Abstract
Considerable research has examined plant responses to concurrent attack by herbivores and pathogens, but the effects of attack by parasitic plants, another important class of plant-feeding organisms, on plant defenses against other enemies has not been explored. We investigated how attack by the parasitic plant Cuscuta pentagona impacted tomato (Solanum lycopersicum) defenses against the chewing insect beet armyworm (Spodoptera exigua; BAW). In response to insect feeding, C. pentagona-infested (parasitized) tomato plants produced only one-third of the antiherbivore phytohormone jasmonic acid (JA) produced by unparasitized plants. Similarly, parasitized tomato, in contrast to unparasitized plants, failed to emit herbivore-induced volatiles after 3 d of BAW feeding. Although parasitism impaired antiherbivore defenses, BAW growth was slower on parasitized tomato leaves. Vines of C. pentagona did not translocate JA from BAW-infested plants: amounts of JA in parasite vines grown on caterpillar-fed and control plants were similar. Parasitized plants generally contained more salicylic acid (SA), which can inhibit JA in some systems. Parasitized mutant (NahG) tomato plants deficient in SA produced more JA in response to insect feeding than parasitized wild-type plants, further suggesting cross talk between the SA and JA defense signaling pathways. However, JA induction by BAW was still reduced in parasitized compared to unparasitized NahG, implying that other factors must be involved. We found that parasitized plants were capable of producing induced volatiles when experimentally treated with JA, indicating that resource depletion by the parasite does not fully explain the observed attenuation of volatile response to herbivore feeding. Collectively, these findings show that parasitic plants can have important consequences for host plant defense against herbivores.
Plants have evolved the ability to perceive attack and respond by activating induced defenses (Karban and Baldwin, 1997; Dangl and Jones, 2001). The defensive strategy utilized is dependent on the attacker and can be highly specific. For example, plants can distinguish feeding by closely related herbivore species and tailor induced volatiles to attract specialist parasitoids (De Moraes et al., 1998). The induced physiological changes of plants in response to herbivores and pathogens are well studied and result from complex defense signaling networks regulated by the plant hormones jasmonic acid (JA) and salicylic acid (SA). In general, the JA pathway is activated in response to herbivores and regulates production of compounds that impair digestion (Chen et al., 2005, 2007) and of induced plant volatiles that attract natural enemies (Turlings et al., 1990) and repel ovipositing moths (De Moraes et al., 2001). The SA pathway is typically activated in response to pathogens and mediates a hypersensitive response and the production of an array of antimicrobial phytoalexins and pathogenesis-related proteins that results in systemic acquired resistance to a broad spectrum of pathogens (Durrant and Dong, 2004). However, the categorization of JA as an herbivore defense signal and SA as a pathogen defense signal is imperfect, as JA-mediated defenses are induced by some pathogens and SA-mediated defenses by some herbivores (Moran and Thompson, 2001; Glazebrook, 2005).
The defenses that plants deploy against one enemy may or may not be effective against other enemies (Stout et al., 2006). Moreover, the JA and SA signaling pathways can negatively interact, so that resistance to one pest may increase the vulnerability to another. For example, SA-mediated responses to pathogens have been found to negatively affect subsequent JA-mediated defenses against herbivores, resulting in increased performance of insects that feed on infected plants (Felton et al., 1999; Preston et al., 1999; Thaler et al., 1999, 2002; Stout et al., 2006). Although it is well established that SA can inhibit production of JA and the expression of JA-induced defenses (Peña-Cortés et al., 1993; Doares et al., 1995; Thaler et al., 1999; Cipollini et al., 2004), predicting positive or negative effects on subsequent enemies has proved difficult because a strict dichotomy between the defense pathways for pathogen and insect attack does not always exist and the range of organisms affected by each pathway varies (Felton and Korth, 2000; Thaler et al., 2002, 2004; Cardoza et al., 2003; Stout et al., 2006). Defense signaling cross talk may allow plants to minimize costly, ineffective defenses and fine-tune responses to specific enemies (Reymond and Farmer, 1998; Kunkel and Brooks, 2002), but the mechanisms underlying JA/SA cross talk are not understood.
To date, research on induced plant defenses and defense signaling cross talk has focused almost exclusively on herbivorous arthropods and pathogens, but plants also must defend themselves from attack by other plants. Approximately 4,500 species of flowering plants (about 1%) are parasitic (Nickrent, 2007) and attach to other plants to obtain water and nutrients (Kuijt, 1969). Parasitic plants can severely impact host growth and reproduction (Wolswinkel, 1974; Press and Graves, 1995) and have significant effects on the structure and productivity of ecosystems in which they occur (Press and Phoenix, 2005; Bardgett et al., 2006). Parasitic plants also account for some of the world's most destructive agricultural pests (Parker and Riches, 1993; Musselman et al., 2001). Dodders, genus Cuscuta (Convolvulaceae), are one of the most ecologically and economically significant groups of parasitic plants (Kuijt, 1969). Cuscuta spp. have yellow-to-orange vines that lack obvious chlorophyll, roots, and expanded leaves, and thus are completely dependent on aboveground attachment to other plants for survival and reproduction (Dawson et al., 1994). We recently demonstrated that Cuscuta pentagona seedlings use plant volatiles to locate and choose among hosts (Runyon et al., 2006). Once a host is located, C. pentagona vines twine around the host stem and produce haustoria, specialized organs that grow into the host to extract nutrients from both xylem and phloem (Dawson et al., 1994). Cuscuta spp. cause extensive damage each year to numerous agricultural crops (e.g. tomato [Solanum lycopersicum], alfalfa [Medicago sativa], potato [Solanum tuberosum], soybean [Glycine max], onion [Allium cepa], and cranberry [Vaccinum macrocarpon]) and, because of their close physiological connection to hosts, are difficult to control without also impacting the crop plants (Nadler-Hassar and Rubin, 2003). Despite their economic importance and the profound effects they have on host plants and community dynamics, relatively little is known about the defenses induced by parasitic plant attack or how these defenses affect host plant interactions with other organisms.
Trade-offs in plant defenses against different attackers are likely central to the ecology and evolution of induced defenses. Moreover, understanding such tradeoffs is key to avoiding unwanted side effects if these pathways are to be manipulated to control pests in agriculture. In this study, we examined how parasitism by C. pentagona affects tomato plants' induced defenses against a chewing insect, the beet armyworm (Spodoptera exigua; BAW), by comparing production of JA and plant volatiles from parasitized and unparasitized tomato plants. We also determined the growth rate of BAW caterpillars on parasitized and unparasitized plants. Finally, we investigated several mechanisms that might explain the observed impact of C. pentagona parasitism on tomato herbivore defenses, including the removal of JA by Cuscuta, negative cross talk between the JA and SA pathways, and the availability of resources needed for induced defenses.
RESULTS
Production of JA and SA by Parasitized and Unparasitized Tomato Plants
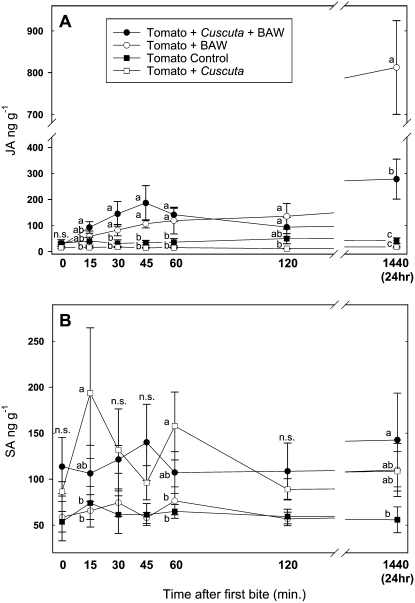
To investigate how C. pentagona infestation affected herbivore-induced defenses of the tomato host, we first constructed a time-course tracking concentrations of JA and SA during the first 24 h of BAW feeding (Figs. 1 and 2). Amounts of JA began to increase as soon as 15 min after insect feeding began, and the highest JA concentrations occurred after BAW had fed for 24 h (Fig. 2A). The production of JA by parasitized and unparasitized plants was not statistically different during the first 2 h of insect feeding, but after 24 h of feeding Cuscuta-infested tomato plants contained only about 30% of the JA found in unparasitized plants (mean ± se ng/g JA: 278 ± 77 parasitized, 812 ± 112 unparasitized; Fig. 2A). Parasitized and unparasitized control plants, which received no insect feeding, did not differ in JA content (Fig. 2A). C. pentagona-infested plants generally contained greater amounts of SA than unparasitized plants (Fig. 2B), but this difference was not consistently significant due to the large variability in SA content in parasitized plants (Fig. 2B).
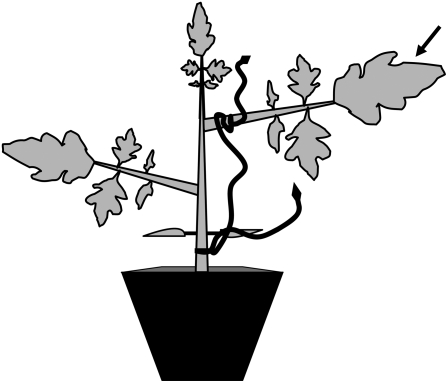
Figure 1.
Schematic showing a 25-d-old tomato plant with attached C. pentagona as used in this study. Tomato plants were first parasitized by C. pentagona seedlings when 10 d old (attachment point below cotyledons). The parasite vine was allowed to grow for 10 d and to attach again to the petiole of the second expanded leaf of the now-20-d-old tomato. Five days later, the leaf of the parasitized petiole (indicated with arrow) of the 25-d-old plant received caterpillar feeding for volatile collection or phytohormone analysis.
Figure 2.
Time course of changes in JA (A) and SA (B) in unparasitized tomato plants and plants parasitized by C. pentagona in response to BAW feeding. Parasitized and unparasitized plants that did not receive insect feeding served as controls. Note breaks in the x axis (A and B) and the y axis (A). Data show the mean and se of untransformed values from six replicates. Different letters indicate significance differences within each time point (P < 0.05); n.s., no significance between treatments.
Production of Herbivore-Induced Volatiles by Parasitized and Unparasitized Tomato Plants
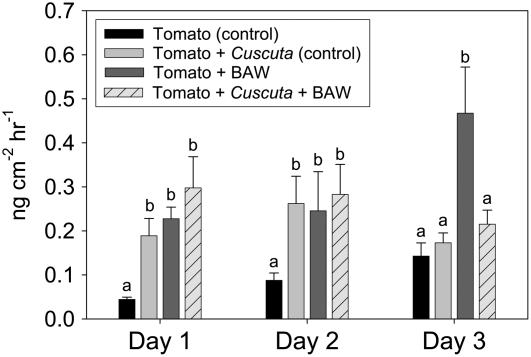
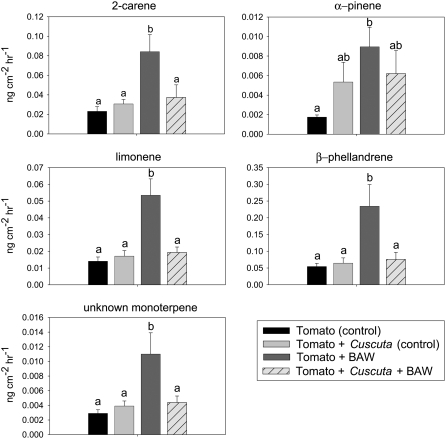
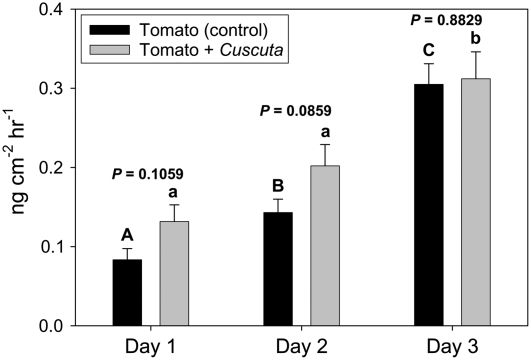
We next examined the impact of parasitism on host-plant volatile production induced by BAW feeding. Undamaged tomato plants released 13 volatile compounds, which included the monoterpenes α-pinene, β-pinene, β-myrcene, 2-carene, p-cymene, β-phellandrene, limonene, (E)-β-ocimene, linalool, and two others that are unidentified; the sesquiterpene β-caryophyllene; and the homoterpene (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene. Aside from small, inconsistent amounts of several six-carbon green leaf volatiles [(Z)-3-hexenal, (Z)-3-hexenol, and (Z)-3-hexenyl acetate], plants that received BAW feeding produced these same 13 volatile compounds, but in greater amounts. Total volatile production induced by BAW feeding did not differ between parasitized and unparasitized plants during the first 2 d; however, unparasitized plants released significantly more total volatiles than parasitized plants on day 3 of feeding (Fig. 3). Moreover, BAW feeding induced a significant increase in total volatiles produced by unparasitized plants (P = 0.0371), while total volatile production by parasitized plants damaged by BAW did not differ among the 3 d (P = 0.6590). At no time did C. pentagona-parasitized plants fed on by BAW produce more volatiles than parasitized plants without BAW (Fig. 3). Among individual volatile compounds, 3 d of BAW feeding on unparasitized plants induced significant increases in α-pinene, 2-carene, β-phellandrene, limonene, and one unidentified monoterpene (Fig. 4). None of these volatile compounds was induced by caterpillar feeding on parasitized plants (Fig. 4). Interestingly, Cuscuta-infested control plants released greater total volatiles (encompassing the same individual volatiles induced by BAW) during the first 2 d of the experiment than unparasitized control plants (Fig. 3).
Figure 3.
Total volatile production (mean ± se) by unparasitized tomato plants and plants parasitized by C. pentagona on days 1 to 3 of BAW feeding. Parasitized and unparasitized plants that did not receive insect feeding served as controls. Data show untransformed values from six replicates. Different letters indicate significance differences within each day (P < 0.05).
Figure 4.
Amounts (mean ± se) of α-pinene, 2-carene, β-phellandrene, limonene, and one unidentified monoterpene produced by unparasitized tomato plants and plants parasitized by C. pentagona on day 3 of BAW feeding. Parasitized and unparasitized plants that did not receive insect feeding served as controls. These five volatile compounds are induced by BAW feeding on unparasitized plants. Data show untransformed values from six replicates. Different letters indicate significance differences between treatments (P < 0.05).
BAW Feeding and Growth on Parasitized and Unparasitized Tomato
Because JA content and volatile production can be positively correlated with amounts of damage (Ohnmeiss et al., 1997; Gouinguené et al., 2003; Dean and De Moraes, 2006; Tooker and De Moraes, 2007), we compared the leaf area consumed by BAW on parasitized and unparasitized tomato plants over a 24-h period. Although BAW tended to remove more leaf area from unparasitized than parasitized plants (44 ± 8 and 33 ± 7 cm2 24 h−1, respectively), this difference was not significant (t test, P = 0.304), nor did the proportion of total leaf area eaten differ (0.041 ± 0.005 unparasitized, 0.048 ± 0.009 parasitized; t test, P = 0.456). There were no noticeable differences in the feeding pattern of BAW on leaves of parasitized and unparasitized plants.
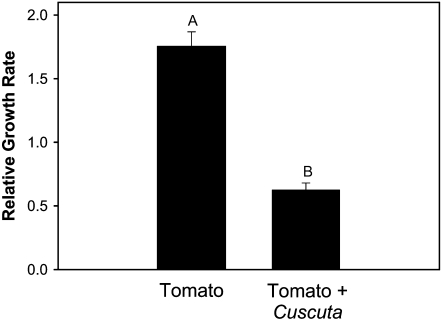
BAW caterpillars feeding on leaves of parasitized tomato plants grew much slower than those feeding on unparasitized tomato leaves (Fig. 5; mean ± se relative growth rate: 0.63 ± 0.05 and 1.76 ± 0.11, respectively; t test, P < 0.0001).
Figure 5.
Relative growth rate of BAW on unparasitized tomato and tomato parasitized by C. pentagona. Data show means and se of untransformed values from 15 replicates. Different letters indicate significance differences between treatments (P < 0.05).
Translocation of Herbivore-Induced JA by Cuscuta
The haustoria of Cuscuta form vascular connections with the host, creating a powerful sink that transports sugars, amino acids, and other nutrients from host to parasite (Dawson et al., 1994; Birschwilks et al., 2007). We investigated the possibility that C. pentagona might withdraw JA from BAW-infested tomato plants. After 24 h of BAW feeding, the amounts of JA in C. pentagona vines growing on caterpillar-fed plants were not different from those growing on uninfested plants (mean ± se ng/g JA, 26.5 ± 3.5 and 23.5 ± 2.5, respectively; t test, P = 0.624; n = 12).
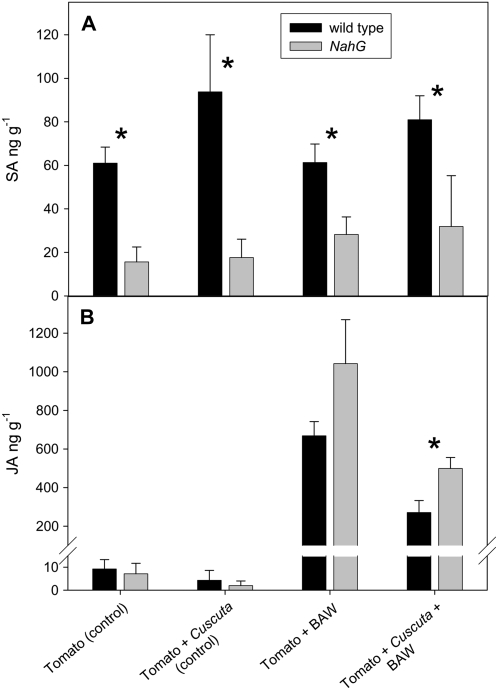
Parasitized NahG Tomato Plants Produce More Herbivore-Induced JA Than Parasitized Wild-Type Plants
We used SA-deficient NahG tomato plants to explore the possible inhibition of JA by SA induction, comparing JA in parasitized transgenic and wild-type plants after 24 h of BAW feeding. In all treatments, transgenic NahG plants produced significantly less SA than wild-type plants (Fig. 6A). However, degradation of SA by the enzyme salicylate hydroxylase was not complete in NahG plants, which contained about 20 ng g−1 of SA, amounts similar to those reported by Li et al. (2006). Parasitized NahG plants produced more JA in response to BAW feeding than parasitized wild-type plants (Fig. 6B). Unparasitized NahG plants tended to produce more herbivore-induced JA than unparasitized wild-type plants, but this difference was not significant (Fig. 6B; P = 0.096). In both NahG and wild-type plants, parasitism by C. pentagona significantly reduced production of JA in response to BAW feeding (Fig. 6B; P = 0.033 and 0.004, respectively).
Figure 6.
Comparison of SA (A) and JA (B) in unparasitized NahG and wild-type tomato plants and plants parasitized by C. pentagona after 24 h of BAW feeding. Data represent means and se of untransformed values from six replicates. *, Significant differences in SA or JA between NahG and wild-type plants within treatments (P < 0.05).
Parasitized Tomato Plants Produce Induced Volatiles When Treated with JA
To determine if C. pentagona-infested tomato plants could produce induced volatiles, we treated parasitized and unparasitized wild-type plants with synthetic JA and compared subsequent volatile production. Application of JA induced a significant increase in volatiles after 3 d in both parasitized and unparasitized plants (Fig. 7). Total JA-induced volatile production by parasitized plants was not different from that of unparasitized plants for any day (Fig. 7).
Figure 7.
Total volatile production (means ± se) by unparasitized tomato plants and plants parasitized by C. pentagona on days 1 to 3 after treatment with JA. Data show untransformed values from six replicates. Different uppercase letters indicate significance differences among days for unparasitized plants; lowercase letters indicate significance differences among days for parasitized plants (P < 0.05); P values indicate differences between unparasitized and parasitized plants within days.
DISCUSSION
C. pentagona Impacts on Herbivore-Induced JA and Volatiles
Tomato plants parasitized by C. pentagona contained only about one-third as much JA as unparasitized plants after 24 h of caterpillar feeding (Fig. 1A). The role of JA in regulating induced plant defenses against chewing insects (e.g. proteinase inhibitors) is well established, and tomato served as a model system for much of this work. For example, loss-of-function tomato mutants for JA production have been shown to be more susceptible to insect feeding (Orozco-Cardenas et al., 1993; Howe et al., 1996; Li et al., 2003), whereas gain-of-function mutants have increased resistance to herbivores (Li et al., 2002; Chen et al., 2005). Furthermore, application of exogenous jasmonate has been shown to promote resistance of tomato plants to BAW in agricultural fields (Thaler, 1999). Although not verified in this study, reduced production of caterpillar-induced JA in parasitized tomato plants should translate into fewer proteinase inhibitors and other foliar antiherbivore compounds compared to unparasitized plants.
In contrast to unparasitized plants, tomato plants parasitized by C. pentagona failed to produce herbivore-induced volatiles 3 d after insect feeding began (Figs. 3 and 4). Because JA mediates the production of induced plant volatiles in tomato (Ament et al., 2004; Thaler et al., 2005), reduced JA production in parasitized plants may explain the absence of herbivore-induced volatiles. Volatiles induced by insect feeding are known to serve as important cues that can both repel ovipositing herbivores and attract their natural enemies, significantly reducing herbivore pressure in nature (De Moraes et al., 2001; Kessler and Baldwin, 2001). Our results suggest that C. pentagona-infested tomato plants would be unable to gain these benefits of volatile induction. When JA was supplied exogenously, parasitized tomato plants produced amounts of volatiles similar to unparasitized plants (Fig. 7), suggesting that the absence of induced volatiles cannot be explained solely by the removal of resources by the parasite.
Despite the attenuation of herbivore-induced JA and volatiles, the growth rate of BAW was greatly reduced on parasitized plants (Fig. 5). Slower growth of BAW may be explained by reduced water and nutrient availability in parasitized plants. C. pentagona acts as a strong sink withdrawing water and nutrients from the host plant, which can reduce sugar and nitrogen content of host plant leaves (Jeschke et al., 1994). Nutritional inadequacy of the host plant may also explain the slower growth rate of Chilo partellus (Swinhoe), a lepidopteran stem borer, on maize (Zea mays) infested by the parasitic plant Striga hermonthica (Del.) Benth. (Mohamed et al., 2007). However, we cannot rule out the possibility that compound(s) produced in the course of defense against Cuscuta might negatively affect BAW caterpillars.
Cuscuta Does Not Translocate Herbivore-Induced JA
In response to herbivory, plant volatiles are released not only at the site of feeding but also systemically from undamaged leaves (Paré and Tumlinson, 1997). Systemic responses in tomato are mediated by a phloem-mobile signal originating at the site of damage, and recent work indicates that this signal is likely to be JA (Schilmiller and Howe, 2005). We hypothesized that phloem-feeding parasites might withdraw JA from BAW-fed plants, reducing JA levels in leaves and precluding a systemic volatile response. However, we found no evidence that C. pentagona removed JA because parasite vines grown on uninfested and BAW-infested tomato contained the same amount of JA.
Cuscuta-Induced SA May Inhibit JA
Studies using tomato have shown that SA, either applied exogenously or induced by pathogens, can inhibit production of herbivore-induced JA (Doares et al., 1995; Stout et al., 1999; Thaler et al., 2002). Several lines of evidence from this study and others indicate that plant defenses induced by Cuscuta spp. attack are pathogen-like and might be mediated by SA. For example, reported host plant responses to Cuscuta spp. include hypersensitive reactions and phytoalexin production (Bringmann et al., 1999) as well as the expression of pathogenesis-related genes (Borsics and Lados, 2002). In this study, we also observed localized cell death at the point of Cuscuta attachment. Furthermore, parasitized plants tended to contain more SA than unparasitized plants (Fig. 2B). To examine whether Cuscuta-induced SA might be inhibiting JA, we compared BAW-induced JA production in parasitized wild-type and SA-deficient NahG tomato plants. Parasitized NahG plants produced more JA than parasitized wild-type plants in response to BAW feeding (Fig. 6), implying that SA inhibited JA production. However, JA/SA cross talk alone does not fully explain these results because Cuscuta still reduced BAW-induced JA production in NahG plants (Fig. 6B). Though the effect was not statistically significant, BAW-induced JA appeared to be elevated in uninfested NahG plants (Fig. 6B), a pattern that might result from reduced SA suppression (Spoel et al., 2003).
Some plant pathogens and herbivores are known to manipulate host defenses by interfering with plant defense signaling. For example, Pseudomonas syringae injects the JA mimic coronatine into tomato, eliciting JA responses and suppressing effective SA responses to promote pathogenesis (Zhao et al., 2003). Moreover, silverleaf whitefly feeding activates SA defenses and reduces operative JA defenses in Arabidopsis (Zarate et al., 2007). In broad terms, Cuscuta feeding resembles that of whiteflies; both are stealthy phloem feeders (i.e. cause little tissue damage) that feed continuously from the same location over an extended period of time. We cannot rule out the possibility that, like some pathogens and insects, C. pentagona co-opts JA/SA cross talk to manipulate host defenses. We are currently investigating which defense pathways are activated in parasitized tomato plants and the efficacy of JA and SA responses in defense against C. pentagona.
In summary, herbivore-induced production of JA and volatiles are compromised when tomato plants are infested by the parasitic plant C. pentagona. SA-mutant (NahG) tomato plants deficient in SA production contained significantly more BAW-induced JA when parasitized than wild-type plants, providing some evidence of SA-JA antagonism in host plant defense signaling. Our results further suggest that parasitism by C. pentagona induces plant volatiles and may elicit an SA-mediated pathogen-like response in tomato. However, a better understanding of host plant perception and physiological responses to attack by parasitic plants is needed to identify the mechanisms underlying C. pentagona-mediated effects on host plant defenses against herbivores.
MATERIALS AND METHODS
Plant/Insect Material and Growth Conditions
Seeds of Cuscuta pentagona collected from an infested tomato field in Yolo County, CA, were obtained from Dr. Tom Lanini (University of California, Davis). Seeds were soaked in concentrated sulfuric acid for 1 h using a Gooch crucible, rinsed for 1 min with distilled water, and placed in a petri dish on moist filter paper to germinate. Tomato plants (Solanum lycopersicum) ‘Halley 3155’ were grown in an insect-free growth chamber (25°C, 16-h photoperiod at 250 μmol m−2 s−1 provided by cool-white fluorescent tubes) in 9-cm-tall × 10-cm-wide square plastic pots filled with a peat-based general-purpose potting soil with fertilizer (Osmocote; The Scotts Company). Seeds of NahG tomato plants and the corresponding wild type (‘MoneyMaker’) were obtained from Dr. Harry Klee (University of Florida) and grown similarly, except that they received low light intensity (75 μmol m−2 s−1) to prevent development of necrotic leaf spots. BAW (Spodoptera exigua) eggs were obtained from the U.S. Department of Agriculture/Agricultural Research Service Research Laboratory in Tifton, GA, and reared on a casein-based artificial diet in a growth chamber (25°C/22°C day/night, 16-h photoperiod).
C. pentagona Attachment and Growth on Tomato
Newly germinated C. pentagona seedlings, approximately 4 cm long, were allowed to attach to 10-d-old tomato seedlings (first true leaves just beginning to expand) by leaning the C. pentagona seedling against the right side of the tomato meristem (Cuscuta are left-handed and coil from right to left). Because far-red light promotes tight coiling of Cuscuta spp. (Haidar and Orr, 1999), two incandescent 75-W bulbs (75A/CL/DL/RP 120V; Orsam Sylvania) per 15 pots in 30-cm × 50-cm flats were placed 1 m above plants and left on for 24 h (off for 8 h of scotophase). Using this setup, C. pentagona seedlings coiled tightly around the tomato seedlings within 6 h and haustorial swellings at points of contact with the host were evident within 24 h. Control plants received the same treatment, and plants exposed to incandescent light for this short period showed no noticeable physiological effects. Ten days later, the growing Cuscuta vine was allowed to attach a second time to the petiole of the second expanded true leaf (the youngest expanded leaf) of the same now-20-d-old tomato host (Fig. 1). To control the site of attachment, the parasite vine at approximately 2 cm from apex was placed against the right side of the appropriate tomato petiole. Subsequent brief exposure to incandescent light (as above) usually induced coiling around the petiole at this point. Five days later, the apical leaflet attached to the parasitized petiole of the 25-d-old tomato received insect feeding for phytohormone analysis, volatile collection, and caterpillar growth trials (Fig. 1, arrow).
Extraction and Quantification of JA and SA
A time course of changes in JA and SA in 25-d-old tomato was conducted for the following treatments: (1) tomato control (no parasitism or BAW feeding), (2) tomato + parasite control (C. pentagona parasitism only), (3) tomato + BAW (BAW feeding only), and (4) tomato + C. pentagona + BAW (parasitized tomato with BAW feeding). For treatments with insect feeding, one third-instar BAW was confined to the apical leaflet of the parasitized petiole leaf (Fig. 1, arrow) using a round 3-cm-diameter clip-cage. The corresponding leaf of plants in insect-free treatments received empty cages. Insects were watched until they began to feed. At 0, 15, 30, 45, 60, 120 min, and 24 h after feeding began, approximately 100 mg of the leaf (incorporating the feeding site) was removed, immediately snap-frozen in liquid nitrogen in FastPrep tubes (Q-BIOgene) with 1 g of Zirmil beads (1.1 mm; Saint-Gobain ZirPro), weighed, and held at −80°C until processed. We used vapor phase extraction to extract and measure JA and SA following the method of Schmelz et al. (2003, 2004). Briefly, plant tissue was homogenized using Zirmil beads in a FastPrep shaker, and the phytohormones were partitioned into an organic layer (dichloromethane), transferred to a 4-mL glass vial, and derivatized from carboxylic acids to methyl esters using trimethylsilyldiazomethane (Sigma-Aldrich). The solvent was evaporated under an air stream, and the dry vial was heated to 200°C for 2 min to expedite volatilization of analytes, which were collected at this time from the headspace using volatile traps containing 30 mg of Super-Q (Alltech) attached to a vacuum (1 L/min). The phytohormones were eluted from the traps using 150 μL of dichloromethane and analyzed by gas chromatography-mass spectrometry with isobutane chemical ionization with select-ion monitoring (settings described by Schmelz et al., 2004). Amounts of methyl jasmonate and methyl salicylate were quantified using standard curves made with pure standards (Sigma-Aldrich); internal standards were used to confirm derivatization and recovery.
Collection and Analysis of Plant Volatiles
Volatiles were collected from the four plant treatments described above from intact, potted 25-d-old tomato plants using a closed push/pull system. A guillotine Teflon base with a small hole in the center for the plant stem rested on the pot, and plants were enclosed in a glass dome (15 cm tall × 16 cm wide at base). Filtered air was pushed into the top of the chamber (2 L/min), passed over the plant, and was pulled out the side (1 L/min) through volatile traps containing a 30-mg bed of the adsorbent Super-Q. Volatiles were eluded from traps with 150 μL of dichloromethane; 200 ng of n-octane and 400 ng of n-nonyl-acetate were added as internal standards. Samples were analyzed with an Agilent 6890 gas chromatograph (injector, splitless mode, 220°C, 1 μL sample volume) equipped with a flame ionization detector. Compounds were separated on a HP-1 (15 m × 0.25 i.d., 0.1-μm film thickness) column held at 35°C for 1 min after injection, and then programmed at 4°C min−1 to 140°C, then 20°C min−1 to 220°C. Quantifications were made relative to internal standards using ChemStation software (Agilent Technologies). Identifications of compounds were confirmed using mass spectrometry (HP 5973) by comparing retention times and mass spectra to commercial standards (De Moraes and Mescher, 2004). To investigate herbivore-induced volatiles, one third-instar BAW was confined, using a round 3-cm diameter clip-cage, to the apical leaflet of the parasitized tomato petiole leaf (Fig. 1) or to the corresponding leaf of unparasitized plants. Empty cages were clipped on parasitized and unparasitized control plants. Volatiles were collected for 3 d between 1,000 and 2,200 h (light period, 6 am–10 pm).
BAW Feeding and Performance on Parasitized and Unparasitized Tomato
Third-instar BAW caterpillars were caged individually on the parasitized petiole leaf (Fig. 1) or on the corresponding leaf of unparasitized 25-d-old tomato plants. At the beginning and end of the experiment, caterpillars were starved for 24 h to void gut contents and then weighed. Caterpillars were allowed to feed for 24 h and the relative growth rate [(final weight − initial weight)/(initial weight × no. of days)] was calculated (Waldbauer, 1968). In a separate experiment, we compared the total amount and proportion of total leaf area consumed by BAW on parasitized and unparasitized plants. Caterpillars were allowed to feed as above for 24 h, then all leaves were removed, taped to a white piece of paper, digitally scanned, and leaf area was determined using the imaging analysis software SigmaScan Pro 5 (SPSS).
Translocation of JA by C. pentagona from BAW-Infested Tomato
One third-instar BAW was allowed to feed on parasitized and unparasitized tomato plants as described above. After 24 h of feeding, the entire C. pentagona plant (approximately 300 mg fresh weight) was removed, immediately frozen in liquid nitrogen, and stored at −80°C until processed. The vines were ground in liquid nitrogen with a mortar and pestle to a fine powder, and an aliquot of approximately 100 mg (fresh weight) was used for JA extraction and measurement (as described above).
JA Production by NahG Tomato in Response to BAW Feeding
To examine the possibility that SA inhibits BAW-induced JA production in parasitized plants, the production of JA and SA by NahG and wild-type (‘MoneyMaker’) tomato plants in response to BAW feeding was determined. NahG plants express a gene encoding a bacterial enzyme, salicylate hydroxylase, that converts SA immediately to inactive catechol, and are thus deficient in accumulation of this plant hormone (Brading et al., 2000). Caterpillars were allowed to feed for 24 h on 25-d-old transgenic and wild-type plants, and amounts of JA and SA were measured as described above.
Induction of Volatiles with Synthetic JA
The ability of parasitized and unparasitized tomato plants to produce induced volatiles upon treatment with synthetic JA was investigated. JA was synthesized from methyl jasmonate (Farmer et al., 1992) and suspended in 70% ethanol:water. The average fresh weight of the appropriate apical leaflet was determined and the amount of JA typically found in unparasitized plants after 24 h of caterpillar feeding (about 800 ng g−1; Fig. 2A) was evenly applied with a pipette to the apical leaflet attached to the parasitized petiole of the 25-d-old tomato (Fig. 1). JA was applied on the morning of day 1 and volatiles were collected for 3 d.
Statistical Analyses
Comparisons were made among treatments for each sampling period in the JA/SA time-courses and to test for treatment effects on volatile production, using ANOVA; individual means were compared with Tukey's honestly significantly different means separation test. All statistics were done using SAS (version 8.2; SAS Institute). Amounts of JA and SA were analyzed on a per-gram fresh weight basis and were natural log transformed to stabilize variance. Volatile data were square-root transformed to meet variance assumptions. Because parasitized tomato plants were typically smaller than unparasitized plants, volatiles were analyzed by leaf area (ng/cm2). Leaf area was determined using SigmaScan Pro 5 (as described above). The relative growth rate of BAW on healthy and Cuscuta-infested tomato leaves and JA in Cuscuta after 24 h BAW feeding were compared using t-tests.
Acknowledgments
We thank T. Lanini for providing seeds of C. pentagona and tomato ‘Halley 3155’; H. Klee for NahG and ‘MoneyMaker’ tomato seeds; J. Ruberson for supplying BAW eggs; J. Zhu for help with statistics; J. Saunders and E. Bogus for technical assistance; and C. Delphia and J. Tumlinson for comments on the manuscript.
This work was supported by the David and Lucile Packard Foundation, the DuPont Foundation, and the National Science Foundation (Doctoral Dissertation Improvement grant no. 0608345 and NSF CAREER no. 0643966).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Consuelo M. De Moraes (czd10@psu.edu).
References
- Ament K, Kant MR, Sabelis MW, Haring MA, Schuurink RC (2004) Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol 135 2025–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett RD, Smith RS, Shiel RS, Peacock S, Simkin JM, Quirk H, Hobbs PJ (2006) Parasitic plants indirectly regulate below-ground properties in grassland ecosystems. Nature 439 969–972 [DOI] [PubMed] [Google Scholar]
- Birschwilks M, Sauer N, Scheel D, Neumann S (2007) Arabidopsis thaliana is a susceptible host plant for the holoparasite Cuscuta spec. Planta 226 1231–1241 [DOI] [PubMed] [Google Scholar]
- Borsics T, Lados M (2002) Dodder infection induces the expression of a pathogenesis-related gene of the family PR-10 in alfalfa. J Exp Bot 53 1831–1832 [DOI] [PubMed] [Google Scholar]
- Brading PA, Hammond-Kosack KE, Parr A, Jones JDG (2000) Salicylic acid is not required for Cf-2- and Cf-9-dependent resistance of tomato to Cladosporium fulvum. Plant J 23 305–318 [DOI] [PubMed] [Google Scholar]
- Bringmann G, Schlauer J, Ruckert M, Wiesen B, Ehrenfeld K, Proksch P, Czygan FC (1999) Host-derived acetogenins involved in the incompatible parasitic relationship between Cuscuta reflexa (Convolvulaceae) and Ancistrocladus heyneanus (Ancistrocladaceae). Plant Biol 1 581–584 [Google Scholar]
- Cardoza YJ, Lait CG, Schmelz EA, Huang J, Tumlinson JH (2003) Fungus-induced biochemical changes in peanut plants and their effect on development of beet armyworm, Spodoptera exigua Hübner (Lepidoptera: Noctuidae) larvae. Environ Entomol 32 220–228 [Google Scholar]
- Chen H, Gonzales-Vigil E, Wilkerson CG, Howe GA (2007) Stability of plant defense proteins in the gut of insect herbivores. Plant Physiol 143 1954–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wilkerson CG, Kuchar JA, Phinney BS, Howe GA (2005) Jasmonate-inducible plant enzymes degrade essential amino acids in the herbivore midgut. Proc Natl Acad Sci USA 102 19237–19242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipollini D, Enright S, Traw MB, Bergelson J (2004) Salicylic acid inhibits jasmonic acid-induced resistance of Arabidopsis thaliana to Spodoptera exigua. Mol Ecol 13 1623–1653 [DOI] [PubMed] [Google Scholar]
- Dangl JL, Jones JDG (2001) Plant pathogens and integrated defence responses to infection. Nature 411 826–833 [DOI] [PubMed] [Google Scholar]
- Dawson JH, Musselman LJ, Wolswinkel P, Dörr I (1994) Biology and control of Cuscuta. Rev Weed Sci 6 265–317 [Google Scholar]
- De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393 570–573 [Google Scholar]
- De Moraes CM, Mescher MC (2004) Biochemical crypsis in the avoidance of natural enemies by an insect herbivore. Proc Natl Acad Sci USA 101 8993–8997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moraes CM, Mescher MC, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410 577–580 [DOI] [PubMed] [Google Scholar]
- Dean JM, De Moraes CM (2006) Effects of genetic modification on herbivore-induced volatiles from maize. J Chem Ecol 32 713–724 [DOI] [PubMed] [Google Scholar]
- Doares SH, Narváez-Vásquez J, Conconi A, Ryan CA (1995) Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol 108 1741–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42 185–209 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Johnson RR, Ryan CA (1992) Regulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic acid. Plant Physiol 98 995–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton GW, Korth KL (2000) Trade-offs between pathogen and herbivore resistance. Curr Opin Plant Biol 3 309–314 [DOI] [PubMed] [Google Scholar]
- Felton GW, Korth KL, Bi JL, Wesley SV, Huhman DV, Mathews MC, Murphy JB, Lamb C, Dixon RA (1999) Inverse relationship between systemic resistance of plants to microorganisms and insect herbivory. Curr Biol 9 317–320 [DOI] [PubMed] [Google Scholar]
- Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43 205–227 [DOI] [PubMed] [Google Scholar]
- Gouinguené S, Alborn H, Turlings TC (2003) Induction of volatile emissions in maize by different larval instars of Spodoptera littoralis. J Chem Ecol 29 145–162 [DOI] [PubMed] [Google Scholar]
- Haidar MA, Orr GL (1999) The response of Cuscuta planiflora seedlings to red and far-red, blue light and end-of-day irradiations. Ann Appl Biol 134 117–120 [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA (1996) An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke WD, Räth N, Bäumel P, Czygan FC, Proksch P (1994) Modelling the flow and partitioning of carbon and nitrogen in the holoparasite Cuscuta reflexa Roxb. and its host Lupinus albus L. I. Methods for estimating net flows. J Exp Bot 45 791–800 [Google Scholar]
- Karban R, Baldwin IT (1997) Induced Responses to Herbivory. University of Chicago Press, Chicago
- Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291 2141–2144 [DOI] [PubMed] [Google Scholar]
- Kuijt J (1969) The Biology of Parasitic Flowering Plants. University of California Press, Berkeley, CA
- Kunkel BN, Brooks DM (2002) Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 5 325–331 [DOI] [PubMed] [Google Scholar]
- Li C, Liu G, Xu C, Lee GI, Bauer P, Ling HQ, Ganal MW, Howe GA (2003) The tomato Suppressor of prosystemin-mediated responses2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. Plant Cell 15 1646–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Williams MM, Loh YT, Lee GI, Howe GA (2002) Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol 130 494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Xie QG, Smith-Becker J, Navarre DA, Kaloshian I (2006) Mi-1-mediated aphid resistance involved salicylic acid and mitogen-activated protein kinase signaling cascades. Mol Plant Microbe Interact 19 655–664 [DOI] [PubMed] [Google Scholar]
- Mohamed HM, Khan ZR, Mueke JM, Hassanali A, Kairu E, Pickett JA (2007) Behaviour and biology of Chilo partellus (Swinhoe) on Striga hermonthica (Del.) Benth. infested and uninfested maize plants. Crop Prot 26 998–1005 [Google Scholar]
- Moran PJ, Thompson GA (2001) Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol 125 1074–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman LJ, Yoder JI, Westwood JH (2001) Parasitic plants major problem of food crops. Science 293 1434. [DOI] [PubMed] [Google Scholar]
- Nadler-Hassar T, Rubin B (2003) Natural tolerance of Cuscuta campestris to herbicides inhibiting amino acid biosynthesis. Weed Res 43 341–347 [Google Scholar]
- Nickrent DL (2007) Parasitic plant genera and species. Parasitic Plant Connection. http://www.parasiticplants.siu.edu/ (September 15, 2007)
- Ohnmeiss TE, McCloud ES, Lynds GY, Baldwin IT (1997) Within-plant relationships among wounding, jasmonic acid, and nicotine: implications for defence in Nicotiana sylvestris. New Phytol 137 441–452 [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas M, McGurl B, Ryan CA (1993) Expression of an antisense prosystemin gene in tomato plants reduces resistance toward Manduca sexta larvae. Proc Natl Acad Sci USA 90 8273–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH (1997) De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol 114 1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C, Riches CR (1993) Parasitic Weeds of the World: Biology and Control. CAB International, Wallingford, UK
- Peña-Cortés H, Albrecht T, Prat S, Weiler EW, Willmitzer L (1993) Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta 191 123–128 [Google Scholar]
- Press MC, Graves JD (1995) Parasitic Plants. Chapman and Hall, London
- Press MC, Phoenix GK (2005) Impacts of parasitic plants on natural communities. New Phytol 166 737–751 [DOI] [PubMed] [Google Scholar]
- Preston CA, Lewandowski C, Enyedi AJ, Baldwin IT (1999) Tobacco mosaic virus inoculation inhibits wound-induced jasmonic acid-mediated responses within but not between plants. Planta 209 87–95 [DOI] [PubMed] [Google Scholar]
- Reymond P, Farmer EE (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol 1 404–411 [DOI] [PubMed] [Google Scholar]
- Runyon JB, Mescher MC, De Moraes CM (2006) Volatile chemical cues guide host location and host selection by parasitic plants. Science 313 1964–1967 [DOI] [PubMed] [Google Scholar]
- Schilmiller AL, Howe GA (2005) Systemic signaling in the wound response. Curr Opin Plant Biol 8 369–377 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Engelberth J, Alborn HT, O'Donnell P, Sammons M, Toshima H, Tumlinson JH (2003) Simultaneous analysis of phytohormones, phytotoxins, and volatile organic compounds in plants. Proc Natl Acad Sci USA 100 10552–10557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Engelberth J, Tumlinson JH, Block A, Alborn HT (2004) The use of vapor phase extraction in metabolic profiling of phytohormones and other metabolites. Plant J 39 790–808 [DOI] [PubMed] [Google Scholar]
- Spoel SH, Koornneef A, Claessens SMC, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Métraux JP, Brown R, Kazan K, et al (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15 760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout MJ, Fidantsef AL, Duffey SS, Bostock RM (1999) Signal interactions in pathogen and insect attack: systemic plant-mediated interactions between pathogens and herbivores of the tomato, Lycopersicon esculentum. Physiol Mol Plant Pathol 54 115–130 [Google Scholar]
- Stout MJ, Thaler JS, Thomma BPHJ (2006) Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu Rev Entomol 51 663–689 [DOI] [PubMed] [Google Scholar]
- Thaler JS (1999) Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 399 686–688 [Google Scholar]
- Thaler JS, Farag MA, Paré PW, Dicke M (2005) Jasmonate-deficient plants have reduced direct and indirect defences against herbivores. Ecol Lett 5 764–774 [Google Scholar]
- Thaler JS, Fidantsef AL, Duffey SS, Bostock RM (1999) Trade-offs in plant defense against pathogens and herbivores: a field demonstration of chemical elicitors of induced resistance. J Chem Ecol 25 1597–1609 [Google Scholar]
- Thaler JS, Karban R, Ullman DE, Boege K, Bostock RM (2002) Cross-talk between jasmonate and salicylate plant defense pathways: effects on several plant parasites. Oecologia 131 227–235 [DOI] [PubMed] [Google Scholar]
- Thaler JS, Owen B, Higgins VJ (2004) The role of the jasmonate responses in plant susceptibility to diverse pathogens with a range of lifestyles. Plant Physiol 135 530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooker JF, De Moraes CM (2007) Feeding by Hessian fly [Mayetiola destructor (Say)] larvae does not induce plant indirect defenses. Ecol Entomol 32 153–161 [Google Scholar]
- Turlings TCJ, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250 1251–1253 [DOI] [PubMed] [Google Scholar]
- Waldbauer GB (1968) The consumption and utilization of food by insects. Adv Insect Physiol 5 229–289 [Google Scholar]
- Wolswinkel P (1974) Complete inhibition of setting and growth of fruits of Vicia faba L. resulting from the draining of the phloem system by Cuscuta species. Acta Bot Neerl 23 48–60 [Google Scholar]
- Zarate SI, Kempema LA, Walling LL (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Thilmony R, Bender CL, Schaller A, He SY, Howe GA (2003) Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate pathway. Plant J 36 485–499 [DOI] [PubMed] [Google Scholar]