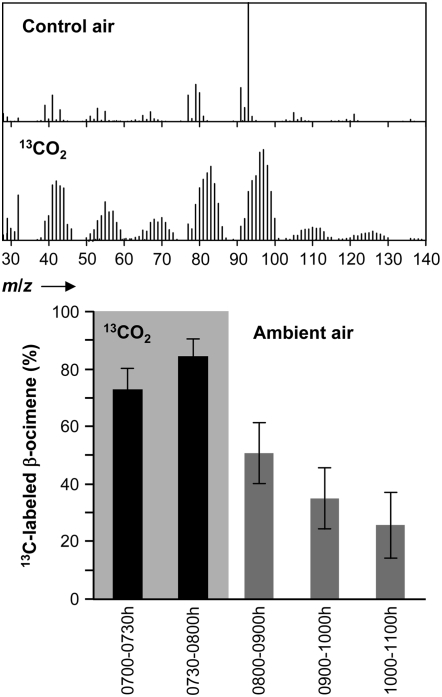
The supply of C5 precursors for monoterpene biosynthesis via the plastidial MEP pathway is directly linked to CO2 fixation (Paré and Tumlinson, 1997). The Calvin cycle delivers the starting substrates for the MEP pathway, namely, glyceraldehyde-3-P and pyruvate. To assess how fast and to what extent CO2 fixation contributed to the light-dependent emission of β-ocimene in induced plants, we supplied air with 13CO2 (at 380 μg mL−1) to damaged lima bean leaves and monitored the incorporation of 13C into β-ocimene (Fig. 4). 13CO2 was introduced 30 min before the end of the dark phase (6:30 am–7 am), and the emitted volatiles were collected at 30-min intervals and analyzed by mass spectroscopy. Analysis showed that, during the first 30 min (7 am–7:30 am), (E)-β-ocimene had incorporated almost 75% of 13C. This level rose during the second interval (7:30 am–8 am) to about 85%. After 90 min, 13CO2 gas was exchanged with ambient air (12CO2) and the degree of labeling in (E)-β-ocimene leveled off to an average of 25%. We therefore conclude that the light-dependent synthesis of β-ocimene was strictly linked to the photosynthetic fixation of CO2. This differs from previous findings, namely, that monoterpenes and sesquiterpenes are not labeled with the 13CO2 gas in tomato (Solanum lycopersicum) plants infested with tobacco hornworms during the photophase (Farag and Paré, 2002). As mentioned above, the induced emission of Hex-Ac depended strictly on the timing of damage, regardless of light or dark conditions (Fig. 3, A and B). Because Hex-Ac is derived from the oxidative degradation of lipids from the plasma membrane rather than from de novo biosynthesis, no incorporation of 13CO2 into Hex-Ac was observed (data not shown).
Figure 4.
Pulse labeling of (E)-β-ocimene after administration of 13CO2. Ambient air in the cabinet was exchanged by synthetic air containing 13CO2 gas (380 μg mL−1) 0.5 h before the onset of the light phase. Headspace analysis was started at its onset. The shaded box corresponds to the presence of 13CO2. One hour after the onset of the photophase, the cabinet was purged with ambient air to remove 13CO2. Collection periods for volatiles are indicated. Mass spectra of β-ocimene from leaves in ambient air or 13CO2-containing air are shown on top. Data represent the mean ± se (n = 4).
Effect of Diurnal and Nocturnal Damage on JA Production and Gene Regulation
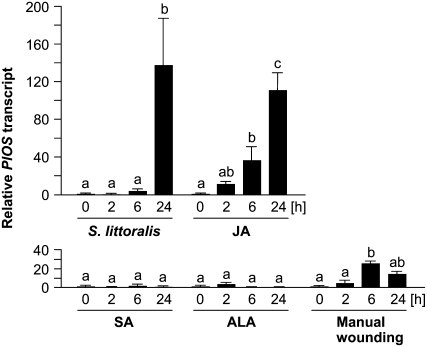
Damage by feeding herbivores and the external application of JA (0.5 mm) to lima bean leaves (Dicke et al., 1999) significantly induced the PlOS transcript (Fig. 5) and resulted in β-ocimene emission. In line with these observations, continuous wounding with MecWorm triggered rapid production of the phytohormone during both the light and dark phases, which was accompanied by a correspondingly elevated level of the PlOS transcript (Fig. 3, C and D). A close connection between the increased level of JA and the elevated transcript level of PlOS was observed for both diurnal and nocturnal damage. When leaves were damaged by MecWorm for 6 h starting at 11 pm (dark phase), induced JA levels were significantly higher (approximately 500 ng JA g−1 leaf fresh weight) than after they had been damaged for 6 h during the day starting at 9 am (approximately 200 ng JA g−1 fresh weight). On average, the nocturnal levels of JA we observed were 2 to 3 times higher than those measured after damage during the photophase (Fig. 3, C and D). Interestingly, the day-induced JA level dropped immediately after the mechanical wounding ended, whereas nocturnally induced JA continued to increase for about 2 h after the mechanical wounding ended. Manual wounding (single events) raised the level of JA only moderately after 30 min (approximately 100 ng g−1 fresh weight; Supplemental Fig. S1) along with the transcript levels of PlOS after 6 h (Fig. 5); emission of (E)-β-ocimene as a consequence of manual wounding was only occasionally observed. Treatment of the leaves with the phytohormone salicylic acid or the channel-forming fungal elicitor alamethicin, which stimulates internal salicylic acid biosynthesis (Engelberth et al., 2001), did not affect transcript levels of PlOS.
Figure 5.
Effect of herbivory, wounding, and chemical treatment on the expression level of PlOS in lima bean leaves. Chemical treatments: JA (0.5 mm), salicylic acid (SA; 0.5 mm), and alamethicin (ALA; 1 mm). Means with small letters are significantly different according to ANOVA followed by Scheffe's test (P < 0.05). Data represent the mean ± se (n = 4).
Localization of JA and PlOS Transcript Accumulation in Damaged Leaves
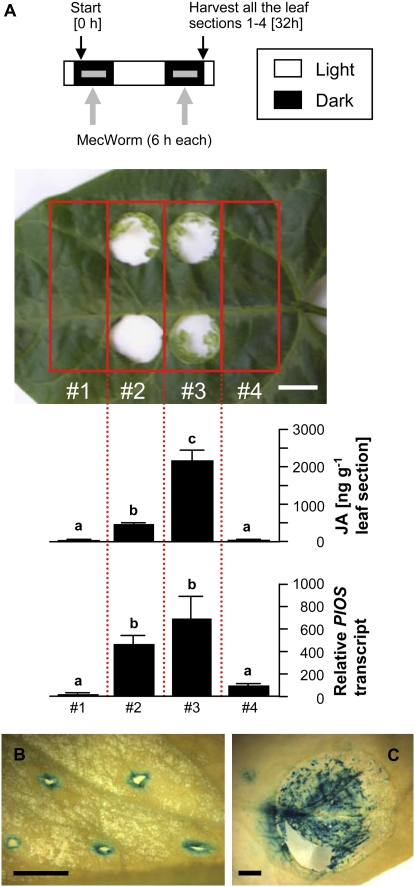
Localization patterns of JA and the PlOS transcript in leaf sections in relation to the first and second instances of damage in the dark were then investigated (Fig. 6A). Thirty-two hours after onset of the first nocturnal damage by MecWorm, high JA levels were found in segments 2 and 3. Increased JA levels in segments 2 and 3 reflect the increased JA levels of the consecutive treatments shown in Figure 3D. JA and PlOS transcript levels in undamaged neighbor segments 1 and 4 corresponded to the resting level, showing that JA accumulated only locally at the wounding site. PlOS expression showed a similar localization pattern; the difference in expression levels in leaf sections 2 and 3 was statistically insignificant. That PlOS was expressed only at the sites of wounding was further demonstrated with transgenic Arabidopsis (Arabidopsis thaliana) stably transformed with PlOS promoter∷GUS. As shown in Figure 6A, 2 h of manual wounding led to the expression of the inducible GUS reporter gene only at the wounding sites (Fig. 6B). Treatment with MecWorm led to the same result (Fig. 6C). Overall, these results indicate that JA-induced PlOS expression is localized at the wounding site, thus facilitating terpenoid emission locally and temporally. Similar herbivory-linked local patterns were observed for TPS genes AtTPS12 (At4g13280), AtTPS13 (At4g13300; Ro et al., 2006), and LjEβOS (Arimura et al., 2004b).
Figure 6.
Localization of PlOS transcript and accumulation of JA in mechanically damaged lima bean leaves. A, Leaf damaged for 6 h by MecWorm during the first and second dark phases. See Figure 3D for details. Leaf segments were harvested 32 h after initiation of the first MecWorm damage. Sections 2 and 3 correspond to the first and the second nocturnal damage. Undamaged sections 1 and 4 served as control. Data represent the mean ± se (n = 3–4). Scale bar = 10 mm. Means followed with small letters are significantly different according to ANOVA followed by Scheffe's test (P < 0.05). B and C, Wound-induced expression of PlOS promoter∷GUS reporter gene constructs in leaves of 6-week-old Arabidopsis plants. Wounding by a needle (B) or MecWorm treatment (C) induced GUS activity after 2 h in areas in close proximity to wounding sites. Histochemical GUS assays were performed with two independent lines. Scale bar = 2 mm.
DISCUSSION
Analyzing the volatiles from lima bean plants damaged by MecWorm for 6 h during the day or night over a period of 3 d, we observed a clear difference between the emission of the terpenoid β-ocimene and the emission of the fatty acid-derived volatile Hex-Ac. However, emission patterns for both compounds were clearly periodic (Fig. 7). The total amount of volatiles increased during the day (independent of whether damage occurred in the light or dark), whereas the amount dropped at night. This pattern corresponded to the results of Loughrin et al. (1994) and Turlings et al. (1995), who studied corn (Zea mays) and cotton (Gossypium hirsutum) plants infested by caterpillars. However, our study has revealed additional details due to two major technical improvements. First, the completely reproducible mechanical damage inflicted by MecWorm when applied during the day or night to the same plant species allowed us to compare even subtle differences in the biosynthesis of HIPVs, JA, and the expression level of PlOS. Second, the high time resolution of zNose (Kunert et al., 2002) allowed close correlation to be made between the emission profiles of individual volatiles and their gene expression, as well as their levels of JA.
Figure 7.
Schematic representation of the signaling and metabolic pathways required for herbivore-induced β-ocimene and Hex-Ac emissions in lima bean leaves in a daily cycle. LOX, Lipoxygenase.
In lima bean, herbivore-induced emission of β-ocimene was found to be exclusively linked to light and not to the circadian clock. Rapidly diminishing levels of β-ocimene after an AD period was introduced into the photophase strikingly demonstrated this link. The light-dependent β-ocimene emission was hampered by the level of the early terpenoid precursors delivered to the MEP pathway by the photosynthetic fixation of CO2. Because the monoterpene β-ocimene is synthesized in the lima bean via the MEP pathway (Piel et al., 1998; Bartram et al., 2006), the very high degree of 13C-labeling of the monoterpene (70% within the first 30 min and 85% in the second 30 min after the beginning of the light phase) synthesized de novo from 13CO2 after the onset of the light period strongly supported this view. Thus, the metabolic flux from photosynthesis to terpenoid biosynthesis is a key regulatory element of the light-dependent emission of β-ocimene. This view is complemented by the observation that, after the first instance of damage, the high transcript level of nocturnally induced PlOS followed the high nocturnal level of JA. Also, all other enzymes along the MEP pathway have to be produced during the dark phase. The metabolic flow-through system begins to operate as soon as the very first photosynthates are delivered, and β-ocimene can be emitted within minutes after the onset of the light period. On the other hand, light seems also to be essential to maintain high levels of PlOS expression because an additionally introduced dark phase rapidly reduced PlOS expression levels in herbivore-damaged leaves within 4 h after the dark period began (Fig. 2B). Similar observations were made for the isoprene synthase, the 1-deoxy-d-xylulose 5-P reductoisomerase from gray poplar (Populus x canescens; Loivamäki et al., 2007), and the germin-like protein from mustard (Sinapsis alba; Heintzen et al., 1994). In both plants, the corresponding mRNAs were no longer detected in the dark even when expression was under the control of the endogenous clock.
In addition to increasing levels of JA and the presence/absence of light, other factors, such as the impact of an endogenous clock, seem to contribute to regulating PlOS. Screening the database PLACE (http://www.dna.affrc.go.jp/PLACE/signalscan.html) for cis-acting regulatory DNA elements revealed the presence of a circadian element (CAANNNNATC; Piechulla et al., 1998) in the PlOS promoter. Moreover, a T/G-box AACTGG motif is present in the promoter fragment, which is known to be a JA-responsive MYC2 transcription factor (Boter et al., 2004). Altogether, these findings suggest that PlOS is regulated by multiple elements, including JA and the clock. Such a complex regulatory mode easily explains the diurnal regulation of PlOS expression in lima bean leaves in response to feeding S. littoralis (Fig. 2A). In herbivore-damaged lima bean leaves, the level of endogenous JA stays consistently high and shows no diurnal pattern (Supplemental Fig. S1). However, rapidly fluctuating JA levels, which are typical for the onset of herbivory or during the application of MecWorm, seem to override other factors (Fig. 3, C and D).
Another major difference between nocturnal and daytime damage was observed in the levels of JA. JA is the major plant hormone involved in the up-regulation of volatiles, in particular, terpenoid biosynthesis (Arimura et al., 2005). Unexpectedly, levels of JA were 2 to 3 times higher after nocturnal damage than they were after daytime damage. As shown in Figure 3C, during the day JA production stopped immediately once the damage period ended, whereas JA production at night increased levels for 2 h after MecWorm treatment ended. Because both damage regimes destroyed the same amount of leaf tissue, which in turn should have generated the same amount of JA, we postulate that certain JA-modifying steps exist that occur only during the light phase. Candidates that might be responsible for effecting different regulatory processes according to the different light regimes include (1) inactivation of JA by hydroxylation, (2) transformation into a sulfate ester or glucoside, and (3) conjugation of the phytohormone to amino acids reviewed by Liechti and Farmer (2006). Moreover, the higher diurnal emission of Hex-Ac (Fig. 3, A and B), the compound that competes with JA for the common precursor α-linolenic acid, may also limit JA production during the light phase. Only comprehensive profiling of the different JA derivatives and all other products derived from α-linolenic acid will clarify which compound is responsible.
Production of JA and the emission of the fatty acid-derived volatile Hex-Ac correlated closely with MecWorm damage, regardless of whether damage occurred under light or dark conditions (Fig. 3, A and B). Besides the low nocturnal emission of Hex-Ac (Fig. 3B), a second emission peak occurred during the day. This biphasic emission of Hex-Ac corresponded to previous observations in Arabidopsis. Enhanced transcript levels of CHAT (At3g03480) coincided with the highest levels of Hex-Ac, which were emitted several hours after wounding. However, the first emission of Hex-Ac was observed just 5 min after wounding (Loreto et al., 2006; D'Auria et al., 2007). The release of (Z)-3-hexenal, another initially formed green-leaf volatile, occurred within 20 s after the damage of Arabidopsis leaf tissue (Matsui et al., 2000). The immediate release of green-leaf volatiles is due to the presence of a functional array of enzymes (phospholipase, lipoxygenase, and fatty acid hydroperoxide lyase) and their contact with α-linolenic acid or the corresponding 13-hydroperoxide in the damaged tissue (Matsui et al., 2000). Thus, biphasic emission of Hex-Ac from damaged lima bean leaves has two elements, namely, the immediate wound response based on constitutively expressed enzymes and induced biosynthesis, which is mediated by enhanced levels of JA (Matsui, 2006; D'Auria et al., 2007).
Both Hex-Ac and β-ocimene, volatiles focused on in this research, are known to attract carnivorous arthropods and thus function as indirect plant defenses by increasing predation pressure on attacking herbivores (Dicke et al., 1990) and by plant-plant interactions (Arimura et al., 2000, 2001; Köllner et al., 2004). Particularly intriguing is the recent observation by Heil and Silva Bueno (2007) that the induced volatiles serve as an information highway, speeding communication between remote parts of the attacked plant or bridging vascular unconnected leaves (Orians, 2005). The immediate damage-linked release of Hex-Ac, which has been shown to induce nectar flow in neighboring areas of the same and other plants, is very important. Any damage by an herbivore allows endangered plant areas to transport information on the herbivore attack without the need for delayed induction. Also, the early-morning terpenoid emission burst following nocturnal leaf damage may have an important biological function, namely, to massively attract the natural enemies of herbivores that are present during the morning hours (Kaas et al., 1993; Hirose et al., 2003). Current findings will have to be complemented by creative field experiments and comparative approaches to better understand emerging areas, such as the macroecology or community ecology, of diurnal plant-insect interactions in the ecosystem.
MATERIALS AND METHODS
Plants and Caterpillars
Lima bean (Phaseolus lunatus ‘Ferry Morse’ var. Jackson Wonder Bush) was grown in soil. Individual plants were grown in plastic pots in a growth chamber at 23°C (160 μE m−2 s−1 during a 14-h photoperiod; relative humidity 60%) for 2 weeks. Larvae of Spodoptera littoralis Boisd. (Lepidoptera, Noctuidae) were reared on an artificial diet in a plastic box (25°C ± 1°C; 14:10 LD; Bergomaz and Boppré, 1986).
Plant Treatments
Primary leaves from intact lima bean plants were used for all treatments and analyses. For S. littoralis infestation, three second- and third-instar larvae were placed on a leaf. Manual wounding was performed by punching 18 6-mm-diameter holes into a leaf for a total of 508 mm2 of damaged area. MecWorm operation was programmed to generate within 6 h circles of damaged leaf area in the primary leaf, yielding 265 mm2 of total damaged area (two circle sectors; φ = 13.0 mm) using 12 punches min−1. For chemical treatment, JA or salicylic acid (0.5 mm, pH 5.8–6.0) in 2 mL of water was sprayed onto intact plants in plastic pots. Alamethicin (1 mm) was applied to the petioles of detached plantlets in aqueous solutions. All treatments, except MecWorm damage in the darkness, started at 9 am. During treatments, temperature was maintained at 25°C ± 1°C with a photoperiod of 14 h (160 μE m−2 s−1). The light period ran from 7 am to 9 pm.
Labeling Experiments with 13CO2
Leaves were damaged by MecWorm during the first and second dark phases (11 pm–5 am) and covered by a Plexiglas cabinet (approximately 500 mL) with air constantly passing through the system at 50 mL min−1. For pulse labeling, synthetic air with 380 μg mL−113CO2, 20.9% O2, and 79% N2 (Westfalen A.G.) was introduced into the cabinet with a starting flow of 200 mL min−1 for 10 min (6 am–6:10 am) to replace the natural 12CO2 followed by a reduced flow at 50 mL min−1. The emitted volatiles were collected by solid-phase microextraction on a polydimethylsiloxane 100 fiber (Supelco). Samples were analyzed on an Agilent 6580 gas chromatograph coupled to a Micromass GCT time-of-flight mass spectrometer (Micromass UK Ltd.). Separation was performed on a Zebron ZB-5ms capillary column (30 m × 0.25 mm i.d. × 0.25 μm; Phenomenex). Helium at a flow rate of 1.0 mL min−1 served as carrier gas and a split-mode injection (1:10) was employed. The gas chromatograph injector, transfer line, and ion source were set at 220°C, 280°C, and 280°C, respectively. Spectra were taken in the total ion-scanning mode at 70 eV. Compounds were eluted under programmed conditions starting at 40°C (2-min isotherm) followed by heating at 15°C min−1 to 200°C, then at 40°C min−1 to 280°C. The injected amount was 1 μL. The intense fragment ions at m/z 93 (control plants) and at m/z 94 to 100 (13CO2-fed plants) were used to analyze and calculate the incorporation rate of 13C. The fragment ions at m/z 94 to 100 correspond to the incorporation of one to seven 13C-atoms into β-ocimene (see Fig. 4).
zNose
A primary leaf attached to an intact lima bean plant was enclosed in a Plexiglas cabinet (552 mL) where it was subjected to insect and MecWorm damage (Mithöfer et al., 2005). zNose (model 4100; Electronic Sensor Technology) was connected to the cabinet via a stainless steel needle attached to the Luer inlet on the instrument. The detector (surface acoustic wave detector) of zNose was operated at 30°C (Kunert et al., 2002). Prepurified air (ZeroAir generator [Parker Hannifin Corp.] and an additional charcoal filter) was passed through the plastic cabinet at 40 mL min−1. Every 10 min, zNose trapped an aliquot of the headspace volatiles for 60 s on an internal Tenax precolumn at 30 mL min−1 followed by separation on the main column under programmed conditions from 40°C to 175°C at 5°C s−1. A DB5 column (1-m × 0.25-mm × 0.25-μm film; Electronic Sensor Technology) was used. β-Ocimene and Hex-Ac were quantified after calibration with authentic standards and are presented as ng min−1 g−1 fresh weight.
Analysis of JA
Plant material was weighed (0.2–0.25 g) and shock frozen with liquid nitrogen. For analyses of spatial patterns of JA, we mixed two samples corresponding to each leaf section to obtain 0.2 g of leaf sections. JA was quantified in plant material according to a modified protocol from Schulze et al. (2006). Frozen plant material was mixed with methanol/2,6-di-tert-butyl-4-methylphenol (BHT; Sigma-Aldrich) solution (2.5 mL, 0.05% BHT), followed by the addition of the derivatization agent pentafluorobenzylhydroxylamine hydrochloride, 2 mL, 0.05 m in methanol (Sigma-Aldrich). For quantification, 9,10-[2H2]-dihydrojasmonic acid (250 ng) was added as an internal standard. Next, the mixture, cooled on ice and kept under argon atmosphere, was homogenized for 5 min with a high-performance dispenser at 24,000 rpm (Ultra-Turrax T-25; IKA-Werk). Derivatization was completed by shaking the samples for 2 h at room temperature. After acidification with 0.1 m HCl (approximate pH 3), the methanol/water phase was quantitatively extracted with hexane (3 × 5 mL). The combined organic layers were subsequently passed through preconditioned (methanol, 5 mL; hexane, 5 mL) Chromabond NH2 cartridges (3 mL/0.5 g; Macherey-Nagel). Cartridges were washed with i-propanol:dichloromethane (5 mL, 2:1 [v/v]) and eluted with diethyl ether:formic acid (10 mL, 98:2 [v/v]). The solvent was then removed under a gentle stream of argon and the sample was esterified with an etheral solution of diazomethane for 5 min. After removal of excess diazomethane, the sample was taken up in 45 μL of dichloromethane. Samples were analyzed on a Finnigan GCQ Instrument (Thermoelectron) running in a CI-negative ion mode, as described by Schulze et al. (2006).
cDNA Cloning and Terpene Synthase Assay
A whole leaf was crushed in liquid N2 and the tissue (100 mg) was used for RNA extraction. First-strand cDNA was synthesized using the SuperScript II (Invitrogen) oligo(dT)12-18 primer and 1 μg of total RNA at 50°C for 50 min. For PCR, primers for the PlOS cDNA fragment were designed using a LjEβOS cDNA sequence (GenBank accession no. AY575970). PCR was run for 2 min at 95°C, 35 cycles of 15 s at 94°C, 30 s at 50°C, and 60 s at 72°C. Further cloning of 5′- and 3′-ends was accomplished by RACE-PCR using the first-choice RLM-RACE kit (Ambion) according to the manufacturer's protocol. For functional identification of the full-length genes, the region of an open reading frame of PlOS was subcloned into pHis-8-3 expression vectors (Jez et al., 2000). Terpene synthase enzyme assays were performed according to Arimura et al. (2008).
Real-Time PCR
First-strand cDNA was synthesized from isolated total RNA (see above) using SuperScript II reverse transcriptase, oligo(dT)12-18 primer, and 1 μg of total RNA at 42°C for 50 min. Real-time PCR was done on a Mx3000P real-time PCR system (Stratagene). The process was performed with 25 μL of reaction mixture containing 12.5 μL of 2× Brilliant SYBR Green QPCR master mix (Stratagene), cDNA (1 μL from 20 μL of each reverse transcriptase product pool), 100 nm primers, and 30 mm ROX as a passive reference dye. Initial polymerase activation: 10 min at 95°C; 40 cycles of 30 s at 95°C, 60 s at 55°C, and 30 s at 72°C. PCR conditions were determined by comparing threshold values in a dilution series of the RT product, followed by non-RT template control and nontemplate control for each primer pair. Relative RNA levels were calibrated and normalized with the level of PlACT1 mRNA (GenBank accession no. DQ159907). Primers are shown in Supplemental Table S1.
Cloning the PlOS Promoter and PlOS Promoter∷GUS Fusion Construct
Genomic DNA was extracted from lima bean leaves using the Qiagen plant DNA extraction kit. Genomic DNA was digested with EcoRV and then circularized with T4 DNA ligase (New England Biolabs). Nested, inverse PCR was performed with circularized genomic DNA as the template, using Taq DNA polymerase (New England Biolabs). PCR protocol: 40 cycles of 15 s at 95°C, 30 s at 55°C, and 60 s at 72°C. The PCR product was ligated to a TOPO TA cloning kit (Invitrogen). Primers are shown in Supplemental Table S1. The 517-bp PlOS promoter region upstream of the start codon (GenBank accession no. EU194554) was reamplified by PCR using Pfu DNA polymerase with a pair of primers introducing restriction sites. The amplified promoter fragments were digested with BamHI and EcoRI, and digested fragments were ligated to the corresponding site of pCAMBIA1391 upstream of the GUS gene (Cambia). Constructs were transformed into the Agrobacterium GV3101 strain, which was then used to transform Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 according to the floral-dip method (Clough and Bent, 1998). Arabidopsis T1 or T2 lines were used for histochemical analysis according to the method described by Jefferson et al. (1987).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers EU194553 and EU194554.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Levels of JA in leaves after feeding by S. littoralis or manual wounding in a LD cycle.
Supplemental Table S1. Primers used for this study.
Supplementary Material
Acknowledgments
We thank Dr. A. Mithöfer and Dr. S. Bartram for valuable discussions and H. Uchtenhagen and S. Garms for technical assistance.
This work was supported in part by the Center of Excellence for Plant and Microbial Biosensing of the University of Turin.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Wilhelm Boland (boland@ice.mpg.de).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Arimura G, Garms S, Maffei M, Bossi S, Schulze B, Leitner M, Mithöfer A, Boland W (2008) Herbivore-induced terpenoid emission in Medicago truncatula: concerted action of jasmonate, ethylene and calcium signaling. Planta 227 453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura G, Huber DPW, Bohlmann J (2004. a) Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa x deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (-)-germacrene D synthase, PtdTPS1. Plant J 37 603–616 [DOI] [PubMed] [Google Scholar]
- Arimura G, Kost C, Boland W (2005) Herbivore-induced, indirect plant defences. Biochim Biophys Acta 1734 91–111 [DOI] [PubMed] [Google Scholar]
- Arimura G, Ozawa R, Horiuchi J, Nishioka T, Takabayashi J (2001) Plant-plant interactions mediated by volatiles emitted from plants infested by spider mites. Biochem Syst Ecol 29 1049–1061 [Google Scholar]
- Arimura G, Ozawa R, Kugimiya S, Takabayashi J, Bohlmann J (2004. b) Herbivore-induced defense response in a model legume. Two-spotted spider mites induce emission of (E)-β-ocimene and transcript accumulation of (E)-β-ocimene synthase in Lotus japonicus. Plant Physiol 135 1976–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J (2000) Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406 512–515 [DOI] [PubMed] [Google Scholar]
- Arimura G, Volpe V, Kunert M, Maffei M, Boland W (2007) Terpenoid biosynthesis and signaling in legume plants in response to herbivorous damage. In A Ramina, C Chang, J Giovannoni, H Klee, P Perata, E Woltering, eds, Advances in Plant Ethylene Research. Springer, Dordrecht, The Netherlands, pp 353–358
- Bartram S, Jux A, Gleixner G, Boland W (2006) Dynamic pathway allocation in early terpenoid biosynthesis of stress-induced lima bean leaves. Phytochemistry 67 1661–1672 [DOI] [PubMed] [Google Scholar]
- Bergomaz R, Boppré M (1986) A simple instant diet for rearing Arctiidae and other moths. J Lepid Soc 40 131–137 [Google Scholar]
- Boter M, Ruíz-Rivero O, Abdeen A, Prat S (2004) Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes Dev 18 1577–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- D'Auria JC, Pichersky E, Schaub A, Hansel A, Gershenzon J (2007) Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J 49 194–207 [DOI] [PubMed] [Google Scholar]
- De Moraes CM, Mescher MC, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410 577–580 [DOI] [PubMed] [Google Scholar]
- Dicke M, Gols R, Ludeking D, Posthumus MA (1999) Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. J Chem Ecol 25 1907–1922 [Google Scholar]
- Dicke M, van Beek TA, Posthumus MA, Ben Dom N, van Bokhoven H, de Groot AE (1990) Isolation and identification of volatile kairomone that affects acarine predator-prey interactions. J Chem Ecol 16 381–396 [DOI] [PubMed] [Google Scholar]
- Dudareva N, Andersson S, Orlova I, Gatto N, Reichelt M, Rhodes D, Boland W, Gershenzon J (2005) The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc Natl Acad Sci USA 102 933–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Martin D, Kish CM, Kolosova N, Gorenstein N, Fäldt J, Miller B, Bohlmann J (2003) (E)-β-Ocimene and myrcene synthase genes of floral scent biosynthesis in snapdragon: function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell 15 1227–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberth J, Koch T, Schüler G, Bachmann N, Rechtenbach J, Boland W (2001) Ion channel-forming alamethicin is a potent elicitor of volatile biosynthesis and tendril coiling. Cross talk between jasmonate and salicylate signaling in lima bean. Plant Physiol 125 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag MA, Paré PW (2002) C6-Green leaf volatiles trigger local and systemic VOC emissions in tomato. Phytochemistry 61 545–554 [DOI] [PubMed] [Google Scholar]
- Heil M, Silva Bueno JC (2007) Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA 104 5467–5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzen C, Fischer R, Melzer S, Kappeler K, Apel K, Staiger D (1994) Circadian oscillations of a transcript encoding a germin-like protein that is associated with cell walls in young leaves of the long-day plant Sinapis alba L. Plant Physiol 106 905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose Y, Ehler LE, Hirose Y (2003) Influence of host age on patch use by a quasi-gregarious egg parasitoid. Popul Ecol 32 789–796 [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jez JM, Ferrer JL, Bowman ME, Dixon RA, Noel JP (2000) Dissection of malonyl-coenzyme A decarboxylation from polyketide formation in the reaction mechanism of a plant polyketide synthase. Biochemistry 39 890–902 [DOI] [PubMed] [Google Scholar]
- Kaas JP, Ramaswamy SB, Elzen GW (1993) Behavioral time budget and periodicity exhibited by Microplitis croceipes in field cages with Heliothis virescens on spring host plants. Entomophaga 38 143–154 [Google Scholar]
- Köllner TG, Schnee C, Gershenzon J, Degenhardt J (2004) The variability of sesquiterpenes emitted from two Zea mays cultivars is controlled by allelic variation of two terpene synthase genes encoding stereoselective multiple product enzymes. Plant Cell 16 1115–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunert M, Biedermann A, Koch T, Boland W (2002) Ultrafast sampling and analysis of plant volatiles by a hand-held miniaturised GC with pre-concentration unit: kinetic and quantitative aspects of plant volatile production. J Sep Sci 25 677–684 [Google Scholar]
- Liechti R, Farmer EE (2006) Jasmonate biochemical pathway. Sci STKE 2006 cm3. [DOI] [PubMed] [Google Scholar]
- Loivamäki M, Louis S, Cinege G, Zimmer I, Fischbach RJ, Schnitzler JP (2007) Circadian rhythms of isoprene biosynthesis in grey poplar leaves. Plant Physiol 143 540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Barta C, Brilli F, Nogues I (2006) On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant Cell Environ 29 1820–1828 [DOI] [PubMed] [Google Scholar]
- Loughrin JH, Manukian A, Heath RR, Turlings TC, Tumlinson JH (1994) Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plant. Proc Natl Acad Sci USA 91 11836–11840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K (2006) Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol 9 274–280 [DOI] [PubMed] [Google Scholar]
- Matsui K, Kurishita S, Hisamitsu A, Kajiwara T (2000) A lipid-hydrolysing activity involved in hexenal formation. Biochem Soc Trans 28 857–860 [PubMed] [Google Scholar]
- Mithöfer A, Wanner G, Boland W (2005) Effects of feeding Spodoptera littoralis on lima bean leaves. II. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiol 137 1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orians C (2005) Herbivores, vascular pathways, and systemic induction: facts and artifacts. J Chem Ecol 31 2231–2242 [DOI] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH (1997) De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol 114 1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121 325–332 [PMC free article] [PubMed] [Google Scholar]
- Piechulla B, Merforth N, Rudolph B (1998) Identification of tomato Lhc promoter regions necessary for circadian expression. Plant Mol Biol 38 655–662 [DOI] [PubMed] [Google Scholar]
- Piel J, Donath J, Bandemer K, Boland W (1998) Mevalonate-independent biosynthesis of terpenoid volatiles in plants: induced and constitutive emission of volatiles. Angew Chem Int Ed 37 2478–2481 [DOI] [PubMed] [Google Scholar]
- Ro DK, Ehlting J, Keeling CI, Lin R, Mattheus N, Bohlmann J (2006) Microarray expression profiling and functional characterization of AtTPS genes: duplicated Arabidopsis thaliana sesquiterpene synthase genes At4g13280 and At4g13300 encode root-specific and wound-inducible (Z)-gamma-bisabolene synthases. Arch Biochem Biophys 448 104–116 [DOI] [PubMed] [Google Scholar]
- Roeder S, Hartmann AM, Effmert U, Piechulla B (2007) Regulation of simultaneous synthesis of floral scent terpenoids by the 1,8-cineole synthase of Nicotiana suaveolens. Plant Mol Biol 65 107–124 [DOI] [PubMed] [Google Scholar]
- Schulze B, Lauchli R, Sonwa MM, Schmidt A, Boland W (2006) Profiling of structurally labile oxylipins in plants by in situ derivatization with pentafluorobenzyl hydroxylamine. Anal Biochem 348 269–283 [DOI] [PubMed] [Google Scholar]
- Shiojiri K, Ozawa R, Takabayashi J (2006) Plant volatiles, rather than light, determine the nocturnal behavior of a caterpillar. PLoS Biol 4 e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlings TC, Loughrin JH, McCall PJ, Rose US, Lewis WJ, Tumlinson J H (1995) How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci USA 92 4169–4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.