Abstract
Background and purpose:
Paracetamol, a major cause of acute liver failure (ALF) represents a significant clinical problem. Adrenoceptor stimulation or antagonism can modulate chemical-induced hepatotoxicity. We investigated the role of endogenous catecholamines and α1-adrenoceptors in the development of paracetamol- induced hepatotoxicity.
Experimental approach:
Paracetamol (3.5 mmol kg−1) was administered to male CD-1 mice, with and without α1-adrenoceptor antagonists (prazosin, doxazosin, terazosin and tamsulosin; 35.7 μmol kg−1). Serum transaminases and hepatic glutathione (GSH) levels were assessed as markers of hepatic damage. Paracetamol bioactivation was assessed by covalent binding, hepatic and urinary conjugate formation and uridine glucuronosyltransferase activity. Plasma catecholamines levels and hepatic congestion were also analysed.
Key results:
Plasma catecholamine levels were significantly elevated 5 h post paracetamol administration. Prazosin prevented hepatotoxicity when administered 1 h before a toxic paracetamol insult and importantly, when administered up to 1 h post paracetamol injection. Prazosin had no effect on paracetamol-induced depletion of hepatic GSH, paracetamol bioactivation or paracetamol-induced transcription of defence genes. Paracetamol toxicity is associated with marked accumulation of erythrocytes within hepatic sinusoids and prazosin completely prevented this accumulation.
Conclusion and implications:
Paracetamol-induced hepatocellular damage is associated with increased circulating catecholamines. α1-Adrenoceptor antagonists conferred complete protection from paracetamol -induced hepatotoxicity. Protection was associated with absence of hepatic erythrocyte accumulation. Increased catecholamine levels may contribute to the pathophysiology of paracetamol-induced hepatotoxicity by compromising hepatic perfusion. Protection against paracetamol toxicity by α1 antagonists in mice has implications for therapeutic management of patients presenting with paracetamol overdose and ALF.
Keywords: paracetamol, adrenergic, prazosin, toxicity, protection, glutathione, perfusion, murine, stress, adrenaline
Introduction
The widely used analgesic, paracetamol, is extremely safe at therapeutic doses. However, overdose or chronic use of high-dose paracetamol has been the major cause of hospital admissions and indication for emergency liver transplantation for acute liver failure (ALF) in the UK, over the last 30 years (Bernal, 2003). It also currently accounts for approximately 40% of cases of ALF in the USA (Lee, 2003). At therapeutic levels, paracetamol is primarily metabolized in the liver by glucuronidation and sulphation; however, a small proportion undergoes cytochrome P450 (CYP450)-mediated bioactivation to N-acetyl-p-benzoquinoimine (NAPQI), which is rapidly quenched by glutathione (GSH) (Raucy et al., 1989; Thummel et al., 1993).
After an overdose of paracetamol, elevated levels of the toxic NAPQI metabolite are generated, which can extensively deplete hepatocellular GSH and covalently modify cellular proteins resulting in hepatocyte death. The precise biochemical mechanism of cell necrosis is not fully understood. However, it is generally recognized that there is simultaneous involvement of covalent binding, lipid peroxidation and oxidative stress, each contributing to hepatocellular damage (Jollow et al., 1973; Mitchell et al., 1973; Hinson, 1980; Bessems and Vermeulen, 2001). More recent work has demonstrated that liver damage is clearly a multi-cellular event, with clear evidence for a role of cells of the innate immune system in tissue damage (Liu and Kaplowitz, 2006).
Adrenergic modulation of chemical-induced hepatotoxicity has been recognized for several decades. Early studies performed by Calvert and Brody utilized the model hepatotoxin, carbon tetrachloride (CCl4), and demonstrated the ability to diminish hepatotoxicity via adrenergic blockade and adrenalectomy (Brody and Calvert, 1960; Calvert and Brody, 1960; Brody et al., 1961). This led to the hypothesis that catecholamines played a role in CCl4 toxicity. Further studies demonstrated the potentiation of toxicity following administration of catecholamines, adrenergic agonist co-administration, and electrical or physical stimulation of the nervous system possibly involving α2-adrenoceptor stimulation (Larson and Plaa, 1965; Schwetz and Plaa, 1969; Iwai et al., 1986; Iwai and Shimazu, 1988; Roberts et al., 1991, 1994). The mechanism of potentiation of CCl4 hepatotoxicity remains uncertain but does not involve modulation of hepatic GSH content (Roberts et al., 1997).
Kerger and colleagues demonstrated dramatic reductions in bromobenzene-induced hepatotoxicity with concurrent non-selective α-adrenoceptor antagonist administration (Kerger et al., 1988a, 1988b, 1989). Yohimbine, the selective α2-adrenoceptor antagonist was ineffective in providing hepatoprotection (Harbison et al., 1996) implying a role for α1-adrenoceptors, which was later confirmed with prazosin (1-(4-amino-6, 7-dimethoxy-2-quinazolinyl)-4-(2-furoyl) piperazine hydrochloride), a selective α1 antagonist (Harbison et al., 1996).
Previous studies have shown that the α-adrenoceptor agonists phenylpropanolamine (non-selective α) and phenylephrine (α1) potentiate paracetamol-induced hepatotoxicity in mice (Harbison et al., 1991; James et al., 1993) when administered to coincide with maximal GSH depletion. On the basis of these previous observations, we hypothesized that endogenous catecholamine release is induced by toxic doses of paracetamol and that blockade of adrenoceptors will prevent paracetamol-induced hepatotoxicity. In this study, we have tested this hypothesis by measuring catecholamine levels following paracetamol administration and assessed the effect of α-adrenoceptor antagonism with selective α1 antagonists.
Prazosin is a selective α1-adrenoceptor antagonist clinically used to treat hypertension. The peripheral vasodilatory properties of prazosin are due to postsynaptic α blockade. The mode of action of prazosin is confined primarily to the arterioles and is associated with a reduction in peripheral vascular resistance and mean arterial pressure. Doxazosin and terazosin are also quinazoline antihypertensives, structurally related to prazosin with similar metabolic elimination pathways. Tamsulosin ((−)-(R)-5-[2-[[2-(oethoxyphenoxy)ethyl]amino]-propyl]-2-methoxybenzenesulphonamide hydrochloride) is a subtype-selective α1 antagonist (α1A) of a different chemical class used to improve urinary obstruction in benign prostatic hypertrophy (Soeishi et al., 1996). In these studies, we have investigated the role of endogenous catecholamines and α1-adrenoceptors in the modulation of paracetamol-induced hepatotoxicity.
Materials and methods
Animal dosing regimen
Protocols were undertaken in accordance with criteria outlined in a licence (PPL no. 40/2544) granted under the UK Animals (Scientific Procedures) Act 1986 and approved by the University of Liverpool Animal Ethics Committee. Animals were housed in a light controlled room at constant temperature and were supplied with food and water ad libitum.
Dosing regimen
Male CD-1 mice (n=4–12, 25–35 g) were administered a single dose of paracetamol (1 or 3.5mmol kg−1; i.p.) in warmed saline or vehicle control. Animals were killed after 1, 5 or 24 h by CO2 asphyxiation. Blood was collected via cardiac puncture and livers were removed immediately, rinsed in saline (0.9%) and snap frozen in liquid nitrogen. Tissues were stored at −80 °C until use.
Endogenous catecholamine analyses
Male CD-1 mice (n=4) were administered a single dose of prazosin (35.7 μmol kg−1 in corn oil; i.p.) 1 h prior to a subtoxic dose of paracetamol (3.5 mmol kg−1 in saline, i.p.). Animals were monitored in a darkened, quiet room to minimize stress. Animals were individually killed by an overdose of pentobarbital (Sagatal). It is possible that the actual procedure of killing the animal may raise circulating catecholamine levels, which could then potentially mask any (paracetamol±prazosin)-induced changes. However, pilot studies confirmed that this was not the case. Blood was collected immediately via cardiac puncture into lithium heparin tubes containing EGTA and GSH kept on ice. Plasma was separated via centrifugation at 780 g for 10 min. The blood from two animals was pooled for each sample to ensure sufficient sample volume. Samples were stored at −80 °C.
Hepatotoxicity protection experiments
Male CD-1 mice (n=4–12;25–35 g) were administered a single dose of prazosin, doxazosin, terazosin or tamsulosin (35.7 μmol kg−1 emulsion in corn oil; i.p.) 1 h prior to a toxic dose of paracetamol (3.5 mmol kg−1 in saline, i.p.). Mice were also administered a single dose of prazosin (35.7 μmol kg−1; i.p.) 30 min and 1 h after a toxic dose of paracetamol (3.5 mmol kg−1; i.p.). Mice also received multiple doses of prazosin (35.7 μmol kg−1 in corn oil; i.p.) administered as a 15 min pre-dose followed by four further doses at 90-min intervals, alongside a toxic dose of paracetamol (3.5 mmol kg−1; i.p.) at the 0 h time point. Mice receiving vehicle alone were used as controls. Animals were killed after 1, 5 or 24 h by CO2 asphyxiation. Blood was collected via cardiac puncture. Livers were immediately removed and rinsed in saline (0.9%). Half the large lobe was stored in 10% neutral buffered formalin and the remainder of the liver was divided into aliquots and snap frozen in liquid nitrogen. Tissues were stored at −80 °C until use.
Metabolism analyses
Male CD-1 mice were maintained under anaesthesia with pentobarbital (70 mg kg−1, i.p. in saline). The external jugular vein was cannulated (0.40 mm ID, 0.8 mm OD fine bore polythene tubing, Portex Ltd, Kent, UK) to aid dosing. Animals received prazosin (35.7 μmol kg−1, i.p.), 1 h prior to the bolus administration (over 2 min) of paracetamol (1.5 or 3.5 mmol kg−1). Following CO2 asphyxiation, livers were removed for immediate metabolite extraction.
Irreversible binding analyses
Male CD-1 mice (n=10) were given paracetamol (3.5 mmol kg−1 in saline, i.p.) containing 10 μCi [3H] paracetamol per mouse (stock 1.2 μCi μl−1) in combination, in the presence or absence of pretreatment (1 h before paracetamol) with prazosin (35.7 μmol kg−1 in corn oil; i.p.). Livers were collected 5 h after paracetamol and stored at −80 °C.
Determination of serum alanine transaminase levels as a marker of hepatotoxicity
Serum was separated, via pulse centrifugation from blood that had been allowed to clot overnight. Serum alanine transaminase (ALT) levels were determined kinetically at 37 °C using ThermoTrace Infinity ALT Liquid stable reagent, according to the manufacturer's instructions. ALT levels greater than 200 U l−1 that were statistically significant from controls were considered hepatotoxic (Kitteringham et al., 2000; Goldring et al., 2004).
Histopathological examination of H&E-stained hepatic sections
Following hepatic excision, a 4-mm transverse section was taken from the formalin-fixed tissue samples. The samples were dehydrated, and embedded in paraffin prior to 4-μm sections being cut. Sections were stained with haematoxylin and eosin (H&E) for evaluation. All histological examinations were performed blind by a single pathologist evaluating one liver section per animal. Liver sections were scored according to pre-defined criteria, where 0 is normal histology and 4 represents massive hepatocellular necrosis involving entire lobules
Analysis of plasma catecholamines as an indicator of the murine stress response following paracetamol administration
Plasma samples were organically extracted and analysed using HPLC with electrochemical detection according to the method of Forster and Macdonald (1999).
Assessment of hepatic GSH levels following paracetamol administration
Hepatic GSH levels were determined using a 5,5′-dithiobis(2-nitrobenzoic acid)––GSH disulphide recycling assay according to the method of Vandeputte et al. (1994).
Determination of hepatic paracetamol–Glutathione metabolite levels
Paracetamol-SG conjugate levels in liver extracts were quantified by HPLC as described previously (Elsby et al., 2003) using authentic standards.
Determination of irreversible binding of radiolabelled paracetamol to hepatic proteins as a marker of reactive metabolite formation
Radiolabelled material irreversibly bound to precipitated protein was measured after removal of unbound drug by exhaustive solvent extraction according to the method of Pirmohamed et al. (1995). Irreversible binding of radiolabelled paracetamol is expressed as percentage bound per milligram hepatic protein.
Assessment of hepatic defence gene response via reverse transcriptase-PCR of GCLC and HO-1 mRNA
Total cellular hepatic RNA was extracted using the method of Chomczynski and Sacchi (1987). Briefly, liver (100 mg) was homogenized in ultraPURE TRIzol reagent according to the manufacturer's instructions. The purity, integrity and concentration of isolated total RNA was quantified by agarose gel electrophoresis and spectrophotometry, respectively. cDNA was synthesized using the Promega reverse transcription system according to the manufacturer's instructions using 1 μg total RNA as a template.
Glutamate cysteine ligase catalytic subunit (GCLC) was co-amplified with the constitutively expressed housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) according to the method of Kitteringham et al. (2000). The following primer pairs were used:
- GCLC, Forward
5′-GCAATGCATCCTGCACCACC-3′
- Reverse
5′-GGTCTGGGATGGAAATTGTG-3′
- GAPDH, Forward
5′-ATCCTCCAGTTCCTGCACAT-3′
- Reverse
5′-TGTGAATCCAGGGCAGCCTA-3′.
Optimization of reverse transcriptase-PCR assay conditions were performed on cDNA isolated from control and treated mice. Primer concentrations, volume of cDNA and PCR cycle number were optimized during linearity studies. Reactions contained cDNA (5 μl of a 1:10 dilution in nuclease-free water) combined with 4 μM each of the GCLC and GAPDH forward and reverse primers, 50 μM of each dNTP, 1.25 U Taq DNA polymerase and 5 μl of PCR buffer (100 mM Tris-HCl (pH 8.8); 500 mM KCl, 0.8% Nonidet P40) in a total volume of 50 μl. Amplification was initiated for 3 min of denaturation at 94 °C followed by 35 cycles of 30 s at 94 °C (denaturation), 1 min at 53 °C (primer annealing) and 1 min at 72 °C (polymerization) with a final elongation step of 72 °C for 10 min. The GCLC and GAPDH PCR products were 390 and 670 bp, respectively.
Haem-oxygenase 1 (HO-1) was co-amplified with cyclophilin using a method designed in our laboratory. The following primer pairs were used:
- HO-1, Forward
5′-GCTGAGTTCATGAAGAACTT-3′
- Reverse
5′-AGACGATTTACATAGTGCT-3′;
- Cyclophilin, Forward
5′-GTCTGCTTCGAGCTGTTTGCA-3′
- Reverse
5′-GGGTGCTCTCCTGAGCTACAG-3′.
Reactions contained 0.04 μM HO-1 forward and reverse primers along with 0.08 μM forward and reverse cyclophilin primers, 50 μM of each dNTP, 0.4 U Taq DNA polymerase and 5 μl of PCR buffer in a total volume of 50 μl. Amplification was initiated for 5 min of denaturation at 94 °C followed by 35 cycles of 10 s at 94 °C (denaturation), 30 s at 48 °C (primer annealing) and 30 s at 72 °C with a final elongation step of 72 °C for 5 min. The HO-1 and cyclophilin PCR products were 261 and 450 bp, respectively.
The GAPDH and cyclophilin housekeeping genes were not altered by either paracetamol or prazosin treatments and were therefore used to normalize hepatic defence gene expression data.
Quantitation of hepatic congestion using CFSE-labelled erythrocytes
Erythrocytes were purified from heparinized blood, obtained from donor mice, by density centrifugation over Lymphoprep. Erythrocytes were washed extensively, suspended in Hank's Buffered Salt Solution (HBSS) and fluorescently labelled with 10 μM of either carboxyfluorescein diacetate succinimidyl ester (CFSE) or tetramethylrhodamine-5-(and-6)-isothiocyanate (TRITC) at 37 °C for 20 min. Labelling was quenched with 10% fetal bovine serum, cells were washed and CFSE-labelled cells were intravenously injected into the tail vein of CD-1 mice, which were left overnight before administration of test compounds. Animals were killed by CO2 asphyxiation 5 h after paracetamol±prazosin treatment. Blood was collected via cardiac puncture, livers were removed, rinsed briefly in saline and dispersed through a wire mesh strainer with heparinized HBSS to release trapped erythrocytes. Cells were passed through a 40-μm cell strainer to remove tissue debris. Erythrocytes were separated from the filtrate using a Lymphoprep density gradient centrifugation. The erythrocyte pellet was resuspended in 1 ml HBSS to which a fixed quantity of TRITC-labelled erythrocytes were added. These served as an internal control to assist quantitative analysis. Hepatic congestion was quantified using bivariate flow cytometry analysis (Coulter Epics, XL software; Beckman Coulter, High Wycombe, Buckinghamshire, UK) of CFSE-labelled erythrocytes recovered from the livers of treated and control animals. Erythrocytes were electronically gated based on their scatter profiles and the fluorescently labelled erythrocytes were detected and counted. CFSE erythrocytes and TRITC erythrocytes fluoresce at different wavelengths and therefore can be distinguished on the flow cytometer. CFSE-labelled erythrocytes were counted until 4000 TRITC-labelled erythrocytes had been detected. Data are expressed as the mean ratio of CFSE-labelled erythrocytes recovered from treated vs control livers.
Protein concentration
Soluble protein concentration was measured using Bio-Rad protein assay reagent according to the manufacturer's instructions. Concentrations were calculated with reference to a BSA standard curve (0–5 mg ml−1).
Statistical analysis
All results are expressed as mean±s.e.mean. Values to be compared were analysed for non-normality using a Shapiro–Wilk test. Student's unpaired t-test was used for comparing two groups when normality was indicated and Mann–Whitney U-test was used for non-parametric data. For multiple comparisons between three or more groups, one-way ANOVA with Scheffé comparison was used for parametric data and Kruskall–Wallis (Conover–Inman) statistical test for non-parametric data. All calculations were performed using StatsDirect statistical software (Research Solutions, Cambridge, UK); results were considered to be significant when P-values were less than 0.05.
Drugs, chemical reagents and other materials
Prazosin and terazosin were purchased from Sigma (Poole, UK), doxazosin and tamsulosin were kind gifts from Pfizer Global Research and Development (Sandwich, Kent, UK). ThermoTrace Infinity ALT liquid stable reagent was purchased from Alpha Labs (Eastleigh, UK). The protein assay reagent was purchased from Bio-Rad (Hemel Hempstead, UK), Gill 3 haematoxylin and ParaPlast Plus Wax were obtained from ThermoShandon (Cheshire, UK), 10% neutral buffered formalin was obtained from Surgipath Ltd (Peterborough, UK). UltraPURE TRIzol reagent and PCR primers were obtained from Gibco BRL (Life Technologies, Paisley, UK). Reverse transcription kit, T4 polynucleotide kinase (10 U) and 10 × T4 polynucleotide kinase buffer was purchased from Promega (Southampton, UK). Ultima Gold scintillation fluid was purchased from Packard Biosciences (MA, USA). CFSE and TRITC dyes were purchased from Invitrogen (Paisley, UK). All other reagents were of analytical or molecular grade and were supplied by Sigma.
Results
Plasma catecholamine levels are elevated following paracetamol administration
Plasma adrenaline and noradrenaline levels were significantly elevated 5 h post-paracetamol administration (Figure 1). The administration of prazosin 1 h before paracetamol treatment significantly reduced circulating noradrenaline and adrenaline levels, which remained similar to vehicle control values (Figure 1).
Figure 1.
Plasma adrenaline and noradrenaline levels 5 h after giving paracetamol (APAP)±prazosin (PZ) pretreatment. Mice were administered vehicle control, prazosin (35.7 μmol kg−1), paracetamol (3.5 mmol kg−1) or prazosin 1 h before paracetamol. Blood was collected and plasma was separated for HPLC analysis. (a) Results are mean adrenaline concentrations expressed as nmol l−1±s.e.mean, n=4. ANOVA statistical analysis with Scheffé comparisons was used. ***P<0.001. (b) Results are mean noradrenaline concentrations expressed as nmol l−1±s.e.mean, n=4. ANOVA statistical analysis with Scheffé comparisons was used. *P<0.05, **P<0.01.
Antagonism of α1-adrenoceptors prevents paracetamol-induced hepatotoxicity
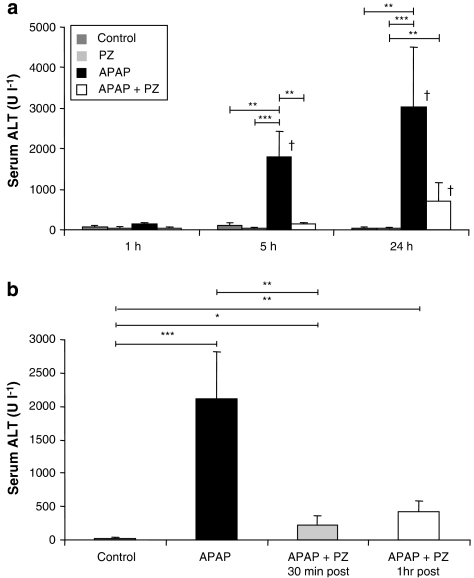
To investigate the role of α1-adrenoceptors in paracetamol-induced toxicity, prazosin was administered pre- and post-paracetamol. Serum ALT activity levels were measured as an indicator of hepatic damage at 1, 5 and 24 h post-dosing. Hepatotoxicity was deemed significant when ALT levels exceeded 200 U l−1 and were statistically different from controls (Kitteringham et al., 2000). Administration of paracetamol to CD-1 mice resulted in severe hepatotoxicity, reflected in significantly elevated ALT activity at 5 and 24 h (Figure 2). Pretreatment with prazosin prevented paracetamol toxicity at 5 h, with ALT levels remaining comparable to controls. Serum sampled at 24 h suggested that a single dose of pretreatment with prazosin was insufficient to completely protect against paracetamol-induced hepatotoxicity, at this later time. Multiple doses of prazosin (mPZ; see Materials and methods) were required to prevent the paracetamol-induced increase in ALT activity at 24 h (mean ALT U l−1±s.e.mean; vehicle control: 21±4; mPZ alone: 27±6; paracetamol alone: 1229±398; paracetamol+mPZ: 35±9; P<0.001 paracetamol vs vehicle control and mPZ alone, P<0.05 paracetamol P vs paracetamol+mPZ). Prazosin alone did not raise ALT levels above 200 U l−1 at any of the time points investigated.
Figure 2.
Effects of prazosin (PZ) on paracetamol (APAP)-induced hepatotoxicity at 1, 5 and 24 h. Serum alanine transaminase (ALT) levels were determined using Infinity ALT reagent. (a) Male CD-1 mice were dosed for 1, 5 and 24 h following prazosin pretreatment. Mice were administered vehicle control, prazosin (35.7 μmol kg−1), paracetamol (3.5 mmol kg−1) or prazosin 1 h before paracetamol. Results are expressed as mean serum ALT U l−1±s.e.mean, n=8–12. (b) Male CD-1 mice were dosed with prazosin (35.7 μmol kg−1) 30 min and 1 h post-paracetamol (3.5 mmol kg−1; 5 h). CD-1 mice received vehicle control, paracetamol, paracetamol+prazosin 30 min post and paracetamol+prazosin 1 h post. Results are mean ALT±s.e.mean, n=4. One-way ANOVA with Scheffé comparison statistical analysis for normally distributed data and Kruskall–Wallis analysis for non-normally distributed data were used. *P<0.05, **P<0.01, ***P<0.001, when compared with time-matched controls; †P<0.05 when comparing identical treatment to values at 1 h.
Mice were also administered prazosin, post-paracetamol administration to assess the timescale of protection with regard to the onset of paracetamol-induced injury. Serum ALT activity levels indicated that prazosin significantly reduced hepatic damage when given 30 min after paracetamol. However, when prazosin was given 1 h after paracetamol, the reduction in ALT activity failed to reach statistical significance (P=0.08; Figure 2b).
Histopathological examination of H&E-stained hepatic sections from treated animals
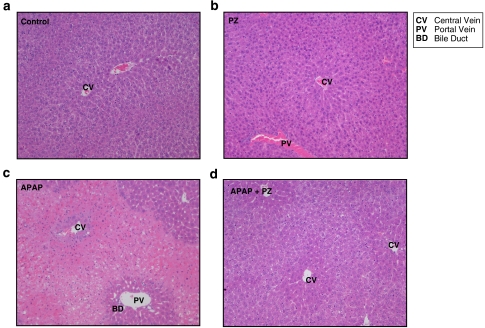
Histological analysis was performed to confirm prazosin-mediated hepatoprotection. H&E-stained hepatic sections from multiple vehicle controls and multiple prazosin-treated animals were microscopically normal (grade 0; Figure 3a and b, respectively). In agreement with the serum ALT data, livers taken 24 h after paracetamol displayed severe multi-focal, haemorrhagic necrosis extending from the centrilobular region to portal areas and adjacent lobules (Figure 3c). Parenchymal preservation was observed in the prazosin pretreated livers, with all the livers displaying normal histology (grade 0; Figure 3d). The centrilobular hepatocytes were observed to be paler and larger within this treatment group, with fewer intercellular spaces, suggesting hypertrophy, possibly to compensate for damaged hepatocytes.
Figure 3.
Representative haemotoxylin and eosin (H&E)-stained liver sections from mice that had received paracetamol (APAP) and/or multiple corn oil (24 h). CD-1 mice were administered multiple corn oil vehicle control (a), prazosin (PZ; 35.7 μmol kg−1) in corn oil at 90-min intervals up to five doses during the dosing period (b), paracetamol (3.5 mmol kg−1) (c) and paracetamol+multiple prazosin (d) ( × 10 objective lens magnification).
Prazosin does not suppress paracetamol-induced GSH depletion
Hepatic GSH levels were rapidly and extensively depleted by paracetamol alone and in combination with prazosin (Figure 4). Replenishment of hepatic GSH was evident at 5 h within both the paracetamol-treated groups. By 24 h there were no discernible differences in GSH levels between dosing groups, despite the severity of the liver damage induced by paracetamol. Prazosin given alone failed to deplete GSH at any of the time points.
Figure 4.
Effects of prazosin (PZ) on decreased hepatic glutathione (GSH) following paracetamol (APAP)-induced hepatotoxicity at 1, 5 and 24 h. GSH levels were determined according to the method of Vandeputte et al. (1994). Male CD-1 mice were dosed for 1, 5 and 24 h following a single prazosin pretreatment. Mice were administered vehicle control, prazosin (35.7 μmol kg−1), paracetamol (3.5 mmol kg−1) or prazosin 1 h before paracetamol. Results are expressed as mean GSH levels±s.e.mean, n=4–8. One-way ANOVA with Scheffé comparison analysis for normally distributed data and Kruskall–Wallis analysis for non-normally distributed data were used. *P<0.05, **P<0.01, ***P<0.001, †P<0.05, ‡P<0.001 vs 1 h, §P<0.01 vs 5 h.
Prazosin does not alter paracetamol GSH metabolite formation
APAP–glutathione conjugate formation was monitored to determine whether prazosin was altering the elimination of paracetamol and NAPQI. There was no difference in the recovery of hepatic paracetamol–SG conjugate 1 h after receiving paracetamol±prazosin (% dose recovered±s.e.mean; paracetamol 11.03±1.83, paracetamol+prazosin 14.58±2.00). Data previously generated within this laboratory has also confirmed that prazosin has no effect on paracetamol–glucuronide formation and therefore is unlikely to alter elimination via phase II metabolism (Clement and Williams, 2005).
Prazosin does not alter the level of paracetamol irreversibly bound to hepatic proteins
Covalent modification of critical cellular proteins has been shown to be important to the development of paracetamol-induced toxicity (Park et al., 2005). [3H]paracetamol irreversibly bound to hepatic proteins was assessed following the removal of unincorporated radiolabel by extensive solvent extraction. There was no difference observed in the mean percentage of [3H]paracetamol bound per milligram protein in prazosin pretreated (0.22±0.05) vs paracetamol alone at 1 h (0.25±0.10; n=10 throughout these assays). Covalent binding increased significantly with the onset of toxicity during the 5 h experimental period. A similar amount of [3H]paracetamol was bound to hepatic proteins in both treatment groups (% bound per milligram protein±s.e.mean, paracetamol 1.41±0.23 vs paracetamol±prazosin 1.30±0.39), indicating that prazosin was not altering the ability of NAPQI to become covalently bound.
Prazosin does not induce hepatocellular defence genes to prevent paracetamol-induced toxicity
Hepatic HO-1 and GCLC gene expression levels were investigated as biomarkers of hepatocellular defence. There were no statistical differences in GCLC expression at 1 h in any of the treatment groups (1 h GCLC/GAPDH ratio±s.e.mean; vehicle control 0.54±0.07, prazosin 0.38±0.09, paracetamol 0.41±0.12, paracetamol+prazosin 0.39±0.02; n=6 throughout). Paracetamol significantly increased GCLC mRNA levels at 5 h in an attempt to replenish GSH lost during bioactivation (5 h GCLC/GAPDH ratio±s.e.mean; vehicle control 0.40±0.04, prazosin 0.47±0.10, paracetamol 0.71±0.13, paracetamol+prazosin 0.45±0.03; P<0.01 paracetamol vs control, P<0.01 paracetamol vs prazosin, P<0.05 paracetamol vs paracetamol+prazosin). When dosed in combination, prazosin attenuated paracetamol-induced GCLC upregulation.
Similar findings were observed with HO-1 mRNA levels, which remained at control levels in all treatment groups 1 h post-dosing (1 h HO-1/cyclophilin ratio±s.e.mean; vehicle control 0.52±0.04, prazosin 0.41±0.04, paracetamol 0.51±0.03, paracetamol+prazosin 0.45±0.06; n=6 throughout). Mice that received paracetamol alone for 5 h showed a significant induction in HO-1 levels, which was prevented with the prazosin pretreatment (5 h HO-1/cyclophilin ratio±s.e.mean; vehicle control 0.48±0.08, prazosin 0.47±0.11, paracetamol 1.44±0.10, paracetamol+prazosin 0.60±0.13; P<0.001 paracetamol vs control, P<0.001 paracetamol vs prazosin, P<0.001 paracetamol vs paracetamol+prazosin). Prazosin alone did not alter HO-1 mRNA levels at either time point.
Prazosin prevents paracetamol-induced hepatic congestion
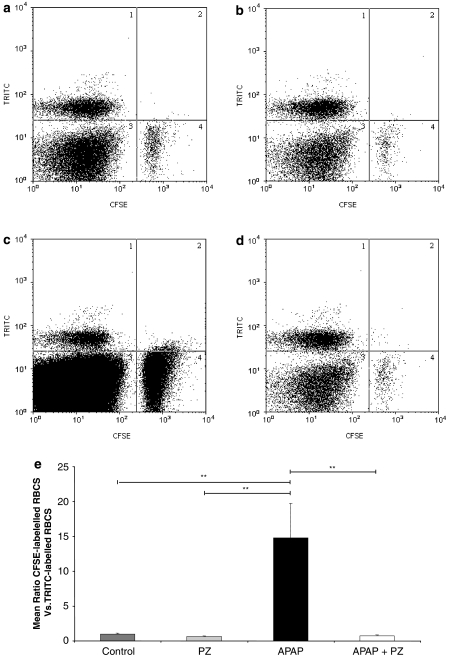
Labelled erythrocyte recovery from the livers of treated animals was used as an indicator of hepatocellular congestion and blood flow stagnation. Fluorescence-activated cell sorting analysis was used to determine the ratio of CFSE-labelled erythrocytes recovered from 5 h treated liver compared with the TRITC-labelled erythrocyte internal standard (Figure 5a–d, region 1). A significantly larger population of CFSE-labelled erythrocytes was recovered from paracetamol-treated livers (Figure 5c, region 4) when compared with other treatment groups (Figure 5e).
Figure 5.
Effect of prazosin (PZ) on carboxyfluorescein diacetate succinimidyl ester (CFSE)- and tetramethylrhodamine-5-(and-6)-isothiocyanate (TRITC)-labelled hepatic red blood cells (RBCs) recovered from paracetamol (APAP)-treated mice at 5 h. CFSE-labelled RBCs were administered i.v. to male CD-1 mice 16 h before paracetamol±prazosin treatment. Mice were then given either vehicle control (a), prazosin (35.7 μmol kg−1; b), paracetamol (3.5 mmol kg−1; c) or prazosin 1 h before paracetamol (d) for 5 h. Representative dot plots of RBCs recovered from the livers of treated mice after 5 h are shown. TRITC-labelled RBCs are shown in region 1 with CFSE-labelled RBCs in region 4. RBCs were assayed using fluorescence-activated cell sorting analysis, CFSE-labelled cells were counted until 4000 TRITC-labelled cells had been counted (e). Results are expressed as mean ratio of CFSE-labelled RBCs vs TRITC-labelled RBCs, n=6. ANOVA statistical analysis with Scheffé comparisons was used. **P<0.01.
Other α-adrenoceptor antagonists also provide protection from paracetamol-induced hepatocellular damage
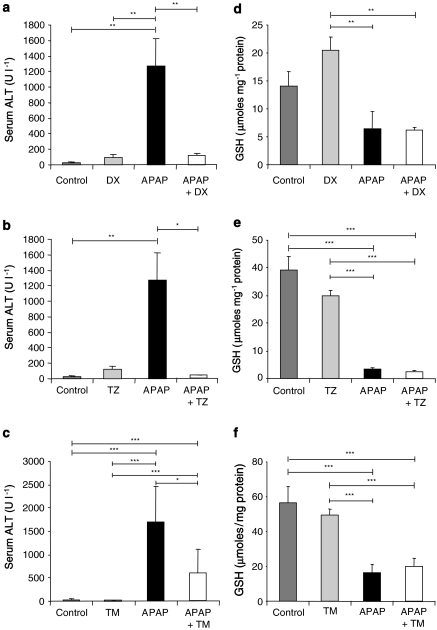
The structurally related adrenoceptor antagonists, doxazosin and terazosin, and the structurally unrelated adrenoceptor antagonist, tamsulosin, were administered 1 h before paracetamol to CD-1 mice. Doxazosin and terazosin also prevented the paracetamol-induced rise in ALT activity at 5 h (Figure 6a–c). Tamsulosin significantly reduced serum ALT activity after paracetamol administration; however, ALT levels were significantly different from controls and exceeded the toxic threshold, suggesting mild hepatocellular damage and incomplete protection.
Figure 6.
The effect of doxazosin (DX), terazosin (TZ) and tamsulosin (TM) on 5 h serum alanine transaminase (ALT) activity and hepatic glutathione (GSH) levels following paracetamol (APAP)-induced hepatotoxicity. Serum ALT levels were determined using Infinity ALT reagent (a–c). Hepatic GSH was determined according to the method of Vandeputte et al. (1994) (d–f). Male CD-1 mice were administered vehicle control, α antagonist (35.7 μmol kg−1), paracetamol (3.5 mmol kg−1) or α antagonist 1 h before paracetamol. DX (a, d), terazosin (b, e) and tamsulosin (c, f). Results are expressed as either mean serum ALT U l−1±s.e.mean or mean GSH±s.e.mean, n=4–12. One-way ANOVA with Scheffé comparison analysis for normally distributed data and Kruskall–Wallis analysis for non-normally distributed data were used. *P<0.05, **P<0.01, ***P<0.001.
Doxazosin and terazosin alone had no significant effects on hepatic GSH levels (Figure 6d–e). However, neither doxazosin nor terazosin prevented the depletion of GSH attributable to paracetamol. Similarly, tamsulosin alone had no effect on hepatic GSH at 5 h but when given in combination with paracetamol, GSH levels were significantly depleted to the same level as after paracetamol alone (Figure 6f).
Discussion and conclusions
Acute liver failure due to paracetamol overdose is a significant clinical problem and could benefit from new therapeutic strategies. Understanding the pathophysiological processes that underlie paracetamol-induced toxicity is an integral part of devising such strategies. In this study, using the mouse as an established experimental model, we provide evidence of a role for α-adrenoceptors in paracetamol-mediated hepatotoxicity in mice. Our data demonstrate that the induction of paracetamol-mediated toxicity is accompanied by increased levels of circulating catecholamines. Chemicals such as bromobenzene and CCl4 have previously been shown to alter circulating catecholamine levels. It has been demonstrated that serum adrenaline levels peak within 2 h of CCl4 administration, which occurred before significant toxicity had developed (Rubenstein, 1962). Bromobenzene elevated serum adrenaline, but not noradrenaline levels in mice at 4 h, which coincided with the bromobenzene-induced depletion of GSH prior to the onset of toxicity (Kerger et al., 1988a).
Our observations led us to investigate whether antagonists of adrenoceptors could prevent or reverse the toxicity mediated by paracetamol, when given either before or after the hepatotoxin. We were able to confirm that the α1 antagonist, prazosin was able to completely protect mice against paracetamol toxicity. Toxicological analysis revealed that prazosin could provide protection against a toxic, sublethal paracetamol dose for up to 5 h but offered only incomplete protection with a single dose at 24 h. Multiple doses of antagonist, administered during the first 6 h, as previously described for hepatotoxic modulation with other adrenergic antagonists (Kerger et al., 1988a), were required to maintain complete protection for 24 h, consistent with the short half-life of the α-blocker in this species.
α-Adrenoceptor antagonists of pharmacological and chemical classes, related and unrelated to prazosin were equally capable of attenuating paracetamol toxicity. Doxazosin and terazosin were able to suppress ALT activity at 5 h; however, there was evidence of minor ALT release with the α1A subtype-selective antagonist tamsulosin, indicative of mild hepatocellular damage at 5 h. This reduction of protection may be related to the relative affinities of these drugs for the adrenoceptor subtypes. Transgenic mice have revealed that the α1B-adrenoceptors play a role in the pressor response to phenylephrine (Cavalli et al., 1997). The murine liver contains an almost homogenous population of α1B receptors (Garcia-Sainz et al., 1995; Yang et al., 1998), in contrast to the human liver that contains predominantly α1A-adrenoceptors. It is thus possible that tamsulosin may be more effective in protecting against hepatotoxicity in man. However, prazosin would still be predicted to offer protection as it has sub-nanomolar affinity for α1A, α1B and α1D subtypes.
Protection was not afforded by simply altering paracetamol metabolism or by inducing a hepatic defence response to overcome the hepatocellular insult. The progression of toxic liver injury is thought to involve inflammatory mediators (Laskin et al., 1986; Michael et al., 1999). Use of high-throughput cytokine protein arrays also revealed that prazosin was not preventing toxicity by modulating the cytokine-mediated progression of toxicity (see Supplementary data). Paracetamol intoxication initiated an immune response within the liver at 5 h, as cytokine and chemokine expression levels were elevated. Generally, these levels were diminished in paracetamol+prazosin-treated mice possibly due to the prevention of toxicity and hepatic damage. Prazosin did not provide protection by inhibition of bioactivation to NAPQI, as depletion of hepatic GSH levels was unaltered by prazosin pretreatment. Equally, analysis of hepatic paracetamol–protein adducts indicated that similar levels of covalent modification were observed between treatment groups regardless of prazosin pretreatment. α1-Adrenoceptor antagonism did not assist paracetamol bio-inactivation by increasing conjugation to GSH. There was also no upregulation of hepatic defence genes following prazosin pretreatment, suggesting that defence genes are upregulated as a consequence of toxicity and are not the mechanism of hepatoprotection.
We hypothesized that prazosin was providing protection through its pharmacological effect on haemodynamic regulation. Within the liver, it was demonstrated that paracetamol-induced toxicity was accompanied by a perturbation of the hepatic microcirculation. Histopathological analysis revealed that toxic livers were congested, with a profound accumulation of erythrocytes within the sinusoids. This is consistent with previous studies where paracetamol-induced microvascular disturbances, such as reduced sinusoidal perfusion and congestion, have been observed (Walker et al., 1980; Bauer et al., 2000; Coen et al., 2003; Ito et al., 2003). To quantify the degree of microvascular circulatory stasis, we developed a method that utilizes fluorescently labelled erythrocytes as markers of erythrocyte accumulation within the liver. Using this methodology, it was observed that paracetamol toxicity is associated with a significant increase in the number of CFSE-labelled red blood cells (RBCs) trapped within the liver. This signified a compromise in the microvascular circulation induced by paracetamol toxicity. The administration of prazosin prevented such a microcirculatory dysfunction, observed through the lack of RBC accumulation, analysed by histology as well as through the use of labelled erythrocytes. Maintenance of the hepatic microcirculation in the presence of a continuing toxic insult may be critical to halt the progression of paracetamol toxicity. It is possible that microcirculatory stasis and RBC accumulation within the sinusoids are due to catecholamine-induced constriction of the hepatic central veins. The ability of prazosin to prevent vasoconstriction and lower portal pressure through a reduction in intra-hepatic vascular resistance (Cummings et al., 1988; Albillos et al., 1994, 1995) would help to maintain an adequate blood flow to the liver during xenobiotic bioactivation and the development of toxicity. The delivery of oxygen, amino acids, cofactors and substrates to the liver would aid GSH replenishment, the maintenance of mitochondrial function and energy production to facilitate repair and regeneration, consistent with our findings. This would suggest that prevention of hepatic microvascular constriction is critical for preventing toxicity. Recent studies by Liu et al. (2003) and Fiorucci et al. (2004) demonstrate that agents such as NO donors that support vasodilation help to improve haemodynamics and provide hepatoprotection from paracetamol toxicity.
In view of our findings, we propose that paracetamol-induced toxicity requires the following sequence of events to occur. First, endogenous catecholamines are released as a consequence of paracetamol-induced hepatocellular damage. This is likely to be due to a physiological stress response elicited as a result of afferent signals arising from nerve endings within the liver. The precise innervation of the hepatic infrastructure is not well documented. However, studies have demonstrated extensive parenchymal innervation, with presumptive sensory nerve endings, and it has been postulated that adrenergic, cholinergic and peptidergic neurons register information from osmoreceptors, baroreceptors, ionic receptors, metabolic receptors and nociceptive receptors (Hall and Leach, 1999; Hartmann and Beckh, 1992). The liver capsule is a visceral structure with afferent input into the central nervous system, probably routed via the sympathetic nervous system (Hall and Leach, 1999). It has been noted that data from animal studies suggest that excessive afferent activity after chemical irritation may be assimilated and interpreted as pain (Hall and Leach, 1999). Second, raised catecholamine levels cause hepatic microvascular vasoconstriction, leading to a compromise in the perfusion of the hepatic microvascular bed. Third, poor perfusion coupled with direct cellular toxic effects causes hepatocyte dysfunction and ultimately necrosis. Finally, this sets up a positive feedback mechanism resulting in amplification of local damage to include the whole organ. Prazosin may thus interrupt the earliest stages of this toxicity cycle by maintaining vascular integrity, thereby preventing sinusoidal congestion, blood stasis and the ensuing cascade of ischaemic hypoxia preventing the progression of toxic damage.
Our findings indicate that prazosin administration significantly modifies the hepatic response to paracetamol intoxication, which may have implications for the therapeutic management of ALF due to paracetamol overdose. Current treatment regimens depend on the repletion of hepatic GSH stores with N-acetylcysteine. Treatment with N-acetylcysteine may be used up to 24 h after ingestion of paracetamol; however, the maximum protective effect is obtained up to 8 h post-ingestion (Prescott et al., 1979). The effectiveness of this antidote declines sharply after this time. A significant proportion of patients are therefore lost due to the inability to initiate N-acetylcysteine administration within the therapeutic time window (Prescott et al., 1979; Wallace et al., 2002). The use of additional agents that could be administered at later times would be of considerable clinical advantage. It is tempting to speculate that provision of prazosin or other agents that maintain hepatic perfusion in conjunction with conventional treatments could extend the therapeutic time window and improve survival rates. Such potential adjuncts to conventional therapy could expand the range of therapeutic strategies currently at the disposal of the clinician. There is pressing need for such combination therapies to be tested and validated in animal models of paracetamol toxicity.
Acknowledgments
We wish to acknowledge the help and support of Peter J O'Brien and Virgile Richard, SSEU, Pfizer Sandwich. Karen Turner, The University of Nottingham medical school for her assistance with the catecholamine analyses. Carrie Greenough for help with the in vivo assays and Robert Elsby for his assistance with the reverse transcriptase-PCR.
Glossary
- ALF
acute liver failure
- ALT
alanine transaminase
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- GCLC
glutamate cysteine ligase catalytic subunit
- GSH
glutathione
- HO-1
haem-oxygenase 1
- RBC
red blood cell
Footnotes
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
Conflict of interest
The authors state no conflict of interest.
Supplementary Material
References
- Albillos A, Lledo JL, Banares R, Rossi I, Iborra J, Calleja JL, et al. Hemodynamic effects of alpha-adrenergic blockade with prazosin in cirrhotic patients with portal hypertension. Hepatology. 1994;20:611–617. [PubMed] [Google Scholar]
- Albillos A, Lledo JL, Rossi I, Perez-Paramo M, Tabuenca MJ, Banares R, et al. Continuous prazosin administration in cirrhotic patients: effects on portal hemodynamics and on liver and renal function. Gastroenterology. 1995;109:1257–1265. doi: 10.1016/0016-5085(95)90586-3. [DOI] [PubMed] [Google Scholar]
- Bauer I, Vollmar B, Jaeschke H, Rensing H, Kraemer T, Larsen R, et al. Transcriptional activation of heme oxygenase-1 and its functional significance in acetaminophen-induced hepatitis and hepatocellular injury in the rat. J Hepatol. 2000;33:395–406. doi: 10.1016/s0168-8278(00)80275-5. [DOI] [PubMed] [Google Scholar]
- Bernal W. Changing patterns of causation and the use of transplantation in the United Kingdom. Semin Liver Dis. 2003;23:27–37. doi: 10.1055/s-2003-42640. [DOI] [PubMed] [Google Scholar]
- Bessems JG, Vermeulen NP. Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol. 2001;31:55–138. doi: 10.1080/20014091111677. [DOI] [PubMed] [Google Scholar]
- Brody TM, Calvert DN. Release of catechol amines from the adrenal medulla by CCl4. Am J Physiol. 1960;198:682–685. doi: 10.1152/ajplegacy.1960.198.3.682. [DOI] [PubMed] [Google Scholar]
- Brody TM, Calvert DN, Schneider AF. Alteration of carbon tetrachloride-induced pathologic changes in the rat by spinal transection, adrenalectomy and adrenergic blocking agents. J Pharmacol Exp Ther. 1961;131:341–345. [Google Scholar]
- Calvert DN, Brody TM. Role of the sympathetic nervous system in CCl4 hepatotoxicity. Am J Physiol. 1960;198:669–676. doi: 10.1152/ajplegacy.1960.198.3.669. [DOI] [PubMed] [Google Scholar]
- Cavalli A, Lattion A, Hummler E, Nenninger M, Pedrazzini T. Decreased blood pressure response in mice deficient of the alpha 1b-adrenergic receptor. Proc Natl Acad Sci USA. 1997;94:11589–11595. doi: 10.1073/pnas.94.21.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clement YN, Williams AF. Protection against paracetamol-induced hepatic injury by prazosin pre-treatment in CD-1 mice. Mutat Res. 2005;579:182–188. doi: 10.1016/j.mrfmmm.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Coen M, Lenz EM, Nicholson JK, Wilson ID, Pognan F, Lindon JC. An integrated metabonomic investigation of acetaminophen toxicity in the mouse using NMR spectroscopy. Chem Res Toxicol. 2003;16:295–303. doi: 10.1021/tx0256127. [DOI] [PubMed] [Google Scholar]
- Cummings SA, Kaumann AJ, Groszmann RJ. Comparison of the hemodynamics responses to ketanserin and prazosin in portal hypertensive rats. Hepatology. 1988;8:1112–1115. doi: 10.1002/hep.1840080523. [DOI] [PubMed] [Google Scholar]
- Elsby R, Kitteringham NR, Goldring CE, Lovatt CA, Chamberlain M, Henderson CJ, et al. Increased constitutive c-Jun N-terminal kinase signaling in mice lacking glutathione S-transferase Pi. J Biol Chem. 2003;278:22243–22249. doi: 10.1074/jbc.M301211200. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Distrutti E, Mencarelli A, Farneti S, Del Soldato P, et al. Liver delivery of NO by NCX-1000 protects against acute liver failure and mitochondrial dysfunction induced by paracetamol in mice. Br J Pharmacol. 2004;143:33–42. doi: 10.1038/sj.bjp.0705780. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Forster CD, Macdonald IA. The assay of the catecholamine content of small volumes of human plasma. Biomed Chromatogr. 1999;13:209–215. doi: 10.1002/(SICI)1099-0801(199905)13:3<209::AID-BMC820>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Garcia-Sainz JA, Romero-Avila MT, Villalobos-Molina R, Minneman KP. Alpha 1-adrenergic subtype selectivity of tamsulosin: studies using livers from different species. Eur J Pharmacol. 1995;1289:1–7. doi: 10.1016/0922-4106(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Goldring CE, Kitteringham NR, Elsby R, Randle LE, Clement YN, Williams DP, et al. Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology. 2004;39:1267–1276. doi: 10.1002/hep.20183. [DOI] [PubMed] [Google Scholar]
- Hall H, Leach A. Paravertebral block in the management of liver capsule pain after blunt trauma. Br J Anaesth. 1999;83:819–821. doi: 10.1093/bja/83.5.819. [DOI] [PubMed] [Google Scholar]
- Harbison RD, Gill J, Roberts SM, Demott RP.1996Reevaluation of adrenoceptor mediated hepatoprotection from bromobenzene Fund Appl Toxicol 290Suppl 30(Abstract 1486). [Google Scholar]
- Harbison RD, James RC, Roberts SM. Hepatic glutathione suppression by the alpha-adrenoreceptor stimulating agents phenylephrine and clonidine. Toxicology. 1991;69:279–290. doi: 10.1016/0300-483x(91)90187-6. [DOI] [PubMed] [Google Scholar]
- Hartmann H, Beckh K.1992Nerve supply and nervous control of liver functionIn: McIntyre N, Benhamou JP, Bircher J (eds).Oxford Textbook of Clinical Hepatology Oxford University Press: Oxford [Google Scholar]
- Hinson J. Biochemical toxicology of acetaminophen. Rev Biochem Toxicol. 1980;69:279–290. [Google Scholar]
- Ito Y, Bethea NW, Abril ER, Mccuskey RS. Early hepatic microvascular injury in response to acetaminophen toxicity. Microcirculation. 2003;10:391–400. doi: 10.1038/sj.mn.7800204. [DOI] [PubMed] [Google Scholar]
- Iwai M, Saheki S, Ohta Y, Shimazu T. Footshock stress accelerates carbon tetrachloride-induced liver injury in rats: implication of the sympathetic nervous system. Biomed Res. 1986;7:145–154. [Google Scholar]
- Iwai M, Shimazu T. Effects of ventromedial and lateral hypothalamic stimulation on chemically-induced liver injury in rats. Life Sci. 1988;42:1833–1840. doi: 10.1016/0024-3205(88)90021-5. [DOI] [PubMed] [Google Scholar]
- James RC, Harbison RD, Roberts SM. Phenylpropanolamine potentiation of acetaminophen-induced hepatotoxicity: evidence for a glutathione-dependent mechanism. Toxicol Appl Pharmacol. 1993;118:159–168. doi: 10.1006/taap.1993.1021. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973;187:195–202. [PubMed] [Google Scholar]
- Kerger BD, Gandy J, Bucci TJ, Roberts SM, Harbison RD, James RC. Antagonism of bromobenzene-induced hepatotoxicity by the alpha-adrenergic blocking agents, phentolamine and idazoxan. Toxicol Appl Pharmacol. 1988a;95:12–23. doi: 10.1016/s0041-008x(88)80003-6. [DOI] [PubMed] [Google Scholar]
- Kerger BD, Roberts SM, Harbison RD, James RC. Antagonism of bromobenzene-induced hepatotoxicity by the alpha-adrenoreceptor blocking agents phentolamine and idazoxan: role of hypothermia. Toxicol Appl Pharmacol. 1989;97:360–369. doi: 10.1016/0041-008x(89)90340-2. [DOI] [PubMed] [Google Scholar]
- Kerger BD, Roberts SM, Hinson JA, Gandy J, Harbison RD, James RC. Antagonism of bromobenzene-induced hepatotoxicity by phentolamine: evidence for a metabolism-independent intervention. Toxicol Appl Pharmacol. 1988b;95:24–31. doi: 10.1016/s0041-008x(88)80004-8. [DOI] [PubMed] [Google Scholar]
- Kitteringham NR, Powell H, Clement YN, Dodd CC, Tettey JN, Pirmohamed M, et al. Hepatocellular response to chemical stress in CD-1 mice: induction of early genes and gamma-glutamylcysteine synthetase. Hepatology. 2000;32:321–333. doi: 10.1053/jhep.2000.9602. [DOI] [PubMed] [Google Scholar]
- Larson RE, Plaa GL. A correlation of the effects of cervical cordotomy, hypothermia, and catecholamines and carbon tetrachloride-induced hepatic necrosis. J Pharmacol Exp Ther. 1965;147:103–111. [PubMed] [Google Scholar]
- Laskin DL, Pilaro AM, Ji S. Potential role of activated macrophages in acetaminophen hepatotoxicity. II. Mechanism of macrophage accumulation and activation. Toxicol Appl Pharmacol. 1986;86:216–226. doi: 10.1016/0041-008x(86)90052-9. [DOI] [PubMed] [Google Scholar]
- Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23:217–226. doi: 10.1055/s-2003-42641. [DOI] [PubMed] [Google Scholar]
- Liu J, Li C, Waalkes MP, Clark J, Myers P, Saavedra JE, et al. The nitric oxide donor, V-PYRRO/NO, protects against acetaminophen-induced hepatotoxicity in mice. Hepatology. 2003;37:324–333. doi: 10.1053/jhep.2003.50063. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2006;2:493–503. doi: 10.1517/17425255.2.4.493. [DOI] [PubMed] [Google Scholar]
- Michael SL, Pumford NR, Mayeux PR, Niesman MR, Hinson JA. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology. 1999;30:186–195. doi: 10.1002/hep.510300104. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187:211–217. [PubMed] [Google Scholar]
- Park BK, Kitteringham NR, Maggs JL, Pirmohamed M, Williams DP. The role of metabolic activation in drug-induced hepatotoxicity. Annu Rev Pharmacol Toxicol. 2005;45:177–202. doi: 10.1146/annurev.pharmtox.45.120403.100058. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M, Williams D, Madden S, Templeton E, Park BK. Metabolism and bioactivation of clozapine by human liver in vitro. J Pharmacol Exp Ther. 1995;272:984–990. [PubMed] [Google Scholar]
- Prescott LF, Illingworth RN, Critchley JA, Stewart MJ, Adam RD, Proudfoot AT. Intravenous N-acetylcysteine: the treatment of choice for paracetamol poisoning. Br Med J. 1979;2:1097–1100. doi: 10.1136/bmj.2.6198.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucy JL, Lasker JM, Lieber CS, Black M. Acetaminophen activation by human liver cytochromes P450IIE1 and P450IA2. Arch Biochem Biophys. 1989;271:270–283. doi: 10.1016/0003-9861(89)90278-6. [DOI] [PubMed] [Google Scholar]
- Roberts SM, Demott RP, James RC. Adrenergic modulation of hepatotoxicity. Drug Metab Rev. 1997;29:329–353. doi: 10.3109/03602539709037587. [DOI] [PubMed] [Google Scholar]
- Roberts SM, Harbison RD, James RC. Methamphetamine potentiation of carbon tetrachloride hepatotoxicity in mice. J Pharmacol Exp Ther. 1994;271:1051–1057. [PubMed] [Google Scholar]
- Roberts SM, Harbison RD, Seng JE, James RC. Potentiation of carbon tetrachloride hepatotoxicity by phenylpropanolamine. Toxicol Appl Pharmacol. 1991;111:175–188. doi: 10.1016/0041-008x(91)90022-7. [DOI] [PubMed] [Google Scholar]
- Rubenstein D. Epinephrine release and liver glycogen levels after carbon tetrachloride administration. Am J Physiol. 1962;203:1033–1037. doi: 10.1152/ajplegacy.1962.203.6.1033. [DOI] [PubMed] [Google Scholar]
- Schwetz BA, Plaa GL. Catecholamine potentiation of carbon tetrachloride-induced hepatotoxicity in mice. Toxicol Appl Pharmacol. 1969;14:495–509. doi: 10.1016/0041-008x(69)90011-8. [DOI] [PubMed] [Google Scholar]
- Soeishi Y, Matsushima H, Teraya Y, Watanabe T, Higuchi S, Kaniwa H. Metabolism of tamsulosin in rat and dog. Xenobiotica. 1996;26:355–365. doi: 10.3109/00498259609046714. [DOI] [PubMed] [Google Scholar]
- Thummel KE, Lee CA, Kunze KL, Nelson SD, Slattery JT. Oxidation of acetaminophen to N-acetyl-p-aminobenzoquinone imine by human CYP3A4. Biochem Pharmacol. 1993;45:1563–1569. doi: 10.1016/0006-2952(93)90295-8. [DOI] [PubMed] [Google Scholar]
- Vandeputte C, Guizon I, Genestie-Denis I, Vannier B, Lorenzon G. A microtiter plate assay for total glutathione and glutathione disulfide contents in cultured/isolated cells: performance study of a new miniaturized protocol. Cell Biol Toxicol. 1994;10:415–421. doi: 10.1007/BF00755791. [DOI] [PubMed] [Google Scholar]
- Walker R, Racz W, Mcelligott T. Acetaminophen-induced hepatotoxicity in mice. Lab Invest. 1980;42:181–189. [PubMed] [Google Scholar]
- Wallace CI, Dargan PI, Jones AL. Paracetamol overdose: an evidence based flowchart to guide management. Emerg Med J. 2002;19:202–205. doi: 10.1136/emj.19.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Reese J, Cotechia S, Michel MC. Murine alpha1-adrenoceptor subtypes. I. Radioligand binding studies. J Pharmacol Exp Ther. 1998;286:841–847. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.