Plants are equipped with an array of defense mechanisms to protect themselves against attack by herbivorous insects and microbial pathogens. Some of these defense mechanisms are preexisting, whereas others are only activated upon insect or pathogen invasion. Induced defense responses entail fitness costs. Therefore, plants possess elaborate regulatory mechanisms that efficiently coordinate the activation of attacker-specific defenses so that fitness costs are minimized, while optimal resistance is attained (Pieterse and Dicke, 2007). A major focus in plant defense-signaling research is to uncover key mechanisms by which plants tailor their responses to different attackers and to investigate how plants cope with simultaneous interactions with multiple aggressors.
During their lifetime, plants encounter numerous herbivorous insects and microbial pathogens with diverse modes of attack. To survive, plants have to perceive attack by these deleterious organisms and respond adequately by activating appropriate defense responses. The primary immune response has evolved to recognize common features of organisms that interact with the plant and to translate this recognition into a defense response that is specifically directed against the invader encountered (Jones and Dangl, 2006). In addition to this attacker-specific primary immune response, plants can activate another line of defense that is referred to as induced resistance. This type of resistance often acts systemically throughout the plant and is typically effective against a broad spectrum of attackers (Walters et al., 2007). Plants are able to activate different types of induced resistance, depending on the organism that interacts with the plant. Well-studied examples of induced resistance are systemic acquired resistance, which is triggered by pathogens causing limited infection, such as hypersensitive necrosis (Durrant and Dong, 2004), rhizobacteria-induced systemic resistance, which is activated upon colonization of roots by selected strains of nonpathogenic rhizobacteria (Van Loon et al., 1998), and wound-induced resistance, which is typically elicited upon tissue damage such as that caused by insect feeding (Kessler and Baldwin, 2002; Howe, 2004). The role of phytohormones in the regulation of these induced defenses is well established. Salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) are recognized as key players in the regulation of the signaling pathways involved (Howe, 2004; Pozo et al., 2004; Lorenzo and Solano, 2005; Grant and Lamb, 2006; Van Loon et al., 2006a; Von Dahl and Baldwin, 2007). Other plant hormones, including abscisic acid (ABA; Mauch-Mani and Mauch, 2005), brassinosteroids (Nakashita et al., 2003), and auxin (Navarro et al., 2006; Wang et al., 2007), have also been implicated in plant defense, but their significance is less well studied.
SPECIFICITY OF PLANT SELF-DEFENSE
Upon pathogen or insect attack, plants respond with production of a specific blend of the alarm signals SA, JA, and ET, which varies greatly in quantity, composition, and timing. It is thought that this so-called signal signature contributes to the specificity of the plant's primary induced defense response (Reymond and Farmer, 1998; De Vos et al., 2005). The signaling pathways that are activated upon endogenous accumulation of these signals regulate different defense responses that are effective against partially distinct classes of attackers. Although there are exceptions (Thaler et al., 2004), generally it can be stated that pathogens with a biotrophic lifestyle are more sensitive to SA-mediated induced defenses, whereas necrotrophic pathogens and herbivorous insects are resisted more through JA/ET-mediated defenses (Thomma et al., 2001; Kessler and Baldwin, 2002; Glazebrook, 2005). In nature, however, plants often deal with simultaneous or subsequent invasion by multiple aggressors, which can influence the primary induced defense response of the host plant (Van der Putten et al., 2001; Bezemer and Van Dam, 2005; Stout et al., 2006). Hence, plants need regulatory mechanisms to effectively adapt to changes in their hostile environment. Cross talk between induced defense-signaling pathways is thought to provide the plant with such a powerful regulatory potential. Signaling interactions can be either mutually antagonistic or synergistic, resulting in negative or positive functional outcomes. Hence, cross talk can be interpreted as an inclusive term for the interaction between signaling pathways (Bostock, 2005). Cross talk helps the plant to minimize energy costs and create a flexible signaling network that allows the plant to finely tune its defense response to the invaders encountered (Reymond and Farmer, 1998; Pieterse et al., 2001; Bostock, 2005). Yet, it seems that insect herbivores and pathogens have also evolved to manipulate plants for their own benefit by suppressing induced defenses through modulation of the plant's defense-signaling network (Pieterse and Dicke, 2007).
DEFENSE SIGNAL INTERACTIONS TO FINE-TUNE DEFENSE
Molecular and genomic tools are now being used to uncover the complexity of the induced defense-signaling networks that have evolved during the arms race between plants and their attackers (Pieterse and Dicke, 2007). Global expression-profiling studies provided ample evidence that SA, JA, and ET pathways interact, either positively or negatively (Glazebrook et al., 2003; De Vos et al., 2005). One of the best-characterized examples of defense-related signal cross talk is the interaction between the SA and JA response pathways (Rojo et al., 2003; Bostock, 2005; Beckers and Spoel, 2006). Although most reports indicate a mutually antagonistic interaction between SA- and JA-dependent signaling, synergistic interactions have been described as well (Schenk et al., 2000; Van Wees et al., 2000; Mur et al., 2006). As a result of negative cross talk between SA and JA, activation of the SA response should render a plant more susceptible to attackers that are resisted via JA-dependent defenses and vice versa (Fig. 1). Indeed, many examples of trade-offs between SA-dependent resistance against biotrophic pathogens and JA-dependent defense against insect herbivory and necrotrophic pathogens have been reported (Pieterse et al., 2001; Bostock, 2005; Stout et al., 2006). In Arabidopsis (Arabidopsis thaliana), Spoel et al. (2007) recently showed that SA-mediated defenses that are triggered upon infection by a virulent strain of the biotrophic pathogen Pseudomonas syringae rendered infected tissues more susceptible to infection by the necrotrophic pathogen Alternaria brassicicola by suppressing the JA-signaling pathway. Similarly, infection by the biotrophic pathogen Hyaloperonospora parasitica strongly suppressed JA-mediated defenses that were activated upon feeding by caterpillars of the small cabbage white Pieris rapae (H.A. Leon-Reyes and C.M.J. Pieterse, unpublished data).
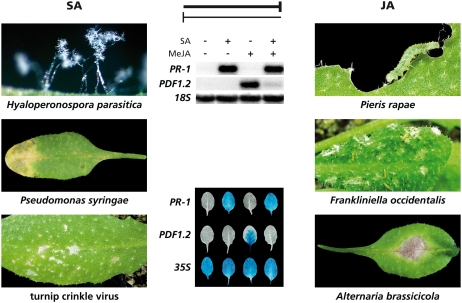
Figure 1.
Schematic representation of mutually antagonistic cross talk between SA- and JA-dependent defense-signaling pathways in Arabidopsis. SA-mediated defenses are predominantly effective against biotrophic pathogens, such as H. parasitica, P. syringae, and Turnip crinkle virus, whereas JA-mediated defenses are primarily effective against herbivorous insects, such as P. rapae caterpillars and thrips (Frankliniella occidentalis), and against necrotrophic pathogens, such as A. brassicicola. Cross talk between SA and JA signaling is typically visualized by monitoring the expression of SA-responsive genes, such as PR-1, and JA-responsive genes, such as PDF1.2. In the middle of the figure, a pharmacological experiment is shown in which exogenous application of 1 mm SA to the leaves of wild-type Arabidopsis Col-0 plants resulted in the accumulation of PR-1 mRNA and activation of the PR-1 promoter that is fused to the GUS reporter gene. Application of 100 μm MeJA resulted in the accumulation of PDF1.2 mRNA and the activation of the PDF1.2 promoter that is fused to the GUS reporter gene. The combined treatment with SA and MeJA resulted in strong SA-mediated suppression of the JA-responsive PDF1.2 gene, thereby exemplifying the antagonistic effect of SA on JA signaling. Depending on the plant-attacker interaction, SA/JA cross talk has been demonstrated in both directions. Photos: Hans van Pelt.
Besides SA/JA cross talk, interactions between SA and ET, JA and ABA, and JA and ET have been shown to function in the adaptive response of plants to herbivores and pathogens with different lifestyles. For instance, ET produced by Arabidopsis upon herbivory by P. rapae was demonstrated to prime the plant for enhanced SA-mediated defenses that are activated upon infection by Turnip crinkle virus, resulting in enhanced resistance against this biotrophic pathogen (De Vos et al., 2006). Furthermore, transcription factors MYC2 and ETHYLENE RESPONSE FACTOR1 (ERF1) were found to function as important signaling nodes that integrate signals from the JA, ABA, and ET pathways (Anderson et al., 2004; Lorenzo et al., 2004). MYC2-dependent gene expression is synergistically induced by JA and ABA in response to wounding, whereas ERF1-dependent gene induction is controlled by the combined action of JA and ET in response to pathogen attack. Depending on the signal input, these transcription factors differentially activate JA-responsive pathogen defense and wound response genes (Lorenzo and Solano, 2005; Dombrecht et al., 2007), thereby modulating the primary JA response to enhanced defense against necrotrophic pathogens or insect herbivores, respectively.
DECOY OF PLANT DEFENSE
Cross talk between defense-signaling pathways is thought to help the plant decide which defensive strategy to follow, depending on the type of attacker it is encountering. Yet, it seems that attackers have also evolved to manipulate plants for their own benefit by suppressing induced defenses or modulating the defense-signaling network (Pieterse and Dicke, 2007). For instance, herbivorous nymphs of the silverleaf whitefly (Bemisia tabaci) may activate the SA-signaling pathway as a decoy strategy because effectual JA-dependent defenses are consequently suppressed, resulting in enhanced insect performance (Zarate et al., 2007). Similarly, egg-derived elicitors from P. rapae and the large cabbage white Pieris brassicae have been suggested to suppress JA-dependent defenses via SA/JA cross talk to benefit hatching larvae (Little et al., 2007). Microbial pathogens have acquired the ability to manipulate the plant's signaling infrastructure by producing phytohormones or their functional mimics to trick the plant into activating inappropriate defenses (Robert-Seilaniantz et al., 2007). For instance, virulent P. syringae bacteria produce coronatine that functions as a potent mimic of JA-Ile (Nomura et al., 2005). It is assumed that coronatine triggers hypersensitive induction of JA-Ile responses, resulting in suppression of effectual SA-dependent defenses through pathway cross talk, as well as enhanced growth of the pathogen (Zhao et al., 2003; Brooks et al., 2005; Cui et al., 2005; Laurie-Berry et al., 2006).
MOLECULAR PLAYERS IN SA/JA CROSS TALK
Several studies have demonstrated that exogenous application of SA suppresses the expression of JA biosynthesis genes, suggesting that SA may target the JA biosynthesis pathway to suppress downstream JA signaling (Peña-Cortés et al., 1993; Doares et al., 1995; Spoel et al., 2003). However, Doares et al. (1995) provided evidence that exogenous application of SA to tomato (Solanum lycopersicum) plants strongly inhibited the JA-induced expression of genes encoding proteinase inhibitors I and II, suggesting that SA also targets the JA pathway downstream of JA biosynthesis. Over the past years, various regulatory components with a role in SA/JA cross talk have been identified. Below, we will discuss the most prominent molecular players of SA/JA cross talk in more detail.
NPR1
SA plays a central role in the regulation of systemic acquired resistance, which is predominantly effective against biotrophic pathogens. Transduction of the SA signal leads to activation of genes encoding pathogenesis-related (PR) proteins, some of which have antimicrobial activity (Van Loon et al., 2006b). The regulatory protein NONEXPRESSOR OF PR GENES1 (NPR1) is required for transduction of the SA signal because mutations in the NPR1 gene render the plant largely unresponsive to pathogen-induced SA production (Dong, 2004). In wild-type Arabidopsis cells, SA-mediated changes in the redox status regulate the nucleocytoplasmic localization of NPR1. Upon localization to the nucleus, NPR1 interacts with TGA transcription factors, resulting in the activation of SA-responsive PR genes (Dong, 2004). SA-mediated suppression of JA-inducible gene expression is blocked in mutant npr1 plants, demonstrating a crucial role for NPR1 in the cross talk between SA and JA signaling (Spoel et al., 2003, 2007). Using npr1 plants expressing recombinant NPR1 protein with a glucocorticoid receptor hormone-binding domain to control the nucleocytoplasmic localization of the NPR1 protein, Spoel et al. (2003) showed that nuclear localization of NPR1 is not required for SA-mediated suppression of the JA response. This indicates that the SA-induced suppression of the JA response is controlled by a novel function of NPR1 in the cytosol. Recently, a similar function of NPR1 in cross talk was reported in rice (Oryza sativa; Yuan et al., 2007). Overexpression of cytosolic OsNPR1 suppressed JA-responsive transcription and enhanced the level of susceptibility to insect herbivory. Moreover, NPR1-dependent suppression of the JA response was no longer present in plants expressing OsNPR1 that was constitutively targeted to the nucleus. Interestingly, a recent report on NPR1-silenced wild tobacco (Nicotiana attenuata) plants demonstrated that these transgenic plants accumulated increased levels of SA upon insect herbivory and were highly susceptible to herbivore attack (Rayapuram and Baldwin, 2007). It was proposed that in wild-type plants NPR1 is required to negatively regulate SA production during herbivore attack and thus suppress SA/JA cross talk to allow induction of JA-mediated defenses against herbivores. These results highlight the regulatory role of NPR1 in cross talk. Yet, it also demonstrates that molecular mechanisms of induced defense as identified in model systems should be tested in the ecological context in which the plant species under study coevolved with its natural enemies to fully understand its biological function.
WRKY TRANSCRIPTION FACTORS
WRKY transcription factors are important regulators of SA-dependent defense responses (Maleck et al., 2000; Wang et al., 2006) and some of them have been implicated in SA/JA cross talk. Arabidopsis WRKY70 was identified as a node of convergence between SA and JA signaling when Li et al. (2004) showed that overexpression of WRKY70 caused enhanced expression of SA-responsive PR genes and concomitantly suppressed methyl jasmonate (MeJA)-induced expression of the JA-responsive marker gene PLANT DEFENSIN1.2 (PDF1.2). Hence, WRKY70 acts as a positive regulator of the SA-mediated defenses while repressing the JA response. Besides WRKY70, WRKY11 and WRKY17 of Arabidopsis have also been implicated in SA/JA cross talk. In a double mutant in which WRKY11 and WRKY17 were knocked out, transcripts of SA-responsive genes accumulated to higher levels, whereas those of JA-responsive genes were notably lower. Expression of WRKY70 was up-regulated in this double mutant, suggesting that WRKY11 and WRKY17 function as negative regulators of WRKY70 (Journot-Catalino et al., 2006). Recently, WRKY62 was added to the list of WRKY transcription factors with a putative role in SA/JA cross talk. Mao et al. (2007) reported that the expression of WRKY62 was synergistically induced by SA and JA in wild-type Columbia-0 plants, but not in mutant npr1-3. Furthermore, transposon-tagged wrky62 plants showed enhanced MeJA-induced transcription of the JA-responsive genes LIPOXYGENASE2 (LOX2) and VEGETATIVE STORAGE PROTEIN2 (VSP2), whereas overexpression of WRKY62 resulted in suppression of these genes. These findings point to a repressive effect of WRKY62 on the JA response.
GLUTAREDOXIN GRX480
Another putative regulator in SA/JA cross talk is the glutaredoxin GRX480. Glutaredoxins catalyze thiol disulfide reductions and have been implicated in redox-dependent regulation of protein activities (Lemaire, 2004). Recently, Ndamukong et al. (2007) identified this glutaredoxin in a two-hybrid screen for interactors with TGA transcription factors. Expression of GRX480 was found to be inducible by SA and dependent on NPR1. Interestingly, overexpression of GRX480 completely abolished MeJA-induced PDF1.2 expression, but hardly affected the induction of the JA-responsive genes LOX2 and VSP2. This suggests that GRX480 affects only a subset of the JA-responsive genes that are sensitive to SA-mediated suppression. The suppressive effect of GRX480 on PDF1.2 induction was abolished in the tga2 tga5 tga6 triple mutant, indicating that the interaction between GRX480 and TGA transcription factors is essential for GRX480-dependent cross talk (Ndamukong et al., 2007). These results suggest a model in which SA-activated NPR1 induces GRX480, which in turn interacts with TGA transcription factors to suppress JA-responsive gene induction. Recently, we found that the antagonistic effect of SA on JA-responsive gene transcription is linked to SA-induced changes in the cellular redox potential (A. Koornneef, T. Ritsema, and C.M.J. Pieterse, unpublished data), corroborating the notion that SA/JA cross talk is redox regulated.
MPK4
Mitogen-activated protein (MAP) kinases transfer information from sensors to cellular responses in all eukaryotes. It is therefore not surprising that several MAP kinases have been implicated in plant defense signaling (Menke et al., 2004; Nakagami et al., 2005). Petersen et al. (2000) identified MAP KINASE4 (MPK4) as a negative regulator of SA signaling and a positive regulator of JA signaling in Arabidopsis. Inactivation of MPK4 in mutant mpk4 plants resulted in elevated SA levels and constitutive expression of SA-responsive PR genes, suppression of JA-responsive genes, and enhanced susceptibility to the necrotroph A. brassicicola. Interestingly, the mpk4 mutation blocked JA-responsive gene expression independently of SA accumulation, as SA-nonaccumulating mpk4/NahG transgenics still exhibited increased susceptibility to A. brassicicola and suppression of MeJA-induced PDF1.2 expression (Petersen et al., 2000; Brodersen et al., 2006). ENHANCED DISEASE SUSCEPTIBILITY1 (EDS1) and PHYTOALEXIN-DEFICIENT4 (PAD4) were identified as downstream effectors of MPK4 function, having the opposite effect of MPK4 by behaving as activators of SA signaling and repressors of JA signaling (Brodersen et al., 2006). Another target of MPK4 is its substrate MAP KINASE 4 SUBSTRATE1 (MKS1). Phosphorylation of MKS1 is thought to repress SA signaling because MKS1-RNAi could partially rescue the PR-1-overexpressing phenotype of mpk4. However, over- or underexpression of MKS1 did not affect PDF1.2 gene expression, indicating that other downstream targets of MPK4 are involved in JA signaling. MKS1 was demonstrated to interact with two WRKY transcription factors, WRKY25 and WRKY33, which can both be phosphorylated by MPK4 (Andreasson et al., 2005). These WRKYs might be downstream targets of MPK4 that contribute to the repression of SA responses because overexpression of both WRKY25 and WRKY33 resulted in decreased pathogen-induced PR-1 expression and enhanced susceptibility to P. syringae (Zheng et al., 2006, 2007). Interestingly, wrky33 mutant plants showed increased susceptibility to the necrotrophs Botrytis cinerea and A. brassicicola and reduced PDF1.2 expression (Zheng et al., 2006), again highlighting the role of WRKY transcription factors in SA/JA cross talk.
CONCLUDING REMARKS
In the past years, significant progress was made in elucidating the molecular mechanism underlying the interplay between hormone-regulated defense-signaling pathways. Several molecular players in pathway cross talk have been identified. However, translation of molecular mechanisms to predictability of trade-offs between herbivore and pathogen resistance requires additional research. To date, studies on trade-offs between induced insect and pathogen resistance have often been performed with individual plant-attacker interactions under a limited set of abiotic conditions. This type of research is highly valuable because only under controlled conditions can the highly flexible induced defense-signaling network be uncovered and novel mechanisms of regulation elucidated. However, because plant defense mechanisms evolved during the coevolutionary arms race between plants and their natural enemies and come with costs in addition to benefits, insights into their biological significance should ideally come from ecological studies. Therefore, to understand the functioning of the complex defense-signaling network in nature, molecular biologists and ecologists should join forces to place molecular mechanisms of induced plant defenses in an ecological perspective.
This work was supported in part by the Earth and Life Sciences Foundation (grant nos. 865.04.002 and 813.06.002), which is subsidized by the Netherlands Organization of Scientific Research.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Corné M.J. Pieterse (c.m.j.pieterse@uu.nl).
References
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K (2004) Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16 3460–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson E, Jenkins T, Brodersen P, Thorgrimsen S, Petersen NHT, Zhu SJ, Qiu JL, Micheelsen P, Rocher A, Petersen M, et al (2005) The MAP kinase substrate MKS1 is a regulator of plant defense responses. EMBO J 24 2579–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers GJM, Spoel SH (2006) Fine-tuning plant defence signalling: salicylate versus jasmonate. Plant Biol 8 1–10 [DOI] [PubMed] [Google Scholar]
- Bezemer TM, Van Dam NM (2005) Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol Evol 20 617–624 [DOI] [PubMed] [Google Scholar]
- Bostock RM (2005) Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annu Rev Phytopathol 43 545–580 [DOI] [PubMed] [Google Scholar]
- Brodersen P, Petersen M, Bjorn Nielsen H, Zhu S, Newman M-A, Shokat KM, Rietz S, Parker J, Mundy J (2006) Arabidopsis MAP kinase 4 regulates salicylic acid- and jasmonic acid/ethylene-dependent responses via EDS1 and PAD4. Plant J 47 532–546 [DOI] [PubMed] [Google Scholar]
- Brooks DM, Bender CL, Kunkel BN (2005) The Pseudomonas syringae phytotoxin coronatine promotes virulence by overcoming salicylic acid-dependent defences in Arabidopsis thaliana. Mol Plant Pathol 6 629–639 [DOI] [PubMed] [Google Scholar]
- Cui J, Bahrami AK, Pringle EG, Hernandez-Guzman G, Bender CL, Pierce NE, Ausubel FM (2005) Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc Natl Acad Sci USA 102 1791–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M, Van Oosten VR, Van Poecke RMP, Van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Métraux JP, Van Loon LC, Dicke M, et al (2005) Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact 18 923–937 [DOI] [PubMed] [Google Scholar]
- De Vos M, Van Zaanen W, Koornneef A, Korzelius JP, Dicke M, Van Loon LC, Pieterse CMJ (2006) Herbivore-induced resistance against microbial pathogens in Arabidopsis. Plant Physiol 142 352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doares SH, Narváez-Vásquez J, Conconi A, Ryan CA (1995) Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol 108 1741–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrecht B, Xue GP, Sprague SJ, Kirkegaard JA, Ross JJ, Reid JB, Fitt GP, Sewelam N, Schenk PM, Manners JM, et al (2007) MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 19 2225–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X (2004) NPR1, all things considered. Curr Opin Plant Biol 7 547–552 [DOI] [PubMed] [Google Scholar]
- Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42 185–209 [DOI] [PubMed] [Google Scholar]
- Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43 205–227 [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Chen W, Estes B, Chang HS, Nawrath C, Métraux JP, Zhu T, Katagiri F (2003) Topology of the network integrating salicylate and jasmonate signal transduction derived from global expression phenotyping. Plant J 34 217–228 [DOI] [PubMed] [Google Scholar]
- Grant M, Lamb C (2006) Systemic immunity. Curr Opin Plant Biol 9 414–420 [DOI] [PubMed] [Google Scholar]
- Howe GA (2004) Jasmonates as signals in the wound response. J Plant Growth Regul 23 223–237 [Google Scholar]
- Jones JDG, Dangl JL (2006) The plant immune system. Nature 444 323–329 [DOI] [PubMed] [Google Scholar]
- Journot-Catalino N, Somssich IE, Roby D, Kroj T (2006) The transcription factors WRKY11 and WRKY17 act as negative regulators of basal resistance in Arabidopsis thaliana. Plant Cell 18 3289–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53 299–328 [DOI] [PubMed] [Google Scholar]
- Laurie-Berry N, Joardar V, Street IH, Kunkel BN (2006) The Arabidopsis thaliana JASMONATE INSENSITIVE 1 gene is required for suppression of salicylic acid-dependent defenses during infection by Pseudomonas syringae. Mol Plant Microbe Interact 19 789–800 [DOI] [PubMed] [Google Scholar]
- Lemaire S (2004) The glutaredoxin family in oxygenic photosynthetic organisms. Photosynth Res 79 305–318 [DOI] [PubMed] [Google Scholar]
- Li J, Brader G, Palva ET (2004) The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 16 319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little D, Gouhier-Darimont C, Bruessow F, Reymond P (2007) Oviposition by pierid butterflies triggers defense responses in Arabidopsis. Plant Physiol 143 784–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Chico JM, Sanchez-Serrano JJ, Solano R (2004) JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16 1938–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Solano R (2005) Molecular players regulating the jasmonate signalling network. Curr Opin Plant Biol 8 532–540 [DOI] [PubMed] [Google Scholar]
- Maleck K, Levine A, Eulgem T, Morgan A, Schmid J, Lawton KA, Dangl JL, Dietrich RA (2000) The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat Genet 26 403–410 [DOI] [PubMed] [Google Scholar]
- Mao P, Duan M, Wei C, Li Y (2007) WRKY62 transcription factor acts downstream of cytosolic NPR1 and negatively regulates jasmonate-responsive gene expression. Plant Cell Physiol 48 833–842 [DOI] [PubMed] [Google Scholar]
- Mauch-Mani B, Mauch F (2005) The role of abscisic acid in plant-pathogen interactions. Curr Opin Plant Biol 8 409–414 [DOI] [PubMed] [Google Scholar]
- Menke FLH, Van Pelt JA, Pieterse CMJ, Klessig DF (2004) Silencing of the mitogen-activated protein kinase MPK6 compromises disease resistance in Arabidopsis. Plant Cell 16 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur LAJ, Kenton P, Atzorn R, Miersch O, Wasternack C (2006) The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol 140 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami H, Pitzschke A, Hirt H (2005) Emerging MAP kinase pathways in plant stress signaling. Trends Plant Sci 10 339–346 [DOI] [PubMed] [Google Scholar]
- Nakashita H, Yasuda M, Nitta T, Asami T, Fujioka S, Arai Y, Sekimata K, Takatsuto S, Yamaguchi I, Yoshida S (2003) Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J 33 887–898 [DOI] [PubMed] [Google Scholar]
- Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JDG (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312 436–439 [DOI] [PubMed] [Google Scholar]
- Ndamukong I, Abdallat AA, Thurow C, Fode B, Zander M, Weigel R, Gatz C (2007) SA-inducible Arabidopsis glutaredoxin interacts with TGA factors and suppresses JA-responsive PDF1.2 transcription. Plant J 50 128–139 [DOI] [PubMed] [Google Scholar]
- Nomura K, Melotto M, He SY (2005) Suppression of host defense in compatible plant-Pseudomonas syringae interactions. Curr Opin Plant Biol 8 361–368 [DOI] [PubMed] [Google Scholar]
- Peña-Cortés H, Albrecht T, Prat S, Weiler EW, Willmitzer L (1993) Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta 191 123–128 [Google Scholar]
- Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, Nielsen HB, Lacy M, Austin MJ, Parker JE, et al (2000) Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell 103 1111–1120 [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Dicke M (2007) Plant interactions with microbes and insects: from molecular mechanisms to ecology. Trends Plant Sci 12 564–569 [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Ton J, Van Loon LC (2001) Cross-talk between plant defence signalling pathways: boost or burden? AgBiotechNet 3 ABN 068
- Pozo MJ, Van Loon LC, Pieterse CMJ (2004) Jasmonates-signals in plant-microbe interactions. J Plant Growth Regul 23 211–222 [Google Scholar]
- Rayapuram C, Baldwin IT (2007) Increased SA in NPR1-silenced plants antagonizes JA and JA-dependent direct and indirect defenses in herbivore-attacked Nicotiana attenuata in nature. Plant J 52 700–715 [DOI] [PubMed] [Google Scholar]
- Reymond P, Farmer EE (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol 1 404–411 [DOI] [PubMed] [Google Scholar]
- Robert-Seilaniantz A, Navarro L, Bari R, Jones JDG (2007) Pathological hormone imbalances. Curr Opin Plant Biol 10 372–379 [DOI] [PubMed] [Google Scholar]
- Rojo E, Solano R, Sanchez-Serrano JJ (2003) Interactions between signaling compounds involved in plant defense. J Plant Growth Regul 22 82–98 [Google Scholar]
- Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA 97 11655–11660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Johnson JS, Dong X (2007) Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc Natl Acad Sci USA 104 18842–18847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Koornneef A, Claessens SMC, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Métraux JP, Brown R, Kazan K, et al (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15 760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout MJ, Thaler JS, Thomma BPHJ (2006) Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu Rev Entomol 51 663–689 [DOI] [PubMed] [Google Scholar]
- Thaler JS, Owen B, Higgins VJ (2004) The role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles. Plant Physiol 135 530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Penninckx IAMA, Broekaert WF, Cammue BPA (2001) The complexity of disease signaling in Arabidopsis. Curr Opin Immunol 13 63–68 [DOI] [PubMed] [Google Scholar]
- Van der Putten WH, Vet LEM, Harvey JA, Wäckers FL (2001) Linking above- and below-ground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends Ecol Evol 16 547–554 [Google Scholar]
- Van Loon LC, Bakker PAHM, Pieterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36 453–483 [DOI] [PubMed] [Google Scholar]
- Van Loon LC, Geraats BPJ, Linthorst HJM (2006. a) Ethylene as a modulator of disease resistance in plants. Trends Plant Sci 11 184–191 [DOI] [PubMed] [Google Scholar]
- Van Loon LC, Rep M, Pieterse CMJ (2006. b) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44 135–162 [DOI] [PubMed] [Google Scholar]
- Van Wees SCM, De Swart EAM, Van Pelt JA, Van Loon LC, Pieterse CMJ (2000) Enhancement of induced disease resistance by simultaneous activation of salicylate- and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc Natl Acad Sci USA 97 8711–8716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Dahl CC, Baldwin IT (2007) Deciphering the role of ethylene in plant-herbivore interactions. J Plant Growth Regul 26 201–209 [Google Scholar]
- Walters D, Newton A, Lyon G (2007) Induced Resistance for Plant Defence: A Sustainable Approach to Crop Protection. Blackwell, Oxford
- Wang D, Amornsiripanitch N, Dong X (2006) A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog 2 1042–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Pajerowska-Mukhtar K, Hendrickson Culler A, Dong X (2007) Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr Biol 17 1784–1790 [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zhong S, Li Q, Zhu Z, Lou Y, Wang L, Wang J, Wang M, Li Q, Yang D, He Z (2007) Functional analysis of rice NPR1-like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotechnol J 5 313–324 [DOI] [PubMed] [Google Scholar]
- Zarate SI, Kempema LA, Walling LL (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Thilmony R, Bender CL, Schaller A, He SY, Howe GA (2003) Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant J 36 485–499 [DOI] [PubMed] [Google Scholar]
- Zheng Z, AbuQamar S, Chen Z, Mengiste T (2006) Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J 48 592–605 [DOI] [PubMed] [Google Scholar]
- Zheng Z, Mosher SL, Fan B, Klessig DF, Chen Z (2007) Functional analysis of Arabidopsis WRKY25 transcription factor in plant defense against Pseudomonas syringae. BMC Plant Biol 7 2. [DOI] [PMC free article] [PubMed] [Google Scholar]