Abstract
The 70-kD heat shock proteins (Hsp70s) have been shown to be important for protein folding, protein translocation, and stress responses in almost all organisms and in almost all subcellular compartments. However, the function of plastid stromal Hsp70s in higher plants is still uncertain. Genomic surveys have revealed that there are two putative stromal Hsp70s in Arabidopsis thaliana, denoted cpHsc70-1 (At4g24280) and cpHsc70-2 (At5g49910). In this study, we show that cpHsc70-1 and cpHsc70-2 could indeed be imported into the chloroplast stroma. Their corresponding T-DNA insertion knockout mutants were isolated and designated as Δcphsc70-1 and Δcphsc70-2. No visible phenotype was observed in the Δcphsc70-2 mutant under normal growth conditions. In contrast, Δcphsc70-1 mutant plants exhibited variegated cotyledons, malformed leaves, growth retardation, and impaired root growth, even though the protein level of cpHsc70-2 was up-regulated in the Δcphsc70-1 mutant. After heat shock treatment of germinating seeds, root growth from Δcphsc70-1 seeds was further impaired, indicating that cpHsc70-1 is important for thermotolerance of germinating seeds. No Δcphsc70-1 Δcphsc70-2 double mutant could be obtained, suggesting that the Δcphsc70 double knockout was lethal. Genotype analyses of F1 seedlings from various crosses indicated that double-knockout mutation was lethal to the female gametes and reduced the transmission efficiency of the male gametes. These results indicate that cpHsc70s are essential for plant development and the two cpHsc70s most likely have redundant but also distinct functions.
The 70-kD heat shock proteins (Hsp70s) are molecular chaperones involved in a variety of cellular processes including protein folding, protein transport across membranes, modulation of protein activity, regulation of protein degradation, and prevention of irreversible protein aggregation. Plant Hsp70s are encoded by a multiple-gene family. Sequence analyses of plant Hsp70 gene families have revealed four major subgroups, each localized to one of the subcellular compartments: cytosol, endoplasmic reticulum (ER), plastids, and mitochondria (Sung et al., 2001a). Functions of plant Hsp70s in most compartments have been studied extensively. It has been shown that Arabidopsis (Arabidopsis thaliana) cytosolic Hsp70-antisense transgenic plants have reduced thermotolerance (Lee and Schöffl, 1996). Overexpression of Arabidopsis cytosolic Hsc70-1 resulted in enhanced heat tolerance under certain conditions (Sung and Guy, 2003). Overexpression of a tobacco (Nicotiana tabacum) ER Hsp70, Bip, alleviates ER stress (Leborgne-Castel et al., 1999) and enhances drought, but not heat tolerance (Alvim et al., 2001). In yeast (Saccharomyces cerevisiae), ER Bip and mitochondrial matrix Hsp70 function as a molecular motor driving precursor-protein translocation into the organelle through repeated cycles of ATP hydrolysis (Pilon and Schekman, 1999; Strub et al., 2000). It is likely that higher plant ER Bip and mitochondrial Hsp70 also have a similar function.
In comparison, the function of higher plant plastid Hsp70 is still uncertain. Two plastid Hsp70 proteins, Com70 and IAP70, localized at the outer envelope membrane of pea chloroplasts, have been proposed to be involved in protein import into chloroplasts (Schnell et al., 1994; Kourtz and Ko, 1997). However, their molecular function and identity are still unknown. Nielsen et al. (1997) found that the stromal Hsp70 of pea (Pisum sativum) chloroplasts, S78, could be coimmunoprecipitated with importing precursor proteins, but S78 did not associate with the Toc/Tic (translocon at the outer and inner envelope membranes of chloroplasts) translocon complex that is responsible for protein import into chloroplasts. On the other hand, in vitro binding assays showed physical interactions between S78 and recombinant transit peptides of precursors to the small subunit of Rubisco (prRBCS; Ivey et al., 2000). However, precursors with altered affinities for Hsp70 in their transit peptides are still efficiently imported into chloroplasts (Rial et al., 2003, 2006). Therefore, whether stromal Hsp70s are involved in chloroplast protein import requires further studies. In the green algae Chlamydomonas and Dunaliella, chloroplast stromal Hsp70B has been shown to function in PSII protection and repair during and after photoinhibition (Schroda et al., 1999; Yokthongwattana et al., 2001). Chlamydomonas stromal Hsp70B regulates the assembly state of VIPP1 (vesicle inducing protein in plastid 1), which may be important for thylakoid biogenesis or maintenance (Liu et al., 2005, 2007). It is not known whether higher plant stromal Hsp70 has any similar function.
Genomic surveys revealed that there are 14 genes encoding Hsp70s in Arabidopsis (Sung et al., 2001a). Among them, two genes encode putative plastid stromal Hsp70s, cpHsc70-1 (At4g24280), and cpHsc70-2 (At5g49910). They harbor a predicted chloroplast-targeting transit peptide and a C-terminal motif (PEGDVIDADFTDSK) conserved among plastid Hsp70s (Guy and Li, 1998). Their polypeptide sequences share a 90.7% identity. Although reverse transcription (RT)-PCR analyses indicated that the amplified signal for cpHsc70-1 was very low and the transcripts of cpHsc70-2 was much higher than cpHsc70-1 in all organs (Sung et al., 2001b), according to the public microarray and MPSS databases (Brenner et al., 2000 [MPSS, http://mpss.udel.edu/at/]; Zimmermann et al., 2004 [Genevestigator, http://www.genevestigator.ethz.ch/]; Toufighi et al., 2005 [Botany Array Resource, http://bbc.botany.utoronto.ca/]), both transcripts are quite abundant in almost all tissues, with the level of cpHsc70-1 slightly higher than cpHsc70-2 in most tissues.
In this report, we show that the two putative plastid Hsp70s were indeed imported into the chloroplast stroma. T-DNA insertion knockout mutants of the two genes were analyzed. Our data indicate that the stromal Hsp70s are important for plant development under both normal and heat-stress conditions.
RESULTS
Arabidopsis cpHsc70-1 and cpHsc70-2 Could Be Imported into the Stroma of Chloroplasts
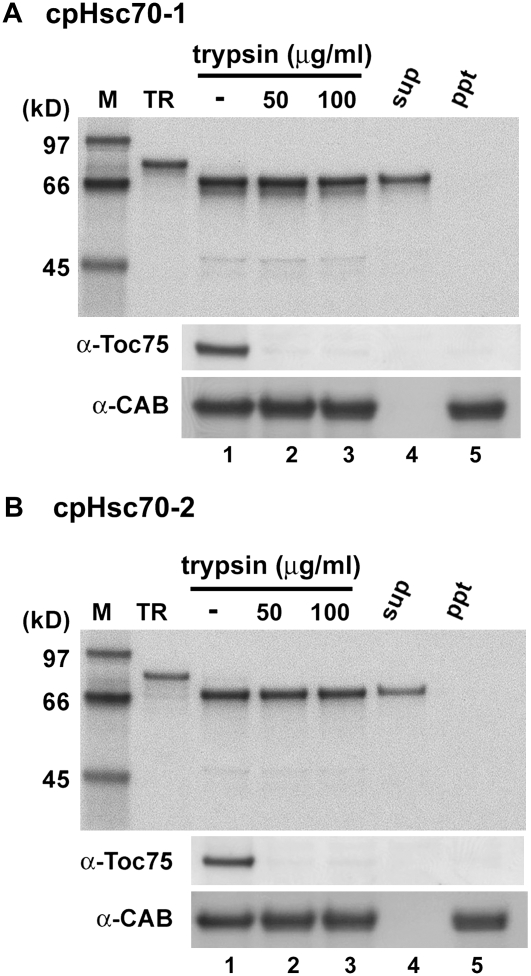
To verify the plastid localization of Arabidopsis cpHsc70-1 and cpHsc70-2, [35S]Met-labeled cpHsc70-1 and cpHsc70-2 were synthesized by in vitro translation and incubated with isolated pea chloroplasts. As shown in Figure 1, the precursor form of both cpHsc70-1 and cpHsc70-2 was about 80 kD. After import, a mature protein of approximately 71 kD was produced, suggesting the cleavage of a transit peptide. The imported mature cpHsc70s were present in the soluble fraction of chloroplasts (lane 4) and were resistant to thermolysin (data not shown) and trypsin digestion (lanes 2 and 3). Trypsin can penetrate the outer but not the inner envelope membrane (Jackson et al., 1998), and resulted in complete digestion of the outer membrane protein Toc75 but not the thylakoid membrane protein chlorophyll a/b-binding protein of PSII (CAB; Fig. 1). These data suggested that the two cpHsc70s were located in the chloroplast stroma. It is less likely that they were located in the thylakoid lumen because their transit peptides do not resemble the typical bipartite luminal-targeting sequence (Gutensohn et al., 2006).
Figure 1.
Arabidopsis cpHsc70s were imported into the stroma of isolated chloroplasts. [35S]Met-labeled precursors of cpHsc70-1 and cpHsc70-2 were incubated with isolated pea chloroplasts under import conditions. After 30 min, chloroplasts were pelleted and digested with the indicated concentration of trypsin. Intact chloroplasts were recovered through a 40% Percoll cushion after the digestion (lanes 1–3), lysed hypotonically, and separated into soluble (sup) and membrane (ppt) fractions (lanes 4 and 5). An equal amount of chloroplasts was loaded in lanes 1 to 3 and the soluble and membrane proteins from the same amount of chloroplasts were loaded in lanes 4 and 5. Samples were analyzed by SDS-PAGE and fluorography. α-Toc75 and α-CAB are the same set of samples decorated with antibodies against Toc75 and CAB on immunoblots. M, Molecular mass markers; TR, in vitro-translated cpHsc70 precursor proteins before import.
Identification of T-DNA Insertion Mutants of cpHsc70s
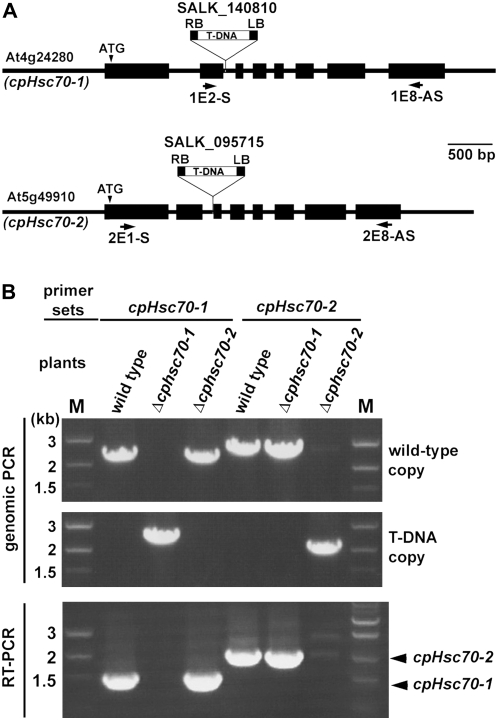
To study the function of cpHsc70s, we obtained their putative T-DNA insertion mutants, SALK_140810 and SALK_095715, from the SALK T-DNA collection (Alonso et al., 2003). The T-DNA insertion sites were confirmed by genomic PCR and DNA sequencing. As shown in Figure 2A, both SALK_140810 and SALK_095715 have a T-DNA insertion in the intron II of cpHsc70-1 and cpHsc70-2, respectively. RT-PCR analyses indicated that both T-DNA insertion lines were null mutants (Fig. 2B). We designated these two mutants as Δcphsc70-1 (SALK_140810) and Δcphsc70-2 (SALK_095715). Genomic DNA analyses further indicated that both Δcphsc70-1 and Δcphsc70-2 contained T-DNA insertion in a single locus and the variegated-cotyledon phenotype Δcphsc70-1 (see below) was linked to the T-DNA insertion (Supplemental Fig. S1; Supplemental Table S1).
Figure 2.
Confirmation of the T-DNA insertion lines for cpHsc70s. A, Schematic representation for the genomic fragments of cpHsc70-1 (At4g24280) and cpHsc70-2 (At5g49910). Black boxes and lines represent exons and introns, respectively. ATG represents the translation initiation sites. The locations of the T-DNA insertions in Δcphsc70-1 and Δcphsc70-2 mutants are illustrated. Positions and directions of primers used in B are also indicated. B, Δcphsc70-1 and Δcphsc70-2 are null mutants. Primer sets for cpHsc70-1 are: 1E2-S + 1E8-AS for wild-type copy of cpHsc70-1 DNA and RT-PCR of RNA, and SALK-Lba1 + 1E8-AS for Δcphsc70-1 T-DNA insertion. Primer sets for cpHsc70-2 are: 2E1-S + 2E8-AS for wild-type copy of cpHsc70-2 DNA and RT-PCR of RNA, and SALK-Lba1 + 2E8-AS for Δcphsc70-2 T-DNA insertion.
Δcphsc70-1 Plants Exhibited Variegated Cotyledons, Malformed Leaves, and Growth Retardation
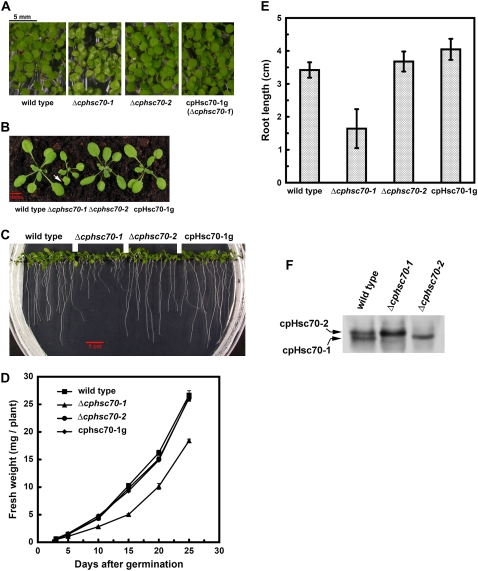
The cotyledons of Δcphsc70-1 plants were variegated (Fig. 3A). Furthermore, in the early vegetative stage, the true leaves of Δcphsc70-1 seedlings often had irregular leaf margins and small lesions (Fig. 3B). The mutant was also smaller than the wild type. At 15 d after germination, the fresh weight of Δcphsc70-1 plants was about 50% of wild-type plants (Fig. 3D). We also measured root growth on vertical plates. Root length of Δcphsc70-1 plants was also about 50% of wild type (Fig. 3, C and E). In contrast, there was no visible phenotype in the Δcphsc70-2 mutant. These results suggest that cpHsc70-1 is important for both shoot and root growth. Because there is only one mutant allele available for Δcphsc70-1, we tried to complement Δcphsc70-1 with a cpHsc70-1 genomic fragment to confirm that the mutant phenotypes were caused by the loss of cpHsc70-1. As shown in Figure 3, Δcphsc70-1 transformed with a 5-kb cpHsc70-1 genomic fragment (transformants designated as cpHsc70-1g) fully recovered the wild-type phenotype.
Figure 3.
Phenotypes of the Δcphsc70 knockout mutants. A, Δcphsc70-1 mutants exhibited variegated cotyledons, which were complemented by a genomic fragment of cpHsc70-1 (cpHsc70-1g). Plants were grown on MS plates for 7 d. B, Δcphsc70-1 had a smaller size and malformed leaves (white arrowhead). Plants were grown on soil for 28 d. C, Δcphsc70-1 had shorter roots. Plants were grown vertically on an MS plate for 7 d. D, Growth curves of mutants and wild type. Fresh weights of plate-grown seedlings were measured. Values are the average ± sd of two independent experiments. For each experiment, three batches of 10 seedlings each were measured. E, Average root length of plants shown in C. Values are the average ± sd of three independent experiments. For each experiment, the root length of more than 10 seedlings was measured. F, cpHsc70 protein levels in the mutants and wild type. Total stromal proteins (∼40 μg) were analyzed by an IEF gel and immunoblotting decorated with anti-S78 antibody.
We further analyzed the cpHsc70 protein level in the mutants and wild type using an antibody specifically recognizing the pea stromal Hsp70, S78 (Akita et al., 1997). This antibody was made against the C-terminal region of S78. This region of S78 shares a 75% sequence identity to the two Arabidopsis cpHsc70s, but only a 22% to 32% identity to other Arabidopsis Hsp70s. The antibody recognized a single band in Arabidopsis total leaf proteins on SDS-PAGE and immunoblots (Supplemental Fig. S2). However, the two Arabidopsis cpHsc70s could be resolved on isoelectric focusing (IEF) gels and each mutant was indeed missing the corresponding cpHsc70 (Fig. 3F). Interestingly, the level of cpHsc70-2 was up-regulated in the Δcphsc70-1 mutant. This result suggested that in the Δcphsc70-1 mutant, even though cpHsc70-2 was up-regulated, cpHsc70-2 still could not rescue the Δcphsc70-1 phenotypes. In comparison, the Δcphsc70-2 mutant had a lower amount of total cpHsc70 (presumably cpHsc70-1) than the wild type and this amount of cpHsc70-1 was sufficient for normal plant development.
cpHsc70s Are Essential for Plant Development
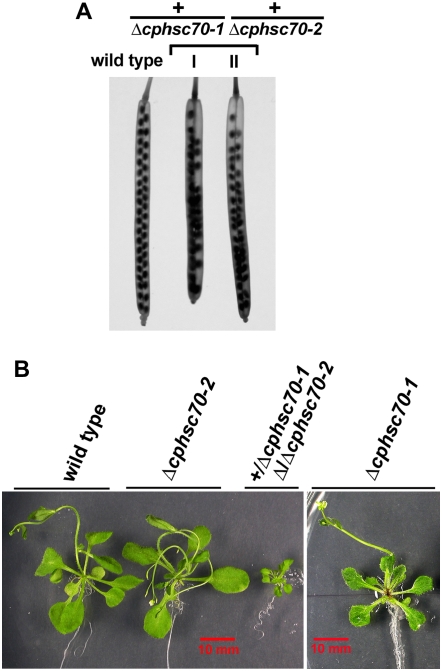
As a first step toward investigating whether cpHsc70-1 and cpHsc70-2 had different or redundant functions, we tried to generate a Δcphsc70-1 Δcphsc70-2 double mutant. As shown in Table I, in the F2 progenies of Δcphsc70-2 flowers crossed with Δcphsc70-1 pollen, no double-knockout seedling was identified. In the meantime, reduced seed sets in the siliques of F1 plants were observed (Fig. 4A). Siliques of F1 plants from a cross of the reverse direction had the same reduced seed sets (Fig. 4A). In addition, plants with the genotype +/Δcphsc70-1 Δcphsc70-2/Δcphsc70-2 were extremely small (Fig. 4B). No plants with the genotypes Δcphsc70-1/Δcphsc70-1 +/Δcphsc70-2 were identified (Table I). To confirm the absence of plants with this genotype, we crossed the Δcphsc70-1 single mutant flowers with pollen from plants with the genotype +/Δcphsc70-1 Δcphsc70-2/Δcphsc70-2 (Table II, third cross). Half of F1 progenies were expected to have the genotype Δcphsc70-1/Δcphsc70-1 +/Δcphsc70-2. However, after analyzing 104 F1 plants, all of them had the genotype heterozygous for both Δcphsc70s. This result indicated that plants with only one copy of cpHsp70-2 are not viable. This may suggest that a threshold level of cpHsc70 is critical for plant development, although the cause of this lethality is currently unknown.
Table I.
Progeny genotypes of the cross Δcphsc70-2 ♀ × Δcphsc70-1 ♂
| Generation | Gene
|
Genotypea
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cpHsc70-1 | +/+ | +/+ | +/+ | +/Δ | +/Δ | +/Δ | Δ/Δ | Δ/Δ | Δ/Δ | |
| cpHsc70-2 | +/+ | +/Δ | Δ/Δ | +/+ | +/Δ | Δ/Δ | +/+ | +/Δ | Δ/Δ | |
| F2b | Observed no. | 36 | 71 | 36 | 86 | 103 | 11 | 35 | 0 | 0 |
| Observed ratio | 1 | 1.97 | 1 | 2.39 | 2.86 | 0.31 | 0.97 | – | – | |
| Theoretical ratio | 1 | 2 | 1 | 2 | 4 | 2 | 1 | 2 | 1 | |
| F3c | Observed no. | 41 | 14 | 0 | ||||||
| Observed ratio | 1 | 0.34 | – | |||||||
| Theoretical ratio | 1 | 2 | 1 | |||||||
+, Wild-type copy of the gene; Δ, gene with the T-DNA insertion.
The parental F1 genotype is +/Δcphsc70-1 +/Δcphsc70-2. The F2 plants were from self-pollination of the F1 parents.
The parental F2 genotype is +/Δcphsc70-1 Δcphsc70-2/Δcphsc70-2. The F3 plants were from self-pollination of the F2 parents.
Figure 4.
The two cpHsc70s together are essential for plant development. A, Siliques produced by plants of the genotype +/Δcphsc70-1 +/Δcphsc70-2 showed reduced seed sets compared to the wild type. Silique I is from an F1 plant produced by crossing Δcphsc70-1 flowers with Δcphsc70-2 pollen. Silique II is from an F1 plant of a cross of the reverse direction. Siliques were bleached overnight before photographs were taken. B, A plant with the genotype +/Δcphsc70-1 Δcphsc70-2/Δcphsc70-2 exhibited severely stunted growth in comparison to Δcphsc70-1 and Δcphsc70-2 single mutants and wild type. Plants are 28-d-old plate-grown seedlings. [See online article for color version of this figure.]
Table II.
Genotypes of F1 plants from various crosses
| Cross
|
Gene
|
F1 Genotypea
|
||
|---|---|---|---|---|
| ♀ × ♂ | cpHsc70-1 |  |
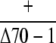 |
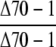 |
| cpHsc70-2 | 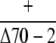 |
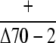 |
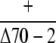 |
|
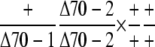 |
Observed no. | 79 | 0b | – |
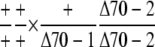 |
Observed no. | 56 | 17c | – |
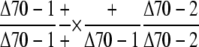 |
Observed no. | – | 104 | 0 |
+, Wild-type copy of the gene; Δ, gene with the T-DNA insertion.
No plant with the genotype +/Δcphsc70-1 +/Δcphsc70-2 was observed, indicating that no viable ovule with the genotype Δcphsc70-1 Δcphsc70-2 was produced.
The transmission efficiency of the double-knockout pollen was reduced compared to pollen with the Δcphsc70-2 single mutation.
The lethality of the double mutation was further confirmed by the absence of double mutants in the self-pollinated progenies of plants with the genotype +/Δcphsc70-1 Δcphsc70-2/Δcphsc70-2 (Table I, bottom half); no double mutant was observed even though one-quarter of the progenies was expected to be double mutants. To further analyze if the absence of the double mutant was caused by gametophytic defects, we performed reciprocal crosses of the +/Δcphsc70-1 Δcphsc70-2/Δcphsc70-2 mutant with wild type (Table II, first two crosses). Genotype analyses of F1 seedlings showed that when the +/Δcphsc70-1 Δcphsc70-2/Δcphsc70-2 mutant flowers were crossed with wild-type pollen, no plant containing the Δcphsc70-1 T-DNA insertion was obtained. This result indicated that there was no viable ovule with the double-knockout mutation. Genotype analysis of F1 seedlings from crosses of the reverse direction was expected to show 50% of the seedlings with the genotype +/+ +/Δcphsc70-2 and 50% with the genotype +/Δcphsc70-1 +/Δcphsc70-2. However, we found 56 plants with the former genotype and only 17 plants with the latter genotype. This result suggested that pollen with the double-knockout mutation had reduced transmission efficiency. These data indicated that cpHsc70s were essential for ovule development and the two cpHsc70s had at least partially redundant functions.
Effect of Individual Δcphsc70 Mutations on Chloroplast Protein Import
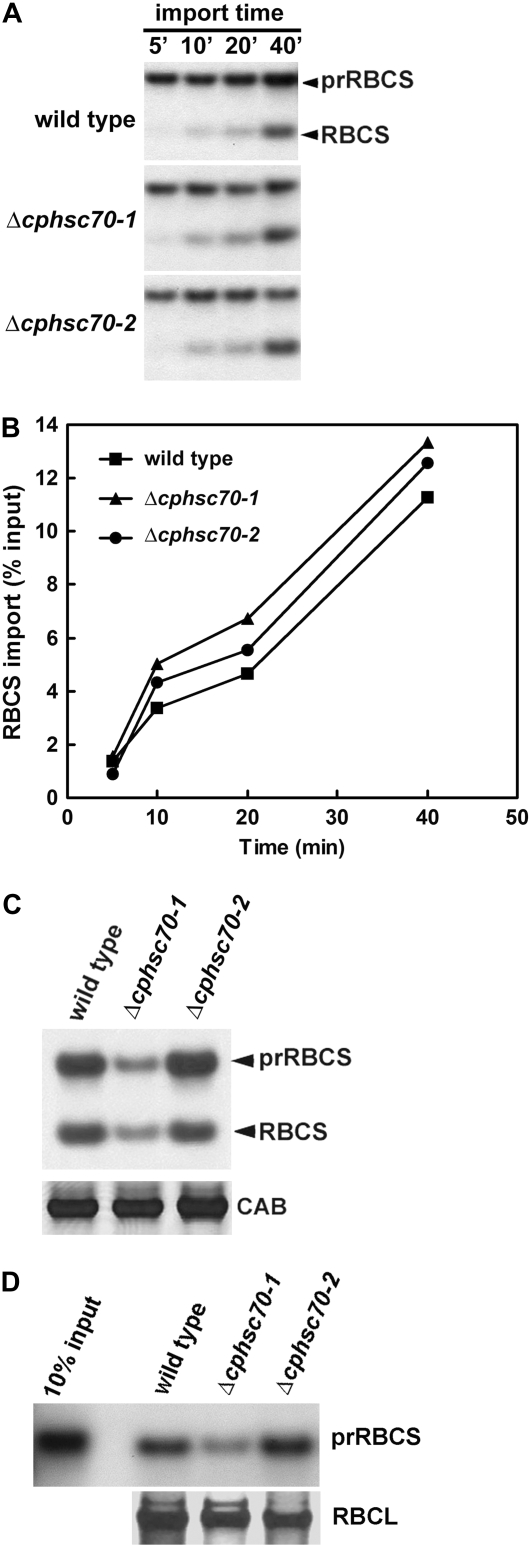
We next tested whether the Δcphsc70 knockout mutants had reduced protein import efficiency. As shown in Figure 5, A and B, chloroplasts isolated from adult mutant plants did not show impaired prRBCS import compared to the wild type. Because Δcphsc70-1 had the most apparent phenotype in cotyledons, we further compared protein import efficiency of chloroplasts isolated from 7-d-old seedlings, in which cotyledons represent the majority of green tissue. As shown in Figure 5C, Δcphsc70-1 chloroplasts had a greatly reduced amount of imported mature RBCS. However, noticeably the amount of envelope-associated prRBCS was also greatly reduced. We, therefore, further analyzed association of prRBCS with the chloroplast surface when no or little ATP was present in the import system. As shown in Figure 5D, fewer prRBCS associated with the Δcphsc70-1 mutant chloroplast surface in the absence of ATP. This result suggested that the Δcphsc70-1 mutation might have some secondary effects that caused damage to the chloroplast surface when isolated, which resulted in less efficient precursor association with the chloroplast. However, the amount of major translocon components like Toc159, Toc75, and Tic110 was not reduced in Δcphsc70-1 compared to the wild type (Supplemental Fig. S2). Therefore, the reason for the reduced precursor association of Δcphsc70-1 chloroplasts is not clear.
Figure 5.
In vitro import assays using chloroplasts isolated from mutants and wild type. A, [35S]Met-labeled prRBCS was imported into chloroplasts isolated from 30-d-old plate-grown plants. At the time point indicated, aliquots of the import reaction were removed and centrifuged through a 40% Percoll cushion. An equal amount of proteins from each sample was analyzed by SDS-PAGE and fluorography. B, Quantification of the amount of imported mature RBCS from the experiment shown in A. C, Import of [35S]Met-labeled prRBCS into chloroplast isolated from 7-d-old seedlings. Import was performed for 15 min in the light with 3 mm ATP. The amount of CAB, as determined by silver staining, was used as a loading control. D, Association of prRBCS with the chloroplast surface in the absence of ATP. Chloroplasts were isolated from 7-d-old seedlings. ATP-depleted precursors and chloroplasts were incubated in the dark for 15 min. The amount of the large subunit of Rubisco (RBCL) was used as a loading control.
cpHsc70-1 Is Important for Thermotolerance of Germinating Seeds
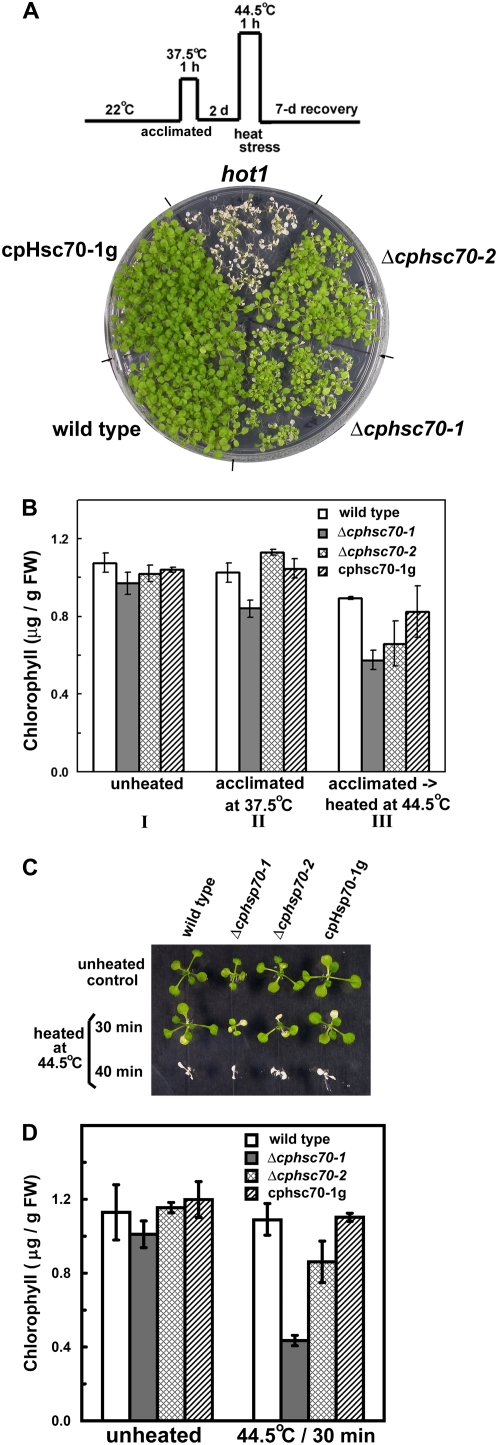
Many Hsp70s have been shown to be important for protection of organisms against heat stress. Expression of Arabidopsis cpHsc70-1 and pea stromal Hsp70 S78 were 5 and 9 times higher, respectively, after heat shock (Marshall and Keegstra, 1992; Busch et al., 2005). We, therefore, investigated if cpHsc70s were involved in plant thermotolerance. In acquired thermotolerance tests (Fig. 6A), in which plants were acclimated at 37.5°C before they underwent heat shock at 44.5°C, the survival rate of the two Δcphsc70 mutants was similar to the wild type. In comparison, the hot1 mutant (SALK_099583c), which has a T-DNA insertion in the gene-encoding cytosolic ClpB (At1g74310), was totally killed. Interestingly, both Δcphsc70-1 and Δcphsc70-2 exhibited pale-green leaves after recovery. When the chlorophyll content was determined, both mutants had a reduced amount of total chlorophylls compared to the wild-type and cpHsc70-1g plants (Fig. 6B). For basal thermotolerance tests, seedlings were directly heated at 44.5°C for 30 or 40 min, and then allowed to recover for 7 d. Almost all seedlings, mutants and wild type alike, were killed by the 40-min heat shock, but all survived the 30-min heat shock (Fig. 6C). Again the Δcphsc70 mutants had a reduced chlorophyll content after the recovery (Fig. 6D).
Figure 6.
Acquired and basal thermotolerance analyses. A, Seedlings were heated to 37.5°C for 1 h, returned to 22°C for 2 d, heated to 44.5°C for 1 h, and then allowed to recover for 7 d. B, Quantification of seedling chlorophyll contents after the 7-d recovery as shown in A. Group I did not undergo any heat treatment. Group II was only acclimated at 37.5°C for 1 h and did not undergo the 44.5°C 1-h heat shock. C, Seedlings 7-d-old were directly heated at 44.5°C for the amount of time indicated. After the heat shock treatments, seedlings were allowed to recover for 7 d. D, Quantification of chlorophyll contents of seedlings that went through the 30-min heat shock treatment in C. [See online article for color version of this figure.]
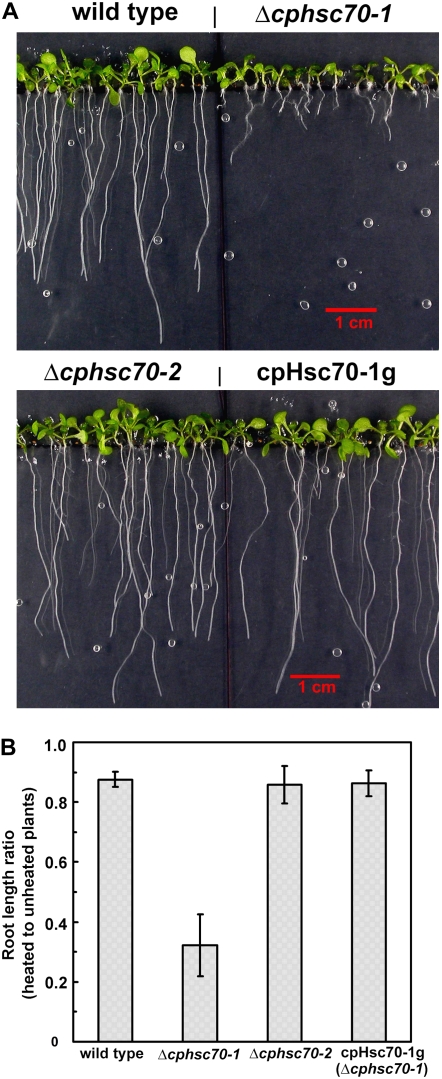
The cpHsc70-1 gene has its highest expression level in seeds (Genevestigator; Zimmermann et al., 2004). We therefore tested seed basal thermotolerance by heating imbibed seeds at 44.5°C for 150 min. We did not observe a consistent difference in the germination rate between the mutants and the wild type. However, the root growth of the Δcphsc70-1 was further impaired by the heat treatment (Fig. 7). After growing on vertical plates for 7 d, for both wild type and Δcphsc70-2, heated seeds exhibited a root growth approximately 90% of the unheated wild type and Δcphsc70-2. In contrast, root length of plants from heat-treated Δcphsc70-1 seeds was only approximately 30% of the root length of plants from unheated Δcphsc70-1 seeds (Fig. 7B). This result indicates that cpHsc70-1 is important for root growth from heat-stressed seeds.
Figure 7.
Δcphsc70-1 had reduced seed thermotolerance. A, Seeds were sown on MS plates. After a 3-d cold stratification, plates were heated at 44.5°C for 150 min. Plates were then placed vertically and seeds were allowed to germinate for 7 d. Unheated controls were the same as those shown in Figure 3C. B, Root length of plants from heated seeds as shown in A, and unheated control seeds as shown in Figure 3C, was measured. The ratio of heated to unheated plants of the same genotype was plotted. The average of two independent experiments ± sd is shown. [See online article for color version of this figure.]
DISCUSSION
Mutant phenotype analyses revealed that Δcphsc70-1 plants had the most apparent phenotypes in altered growth of cotyledons and roots, and also in basal seed thermotolerance. Interestingly, expression of cpHsc70-1 also happens to be significantly higher than cpHsc70-2 only in cotyledons, root tips and seeds (Genevestigator; Zimmermann et al., 2004). This correlation may suggest that the differences observed between the Δcphsc70-1 and Δcphsc70-2 mutants result from the different promoter activities of the two genes, rather than different functions of the two proteins. Furthermore, plants that were homozygous for Δcphsc70-1 and heterozygous for Δcphsc70-2 were not found (Tables I and II), suggesting that in the absence of cpHsc70-1, the level of cpHsc70-2 became critical for plant growth. Δcphsc70-1 Δcphsc70-2 double-knockout mutation was lethal for the development of ovules and reduced the transmission efficiency of pollen. These results support that the two cpHsc70s have overlapping essential functions. It has been suggested that this redundancy may serve as a safety net against mutations that would otherwise be lethal (Sung et al., 2001a).
However, in the Δcphsc70-1 mutant, cpHsp70-2 was up-regulated to a level higher than the two cpHsc70s combined in the wild type (Fig. 3F; Supplemental Fig. S2) and Δcphsc70-1 still showed clear growth defects. This result suggests that cpHsc70-1 may have some specific functions that cannot be substituted by cpHsc70-2. Therefore it is most likely that the two cpHsc70s have overlapping but distinct functions. The two cpHsc70s share more than a 95% identity in both the ATPase domain and the substrate-binding domain, but only a 63% identity in the C-terminal 5-kD subdomain. The C terminus of Hsc70s has been shown to be important for interacting with cochaperones like Hop (Hsp70/Hsp90 organizing protein). It is possible that the two cpHsc70s may interact with different cochaperones, which lead them to different substrates.
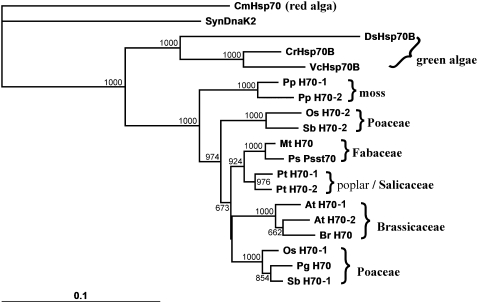
It is interesting that there are also two putative stromal Hsp70s in the fully sequenced genomes of other land plants, such as moss (Physcomitrella spp.), poplar (Populus spp.), rice (Oryza sativa), and sorghum (Sorghum bicolor; Supplemental Table S2), but green algae only harbor a single cpHsp70. A second copy of cpHsc70 may be beneficial to cope with the more stressful and variable environment on land. However, phylogenetic analyses (Fig. 8) suggested that land-plant cpHsc70s were duplicated recently during evolution because the two copies of cpHsc70s could not be grouped into two protein subfamilies among different plant families. In most cases, the two copies from the same family are more similar to each other than to cpHsc70s from other families, although the two monocot cpHsc70s have a more divergent relationship. Experiments are required to first define the elements for specific expression of the two genes and then to perform promoter swap experiments to determine the functional specificity/redundancy of the two proteins.
Figure 8.
Phylogenetic analysis of plastid Hsp70s. The amino acid sequences of plastid Hsp70s were aligned (Supplemental Fig. S3) and used to construct a gene tree by the Neighbor-Joining method with PHYLIP program version 3.67 (Felsenstein, 1989). The Dayhoff-PAM model was employed to compute the distance matrix, and Synechocystis DnaK2 (SynDnaK2) was set as an out-group. The branch length is proportional to the difference between protein sequences at the beginning and end of the branch. The bootstrap values out of 1,000 trees were given beside the branches. Syn, Synechocystis sp.; At, Arabidopsis; Br, Brassica rapa; Cm, Cyanidioschyzon merolae; Cr, Chlamydomonas reinhardtii; Ds, Dunaliella salina; Mt, Medicago truncatula; Os, Oryza sativa; Pg, Pennisetum glaucum; Pp, Physcomitrella patens; Ps, Pisum sativum; Pt, Populus trichocarpa; Sb, Sorghum bicolor; Vc, Volvox carteri. More information on accession numbers and sources of sequences is given in Supplemental Table S2.
The isolation of the cpHsc70-knockout mutants enabled us to directly test if plastid Hsp70s was involved in chloroplast protein import. Mature chloroplasts isolated from either single Δcphsc70-knockout mutant showed no import defect. Under normal import conditions, both the amount of precursor bound and the amount of mature protein imported were reduced in chloroplasts isolated from cotyledons of Δcphsc70-1 (Fig. 5C). For isolated Arabidopsis chloroplasts, most of the precursor proteins that were still bound to the envelope after a 20- to 30-min import could not be imported even after prolonged incubation and were thought to represent nonspecific sticking of precursors on the chloroplast surface (Fitzpatrick and Keegstra, 2001; data not shown). Indeed, in all translocon mutants reported so far, only the amount of imported mature proteins, but not the amount of envelope-bound precursor proteins, was reduced (Jarvis et al., 1998; Sun et al., 2001; Kubis et al., 2003; Constan et al., 2004; Hung et al., 2004; Teng et al., 2006; Kovacheva et al., 2007). Hence, there seem to be some differences between the surface of isolated Δcphsc70-1 cotyledon chloroplasts and that of other mutant chloroplasts. Indeed, when analyzed under conditions in which almost no ATP was present, precursor association with the mutant chloroplasts was reduced. Therefore, it is likely that defects in chloroplast physiology had resulted in damage to the surface of Δcphsc70-1 cotyledon chloroplasts when isolated. More informative results on the function of cpHsp70 in chloroplast protein import await conditional double knockout or extreme double knockdown of the cpHsc70s. Another chaperone, Hsp100 (ClpC), has been shown to stably associate with the translocon complex (Akita et al., 1997; Nielsen et al., 1997). In addition, Cpn60 also has been found in isolated protein-import complexes (Kessler and Blobel, 1996). Therefore, it would be interesting to test whether multiple chaperones are involved in chloroplast protein transport across the inner membrane, with each chaperone assisting the import of certain precursors or under certain conditions.
Heat shock proteins have been shown to play important roles in helping cells to cope with environmental stress. The cytosolic Hsp100/ClpB plays major roles in acquired thermotolerance (Hong and Vierling, 2000, 2001; Nieto-Sotelo et al., 2002; Hong et al., 2003; Katiyar-Agarwal et al., 2003). Cytosolic Hsp70 and plastid ClpB and small heat shock proteins have also been implicated in heat sensitivity of plants (Lee and Schöffl, 1996; Sung and Guy, 2003; Wang and Luthe, 2003; Miroshnichenko et al., 2005; Myouga et al., 2006; Yang et al., 2006; Lee et al., 2007). However, no such role has been proposed for plastid Hsp70s. Here we have provided genetic evidence to show that Hsp70s in the plastid stroma are important for thermotolerance of germinating seeds, indicating that plastid physiology is important for seeds to endure heat stress. Plastids are involved in the assimilation of nitrogen and in the syntheses of lipids and many hormones, which are important for seed germination and root growth. It is therefore reasonable to have chaperones protecting the biosynthesis enzymes during heat stress. Accumulation of toxic intermediates due to heat inactivation of the enzymes may also have contributed to the inhibition of root growth observed. For acquired and basal thermotolerance in seedlings, we could not detect a difference in the survival rate between the mutants and the wild type. However, both Δcphsc70 mutants had reduced chlorophyll contents during heat shock recovery (Fig. 6, B and D). These results suggest that cpHsc70s may play a role in protection of the photosystems during heat shock and/or recovery. In green algae, the chloroplast Hsp70B has been shown to participate in the protection and repair of the PSII during and after photoinhibition (Schroda et al., 1999; Yokthongwattana et al., 2001). Therefore the involvement of stromal Hsp70s in the maintenance of chloroplast photosystems during stress conditions may be a general mechanism in plants. The mild phenotypes in seedling thermotolerance of the Arabidopsis Δcphsc70 mutants could be due to the presence of two homologous genes. It would be interesting to reduce the total level of cpHsc70s by antisense or RNAi techniques, coupled with inducible promoters to circumvent the essential nature of the proteins to further analyze the function of stromal Hsp70s in stress protection, chloroplast protein import, thylakoid formation, or even other novel functions.
MATERIALS AND METHODS
Plant Growth Conditions
All Arabidopsis (Arabidopsis thaliana) plants used in this study were of the ecotype Columbia. Sterilized Arabidopsis seeds were plated on 0.3% Gelrite-solidified 1× Murashige and Skoog (MS) medium containing Gamborg's B5 vitamin and 0.5% Suc. After a 3-d cold stratification, seeds were grown in growth chambers under a 16-h photoperiod with a light intensity approximately 80 μmol m−2 s−1 at 22°C. Soil-grown Arabidopsis plants were grown on a 9:1:1 mixture of peat, vermiculite, and perlite at 22°C under a constant light with an intensity approximately 120 μmol m−2 s−1, except all plants used for cross-experiments were grown on soil to maturity under a 16-h photoperiod with a light intensity approximately 120 μmol m−2 s−1 at 22°C. For growing pea seedlings (Pisum sativum ‘Little Marvel’), imbibed seeds were grown on vermiculite for 9 to 12 d under a 12-h photoperiod at 20°C with a light intensity of approximately 150 μmol m−2 s−1.
Identification of Δcphsc70-1 and Δcphsc70-2 T-DNA Insertion Mutant Lines
Mutant lines SALK_140810 (Δcphsc70-1), SALK_095715 (Δcphsc70-2), and SALK_099583c (hot1) were obtained from the SALK T-DNA collections (Alonso et al., 2003) via the Arabidopsis Biological Resource Center; they were screened by PCR amplification of genomic DNA because Δcphsc70-1 had lost kanamycin resistance and Δcphsc70-2 showed heterogeneous growth on kanamycin plates. Primers used for amplifying the wild-type copy of cpHsc70-1 were 1E2-S (5′-gattcacagaggacagctac-3′) and 1E8-AS (5′-tgaatctcctgatgaagcac-3′). For amplifying the wild-type copy of cpHsc70-2, the primers used were 2E1-S (5′-gtcctttcactactccaacc-3′) and 2E8-AS (5′-ctgaggatgatgtgtcagac-3′). To identify the T-DNAs inserted in cpHsc70-1 and cpHsc70-2, the SALK Lba1 primer (5′-tggttcacgtagtgggccatcg-3′) was combined with 1E8-AS and 2E8-AS, respectively. The T-DNA insertion sites were verified by sequencing the PCR products. Primer pairs used for checking cpHsc70-1 and cpHsc70-2 transcripts by RT-PCR were 1E2-S + 1E8-AS and 2E1-S + 2E8-AS, respectively. To test the expression of the cpHsc70 protein in each mutant, total stromal proteins were analyzed in IEF gels (pI 3–7) and immunoblots decorated with the anti-S78 antibody.
Vector Construction and Plant Transformation
Using genomic DNA extracted from wild-type Arabidopsis seedlings as the template, a 5-kb genomic fragment containing the promoter and coding regions of cpHsc70-1 was amplified by PCR with primers H70A-PS (5′-aaaagctttcgtaaaggcttgtaagc-3′) and H70At-AS (5′-gaaggtagatggtcaccggtgaagcgag-3′). The PCR product was first cloned into the pGEM-T vector (Promega), and then subcloned into the binary vector pCambia1390. The resulting plasmid was named pCambia1390/cpHsc70-1g and was transformed into Agrobacterium GV3101. The Δcphsc70-1 mutant plants were infected by the floral spray method (Chung et al., 2000). Transgenic plants harboring the introduced cpHsc70-1 genomic fragments were screened on MS media containing 50 mg L−1 hygromycin.
In Vitro Translation and Protein Import Assays
T3 promoter fused cpHsc70-1 and cpHsc70-2 linear DNAs were amplified by PCR with pfu DNA polymerase (Stratagene) using plasmids containing full-length cDNA as templates and primers containing the T3 promoter sequence. Primers used for amplifying T3:cpHsc70-1 were 5′-ccaattaaccctcactaaagggagccaccatggcatcttcagccgcccaa-3′ and 5′-gtctctattggctgtctgtgaagtcag-3′. For amplifying T3:cpHsc70-2, the primers used were 5′-ccaattaaccctcactaaagggattctcatttccaaccatgg-3′ and 5′-ttctctaattgctgtctgtgaagtcag-3′. [35S]Met-labeled precursors of cpHsc70-1 and cpHsc70-2 were in vitro translated in a TNT reticulocyte lysate system (Promega), with the addition of PCR-generated T3:cpHsc70-1 and T3:cpHsc70-2 DNA templates. [35S]Met-prRBCS synthesis, pea and Arabidopsis chloroplast isolation, and import assays were conducted as described (Perry et al., 1991), except the grinding buffer for Arabidopsis chloroplast isolation was modified to 50 mm HEPES-KOH (pH 8.0), 330 mm sorbitol, 2 mm EDTA, and 0.5% bovine serum albumin. Trypsin digestion of chloroplasts and separation of chloroplasts after digestion into soluble and membrane fractions were performed as described (Jackson et al., 1998; Hung et al., 2004). For import in the absence of ATP (Fig. 5D), ATP was removed from the in vitro-translated precursors by gel filtration (Perry et al., 1991) and chloroplasts were depleted of internal ATP by incubation in the dark at 4°C for 1 h. Import was performed under a green-safe light at room temperature for 15 min. Quantification of gel bands was performed using the Fuji FLA5000 phosphoimager (Fujifilm). Antibodies against Tic and Toc proteins were prepared as described (Chou et al., 2003; Tu et al., 2004).
Heat-Stress Treatments and Measurement of Chlorophylls
Thermotolerance assays of seeds and seedlings on plates were performed according to Charng et al. (2006) with minor modifications. To make the heat treatment more uniform, heat shock was conducted by heating tape-sealed agar plate in water bath in the dark. Plates were then cooled down to room temperature by floating on tap water, and kept in the dark at room temperature for 2 h to avoid possible additional effects of light stress. Seedlings were allowed to recover for an additional 7 d in the original growth conditions prior to calculating the survival rate. Plants that were still green and producing new leaves were scored as survived. For the acquired thermotolerance test, 5- to 7-d-old seedlings were initially acclimated at 37.5°C for 1 h and returned to the original growth condition for 2 d, then challenged at the lethal temperature 44.5°C for 1 h. Total chlorophyll was determined by the method of Lichtenthaler (1987). Root length was measured using pictures of seedlings and the software ImageJ (National Institutes of Health image).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AL161561 (cpHsc70-1) and AB024032 (cpHsc70-2).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Genomic Southern analyses of the T-DNA in Δcphsc70 mutants.
Supplemental Figure S2. Δcphsc70-1 had a higher amount of cpHsc70-2 but a normal amount of chloroplast translocon proteins.
Supplemental Figure S3. Alignment of the amino acid sequences of plastid Hsp70s used for constructing the phylogenetic tree.
Supplemental Table S1. Progeny segregation of the heterozygous Δcphsc70-1 mutant.
Supplemental Table S2. Plastid Hsp70s used for the phylogenetic analysis.
Supplementary Material
Acknowledgments
We thank Dr. Chung-Yen Lin for advice on phylogenetic analyses, Dr. Ken Keegstra for the antibody against S78, Dr. Hwa Dai for the monoclonal antibody against maize mitochondrial porin, and Dr. Neil Hoffman for the antibody against CAB. We thank the Arabidopsis Biological Resource Center and the Salk Institute for providing the Δcphsc70-1, Δcphsc70-2, and hot1 mutants. We also thank Dr. Harry Wilson for English editing, Dr. Yi-Fang Tsay for helpful discussions, and Drs. Shih-Long Tu, Yee-yung Charng, and Tien-Shin Yu for critical reading of the manuscript.
This work was supported by the National Science Council (grant no. NSC–95–2321–B–001–004 to H.M.L.) and the Academia Sinica of Taiwan.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Hsou-min Li (mbhmli@gate.sinica.edu.tw).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Akita M, Nielsen E, Keegstra K (1997) Identification of protein transport complexes in the chloroplastic envelope membranes via chemical cross-linking. J Cell Biol 136 983–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 [DOI] [PubMed] [Google Scholar]
- Alvim FC, Carolino SM, Cascardo JC, Nunes CC, Martinez CA, Otoni WC, Fontes EP (2001) Enhanced accumulation of BiP in transgenic plants confers tolerance to water stress. Plant Physiol 126 1042–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S, Johnson M, Bridgham J, Golda G, Lloyd DH, Johnson D, Luo S, McCurdy S, Foy M, Ewan M, et al (2000) Gene expression analysis by massively parallel signature sequencing (MPSS) on microbead arrays. Nat Biotechnol 18 630–634 [DOI] [PubMed] [Google Scholar]
- Busch W, Wunderlich M, Schöffl F (2005) Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. Plant J 41 1–14 [DOI] [PubMed] [Google Scholar]
- Charng YY, Liu HC, Liu NY, Hsu FC, Ko SS (2006) Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation. Plant Physiol 140 1297–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou ML, Fitzpatrick LM, Tu SL, Budziszewski G, Potter-Lewis S, Akita M, Levin JZ, Keegstra K, Li HM (2003) Tic40, a membrane-anchored co-chaperone homolog in the chloroplast protein translocon. EMBO J 22 2970–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MH, Chen MK, Pan SM (2000) Floral spray transformation can efficiently generate Arabidopsis transgenic plants. Transgenic Res 9 471–476 [DOI] [PubMed] [Google Scholar]
- Constan D, Froehlich JE, Rangarajan S, Keegstra K (2004) A stromal Hsp100 protein is required for normal chloroplast development and function in Arabidopsis. Plant Physiol 136 3605–3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J (1989) PHYLIP—phylogeny inference package (version 3.2). Cladistics 5 164–166 [Google Scholar]
- Fitzpatrick LM, Keegstra K (2001) A method for isolating a high yield of Arabidopsis chloroplasts capable of efficient import of precursor proteins. Plant J 27 59–65 [DOI] [PubMed] [Google Scholar]
- Gutensohn M, Fan E, Frielingsdorf S, Hanner P, Hou B, Hust B, Klösgen RB (2006) Toc, Tic, Tat et al.: structure and function of protein transport machineries in chloroplasts. J Plant Physiol 163 333–347 [DOI] [PubMed] [Google Scholar]
- Guy CL, Li QB (1998) The organization and evolution of the spinach stress 70 molecular chaperone gene family. Plant Cell 10 539–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SW, Lee U, Vierling E (2003) Arabidopsis hot mutants define multiple functions required for acclimation to high temperatures. Plant Physiol 132 757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SW, Vierling E (2000) Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc Natl Acad Sci USA 97 4392–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SW, Vierling E (2001) Hsp101 is necessary for heat tolerance but dispensable for development and germination in the absence of stress. Plant J 27 25–35 [DOI] [PubMed] [Google Scholar]
- Hung WF, Chen LJ, Boldt R, Sun CW, Li Hm (2004) Characterization of Arabidopsis glutamine phosphoribosyl pyrophosphate amidotransferase-deficient mutants. Plant Physiol 135 1314–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey RA, Subramanian C, Bruce BD (2000) Identification of a Hsp70 recognition domain within the Rubisco small subunit transit peptide. Plant Physiol 122 1289–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DT, Froehlich JE, Keegstra K (1998) The hydrophilic domain of Tic110, an inner envelope membrane component of the chloroplastic protein translocation apparatus, faces the stromal compartment. J Biol Chem 273 16583–16588 [DOI] [PubMed] [Google Scholar]
- Jarvis P, Chen LJ, Li Hm, Peto CA, Fankhauser C, Chory J (1998) An Arabidopsis mutant defective in the plastid general protein import apparatus. Science 282 100–103 [DOI] [PubMed] [Google Scholar]
- Katiyar-Agarwal S, Agarwal M, Grover A (2003) Heat-tolerant basmati rice engineered by over-expression of hsp101. Plant Mol Biol 51 677–686 [DOI] [PubMed] [Google Scholar]
- Kessler F, Blobel G (1996) Interaction of the protein import and folding machineries in the chloroplast. Proc Natl Acad Sci USA 93 7684–7689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtz L, Ko K (1997) The early stage of chloroplast protein import involves Com70. J Biol Chem 272 2808–2813 [DOI] [PubMed] [Google Scholar]
- Kovacheva S, Bédard J, Wardle A, Patel R, Jarvis P (2007) Further in vivo studies on the role of the molecular chaperone, Hsp93, in plastid protein import. Plant J 50 364–379 [DOI] [PubMed] [Google Scholar]
- Kubis S, Baldwin A, Patel R, Razzaq A, Dupree P, Lilley K, Kurth J, Leister D, Jarvis P (2003) The Arabidopsis ppi1 mutant is specifically defective in the expression, chloroplast import, and accumulation of photosynthetic proteins. Plant Cell 15 1859–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leborgne-Castel N, Jelitto-Van Dooren EP, Crofts AJ, Denecke J (1999) Overexpression of BiP in tobacco alleviates endoplasmic reticulum stress. Plant Cell 11 459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Schöffl F (1996) An Hsp70 antisense gene affects the expression of HSP70/HSC70, the regulation of HSF, and the acquisition of thermotolerance in transgenic Arabidopsis thaliana. Mol Gen Genet 252 11–19 [DOI] [PubMed] [Google Scholar]
- Lee U, Rioflorido I, Hong SW, Larkindale J, Waters ER, Vierling E (2007) The Arabidopsis ClpB/Hsp100 family of proteins: chaperones for stress and chloroplast development. Plant J 49 115–127 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148 350–382 [Google Scholar]
- Liu C, Willmund F, Golecki JR, Cacace S, Hess B, Markert C, Schroda M (2007) The chloroplast HSP70B-CDJ2-CGE1 chaperones catalyse assembly and disassembly of VIPP1 oligomers in Chlamydomonas. Plant J 50 265–277 [DOI] [PubMed] [Google Scholar]
- Liu C, Willmund F, Whitelegge JP, Hawat S, Knapp B, Lodha M, Schroda M (2005) J-domain protein CDJ2 and HSP70B are a plastidic chaperone pair that interacts with vesicle-inducing protein in plastids 1. Mol Biol Cell 16 1165–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JS, Keegstra K (1992) Isolation and characterization of a cDNA clone encoding the major Hsp70 of the pea chloroplastic stroma. Plant Physiol 100 1048–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroshnichenko S, Tripp J, Nieden U, Neumann D, Conrad U, Manteuffel R (2005) Immunomodulation of function of small heat shock proteins prevents their assembly into heat stress granules and results in cell death at sublethal temperatures. Plant J 41 269–281 [DOI] [PubMed] [Google Scholar]
- Myouga F, Motohashi R, Kuromori T, Nagata N, Shinozaki K (2006) An Arabidopsis chloroplast-targeted Hsp101 homologue, APG6, has an essential role in chloroplast development as well as heat-stress response. Plant J 48 249–260 [DOI] [PubMed] [Google Scholar]
- Nielsen E, Akita M, Davila-Aponte J, Keegstra K (1997) Stable association of chloroplastic precursors with protein translocation complexes that contain proteins from both envelope membranes and a stromal Hsp100 molecular chaperone. EMBO J 16 935–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Sotelo J, Martinez LM, Ponce G, Cassab GI, Alagon A, Meeley RB, Ribaut JM, Yang R (2002) Maize HSP101 plays important roles in both induced and basal thermotolerance and primary root growth. Plant Cell 14 1621–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SE, Li Hm, Keegstra K (1991) In vitro reconstitution of protein transport into chloroplasts. Methods Cell Biol 34 327–344 [DOI] [PubMed] [Google Scholar]
- Pilon M, Schekman R (1999) Protein translocation: how Hsp70 pulls it off. Cell 97 679–682 [DOI] [PubMed] [Google Scholar]
- Rial DV, Arakaki AK, Almara AM, Orellano EG, Ceccarelli EA (2006) Chloroplast Hsp70s are not involved in the import of ferredoxin-NADP(+) reductase precursor. Physiol Plant 128 618–632 [Google Scholar]
- Rial DV, Ottado J, Ceccarelli EA (2003) Precursors with altered affinity for Hsp70 in their transit peptides are efficiently imported into chloroplasts. J Biol Chem 278 46473–46481 [DOI] [PubMed] [Google Scholar]
- Schnell DJ, Kessler F, Blobel G (1994) Isolation of components of the chloroplast protein import machinery. Science 266 1007–1012 [DOI] [PubMed] [Google Scholar]
- Schroda M, Vallon O, Wollman FA, Beck CF (1999) A chloroplast-targeted heat shock protein 70 (HSP70) contributes to the photoprotection and repair of photosystem II during and after photoinhibition. Plant Cell 11 1165–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strub A, Lim JH, Pfanner N, Voos W (2000) The mitochondrial protein import motor. Biol Chem 381 943–949 [DOI] [PubMed] [Google Scholar]
- Sun CW, Chen LJ, Lin LC, Li Hm (2001) Leaf-specific upregulation of chloroplast translocon genes by a CCT motif-containing protein, CIA 2. Plant Cell 13 2053–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung DY, Guy CL (2003) Physiological and molecular assessment of altered expression of Hsc70-1 in Arabidopsis. Evidence for pleiotropic consequences. Plant Physiol 132 979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung DY, Kaplan F, Guy CL (2001. a) Plant Hsp70 molecular chaperones: protein structure, gene family, expression and function. Physiol Plant 113 443–451 [Google Scholar]
- Sung DY, Vierling E, Guy CL (2001. b) Comprehensive expression profile analysis of the Arabidopsis Hsp70 gene family. Plant Physiol 126 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YS, Su YS, Chen LJ, Lee YJ, Hwang I, Li Hm (2006) Tic21 is an essential translocon component for protein translocation across the chloroplast inner envelope membrane. Plant Cell 18 2247–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufighi K, Brady SM, Austin R, Ly E, Provart NJ (2005) The botany array resource: e-northerns, expression angling, and promoter analyses. Plant J 43 153–163 [DOI] [PubMed] [Google Scholar]
- Tu SL, Chen LJ, Smith MD, Su YS, Schnell DJ, Li Hm (2004) Import pathways of chloroplast interior proteins and the outer-membrane protein OEP14 converge at Toc75. Plant Cell 16 2078–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Luthe DS (2003) Heat sensitivity in a bentgrass variant. Failure to accumulate a chloroplast heat shock protein isoform implicated in heat tolerance. Plant Physiol 133 319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JY, Sun Y, Sun AQ, Yi SY, Qin J, Li MH, Liu J (2006) The involvement of chloroplast HSP100/ClpB in the acquired thermotolerance in tomato. Plant Mol Biol 62 385–395 [DOI] [PubMed] [Google Scholar]
- Yokthongwattana K, Chrost B, Behrman S, Casper-Lindley C, Melis A (2001) Photosystem II damage and repair cycle in the green alga Dunaliella salina: involvement of a chloroplast-localized HSP70. Plant Cell Physiol 42 1389–1397 [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol 136 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.