Abstract
Background and purpose:
β1 and β2-adrenoceptors coexist in murine heart but β2-adrenoceptor-mediated effects have not been detected in atrial and ventricular tissues, possibly due to marked phosphodiesterase (PDE) activity. We investigated the influence of the PDE3 inhibitor cilostamide and PDE4 inhibitor rolipram on the effects of (−)-adrenaline in three regions of murine heart.
Experimental approach:
(−)-Adrenaline-evoked cardiostimulation was compared on sinoatrial beating rate, left atrial and right ventricular contractile force in isolated tissues from 129SvxC57B1/6 cross mice. Ventricular arrhythmic contractions were also assessed.
Key results:
Both rolipram (1 μM) and cilostamide (300 nM) caused transient sinoatrial tachycardia but neither enhanced the chronotropic potency of (−)-adrenaline. Rolipram potentiated 19-fold (left atrium) and 7-fold (right ventricle) the inotropic effects of (−)-adrenaline. (−)-Adrenaline elicited concentration-dependent ventricular arrhythmias that were potentiated by rolipram. All effects of (−)-adrenaline were antagonized by the β1-adrenoceptor-selective antagonist CGP20712A (300 nM). Cilostamide (300 nM) did not increase the chronotropic and inotropic potencies of (−)-adrenaline, but administered jointly with rolipram in the presence of CGP20712A, uncovered left atrial inotropic effects of (−)-adrenaline that were prevented by the β2-adrenoceptor-selective antagonist ICI118551.
Conclusions and implications:
PDE4 blunts the β1-adrenoceptor-mediated effects of (−)-adrenaline in left atrium and right ventricle but not in sinoatrial node. Both PDE3 and PDE4 reduce basal sinoatrial rate in a compartment distinct from the β1-adrenoceptor compartment. PDE3 and PDE4, acting in concert, prevent left atrial β2-adrenoceptor-mediated inotropy. PDE4 partially protects the right ventricle against (−)-adrenaline-evoked arrhythmias.
Keywords: phosphodiesterase-4, murine heart, arrhythmias, β1- and β2-adrenoceptors, (−)-adrenaline
Introduction
The mouse heart has been used as a model for human β1-and β2-adrenoceptors. The murine ventricular β-adrenoceptor population consists of 70% β1-adrenoceptor and 30% β2-adrenoceptor (Heubach et al., 1999), similar to the non-failing human ventricle (Molenaar et al., 2000) and atrium (Molenaar et al., 1997). It has been proposed that murine cardiac β2-adrenoceptors couple concurrently to Gs and Gi proteins in murine hearts and that Gs protein-mediated cardiostimulant effects only become apparent after inactivating Gi protein with Pertussis toxin (PTX) in ventricular cardiomyocytes (Xiao et al., 1999). However, the work of Oostendorp and Kaumann (2000) in murine left atria and Heubach et al. (2002) in murine ventricle and sinoatrial node has failed to detect cardiostimulant effects of adrenaline through β2-adrenoceptors, even after treatment with PTX. Furthermore, these findings agree with recent work on murine ventricular myocytes, demonstrating that PTX failed to affect the β2-adrenoceptor-mediated increase in cAMP (Nikolaev et al., 2006). A plausible reason for the lack of detectable function of β2-adrenoceptors in the murine heart could be avid phosphodiesterase-catalysed hydrolysis of the cAMP produced through agonist-evoked receptor activation.
The ryanodine receptor 2 (RyR2) is the main cardiac intracellular channel for Ca2+ release from the sarcoplasmic reticulum of myocytes from the sinoatrial node (Rigg et al., 2000; Vinogradova et al., 2005a), left atrium (Vest et al., 2005) and ventricle (Li et al., 2002; Wehrens et al., 2005). The cardiomyocyte RyR2 is crucial in mediating excitation–contraction coupling, which in turn is strongly modulated by the sympathetic nervous system. Upon catecholamine-evoked stimulation through β-adrenoceptors, cAMP activates the cAMP-dependent PKA, which phosphorylates several proteins including phospholamban and RyR2. Phosphorylated phospholamban dis-inhibits the sarcoplasmic reticulum (SR) calcium pump allowing refilling of the SR calcium stores and making calcium available for release through the RyR2 channel. Murine RyR2 can be phosphorylated by PKA at Serine2008 (Wehrens et al., 2006) and Serine2030 (Xiao et al., 2006). Murine RyR2 phosphorylation, for example, at Serine2008 (Wehrens et al., 2006), appears to reduce binding of the channel-stabilizing subunit calstabin 2 (formerly FKBP12.6) thereby facilitating calcium leak and arrhythmias (Vest et al., 2005). Rolipram-sensitive phosphodiesterase-4D3 (PDE4D3) forms a complex with RyR2. It has been reported that reduction of PDE4D3 levels in heart failure contributes to PKA-induced hyperphosphorylation, resulting in leaky RyR2 channels that facilitate cardiac arrhythmias (Lehnart et al., 2005).
To elucidate whether the activity of PDE3 and/or PDE4 prevent the manifestation of cardiostimulation through murine β2-adrenoceptor, we investigated the effects of the PDE3 inhibitor cilostamide and PDE4 inhibitor rolipram (Vargas et al., 2006) on the responses to (−)-adrenaline under conditions of β1-adrenoceptor blockade in murine cardiac tissues. To investigate whether there are regional differences in the roles of PDE3 and PDE4, we first studied the effects of the PDE inhibitors on the responses to (−)-adrenaline, mediated through β1-adrenoceptor in three cardiac regions: sinoatrial node, left atrium and right ventricle. Right ventricular walls tend to become arrhythmic with high catecholamine concentrations (Heubach et al., 2004). To inquire whether inhibition of PDE4 increases catecholamine-evoked arrhythmias, we investigated the influence of rolipram on concentration-dependent arrhythmias elicited by (−)-adrenaline on murine right ventricular wall.
Methods
Mice
All mice were bred and used in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986. We used genetically heterogenous, outbred 129Sv × C57Bl/6 cross mice of either sex. The mice were studied at 6 months of age. All animals were maintained at 21 °C on a 12 h light/dark cycle and allowed free access to standard rodent chow and water.
Isolated cardiac tissues
Mice of either sex were killed by dislocation of the neck and the hearts were dissected and placed in oxygenated, modified Tyrode's solution at room temperature containing (in mM): NaCl 136.9, KCl 5.0, CaCl2 1.8, MgCl2 1.5, NaHCO3 11.9, NaH2PO4 0.4, EDTA 0.04, ascorbic acid 0.2, pyruvate 5 and glucose 5.0. The pH of the solution was maintained at pH 7.4 by bubbling a mixture of 5% CO2 and 95% O2. Spontaneously beating right atria, left atria and the free wall of the right ventricle were rapidly dissected, mounted in pairs and attached to Swema 4-45 strain gauge transducers in an apparatus containing modified Tyrode's solution at 37 °C. Left atria and right ventricular walls were paced at 2 Hz and stretched as described (Oostendorp and Kaumann, 2000; Heubach et al., 2002). Contractile force was recorded through PowerLab amplifiers on a Chart for Windows, version 5.0 recording programme (ADInstruments, Castle Hill, NSW, Australia).
All tissues were exposed to phenoxybenzamine (5 μM) for 90 min followed by washout, to irreversibly block α-adrenoceptors and tissue uptake of (−)-adrenaline (Gille et al., 1985; Heubach et al., 2002). Some experiments were carried out in the presence of 2-hydroxy-5-[2-[[2-hydroxy-3-[4-[1-methyl-4-(trifluoromethyl)-1H-imidazol-2-yl]phenoxy]propyl]amino]ethoxy]-benzamide (CGP20712A) (300 nM) to selectively block β1-adrenoceptor (Oostendorp and Kaumann, 2000) and conceivably uncover CGP20712A-resistant effects, mediated through β2-adrenoceptor (Heubach et al., 2002, 2003). To corroborate that CGP20712A-resistant effects were mediated through β2-adrenoceptor, the β2-adrenoceptor-selective antagonist 1-[2,3-dihydro-7-methyl-1H-inden-4-yl)oxy-3-[(1-methylethyl)amino]-2-butanol (ICI118551) (50 nM; Oostendorp and Kaumann, 2000) was used in the presence of CGP20712A.
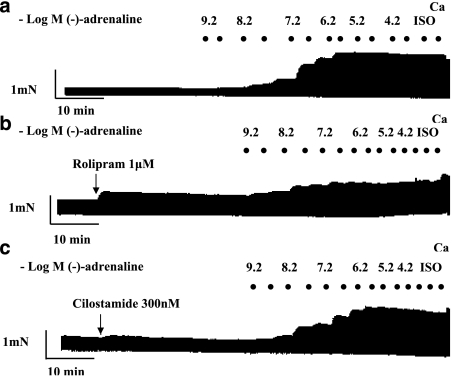
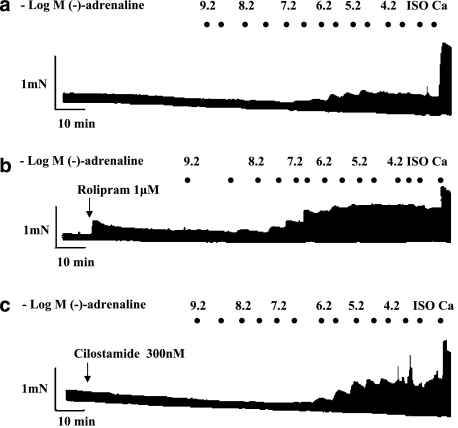
Cumulative concentration–effect curves for (−)-adrenaline were carried out in the absence and presence of the PDE3 inhibitor cilostamide (300 nM) or PDE4 inhibitor rolipram (1 μM) (Vargas et al., 2006), followed by the administration of a saturating concentration of (−)-isoprenaline (200 μM). For inotropic studies, the experiments were terminated by elevating the CaCl2 concentration to 9 mM as shown in representative experiments for left atrium (Figure 1) and right ventricular wall (Figure 2).
Figure 1.
Potentiation of the effects of (−)-adrenaline by rolipram but not by cilostamide on left atrium. Representative experiments, depicting cumulative concentration–effect curves for (−)-adrenaline in the absence of PDE inhibitors (a), in the presence of rolipram (b) and in the presence of cilostamide (c). Black spots indicate −log(−)-adrenaline concentrations, achieved by cumulative administration. Ca, CaCl2 (9 mM); Iso, (−)-isoprenaline (200 μM).
Figure 2.
Positive inotropic and arrhythmic effects of (−)-adrenaline on right ventricular walls; influence of PDE inhibitors. Representative experiments, depicting cumulative concentration–effect curves for (−)-adrenaline in the absence of PDE inhibitors (a), presence of rolipram (b) and presence of cilostamide (c). Black spots indicate −log(−)-adrenaline concentrations, achieved by cumulative administration. Ca, CaCl2 (9 mM); Iso, (−)-isoprenaline (200 μM).
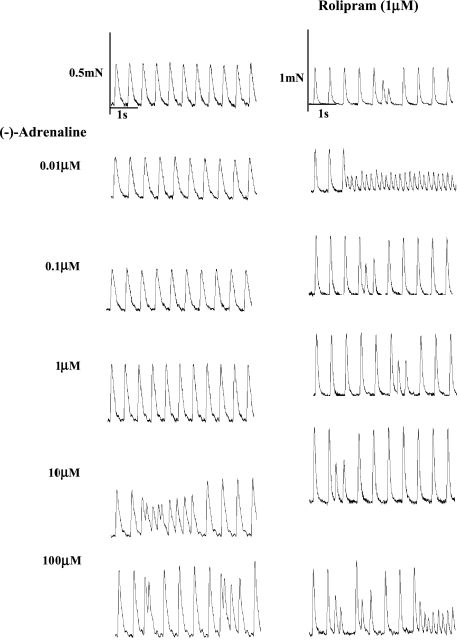
Paced right ventricular walls tend to become arrhythmic with high catecholamine concentrations (Heubach et al., 2004). Arrhythmic contractions consisted of extrasystoles and ventricular tachycardia (Figure 3). The incidence of arrhythmic contractions was assessed from fast-speed tracings as a function of (−)-adrenaline concentration as shown in the representative experiments of Figure 3. The percentage incidence of these arrhythmic events, regardless of whether they were extrasystoles or tachycardia or both, was computed for each (−)-adrenaline concentration across all used right ventricles. The number of preparations with arrhythmic contraction was divided by the total number of preparations, and a standard error calculated. Positive inotropic effects of (−)-adrenaline were measured only from non-arrhythmic ventricles or during periods of stable non-arrhythmic contractions.
Figure 3.
Rolipram potentiates the positive inotropic and arrhythmic effects of (−)-adrenaline on murine right ventricular wall. Comparison of the effects of increasing (−)-adrenaline concentrations on a right ventricular wall in the absence (left hand panels) and another right ventricular wall in the presence of rolipram (right hand panels). Please note extrasystoles at 10 and 100 μM (−)-adrenaline in the absence of rolipram and with rolipram and all (−)-adrenaline concentrations in the presence of rolipram. (−)-Adrenaline 10 μM in the absence of rolipram and (−)-adrenaline 0.01 and 100 μM in the presence of rolipram caused episodes of tachycardia.
Statistics
−log EC50 M values of (−)-adrenaline were estimated from fitting a Hill function with variable slopes to concentration–effect curves from individual experiments. When appropriate, we used an equation for two receptor populations, taken from GraphPad Prism 4 for Windows. The data are expressed as mean±s.e.mean of n=number of mice. Significance of differences between means was assessed with paired and unpaired Student's t-test using GraphPad 4 Software Inc. (San Diego, CA, USA). The distribution of the arrhythmia data is a Bernoulli (0, 1) distribution. The statistical data are the sum of Bernoulli distributions that yields a binomial distribution (Feller, 1968). Since the sample size was sufficiently large, the binomial distribution was approximated to a normal distribution (Feller, 1968). ANOVA with repeated measurements was applied, using the SPSS programme (Chicago, IL, USA). P<0.05 was considered significant.
Drugs
CGP20712A was from Novartis (Basel, Switzerland). ICI118551 was from Tocris (Bristol, UK); (−)-adrenaline, (−)-isoprenaline, rolipram, phenoxybenzamine and cilostamide were from Sigma (Poole, Dorset, UK).
Results
Basal cardiac force and rate. Effects of (−)-isoprenaline and high calcium
Basal left atrial and right ventricular force, as well as sinoatrial rate, are shown in Table 1. The high Ca2+ concentration (9 mM) did not increase further the maximum (−)-isoprenaline response in left atrium (Figure 1 and Table 1). However, 9 mM Ca2+ caused a considerably greater increase of ventricular force than 200 μM (−)-isoprenaline (Figure 2 and Table 1). CGP20712A (300 nM) did not significantly modify atrial force but reduced ventricular force. However, (−)-isoprenaline and 9 mM Ca2+ elevated contractile force to values similar to those in the absence of CGP20712A (Table 1). The combination of CGP20712A (300 nM) and ICI118551 (50 nM) did not affect basal force and the responses to (−)-isoprenaline and 9 mM Ca2+ in left atrium and right ventricle. CGP20712A and CGP20712A plus ICI118551 affected neither basal sinoatrial rate nor the response to (−)-isoprenaline (200 μM).
Table 1.
Contractile force of the left atria and right ventricles as well as sinoatrial rate of right atria
|
Force (mN) |
||||
|---|---|---|---|---|
| n | Basal | (−)-Isoprenaline (200 μM) | Ca2+(9 mM) | |
| Left atrium | ||||
| No β-antagonist | 57 | 0.94±0.11 | 2.10±0.20 | 2.19±0.20 |
| CGP20712A | 26 | 0.80±0.18 | 2.14±0.29 | 2.27±0.28 |
| CGP20712A+ici 118551 | 5 | 1.01±0.24 | 2.40±0.49 | 3.49±0.31 |
| Right ventricle | ||||
| No β-antagonist | 49 | 0.53±0.07 | 1.08±0.12 | 2.11±0.23* |
| CGP20712A | 17 | 0.23±0.04# | 1.14±0.28 | 2.32±0.47 |
| CGP20712A+ici 118551 | 4 | 0.54±0.11 | 1.50±0.52 | 2.67±0.95 |
|
Sinoatrial rate (beats min−1) | ||||
| No β-antagonist | 42 | 294±8 | 508±10 | |
| CGP20712A | 24 | 282±9** | 499±12 | |
| CGP20712A+ici 118551 | 5 | 319±36 | 521±41 | |
#P<0.05 with respect to the absence of β-antagonist.
*P<0.01 with respect to (−)-isoprenaline.
**P=0.06 with respect to the absence of β-antagonist.
Rolipram potentiates the effects of (−)-adrenaline on left atria but not on the sinoatrial node
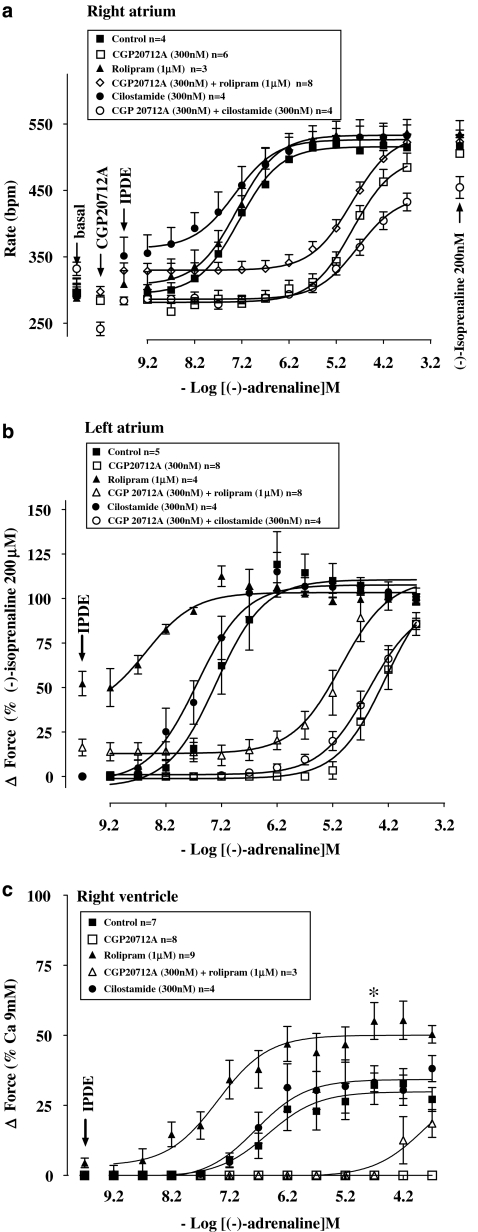
Rolipram (1 μM) increased left atrial contractile force (Figures 1 and 4b). Rolipram tended to transiently increase sinoatrial rate maximally from 288±22 to 313±21 beats min−1 (n=5, P=0.08, paired Student's t-test). Rolipram potentiated 19-fold the positive inotropic effects of (−)-adrenaline on left atrium (Figure 4b and Table 2) but did not potentiate the positive chronotropic effects of (−)-adrenaline on sinoatrial node (Figure 4a and Table 2).
Figure 4.
Potentiation of the effects of (−)-adrenaline by rolipram, mediated through β1-adrenoceptors, on left atria (b) and right ventricular walls (c), but not on sinoatrial pacemaker (a) in the absence and presence of CGP20712A. Lack of effect of cilostamide on (−)-adrenaline potency. A single concentration–effect curve for (−)-adrenaline was determined in the absence or presence of CGP20712A. IPDE, PDE inhibitor. *Increase of the Emax of (−)-adrenaline by rolipram (*P<0.03).
Table 2.
Cardiostimulant potencies (−log EC50 M) of (−)-adrenaline
| −log EC50 M |
||||
|---|---|---|---|---|
|
Control |
CGP20712A (300 nM) |
|||
| n | n | |||
| Right atrium (sinus rate) | ||||
| Control | 4 | 7.24±0.19 | 6 | 4.83±0.07 |
| Rolipram | 3 | 7.26±0.24 | 8 | 4.87±0.06 |
| Cilostamide | 4 | 7.35±0.11 | 4 | 4.71±0.11 |
| Rolipram+cilostamide | 5 | 8.11±0.11* | 6 | 4.95±0.05 |
| Left atrium (contractile force) | ||||
| Control | 5 | 7.19±0.12 | 8 | 4.22±0.19 |
| Rolipram | 4 | 8.47±0.11*** | 8 | 4.97±0.16** |
| Cilostamide | 4 | 7.66±0.20 | 4 | 4.55±0.14 |
| Rolipram+cilostamide | 13 | 5.53a±0.16***# | ||
| Rolipram+cilostamide | 13 | 7.93b±0.30 | ||
| Ventricle (contractile force) | ||||
| Control | 7 | 6.54±0.12 | ||
| Rolipram | 9 | 7.40±0.14** | ||
| Cilostamide | 4 | 6.37±0.41 | ||
| Rolipram+cilostamide | 8 | 7.42±0.30** | 6 | 4.32±0.05 |
*P<0.05, **P<0.01, ***P<0.001 compared to control.
#P<0.01, compared to rolipram.
β1-adrenoceptor component.
β2-adrenoceptor component.
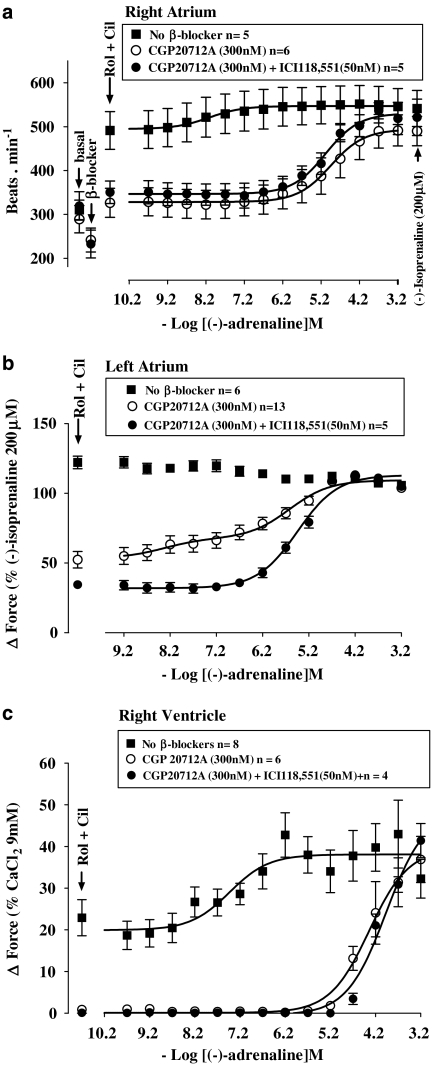
As observed previously (Heubach et al., 2002), CGP20712A tended to cause bradycardia (Figures 4a and 6a and Table 1) but the effect did not reach statistical significance (Table 2). CGP20712A caused a 3 log unit rightward and surmountable shift of the concentration–effect curve of (−)-adrenaline for sinoatrial tachycardia (Figure 4a and Table 2) and left atrial contractile force (Figure 4b and Table 2). Rolipram, in the presence of CGP20712A, increased sinoatrial rate from 297±7 to 329±11 beats min−1 (n=8, P<0.005). Rolipram, in the presence of CGP20712A, potentiated sixfold the positive inotropic effects of (−)-adrenaline on left atria (Figure 4b and Table 2) but did not affect the positive chronotropic effects of (−)-adrenaline on sinoatrial node (Figure 4a and Table 2).
Rolipram potentiates ventricular effects of (−)-adrenaline
Rolipram tended to increase basal force (Figures 2 and 4c) to a variable extent by an average of 4.5±1.7% of the response to 9 mM CaCl2 but the effect did not reach significance (P=0.056). Rolipram potentiated sevenfold the positive inotropic effects of (−)-adrenaline (Table 2, Figure 4c) and increased the (−)-adrenaline-evoked maximum contraction (Emax as % of the response to 9 mM CaCl2, P<0.03; Figure 4c). CGP20712A caused insurmountable blockade of the inotropic effects of (−)-adrenaline on ventricle in the absence of rolipram or partially surmountable blockade in the presence of rolipram (Figure 4c).
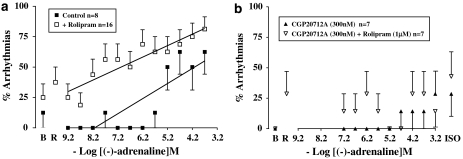
High (−)-adrenaline concentrations tended to produce arrhythmic contractions on the free wall of the right ventricle (Figures 3 and 5a). The arrhythmic contractions consisted of extrasystoles and/or episodes of ventricular tachycardia. A contraction due to an extrasystole appeared prematurely with reduced force and also reduced the force of the following paced contraction (Figure 3). Ventricular tachycardia appeared as a sequence of non-paced contractions at a faster rhythm than the paced contractions and with decreased contractile force. Some ventricular preparations on occasion produced spontaneous arrhythmic contractions in the absence or presence of rolipram (Figures 5a and b). The incidence of spontaneous arrhythmic contractions was not significantly enhanced by rolipram in the absence of CGP20712A (P=0.164, n=16, paired Student's test; P=0.258, n=24 controls vs n=16 rolipram-treated, unpaired Student's test; Figure 5a) or presence of CGP20712A (P=0.172, n=7, paired Student's test; Figure 5b). The increase of (−)-adrenaline-evoked arrhythmic contractions was linearly related to −log concentration but the slope was steeper in the absence of rolipram (slope 0.126, r2 0.715) than in the presence of rolipram (slope 0.096, r2 0.831) (Figure 5a). CGP20712A prevented the incidence of the (−)-adrenaline-evoked arrhythmias, both in the absence and presence of rolipram (Figure 5b).
Figure 5.
Increased incidence of (−)-adrenaline-evoked arrhythmias by rolipram (a) and blockade by CGP20712A (b). %Arrythmias is the % of ventricles that showed extrasystoles and/or ventricular tachycardia at each (−)-adrenaline concentration. Lines depict the dependence of arrhythmias on −log(−)-adrenaline concentration. The slopes of the lines were 0.126 and 0.096 in the absence and presence of rolipram, respectively. B, basal; ISO, (−)-isoprenaline 200 μM;R, rolipram.
Cilostamide does not potentiate the positive chronotropic and inotropic effects of (−)-adrenaline
Cilostamide (300 nM) did not significantly increase left atrial contractility (Figure 1c); force was 1.13±0.52 and 1.19±0.53 mN in the absence and presence of cilostamide. Cilostamide did not affect ventricular contractility (Figure 2d). Cilostamide transiently increased sinoatrial rate maximally from a basal rate of 294±23 to 351±28 beats min−1 (P<0.01, n=4, paired Student's test). In the presence of CGP20712A, cilostamide increased sinoatrial rate from 241±10 to 283±6 beats min−1 (P<0.005, n=4). Cilostamide did not significantly affect the potency of (−)-adrenaline on sinoatrial node (Figure 4a) and left atrium (Figure 4b) in the absence or presence of CGP20712A (Figures 4a and b and Table 2). Cilostamide did not affect the potency of (−)-adrenaline in right ventricle (Figure 4c and Table 2).
Concurrent cilostamide and rolipram uncover functional β2-adrenoceptors in left atrium but not in sinoatrial node and right ventricle
Cilostamide and rolipram, administered together, caused marked increases in sinoatrial rate as well as of left atrial and right ventricular force (Figures 6a–c). Concurrent cilostamide and rolipram potentiated the sinoatrial and ventricular effects of (−)-adrenaline (compare Figures 6a and c with Figures 4a and c; Table 2). However, the ventricular potency of (−)-adrenaline in the presence of both cilostamide and rolipram was not different from the potency of (−)-adrenaline in the presence of rolipram alone (Table 2). The maximum increase in contractility by combined cilostamide and rolipram prevented further effects of (−)-adrenaline in left atrium (Figure 6b). In the presence of CGP20712A, the increases in left atrial and right ventricular force and sinoatrial rate caused by the combination of cilostamide and rolipram were smaller (P<0.001, P<0.01 and P=0.02, respectively) than in the absence of CGP20712A and the concentration–effect curves of (−)-adrenaline were shifted to the right by 3 log units (Figure 6 and Table 2). CGP20712A-resistant effects of (−)-adrenaline were detected on left atrium (Figure 6b) but not on sinoatrial node and right ventricle (Figures 6a and c). The concentration–effect curve for (−)-adrenaline on left atrium in the presence of CGP20712A was biphasic (Figure 6b) with a high-potency and low-potency component (Table 2). The CGP20712A-resistant effects of (−)-adrenaline were prevented by the β2-adrenoceptor-selective antagonist ICI118551 (50 nM) (Figure 6b), consistent with mediation through β2-adrenoceptors. The fractions of left atrial β1- and β2-adrenoceptor-mediated effects of (−)-adrenaline in the presence of cilostamide, rolipram and CGP20712A amounted to 0.74±0.07 vs 0.26±0.07, respectively (Table 2).
Figure 6.
Cilostamide (Cil) and rolipram (Rol), administered together, potentiate the chronotropic effects of (−)-adrenaline (a) and uncover β2AR-mediated inotropic effects in the presence of CGP20712A, sensitive to blockade by ICI118551, in left atrium (b) but not in right ventricle (c). Data from left atria in the presence of CGP20712A were fitted for two β-adrenoceptor populations with −log EC50 M of 7.93±0.30 (β2-adrenoceptor) and 5.53±0.16 (β1-adrenoceptor) and fractional Emax of 0.74±0.07 (β1-adrenoceptor) and 0.26±0.07 (β2-adrenoceptor). The left atrial −log EC50 M of the curve for (−)-adrenaline in the presence of both CGP20712A and ICI118551 was 5.44±0.08.
Discussion
Our results point to regional differences in the role of PDE4 in murine heart. First, (−)-adrenaline-evoked sinoatrial tachycardia was not potentiated by either cilostamide or rolipram, inconsistent with modulation by either PDE3 or PDE4 alone. However, both isoenzymes appear to control basal sinoatrial beating rate. Second, in contrast, the positive inotropic effects of (−)-adrenaline, mediated through left atrial and right ventricular β1-adrenoceptors, were potentiated by rolipram, but not by cilostamide, suggesting hydrolysis of inotropically relevant cAMP by PDE4 but not by PDE3. Third, the effects of (−)-adrenaline in the three cardiac regions were antagonized by CGP20712A, consistent with mediation through β1-adrenoceptors. Next, CGP20712A-resistant effects, mediated through β2-adrenoceptor, were only observed on left atrium in the presence of both cilostamide and rolipram, suggesting that PDE3 and PDE4 act in concert to prevent manifestation of β2-adrenoceptor function. Finally, rolipram potentiated (−)-adrenaline-evoked right ventricular arrhythmias.
PDE3 and PDE4 modulate basal sinoatrial beating but not β1-adrenoceptor-mediated tachycardia of (−)-adrenaline
Sinoatrial cells exhibit a considerably higher basal cAMP content and basal PKA-mediated phosphorylation of phospholamban than atrial or ventricular cells (Vinogradova et al., 2006). Submaximal PKA inhibition slows the spontaneous firing rate of sinoatrial action potentials and it has been postulated that basal PKA activity is obligatory for rhythmical Ca2+ release from RyR2 channels involved in generating spontaneous sinoatrial beating (Maltsev et al., 2006; Vinogradova et al., 2006). Interestingly, basal PDE activity also appears to be elevated in sinoatrial cells (Vinogradova et al., 2005b) and our data, showing that cilostamide and rolipram increase sinoatrial rate, suggest that both PDE3 and PDE4 reduce tonically sinoatrial beating rate by hydrolysing cAMP. The tachycardia elicited by either cilostamide or rolipram suggests mediation through an increase of cAMP in sinoatrial cells. Blockade of β1-adrenoceptors with CGP20712A did not prevent the tachycardia of cilostamide or rolipram, ruling out an interaction of endogenously released noradrenaline with β1-adrenoceptors. The tachycardia of cilostamide or rolipram is likely to result from the inhibition of either PDE3 or PDE4 respectively, followed by elevation of sinoatrial cAMP and increase of PKA-dependent beating rate. In addition, the increased sinoatrial cAMP in the presence of cilostamide or rolipram may also directly bind to, and open the channels responsible for the current activated by hyperpolarization, If (DiFrancesco and Tortora, 1991). Our evidence is consistent with the tachycardia produced by several other PDE3-selective inhibitors in a variety of species (Brunkhorst et al., 1989; Sato et al., 1999; Herring and Paterson, 2001).
The modulation of sinoatrial beating rate by PDE3 is in marked contrast to the lack of influence of cilostamide on the β1-adrenoceptor-mediated increase in sinoatrial rate elicited by (−)-adrenaline. This discrepancy suggests that the PDE3-sensitive pool of cAMP that modulates sinoatrial beating frequency is separated from a PDE3-insensitive pool of cAMP through which (−)-adrenaline increases sinoatrial rate. The rolipram-evoked tachycardia is also at variance with the lack of potentiation of the positive chronotropic effects of (−)-adrenaline by rolipram, suggesting the existence of a PDE4-sensitive cAMP compartment for basal heart rate but not for sinoatrial β1-adrenoceptor stimulation by (−)-adrenaline.
Does inhibition of both PDE3 and PDE4 potentiate (−)-adrenaline-evoked tachycardia?
Neither inhibition of PDE3 nor PDE4 alone affected the chronotropic potency of (−)-adrenaline. In contrast, the combined administration of rolipram (1 μM) and cilostamide (300 nM) caused tachycardia and appeared to potentiate the positive chronotropic effects of (−)-adrenaline (Figure 6a and Table 2). However, in the presence of CGP20712A, which shifted the concentration–effect curve of (−)-adrenaline by 3 log units to the right, the combination of rolipram and cilostamide failed to potentiate the positive chronotropic effects of (−)-adrenaline (Figure 6a). The tachycardia caused by the combination of rolipram and cilostamide was less marked in the presence than in the absence of CGP20712A (Figure 6a), and could be plausibly related to an inverse agonist effect of CGP20712A and perhaps to blockade of β1-adrenoceptors activated by traces of endogenously released noradrenaline. Since the chronotropic potency of (−)-adrenaline was not significantly increased by the combination of rolipram and cilostamide in the presence of CGP20712A, the apparent potentiation of the effects of (−)-adrenaline in the absence of CGP20712A appears mainly due to the additivity of the effects of rolipram, cilostamide and (−)-adrenaline. However, since both PDE3 and PDE4 appear to reduce the cAMP required for basal sinoatrial rate, it cannot be excluded that some cAMP from this compartment may leak into the β1-adrenoceptor compartment of cAMP when both isoenzymes are inhibited. Alternatively, both PDE3 and PDE4 may actually hydrolyse cAMP in the β1-adrenoceptor compartment but, when one enzyme is inhibited, the resulting increase of cAMP induced PKA-catalysed phosphorylation of the other enzyme, thereby facilitating cAMP hydrolysis and reducing both increases of cAMP and sinoatrial rate by (−)-adrenaline. The hydrolytic activity of both PDE3 (Gettys et al., 1987; Smith et al., 1991) and PDE4 (MacKenzie et al., 2002) are enhanced by PKA-dependent phosphorylation. Only when both PDE3 and PDE4 were inhibited in the sinoatrial β1-adrenoceptor compartment was cAMP enhanced and some potentiation of (−)-adrenaline-evoked tachycardia followed. Further evidence is needed to support or reject these interpretations.
PDE4 limits the β1-adrenoceptor inotropic function and PDE3 and PDE4 jointly prevent β2-adrenoceptor function in left atrium
Rolipram caused marked potentiation of the effects of (−)-adrenaline on left atrium in the absence and presence of CGP20712A but cilostamide failed to affect the inotropic potency. These effects are consistent with an exclusive role of PDE4 in controlling the inotropically relevant cAMP generated through left atrial β1-adrenoceptor stimulation.
Inhibition of both PDE3 and PDE4 uncovered functional β2-adrenoceptors in left atrium. The CGP20712A-resistant effects of (−)-adrenaline in the presence of both cilostamide and rolipram were prevented by ICI118551, consistent with mediation through β2AR. Our results indicate that PDE3 and PDE4, acting in concert, prevent the manifestation of β2AR-mediated effects of (−)-adrenaline in murine left atrium. The potentiation by rolipram of the effects of (−)-adrenaline in the presence of CGP20712A (Figure 4b) is similar to that observed in the absence of CGP20712A. Importantly, the leftward shift of the concentration–effect curve was parallel and expected from the high affinity of CGP20712A for β1-adrenoceptors without evidence for CGP20712A-resistant effects. Therefore inhibition of PDE4 alone does not appear to uncover β2-adrenoceptor-mediated effects of (−)-adrenaline. Previous work failed to detect β2-adrenoceptor-mediated effects in murine left atrium, even after inactivation of Gi protein with PTX (Oostendorp and Kaumann, 2000; Heubach et al., 2002). On the other hand, β2-adrenoceptor stimulation increases cAMP but this was not affected by PTX (Nikolaev et al., 2006). Taken together, this evidence is inconsistent with the concept that activation of Gi protein through β2-adrenoceptor blunts Gs protein-mediated effects in murine heart (Xiao et al., 1999). β2-Adrenoceptor-mediated increases of cAMP are spatially confined and do not propagate and, further, β2-adrenoceptor-mediated effects are mainly blunted by both PDE3 and PDE4 in murine myocytes (Nikolaev et al., 2006). Our present results with β2-adrenoceptor-mediated inotropic effects on left atrium are in line with the conclusions of Nikolaev et al. (2006).
Under our conditions, the β2-adrenoceptor function was uncovered only with the concurrent use of cilostamide and rolipram under β1-adrenoceptor blockade with CGP20712A in left atrium but not in sinoatrial node and right ventricle. Genetic deletion of the β2-adrenoceptor does not modify the chronotropic response to (−)-isoprenaline, which is entirely mediated through β1-adrenoceptors (Chruscinski et al., 1999). It is therefore unknown whether the murine sinoatrial node possesses functional β2-adrenoceptors. However, 30% of murine ventricular β-adrenoceptors are β2-adrenoceptors (Heubach et al., 1999). A possible reason for the lack of β2-adrenoceptor-mediated responses in right ventricle could be an involvement of PDE2, an option which requires further research.
The −log EC50 M of the left atrial β1AR-mediated component of the inotropic effects of (−)-adrenaline in the presence of rolipram, cilostamide and CGP20712A was 3.6-fold (that is 0.56 log units) larger than the −log EC50 M in the presence of rolipram and CGP20712 (Table 2). These results suggest that when PDE4 is inhibited, PDE3 may become activated, by an excess of cAMP, possibly through PKA-catalysed phosphorylation, and contribute to hydrolyse inotropically relevant cAMP.
The effects of (−)-adrenaline, mediated through β1-adrenoceptors, are blunted by PDE4 but not by PDE3 in right ventricle
The effects of (−)-adrenaline were antagonized by CGP20712A, consistent with mediation through β1-adrenoceptors and the 3 log surmountable shift of the concentration–effect curves did not reveal CGP20712A-resistant effects of (−)-adrenaline in right ventricular wall, inconsistent with the participation of β2-adrenoceptors. The addition of cilostamide to rolipram did not cause additional potentiation (Table 2), pointing towards an exclusive function of PDE4, and no role of PDE3, in murine ventricle. The potentiation of the positive inotropic effects of (−)-adrenaline by rolipram, but not by cilostamide, and the lack of additional potentiation by cilostamide in the presence of rolipram, are consistent with hydrolysis of inotropically relevant cAMP by PDE4 but not by PDE3 in right ventricle. Our results are consistent with the conclusion of recent work by Nikolaev et al. (2006) demonstrating that β1-adrenoceptor-mediated cAMP signals are entirely controlled by PDE4 in murine ventricular myocytes.
Our results are at variance with data of Xiang et al. (2005), showing that PDE4 blunted the effects of isoprenaline mediated through β2-adrenoceptor in spontaneously beating ventricular myocytes from new-born mice. These authors demonstrated that the fade of (−)-isoprenaline-induced increase in myocyte beating rate was prevented by a PDE4-selective inhibitor and that fade did not occur in PDE4D3-KO mice, clearly proving involvement of this PDE4 isoenzyme. However, in contrast to our demonstration that the effects of (−)-adrenaline are mediated through β1-adrenoceptors and potentiated by the PDE4 inhibitor rolipram, in their work, the effects of (−)-isoprenaline, mediated through β1-adrenoceptors, were not affected by PDE4 inhibition (Xiang et al., 2005). Xiang et al. (2005) also found that cilostamide did not affect the responses to (−)-isoprenaline, mediated through β2-adrenoceptors in their experimental model, ruling out the role of PDE3. Comparison of the results of the experiments of Xiang et al. (2005) in ventricular myocardium from new-born mice and our results from myocardium of adult mice suggests that the β2AR inotropic function is reduced (left atrium) or lost (right ventricle) in adult mice. Consistent with this suggestion are the results of Heubach et al. (2002), as well as our present results, demonstrating a lack of functional β2-adrenoceptors in adult murine ventricular myocardium, even after inactivation of Gi protein with PTX (Heubach et al., 2002). Furthermore, the results of Kuznetsov et al. (1995) in rat myocardium is also in agreement with work on murine hearts, because activation of β2-adrenoceptor, at low agonist concentrations that cause positive inotropic and lusitropic effects in neonatal cardiomyocytes, is lost in adult myocytes. In the adult rat, the ventricular inotropic responses to (−)-noradrenaline, mediated through β1-adrenoceptors, are potentiated by rolipram but not by cilostamide (Vargas et al., 2006), findings compatible with our present work using (−)-adrenaline in adult murine right ventricle and left atrium.
Unlike murine and rat heart, human atrial and ventricular myocardium respond to catecholamines with positive inotropic effects, mediated through β-adrenoceptors, which are potentiated by cilostamide but not by rolipram, that is, modulated by PDE3 but not by PDE4 (Kaumann et al., 2007; Christ et al., 2006a, 2006b). Moreover, in human isolated myocardium, cilostamide potentiates the effects of (−)-adrenaline, mediated through β2-adrenoceptors, more than the effects of (−)-noradrenaline, mediated through β1-adrenoceptors, perhaps suggesting a more marked phosphorylation of PDE3 by PKA via β2-adrenoceptors than via β1-adrenoceptors (Christ et al., 2006a, 2006b). In contrast to murine and rat myocardium, in human myocardium, rolipram does not affect the positive inotropic effects of physiological catecholamines, mediated through either β1AR (Christ et al., 2006a; Kaumann et al., 2007) or β2AR (Christ et al., 2006a, 2006b). Thus, murine and rat cardiac myocardial models do not mimic the control by specific PDE isoenzymes of the positive inotropic responses to physiological catecholamines, mediated through β1- and β2-adrenoceptors in human myocardium.
Murine right ventricle, a model for catecholaminergic polymorphic ventricular tachycardia
(−)-Adrenaline caused concentration-dependent arrhythmias in the right ventricular wall. The extrasystolic contractions showed reduced force. The contractions of ventricular tachycardia, initiated by an extrasystole, also exhibited markedly reduced force. Larger mammals including man with non-failing hearts, increase cardiac contractile force when heart rate is increased, the Bowditch staircase. In rodents, however, contractile force of cardiac tissues and myocytes is decreased when heart rate is increased and this negative staircase has also been demonstrated in mice (Wussling et al., 1987; Ceylan-Isik et al., 2006). The reduced contractile force of ventricular extrasystoles and tachycardia is probably a manifestation of negative staircase.
The arrhythmias were greatly attenuated by CGP20712A and are therefore mediated through β1-adrenoceptors. In the presence of rolipram, (−)-adrenaline elicited arrhythmic contractions at lower concentrations than in the absence of rolipram (Figure 5a), consistent with potentiation. However, the concentration–effect curve for (−)-adrenaline was flatter in the presence than in the absence of rolipram, so that an additive effect of rolipram cannot be ruled out, despite our finding that rolipram-evoked arrhythmias did not reach statistical significance. Taken together, these results are consistent with a protective role of PDE4 through hydrolysis of cAMP, thereby preventing both PKA-catalysed phosphorylation of the RyR2 channels (Wehrens et al., 2006; Xiao et al., 2006) and Ca2+ leak that would lead to ventricular arrhythmias. Our results with ventricular arrhythmias are consistent with results in isolated cardiomyocytes from adult mice in which the PDE4D3 isoform was found to participate in a macromolecular complex including RyR2 and PKA (Lehnart et al., 2005). PDE4D3 ablation in mice hastened the appearance of heart failure after myocardial infarction and of arrhythmias, associated with increased cAMP-dependent signals at RyR2 sites after low catecholamine concentrations, compared to wild-type mice (Lehnart et al., 2005).
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a cardiac arrhythmia that occurs under conditions of adrenergic stimulation during exercise or under emotional stress. Affected individuals present syncope and/or sudden cardiac death in childhood and adolescence (Coumel et al., 1978; Leenhardt et al., 1995). The mortality rate is approximately one-third of CPVT patients by age of 35 years (Lehnart et al., 2004). The disease was linked to chromosome 1q42–q43 (Rampazzo et al., 1995) and subsequently both Priori et al. (2001) and Laitinen et al. (2001) showed that individuals with the autosomal dominant form of CPVT had mutations in the human cardiac RyR2. Over 30 mutations have been identified, which cluster within three regions of the RyR2 receptor, the first 450 amino acids at N terminus, a central region (amino acids 2240–2510) and the C terminus (amino acids 3378–5000) (Jiang et al., 2005).
Some clinically relevant RyR2 mutations of patients with CPVT have been reproduced in mice. These include RyR2R4496C mice that exhibit proarrhythmic delayed afterdepolarizations (Liu et al., 2006) and RyR2R176Q mice that exhibit a high incidence of ventricular tachycardia and cardiocyte Ca2+ oscillations (Kannankeril et al., 2006). Ventricular arrhythmias, including tachycardia and fibrillation, which occur in patients with RyR2 mutations have been attributed to enhanced store overload-induced Ca2+ release from the RyR2 (Jiang et al., 2004, 2005).
We attribute the right ventricular arrhythmias, observed as a function of (−)-adrenaline concentration, to pro-arrhythmic Ca2+ leaking out from the RyR2 channels, due to cAMP and resultant PKA-catalysed RyR2 phosphorylation (Lehnart et al., 2005; Wehrens et al., 2006; Xiao et al., 2006). The potentiation of the (−)-adrenaline-evoked arrhythmias we observed with rolipram is consistent with the work of Lehnart et al. (2005). We suggest that the murine right ventricular wall would provide an experimental model for CPVT. Mice generated to carry human CPVT mutations of RyR2 channels should exhibit a greater sensitivity to (−)-adrenaline-evoked arrhythmias, mediated through β1-adrenoceptors.
Conclusions
Rolipram revealed regional differences in the role of PDE4 in murine heart. (−)-Adrenaline-evoked cardiostimulation was blunted considerably by PDE4, but not by PDE3, in murine left atrium and in right ventricle. Although both PDE3 and PDE4 modulated basal sinoatrial beating rate, inhibition of either of these phosphodiesterases did not potentiate the β1-adrenoceptor-mediated tachycardia elicited by (−)-adrenaline. Concurrent inhibition of both PDE3 and PDE4 uncovered cardiostimulant effects of (−)-adrenaline mediated through β2-adrenoceptors of left atrium but not of sinoatrial node or right ventricle. Rolipram potentiated (−)-adrenaline-evoked arrhythmias, mediated through β1-adrenoceptors in right ventricular wall. The murine right ventricle may serve as a model for (−)-adrenaline-evoked arrhythmias in mice carrying RyR2 mutations corresponding to human CPVT.
Acknowledgments
This work was supported by the British Heart Foundation and the Seneca Foundation. We thank Dr Manuel Canteras, Department of Biostatistics, University of Murcia, Spain, for help with the statistics.
Abbreviations
- CGP20712A
(2-hydroxy-5-[2-[[2-hydroxy-3-[4-[1-methyl-4-(trifluoromethyl)-1H-imidazol-2-yl]phenoxy]propyl]amino]ethoxy]-benzamide)
- ICI118551
(1-[2,3-dihydro-7-methyl-1H-inden-4-yl)oxy-3-[(1-methylethyl)amino]-2-butanol)
- RyR2
ryanodine receptor 2
Conflict of interest
The authors state no conflict of interest.
References
- Brunkhorst D, v der Leysen H, Meyer W, Nigbur R, Schmidt-Schumacher C, Scholz H. Relation of positive inotropic effects of pimobendan, UD-CG212Cl, milrinone and other phosphodiesterase inhibitors to phosphodiesterase III inhibition in guinea-pig heart. Naunyn Schmiedebergs Arch Pharmacol. 1989;339:575–583. doi: 10.1007/BF00167264. [DOI] [PubMed] [Google Scholar]
- Ceylan-Isik AF, LaCour KH, Ren J. Gender disparity of streptozotocin-induced intrinsic contractile dysfunction in murine ventricular myocytes: role of chronic activation of AKT. Clin Exp Pharmacol Physiol. 2006;33:102–108. doi: 10.1111/j.1440-1681.2006.04331.x. [DOI] [PubMed] [Google Scholar]
- Christ T, Engel A, Ravens U, Kaumann AJ. Cilostamide potentiates more the positive inotropic effects of (−)-adrenaline through β2-adrenoceptors than the effects of (−)-noradrenaline through β1-adrenoceptors in human atrial myocardium. Naunyn Schmiedebergs Arch Pharmacol. 2006a;374:249–253. doi: 10.1007/s00210-006-0119-5. [DOI] [PubMed] [Google Scholar]
- Christ T, Molenaar P, Galindo-Tovar A, Ravens U, Kaumann AJ.Contractile responses through Gs-coupled receptors are reduced by phosphodiesterase3 activity in human isolated myocardium Biochemical Society Focusing Meeting. Compartmentalization of Cyclic AMP Signaling 2006bKing's College: Cambridge, UK; 29–30.March: P014 [Google Scholar]
- Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Berstein D, Kobilka BK. Targeted disruption of the β2-adrenergic receptor gene. J Biol Chem. 1999;274:16694–16700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- Coumel P, Fidelle J, Lucet V, Attuel P, Bouvrain Y. Catecholaminergic-induced severe ventricular arrhythmias with Adam-Stokes syndrome in children: report of four cases. Br Heart J. 1978;40 Suppl:28–37. [Google Scholar]
- DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- Feller W. An Introduction to Probability Theory and its Application 1968John Wiley & Sons Inc.: New York; Vol 1 [Google Scholar]
- Gettys TW, Blackmore PF, Redmon JB, Beebe SJ, Corbin JD. Short-term feedback regulation of cAMP by accelerated degradation in rat tissues. J Biol Chem. 1987;262:333–339. [PubMed] [Google Scholar]
- Gille E, Lemoine H, Ehle B, Kaumann AJ. The affinity of (−)-propranolol for β1- and β2-adrenoceptors of human heart. Differential antagonism of the positive inotropic effects and adenylate cyclase stimulation by (−)-noradrenaline and (−)-adrenaline. Naunyn Schmiedebergs Arch Pharmacol. 1985;331:60–70. doi: 10.1007/BF00498852. [DOI] [PubMed] [Google Scholar]
- Herring N, Paterson DJ. Nitric oxide–cGMP pathway facilitates acetylcholine release and bradycardia during vagal nerve stimulation in the guinea-pig in vitro. J Physiol. 2001;535:507–518. doi: 10.1111/j.1469-7793.2001.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heubach J, Blaschke M, Harding SE, Ravens U, Kaumann AJ. Cardiostimulant and cardiodepressant effects through overexpressed human β2-adrenoceptors in murine heart: regional differences and functional role of β1-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:380–390. doi: 10.1007/s00210-002-0681-4. [DOI] [PubMed] [Google Scholar]
- Heubach JF, Rau T, Eschenhagen T, Ravens U, Kaumann AJ. Physiological antagonism between ventricular β1-adrenoceptors and α1-adrenoceptors but no evidence for β2- and β3-adrenoceptor function in murine heart. Br J Pharmacol. 2002;136:217–229. doi: 10.1038/sj.bjp.0704700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heubach JF, Ravens U, Kaumann AJ. Epinephrine activates both Gs and Gi pathways, but norepinephrine activates only the Gs pathway through human β2-adrenoceptors overexpressed in mouse heart. Mol Pharmacol. 2004;65:1313–1322. doi: 10.1124/mol.65.5.1313. [DOI] [PubMed] [Google Scholar]
- Heubach JF, Trebeß T, Wettwer E, Himmel HM, Michel MC, Kaumann AJ, et al. L-type Ca2+ current and contractility in ventricular myocytes from mice overexpressing the cardiac β2-adrenoceptor. Cardiovasc Res. 1999;42:173–182. doi: 10.1016/s0008-6363(98)00262-4. [DOI] [PubMed] [Google Scholar]
- Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, et al. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, et al. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci USA. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannankeril PJ, Mitchell BM, Goonasekera SA, Chelu MG, Zhang W, Sood S, et al. Mice with R176Q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc Natl Acad Sci USA. 2006;103:12179–12184. doi: 10.1073/pnas.0600268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaumann AJ, Semmler AB, Molenaar P. The effects of both noradrenaline and CGP12177, mediated through human β1-adrenoceptors, are reduced by PDE3 in human atrium but PDE4 in CHO cells. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:123–131. doi: 10.1007/s00210-007-0140-3. [DOI] [PubMed] [Google Scholar]
- Kuznetsov V, Pak E, Robinson RB, Steinberg SF. β2-Adrenergic receptor actions in neonatal and adult rat ventricular myocytes. Circ Res. 1995;76:40–52. doi: 10.1161/01.res.76.1.40. [DOI] [PubMed] [Google Scholar]
- Laitinen PJ, Brown KM, Piippo K, Swan H, Devaney JM, Brahmbhatt B, et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–490. doi: 10.1161/01.cir.103.4.485. [DOI] [PubMed] [Google Scholar]
- Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children: a 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Wehrens XHT, Laitinen P, Reiken SR, Den SX, Cheng Z, et al. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109:3208–3214. doi: 10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Wehrens XHT, Reiken S, Warrier S, Belevych AE, Harvey RD, et al. Phosphodiesterase 4D deficiency in the ryanodine–receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kranias EG, Mignery GA, Bers DM. Protein kinase A phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res. 2002;90:309–316. doi: 10.1161/hh0302.105660. [DOI] [PubMed] [Google Scholar]
- Liu N, Colombi B, Memmi M, Zissimopoulos S, Rizzi N, Negri S, et al. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia. Insights from a RyR2 R4496C knock-in mouse model. Circ Res. 2006;99:292–298. doi: 10.1161/01.RES.0000235869.50747.e1. [DOI] [PubMed] [Google Scholar]
- MacKenzie SJ, Baillie GS, MacPhee I, MacKenzie C, Seamons R, McSorley T, et al. Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in upstream conserved region 1 (UCR1) Br J Pharmacol. 2002;136:421–433. doi: 10.1038/sj.bjp.0704743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev VA, Vinogradova TM, Lakatta EG. The emergence of a general theory of the initiation and strength of the heartbeat. J Pharmacol Sci. 2006;100:338–369. doi: 10.1254/jphs.cr0060018. [DOI] [PubMed] [Google Scholar]
- Molenaar P, Bartel S, Cochrane A, Vetter D, Jalali H, Pohlner P, et al. Both β2 and β1-adrenergic receptors mediate hastened relaxation and phosphorylation of phospholamban and troponin I in ventricular myocardium of Fallot infants, consistent with selective coupling of β2-adrenergic receptors to Gs-protein. Circulation. 2000;102:1814–1821. doi: 10.1161/01.cir.102.15.1814. [DOI] [PubMed] [Google Scholar]
- Molenaar P, Sarsero D, Arch JRS, Kelly J, Henson SM, Kaumann AJ. Effects of (−)-RO363 at human atrial β-adrenoceptor subtypes, the human cloned β3-adrenoceptor and rodent intestinal β3-adrenoceptors. Br J Pharmacol. 1997;120:165–176. doi: 10.1038/sj.bjp.0700850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev O, Bünemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching β1-adrenergic receptor-mediated signalling. Circ Res. 2006;99:1084–1091. doi: 10.1161/01.RES.0000250046.69918.d5. [DOI] [PubMed] [Google Scholar]
- Oostendorp J, Kaumann AJ. Pertussis toxin suppresses carbachol-evoked cardiodepression but does not modify cardiostimulation mediated through β1- and putative β4-adrenoceptors in mouse left atria: no evidence for β2- and β3-adrenoceptor function. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:134–145. doi: 10.1007/s002109900156. [DOI] [PubMed] [Google Scholar]
- Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- Rampazzo A, Nava A, Erne P, Eberhard M, Vian E, Slomp P, et al. A new locus for arrhythmogenic right ventricular cardiomyopathy (ARVD2) maps to chromosome 1q42–q43. Hum Mol Genet. 1995;4:2151–2154. doi: 10.1093/hmg/4.11.2151. [DOI] [PubMed] [Google Scholar]
- Rigg L, Heath BM, Cui Y, Terrar DA. Localisation and functional significance of ryanodine receptors during β-adrenoceptor stimulation in the guinea-pig sino-atrial node. Cardiovasc Res. 2000;48:254–264. doi: 10.1016/s0008-6363(00)00153-x. [DOI] [PubMed] [Google Scholar]
- Sato N, Asai K, Okumura S, Takagi G, Shannon RP, Fujita Y, et al. Mechanisms of desensitization to a PDE inhibitor (milrinone) in conscious dogs with heart failure. Am J Physiol. 1999;276 Heart Circ Physiol 45:H1699–H1705. doi: 10.1152/ajpheart.1999.276.5.H1699. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Vasta V, Degerman E, Belfrage P, Manganiello VC. Hormone sensitive cyclic GMP-inhibited cylic AMP phosphodiesterases in rat adipocytes. J Biol Chem. 1991;266:13385–13390. [PubMed] [Google Scholar]
- Vargas ML, Hernandez J, Kaumann AJ. Phosphodiesterase PDE3 blunts the positive inotropic and cyclic AMP enhancing effects of CGP12177 but not of noradrenaline in rat ventricle. Br J Pharmacol. 2006;147:158–163. doi: 10.1038/sj.bjp.0706498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest JA, Wehrens XHT, Reiken SR, Lehnart SE, Dobrev D, Chandra P, et al. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006;98:505–514. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- Vinogradova TM, Lyashkov AE, Zhu W, Spurgeon H, Maltsev VA, Lakatta EG. Constitutive phosphodiesterase activity confers negative feedback on intrinsic cAMP-PKA regulation of local rhythmic subsarcolemmal calcium release and spontaneous beating in rabbit sinoatrial node. Biophys J. 2005b;88:303a. [Google Scholar]
- Vinogradova TM, Maltsev VA, Bogdanov K, Lyashkov AE, Lakatta EG. Rhythmic Ca2+ oscillations drive sinoatrial nodal cell pacemaker function to make the heart tick. Ann NY Acad Sci. 2005a;1047:138–156. doi: 10.1196/annals.1341.013. [DOI] [PubMed] [Google Scholar]
- Wehrens XHT, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annu Rev Physiol. 2005;67:69–98. doi: 10.1146/annurev.physiol.67.040403.114521. [DOI] [PubMed] [Google Scholar]
- Wehrens XHT, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Soc Natl Acad Sci USA. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wussling M, Schenk W, Nilius B. A study of dynamic properties in isolated myocardial cells by laser diffraction method. J Mol Cell Cardiol. 1987;19:897–907. doi: 10.1016/s0022-2828(87)80618-1. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Naro F, Zoudilova M, Jin SLC, Conti M, Kobilka B. Phosphodiesterase 4D is required for β2 adrenoceptor subtype-specific signalling in cardiac myocytes. Proc Natl Acad Sci USA. 2005;102:909–914. doi: 10.1073/pnas.0405263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Zhong G, Obayashi M, Yang D, Chen K, Walsh MP, et al. Ser-2030, but not Ser-2808, is the major phosphorylation site in cardiac ryanodine receptors responding to protein kinase A activation upon β-adrenergic stimulation in normal and failing hearts. Biochem J. 2006;396:7–16. doi: 10.1042/BJ20060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao RP, Avdonin P, Zhou YY, Cheng H, Akhter SA, Eschenhagen T, et al. Coupling of β2-adrenoceptor Gi protein and its physiological relevance in murine cardiac myocytes. Circ Res. 1999;84:43–52. doi: 10.1161/01.res.84.1.43. [DOI] [PubMed] [Google Scholar]