Plants can be attacked by arthropods both above and below ground. The ensuing systemic defense response of the plant can affect even the most distant tissues. Both primary and secondary metabolic profiles of shoots can be altered upon root herbivory and vice versa (Gange and Brown, 1989; Bezemer et al., 2003; Hol et al., 2004; Schwachtje et al., 2006), making plants powerful mediators of interactions between otherwise loosely connected food webs (van der Putten et al., 2001; Bardgett and Wardle, 2003). Whereas the ecological relevance of such processes has been recognized and the role of primary and secondary metabolites acknowledged (for review, see Blossey and Hunt-Joshi, 2003; van Dam et al., 2003; Bezemer and van Dam, 2005), it remains to be explored exactly how plants coordinate their root and shoot responses against herbivores.
We propose that results from current research into the mechanisms governing plant stress responses might provide several starting points to explore the physiological basis of plant-mediated aboveground and belowground interactions. Priming (Ryals et al., 1996; van Wees et al., 1999; Ton et al., 2005; Conrath et al., 2006; Frost et al., 2008) and plant volatile signaling (Engelberth et al., 2004; Heil and Kost, 2006; Ton et al., 2007) may be particularly relevant, and we attempt to place these novel insights in the context of interactions between aboveground and belowground plant defense responses.
Because of the scope of this Focus Issue, we limit our review to arthropod-induced plant defense responses. We do not discuss induced changes in primary metabolites, which can be of substantial importance (Mattson, 1980; Gange and Brown, 1989; Babst et al., 2005; Schwachtje et al., 2006; Schwachtje and Baldwin, 2008). We also acknowledge the importance of putting the current findings in an appropriate ecological context (Rasmann and Agrawal, 2008) and the necessity of including microorganisms as important players in both rhizosphere and phyllosphere interactions. Several excellent reviews cover these and other intricacies of aboveground and belowground interactions (van der Putten et al., 2001; Blossey and Hunt-Joshi, 2003; van Dam et al., 2003; Bonkowski, 2004; Wardle et al., 2004).
PLANT DEFENSE RESPONSES UPON ABOVEGROUND AND BELOWGROUND HERBIVORY
Changes of Defenses in Nonattacked Tissues
Various studies on interactions between aboveground and belowground plant responses have found an increase in basal levels of shoot defenses (defined here as the level of shoot defenses in the absence of aboveground herbivores) following root herbivory, artificial damage, and plant defense hormone application (Table I). Root treatments have been shown to increase shoot concentrations of terpenoids in Gossypium herbaceum and maize (Zea mays; Bezemer et al., 2003, 2004; Rasmann et al., 2005), phenolics in Brassica nigra (van Dam et al., 2005), pyrrolizidine alkaloids in Senecio jacobea (Hol et al., 2004), certain glucosinolates in Brassica spp. (Birch et al., 1992; van Dam et al., 2004; Soler et al., 2005, 2007; van Dam and Raaijmakers, 2006), phytoectosteroids in spinach (Spinacia oleracea; Schmelz et al., 1998), proteinase inhibitors in Nicotiana attenuata (van Dam et al., 2001), and extrafloral nectar in G. herbaceum (Wäckers and Bezemer, 2003). Within this wide array of defensive metabolites, negative effects of root herbivory on basal levels of shoot defenses are also possible in some plant genotypes (Hol et al., 2004) and under certain experimental conditions (van Dam et al., 2005). Current results are as yet inconclusive about whether the generally observed increase of shoot defensive compounds is a result of active defense signaling and de novo synthesis in the shoot or whether the metabolites are translocated from the root to the shoot. We discuss both possibilities below.
Table I.
Summary of the literature on effects of root treatments (herbivory, mechanical damage, or defense hormone application) on shoot defenses and vice versa
MD, Mechanical damage; ST, shoot treatment; RT, root treatment; AB, above ground; BG, below ground; n.a., not applicable. For a complementary table, see Rasmann and Agrawal (2008).
| Plant | Root Treatment | Induced Root Defense | Altered Basal Shoot Defense | Shoot Treatment | Altered ST-Induced Shoot Defense | Influences on Herbivore AG | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Effects of root treatments on shoot defenses | |||||||||
| G. herbaceum | Agriotes lineatus | Terpenoids | + | Increase of terpenoids | S. exigua | 0 | None | Reduced growth | Bezemer et al. (2003) |
| G. herbaceum | A. lineatus, MD | Terpenoid aldehydes | + | Higher terpenoid aldehyde levels | S. exigua | 0 | None | n.a. | Bezemer et al. (2004) |
| B. oleracea, Brassica napus | Delia floralis | Glucosinolates, indole-based compounds | +/− | Higher glucosinolate contents, lower indole- based compounds | n.a | n.a. | n.a. | Birch et al. (1992) | |
| S. jacobea | MD | Pyrrolizidine alkaloids | + | Partially increased pyrrolizidine alkaloids (genotype) | M. brassicae | 0 | None | Partially reduced survival | Hol et al. (2004) |
| B. campestris | D. radicum | Unknown volatiles | + | Induced volatiles | n.a. | n.a. | n.a. | Neveu et al. (2002) | |
| Maize | D. virgifera | (E)-β-caryophyllene | + | Increased (E)-β-caryophyllene (foliage) | n.a. | n.a | n.a. | Rasmann et al. (2005) | |
| Maize | D. virgifera | (E)-β-caryophyllene | 0 | None (headspace) | S. littoralis | − | Reduced volatiles (trend) | n.a. | Rasmann and Turlings (2007) |
| Spinach | MD, MeJA | 20-Hydroxyecdysone | + | Small induction of 20E | S. exigua, MD, MeJA | 0 | None | n.a | Schmelz et al. (1998) |
| Spinach | Otiorhynchus sulcatus | 20-Hydroxyecdysone | 0 | None | n.a. | n.a. | n.a. | Schmelz et al. (1999) | |
| B. nigra | D. radicum | n.a. | + | Higher sinigrin levels | P. brassicae | 0/+ | None/trend for increased sinigrin levels (young leaves) | Reduced growth | Soler et al. (2005) |
| B. nigra | D. radicum | n.a. | + | More volatile sulfides (headspace) | P. brassicae | −/+ | Altered volatile profile | n.a. | Soler et al. (2007) |
| N. attenuata | MeJA | Proteinase inhibitors | + | Higher proteinase inhibitor levels | n.a. | n.a. | n.a. | van Dam et al. (2001) | |
| B. oleracea, B. nigra | JA/SA | Glucosinolates (JA) | + | Induced glucosinolates (JA) | JA, SA | + | More total glucosinolates (JA/JA) | n.a. | van Dam et al. (2004) |
| B. nigra | D. radicum | n.a. | −/+ | Less total glucosinolates, more phenolics | P. rapae | + | More total phenolics | Reduced growth and survival | van Dam et al. (2005) |
| B. oleracea, B. nigra | D. radicum | Indole glucosinolates | 0/+ | None/higher glucosinolate levels (plant species) | n.a. | n.a. | n.a. | van Dam and Raaijmakers (2006) | |
| G. herbaceum | A. lineatus, MD | n.a. | + | Induced extrafloral nectar | n.a. | n.a. | n.a. | Wäckers and Bezemer (2003) | |
| Plant
|
Shoot Treatment
|
Induced Shoot Defense
|
Altered Basal Root Defense
|
Root Treatment
|
Altered RT-Induced Root Defense
|
Influences on Herbivore BG
|
References
|
||
| Effects of shoot treatments on root defenses | |||||||||
| N. attenuata | MD | Nicotine | + | Nicotine | n.a. | n.a. | n.a. | Baldwin et al. (1994) | |
| G. herbaceum | S. exigua | Terpenoids | 0 | None | A. lineatus | − | Nonsignificant reduction of terpenoids | None | Bezemer et al. (2003) |
| G. herbaceum | S. exigua | Terpenoid aldehydes | 0 | None | A. lineatus, MD | − | Reduced terpenoid aldehyde levels | n.a. | Bezemer et al. (2004) |
| S. jacobea | Mamestra brassicae | None (pyrrolizidine alkaloids) | − | Reduced pyrrolizidine alkaloids | MD | 0/− | Partially reduced pyrrolizidine alkaloids (genotype) | n.a. | Hol et al. (2004) |
| B. campestris | JA, SA | Glucosinolates | + | Higher level of glucosinolates | n.a. | n.a. | n.a. | Ludwig-Müller et al. (1997) | |
| Maize | S. littoralis | (E)-β-caryophyllene | 0 | None | D. virgifera | − | Reduced (E)-β-caryophyllene | n.a. | Rasmann and Turlings (2007) |
| Spinach | S. exigua, MD, MeJA | None (20E) | 0 | None | n.a. | n.a. | n.a. | Schmelz et al. (1998) | |
| B. nigra | P. brassicae | n.a. | + | Higher indole glucosinolate levels | D. radicum | n.a. | Reduced survival and size | Soler et al. (2007) | |
| N. attenuata | MJ, MD | Proteinase inhibitors | + | Higher level of trypsin proteinase inhibitors | n.a. | n.a. | n.a. | van Dam et al. (2001) | |
| B. oleracea, B. nigra | JA, SA | Glucosinolates | 0 | None | JA, SA | 0 | None discussed | n.a. | van Dam et al. (2004) |
| C. officinale | MD | Pyrrolizidine alkaloids | +/− | Higher/lower level of pyrrolizidine alkaloids (genotype) | n.a. | n.a. | n.a. | van Dam and Vrieling (1994) | |
In the reverse direction, effects of shoot herbivores on basal levels of root defenses have been observed (Table I). Shoot herbivory or treatment with jasmonic acid can increase root concentrations of nicotine and proteinase inhibitors in N. attenuata (Baldwin et al., 1994; van Dam et al., 2001), as well as glucosinolates in Brassica campestris and B. nigra (Ludwig-Müller et al., 1997; Soler et al., 2007). In contrast, reduced concentrations of other defense-related compounds can also be observed, such as in the case of pyrrolizidine alkaloids in the roots of S. jacobea after herbivory on shoots (Hol et al., 2004). Other studies found no clear effects of shoot treatments on basal levels of root defensive compounds, including terpenoids in G. herbaceum and maize (Bezemer et al., 2003, 2004; Rasmann and Turlings, 2007), phytoectosteroids in spinach (Schmelz et al., 1998), pyrrolizidine alkaloids in Cynoglossum officinale (van Dam and Vrieling, 1994), and glucosinolates in Brassica oleracea and B. nigra (van Dam et al., 2004). Various patterns can be found, even for different genotypes of the same species (van Dam and Vrieling, 1994), making it difficult to draw general conclusions on how shoot treatments affect basal levels of root defenses.
Aboveground and Belowground Changes of Induced Defenses
The above examples deal with single challenges of plant tissue that affect nonattacked parts of the plant. However, recent studies show that effects of herbivory on distant tissues do not always result in changes of defense substances, but rather in how these tissues respond when they themselves are subsequently attacked (Table I). This is the principle of priming for defense, a cost-effective way of “getting ready for battle” that results in faster and stronger defense responses upon attack (Conrath et al., 2006; van Hulten et al., 2006; Frost et al., 2008). Whereas several studies indicate that root herbivory results in enhanced resistance against aboveground attackers (Bezemer et al., 2003; Hol et al., 2004; Soler et al., 2005; van Dam et al., 2005), the importance of priming has not been thoroughly investigated in this context. van Dam et al. (2005) found that Delia radicum attack of the roots resulted in lower initial glucosinolate levels in the shoot of B. nigra. Upon leaf damage by Pieris rapae, however, aboveground glucosinolate levels increased more strongly in these plants, suggesting that B. nigra leaves were primed for defense. In contrast, Soler et al. (2005) found no clear effect of belowground herbivory on glucosinolate levels in B. nigra leaves attacked by Pieris brassicae, implying that aboveground and belowground responses may depend on the herbivore combination. Because priming often merely involves a faster defense reaction upon attack, its occurrence can easily be missed if measurements are taken only at one time point. Intensity and timing of direct defenses might be most easily observed by measuring the expression of defense marker genes and hormone levels (Engelberth et al., 2004; Ton et al., 2007) rather than a small subsample of defense-related secondary metabolites present in a plant. It has also been found that root herbivory can reduce herbivore-induced defense responses in the shoot, specifically the production of volatile terpenoids as shown for B. nigra (Soler et al., 2007) and maize (Rasmann and Turlings, 2007). Suppression of inducible plant defenses could be of benefit if the plant has to “set priorities” in cases of resource limitations and differential effects on fitness.
The effects of shoot herbivory on belowground herbivore-induced root defenses have received little attention. Bezemer et al. (2003, 2004) found that shoot attack leads to a reduction of root treatment-induced terpenoids and terpenoid aldehydes in G. herbaceum. A similar phenomenon was observed for terpenoid volatiles in maize (Rasmann and Turlings, 2007). We are not aware of any study that reports an increase of belowground herbivore-induced root defenses upon shoot herbivory, and it has been speculated that when attacked by both aboveground and belowground herbivores simultaneously, plants preferentially allocate their defenses to the shoot (Bezemer et al., 2004; Rasmann and Turlings, 2007). This hypothesis awaits further testing. Another exciting possibility is that herbivores themselves manipulate plant defenses in their favor, which could also result in changes in distant tissues. This could simply be suppression of defense responses (Musser et al., 2002) or activation of defenses that are ineffective against the herbivore itself, but might affect other attackers. Such “decoy strategies” could be of major ecological significance and should be kept in mind when investigating aboveground and belowground interactions.
THE PHYSIOLOGICAL BASIS OF ROOT-SHOOT INTERACTIONS
The findings discussed in the previous section strongly suggest that signals are exchanged between roots and shoot upon herbivore attack. Root-shoot communication likely follows either the internal vascular network of the plant (i.e. phloem and xylem bundles; Orians, 2005; Atkins and Smith, 2007) or the external route via volatile signaling. These possible routes and preferential flows are depicted in Figure 1. It remains largely unclear which signals and/or compounds are mediating the interactions between root and shoot. The extremely variable effects of root herbivores on shoot responses and vice versa make it unlikely that one specific signal or process is involved. We discuss three classes of compounds that could be of major importance in this context: plant hormones, volatile organic compounds, and nonhormonal secondary metabolites.
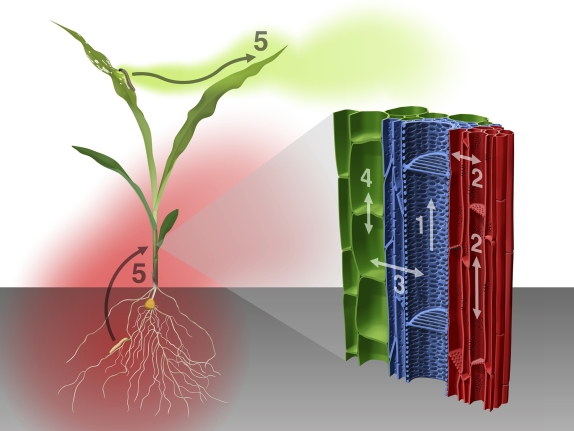
Figure 1.
Model of the signaling processes behind plant-mediated aboveground and belowground interactions. Herbivores attack roots and shoot of a plant, resulting in the production of various stress-related signals. As depicted in the enlarged section of a monocotyledonous vascular bundle (right), aboveground and belowground signaling will most probably involve root-to-shoot transport via xylem vessels (1), bidirectional translocation via the phloem (2), exchange between the vascular tissue and the surrounding cells (3), and nonvascular cell-to-cell signaling (4). External communication with volatile compounds that can reach distant parts of the plant is also possible (5), as illustrated for a maize seedling (left). Possible mediators of the interactions are typical stress signals such as plant hormones and volatiles, as well as bioactive nonhormonal metabolites.
Plant Hormones
Plant hormones are crucial components of the regulatory network underlying plant growth, development, and defense reactions. Several hormones have been implicated in root-shoot communication and might therefore mediate aboveground and belowground interactions in response to herbivory.
Auxin is readily translocated from the shoot to the roots (Reed et al., 1998), where it promotes root cell proliferation and elongation (Hager et al., 1971). Belowground attack can result in compensatory root growth (Steinger and Müller-Schärer, 1992), thereby likely affecting the auxin-cytokinin balance (Woodward and Bartel, 2005), which is of major importance in regulating aboveground and belowground metabolic states. Application of synthetic auxin (1-naphtaleneaic acid) to spinach roots has been found to enhance levels of root phytoecdysteroids (Schmelz et al., 1999) and causes root to shoot dry mass ratios to shift. This shift indicates higher resource allocation to the roots (Schmelz et al., 1999) and implicates auxin's role as a possible regulator of aboveground and belowground feedback. Indeed, transcriptional up-regulation of the auxin marker gene ZmSAUR2 in the roots of maize upon belowground feeding by Diabrotica virgifera was found (M. Erb, J. Ton, and T.C.J. Turlings, unpublished data), indicative of increased auxin shoot-root translocation or biosynthesis in the roots.
Abscisic acid (ABA) represents a classic example of a xylem-translocated root-shoot hormone (Davies and Zhang, 1991; Jackson, 1997; see also Christmann et al., 2005, 2007). Whereas ABA is traditionally associated with responses to drought stress (Davies and Zhang, 1991), it is becoming evident that it may also have an important role in herbivore defense (Anderson et al., 2004). Schmelz et al. (1999) found that application of ABA to the roots of spinach decreased the concentration of the defensive phytoecdysteroid 20E in the shoot. ABA deficiency has been shown to enhance the performance of both Spodoptera exigua on Solanum lycopersicum and Spodoptera littoralis on Arabidopsis (Arabidopsis thaliana; Thaler and Bostock, 2004; Bodenhausen and Reymond, 2007). Furthermore, root herbivory can elicit drought-like responses in plants (Gange and Brown, 1989), which may represent an additional link between ABA and aboveground and belowground interactions. This is expected to be especially important when herbivores severely damage root systems, as is the case for various chewing insects. Hence, further research into the role of ABA in plant-mediated interactions between root and shoot herbivores is certainly warranted.
Jasmonic acid (JA) is often considered to be the central hormone governing systemic plant responses to herbivory above ground (Farmer and Ryan, 1992; Howe et al., 1996; McConn et al., 1997) and probably has a similar role below ground (McConn et al., 1997; Schmelz et al., 1999; Puthoff and Smigocki, 2007). Compounds of the JA family are suggested to be responsible for long-distance wound signaling (Stratmann, 2003; Wasternack et al., 2006), a fact supported by the ability of methyl jasmonate (MeJA) to move readily along both xylem and phloem pathways (Thorpe et al., 2007), as well as through the air (Farmer and Ryan, 1990). The potential of JA as an aboveground and belowground regulator is indicated by the fact that, when applied to the leaves of Nicotiana sylvestris, it seems to be transported to the roots, where it induces nicotine synthesis (Zhang and Baldwin, 1997). Furthermore, application of JA (or MeJA) to roots induces shoot defenses (Baldwin, 1996; van Dam et al., 2001, 2004), providing additional evidence for its key role in root-shoot interactions.
Salicylic acid (SA) is usually implicated in defense responses to pathogens, but can also be involved in plant responses upon herbivore attack (Zarate et al., 2007). It is not clear, however, in what respect SA functions as a systemic signal. It is unlikely that SA is the translocated signal inducing resistance in plant-pathogen interactions (Ryals et al., 1996), and van Dam et al. (2004) found no systemic effects of SA applied to either roots or shoots on glucosinolate levels in two Brassica species. However, the methylated form of SA (MeSA) is a mobile signal that is required for systemic resistance induction in tobacco (Nicotiana tabacum) plants (Park et al., 2007). MeSA and may also function as an airborne signal (Shulaev et al., 1997). Root systems damaged by herbivores can be assumed to have an increased risk of colonization by microorganisms, be it from the oral secretions of the attacker itself or from the rhizosphere. Hence, SA-related defenses induced in response to herbivory could be adaptive and also modulate aboveground defenses, for example, via SA/JA cross talk (Niki et al., 1998).
Finally, ethylene and its precursor 1-amino-cyclopropane-1-carboxylic acid have a well-known function in positive root-shoot signaling (Bradford and Yang, 1980; Jackson, 1997). Research focusing on plant hormonal cross talk has shown the importance of ethylene in modulating responses to biotic stress above ground (Xu et al., 1994; Odonnell et al., 1996; van Loon et al., 2006), which includes activity upon attack by arthropod herbivores (Kendall and Bjostad, 1990; von Dahl and Baldwin, 2007). Puthoff and Smigocki (2007) found an up-regulation of genes responsive to root herbivory in Beta vulgaris upon ethylene treatment, a first indication that ethylene is also involved in root defenses. Because of its volatility, ethylene can either diffuse through the vascular tissue directly into the shoot (Jackson and Campbell, 1975) or travel externally, diffusing from the rhizosphere (Jackson and Campbell, 1975) to the phyllosphere. Because it is likely that ethylene is involved in volatile defense signaling within and between plants (Ruther and Kleier, 2005; J. Ton, unpublished data), it is imperative to study this compound as a possible root-shoot signal in plant-arthropod interactions.
Volatile Organic Compounds as Root-Shoot Signals
Apart from ethylene, a wide range of other volatile organic compounds are synthesized and released after herbivore attack above and below ground (e.g. Rasmann et al., 2005; D'Alessandro et al., 2006). Plant volatiles, in particular induced volatiles, have long been implicated in plant-plant communication. The benefit of such communication for the emitting plant is questionable, unless the information is passed on to a closely related plant. Moreover, volatile signals can be exploited by herbivores (Carroll et al., 2006; Halitschke et al., 2008) and even parasitic plants (Runyon et al., 2006). A more adaptive functioning of volatiles is in overcoming the plant's vascular constraints and communicating between parts of the same plant (Frost et al., 2007; Heil and Silva Bueno, 2007). There is increasing evidence that green-leaf volatiles (GLVs) play an important role in this context (Arimura et al., 2001; Engelberth et al., 2004; Ruther and Furstenau, 2005). Some GLVs belong to the family of reactive electrophile species, which have recently been implicated as stress and defense signals (Farmer and Davoine, 2007). Several reactive electrophile species are very short-lived and therefore could be ideal short-range signals. We have found evidence that GLVs, despite their name, are also released from crushed roots of maize (M. Erb and T.C.J. Turlings, unpublished data). In the only study that looked for belowground GLVs, Steeghs et al. (2004) did not detect any emission from artificially damaged Arabidopsis roots, possibly because the ecotype they used (Columbia-0) carries a mutation severely affecting hydroperoxide lyase activity and C6 volatile synthesis (Duan et al., 2005). GLVs, if indeed produced by the roots, and other volatiles are likely to diffuse into the phyllosphere and change the physiological state of plants above ground (Fig. 1). Research on the biochemistry of GLVs and other volatile organic compounds is progressing rapidly (Matsui, 2006; Schnee et al., 2006; D'Auria et al., 2007), revealing new experimental approaches to test for their effects.
Translocation of Nonhormonal Secondary Metabolites
Secondary metabolites with defensive properties are by no means bound to either the roots or the shoot of a plant, and their translocation could account for many of the observed effects of cross-resistance and interactions between aboveground and belowground plant defenses. Nicotine is the prime example of a secondary metabolite that it synthesized in the roots of Nicotiana spp. and then translocated to the shoots to unleash its antiherbivore properties (Shoji et al., 2000, and refs. therein). van Dam and Vrieling (1994) report a negative relationship between changes in wound-induced pyrrolizidine alkaloid content in the roots and shoots of C. officinale, which can be seen as an indication for within-plant transport of this class of compounds. Rasmann et al. (2005) found increased levels of (E)-β-caryophyllene in maize shoots upon root feeding by D. virgifera. Koellner et al. (2008) found no indication of higher transcriptional activity of the corresponding terpene synthase in the shoot upon D. virgifera feeding on the roots, indicating that it is the compound itself that is translocated from the roots to the shoot. A recent study on terpenoid synthesis in carrots (Daucus carota) found (E)-β-caryophyllene to be independently synthesized in the roots and shoots (Hampel et al., 2005). These indicative results underpin the possibility that it is not necessarily only the activation of aboveground defenses that leads to higher concentrations of secondary compounds in the shoot upon root herbivory, but also simple translocation, be it active transport or passive diffusion.
CONCLUSION
Plant-mediated interactions between aboveground and belowground arthropod herbivores can have profound effects on natural and agricultural food webs. Although only few studies have specifically looked at defense responses of plants that have been subjected to both root and shoot herbivory, it is clear that there is considerable complexity, which depends on a variety of biotic and abiotic factors. Even with our limited knowledge, we can conclude that it is unlikely that all effects are the result of the same physiological processes. Research into the mechanisms as well as the ecological significance of root-shoot feedback effects is sorely needed, and current progress in plant biochemistry and targeted molecular manipulation is likely to reveal which genes and pathways are involved. Recent discoveries focusing on priming for defense and the role of volatiles as external cues involved in plant defense responses show great promise for better understanding of within-plant signaling. Applying this knowledge for comprehensive insight into the ecological relevance of cross-effects between aboveground and belowground interactions requires close collaboration between plant physiologists and ecologists.
Acknowledgments
We thank Gregg Howe and Georg Jander for the invitation to contribute to this Focus Issue. Sarah Kenyon, Marco D'Alessandro, and Claudia Zwahlen provided valuable comments on an earlier version of the manuscript. We are grateful to two anonymous reviewers that helped to improve this review. Figure 1 was created by Thomas Degen (www.thomas-degen.ch).
This work was supported by the Swiss National Science Foundation (grant no. 31–058865.99), the Swiss National Centre of Competence in Research “Plant Survival,” and the Biotechnology and Biological Sciences Research Council (Institute Career Path Fellowship no. BB/E023959/1 to J.T.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Ted C.J. Turlings (ted.turlings@unine.ch).
References
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K (2004) Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16 3460–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura G, Ozawa R, Horiuchi J, Nishioka T, Takabayashi J (2001) Plant-plant interactions mediated by volatiles emitted from plants infested by spider mites. Biochem Syst Ecol 29 1049–1061 [Google Scholar]
- Atkins CA, Smith PMC (2007) Translocation in legumes: assimilates, nutrients, and signaling molecules. Plant Physiol 144 550–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst BA, Ferrieri RA, Gray DW, Lerdau M, Schlyer DJ, Schueller M, Thorpe MR, Orians CM (2005) Jasmonic acid induces rapid changes in carbon transport and partitioning in Populus. New Phytol 167 63–72 [DOI] [PubMed] [Google Scholar]
- Baldwin IT (1996) Methyl jasmonate-induced nicotine production in Nicotiana attenuata: inducing defenses in the field without wounding. Entomol Exp Appl 80 213–220 [Google Scholar]
- Baldwin IT, Schmelz EA, Ohnmeiss TE (1994) Wound-induced changes in root and shoot jasmonic acid pools correlate with induced nicotine synthesis in Nicotiana sylvestris Spegazzini and Comes. J Chem Ecol 20 2139–2157 [DOI] [PubMed] [Google Scholar]
- Bardgett RD, Wardle DA (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84 2258–2268 [Google Scholar]
- Bezemer TM, van Dam NM (2005) Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol Evol 20 617–624 [DOI] [PubMed] [Google Scholar]
- Bezemer TM, Wagenaar R, Van Dam NM, Van Der Putten WH, Wäckers FL (2004) Above- and below-ground terpenoid aldehyde induction in cotton, Gossypium herbaceum, following root and leaf injury. J Chem Ecol 30 53–67 [DOI] [PubMed] [Google Scholar]
- Bezemer TM, Wagenaar R, Van Dam NM, Wäckers FL (2003) Interactions between above- and belowground insect herbivores as mediated by the plant defense system. Oikos 101 555–562 [Google Scholar]
- Birch ANE, Griffiths DW, Hopkins RJ, Smith WHM, McKinlay RG (1992) Glucosinolate responses of swede, kale, forage and oilseed rape to root damage by turnip root fly (Delia floralis) larvae. J Sci Food Agric 60 1–9 [Google Scholar]
- Blossey B, Hunt-Joshi TR (2003) Belowground herbivory by insects: influence on plants and aboveground herbivores. Annu Rev Entomol 48 521–547 [DOI] [PubMed] [Google Scholar]
- Bodenhausen N, Reymond P (2007) Signaling pathways controlling induced resistance to insect herbivores in Arabidopsis. Mol Plant Microbe Interact 20 1406–1420 [DOI] [PubMed] [Google Scholar]
- Bonkowski M (2004) Protozoa and plant growth: the microbial loop in soil revisited. New Phytol 162 617–631 [DOI] [PubMed] [Google Scholar]
- Bradford KJ, Yang SF (1980) Xylem transport of 1-aminocyclopropane-1-carboxylic acid, an ethylene precursor, in waterlogged tomato plants. Plant Physiol 65 322–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MJ, Schmelz EA, Meagher RL, Teal PEA (2006) Attraction of Spodoptera frugiperda larvae to volatiles from herbivore-damaged maize seedlings. J Chem Ecol 32 1911–1924 [DOI] [PubMed] [Google Scholar]
- Christmann A, Hoffmann T, Teplova I, Grill E, Muller A (2005) Generation of active pools of abscisic acid revealed by in vivo imaging of water-stressed Arabidopsis. Plant Physiol 137 209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmann A, Weiler EW, Steudle E, Grill E (2007) A hydraulic signal in root-to-shoot signalling of water shortage. Plant J 52 167–174 [DOI] [PubMed] [Google Scholar]
- Conrath U, Beckers GJM, Flors V, Garcia-Agustin P, Jakab G, Mauch F, Newman MA, Pieterse CMJ, Poinssot B, Pozo MJ, et al (2006) Priming: getting ready for battle. Mol Plant Microbe Interact 19 1062–1071 [DOI] [PubMed] [Google Scholar]
- D'Alessandro M, Held M, Triponez Y, Turlings TCJ (2006) The role of indole and other shikimic acid derived maize volatiles in the attraction of two parasitic wasps. J Chem Ecol 32 2733–2748 [DOI] [PubMed] [Google Scholar]
- D'Auria JC, Pichersky E, Schaub A, Hansel A, Gershenzon J (2007) Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J 49 194–207 [DOI] [PubMed] [Google Scholar]
- Davies WJ, Zhang JH (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42 55–76 [Google Scholar]
- Duan H, Huang MY, Palacio K, Schuler MA (2005) Variations in CYP74B2 (hydroperoxide lyase) gene expression differentially affect hexenal signaling in the Columbia and Landsberg erecta ecotypes of Arabidopsis. Plant Physiol 139 1529–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH (2004) Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci USA 101 1781–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Davoine C (2007) Reactive electrophile species. Curr Opin Plant Biol 10 380–386 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA (1990) Interplant communication: Airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci USA 87 7713–7716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA (1992) Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 4 129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost CJ, Appel M, Carlson JE, De Moraes CM, Mescher MC, Schultz JC (2007) Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol Lett 10 490–498 [DOI] [PubMed] [Google Scholar]
- Frost CJ, Mescher MC, Carlson JE, De Moraes CM (2008) Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol 146 818–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gange AC, Brown VK (1989) Effects of root herbivory by an insect on a foliar-feeding species, mediated through changes in the host plant. Oecologia 81 38–42 [DOI] [PubMed] [Google Scholar]
- Hager A, Menzel H, Krauss A (1971) Experiments and hypothesis concerning primary action of auxin in elongation growth. Planta 100 47–75 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Stenberg JA, Kessler D, Kessler A, Baldwin IT (2008) Shared signals—‘alarm calls’ from plants increase apparency to herbivores and their enemies in nature. Ecol Lett 11 24–34 [DOI] [PubMed] [Google Scholar]
- Hampel D, Mosandl A, Wust M (2005) Biosynthesis of mono- and sesquiterpenes in carrot roots and leaves (Daucus carota L.): metabolic cross talk of cytosolic mevalonate and plastidial methylerythritol phosphate pathways. Phytochemistry 66 305–311 [DOI] [PubMed] [Google Scholar]
- Heil M, Kost C (2006) Priming of indirect defences. Ecol Lett 9 813–817 [DOI] [PubMed] [Google Scholar]
- Heil M, Silva Bueno JC (2007) Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA 104 5467–5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol WHG, Macel M, van Veen JA, van der Meijden E (2004) Root damage and aboveground herbivory change concentration and composition of pyrrolizidine alkaloids of Senecio jacobaea. Basic Appl Ecol 5 253–260 [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA (1996) An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M (1997) Hormones from roots as signals for the shoots of stressed plants. Trends Plant Sci 2 22–28 [Google Scholar]
- Jackson MB, Campbell DJ (1975) Movement of ethylene from roots to shoots, a factor in responses of tomato plants to waterlogged soil conditions. New Phytol 74 397–406 [Google Scholar]
- Kendall DM, Bjostad LB (1990) Phytohormone ecology—herbivory by Thrips tabaci induces greater ethylene production in intact onions than mechanical damage alone. J Chem Ecol 16 981–991 [DOI] [PubMed] [Google Scholar]
- Koellner TG, Held M, Lenk C, Hiltpold I, Turlings TCJ, Gershenzon J, Degenhardt J (2008) A maize (E)-β-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell (in press) [DOI] [PMC free article] [PubMed]
- Ludwig-Müller J, Schubert B, Pieper K, Ihmig S, Hilgenberg W (1997) Glucosinolate content in susceptible and resistant Chinese cabbage varieties during development of clubroot disease. Phytochemistry 44 407–414 [Google Scholar]
- Matsui K (2006) Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol 9 274–280 [DOI] [PubMed] [Google Scholar]
- Mattson WJ (1980) Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst 11 119–161 [Google Scholar]
- McConn M, Creelman RA, Bell E, Mullet JE, Browse J (1997) Jasmonate is essential for insect defense Arabidopsis. Proc Natl Acad Sci USA 94 5473–5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser RO, Hum-Musser SM, Eichenseer H, Peiffer M, Ervin G, Murphy JB, Felton GW (2002) Herbivory: caterpillar saliva beats plant defences—a new weapon emerges in the evolutionary arms race between plants and herbivores. Nature 416 599–600 [DOI] [PubMed] [Google Scholar]
- Neveu N, Grandgirard J, Nenon JP, Cortesero AM (2002) Systemic release of herbivore-induced plant volatiles by turnips infested by concealed root-feeding larvae Delia radicum L. J Chem Ecol 28 1717–1732 [DOI] [PubMed] [Google Scholar]
- Niki T, Mitsuhara I, Seo S, Ohtsubo N, Ohashi Y (1998) Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis-related (PR) protein genes in wounded mature tobacco leaves. Plant Cell Physiol 39 500–507 [Google Scholar]
- Odonnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ (1996) Ethylene as a signal mediating the wound response of tomato plants. Science 274 1914–1917 [DOI] [PubMed] [Google Scholar]
- Orians C (2005) Herbivores, vascular pathways, and systemic induction: facts and artifacts. J Chem Ecol 31 2231–2242 [DOI] [PubMed] [Google Scholar]
- Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF (2007) Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318 113–116 [DOI] [PubMed] [Google Scholar]
- Puthoff DP, Smigocki AC (2007) Insect feeding-induced differential expression of Beta vulgaris root genes and their regulation by defense-associated signals. Plant Cell Rep 26 71–84 [DOI] [PubMed] [Google Scholar]
- Rasmann S, Agrawal AA (2008) In defense of roots: a research agenda for studying plant resistance to belowground herbivory. Plant Physiol 146 875–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TCJ (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434 732–737 [DOI] [PubMed] [Google Scholar]
- Rasmann S, Turlings TCJ (2007) Simultaneous feeding by aboveground and belowground herbivores attenuates plant-mediated attraction of their respective natural enemies. Ecol Lett 10 926–936 [DOI] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday GK (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118 1369–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyon JB, Mescher MC, De Moraes CM (2006) Volatile chemical cues guide host location and host selection by parasitic plants. Science 313 1964–1967 [DOI] [PubMed] [Google Scholar]
- Ruther J, Furstenau B (2005) Emission of herbivore-induced volatiles in absence of a herbivore—response of Zea mays to green leaf volatiles and terpenoids. Z Naturforsch [C] 60 743–756 [DOI] [PubMed] [Google Scholar]
- Ruther J, Kleier S (2005) Plant-plant signaling: ethylene synergizes volatile emission in Zea mays induced by exposure to (Z)-3-hexen-1-ol. J Chem Ecol 31 2217–2222 [DOI] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Grebenok RJ, Galbraith DW, Bowers WS (1998) Damage-induced accumulation of phytoecdysteroids in spinach: a rapid root response involving the octadecanoic acid pathway. J Chem Ecol 24 339–360 [Google Scholar]
- Schmelz EA, Grebenok RJ, Galbraith DW, Bowers WS (1999) Insect-induced synthesis of phytoecdysteroids in spinach, Spinacia oleracea. J Chem Ecol 25 1739–1757 [Google Scholar]
- Schnee C, Kollner TG, Held M, Turlings TCJ, Gershenzon J, Degenhardt J (2006) The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA 103 1129–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwachtje J, Baldwin IT (2008) Why does herbivore attack reconfigure primary metabolism? Plant Physiol 146 845–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwachtje J, Minchin PEH, Jahnke S, van Dongen JT, Schittko U, Baldwin IT (2006) SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proc Natl Acad Sci USA 103 12935–12940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T, Yamada Y, Hashimoto T (2000) Jasmonate induction of putrescine N-methyltransferase genes in the root of Nicotiana sylvestris. Plant Cell Physiol 41 831–839 [DOI] [PubMed] [Google Scholar]
- Shulaev V, Silverman P, Raskin I (1997) Airborne signalling by methyl salicylate in plant pathogen resistance. Nature 385 718–721 [Google Scholar]
- Soler R, Bezemer TM, Cortesero AM, Van der Putten WH, Vet LEM, Harvey JA (2007) Impact of foliar herbivory on the development of a root-feeding insect and its parasitoid. Oecologia 152 257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler R, Bezemer TM, Van der Putten WH, Vet LEM, Harvey JA (2005) Root herbivore effects on above-ground herbivore, parasitoid and hyperparasitoid performance via changes in plant quality. J Anim Ecol 74 1121–1130 [Google Scholar]
- Soler R, Harvey JA, Kamp AFD, Vet LEM, Van der Putten WH, Van Dam NM, Stuefer JF, Gols R, Hordijk CA, Bezemer TM (2007) Root herbivores influence the behaviour of an aboveground parasitoid through changes in plant-volatile signals. Oikos 116 367–376 [Google Scholar]
- Steeghs M, Bais HP, de Gouw J, Goldan P, Kuster W, Northway M, Fall R, Vivanco JM (2004) Proton-transfer-reaction mass spectrometry as a new tool for real time analysis of root-secreted volatile organic compounds in Arabidopsis. Plant Physiol 135 47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinger T, Müller-Schärer H (1992) Physiological and growth-responses of Centaurea maculosa (Asteraceae) to root herbivory under varying levels of interspecific plant competition and soil-nitrogen availability. Oecologia 91 141–149 [DOI] [PubMed] [Google Scholar]
- Stratmann JW (2003) Long distance run in the wound response—jasmonic acid is pulling ahead. Trends Plant Sci 8 247–250 [DOI] [PubMed] [Google Scholar]
- Thaler JS, Bostock RM (2004) Interactions between abscisic-acid-mediated responses and plant resistance to pathogens and insects. Ecology 85 48–58 [Google Scholar]
- Thorpe MR, Ferrieri AP, Herth MM, Ferrieri RA (2007) C-11-imaging: methyl jasmonate moves in both phloem and xylem, promotes transport of jasmonate, and of photoassimilate even after proton transport is decoupled. Planta 226 541–551 [DOI] [PubMed] [Google Scholar]
- Ton J, D'Alessandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TCJ (2007) Priming by airborne signals boosts direct and indirect resistance in maize. Plant J 49 16–26 [DOI] [PubMed] [Google Scholar]
- Ton J, Jakab G, Toquin V, Flors V, Iavicoli A, Maeder MN, Metraux JP, Mauch-Mani B (2005) Dissecting the beta-aminobutyric acid-induced priming phenomenon in Arabidopsis. Plant Cell 17 987–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam NM, Harvey JA, Wackers FL, Bezemer TM, van der Putten WH, Vet LEM (2003) Interactions between aboveground and belowground induced responses against phytophages. Basic Appl Ecol 4 63–77 [Google Scholar]
- van Dam NM, Horn M, Mares M, Baldwin IT (2001) Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. J Chem Ecol 27 547–568 [DOI] [PubMed] [Google Scholar]
- van Dam NM, Raaijmakers CE (2006) Local and systemic induced responses to cabbage root fly larvae (Delia radicum) in Brassica nigra and B. oleracea. Chemoecology 16 17–24 [Google Scholar]
- van Dam NM, Raaijmakers CE, van der Putten WH (2005) Root herbivory reduces growth and survival of the shoot feeding specialist Pieris rapae on Brassica nigra. Entomol Exp Appl 115 161–170 [Google Scholar]
- van Dam NM, Vrieling K (1994) Genetic variation in constitutive and inducible pyrrolizidine alkaloid levels in Cynoglossum officinale L. Oecologia 99 374–378 [DOI] [PubMed] [Google Scholar]
- van Dam NM, Witjes L, Svatos A (2004) Interactions between aboveground and belowground induction of glucosinolates in two wild Brassica species. New Phytol 161 801–810 [DOI] [PubMed] [Google Scholar]
- van der Putten WH, Vet LEM, Harvey JA, Wackers FL (2001) Linking above- and belowground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends Ecol Evol 16 547–554 [Google Scholar]
- van Hulten M, Pelser M, van Loon LC, Pieterse CMJ, Ton J (2006) Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci USA 103 5602–5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LC, Geraats BPJ, Linthorst HJM (2006) Ethylene as a modulator of disease resistance in plants. Trends Plant Sci 11 184–191 [DOI] [PubMed] [Google Scholar]
- van Wees SCM, Luijendijk M, Smoorenburg I, van Loon LC, Pieterse CMJ (1999) Rhizobacteria-mediated induced systemic resistance (ISR) in Arabidopsis is not associated with a direct effect on expression of known defense-related genes but stimulates the expression of the jasmonate-inducible gene Atvsp upon challenge. Plant Mol Biol 41 537–549 [DOI] [PubMed] [Google Scholar]
- von Dahl CC, Baldwin IT (2007) Deciphering the role of ethylene in plant-herbivore interactions. J Plant Growth Regul 26 201–209 [Google Scholar]
- Wäckers FL, Bezemer TM (2003) Root herbivory induces an above-ground indirect defence. Ecol Lett 6 9–12 [Google Scholar]
- Wardle DA, Bardgett RD, Klironomos JN, Setala H, van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304 1629–1633 [DOI] [PubMed] [Google Scholar]
- Wasternack C, Stenzel I, Hause B, Hause G, Kutter C, Maucher H, Neumerkel J, Feussner I, Miersch O (2006) The wound response in tomato—role of jasmonic acid. J Plant Physiol 163 297–306 [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot (Lond) 95 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Chang PFL, Liu D, Narasimhan ML, Raghothama KG, Hasegawa PM, Bressan RA (1994) Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell 6 1077–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate SI, Kempema LA, Walling LL (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZP, Baldwin IT (1997) Transport of [2-C-14]jasmonic acid from leaves to roots mimics wound-induced changes in endogenous jasmonic acid pools in Nicotiana sylvestris. Planta 203 436–441 [Google Scholar]