Abstract
Porins from pathogenic Neisseriae are among several bacterial products with immune adjuvant activity. N. meningitidis (Nme) PorB, has been shown to induce immune cells activation in a TLR2-dependent manner and acts as a vaccine immune adjuvant. The PorB porin from Neisseria lactamica (Nlac), a common nasopharyngeal commensal shares significant structural and functional similarities with Nme PorB. In this work we ask whether the immune adjuvant ability of porins from pathogenic Neisserial strains is a characteristic shared with porins from non-pathogenic Neisserial species or whether it is unique for bacterial products derived from microorganisms capable of inducing inflammation and disease. We evaluate the potential immune adjuvant effect of Nlac PorB in mice using ovalbumin (OVA) as a prototype antigen. Immunization with Nlac PorB/OVA induced high OVA-specific IgG and IgM titers compared to OVA alone, similar to other adjuvants such as Nme PorB and alum. High titers of IgG1 and IgG2b were detected as well as production of IL-4, IL-10, IL-12 and INF-γ in response to Nlac PorB, consistent with induction of both a Th1-type and a Th2-type immune response. OVA-specific proliferation was also determined in splenocytes from Nlac PorB/OVA immunized mice. In addition, B cell activation in vitro and cytokine production in response to Nlac PorB was found to be mediated by TLR2, in a similar manner to Nme PorB.
Keywords: Adjuvant, immune stimulation, commensal bacteria, porin
1. Introduction
Adjuvants are used for improving the specific immune response to vaccine antigens and for induction of immunological memory [1,2]. The addition of adjuvants to vaccine formulations makes it possible to reduce the amount of antigen and the number of immunizations needed, while improving the magnitude and the duration of the specific immune response. However, the majority of adjuvants are not accepted for use in humans [2]. Although several potential adjuvants are in experimental stages, the only licensed adjuvant in use for humans in the United States is aluminum and calcium salts [3]. We propose that the PorB porin from commensal N. Lactamica acts as an immune adjuvant and has the potential of being developed as a potent immune modulator derived from a non-pathogenic bacterium, which would positively affect ease of manufacturing. Examples of adjuvants are oil emulsions [4–6], squalene (MF59) [7], immune stimulating complexes (ISCOMs [8,9]) with Quil-A and both gram-negative bacteria and bacterial products [4,10–12]. These include DNA with immunostimulatory CpG motifs (one of the most powerful adjuvants [13]), Bordetella pertussis toxin [2,14], Corynebacterium granulosum derived P40 component [15], MPL [16], Mycobacterium and its components (Freund adjuvant)[4,17], Cholera toxin [18] and porins from various organisms such as Fusobacterium nucleatum [19], Shigella [20–22], Salmonella [22,23] and Neisseria meningitidis and gonorrhoeae [24–29].
Porins from pathogenic Neisseria species have adjuvant activity in humans and animals [34,35]; this is due to activation of antigen presenting cells (APCs), and our group has shown that signaling via Toll-Like receptors (TLRs) [31,32,68] is required for this activity. Increased expression of CD86/CD80, MHC II and CD40 on the APC surface has been shown in response to porins from pathogenic Neisserial species (and other bacteria) [23,26,30–32], as well as B cell proliferation and increased antibody production [33]. Activated APCs release specific cytokines (i.e. IL-12, IL-4, IL-6) that proceed to guide the differentiation of T cells [36].
Toll-Like receptors (TLRs) have recently been described as specific surface receptors expressed by APCs for recognition of pathogen associated molecular patterns (PAMPs) [37–41]. However, TLRs detect specific molecules which are not exclusive to pathogenic organisms. For example, TLR4 recognizes LPS [37], which is present on all Gram-negative bacteria, including non-pathogenic bacteria, and TLR5 [42] recognizes flagellin [43], also expressed by most gut commensal [44]. Various TLR ligands have been shown to modulate the immune response [45–47] and act as vaccine adjuvants regardless of their pathogenic or non-pathogenic origin (i.e LPS, CpG DNA). Examples of non-pathogenic bacteria with adjuvant activity include Bacillus firmus [48], probiotic Bacillus subtilis spores [49] and some non-pathogenic intestinal gram-negative bacteria, including Bacteroides vulgatus or Veillonella parvula [50], which can induce both Th1- and Th2-type immune responses.
Porins from non-pathogenic Neisseria species share structural and functional similarities with porins from pathogenic strains [51]. They belong to the gram-negative porin superfamily and are native trimeric proteins with a predicted 16-strand β-barrel fold structure and eight surface-exposed, variable, hydrophilic loops [51]. A sequence alignment of N. meningitidis and N. lactamica PorB has determined that the intermembrane domains are mostly conserved while some extracellular loops (loop I, IV, V and VI) [51] have more variability. Their pore function in the bacteria is regulated by a similar gating mechanism, only observed in N. meningitidis PorB, N. gonorrhoeae PIA and PIB, N.lactamica and N. polysaccharea. A similar pore function, regulated by ATP and GTP, has been described in host cells upon porin insertion in the cell membrane [75].
In the light of the similarities between porin from pathogenic and non-pathogenic Neisseriae, we asked whether the porin’s ability to act as immune adjuvant is also part of these functional similarities or whether it is specific for pathogenic species. Our work is focused on porin from Neisseria lactamica (a commensal bacterium which most frequently colonizes the nasopharynges of children [52,53]) and is aimed at characterizing its potential effect as an adjuvant of the immune system and its mechanism of action.
2. Materials and Methods
2.1. Bacteria and cell cultures
Neisseria lactamica (Nlac) strain Y92-1009 [54] (a gift from A. Gorringe, HPA, Porton Down, Salisbury, UK) was originally isolated in Northern Ireland and is part of the ST-613 clonal complex, and N. meningitidis (Nme) strain H44/76 Δ1Δ4 [55] were grown on chocolate-agar plates containing 1% Isovitalex in a 37°C in a 5% CO2 incubator. Murine B cells were isolated from C57Bl/6J mice and C3H/HeJ mice as previously described [31]. Cells were grown in RPMI (Mediatech) containing 10% FBS, 2mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 10 mM HEPES and 10 mM β-ME and defined as R10.
2.2. Porin purification
N. lactamica Y92-1009 were plated as above and grown overnight. The next day, colonies were inoculated in liquid medium (50 ml total) and grown for 6h. This step was repeated once using larger volumes of growth medium (250 ml total), and the cultures were then transferred into a total of 6 liters of medium and grown overnight at 37°C [56]. PorB was purified from the bacterial cultures with the method previously described for meningococcal porin [56], which allows for removal of endotoxin and lipoprotein. At the end of the purification, proteosomes (micellar multi-molecular organized structures) free of detergent were obtained [56] and will be referred to as “Nlac PorB”. Purity of Nlac PorB was examined by Coomassie Brilliant blue staining of 12% SDS-PAGE [57] and silver stain of 15% SDS-PAGE [58]. Bacterial endotoxin content was determined with Limulus Amebocyte Lysate (LAL) assay as specified by the manufacturer (Cell Sciences, Canton, MA)
2.3. Immunization of animals
For generation of anti-Nlac PorB antibodies, C57Bl/6 mice (n=5) were immunized with 10 µg of purified Nlac PorB in 100 µl of sterile PBS in the absence of other adjuvants every two weeks via the sub-cutaneous (s.c.) route for a total of three immunizations. Sera were collected prior to the first immunization (week 0) and two weeks after each subsequent immunization (week 2, 4 and 6). Another group of animals was immunized with 10 µg of Nme PorB. For generation of anti-OVA antibodies, C57Bl/6 mice (n=5) and C3H/HeJ mice (n=4) (a mouse strain naturally non-responsive to LOS due to a mutation in the TLR4 gene [59]) were immunized with 10 µg of ovalbumin (OVA) alone or in the presence of 10 µg of Nlac PorB, 10 µg of Nme PorB [30] or with 200 µg of alum (Sigma) [60] as adjuvants. Ovalbumin was obtained from commercial chicken egg whites by freeze-drying followed by lyophilization and resuspension of the total egg white proteins in sterile PBS. Ovalbumin obtained with this method represents ~65% of the total protein content and is LPS-free [32].
2.4. Measurement of anti-PorB specific antibodies
Specific anti-porin antibodies in the mice sera were examined by Western blot and ELISA [24], respectively. For Western blot, Nlac PorB was subjected to 12% SDS-PAGE [57] followed by transfer on PVDF membrane (Millipore, Bedford, MA). The membrane was blocked for 1 h with 5% non-fat dry milk in 25 mM Tris pH 8.0, 125 mM NaCl and 0.1% Tween 20 followed by overnight incubation at 4°C with a 1:1000 dilution of mouse sera raised to Nlac PorB as previously described. For detection of the immunoreactive bands, an alkaline phosphatatse-conjugated anti-mouse secondary antibody (Sigma) was used followed by BCIP/NBT purple substrate (Sigma). For ELISA, briefly, 96-well plates (Immulon) were coated with 2 µg/ml of purified Nlac PorB or Nme PorB in carbonate buffer pH 9.6 at 4°C for 24h, washed three times with PBS/ 0.05% Tween-20 followed by 1h blocking with 5% BSA in PBS at room temperature. Serial dilution of individual mouse sera were incubated at 4°C for 24h washed and incubated with a secondary anti-mouse IgG alkaline-phosphatase (AP) conjugated antibody (Sigma) for 2h at room temperature followed by detection with 1 step PNPP substrate (Pierce) as specified by the manufacturer. The absorbance was measured at O.D.405. To quantify the amount of anti-porin serum IgG in µg/ml, a reference standard curve was used. One plate was coated with 10 µg/ml of a goat anti-mouse IgG F(ab')2-specific antibody (Jackson Laboratories), incubated with serial dilutions of a known amount of mouse total IgG (Sigma) followed by secondary antibody and detection as described above. The amount of anti-porin serum IgG was extrapolated from the standard curve using a linear regression function.
2.5. Measurement of anti-OVA specific antibodies
OVA-specific total IgG and IgM in the mice sera were determined by ELISA as described above. After coating plates with 5µg of OVA, serial dilutions of mice sera were added and incubated as described. Total IgG and IgM were detected using alkaline-phosphatase conjugated secondary goat anti-mouse IgG and anti-mouse IgM (Sigma). Quantification of OVA-specific IgG and IgM was performed as described above using an IgG and an IgM standard curve, respectively. Results are expressed in µg/ml of OVA-specific antibody. For determination of OVA-specific IgG subclass titers, goat anti-mouse specific anti-IgG1, IgG2a, IgG2b and IgG3 were used, followed by AP-conjugated secondary antibody as previously described. Results are expressed as sera titers at O.D. 405.
2.6. Measurement of cytokines
INF-γ and IL-12p70 (Th1 cytokines), IL-4 and IL10 (Th2 cytokines), IL-6 and TNF-α were examined by ELISA using Opt-EIA kit (BD Biosciences) specific for each cytokine according to manufacture's protocol.
2.7. Cell incubations and flow cytometric analysis
Purified mouse B lymphocytes (5 × 106/ml) [26] were stimulated in R10 for 24h with Nlac PorB or Nme PorB (10 µg/ml), the TLR2/TLR1 synthetic ligand Pam3CSK4, the TLR2/TLR6 synthetic ligand Pam2CSK4 (100 and 10 ng/ml) and the TLR4 ligand N. meningitidis LOS (100 ng/ml). The culture supernatants were collected and analyzed for cytokine production as described above. For expression of cell surface antigens, the following anti-murine FITC-labeled MAbs were used: anti-rat IgG, anti-CD86, anti MHC-class II and anti-CD40 (PharMingen, San Diego, CA). Cells were analyzed by flow cytometry on a FACScan(TM) flow cytometer using CellQuest acquisition and analysis software (Becton Dickinson, Mountain View, CA). Gating was used to exclude cellular debris. All the histograms shown are representative of three separate experiments.
2.8. Lymphocytes proliferation assay
Whole splenocytes were obtained from immunized mice and cell proliferation was measured by 3-[4,5-dimethylthiazol-2-y]-2,5-diphenyl tetrazolium bromide (MTT, Sigma) incorporation assay [60]. Briefly, after erythrocytes lysis with NH4Cl, the splenocytes were seeded in R10 medium in 96-well flat-bottom plates in quadruplicate at a concentration of 5 × 106 cell/ml in 100 µl of medium. OVA (10 µg/ml), Neisserial LOS (10 µg/ml) and concanavalin A (ConA) (10 µg/ml) were added and incubated for 72h followed by addition of 10 µl of MTT to each well for further 3–4h. MTT solvent (100 µl) was added and the absorbance was measured in an ELISA reader at 570 nm with a 690 nm reference. Alternatively, splenic B cells from naive C57Bl/6 mice and TLR2 knockout mice [26] were incubated with Nlac PorB and Nme PorB at 10 µg/ml, LOS (100 ng/ml) and ConA (5 µg/ml) for 44h and cell proliferation was determined as described above. Cell proliferation was expressed in arbitrary units as Stimulation Index, calculated by dividing the average cell proliferation in the wells incubated with each stimulus by the average cell proliferation in the wells incubated with medium alone.
2.9. Statistic analysis
ELISA were performed in duplicate and repeated three times to ensure reproducibility. Statistical analysis of the data were performed with Graphpad InStat software.
3. Results
3.1. Purification of PorB from N. lactamica
Neisserial porins are native trimeric proteins composed by monomers of approximate molecular weight of ~ 34–42 kDa [51]. Most of their properties in vitro are dependent on an intact trimeric structure, including their ability to activate immune cells, modulate apoptosis and form functional pores, as shown for Nme PorB by our group [56]. PorB was purified from N. lactamica (Nlac) strain Y92-1009 [54] (a gift from Dr. A. Gorringe, HPA, Porton Down, Salisbury, UK) with a method that we have previously optimized for purification of PorB from N. meningitidis (Nme) and N. gonorrhoeae (Ngo) [56]. Purified Nlac PorB was first examined by SDS-PAGE followed by Coomassie staining (Figure 1A) and it appears to have a m.w. of approximately 35–37 kDa (indicated by the arrow) in reducing conditions (monomeric form) [61,62]. Higher molecular weight bands corresponding to trimers and oligomers were detected by non-denaturing gel electrophoresis performed in the absence of SDS (data not shown), suggesting a presumed native trimeric structure similar to Nme PorB [56]. Traces of a protein contaminant of approx. m.w. of 25 kDa were consistent with Reduction Modifiable Protein (Rmp) [63] (Figure 1A), recognized by an anti-meningococcal RmpM MAb by Western blot (data not shown). Silver stain SDS-PAGE was consistent with negligible presence of LOS contamination (Figure 1B), confirmed by LOS quantification with Limulus Amebocyte Lysate (LAL) assay [56] (data not shown).
Figure 1.
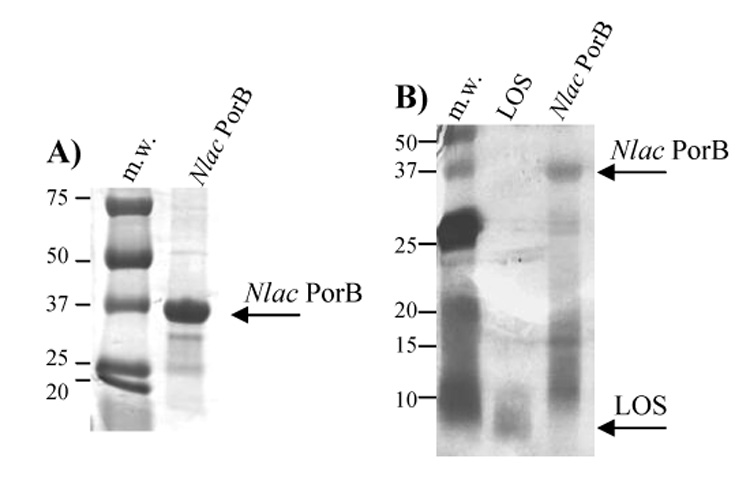
Purification of PorB from N. lactamica. PorB was purified from liquid cultures of N. lactamica as previously described [56]. A) SDS-PAGE and Coomassie staining of purified Nlac PorB. The reduced monomeric form is indicated by the arrow. B) SDS-PAGE and silver staining of Nlac PorB. Neisserial LOS was used as positive control for detection of potential contaminant lipopolysaccharide. Reduced PorB and LOS are indicated by the arrows.
3.2. Immunogenicity of Nlac PorB
C57Bl/6 mice and C3H/HeJ mice were immunized with Nlac PorB in the absence of other adjuvants and aliquots of sera were collected prior to the first immunization (Preimmune) and two weeks after each subsequent immunization for Western blot and ELISA analysis. Nlac PorB is detected by a representative anti-Nlac PorB mouse serum (week 6, 1:1000 dilution) as a band of approx. 35 kDa in Western blot (Figure 2A, lane 2). The pre-immune serum failed to recognize the porin (Figure 2A, Lane 1), indicating that specific antibodies are generated to Nlac PorB. We also examined the mouse sera by ELISA. Plates were coated with Nlac PorB and specific mouse anti-porin total IgG levels were measured. Immunization of mice with Nlac PorB induced production of specific antibodies to Nlac PorB (Figure 2B, open square), while the preimmune sera did not recognize the porin (Figure 2B, closed square). Similar results were obtained when the sera of C3H/HeJ mice were analyzed (data not shown).
Figure 2.
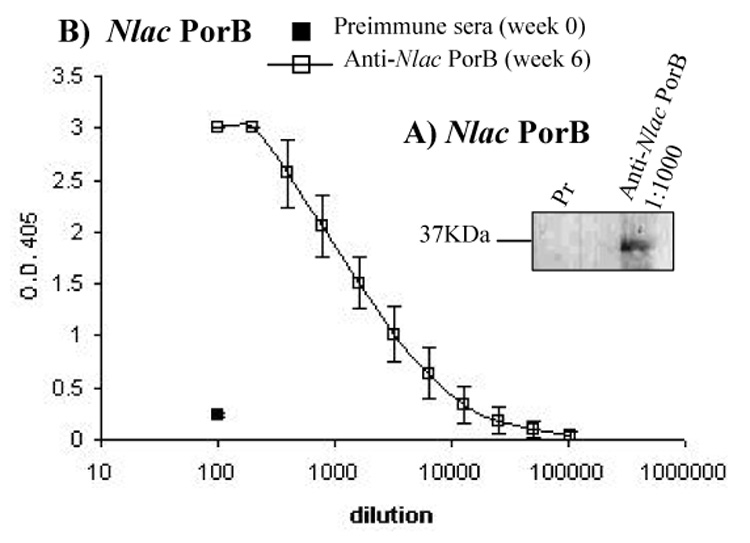
Immunogenicity of Nlac PorB. A) Sera from C57Bl/6 mice immunized s.c. with Nlac PorB were used in Western blot (1:1000 dilution) for detection of Nlac PorB (lane 2). Preimmune serum (Pr) is shown in lane 1. B) Serial dilution (1:1000 initial dilution) of anti-Nlac PorB (open square) were used in ELISA for detection of Nlac PorB. Preimmune serum is shown by the closed square. The results represent the mean of two experiments performed in triplicate wells ± standard deviations.
3.3. Adjuvant effect of Nlac PorB
Porins from several bacterial species have been demonstrated to act as immune adjuvants, including Nme PorB [19,20,25]. To examine whether Nlac PorB has a similar property, its ability to increase antibody production to a prototype antigen, ovalbumin (OVA), was determined. To insure absence of LPS contamination in the OVA preparation, OVA was obtained from chicken egg whites as described [32], and was found to be free of LPS by LAL assay (data not shown). C57Bl/6 mice were immunized with OVA alone or mixed with adjuvants i.e. Nlac PorB, Nme PorB and alum. Sera were collected two weeks after each immunization and specific anti-OVA antibodies production was examined by ELISA. In Figure 3A, the concentration in micrograms per ml of anti-OVA total IgG in the sera of mice immunized with OVA alone, Nlac PorB/OVA and alum/OVA is shown and demonstrates that Nlac PorB significantly enhanced IgG production after two immunizations, similar to alum. Furthermore, Nlac PorB proved to be as efficient as Nme PorB as an adjuvant, since mice immunized with Nme PorB/OVA produced similar amounts of specific anti-OVA IgG (data not shown).
Figure 3.
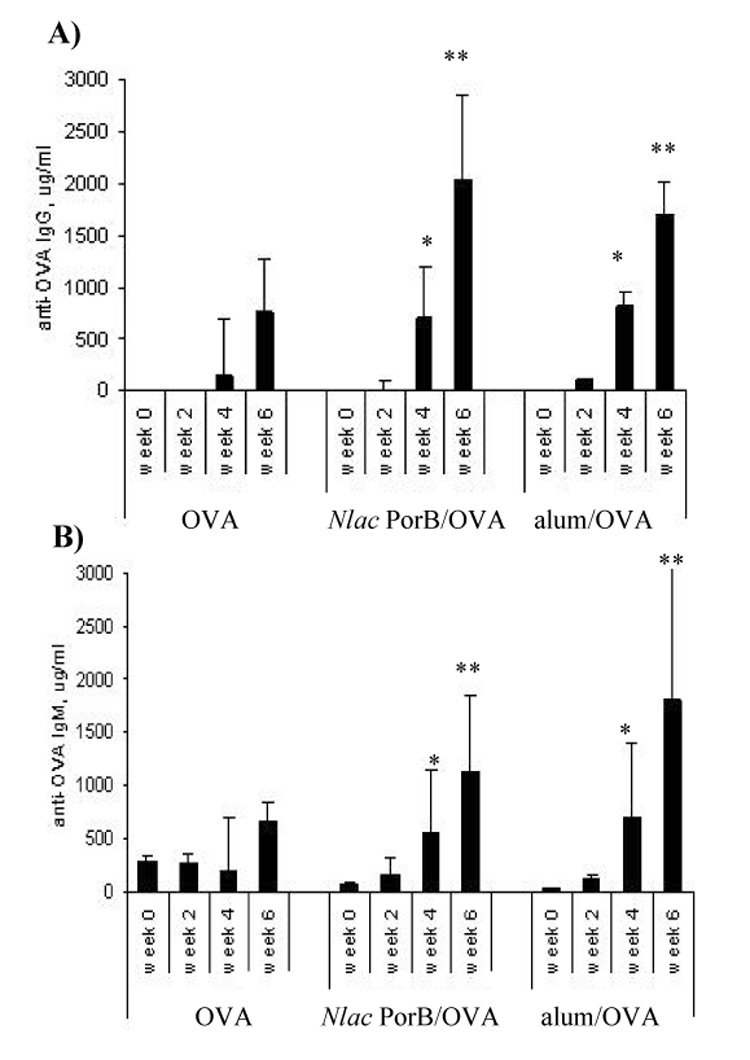
Effect of Nlac PorB on induction of OVA-specific total IgG and IgM in OVA-immunized mice. Groups of five mice were immunized s.c. with 10 µg of OVA alone or dissolved in saline containing 200µg of alum or 10 µg of Nlac PorB every two weeks for a total of three immunizations. Sera were examined prior to the first immunization (week 0) and two weeks after each immunization (week 2, 4 and 6). A) and B) OVA-specific total IgG and IgM antibodies in the sera were measured by ELISA, quantified by using standard IgG and IgM reference curves and expressed in µg/ml. The values represent the mean of triplicate wells for each individual mouse serum ± standard deviation. Significant differences (p = < 0.05) with the OVA group were calculated by Mann Withney test and are indicated by the asterisks.
In Figure 3B, specific anti-OVA IgM levels induced by Nlac PorB and alum are shown, demonstrating that Nlac PorB also elicited a significantly enhanced IgM production to OVA. Collectively these data support the effect of Nlac PorB as a protein with immune adjuvant ability, similar to Nme PorB, with the difference of being derived from a non-pathogenic bacterial specie. We also examined the response of C3H/HeJ mice (LPS-hyporesponsive mice) [59] immunized with OVA alone and Nlac PorB/OVA, and determined a very similar response to C57Bl/6 mice (data not shown).
3.4. Analysis of IgG subclasses and Th1/Th2 response
To determine whether Nlac PorB induced a Th-biased response, we measured IgG subclasses, IgE and Th1 and Th2 cytokines in the sera of immunized mice. The different IgG subclasses were examined by ELISA and are shown in Figure 4. Significantly higher titers (p = < 0.05) of OVA-specific IgG1 were induced by alum (Figure 4, white bars) and by Nlac PorB (Figure 4, black bars) as compared to OVA alone (Figure 4, gray bars). Nme PorB also induced similar IgG1 levels (data not shown). In addition, Nlac PorB elicited significantly higher titers (p = < 0.05) of IgG2b and, to a lesser extent, of IgG3 and IgG2a (Figure 4), which were not induced by alum.
Figure 4.
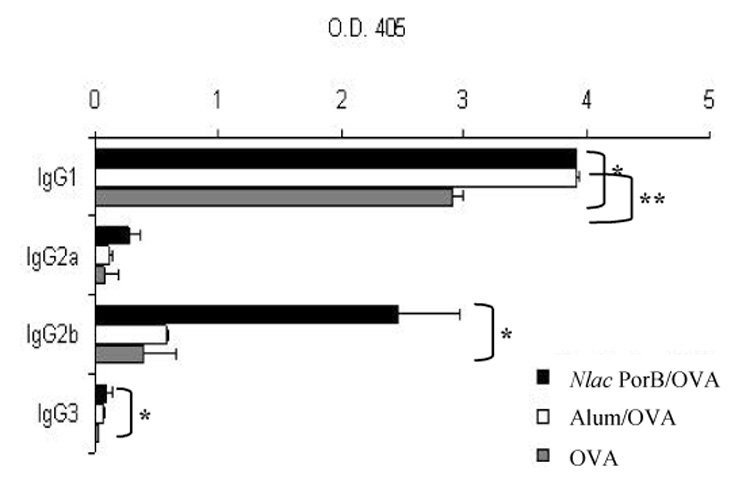
Effect of Nlac PorB on induction of OVA-specific IgG subclasses. OVA-specific IgG1, IgG2a, IgG2b and IgG3 titers were measured by ELISA in the sera of immunized mice. The results represent the mean of triplicate wells ± standard deviation and are expressed as O.D.405. Significant differences (p=<0.05) with the OVA group were calculated by Mann Withney test and are indicated by (*) for Nlac PorB and (**) for alum.
As induction of high IgG1 titers and of IgE is considered indicative of a Th2-type immune response while high IgG2a, IgG2b and IgG3 and low IgE titers are typical of a Th1-type response [64], we examined whether Th1- and Th2-type associated cytokines were induced in the mice sera after receiving two immunizations (week 4). As shown in Table 1, mice immunized with Nlac PorB/OVA exhibited high levels of IL-4 and IL-10 (Th2 cytokines), similar to mice immunized with alum/OVA (and with Nme PorB/OVA, data not shown). Furthermore, the Th1 cytokines INF-γ and IL-12p70 were also augmented in response to Nlac PorB (and Nme PorB, not shown), but not by alum (Table 1), supporting the hypothesis that Nlac PorB can induce both a Th1- and a Th2-type response when is used as adjuvant. Mice immunized with Nlac PorB alone produced cytokine levels equal or slightly higher than mice immunized with OVA alone (data not shown), and we also determined a similar cytokines pattern in the immune sera of C3H/HeJ mice (data not shown).
Table 1. Serum levels of Th1 and Th2 cytokine in immunized mice.
The sera of mice after receiving two immunizations (week 4) were examined by ELISA for production of cytokines. The values are presented as mean ± standard deviation of triplicate wells for each individual mouse serum. Significant differences with the OVA group were calculated by Mann Withney test and are indicated by (*)
| Groups | INF-γ (ng/ml) | IL-12p70 (ng/ml) | IL-10 (pg/ml) | IL-4 (pg/ml) |
|---|---|---|---|---|
| OVA | 17.09 ± 5.8 | 47.2 ± 15.2 | 223.3 ± 171 | 219.1 ± 0.2 |
| Nlac PorB/OVA | 31 ± 9.7* | 89.7 ± 26* | 530 ± 178 | 451.2 ± 3.4* |
| Alum/OVA | 6.36 ± 6.9 | 49.3 ± 40 | 421 ± 148.4 | 441.5 ± 2.5* |
3.5. Splenocyte proliferation in OVA-immunized mice
In vitro proliferation of splenocytes from mice immunized with OVA alone, Nlac PorB/OVA, Nme PorB/OVA and alum/OVA in response to stimulation with OVA was measured. Mice were immunized every two weeks as previously described and the spleens were collected two weeks after the second immunization (week 4). Splenocytes were incubated with OVA (10µg/ml) for induction of proliferation. Neisserial LOS (10 µg/ml) and the mitogen Concanavalin A (10 µg/ml) were used as non-specific inducers of cell proliferation while medium alone was used as a negative control. The cells were incubated for 72h as described in the Methods and proliferation was measured by addition of a chromogenic substrate (MTT), which forms intracellular crystals detectable spectrophotometrically at 570nm with 690nm correction. Cell proliferation was calculated by dividing the average cell proliferation in the wells incubated with each stimulus by the average cell proliferation in the wells incubated with medium alone and expressed in arbitrary units as Stimulation Index, shown in Figure 5. Specific proliferation in response to OVA (Figure 5, black bars) was determined in splenocytes from mice immunized with Nlac PorB/OVA and Nme PorB/OVA, while it was not induced in cells from the OVA alone and alum/OVA mice groups. Non-specific proliferation was induced in all the splenocyte groups by of ConA (Figure 5, white bars) and by LOS (Figure 5, gray bars), appearing to be further increased in cells from mice immunized with Nlac PorB/OVA and Nme PorB/OVA. These data suggest that immunization with a mixture of Nlac PorB and OVA sensitizes mouse splenocytes for induction of Nlac PorB-mediated proliferation in response to OVA stimulation.
Figure 5.
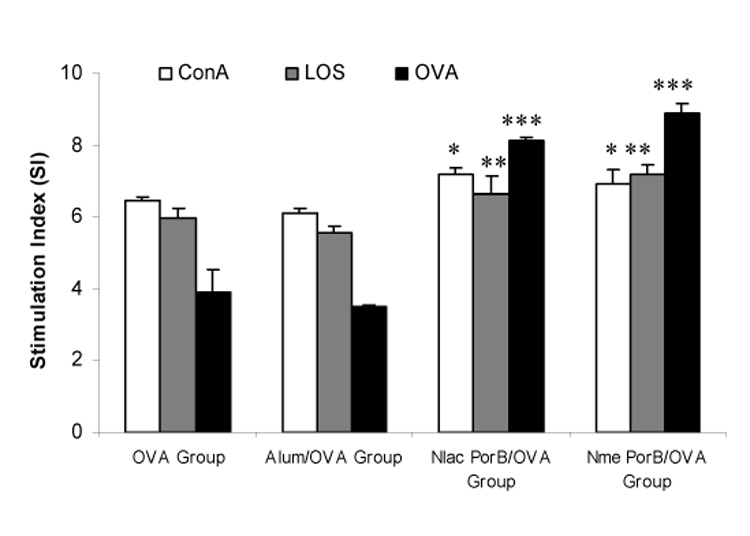
Effect of Nlac PorB on specific and non-specific splenocyte proliferation. Splenocytes from mice immunized with OVA alone, alum/OVA, Nlac PorB/OVA or Nme PorB/OVA were incubated for 72h with OVA (10 µg/ml, black bars) for induction of specific proliferation or with LOS (10 µg/ml, gray bars) and Concanavalin A (ConA, 10 µg/ml, white bars) for induction of non-specific proliferation. Medium alone was used as a negative control. Splenocytes proliferation was determined by the MTT assay. The values represent the mean of quadruplicate wells ± standard deviation and expressed as Stimulation Index (SI) as described in the text. Significant differences (p=<0.05) with the OVA-immunized group were calculated by Mann Withney test and are indicated by (*) for ConA stimulation, (**) for LOS stimulation and (***) for OVA stimulation. No significant differences were detected between the OVA group and the alum/OVA group.
3.6. Activation of immune cells in vitro and role of TLR2
To assess whether the mechanism of adjuvanticity of Nlac PorB was consistent with APC activation, similar to what has been described for Nme PorB [31], we examined its effect on B cells in vitro. Although dendritic cells are mostly responsible for initiation of a primary immune response, activation of both DCs and B cells has been extensively characterized in response to Nme PorB [26,31,32] and is a crucial comparison for the effect of Nlac PorB. Purified mouse splenic B cells were incubated with different concentrations of Nlac PorB (20, 10, 1, 0.1 µg/ml) for 24h and a dose-dependent increase of surface expression of the co-stimulatory molecule CD86, of CD40 and of MHC II was determined by flow cytometry (data not shown). In all the histograms in Figure 6, the isotype control is shown by the gray area and medium-incubated cells are shown by the thin line. In Figure 6A, we compared the effect of Nlac PorB (10 µg/ml, thick line) and Nme PorB (10 µg/ml, dotted line) [26] on B cell activation and a similar up-regulation of CD86, MHC II and CD40 was determined.
Figure 6.
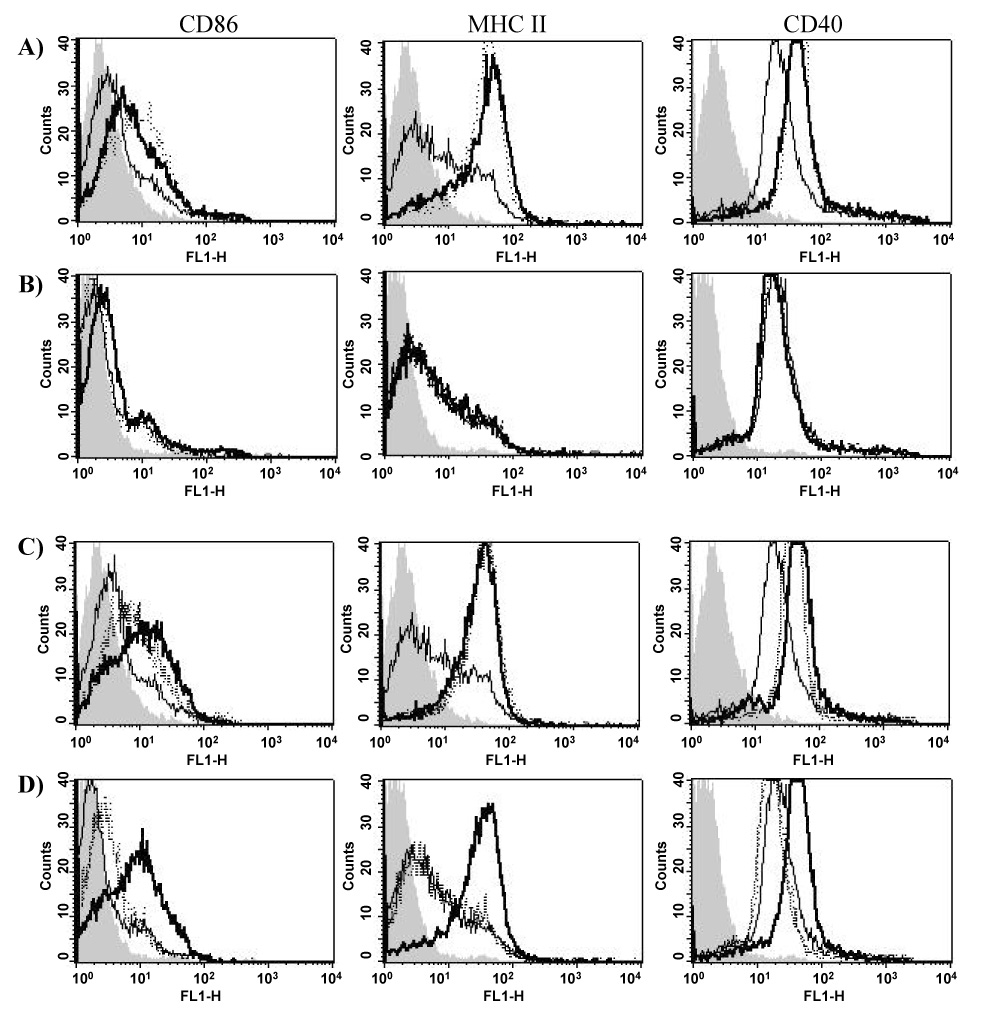
Nlac PorB stimulates B cells in vitro. Surface upregulation of CD86, MHC II and CD40 was measured by flow cytometry. In all the histograms the isotype control is indicated by the gray area and the medium is indicated by the thin line. B cells from A) C57Bl/6 wild type mice and B) TLR2 knockout mice incubated for 24h with Nlac PorB (10 µg/ml, thick line) and Nme PorB (10 µg/ml, dotted line). B cells from C) wild type mice and D) TLR2 knockout mice incubated for 24h with Pam2CSK4 (100 ng/ml, dotted line), Pam3CSK4 (100 ng/ml, dashed line) and LOS (100 ng/ml, thick line).
As a TLR-mediated cell activation has been described for adjuvants of bacterial origin (i.e. TLR9 for CpG DNA [13] and, most importantly, TLR2 for Nme PorB [65]), we examined whether B cell activation mediated by Nlac PorB was also TLR2-dependent using B cells from TLR2 knockout mice. Figure 6B shows that the expression of CD86, MHC II and CD40 was abrogated in B cells from TLR2 knockout mice in response to Nlac PorB (Figure 6B, thick line) similar to Nme PorB (Figure 6B, dotted line). B cells from wild type mice and TLR2 knockout mice were also incubated with the TLR2/TLR6 ligand, Pam2CSK4 (100 ng/ml), the TLR2/TLR1 ligand Pam3CSK4 (100 ng/ml) as positive controls and with the TLR4 ligand LOS (100 ng/ml) as a negative control. Figure 6C shows up-regulation of CD86, MHC II and CD40 in response to Pam2CSK4 (Figure 6C, dotted line), Pam3CSK4 (Figure 6C, dashed line) and LOS (Figure 6C, thick line) in B cells from wild type mice. However, expression of these markers was abrogated in B cells from TLR2 knockout mice in response to Pam2CSK4 and Pam3CSK4 (Figure 6D, dotted line and dashed line, respectively) while the ability of the TLR4 ligand, LOS, to induce surface markers upregulation was unaffected (Figure 6D, thick line). These data suggest that Nlac PorB activates immune cells in vitro in a TLR2-dependent manner.
Next, we determined whether Nlac PorB induced cytokine secretion in vitro in B cells and whether this was also mediated by TLR2. Purified B cells from wild type mice and TLR2 knockout mice were incubated with Nlac PorB, Pam3CSK4 and LOS as described above and IL-6 and TNF-α were measured in the cell supernatant by ELISA. Table 2 shows that Nlac PorB induced increased levels of both IL-6 and TNF-α, similar to Pam3CSK4 and LOS. This response was dependent on TLR2 expression, as B cells from TLR2 knockout mice failed to produce them in response to PorB and Pam3CSK4, although they were responsive to the TLR2-independent positive control, LOS.
Table 2. Levels of Th1 and Th2 cytokine produced by B cell in vitro.
B cells from C57Bl/6 wild type mice and TLR2 knockout mice were incubated for 24h with Nlac PorB, Pam3CSK4, Pam2CSK4 and Neisserial LOS as indicated. The levels of IL-6 and TNF-α were measured by ELISA, quantified by using standard reference curves and expressed in pg/ml. The results represent the mean of triplicate wells ± standard deviations.
| WT B cells | TLR2 KO B cells | |||
|---|---|---|---|---|
| (pg/ml) | TNF-α | IL-6 | TNF-α | IL-6 |
| Medium | n.d. | 0.6 ± 0.3 | n.d | 6.3 ± 1.5 |
| Nlac PorB 10µg/ml | 122.4 ± 16 | 13.6 ± 1.8 | n.d. | 4.8 ± 2.1 |
| Pam3CSK4 0.1 µg/ml | 216.6 ± 39 | 19.2 ± 7.3 | n.d. | 3.4 ± 0.9 |
| Pam2CSK4 0.1 µg/ml | 180 ± 59 | 22.6 ± 12.8 | n.d. | 4.7 ± 5.4 |
| LOS 0.1 µg/ml | 95 ± 31 | 11.8 ± 5.6 | 93.9 ± 47.5 | 12.9 ± 4.9 |
As an additional method for correlating the ability of Nlac PorB to activate immune cells in vitro with its immune adjuvant activity, we measured induction of B cell proliferation. LOS and ConA were used as positive controls and medium alone was used as a negative control. As shown in Figure 7, incubation with Nlac PorB for 48h induced proliferation of B cells from wild type mice (Figure 7, black bars) in a similar manner to Nme PorB, LOS and the mitogen ConA. However, B cells from TLR2 knockout mice failed to proliferate in response to Nlac PorB and Nme PorB, while TLR4-dependent proliferation was induced by LOS and non-specific proliferation was induced by ConA (Figure 7, white bars). Collectively, these data strongly indicate that the immune stimulatory effects of Nlac PorB on mouse B cells are dependent on expression of TLR2.
Figure 7.
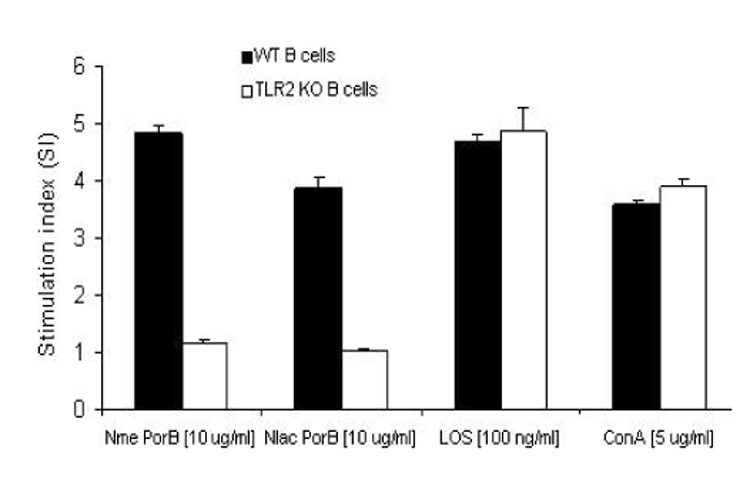
Effect of Nlac PorB on B cell proliferation. B cells from C57Bl/6 mice (black bars) and TLR2 knockout mice (white bars) were incubated for 44h with Nlac PorB or Nme PorB (10 µg/ml), LOS (100 ng/ml) or the mitogen Concanavalin A (ConA, 5 µg/ml) as previously described. Medium alone was used as a negative control. Proliferation was determined by the MTT assay as previously described. The values represent the mean of quadruplicate wells ± standard deviation and expressed as Stimulation Index (SI) as described in the text.
4. Discussion
Although bacterial porins have been characterized as adjuvants, purified N. lactamica porin PorB has not been examined before. In this work we describe the purification of PorB from N. lactamica Y92-1009 (a particularly relevant strain, as outer membrane vesicles derived from it are being explored as an alternative approach for a meningococcal vaccine [54]). Purified Neisserial porins mediate cell activation and signaling pathways in vitro and in vivo; however, if their native trimeric structure is disrupted into monomers, these activities are lost [56]. We have supporting evidence that the presumed native trimeric structure of Nlac PorB is maintained upon purification, suggested by the presence of high molecular weight bands in non-denaturing SDS PAGE, in a similar manner to PorB from N. meningitidis [56]. In fact, a high degree of structural and functional similarities between neisserial porins has been described [51]; they share a high content of β-pleated sheet, a predicted 16-strand β-barrel fold and multiple surface-exposed, variable, hydrophilic loops [51,67]. However, an aminoacid sequence alignment between PorB from N. meningitidis and N. lactamica has determined that the regions of homology do not include some surface exposed loops (loop I, IV, V and VI) [51]. Mice immunized with purified Nlac PorB in the absence of other adjuvants mount a robust specific immune response to the porin.
The immune adjuvant effect of bacterial porins, including Neisserial porins from pathogenic strains, has been previously characterized [19,20,25]. Both native and recombinant pathogenic neisserial porins have demonstrated to induce similar immune responses, in terms of generation of anti-porin antibodies and bactericidal activity and are considered for a potential anti-meningococcal vaccine [28,76,77]. However, potential difficulties in the recombinant porin's expression and structure re-folding or the use of large amounts of pathogenic bacterial cultures (although it could be argued that the use of non-capsulated strains would lower the risks of growing hundred liters of culture), might limit their development as adjuvants. We asked whether porins from non-pathogenic N. lactamica have equivalent immune adjuvant properties and propose that they can be safely used as an alternative to pathogenic strains. Our data demonstrate that Nlac PorB induces increased antibodies production to a prototype antigen (ovalbumin) in mice, similar to other adjuvants such as alum and Nme PorB. Alum typically induces high IgG1 and IgE titers and Th2-type cytokine IL-4 and IL-10 in mice. Nlac PorB not only induces high IgG1 titers, similar to alum, but also higher titers of IgG2b and IgG3, which are events associated with induction of a Th1 type response in mice [64]. We also demonstrate that secretion of both Th1-type cytokine IL-12p70 and INF-γ and Th2-type cytokines IL-4 and IL-10 was increased by Nlac PorB. Induction of both Th1 and Th2 type immune responses is a very desirable characteristic for an immune adjuvant, making it potentially suitable for formulation of vaccines directed not only towards bacterial pathogens but also viral pathogens or helmints.
Achievement of a long-lasting immunity against vaccine antigens via antigen-specific lymphocytes proliferation and induction of memory cells is also induced by immune adjuvants. In our experimental system, Nlac PorB (and Nme PorB) induced antigen-specific splenocytes proliferation, while OVA alone and alum [60] failed to induce such proliferation, suggesting a specific effect for both Nlac PorB and Nme PorB. However, splenocytes from mice immunized with Nlac PorB/OVA (and Nme PorB/OVA) also appeared more susceptible to non-specific proliferation induced by both ConA and LOS, maybe due to a synergistic effect.
There is a growing body of evidence suggesting that bacterial components with immune adjuvant activity, including CpG DNA [13], Nme PorB [31] or Shigella porins[20], act via Toll-like receptors (TLRs) and can induce both a Th1- and a Th2- immune response [45,68–70]. In addition, it has been recently shown that TLR2 mediates Th1 responses via direct stimulation of Th1 cells and via IL-12 production by APCs, and that TLR2-mediated INF-γ production is crucial for protective immunity against several infectious pathogens [71]. As Nme PorB has been shown to activate immune cells via a TLR2-mediated mechanism [31], we have examined whether the immune adjuvant effect of Nlac PorB might have a similar mechanism of action. Nlac PorB appears to induce immune cell activation (i.e. up-regulation of CD86, CD40 and MHC II on murine B cells, in vitro production of IL-6 and TNF-α, in vitro B cell proliferation) similar to other known TLR2 ligands, such as Nme PorB, Pam2CSK4 and Pam3CSK4. However, these effects are abrogated in B cells from TLR2 knockout mice, suggesting a role for TLR2 in Nlac PorB-mediated immune cell activation although it is derived from a non-pathogenic bacterium which does not elicit much inflamation.
TLR ligands are not only expressed by pathogenic organisms; for example the TLR4 ligand, LPS or the TLR5 ligand, flagellin are present on all Gram-negative bacteria and on most gut commensals, respectively, and can activate immune cells. One could speculate that purified bacterial components from both pathogenic and non-pathogenic species could equally signal via TLRs in vitro but they might require different adaptor molecules (i.e. MyD88 or TRIF–TRAM [72]) or even initiate distinct intracellular cascades of events when they are integral part of live bacteria [73]. Furthermore, pathogenic signals might require different co-receptors for TLR signaling: for example, porins from pathogenic N. meningitidis signal via TLR2/TLR1 [65]. Whether TLR2 engagement by Nlac PorB expressed on intact live bacteria is followed by NF-κB nuclear translocation and cytokine secretion [39–41] still remains undetermined, and further analysis of Nlac PorB interaction with TLRs will be required to elucidate its ability to activate cells, to act as an adjuvant and ultimately even potentially relate to its non-pathogenic nature. Finally, commensals also might lack additional virulence factors to initiate pro-inflammatory responses.
In conclusion, development of efficient adjuvants is of great importance for successful vaccinations to improve the control of diseases. Adding adjuvants to vaccines potentiates their immune stimulating effect, as demonstrated by N. meningitidis serogroup C conjugate vaccines, which have almost eliminated serogroup C meningitis in the UK in 1999 [74]. Moreover, adjuvants allow to induce an effective protection using fewer doses of vaccine, which is particularly advantageous for vaccines targeted to infants and elderly. We have characterized PorB from commensal Neisseria lactamica as an immune adjuvant with equivalent properties to PorB from pathogenic N. meningitidis and to alum. An additional advantage of using neisserial porins as adjuvants is that these proteins can be stored at room temperature for prolonged periods of time without losing activity, decreasing cold chain requirements for shipment and storage in sub-optimal conditions (i.e. small spaces with poor refrigeration in Third World countries). There is no doubt that improvement of vaccine formulations is essential to fight numerous diseases and the development of new adjuvants is key to advance the field of vaccination, our understanding of biological systems to improve the control of diseases and to enhance health.
Acknowledgments
The authors thank Dr. Rob S. Heyderman for critically reading the manuscript and helpful discussion. This work was supported by the BUSM/BUMC Pilot Grant 499-85-221 and by the NIH/NIAIAD Grant AI40944-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Chedid L. Adjuvants of immunity. Ann. Inst. Pasteur Immunol. 1985;136D:283–291. doi: 10.1016/s0769-2625(85)80113-6. [DOI] [PubMed] [Google Scholar]
- 2.Gupta RK, Relyveld EH, Lindblad EB, Bizzini B, Ben Efraim S, Gupta CK. Adjuvants–a balance between toxicity and adjuvanticity. Vaccine. 1993;11:293–306. doi: 10.1016/0264-410x(93)90190-9. [DOI] [PubMed] [Google Scholar]
- 3.Lindblad EB. Aluminium compounds for use in vaccines. Immunol. Cell Biol. 2004;82:497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 4.Freund J. The mode of action of immunologic adjuvants. Bibl. Tuberc. 1956:130–148. [PubMed] [Google Scholar]
- 5.Hilleman MR. Critical appraisal of emulsified oil adjuvants applied to viral vaccines. Prog. Med. Virol. 1966;8:131–182. [PubMed] [Google Scholar]
- 6.Ott G, Barchfeld GL, Chernoff D, Radhakrishnan R, van Hoogevest P, Van Nest G. MF59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm. Biotechnol. 1995;6:277–296. doi: 10.1007/978-1-4615-1823-5_10. [DOI] [PubMed] [Google Scholar]
- 7.Asa PB, Wilson RB, Garry RF. Antibodies to squalene in recipients of anthrax vaccine. Exp. Mol. Pathol. 2002;73:19–27. doi: 10.1006/exmp.2002.2429. [DOI] [PubMed] [Google Scholar]
- 8.de Vries P, van Binnendijk RS, van der MP, van Wezel AL, Voorma HO, Sundquist B, UytdeHaag FG, Osterhaus AD. Measles virus fusion protein presented in an immune-stimulating complex (iscom) induces haemolysis-inhibiting and fusion-inhibiting antibodies, virus-specific T cells and protection in mice. J. Gen. Virol. 1988;69(Pt 3):549–559. doi: 10.1099/0022-1317-69-3-549. [DOI] [PubMed] [Google Scholar]
- 9.Lovgren K, Morein B. The ISCOM: an antigen delivery system with built-in adjuvant. Mol. Immunol. 1991;28:285–286. doi: 10.1016/0161-5890(91)90075-u. [DOI] [PubMed] [Google Scholar]
- 10.Allison AC, Gregoriadis G. Liposomes as immunological adjuvants. Recent Results Cancer Res. 1976:58–64. doi: 10.1007/978-3-642-81049-7_8. [DOI] [PubMed] [Google Scholar]
- 11.Alving CR. Lipopolysaccharide, lipid A, and liposomes containing lipid A as immunologic adjuvants. Immunobiology. 1993;187:430–446. doi: 10.1016/S0171-2985(11)80355-4. [DOI] [PubMed] [Google Scholar]
- 12.Balboa JA, Cuello M, Cabrera O, del Campo J, Lastre M, Gil D, Taboada C, Farinas M, Hernandez M, Perez O. Adjuvant properties of lipopolysaccharide from Neisseria meningitidis serogroup B detoxified and conjugated with tetanus toxoid. Vaccine. 2006;24 Suppl 2:S2–S4. doi: 10.1016/j.vaccine.2005.01.125. [DOI] [PubMed] [Google Scholar]
- 13.Klinman DM. Adjuvant activity of CpG oligodeoxynucleotides. Int. Rev. Immunol. 2006;25:135–154. doi: 10.1080/08830180600743057. [DOI] [PubMed] [Google Scholar]
- 14.Ryan M, McCarthy L, Rappuoli R, Mahon BP, Mills KH. Pertussis toxin potentiates Th1 and Th2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the co-stimulatory molecules B7-1, B7-2 and CD28. Int. Immunol. 1998;10:651–662. doi: 10.1093/intimm/10.5.651. [DOI] [PubMed] [Google Scholar]
- 15.Bizzini B, Carlotti M, Fattal-German M. Induction of various cytokines in mice and activation of the complement system in rats as a part of the mechanism of action of the Corynebacterium granulosum-derived P40 immunomodulator. FEMS Microbiol. Immunol. 1992;5:171–180. doi: 10.1111/j.1574-6968.1992.tb05899.x. [DOI] [PubMed] [Google Scholar]
- 16.Thoelen S, Van Damme P, Mathei C, Leroux-Roels G, Desombere I, Safary A, Vandepapeliere P, Slaoui M, Meheus A. Safety and immunogenicity of a hepatitis B vaccine formulated with a novel adjuvant system. Vaccine. 1998;16:708–714. doi: 10.1016/s0264-410x(97)00254-5. [DOI] [PubMed] [Google Scholar]
- 17.McLaughlin CA, Schwartzman SM, Horner BL, Jones GH, Moffatt JG, Nestor JJ, Jr, Tegg D. Regression of tumors in guinea pigs after treatment with synthetic muramyl dipeptides and trehalose dimycolate. Science. 1980;208:415–416. doi: 10.1126/science.7189295. [DOI] [PubMed] [Google Scholar]
- 18.Rappuoli R, Pizza M, Douce G, Dougan G. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol. Today. 1999;20:493–500. doi: 10.1016/s0167-5699(99)01523-6. [DOI] [PubMed] [Google Scholar]
- 19.Takada H, Ogawa T, Yoshimura F, Otsuka K, Kokeguchi S, Kato K, Kotani S. Immunobiological activities of a porin fraction isolated from Fusobacterium nucleatum ATCC 10953. Infect. Immun. 1988;56:855–863. doi: 10.1128/iai.56.4.855-863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray A, Chatterjee NS, Bhattacharya SK, Biswas T. Porin of Shigella dysenteriae enhances mRNA levels for Toll-like receptor 2 and MyD88, up-regulates CD80 of murine macrophage, and induces the release of interleukin-12. FEMS Immunol. Med. Microbiol. 2003;39:213–219. doi: 10.1016/S0928-8244(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 21.Biswas A, Banerjee P, Mukherjee G, Biswas T. Porin of Shigella dysenteriae activates mouse peritoneal macrophage through Toll-like receptors 2 and 6 to induce polarized type I response. Mol. Immunol. 2007;44:812–820. doi: 10.1016/j.molimm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Alurkar V, Kamat R. Immunomodulatory properties of porins of some members of the family Enterobacteriaceae. Infect. Immun. 1997;65:2382–2388. doi: 10.1128/iai.65.6.2382-2388.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Secundino I, Lopez-Macias C, Cervantes-Barragan L, Gil-Cruz C, Rios-Sarabia N, Pastelin-Palacios R, Villasis-Keever MA, Becker I, Puente JL, Calva E, Isibasi A. Salmonella porins induce a sustained, lifelong specific bactericidal antibody memory response. Immunology. 2006;117:59–70. doi: 10.1111/j.1365-2567.2005.02263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wetzler LM, Blake MS, Barry K, Gotschlich EC. Gonococcal porin vaccine evaluation: comparison of Por proteosomes, liposomes and blebs isolated from rmp deletion mutants. J. Inf. Dis. 1992;166:551–555. doi: 10.1093/infdis/166.3.551. [DOI] [PubMed] [Google Scholar]
- 25.Wetzler LM. Immunopotentiating ability of neisserial major outer membrane proteins. Use as an adjuvant for poorly immunogenic substances and potential use in vaccines. Ann. N. Y. Acad. Sci. 1994;730:367–370. doi: 10.1111/j.1749-6632.1994.tb44295.x. [DOI] [PubMed] [Google Scholar]
- 26.Wetzler LM, Ho Y, Reiser H. Neisserial porins induce B lymphocytes to express costimulatory B7-2 molecules and to proliferate. J. Exp. Med. 1996;183:1151–1159. doi: 10.1084/jem.183.3.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fusco PC, Michon F, Laude-Sharp M, Minetti CA, Huang CH, Heron I, Blake MS. Preclinical studies on a recombinant group B meningococcal porin as a carrier for a novel Haemophilus influenzae type b conjugate vaccine. Vaccine. 1998;16:1842–1849. doi: 10.1016/s0264-410x(98)00174-1. [DOI] [PubMed] [Google Scholar]
- 28.Christodoulides M, Brooks JL, Rattue E, Heckels JE. Immunization with recombinant class 1 outer-membrane protein from Neisseria meningitidis: influence of liposomes and adjuvants on antibody avidity, recognition of native protein and the induction of a bactericidal immune response against meningococci. Microbiology. 1998;144(Pt 11):3027–3037. doi: 10.1099/00221287-144-11-3027. [DOI] [PubMed] [Google Scholar]
- 29.Peeters CC, Claassen IJ, Schuller M, Kersten GF, van der Voort EM, Poolman JT. Immunogenicity of various presentation forms of PorA outer membrane protein of Neisseria meningitidis in mice. Vaccine. 1999;17:2702–2712. doi: 10.1016/s0264-410x(99)00011-0. [DOI] [PubMed] [Google Scholar]
- 30.Mackinnon FG, Ho Y, Blake MS, Michon F, Chandraker A, Sayegh MH, Wetzler LM. The role of B/T costimulatory signals in the immunopotentiating activity of neisserial porin. J. Infect. Dis. 1999;180:755–761. doi: 10.1086/314966. [DOI] [PubMed] [Google Scholar]
- 31.Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, Wetzler LM. Cutting edge: Immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J. Immunol. 2002;168:1533–1537. doi: 10.4049/jimmunol.168.4.1533. [DOI] [PubMed] [Google Scholar]
- 32.Singleton TE, Massari P, Wetzler LM. Neisserial Porin-Induced Dendritic Cell Activation Is MyD88 and TLR2 Dependent. J. Immunol. 2005;174:3545–3550. doi: 10.4049/jimmunol.174.6.3545. [DOI] [PubMed] [Google Scholar]
- 33.Snapper CM, Rojas FR, Kerry MM, Mind JJ, Wetzler LM. Neisserial porins may provide critical second signals to polysaccharide- activated murine B cells for induction of immunoglobulin secretion. Infect. Immun. 1997;65:3203–3208. doi: 10.1128/iai.65.8.3203-3208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poolman JT. Meningococcal vaccines. J. Med. Microbiol. 1988;26:170–172. [PubMed] [Google Scholar]
- 35.Zollinger WD. New and improved vaccines against meningococcal disease. In: Woodrow GC, Levine MM, editors. New Generation Vaccines. New York: Marcel Decker, Inc.; 1990. pp. 325–348. [Google Scholar]
- 36.Mosman TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Ainu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 37.Chow JC, Young DW, Golenbock DT, Christ IJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 38.Takeda K, Takeuchi O, Akira S. Recognition of lipopeptides by Toll-like receptors. J. Endotoxin. Res. 2002;8:459–463. doi: 10.1179/096805102125001073. [DOI] [PubMed] [Google Scholar]
- 39.Romagnani S. Development of Th 1- or Th 2-dominated immune responses: what about the polarizing signals? Int. J. Clin. Lab Res. 1996;26:83–98. doi: 10.1007/BF02592350. [DOI] [PubMed] [Google Scholar]
- 40.Zhang G, Ghosh S. Toll-like receptor-mediated NF-kappa activation: a phylogenetically conserved paradigm in innate immunity. J. Clin. Invest. 2001;107:13–19. doi: 10.1172/JCI11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krutzik SR, Sieling PA, Modlin L. The role of Toll-like receptors in host defense against microbial infection. Curr. Opin. Immunol. 2001;13:104–108. doi: 10.1016/s0952-7915(00)00189-8. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll- like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 43.Rumbo M, Nempont C, Kraehenbuhl JP, Sirard JC. Mucosal interplay among commensal and pathogenic bacteria: lessons from flagellin and Toll-like receptor 5. FEMS Let. 2006;580:2976–2984. doi: 10.1016/j.febslet.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 44.Bambou JC, Giraud A, Menard S, Begue B, Rakotobe S, Heyman M, Taddei F, Cerf-Bensussan N, Gaboriau-Routhiau V. In vitro and ex vivo activation of the TLR5 signaling pathway in intestinal epithelial cells by a commensal Escherichia coli strain. J. Biol. Chem. 2004;279:42984–42992. doi: 10.1074/jbc.M405410200. [DOI] [PubMed] [Google Scholar]
- 45.Akira S. Toll-like receptors and innate immunity. Adv. Immunol. 2001;78:1–56. doi: 10.1016/s0065-2776(01)78001-7. [DOI] [PubMed] [Google Scholar]
- 46.Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochem. Biophys. Acta. 2002;1589:1–13. doi: 10.1016/s0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- 47.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol. Rev. 2004;199:227–250. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 48.Mlckova P, Cechova D, Chalupna P, Novotna O, Prokesova L. Enhanced systemic and mucosal antibody responses to a model protein antigen after intranasal and intra tracheal immunization using Bacillus firmus as an adjuvant. Immunol. Let. 2001;77:39–45. doi: 10.1016/s0165-2478(01)00192-4. [DOI] [PubMed] [Google Scholar]
- 49.Barnes AG, Cerovic V, Hobson PS, Klavinskis LS. Bacillus subtilis spores: a novel micro particle adjuvant which can instruct a balanced Th1 and Th2 immune response to specific antigen. Eur. J. Immunol. 2007;37:1538–1547. doi: 10.1002/eji.200636875. [DOI] [PubMed] [Google Scholar]
- 50.Smits HH, van Beelen AJ, Hessle C, Westland R, de Jong E, Soeteman E, Wold A, Wierenga EA, Kapsenberg ML. Commensal Gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. Eur. J. Immunol. 2004;34:1371–1380. doi: 10.1002/eji.200324815. [DOI] [PubMed] [Google Scholar]
- 51.Derrick JP, Urwin R, Suker J, Feavers IM, Maiden MC. Structural and evolutionary inference from molecular variation in Neisseria porins. Infect. Immun. 1999;67:2406–2413. doi: 10.1128/iai.67.5.2406-2413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gold R, Goldschneider I, Lepow ML, Draper GF, Randolph M. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J. Infect. Dis. 1978;137:112–121. doi: 10.1093/infdis/137.2.112. [DOI] [PubMed] [Google Scholar]
- 53.Olsen SF, Djurhuus B, Rasmussen K, Joensen HD, Larsen SO, Zoffman H, Lind I. Pharyngeal carriage of Neisseria meningitidis and Neisseria lactamica in households with infants within areas with high and low incidences of meningococcal disease. Epidemiol. Infect. 1991;106:445–457. doi: 10.1017/s0950268800067492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oliver KJ, Reddin KM, Bracegirdle P, Hudson IJ, Borrow R, Feavers IM, Robinson A, Cartwright K, Gorringe AR. Neisseria lactamica protects against experimental meningococcal infection. Infect. Immun. 2002;70:3621–3626. doi: 10.1128/IAI.70.7.3621-3626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guttormsen HK, Bjerknes R, Halstensen A, Naess A, Hoiby EA, Solberg CO. Cross-reacting serum opsonins to meningococci after vaccination. J. Infect. Dis. 1993;167:1314–1319. doi: 10.1093/infdis/167.6.1314. [DOI] [PubMed] [Google Scholar]
- 56.Massari P, King CA, Macleod H, Wetzler LM. Improved purification of native meningococcal porin PorB and studies on its structure/function. Protein Exp. Purify. 2005;44(2):136–146. doi: 10.1016/j.pep.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 57.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 58.Tsai CM, Frasch CE. A sensitive silver stain for detecting Lipopolysaccharide in polyacrylamide gels. Analyt. Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 59.Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 60.Yang Z, Chen A, Sun H, Ye Y, Fang W. Ginsenoside Rd elicits Th1 and Th2 immune responses to ovalbumin in mice. Vaccine. 2007;25:161–169. doi: 10.1016/j.vaccine.2006.05.075. [DOI] [PubMed] [Google Scholar]
- 61.Troncoso G, Sanchez S, Criado MT, Ferreiros CM. Analysis of Neisseria lactamica antigens putatively implicated in acquisition of natural immunity to Neisseria meningitidis. FEMS Immunol. Med. Microbiol. 2002;34:9–15. doi: 10.1111/j.1574-695X.2002.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 62.Vaughan TE, Skipp PJ, O'connor CD, Hudson MJ, Vipond R, Elmore MJ, Gorringe AR. Proteomic analysis of Neisseria lactamica and Neisseria meningitidis outer membrane vesicle vaccine antigens. Vaccine. 2006;24:5277–5293. doi: 10.1016/j.vaccine.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez S, Arenas J, Abel A, Criado MT, Ferreiros CM. Analysis of outer membrane protein complexes and heat-modifiable proteins in Neisseria strains using two-dimensional diagonal electrophoresis. J. Proteome Res. 2005;4:91–95. doi: 10.1021/pr049846i. [DOI] [PubMed] [Google Scholar]
- 64.Germann T, Rude E, Schmitt E. The influence of IL12 on the development of Th1 and Th2 cells and its adjuvant effect for humoral immune responses. Res. Immunol. 1995;146:481–486. doi: 10.1016/0923-2494(96)83020-3. [DOI] [PubMed] [Google Scholar]
- 65.Massari P, Visintin A, Gunawardana J, Halmen KA, King CA, Golenbock DT, Wetzler LM. Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J. Immunol. 2006;176:2373–2380. doi: 10.4049/jimmunol.176.4.2373. [DOI] [PubMed] [Google Scholar]
- 66.Jitsukawa T, Nakajima S, Sugawara I, Watanabe H. Increased coating efficiency of antigens and preservation of original antigenic structure after coating in ELISA. J. Immunol. Methods. 1989;116:251–257. doi: 10.1016/0022-1759(89)90211-1. [DOI] [PubMed] [Google Scholar]
- 67.Minetti CA, Tai JT, Blake MS, Pullen JK, Liang SM, Remeta DP. Structural and functional characterization of a recombinant PorB class 2 protein from Neisseria meningitidis. Conformational stability and porin activity. J. Biol. Chem. 1997;272:10710–10720. doi: 10.1074/jbc.272.16.10710. [DOI] [PubMed] [Google Scholar]
- 68.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologous of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 69.Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochem. Biophys. Acta. 2002;1589:1–13. doi: 10.1016/s0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- 70.Dillon S, Agrawal A, Van Dyke T, Landreth G, McCauley L, Koh A, Maliszewski C, Akira S, Pulendran B. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-FBS in dendritic cells. J. Immunol. 2004;172:4733–4743. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 71.Imanishi T, Hara H, Suzuki S, Suzuki N, Akira S, Saito T. Cutting Edge: TLR2 directly triggers Th1 effector functions. J. Immunol. 2007;178:6715–6719. doi: 10.4049/jimmunol.178.11.6715. [DOI] [PubMed] [Google Scholar]
- 72.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signaling. Nat. Rev. Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 73.Fischer H, Yamamoto M, Akira S, Beutler B, Svanborg C. Mechanism of pathogen-specific TLR4 activation in the mucosa: fimbriae, recognition receptors and adaptor protein selection. Eur. J. Immunol. 2006;36:267–277. doi: 10.1002/eji.200535149. [DOI] [PubMed] [Google Scholar]
- 74.Balmer P, Borrow R, Miller E. Impact of meningococcal C conjugate vaccine in the UK. J.Med.Microbiol. 2002;51:717–722. doi: 10.1099/0022-1317-51-9-717. [DOI] [PubMed] [Google Scholar]
- 75.Rudel T, Schmid A, Benz R, Kolb HA, Lang F, Meyer TF. Modulation of Neisseria porin (PorB) by cytosolic ATP/GTP of target cells: parallels between pathogen accommodation and mitochondrial endosymbiosis. Cell. 1996;85:391–402. doi: 10.1016/s0092-8674(00)81117-4. [DOI] [PubMed] [Google Scholar]
- 76.Qi HL, Tai JY, Blake MS. Expression of large amounts of neisserial porin proteins in Escherichia coli and refolding of the proteins into native trimers. Infect Immun. 1994;62(6):2432–2439. doi: 10.1128/iai.62.6.2432-2439.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wright JC, Williams JN, Christodoulides M, Heckels JE. Immunization with the recombinant PorB outer membrane protein induces a bactericidal immune response against Neisseria meningitidis. Infect Immun. 2002;70(8):4028–4034. doi: 10.1128/IAI.70.8.4028-4034.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


