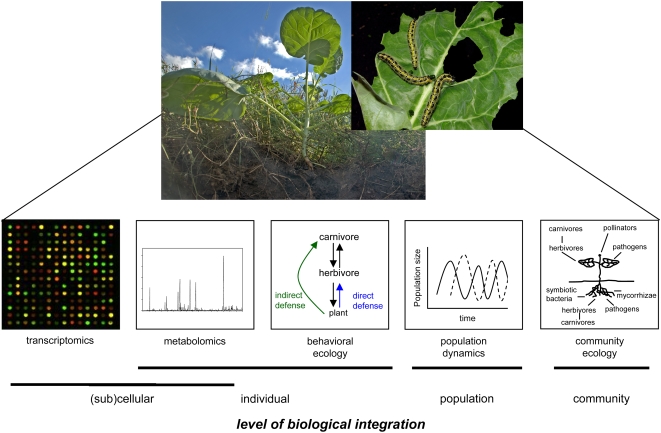
A major challenge for current biology is to integrate research approaches that address different levels of biological organization, from subcellular mechanisms to functions in ecological communities. The study of plant-insect interactions provides interesting options for this. Ample information at the subcellular and the individual level is available on the one hand, while important insight in the (community) ecology of insect-plant interactions is available as well (Kessler and Baldwin, 2002; Bezemer and van Dam, 2005; Schoonhoven et al., 2005; Kessler and Halitschke, 2007; Snoeren et al., 2007). A major step forward will be to connect these research fields, and encouraging steps have been made during the past years.
Insects make up the most diverse and abundant group of plant consumers. A total of 45% of the approximately 1 million described insect species feed on plants (Schoonhoven et al., 2005). Given that the estimated number of insects species is several times higher (Stork, 2007), the number of herbivorous insect species is likely to be much higher too. Herbivorous insects may attack plants below ground as well as above ground, and not a single organ remains free of potential insect attack (Bezemer and van Dam, 2005; Schoonhoven et al., 2005). Plants have evolved a range of defenses to ward off this diversity of attackers, including constitutive and induced defenses (Walling, 2000; Kessler and Baldwin, 2002; Schoonhoven et al., 2005). Because an individual plant may potentially be under the attack of tens or hundreds of consumer species, it is impossible to have constitutive defenses against all these attackers. Furthermore, whether the potential enemies will indeed attack a certain individual is usually unpredictable. Moreover, constitutive defenses may also select for adaptation in herbivorous insects (Agrawal and Karban, 1999). Thus, inducible defenses may tune the defensive needs to the actual presence of attackers and in addition may transform plants into moving fortresses that have a modified phenotype and consequently retard adaptation in herbivores (Agrawal and Karban, 1999; Kahl et al., 2000). For instance, Nicotiana attenuata plants respond to mechanical damage with the induction of nicotine, a neurotoxin affecting most herbivores (Kahl et al., 2000). However, the plant has an attenuated nicotine induction in response to feeding damage by caterpillars of the specialist tobacco hornworm Manduca sexta, which can tolerate considerable levels of nicotine and furthermore can exploit it in its own defense against pathogens and parasitic wasps (Krischik et al., 1988). Instead, N. attenuata responds to Manduca feeding with the production of volatile terpenoids that can attract parasitic wasps that attack the caterpillars (Kahl et al., 2000).
Induced defenses comprise direct defenses, such as secondary metabolites and protease inhibitors that negatively affect herbivore growth and survival, as well as indirect defenses, such as herbivore-induced plant volatiles and herbivore-induced extrafloral nectar that enhance the effectiveness of natural enemies of herbivores, such as parasitoids or predators (Kessler and Baldwin, 2002; Heil et al., 2004; Kappers et al., 2005; D'Alessandro and Turlings, 2006; Mumm and Hilker, 2006). The induction of defenses is often specific for the attacker species (Kahl et al., 2000; Dicke et al., 2003; Arimura et al., 2005) and an individual plant can therefore express a range of different phenotypes where each phenotype has its own effects on the members of the community, such as herbivores, carnivores, and pollinators (Dicke et al., 2004; Kessler et al., 2004; Kessler and Halitschke, 2007; Bruinsma and Dicke, 2008). For instance, previous attack may influence the induction of subsequent defenses in specific ways and thereby affect herbivore and plant fitness (Kessler and Baldwin, 2004; Voelckel and Baldwin, 2004b). As a consequence, the investigation of induced defenses requires an approach that considers the total community as well as individual interactions among community members (Kessler and Baldwin, 2004; Kessler et al., 2004; Bruinsma and Dicke, 2008). Integrating this approach at different levels of biological organization is a major challenge that will link mechanisms in terms of transcriptional induction, metabolite induction, and ecological interactions (Mercke et al., 2004). After identifying genes that are involved in specific interactions, their ecological function should be investigated in in-depth studies addressing e.g. genotypes in which the gene of interest has been silenced (Paschold et al., 2007). In doing so, gene function is investigated at different levels of biological integration, and this provides novel insight into the ecological function of particular genes (Fig. 1).
Figure 1.
Individual interactions such as those between Brassica plants and P. brassicae caterpillars result in effects at different levels of biological integration, from subcellular processes to community dynamics, and from the expression of genes involved in biosynthesis of induced plant volatiles to effects on attraction of parasitic wasps and changes in community dynamics. Pictures courtesy of Hans M. Smid, www.bugsinthepicture.com. [See online article for color version of this figure.]
SIGNAL TRANSDUCTION
Induced defenses involve phytohormone-mediated signal transduction that links the damage with the phenotypic change in the plant. There are three main signal transduction pathways that underlie induced defenses, i.e. the jasmonate pathway, the shikimate pathway, and the ethylene (ET) pathway, characterized by the phytohormones jasmonic acid (JA), salicylic acid (SA), and ET, respectively (Dicke and Van Poecke, 2002; Kessler and Baldwin, 2002). Of these pathways, the jasmonate pathway seems to play a dominant role in insect-induced and wound-induced plant responses both in terms of direct and indirect defense (Dicke and Van Poecke, 2002; Kessler and Baldwin, 2002; Howe, 2004; Thines et al., 2007). For instance, jasmonates have been shown to be induced by M. sexta caterpillars in native tobacco plants (Kahl et al., 2000), by the spider mite Tetranychus urticae in tomato (Solanum lycopersicum; Li et al., 2002), and by Pieris rapae caterpillars or Frankliniella occidentalis thrips in Arabidopsis (Arabidopsis thaliana; Reymond et al., 2004; De Vos et al., 2005), and JA induces direct and/or indirect defenses in these plants (e.g. Thaler, 1999; Kessler and Baldwin, 2002; Reymond et al., 2004). Yet, not all herbivores induce JA in plants: e.g. the silverleaf whitefly Bemisia tabaci suppresses JA-dependent defenses and induces SA-dependent defenses (Kempema et al., 2007). Individual attackers appear to elicit distinct phytohormone signatures consisting of dynamic phytohormone induction patterns (De Vos et al., 2005).
GLOBAL TRANSCRIPTOME CHANGES IN RESPONSE TO HERBIVORY
In response to insect herbivory, plants undergo an extensive rearrangement of gene transcription. Hundreds, up to several thousands, of genes can be up- or down-regulated. The number of studies addressing large-scale transcriptomic changes in response to insect herbivory is steadily increasing (e.g. Heidel and Baldwin, 2004; Reymond et al., 2004; Voelckel and Baldwin, 2004a; De Vos et al., 2005; Bodenhausen and Reymond, 2007; Broekgaarden et al., 2007; Kempema et al., 2007). This provides extensive new information about the first steps of plant responses to insect attack.
It appears that there can be considerable differences in the transcriptomic response of a plant to different attackers (Voelckel and Baldwin, 2004a; De Vos et al., 2005) as well as responses of different plant cultivars in response to the same herbivore (Broekgaarden et al., 2007). For instance, damage by cell content-feeding thrips (F. occidentalis) and chewing-biting caterpillars (P. rapae) results in similar numbers of differentially expressed genes (around 200). Yet, the gene sets differentially responding to these two herbivore species are quite different: of the JA-responsive genes, only 9% to 17% show similar responses to F. occidentalis and P. rapae (De Vos et al., 2005). Even in response to insects with a similar feeding mode, such as aphids (Myzus persicae) and whiteflies (B. tabaci), the transcriptomic changes can be quite different (Kempema et al., 2007). Plant genotypes can also differ in transcriptional responses to the same herbivore. Two white cabbage (Brassica oleracea var. capitata) cultivars differ considerably in the global gene expression patterns induced by P. rapae caterpillar attack (Broekgaarden et al., 2007). The two cultivars also differ in the level of direct defense against caterpillar (P. rapae) feeding. Several of the transcriptomic differences involve genes that may have an impact on P. rapae performance and may underlie the different mechanisms of direct defense present in the two cultivars. Linking studies at the level of transcriptomic changes with studies on metabolite production and expression of different levels of resistance will provide new insight into the mechanisms underlying variation in ecological interactions.
In Arabidopsis, mechanical damage induces a different transcript profile than P. rapae feeding (Reymond et al., 2000). Many wound-induced genes were induced either to a lesser extent or not at all by larval feeding, thus indicating that larval feeding strategies may minimize the induction of defense-related genes (Reymond et al., 2000). In contrast, similar expression patterns in response to feeding by both specialist P. rapae and generalist Spodoptera littoralis caterpillars have been found (Reymond et al., 2004). However, the transcript profiles were different in ET and SA mutants (Bodenhausen and Reymond, 2007). Native tobacco plants, N. attenuata, are able to distinguish between attack by two generalist (S. littoralis and Heliothis virescens) and a specialist (M. sexta) herbivore of the same feeding guild (Voelckel and Baldwin, 2004a). Despite the large overlap in transcriptional responses to all three lepidopteran larvae, these plants responded more similarly to attack from the two generalists than to attack from the specialist. The fatty acid-amino acid conjugate profiles of S. littoralis and H. virescens regurgitates are almost identical, but M. sexta regurgitate differs from the others and is dominated by fatty acid-Glu conjugates (Voelckel and Baldwin, 2004a). It is interesting to note that attack of Arabidopsis by the phloem-feeding aphid M. persicae and the silverleaf whitefly B. tabaci, which inflict relatively low levels of cell damage, results in the differential expression of many more genes than feeding by the chewing-biting caterpillar P. rapae, even when one should be careful in quantitatively comparing studies made in different laboratories (De Vos et al., 2005; Kempema et al., 2007).
Full transcriptomic analyses provide important insight into overall changes in gene expression, usually at a limited number of time points. Yet, quantitative real-time PCR can yield detailed dynamics of transcriptomic changes for a limited number of genes of particular interest (e.g. Zheng et al., 2007). Such analyses are of special interest to ecologists as they can provide information on the phenotypic plasticity in the expression of specific genes of interest that can be linked to the dynamics of interactions of the plant with members of the community.
EFFECTS OF A FIRST ATTACK ON SUBSEQUENT BIOTIC INTERACTIONS
In nature, plants are seldom attacked by a single herbivore species and most likely they are challenged by different herbivores and pathogens sequentially or simultaneously. After having been ignored for a long time, there is a renewed appreciation of the occurrence of plant-mediated competition between herbivorous insects and between insects and pathogens (Kaplan and Denno, 2007). However, investigations on the mechanisms underlying the effects of defenses induced by a first attack on other members of the community have so far been limited. Linking the ecological phenomena of community effects of plant infestation with mechanical information is now in reach with a possibility to understand ecological phenomena in the context of their mechanistic background.
Peanut (Arachis hypogaea) plants that are infected with the white mold fungus Sclerotium rolfsii are preferred by beet armyworm moths (Spodoptera exigua) for oviposition. However, beet armyworm moth larval feeding on fungus-infected plants results in enhanced risks of attack by parasitic wasps; volatiles emitted from plants infested with fungus plus caterpillars were more attractive than volatiles emitted from plants infested only with caterpillars (Cardoza et al., 2003). Arabidopsis plants infested by the caterpillar P. rapae are more resistant to several plant pathogens, including Turnip crinkle virus (De Vos et al., 2006). This was surprising because resistance to Turnip crinkle virus is dependent on SA, while P. rapae induces JA and ET but does not induce SA. It appeared that caterpillar-induced ET primed Arabidopsis for augmented expression of SA-dependent defenses (De Vos et al., 2006). Tomato plants damaged by aphids (Macrosiphum euphorbiae) were preferred for oviposition by beet armyworm moths, and the larvae consumed more on aphid-infested plants than on uninfested control plants (Rodriguez-Saona et al., 2005). Treatment of cultivated tomato plants with the phytohormone JA (as a mimic of herbivory) early in the season had long-lasting effects on community dynamics under field conditions (Thaler, 1999).
Multiple attacks may also affect indirect defenses. For instance, caterpillars of Plutella xylostella experience reduced parasitization by the parasitoid Cotesia plutellae on plants that are also infested by caterpillars of P. rapae as the parasitoids have a reduced attraction to plants infested by both caterpillar species compared to volatiles from plants only infested by their hosts P. xylostella. In this context, it is interesting to see that adult females of P. xylostella prefer to oviposit on plants already infested by P. rapae caterpillars (Shiojiri et al., 2002). N. attenuata plants that are infested by the mirid bug Tupiocoris notatus are better protected against M. sexta caterpillars because of modifications in direct and indirect defenses (Kessler and Baldwin, 2004) that have also been addressed at the transcriptomic and metabolite level (Voelckel and Baldwin, 2004b). Such an integrative approach to effects of infestation by a first attacker at different levels of biological integration will provide a major step forward to understanding multiple plant-attacker interactions.
ARMS RACE BETWEEN PLANT DEFENSES AND INSECT ATTACKERS
Plants and their attackers are involved in an intimate arms race (Schoonhoven et al., 2005). For instance, herbivores may inject salivary components in the plant to overcome plant defenses (Will et al., 2007), while plants may exploit salivary components of herbivores to induce defenses (Kessler and Baldwin, 2002). As mentioned above, plant defense responses are regulated by a network of signal transduction pathways. There is cross talk between the pathways; for instance, SA and JA pathways can negatively interact, while the JA and ET pathways often show positive interaction (Pieterse et al., 2002; Koornneef and Pieterse, 2008). From the plant's point of view, this network of signal transduction pathways may benefit its fine-tuning of defenses against the wide range of attackers it faces (e.g. Reymond and Farmer, 1998; De Vos et al., 2006). However, it may also provide attackers with means to interfere with the induction of defenses. For instance, B. tabaci down-regulate JA-mediated defense responses and induce SA defense responses that enhanced the whiteflies' performance and thus can be seen as a manipulation of the plant's defense responses (Zarate et al., 2007).
PRIMING OF DEFENSES
In addition to induction, plant defenses may also be primed (Frost et al., 2008); as a result, genes are up-regulated faster or stronger in response to attack than in nonprimed plants. Priming may be mediated by attack but also by exposure to volatiles. For instance, caterpillar attack can prime plants for induced resistance responses against a pathogen (De Vos et al., 2006), and induced defenses may also be primed by exposure of the plant's leaves to volatiles from attacked leaves from the same plant or a neighboring plant (e.g. Heil and Bueno, 2007; Ton et al., 2007). Priming is an ecologically interesting phenomenon, as it is likely to be less costly to the plant than induction of defenses (van Hulten et al., 2006). Moreover, priming seems to involve very limited gene expression and phytohormone induction, especially in tissues that have not been exposed to the priming agent (Verhagen et al., 2004). This may also limit the potential for manipulation of the plant's responses by attackers. Priming further adds to the plasticity of the plant's phenotype and thus to the dynamics of plant-insect interactions. This provides exciting new research options to ecologists to address community dynamics (e.g. Kessler et al., 2006).
MOLECULAR ECOLOGY OF INSECT-PLANT INTERACTIONS
The ultimate goal of ecology is to understand how the traits of an individual contribute to its fitness in terms of reproductive success. Until recently, this was investigated by comparing the performance of individuals or populations that differed in certain traits without (much) information on the underlying mechanisms and genes. Recent developments in molecular genetics have opened stimulating new avenues for ecologists through a molecular genetic approach. With the sequencing of plant genomes and the availability of well-characterized mutants and transgenics altered in traits that mediate interactions between plants and their biotic community members, ecologists can now address the ecological function of individual traits in very precise ways (Dicke et al., 2004; Kessler et al., 2004; Steppuhn et al., 2004).
Such tools are most abundantly available for molecular model species such as Arabidopsis and are used to investigate mechanisms of induced defense through a molecular genetic approach (for review, see Van Poecke, 2007). However, Arabidopsis is not the ideal plant for investigating the ecology of insect-plant interactions due to its early-season phenology. Other crucifers are more suitable to investigate the effects of genes on community ecology. There is extensive information on the ecology of brassicaceous plants (e.g. Louda and Rodman, 1996; Agrawal, 2000; Shiojiri et al., 2002; Harvey et al., 2003; van Dam et al., 2004; Gols et al., 2005). The ecology of B. oleracea is not only investigated for agricultural varieties (e.g. Shiojiri et al., 2002) but also for feral and native populations (Moyes et al., 2000; Bukovinszky et al., 2008). This provides interesting options for an ecogenomic approach to community ecology of Brassica-insect interactions. Arabidopsis can be an interesting tool for advancing molecular ecological information on other brassicaceous plant species (e.g. Broekgaarden et al., 2007). Arabidopsis may even aid the understanding of gene functioning in non-brassicaceous plants; for instance, by transferring terpene synthase genes from non-brassicaceous plants to Arabidopsis, the ecological effects of the volatile terpenoid products of the terpene synthase in terms of attraction of carnivorous arthropods can be investigated (Kappers et al., 2005; Schnee et al., 2006).
Moreover, solanaceous plant species such as N. attenuata and Solanum nigrum are being rapidly developed for molecular ecological studies of community dynamics (Kessler et al., 2004; Schmidt et al., 2004). This is done by gaining extensive knowledge on the molecular genetic basis of plant responses to environmental stress and developing tools to alter the expression of specific genes. For instance, blocking the jasmonate cascade by silencing the LIPOXYGENASE3 gene in this species has important consequences for the insect community associated with N. attenuata in its native environment; some herbivores that were never before encountered on this plant were now able to colonize the plant (Kessler et al., 2004). Moreover, silencing the CORONATINE INSENSITIVE1 gene results in changes in a range of defense-related characteristics and changes in interactions with the natural community (Paschold et al., 2007). Such experiments where plants altered in the expression of a single gene are exposed to their natural community provide important new information that can be supplemented with data on experiments in the laboratory that address the effects of the altered gene expression on individual plant-insect interactions (e.g. Li et al., 2002; Paschold et al., 2007).
FUTURE PERSPECTIVES
The rapid development of novel tools to address the ecological function of genes is likely to revolutionize ecology by allowing integration from molecular genetics and community ecology. To date, large numbers of well-characterized mutants and transgenic lines have been available only for model species like Arabidopsis. However, through mutant screens or with the development of RNA interference, and virus-induced gene silencing, generating specific lines in which individual genes have been knocked out is going to provide ecologists working on ecological model plants with important new tools (e.g. Paschold et al., 2007) to investigate the effects of individual genes on individual plant-insect interactions or on community dynamics. So far, these techniques allow for investigating the effects of two extremes, i.e. the presence or absence of a functional expression of the gene of interest. However, ecologists are especially interested in quantitative variation, and, thus, the development of tools to address the effect of quantitative genetic variation should be a next step. One way of proceeding on that route can be to exploit natural variation, and the molecular genetic information on the genes of interest will be very helpful in finding genotypes that differ quantitatively in the functional expression of genes of interest.
At present, ecologists with an interest in molecular ecology have almost exclusively addressed the ecological function of plant genes. However, given the long history of the interest in the reciprocal aspects of plant-insect interactions (Ehrlich and Raven, 1964; Schoonhoven et al., 2005), there is a need to enter the field of molecular ecology from the insect side. Given that several insect genomes are being sequenced, including herbivores and their natural enemies, we may soon see tools being developed to address functional ecological questions at the insect side of plant-insect interactions.
This work was supported by the Netherlands Organization for Scientific Research, NWO (VICI grant no. 865.03.002 to M.D.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Marcel Dicke (marcel.dicke@wur.nl).
Some figures in this article are displayed in color online but in black and white in the print edition.
References
- Agrawal AA (2000) Benefits and costs of induced plant defense for Lepidium virginicum (Brassicaceae). Ecology 81 1804–1813 [Google Scholar]
- Agrawal AA, Karban R (1999) Why induced defenses may be favored over constitutive strategies in plants. In R Tollrian, CD Harvell, eds, The Ecology and Evolution of Inducible Defenses. Princeton University Press, Princeton, pp 45–61
- Arimura G, Kost C, Boland W (2005) Herbivore-induced, indirect plant defences. Biochim Biophys Acta 1734 91–111 [DOI] [PubMed] [Google Scholar]
- Bezemer TM, van Dam NM (2005) Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol Evol 20 617–624 [DOI] [PubMed] [Google Scholar]
- Bodenhausen N, Reymond P (2007) Signaling pathways controlling induced resistance to insect herbivores in Arabidopsis. Mol Plant Microbe Interact 20 1406–1420 [DOI] [PubMed] [Google Scholar]
- Broekgaarden C, Poelman EH, Steenhuis G, Voorrips RE, Dicke M, Vosman B (2007) Genotypic variation in genome-wide transcription profiles induced by insect feeding: Brassica oleracea-Pieris rapae interactions. BMC Genomics 8 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinsma M, Dicke M (2008) Herbivore-induced indirect defence: from induction mechanisms to community ecology. In A Schaller, ed, Induced Plant Resistance to Herbivory. Springer Publishers, Berlin, pp 31–60
- Bukovinszky T, van Veen FJF, Jongema Y, Dicke M (2008) Direct and indirect effects of resource quality on food web structure. Science 319 804–807 [DOI] [PubMed] [Google Scholar]
- Cardoza YJ, Teal PEA, Tumlinson JH (2003) Effect of peanut plant fungal infection on oviposition preference by Spodoptera exigua and on host-searching behavior by Cotesia marginiventris. Environ Entomol 32 970–976 [Google Scholar]
- D'Alessandro M, Turlings TCJ (2006) Advances and challenges in the identification of volatiles that mediate interactions among plants and arthropods. Analyst 131 24–32 [DOI] [PubMed] [Google Scholar]
- De Vos M, Van Oosten VR, Van Poecke RMP, Van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Metraux JP, Van Loon LC, Dicke M, et al (2005) Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact 18 923–937 [DOI] [PubMed] [Google Scholar]
- De Vos M, Van Zaanen W, Koornneef A, Korzelius JP, Dicke M, Van Loon LC, Pieterse CMJ (2006) Herbivore-induced resistance against microbial pathogens in Arabidopsis. Plant Physiol 142 352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicke M, van Loon JJA, de Jong PW (2004) Ecogenomics benefits community ecology. Science 305 618–619 [DOI] [PubMed] [Google Scholar]
- Dicke M, Van Poecke RMP (2002) Signalling in plant-insect interactions: signal transduction in direct and indirect plant defence. In D Scheel, C Wasternack, eds, Plant Signal Transduction. Oxford University Press, Oxford, pp 289–316
- Dicke M, van Poecke RMP, de Boer JG (2003) Inducible indirect defence of plants: from mechanisms to ecological functions. Basic Appl Ecol 4 27–42 [Google Scholar]
- Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution Int J Org Evolution 18 586–608 [Google Scholar]
- Frost CJ, Mescher MC, Carlson JE, De Moraes CM (2008) Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol 146 818–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gols R, Bukovinszky T, Hemerik L, Harvey JA, Van Lenteren JC, Vet LEM (2005) Reduced foraging efficiency of a parasitoid under habitat complexity: implications for population stability and species coexistence. J Anim Ecol 74 1059–1068 [Google Scholar]
- Harvey JA, van Dam NM, Gols R (2003) Interactions over four trophic levels: foodplant quality affects development of a hyperparasitoid as mediated through a herbivore and its primary parasitoid. J Anim Ecol 72 520–531 [Google Scholar]
- Heidel AJ, Baldwin IT (2004) Microarray analysis of salicylic acid- and jasmonic acid-signalling in responses of Nicotiana attenuata to attack by insects from multiple feeding guilds. Plant Cell Environ 27 1362–1373 [Google Scholar]
- Heil M, Bueno JCS (2007) Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA 104 5467–5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, Greiner S, Meimberg H, Kruger R, Noyer JL, Heubl G, Eduard Linsenmair K, Boland W (2004) Evolutionary change from induced to constitutive expression of an indirect plant resistance. Nature 430 205–208 [DOI] [PubMed] [Google Scholar]
- Howe GA (2004) Jasmonates as signals in the wound response. J Plant Growth Regul 23 223–237 [Google Scholar]
- Kahl J, Siemens DH, Aerts RJ, Gäbler R, Kühnemann F, Preston CA, Baldwin IT (2000) Herbivore-induced ethylene suppresses a direct defense but not a putative indirect defense against an adapted herbivore. Planta 210 336–342 [DOI] [PubMed] [Google Scholar]
- Kaplan I, Denno RF (2007) Interspecific interactions in phytophagous insects revisited: a quantitative assessment of competition theory. Ecol Lett 10 977–994 [DOI] [PubMed] [Google Scholar]
- Kappers IF, Aharoni A, van Herpen T, Luckerhoff LLP, Dicke M, Bouwmeester HJ (2005) Genetic engineering of terpenoid metabolism attracts, bodyguards to Arabidopsis. Science 309 2070–2072 [DOI] [PubMed] [Google Scholar]
- Kempema LA, Cui XP, Holzer FM, Walling LL (2007) Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs. Similarities and distinctions in responses to aphids. Plant Physiol 143 849–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53 299–328 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2004) Herbivore-induced plant vaccination. Part I. The orchestration of plant defenses in nature and their fitness consequences in the wild tobacco Nicotiana attenuata. Plant J 38 639–649 [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R (2007) Specificity and complexity: the impact of herbivore-induced plant responses on arthropod community structure. Curr Opin Plant Biol 10 409–414 [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Baldwin IT (2004) Silencing the jasmonate cascade: induced plant defenses and insect populations. Science 305 665–668 [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Diezel C, Baldwin IT (2006) Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 148 280–292 [DOI] [PubMed] [Google Scholar]
- Koornneef A, Pieterse CMJ (2008) Cross talk in defense signaling. Plant Physiol 146 839–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krischik VA, Barbosa P, Reichelderfer CF (1988) Three trophic level interactions: allelochemicals, Manduca sexta (L.), and Bacillus thuringiensis var. kurstaki Berliner. Environ Entomol 17 476–482 [Google Scholar]
- Li CY, Williams MM, Loh YT, Lee GI, Howe GA (2002) Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol 130 494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louda SM, Rodman JE (1996) Insect herbivory as a major factor in the shade distribution of a native crucifer (Cardamine cordifolia A. Gray, bittercress). J Ecol 84 229–237 [Google Scholar]
- Mercke P, Kappers IF, Verstappen FWA, Vorst O, Dicke M, Bouwmeester HJ (2004) Combined transcript and metabolite analysis reveals genes involved in spider mite induced volatile formation in cucumber plants. Plant Physiol 135 2012–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes CL, Collin HA, Britton G, Raybould AE (2000) Glucosinolates and differential herbivory in wild populations of Brassica oleracea. J Chem Ecol 26 2625–2641 [Google Scholar]
- Mumm R, Hilker M (2006) Direct and indirect chemical defence of pine against folivorous insects. Trends Plant Sci 11 351–358 [DOI] [PubMed] [Google Scholar]
- Paschold A, Halitschke R, Baldwin IT (2007) Co(i)-ordinating defenses: NaCOI1 mediates herbivore-induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant J 51 79–91 [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Van Wees SCM, Ton J, Van Pelt JA, Van Loon LC (2002) Signalling in rhizobacteria-induced systemic resistance in Arabidopsis thaliana. Plant Biol 4 535–544 [Google Scholar]
- Reymond P, Bodenhausen N, Van Poecke RMP, Krishnamurthy V, Dicke M, Farmer EE (2004) A conserved transcript pattern in response to a specialist and a generalist herbivore. Plant Cell 16 3132–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Farmer EE (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol 1 404–411 [DOI] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Saona C, Chalmers JA, Raj S, Thaler JS (2005) Induced plant responses to multiple damagers: differential effects on an herbivore and its parasitoid. Oecologia 143 566–577 [DOI] [PubMed] [Google Scholar]
- Schmidt DD, Kessler A, Kessler D, Schmidt S, Lim M, Gase K, Baldwin IT (2004) Solanum nigrum: a model ecological expression system and its tools. Mol Ecol 13 981–995 [DOI] [PubMed] [Google Scholar]
- Schnee C, Kollner TG, Held M, Turlings TCJ, Gershenzon J, Degenhardt J (2006) The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA 103 1129–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonhoven LM, van Loon JJA, Dicke M (2005) Insect-Plant Biology, Ed 2. Oxford University Press, Oxford
- Shiojiri K, Takabayashi J, Yano S, Takafuji A (2002) Oviposition preferences of herbivores are affected by tritrophic interaction webs. Ecol Lett 5 186–192 [Google Scholar]
- Snoeren TAL, De Jong PW, Dicke M (2007) Ecogenomic approach to the role of herbivore-induced plant volatiles in community ecology. J Ecol 95 17–26 [Google Scholar]
- Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT (2004) Nicotine's defensive function in nature. PLoS Biol 2 e217 [DOI] [PMC free article] [PubMed]
- Stork NE (2007) Biodiversity: world of insects. Nature 448 657–658 [DOI] [PubMed] [Google Scholar]
- Thaler JS (1999) Jasmonate-inducible plant defenses cause increased parasitism of herbivores. Nature 399 686–688 [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu GH, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCFCO11 complex during jasmonate signalling. Nature 448 661–665 [DOI] [PubMed] [Google Scholar]
- Ton J, D'Alessandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TCJ (2007) Priming by airborne signals boosts direct and indirect resistance in maize. Plant J 49 16–26 [DOI] [PubMed] [Google Scholar]
- van Dam NM, Witjes L, Svatos A (2004) Interactions between aboveground and belowground induction of glucosinolates in two wild Brassica species. New Phytol 161 801–810 [DOI] [PubMed] [Google Scholar]
- van Hulten M, Pelser M, van Loon LC, Pieterse CMJ, Ton J (2006) Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci USA 103 5602–5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Poecke RMP (2007) Arabidopsis-insect interactions. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, doi/10.1199/tab.0107, www.aspb.org/publications/arabidopsis/ [DOI] [PMC free article] [PubMed]
- Verhagen BWM, Glazebrook J, Zhu T, Chang HS, van Loon LC, Pieterse CMJ (2004) The transcriptome of rhizobacteria-induced systemic resistance in Arabidopsis. Mol Plant Microbe Interact 17 895–908 [DOI] [PubMed] [Google Scholar]
- Voelckel C, Baldwin IT (2004. a) Generalist and specialist lepidopteran larvae elicit different transcriptional responses in Nicotiana attenuata, which correlate with larval FAC profiles. Ecol Lett 7 770–775 [Google Scholar]
- Voelckel C, Baldwin IT (2004. b) Herbivore-induced plant vaccination. Part II. Array-studies reveal the transience of herbivore-specific transcriptional imprints and a distinct imprint from stress combinations. Plant J 38 650–663 [DOI] [PubMed] [Google Scholar]
- Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19 195–216 [DOI] [PubMed] [Google Scholar]
- Will T, Tjallingii WF, Thonnessen A, van Bel AJE (2007) Molecular sabotage of plant defense by aphid saliva. Proc Natl Acad Sci USA 104 10536–10541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate SI, Kempema LA, Walling LL (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ, van Dijk JP, Bruinsma M, Dicke M (2007) Sensitivity and speed of induced defense of cabbage (Brassica oleracea L.): dynamics of BoLOX expression patterns during insect and pathogen attack. Mol Plant Microbe Interact 20 1332–1345 [DOI] [PubMed] [Google Scholar]