Abstract
Ascorbate and glutathione are major antioxidants and redox buffers in plant cells but also play key functions in growth, development, and stress responses. We have studied the regulation of ascorbate and homoglutathione biosynthesis in common bean (Phaseolus vulgaris) nodules under stress conditions and during aging. The expression of five genes of the major ascorbate biosynthetic pathway was analyzed in nodules, and evidence was found that l-galactono-1,4-lactone dehydrogenase, the last committed step of the pathway, is posttranscriptionally regulated. Also, in nodules under stress conditions, γ-glutamylcysteine synthetase was translationally regulated, but homoglutathione synthetase (mRNA and activity) and homoglutathione (content and redox state) were not affected. Most interestingly, in nodules exposed to jasmonic acid, dehydroascorbate reductase activity was posttranslationally suppressed, ascorbate oxidase showed strong transcriptional up-regulation, and dehydroascorbate content increased moderately. These changes were not due to a direct effect of jasmonic acid on the enzyme activities but might be part of the signaling pathway in the response of nodules to stress. We determined ascorbate, homoglutathione, and ascorbate-glutathione pathway enzyme activities in two senescing stages of nodules undergoing oxidative stress. When all parameters were expressed on a nodule fresh weight basis, we found that in the first stage ascorbate decreased by 60% and homoglutathione and antioxidant activities remained fairly constant, whereas in the second stage ascorbate and homoglutathione, their redox states, and their associated enzyme activities significantly decreased. The coexistence in the same plants of nodules at different senescence stages, with different ascorbate concentrations and redox states, indicates that the life span of nodules is in part controlled by endogenous factors and points to ascorbate as one of the key players.
Legume nodules are unique symbiotic organs that develop on the roots and, in a few species, also on the stems, after infection with rhizobia. The plant provides Suc to nodule host cells, where it is oxidized to dicarboxylic acids and used as energy source by the bacteroids to fix atmospheric N2. Nodules have a high potential for production of reactive oxygen species due, among other factors, to the acid pH (5.5–6.5) and high leghemoglobin (Lb) concentration (1–2 mm) in the infected cells, the elevated rates of bacteroid respiration, and the abundance of O2-labile proteins (Dalton et al., 1986). However, nodules possess an array of antioxidant metabolites and enzymes that prevent the generation of highly oxidizing radicals and hence the damage of lipids, proteins, and DNA. In addition, antioxidants regulate the intracellular concentrations of reactive oxygen species, such as the superoxide radical and hydrogen peroxide (H2O2), that serve useful purposes in signaling stressful situations and in activating defense genes (Pastori et al., 2003; Mittler et al., 2004).
In plants, ascorbate and glutathione (GSH; γGlu-Cys-Gly) are major cellular antioxidants and redox buffers but also play important functions in growth, development, and stress responses (Smirnoff, 2000; Pastori et al., 2003). Another thiol tripeptide, homoglutathione (hGSH; γGlu-Cys-βAla), is present in nodules and other organs of some legume species in addition to or in place of GSH (Matamoros et al., 1999) and may share with it some antioxidative and regulatory properties (Zopes et al., 1993; Frendo et al., 2005). Ascorbate, GSH, and hGSH can be found in nodules at concentrations of 0.5 to 2 mm, which are needed, among other purposes, to maintain an active ascorbate-GSH pathway for H2O2 detoxification in the cytosol (Dalton et al., 1986). This pathway involves the activities of four enzymes, ascorbate peroxidase (APX), monodehydroascorbate reductase (MR), dehydroascorbate reductase (DR), and glutathione reductase (GR), which have been found to be positively correlated to N2 fixation during natural (Dalton et al., 1986) and stress-induced (Gogorcena et al., 1997) nodule senescence. Notably, cytosolic APX accounts for up to 0.9% of the total soluble protein of nodules and is particularly abundant in the infected cells and nodule parenchyma (Dalton et al., 1998). Additional APX and MR isoforms are localized in nodule mitochondria and other subcellular compartments (Dalton et al., 1993; Iturbe-Ormaetxe et al., 2001).
Several enzymes involved in the biosynthesis of ascorbate (Matamoros et al., 2006) and thiols (Frendo et al., 1999; Matamoros et al., 1999) are expressed in nodules. These include l-galactono-1,4-lactone dehydrogenase (GalLDH), which catalyzes the last step of ascorbate synthesis in the mitochondria; γ-glutamylcysteine synthetase (γECS), which catalyzes the synthesis of γ-glutamylcysteine in the plastids; and glutathione synthetase (GSHS) and homoglutathione synthetase (hGSHS), which catalyze, respectively, the synthesis of GSH and hGSH in the plastids and cytosol. Despite the multiple and critical functions of ascorbate and thiol tripeptides in plants, there is virtually no information on how these enzymes are regulated in nodules at the mRNA, protein, and activity levels. In this article, we have studied the regulation of ascorbate and hGSH biosynthesis in common bean (Phaseolus vulgaris) nodules under stress conditions and during natural senescence. This legume species was chosen because of its agronomic interest, large nodule production that makes it amenable for senescence studies, and the availability of physiological, biochemical, and molecular data (Gogorcena et al., 1997; Matamoros et al., 1999; Ramírez et al., 2005).
RESULTS
Oxidative Stress Occurs in Nodules Exposed to Stressful Conditions and During Natural Senescence
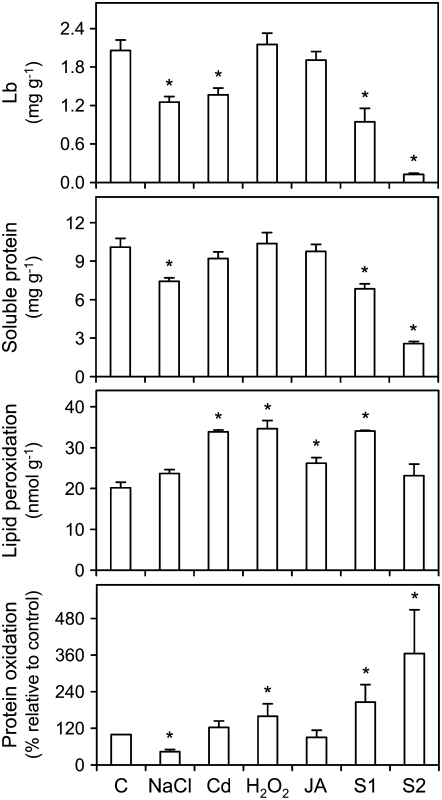
Common bean plants were exposed to abiotic stress (150 mm NaCl for 7 d or 100 μm CdCl2 for 4 d), oxidative stress (10 mm H2O2 for 4 d), or a stress-related signaling compound (100 μm jasmonic acid [JA] for 4 d). The last treatment was included because jasmonates (JA and its ester methyl jasmonate [MeJA]) are involved in the stress and defense response of plants (Wasternack, 2007) and because nothing is known about their effects on nodules. All plants were 30 d of age (late vegetative stage) when nodules were harvested, except plants to be used for studies of natural senescence (aging) of nodules. These plants were left to grow until approximately 53 d of age (late fruiting stage), and then two nodule populations were collected from the same plants (Fig. 1). The two groups of nodules had similar size but different color, which was either pink (S1 nodules) or brown-dark green (S2 nodules). Quantification of Lb and total soluble protein, two markers of nodule activity (Dalton et al., 1986; Gogorcena et al., 1997; Evans et al., 1999), revealed major differences between the S1 and S2 nodules (Fig. 2). Compared to controls, the S1 nodules had only 46% of Lb and 68% of soluble protein, but these values further decreased to 6% and 25%, respectively, in the S2 nodules. The same parameters were measured in nodules under stress conditions (Fig. 2). The Lb content was reduced by 37% in nodules treated with NaCl or cadmium (Cd), but remained unaffected in those treated with H2O2 or JA. The soluble protein content declined by 36% in the NaCl-treated nodules.
Figure 1.
Common bean plant at the fruiting state (53 d old) showing two nodule populations, designated S1 and S2, which were harvested separately based on visual differences in Lb content (pink or brown-dark-green nodules). Insets show transversal sections of representative nodules dissected under a binocular microscope.
Figure 2.
Markers of nodule activity and oxidative damage in nodules exposed to stress and during senescence. The contents of Lb, soluble protein, and malondialdehyde (lipid peroxidation) are expressed per gram of fresh weight. The content of carbonyl groups (protein oxidation) is given in percentage relative to control nodules after densitometric analysis of the corresponding immunoblots. Values are means ± se of four to five samples from at least two series of plants grown independently. Asterisks denote significant differences from control nodules based on the lsd test (P < 0.05).
Lipid peroxidation (estimated as malondialdehyde) and protein oxidation (estimated as carbonyl groups) were used as reliable markers of oxidative stress (Halliwell and Gutteridge, 2007) and were measured in nodules under the same stress and senescence conditions (Fig. 2). The malondialdehyde content of nodules increased approximately by 70% with Cd and H2O2 and by 30% with JA, but was not affected by the salt treatment. It was also increased by 69% in S1 nodules but not in S2 nodules. Protein oxidation was enhanced by 60% in nodules treated with H2O2 but decreased by 57% in response to NaCl. Likewise, there was a significant accumulation of oxidized proteins with advancing age of nodules, the level being approximately 2- to 3.5-fold greater in S1 and S2 nodules than in the control nodules (Fig. 2). The accumulation of lipid peroxides and oxidatively modified proteins allows us to conclude that oxidative stress occurs in nodules under the stress conditions tested in this study, except for the NaCl treatment, as well as during natural senescence.
Evidence That GalLDH Activity Is Posttranscriptionally Regulated But Is Not Determinant for Ascorbate Content in Nodules under Stress Conditions
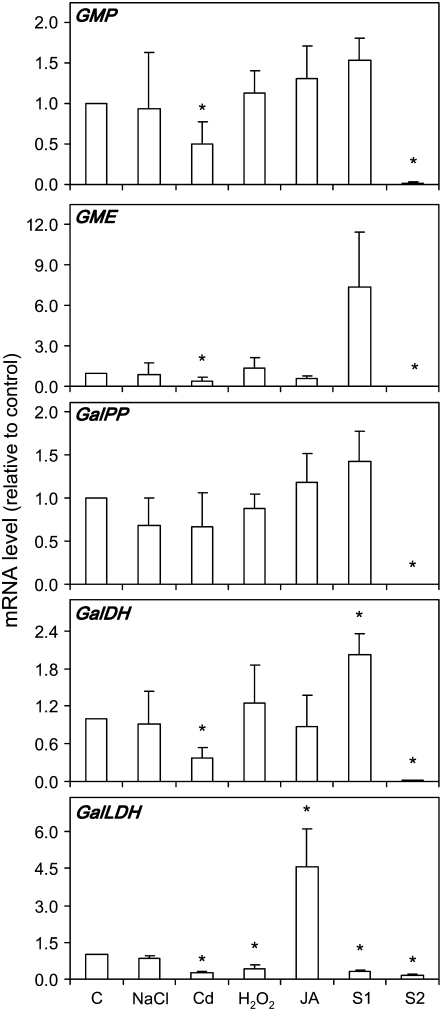
To investigate the regulation of ascorbate metabolism in nodules under stressful conditions and during natural senescence, the mRNA levels of five genes involved in the major ascorbate biosynthetic pathway of plants (Wheeler et al., 1998) were determined by quantitative reverse transcription (qRT)-PCR. As previously shown for the model legume Lotus japonicus (Matamoros et al., 2006), the genes encoding GDP-Man pyrophosphorylase (GMP), GDP-Man-3″,5″-epimerase (GME), l-Gal-1-P phosphatase (GalPP), l-Gal dehydrogenase (GalDH), and GalLDH were found to be expressed in common bean nodules (Fig. 3). The mRNA levels of all these genes, except GalPP, declined in Cd-treated nodules relative to control nodules. The exposure of plants to H2O2 or JA decreased or increased, respectively, the GalLDH mRNA level in nodules, whereas the NaCl treatment had no effect on the expression of any of the five genes examined in this study (Fig. 3). In S1 nodules, there was up-regulation of GalDH and down-regulation of GalLDH, whereas in S2 nodules the mRNAs for all the five genes were virtually undetectable (Fig. 3).
Figure 3.
Steady-state mRNA levels of five genes of the main ascorbate biosynthetic pathway in nodules exposed to stress and during senescence. Expression levels are normalized with respect to those of control nodules (which are given an arbitrary value of 1). Values are means ± se of three to four samples from at least two series of plants grown independently. Asterisks denote up-regulation (R > 2) or down-regulation (R < 0.5) of the genes.
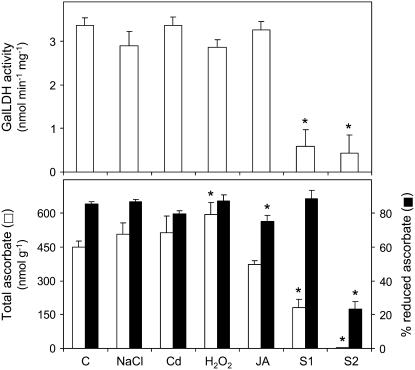
Because several experiments in tobacco (Nicotiana tabacum; Tabata et al., 2002) and Arabidopsis (Arabidopsis thaliana; Tamaoki et al., 2003) suggested that GalLDH activity is responsible for the ascorbate accumulation in leaves, we investigated whether the changes in GalLDH mRNA levels were reflected in the GalLDH activity and in the ascorbate content of nodules (Fig. 4). We found that, despite the Cd-induced decreases in the expression of GalLDH and three other genes of ascorbate biosynthesis (Fig. 3), the GalLDH activity and the total ascorbate content (reduced ascorbate + dehydroascorbate) of nodules remained unaffected (Fig. 4). Furthermore, in nodules exposed to H2O2 or JA, the GalLDH mRNA level and the ascorbate content showed different trends, whereas the GalLDH activity did not change in any of the two treatments. Taken together, these results provide strong evidence that GalLDH activity in nodules is regulated at the posttranscriptional level in response to stressful conditions or JA treatment. They also suggest that GalLDH activity is not determinant for the ascorbate content of nodules. On the other hand, GalLDH activity and ascorbate content declined, respectively, by 83% and 60% in S1 nodules and by 87% and 98% in S2 nodules (Fig. 4). These decreases are probably associated to a progressive switch off of the ascorbate biosynthetic pathway during nodule senescence.
Figure 4.
GalLDH activity, total ascorbate content, and percentage of reduced ascorbate in nodules exposed to stress and during senescence. GalLDH activity is expressed per milligram of protein and total ascorbate is expressed per gram of fresh weight. Values are means ± se of six to seven samples from at least three series of plants grown independently. Asterisks denote significant differences from control nodules based on the lsd test (P < 0.05).
The proportion of reduced ascorbate relative to total ascorbate was 85% in control nodules and in those exposed to NaCl or H2O2, but it decreased moderately with the JA treatment (Fig. 4). Surprisingly, S1 nodules retained 89% of the ascorbate pool in the reduced form, whereas this value declined to only 23% in S2 nodules (Fig. 4). The proportion of reduced ascorbate of control common bean nodules was remarkably higher than that reported by Groten et al. (2006) in pea (Pisum sativum) nodules (approximately 30%) but similar to that found in alfalfa (Medicago sativa) nodules (Naya et al., 2007). In our hands, there was a rapid drop in the ascorbate redox state if nodules were not directly harvested in liquid nitrogen. In fact, in control nodules that were kept on ice for 10 min, the proportion of reduced ascorbate declined from 85% to 46%. This decrease was even more dramatic in leaves because, after only 5 min on ice, virtually all ascorbate became oxidized to dehydroascorbate (data not shown).
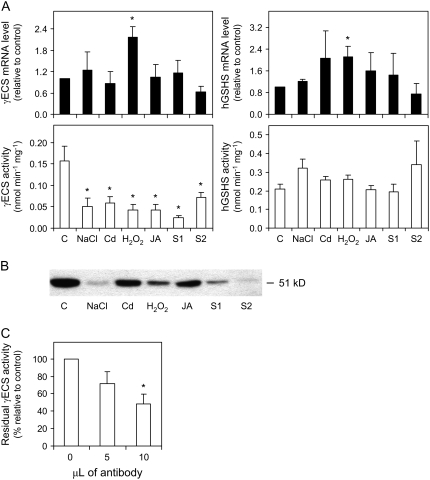
γECS Activity Is Translationally Regulated In Nodules, But Stress Conditions Do Not Have Major Effects on hGSHS Expression or on the Homoglutathione Content and Redox State
The mRNA level of γECS, a key enzyme for GSH biosynthesis (May et al., 1998; Xiang and Oliver, 1998), was increased 2-fold in nodules exposed to H2O2 but remained constant in the other stress treatments and during senescence (Fig. 5A). However, the specific activity (that is, the activity expressed per milligram of protein) of γECS was inhibited by approximately 70% in the stress treatments, by 85% in S1 nodules, and by 54% in S2 nodules (Fig. 5A), indicating that the enzyme is posttranscriptionally regulated. To determine whether γECS is regulated at the levels of protein content, enzyme activity, or both, a polyclonal monospecific antibody was raised against common bean γECS (M.R. Clemente and M. Becana, unpublished data) and used for immunoblot analysis of nodule extracts. The antibody recognized a single protein band of 51 kD (Fig. 5B), which is the expected molecular mass for common bean γECS. The specificity of the antibody was further confirmed by immunoprecipitation of the enzyme activity from nodule extracts (Fig. 5C). Immunoblots revealed a strong reduction in the nodule content of γECS protein with the NaCl or H2O2 treatments and with progression of senescence, as well as moderate decreases with the Cd or JA treatments (Fig. 5B). Therefore, the loss of γECS activity under stress conditions or during senescence is largely explained by the lower amounts of γECS protein, leading us to conclude that the enzyme is down-regulated in nodules, at least in part, at the translational level. The comparison between activities (Fig. 5A) and protein levels (Fig. 5B) of γECS in senescing nodules deserves some comment. Thus, γECS activity was similar in S1 and S2 nodules when expressed on a nodule fresh weight basis (data not shown), but it was significantly higher in S2 nodules than in S1 nodules when expressed as specific activity. This is probably due to a large decrease in the nodule content of soluble protein with advancing senescence (Fig. 2). However, the γECS protein of S2 nodules was barely detectable on western blots in which lanes were also loaded on a protein basis. This, and a similar case with the NaCl treatment (Fig. 5, A and B), suggests that γECS activity may be also subjected to posttranslational regulation.
Figure 5.
Expression analysis of the hGSH biosynthetic pathway enzymes in nodules exposed to stress and during senescence. A, Steady-state mRNA levels and γECS and hGSHS activities. Values are means ± se of three to four samples corresponding to RNA or enzyme extractions from nodules of at least two series of plants grown independently. Expression levels are normalized with respect to values of control nodules (except for values of the JA treatment, which are normalized with respect to DMSO-treated nodules), and asterisks denote up-regulation (R > 2) of the genes. Enzyme activities are expressed per milligram of protein, and asterisks denote significant differences from control nodules based on the lsd test (P < 0.05). B, Immunoblot of γECS using a polyclonal monospecific antibody raised against the recombinant common bean enzyme. SDS-PAGE (10%) gels were loaded with 30 μg of protein per lane. The blot shown is representative of three independent blots. C, Immunoprecipitation of γECS activity from nodule extracts. Values are means ± se of three nodule samples from at least two series of plants grown independently. The 100% value of γECS activity corresponds to 0.17 ± 0.04 nmol of γEC synthesized min−1 mg−1 of protein.
The second step of GSH or hGSH biosynthesis in legumes is catalyzed by specific GSHS and hGSHS enzymes. We could not detect significant GSHS activity in the plant fraction of common bean nodules, in agreement with our previous studies (Matamoros et al., 1999; Moran et al., 2000), but found abundant hGSHS activity (Fig. 5A). The hGSHS mRNA level increased moderately in nodules exposed to H2O2 but was not affected by the remaining treatments or during senescence. Also, the specific activity of hGSHS in nodules remained fairly constant with the stress treatments or with progression of senescence (Fig. 5A).
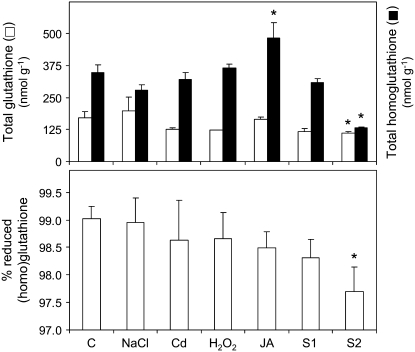
The decreases of γECS protein and activity with stress or senescence did not result in a lower content of thiol tripeptides, with the exception of S2 nodules, in which there was a sharp decline in both the total homoglutathione content and redox state (Fig. 6). Despite the lack of detectable GSHS activity in the nodule plant fraction, we found high concentrations of GSH when nodules were extracted with methanesulfonic acid. These conditions minimized thiol degradation but caused breakage of bacteroids. Thus, most of the GSH found in whole nodules, if not all, originated in the bacteroids, which express γECS and GSHS activities (Moran et al., 2000). We did not investigate further the bacteroid enzymes, but the concentration of GSH in nodules was not affected by the stress treatments and only decreased significantly in S2 nodules (Fig. 6).
Figure 6.
Total (homo)glutathione contents and percentage of (h)GSH in nodules exposed to stress and during senescence. The (homo)glutathione contents are expressed per gram of fresh weight. Values are means ± se of five to six samples from at least three series of plants grown independently. Asterisks denote significant differences from control nodules based on the lsd test (P < 0.05).
The proportion of (h)GSH relative to total (homo)glutathione in nodules remained in the range of 98% to 99% for all stress treatments (Fig. 6). Notably, this value decreased significantly in nodules that were kept on ice for 10 min after harvest (89%) in comparison to nodules that had been immediately immersed in liquid nitrogen (99%). This observation, along with that described above for ascorbate, shows that both antioxidant metabolites are prone to rapid oxidation upon nodule detachment and underscores the need for careful harvest of nodules to be used for determination of redox states.
DR Is Posttranslationally Regulated and Ascorbate Oxidase Is Transcriptionally Regulated in Nodules in Response to JA
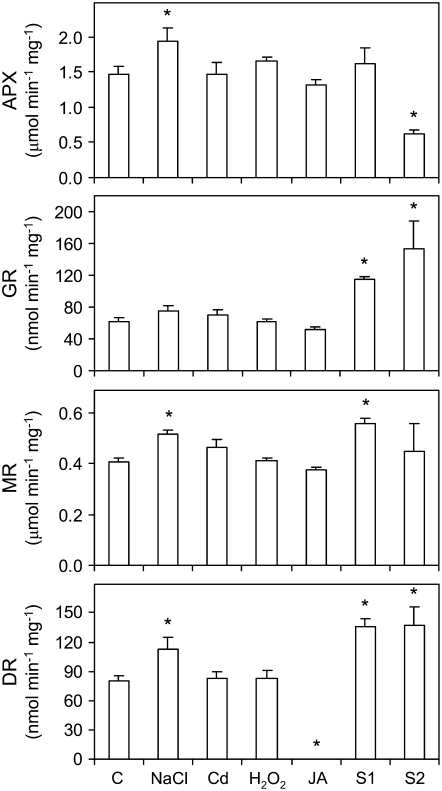
The four enzyme activities of the ascorbate-GSH pathway were determined in senescing nodules and in nodules exposed to stress conditions or to JA (Fig. 7). Multiple isoforms of APX, GR, MR, and DR are expected to occur in nodules (Dalton et al., 1993; Iturbe-Ormaetxe et al., 2001). Given the complexity of this study, we could not assess the contribution of the cytosolic and organellar isoforms to the total activities. However, we found for DR that the mRNA level of the cytosolic isoform (DR2) was 6-fold more abundant than that of the plastidial isoform (DR1) in control nodules (see also below). In S1 nodules, there were increases in the specific activities of GR, MR, and DR, but only the increase of GR was significant when the activity was expressed per fresh weight of nodules (data not shown). In S2 nodules, the specific activity of APX decreased by 57%, whereas that of GR and DR increased by 146% and 72%, respectively (Fig. 7). When expressed per fresh weight of nodules, the APX activity of S2 nodules decreased even more markedly, whereas GR, MR, and DR activities decreased significantly (data not shown). This discrepancy in the trends of the enzyme activities, especially in S2 nodules, can be explained by the considerable loss of soluble protein, especially Lb (Fig. 2), that occurs during senescence. Therefore, the activities of the ascorbate-GSH enzymes expressed on a nodule fresh weight basis are more representative of the overall antioxidant capacity of nodules and are also more suitable for comparison with the patterns of the associated metabolites.
Figure 7.
Activities of the ascorbate-glutathione pathway enzymes in nodules exposed to stress and during senescence. Values are expressed per milligram of protein and are means ± se of five to six samples from at least three series of plants grown independently. Asterisks denote significant differences from control nodules based on the lsd test (P < 0.05). Note the absence of DR activity in JA-treated nodules.
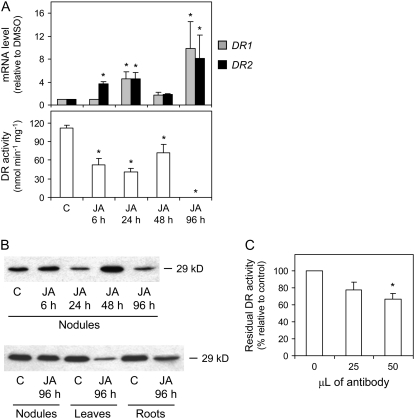
The stress treatments had no effects on the specific activities of the enzymes, with a few exceptions. There were moderate increases of APX, MR, and DR activities with the NaCl treatment (Fig. 7), but these were not statistically significant when the activities were expressed on a nodule fresh weight basis (data not shown). Quite unexpectedly, the treatment with JA completely abolished the DR activity of nodules (Fig. 7). This novel finding was further investigated by time-course determinations of mRNA and enzyme activity levels (Fig. 8A) and by immunoblot analysis (Fig. 8B). Following exposure of plants to JA, the specific activity of DR in nodules decreased by 53% within 6 h, with no additional change after 24 or 48 h and complete inhibition after 96 h. Moreover, we did not observe any change in DR activity after addition of JA to the reaction mixture, ruling out a direct inhibitory effect on the protein. Contrary to what would be expected from the enzyme activity assays, the expression of the DR1 and DR2 genes was increased at variable extents after 6, 24, or 96 h of JA treatment (Fig. 8A). This indicates that DR is posttranscriptionally regulated in nodules as a result of JA treatment. Remarkably, the complete inhibition of DR activity occurred exclusively in nodules, as the corresponding activities in roots and leaves remained unaffected (data not shown). To ascertain whether the inhibition of DR activity in nodules is due to a posttranslational modification of the enzyme or to protein degradation, an immunoblot analysis was performed with a polyclonal antibody raised against the cytosolic DR of Arabidopsis (Eltayeb et al., 2006). Immunoblots of nodule, leaf, and root extracts revealed the presence of a single band at 29 kD (Fig. 8B), which fits in the range of the theoretical molecular masses (28.5–29.5 kD) for the DRs (accession nos. in parentheses) of Arabidopsis (BAD43518), L. japonicus (AAY52461), Medicago truncatula (AAY85185), and Glycine max (AAY85184). The specificity of the antibody was also verified by immunoprecipitation of DR activity from the nodule extracts (Fig. 8C). Immunoblot analysis showed that significant amounts of DR protein were present in nodule extracts following exposure to JA for 6 to 96 h, with small decreases of the DR protein level after 96 h in the leaves and roots relative to the untreated plants (Fig. 8B). We therefore conclude that the DR activity of nodules is regulated at the posttranslational level in response to JA.
Figure 8.
Time-course study of the effect of JA on DR expression in nodules. A, Steady-state mRNA levels of the putative plastidial (DR1) and cytosolic (DR2) isoforms and DR activity (DR1 + DR2) in nodules exposed to 100 μm JA for 6, 24, 48, or 96 h. Values are means ± se of three to four samples from at least two series of plants grown independently. DR1 and DR2 mRNA levels are normalized with respect to those of DMSO-treated nodules (which are given an arbitrary value of 1) for each time point, and asterisks denote up-regulation (R > 2) of the genes. DR activity is expressed per milligram of protein, and asterisks denote significant differences from control nodules based on the lsd test (P < 0.05). B, Immunoblots were carried out using a polyclonal antibody raised against recombinant Arabidopsis cytosolic DR. SDS-PAGE (15%) gels were loaded with 25 μg of protein (nodules and roots) or 50 μg (leaves) per lane. Blots are representative of three replicates of nodule extracts from different plants. C, Immunoprecipitation of DR activity from nodule extracts. The 100% value of DR activity corresponds to 88.1 ± 9.0 nmol of ascorbate produced min−1 mg−1 of protein.
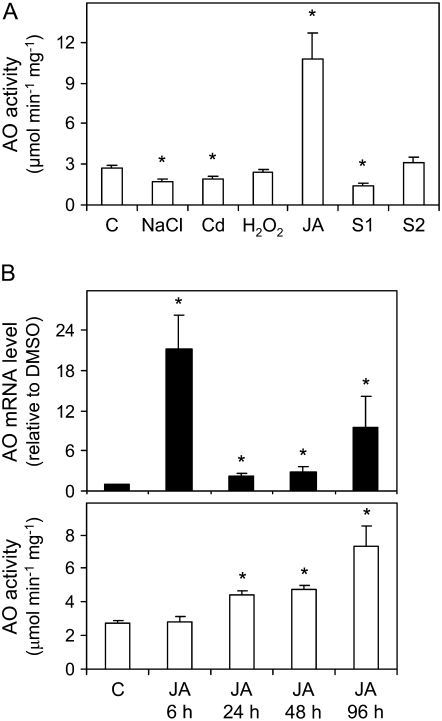
Ascorbate oxidase (AO) is an apoplastic enzyme that catalyzes the oxidation of ascorbate to monodehydroascorbate. Its biological function is poorly understood, but the enzyme is believed to facilitate cell expansion through changes in the ascorbate redox state of the apoplast (Pignocchi et al., 2003). We found that the AO activity of nodules was induced approximately 4-fold by JA and was inhibited by approximately 33% by the NaCl and Cd treatments. Also, the AO activity decreased by 48% in S1 nodules but not in S2 nodules (Fig. 9A). Time-course experiments showed that the AO mRNA level in nodules was maximal within 6 h of JA treatment, decreased after 24 and 48 h but still remained over the control, and increased again after 96 h (Fig. 9B). Consistent with a transcriptional regulation of the enzyme, the maximal increase of AO activity lagged behind that of the transcript, reaching 1.6-fold of the control value after 24 and 48 h of JA treatment and 2.6-fold after 96 h (Fig. 9B).
Figure 9.
A, AO activity in nodules exposed to stress and during senescence. Values are means ± se of three to four samples from at least two series of plants grown independently. AO activity is expressed per milligram of protein, and asterisks denote significant differences from control nodules based on the lsd test (P < 0.05). B, Time-course study of AO mRNA and activity levels in nodules exposed to 100 μm JA for 6, 24, 48, or 96 h. mRNA levels are normalized with respect to those of DMSO-treated nodules (which are given an arbitrary value of 1) for each time point, and asterisks denote up-regulation (R > 2) of the gene. AO activity is expressed and statistically analyzed as in A.
DISCUSSION
The regulatory mechanisms of ascorbate biosynthesis in plants are largely unknown. Most studies have focused on the last enzyme of the pathway, GalLDH, which produces ascorbate from l-galactono-1,4-lactone in the mitochondria (Siendones et al., 1999; Bartoli et al., 2000). However, results appear to be contradictory. Some authors described transcriptional up-regulation of GalLDH in response to light or fruit ripening and proposed that this activity is a major determinant of ascorbate content (Tabata et al., 2002; Tamaoki et al., 2003; Pateraki et al., 2004), whereas others proposed that GalLDH activity is posttranslationally regulated and is not related to the ascorbate content (Bartoli et al., 2005). We found that the expression of some genes involved in ascorbate biosynthesis in nodules is affected by stress conditions and particularly by Cd. In none of the stress treatments (except for NaCl) was there a correlation among GalLDH mRNA level, GalLDH activity, and ascorbate content. Consequently, our results are consistent with a posttranscriptional regulation of GalLDH activity and suggest that it does not determine the ascorbate content of nodules. This conclusion is at odds with the finding that the GalLDH mRNA and activity are correlated and that this enzyme is responsible for the levels of ascorbate accumulation in leaves, but not in roots, of Arabidopsis (Tamaoki et al., 2003). The regulation of GalLDH activity and ascorbate concentration may therefore vary with plant species or tissues and in response to environmental cues.
The γECS activity of nodules decreased markedly under stress conditions or during senescence. These decreases were not caused by down-regulation of mRNA levels, because they remained unaffected or even increased upon H2O2 treatment, but, at least in part, by a decline in γECS protein. In addition, our comparative data between the γECS activity and protein levels, particularly in NaCl-treated nodules and in S2 nodules, support a posttranslational regulation of the enzyme. Hence, it is likely that the γECS of nodules is subjected to both translational and posttranslational control under oxidative stress. This conclusion is fully supported by studies showing that the γECS of Arabidopsis can be activated posttranscriptionally in cell suspensions (May et al., 1998) and modified posttranslationally in vitro and in vivo by the redox state (Hicks et al., 2007).
Jasmonates are ubiquitous stress-signaling compounds of plants but few reports have addressed their effects on ascorbate and GSH metabolism. In Arabidopsis, JA enhanced the mRNA levels of DR, MR, γECS, and GSHS, as well as DR activity and ascorbate (Xiang and Oliver, 1998; Sasaki-Sekimoto et al., 2005), whereas MeJA caused up-regulation of GME (Wolucka et al., 2005). However, in broccoli (Brassica oleracea) florets, MeJA increased the mRNA levels of AO and cytosolic APX, MR, and GR, but decreased those of GalLDH and the chloroplastic enzymes (Nishikawa et al., 2003). We found that JA had some major effects on ascorbate and hGSH metabolism in nodules, including a decrease in the ascorbate redox state, accumulation of hGSH, inhibition of γECS activity, and suppression of DR activity. The complete inhibition of DR activity was unexpected, especially because this phenomenon occurred in nodules but not in roots or leaves, and was accompanied by a strong increase of AO mRNA and activity, two effects that should decrease the ascorbate redox state. Indeed, we found, in nodules exposed to JA, a moderate increase of dehydroascorbate (from 15% to 25%), but greater increases are likely to occur in the apoplast, where AO is specifically located (Pignocchi et al., 2003). We propose that in nodules JA causes a decline of the ascorbate redox state in the apoplast through the transcriptional induction of AO and the posttranslational inhibition of DR. These changes are not due to a direct effect of JA on the two enzyme activities but are mediated by other components of the JA signaling pathway still to be defined. In any case, these effects of JA on ascorbate metabolism might influence nodule cell growth and constitute a signal by which nodules perceive and respond to stress situations.
Several studies of nodule aging have reported the accumulation of oxidatively damaged lipids, proteins, and DNA (Evans et al., 1999; Alesandrini et al., 2003), although in other cases no evidence of oxidative stress was found (Groten et al., 2005). These authors suggested that the decrease in ascorbate transported from the shoot to the nodules was a regulating factor of nodule senescence (Groten et al., 2006). In our study, we have observed a progressive loss of ascorbate and an increase of protein oxidation during common bean nodule senescence. A comparison between the two senescing stages of nodules with respect to the ascorbate and hGSH contents and the ascorbate-GSH enzyme activities shows important differences when all these parameters are expressed on a nodule fresh weight basis, which is more indicative of the whole antioxidant capacity of nodules. In the S1 nodules, the total ascorbate content (reduced ascorbate + dehydroascorbate) had decreased by 60%, but the hGSH content and the antioxidant activities were still similar to those of young nodules. Notably, in S1 nodules, the proportion of reduced ascorbate was not affected, probably because AO activity was decreased and the capacity for ascorbate regeneration through DR activity remained intact. In the S2 nodules, ascorbate levels were almost negligible, the hGSH content decreased by 60%, and their redox states and associated enzyme activities also declined significantly. The coexistence in common bean plants of nodules at different stages of senescence and with different ascorbate concentrations and redox states indicates that the life span of nodules is in part controlled by endogenous factors and points to ascorbate as one of the key players.
MATERIALS AND METHODS
Biological Material and Plant Treatments
Common bean (Phaseolus vulgaris) ‘Contender’ seeds were surface sterilized with 70% ethanol, transferred to pots containing a 1:1 (v:v) mixture of perlite:vermiculite, inoculated 7 d later with Rhizobium leguminosarum bv phaseoli strain 3622, and grown in a controlled-environment chamber (Gogorcena et al., 1997). After 23 d, plants were separated at random into seven groups. Two groups served as controls and received dimethyl sulfoxide (DMSO) diluted 1:1,000 (v/v) in nutrient solution (control for the JA treatment) or nutrient solution (control for the other treatments as well as for senescence studies). Another group of plants was treated with 150 mm NaCl for 7 d, and three other groups were treated 3 d later with 100 μm JA (dissolved in DMSO), 100 μm CdCl2, or 10 mm H2O2 for 4 d. Finally, another group of plants was grown until they reached 52 to 54 d of age for studies of nodule senescence. Nodules from these plants were classified into two populations, S1 and S2 (see “Results”), harvested in liquid nitrogen, and immediately stored at −80°C.
Markers of Nodule Senescence and Oxidative Stress
The concentration of Lb in nodule soluble extracts was determined by the pyridine-hemochrome method (Appleby and Bergersen, 1980). Total protein was quantified in the same extracts by the Bradford microassay (Bio-Rad) using bovine serum albumin as the standard. Oxidative damage of lipids was estimated as the content of malondialdehyde formed from decomposition of lipid peroxides at acidic pH after reaction with thiobarbituric acid (Naya et al., 2007). Oxidative damage of proteins was estimated as the content of total carbonyl groups. Proteins were separated on 12.5% SDS gels, and carbonyls were quantified after derivatization with 2,4-dinitrophenylhydrazine using the OxyBlot Protein Oxidation Detection kit (Chemicon). The dinitrophenyl-hydrazone derivatives of proteins were detected on membranes with the SuperSignal West Pico Chemiluminescence kit (Pierce) using a rabbit antibody specific to the hydrazone moiety of the proteins (1:150 dilution; Chemicon) and a peroxidase-conjugate goat anti-rabbit IgG as the secondary antibody (1:3,000 dilution; Sigma-Aldrich).
Assay of Enzyme Activities
All enzymes were extracted at 0°C and assayed at 25°C within linear range. The enzymes were extracted from 50 mg of nodules (or 100 mg for GalLDH) using 0.5 mL of the following optimized media: APX, 50 mm potassium phosphate buffer, pH 7.0, 0.5% (w/v) polyvinylpyrrolidone-10, and 5 mm ascorbate; MR, DR, and GR, 50 mm potassium phosphate buffer, pH 7.8, 1% polyvinylpyrrolidone-10, 0.2 mm EDTA, and 10 mm β-mercaptoethanol; γECS and hGSHS, 50 mm Tris-HCl, pH 8.0, 0.2 mm EDTA, 10 mm MgCl2, and 10% glycerol; AO, 10 mm sodium phosphate buffer, pH 6.5; GalLDH, 50 mm Tris-HCl, pH 8.0, and 0.15% (v/v) Triton X-100. All extracts were centrifuged at 13,000g for 15 min at 4°C, and the enzyme activities were assayed in the supernatants.
APX and DR activities were determined by following ascorbate oxidation at 290 nm (Asada, 1984) and ascorbate formation at 265 nm (Nakano and Asada, 1981), respectively. MR and GR activities were assayed by following the oxidation of NADH (Dalton et al., 1993) and NADPH (Dalton et al., 1986) at 340 nm, respectively. GalLDH activity was determined by following the reduction of cytochrome c at 550 nm (Bartoli et al., 2000).
γECS and hGSHS activities were measured as described (Matamoros et al., 1999) with minor modifications. The reaction mixtures were incubated at 30°C for 120 min (γECS) or 60 min (hGSHS) and were stopped by transferring an aliquot into monobromobimane derivatization solution. The monobromobimane derivatives were resolved on a C18 (4.6 × 250 mm; 5 μm) column (Mallinckrodt-Baker) and were eluted with 15% methanol containing 0.25% acetic acid, pH 3.5, at a flow rate of 1 mL min−1.
AO activity was measured by following ascorbate oxidation at 265 nm (Pignocchi et al., 2003). Nodule extracts were centrifuged (13,000g, 15 min), and the pellet was resuspended by vigorous shaking for 10 min in 10 mm sodium phosphate buffer, pH 6.5, and 1 m NaCl. The extract was centrifuged again and AO activity was determined in the supernatant.
Quantification and Redox State of Ascorbate and (Homo)glutathione
For ascorbate and (homo)glutathione determinations, only nodules that had been harvested directly in liquid nitrogen and stored at −80°C were used. Thiol compounds were extracted with 0.2 m methanesulfonic acid containing 0.5 mm diethylenetriaminepentaacetic acid, were derivatized with 7 mm monobromobimane at pH 8.0 in the dark, and were quantified as fluorescent adducts by HPLC (Matamoros et al., 1999) using the same column as described above. The (homo)glutathione redox state was determined spectrophotometrically by an enzyme-cycling assay (Griffith, 1980). Two aliquots were made for each nodule extract and one of them was treated with 2-vinylpiridine to block free thiol groups. Both aliquots were then incubated with yeast GR, and the NADPH-dependent reduction of the oxidized (homo)glutathione present in each aliquot was followed at 340 nm for 3 min. The two aliquots allowed the quantification of total (homo)glutathione (not treated with 2-vinylpyridine) and oxidized (homo)glutathione (treated with 2-vinylpyridine) using appropriate standards.
Total ascorbate (reduced ascorbate + dehydroascorbate) was measured after incubation of samples with 0.4 mm dithioerythritol for 15 min at room temperature in the dark (Matamoros et al., 2006). Reduced ascorbate was determined in the same way but omitting the incubation with dithioerythritol, and dehydroascorbate was calculated as the difference between the concentrations of total and reduced ascorbate.
Immunoblots and Immunoprecipitation
Proteins were extracted from nodules, roots, and leaves at 0°C with 50 mm potassium phosphate buffer, pH 7.8, containing 0.15% (v/v) Triton X-100, and were quantified by the Bradford microassay (Bio-Rad). Proteins were resolved in 10% (γECS) or 15% (DR) SDS-PAGE gels and were transferred onto polyvinylidene fluoride membranes (Pall) using a transfer buffer consisting of 25 mm Tris-HCl, pH 8.3, 192 mm Gly, and 20% methanol. Equal loading of lanes and transfer quality were verified by Ponceau staining of membranes. Immunoblot analyses were performed using a rabbit polyclonal antibody raised against bean γECS or a guinea pig polyclonal antibody raised against Arabidopsis cytosolic DR (Eltayeb et al., 2006) at dilutions of 1:1,000 or 1:2,500, respectively. Goat anti-rabbit (γECS) or anti-guinea pig (DR) IgG horseradish peroxidase conjugated antibodies (Sigma-Aldrich) were used as secondary antibodies at a dilution of 1:20,000 or 1:5,000, respectively, with 5% (w/v) skim milk to reduce background signal. Immunoreactive proteins were visualized using the SuperSignal West Pico (Pierce) chemiluminescent reagent for peroxidase detection.
The specificity of the antibodies was confirmed by immunoprecipitation of the corresponding proteins. Nodule extracts were incubated at 4°C for 8 h with different amounts of the primary antibody or with 20 mm sodium phosphate buffer, pH 7.4, containing 0.01% (w/v) thimerosal. The extracts were incubated at 0°C for 16 h with the corresponding secondary antibodies at a dilution of 1:1,000 and were centrifuged. Enzyme activities were measured in the supernatant as described above.
Expression Analysis by qRT-PCR
Total RNA was isolated from 50 to 100 mg of nodules using the RNAqueous isolation kit (Ambion) and was treated with DNase I (Roche) at 37°C for 1 h. Contamination with genomic DNA was tested in RNA samples using common bean ubiquitin as the housekeeping gene (Supplemental Table S1). cDNA was synthesized from DNase-treated RNA using (dT)17 and Moloney murine leukemia virus reverse transcriptase (Promega). qRT-PCR analysis was carried out with the iCycler iQ instrument and iQ SYBR-Green Supermix reagent (Bio-Rad) using specific primers (Supplemental Table S1). These were designed based on common bean ESTs for GMP, GME, GalPP, GalDH, γECS, hGSHS, DR1, DR2, and AO. Because ESTs for GalLDH were not available, a partial sequence was obtained by PCR using common bean nodule cDNA as a template and primers (forward, 5′-TCAGAAACATGCTTCCCTGCT-3′; reverse, 5′-CATCCACAGGAAACCGCTTT-3′) based on the sequence of Lotus japonicus GalLDH (accession no. DQ455608). The qRT-PCR program consisted of a first denaturation and Taq activation step at 95°C for 5 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. To verify PCR specificity, the amplified products were subjected to melting curve analysis. The mRNA levels of each gene were normalized with respect to those of common bean ubiquitin and the R values calculated by the method of Livak and Schmittgen (2001).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Primers used for qRT-PCR.
Supplementary Material
Acknowledgments
We thank Dr. Amin E. Eltayeb and Prof. Kiyoshi Tanaka (Tottori University, Japan) for kindly providing us with the anti-DR antibody and Prof. Federico Sánchez (Universidad Nacional Autónoma de Mexico) for making available to us the common bean DR sequence.
This work was supported by Ministerio de Educación y Ciencia-Fondos Europeos de Desarrollo Regional (grant no. AGL2005–01404) and by Gobierno de Aragón (group E33 and grant no. PIP137/2005; predoctoral fellowship [B041/2004] to J.L.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Manuel Becana (becana@eead.csic.es).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Alesandrini F, Mathis R, Van de Sype G, Hérouart D, Puppo A (2003) Possible roles for a cysteine protease and hydrogen peroxide in soybean nodule development and senescence. New Phytol 158 131–138 [Google Scholar]
- Appleby CA, Bergersen FJ (1980) Preparation and experimental use of leghemoglobin. In FJ Bergersen, ed, Methods for Evaluating Biological Nitrogen Fixation. John Wiley, Chichester, UK, pp 315–335
- Asada K (1984) Chloroplasts: formation of active oxygen and its scavenging. Methods Enzymol 105 422–429 [Google Scholar]
- Bartoli CG, Guiamet JJ, Kiddle G, Pastori GM, Di Cagno R, Theodoulou FL, Foyer CH (2005) Ascorbate content of wheat leaves is not determined by maximal L-galactono-1,4-lactone dehydrogenase (GalLDH) activity under drought stress. Plant Cell Environ 28 1073–1081 [Google Scholar]
- Bartoli CG, Pastori GM, Foyer CH (2000) Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol 123 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DA, Baird LM, Langeberg L, Taugher CY, Anyan WR, Vance CP, Sarath G (1993) Subcellular localization of oxygen defense enzymes in soybean (Glycine max [L.] Merr.) root nodules. Plant Physiol 102 481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DA, Joyner SL, Becana M, Iturbe-Ormaetxe I, Chatfield JM (1998) Antioxidant defenses in the peripheral cell layers of legume root nodules. Plant Physiol 116 37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DA, Russell SA, Hanus FJ, Pascoe GA, Evans HJ (1986) Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci USA 83 3811–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Morishima I, Shibahara T, Inanaga S, Tanaka K (2006) Enhanced tolerance to ozone and drought stresses in transgenic tobacco overexpressing dehydroascorbate reductase in cytosol. Physiol Plant 127 57–65 [Google Scholar]
- Evans PJ, Gallesi D, Mathieu C, Hernández MJ, de Felipe M, Halliwell B, Puppo A (1999) Oxidative stress occurs during soybean nodule senescence. Planta 208 73–79 [Google Scholar]
- Frendo P, Gallesi D, Turnbull R, Van de Sype G, Hérouart D, Puppo A (1999) Localisation of glutathione and homoglutathione in Medicago truncatula is correlated to a differential expression of genes involved in their synthesis. Plant J 17 215–219 [Google Scholar]
- Frendo P, Harrison J, Norman C, Hernández-Jiménez MJ, Van de Sype G, Gilabert A, Puppo A (2005) Glutathione and homoglutathione play a critical role in the nodulation process of Medicago truncatula. Mol Plant Microbe Interact 18 254–259 [DOI] [PubMed] [Google Scholar]
- Gogorcena Y, Gordon AJ, Escuredo PR, Minchin FR, Witty JF, Moran JF, Becana M (1997) N2 fixation, carbon metabolism, and oxidative damage in nodules of dark-stressed common bean plants. Plant Physiol 113 1193–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106 207–212 [DOI] [PubMed] [Google Scholar]
- Groten K, Dutilleul C, Van Heerden PDR, Vanacker H, Bernard S, Finkemeier I, Dietz KJ, Foyer CH (2006) Redox regulation of peroxiredoxin and proteinases by ascorbate and thiols during pea root nodule senescence. FEBS Lett 580 1269–1276 [DOI] [PubMed] [Google Scholar]
- Groten K, Vanacker H, Dutilleul C, Bastian F, Bernard S, Carzaniga R, Foyer CH (2005) The roles of redox processes in pea nodule development and senescence. Plant Cell Environ 28 1293–1304 [Google Scholar]
- Halliwell B, Gutteridge JMC (2007) Free Radicals in Biology and Medicine, Ed 4. Oxford University Press, Oxford
- Hicks LM, Cahoon RE, Bonner ER, Rivard RS, Sheffield J, Jez JM (2007) Thiol-based regulation of redox-active glutamate-cysteine ligase from Arabidopsis thaliana. Plant Cell 19 2653–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturbe-Ormaetxe I, Matamoros MA, Rubio MC, Dalton DA, Becana M (2001) The antioxidants of legume nodule mitochondria. Mol Plant Microbe Interact 14 1189–1196 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔCt method. Methods 25 402–408 [DOI] [PubMed] [Google Scholar]
- Matamoros MA, Loscos J, Coronado MJ, Ramos J, Sato S, Testillano PS, Tabata S, Becana M (2006) Biosynthesis of ascorbic acid in legume root nodules. Plant Physiol 141 1068–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamoros MA, Moran JF, Iturbe-Ormaetxe I, Rubio MC, Becana M (1999) Glutathione and homoglutathione synthesis in legume root nodules. Plant Physiol 121 879–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Sánchez-Fernández R, Van Montagu M, Inzé D (1998) Evidence for posttranscriptional activation of γ-glutamylcysteine synthetase during plant stress responses. Proc Natl Acad Sci USA 95 12049–12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9 490–498 [DOI] [PubMed] [Google Scholar]
- Moran JF, Iturbe-Ormaetxe I, Matamoros MA, Rubio MC, Clemente MR, Brewin NJ, Becana M (2000) Glutathione and homoglutathione synthetases of legume nodules. Cloning, expression, and subcellular localization. Plant Physiol 124 1381–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase is spinach chloroplasts. Plant Cell Physiol 22 867–880 [Google Scholar]
- Naya L, Ladrera R, Ramos J, González EM, Arrese-Igor C, Minchin FR, Becana M (2007) The response of carbon metabolism and antioxidant defenses of alfalfa nodules to drought stress and to the subsequent recovery of plants. Plant Physiol 144 1104–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa F, Kato M, Hyodo H, Ikoma Y, Sugiura M, Yano M (2003) Ascorbate metabolism in harvested broccoli. J Exp Bot 54 2439–2448 [DOI] [PubMed] [Google Scholar]
- Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15 939–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateraki I, Sanmartin M, Kalamaki MS, Gerasopoulos D, Kanellis AK (2004) Molecular characterization and expression studies during melon fruit development and ripening of L-galactono-1,4-lactone dehydrogenase. J Exp Bot 55 1623–1633 [DOI] [PubMed] [Google Scholar]
- Pignocchi C, Fletcher JM, Wilkinson JE, Barnes JD, Foyer CH (2003) The function of ascorbate oxidase in tobacco. Plant Physiol 132 1631–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez M, Graham MA, Blanco-López L, Silvente S, Medrano-Soto A, Blair MW, Hernández G, Vance CP, Lara M (2005) Sequencing and analysis of common bean ESTs. Building a foundation for functional genomics. Plant Physiol 137 1211–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki-Sekimoto Y, Taki N, Obayashi T, Aono M, Matsumoto F, Sakurai N, Suzuki H, Hirai MY, Noji M, Saito K, et al (2005) Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J 44 653–668 [DOI] [PubMed] [Google Scholar]
- Siendones E, González-Reyes JA, Santos-Ocaña C, Navas P, Córdoba F (1999) Biosynthesis of ascorbic acid in kidney bean. l-Galactono-γ-lactone dehydrogenase is an intrinsic protein located at the mitochondrial inner membrane. Plant Physiol 120 907–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N (2000) Ascorbic acid: metabolism and functions of a multi-facetted molecule. Curr Opin Plant Biol 3 229–235 [PubMed] [Google Scholar]
- Tabata K, Takaoka T, Esaka M (2002) Gene expression of ascorbic acid-related enzymes in tobacco. Phytochemistry 61 631–635 [DOI] [PubMed] [Google Scholar]
- Tamaoki M, Mukai F, Asai N, Nakajima N, Kubo A, Aono M, Saji H (2003) Light-controlled expression of a gene encoding l-galactono-γ-lactone dehydrogenase which affects ascorbate pool size in Arabidopsis thaliana. Plant Sci 164 1111–1117 [Google Scholar]
- Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot (Lond) 100 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393 365–369 [DOI] [PubMed] [Google Scholar]
- Wolucka BA, Goossens A, Inzé D (2005) Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions. J Exp Bot 56 2527–2538 [DOI] [PubMed] [Google Scholar]
- Xiang C, Oliver DJ (1998) Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10 1539–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zopes H, Klapheck S, Bergmann L (1993) The function of homoglutathione and hydroxymethylglutathione for the scavenging of hydrogen peroxide. Plant Cell Physiol 34 515–521 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.