Abstract
Background and purpose:
It has recently been reported that oxytocin is produced by some tumour cell types, and that oxytocin receptors, belonging to the G-protein-coupled receptor (GPCR) family, are expressed in a variety of cell types. Among these, human umbilical vein endothelial cells (HUVECs) respond to oxytocin with an increased proliferation, suggesting a possible role for the hormone in the regulation of angiogenesis.
Experimental approach:
We employed chemotaxis and chemoinvasion assays to characterize the effect of oxytocin on HUVEC motility, and immunoblot analysis to study its molecular mechanisms of action.
Key results:
We showed that oxytocin stimulates migration and invasion in HUVECs via oxytocin receptor activation. Searching for the molecular mechanism(s) responsible for oxytocin's pro-migratory effect, we identified the Gq coupling of oxytocin receptors and phospholipase C (PLC) as the main effectors of oxytocin's action in HUVECs. We also found that oxytocin stimulates the phosphorylation of endothelial nitric oxide synthase (eNOS) via the phosphatidylinositol-3-kinase (PI-3-K)/AKT pathway, and that the activation of PI-3-K and formation of nitric oxide (NO) are required for the pro-migratory effect of oxytocin.
Conclusions and implications:
The ability of oxytocin to stimulate HUVEC motility and invasion suggests that the hormone can participate in physiopathological processes where activation of endothelial cells plays an important role, for example, in angiogenesis. Interestingly, both the AKT and eNOS phosphorylation induced by oxytocin receptor activation depended on PLC activity, thus suggesting the existence of a still undefined mechanism connecting PLC to the PI-3-K/AKT pathway, upon oxytocin stimulation.
Keywords: oxytocin, human endothelial cells, motility, invasion, angiogenesis, phospholipase C, nitric oxide, phosphatidylinositol-3-kinase/AKT pathway.
Introduction
Cell migration is a complex phenomenon that plays a crucial role in all stages of development as well as in adult life. It directs and regulates the morphogenesis of tissues and organs during embryogenesis and, in adulthood, is crucial for tissue regeneration and repair, and immune surveillance (Van Haastert and Devreotes, 2004). It is therefore not surprising that a number of soluble factors and receptors contribute to regulating this process in different cell types and environments. Among these factors, G-protein-coupled receptors (GPCRs) and their ligands play an important role: chemoattractants acting on a number of chemokine receptor subtypes regulate leukocyte trafficking in the immune system (Murphy, 1994), and it has been shown that other agents acting at GPCRs, such as lysophosphatidic acid and sphingosine 1-phosphate, are involved in modulating the migration of various cell types (Moolenaar et al., 2004; Rosen and Goetzl, 2005).
One of the most important events depending on cell migration is angiogenesis (that is, the formation of new blood vessels from pre-existing blood vessels), which occurs in adults under both physiological and pathological conditions, such as the menstrual cycle and tumours (Bergers and Benjamin, 2003). The stimulation of endothelial cell proliferation and motility is the initial event in the formation of the new peritumoral blood vessels that allow tumour growth and survival, and this process is driven and sustained by angiogenic factors that are commonly produced by the tumour cells themselves.
The hypothalamic hormone oxytocin is best known for its role in regulating uterine motility at parturition, but it has recently been shown to be produced by some tumour cell types in vitro as well as by cancer tissues (Cassoni et al., 2004). Furthermore, the expression of oxytocin receptors that are structurally identical to the uterine receptors and that belong to the GPCR family has been demonstrated on human vascular endothelial cells, on which oxytocin induces a proliferative response (Thibonnier et al., 1999). It has also been recently reported that oxytocin stimulates the motility of immortalized human dermal microvascular and breast cancer-derived endothelial cells (Cassoni et al., 2006). It is therefore possible to suggest that oxytocin may act as an endocrine/paracrine regulatory factor that, once locally produced, can contribute in vivo to the formation of new blood vessels in some types of cancer.
Expression of the mRNA for oxytocin has been described in the endometrium of non-pregnant women (Steinwall et al., 2004), which is particularly interesting because the female reproductive system is the part of the adult body that undergoes frequent cycles of physiological angiogenesis (during the menstrual cycle). Moreover, a number of diseases affecting the female reproductive tract are associated with angiogenic dysfunctions, including endometriosis (Reynolds et al., 2002), in which it has been proposed that hyperactivity of the endometrial oxytocin/oxytocin receptor system could play an important role (Leyendecker et al., 1998).
We therefore tested whether, in addition to its mitogenic properties, oxytocin could induce migration in primary cultures of human endothelial cells and thus act as a cofactor facilitating angiogenesis. We found that the hormone does indeed stimulate human umbilical vein endothelial cell (HUVEC) migration and invasion, thus suggesting that it may contribute in vivo to the process of angiogenesis not only in some types of cancer, but also in other physiological and pathological conditions characterized by the formation of new blood vessels.
We also investigated possible molecular mechanisms that may be responsible for oxytocin's pro-migratory effect to clarify: (i) the signalling pathways activated by the binding of oxytocin to its GPCR in HUVECs; (ii) the involvement of these pathways in the migratory response; and (iii) the hierarchy between these pathways. Elucidating the signalling events through which oxytocin regulates cell migration represents a fundamental step in the possible pharmacological exploitation of this peptide as a regulator of cell migration.
Methods
Cell cultures
HUVECs were isolated from freshly derived umbilical cords by digestion with collagenase as described by Jaffe et al. (1973). Cells were routinely grown in 199 medium, supplemented with 20% heat-inactivated fetal bovine serum, 25 μg ml−1 endothelial cell growth factor and 50 μg ml−1 heparin, and used at passages 2–7.
Cell migration and invasion assays
HUVEC migration and invasion were evaluated by the means of chemotaxis and chemoinvasion experiments, respectively, in a 48-well modified Boyden chamber, as described previously (Cattaneo et al., 2003). Briefly, the chemotaxis experiments were performed using 8 μm Nuclepore polyvinylpyrrolidine-free polycarbonate filters coated with 10 μg ml−1 of type IV collagen and placed over a lower chamber containing oxytocin, Thr4Gly7-oxytocin (TG-oxytocin) or vascular endothelial growth factor (VEGF) as attractant factor. For the evaluation of the basal motility, 199 medium supplemented with 2% fetal bovine serum was used in the lower chamber. Suspended in 199 media containing 2% fetal bovine serum, the cells, after pretreatment with the vehicle or the indicated drugs, were added to the upper chamber at a density of 5 × 104 cells per well. Drugs were continuously present during the experiments. After 6 h of incubation at 37 °C, the non-migrated cells on the upper surface of the filter were removed by scraping. The cells that had migrated to the lower side of the filter were stained with Diff-Quick stain (VWR Scientific Products, Bridgeport, NJ, USA), and 5 unit fields per filter were counted by a scorer, unaware of the experimental conditions, using a Zeiss microscope. The assays were run in triplicate. Results are reported as the mean number of cells migrated per microscopic field or as chemotactic index, that is, the number of cells that migrated in the presence of the chemoattractant/number of cells that migrated with medium only (basal migration). To distinguish between true chemotaxis (that is, cell migration following a chemical gradient) and chemokinesis (that is random activation of movement due to the presence of a chemical), a ‘checkboard analysis' was performed, in which oxytocin was placed in the upper and/or in the lower compartments of the Boyden chamber. For the chemoinvasion assays, the filters were coated with a layer of Matrigel (100 μg per filter), and the Boyden chamber was assembled with the coated side of the filter facing the cells in the upper compartment. The assays were then performed as described for chemotaxis.
Immunoblot analysis
HUVECs, plated in 35-mm-diameter Petri dishes, were treated for 30 min with the indicated drugs, stimulated for 10 min with TG-oxytocin, washed with phosphate-buffered saline and then directly lysed in sodium dodecyl sulphate-polyacrylamide gel electrophoresis sample buffer (62 mM Tris-HCl, pH 6.8, 2% sodium dodecyl sulphate, 10% glycerol, 5% 2-mercaptoethanol and 0.04% bromophenol blue) containing 1 mM sodium orthovanadate. After sodium dodecyl sulphate-polyacrylamide gel electrophoresis, proteins were transferred onto nitrocellulose membranes that were blocked with 5% (w/v) non-fat dried milk in TBS-T (Tris-buffered saline containing 0.05% Tween-20) and probed with the indicated primary antibodies at a 1:1000 dilution in 5% non-fat dried milk in TBS-T. After incubation with the appropriate peroxidase-conjugated secondary antibody (1:3000 swine anti-rabbit and 1:2000 rabbit anti-mouse antibodies, both diluted in 5% non-fat dried milk in TBS-T; Dako, Glostrup, Denmark), the immunoreactive bands were visualized with chemiluminescence (ECL; Amersham, Buckinghamshire, UK). The densitometric analysis of the immunoblots was performed using the National Institutes of Health (NIH) Image J program.
Statistical procedures
All data were expressed as mean±s.e.mean. Statistical analysis was carried out using one-way ANOVA followed by Bonferroni's multiple comparison test, two-way ANOVA with Tukey's test or Student's t-tests, where applicable. In the two-way ANOVA analysis, we considered as factors the treatment with the antagonists (SR49059 and OTA for Figure 1d, and atosiban for Figure 3b) and the treatment with TG-oxytocin. P-values of <0.05 were considered significant.
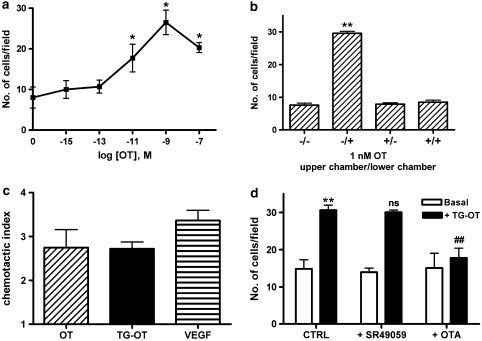
Figure 1.
Oxytocin stimulates HUVEC migration via oxytocin receptors. (a) Oxytocin (OT) was used as the attractant in the lower compartment of a Boyden chamber, and the experiments were performed as described in the Methods section. The results are expressed as the number of migrating cells in the presence of the indicated concentrations of oxytocin. Mean value±s.e.mean of three independent experiments. *P<0.05 compared to basal migration. (b) Checkboard analysis of the chemotactic responsiveness to oxytocin (1 nM) placed in the upper and/or the lower compartments of a Boyden chamber. The results are expressed as the number of migrating cells per field. Mean value±s.e.mean of 15 counts from a representative experiment, repeated once with similar results. **P<0.001 compared to the other treatments. (c) Chemotaxis assays using oxytocin (1 nM), Thr4Gly7-oxytocin (TG-OT, 1 nM) or VEGF (25 ng ml−1) in the lower compartment of a Boyden chamber. The results are expressed as chemotactic index; that is, the number of cells migrating in the presence of the attractants divided by the number of cells migrating in the presence of medium alone. Mean values±s.e.mean of 10–23 independent experiments. One-way ANOVA showed no significant differences between treatments. (d) HUVECs were treated for 5 min with SR49059 (10 nM) or OTA (10 nM) before chemotaxis in the presence of TG-OT (1 nM) as attractant. The results are expressed as the number of migrating cells per field. Mean value±s.e.mean of three independent experiments. **P<0.001 compared to basal migration in the absence of any drug (control cells, CTRL); NS and ##P<0.001 compared to TG-OT; no significant differences between basal values (two-way ANOVA with Tukey's test). HUVEC, human umbilical vein endothelial cell; VEGF, vascular endothelial growth factor.
Reagents and antibodies
All tissue culture reagents (199 medium, fetal bovine serum, endothelial cell growth factor and heparin) were from Sigma Chemicals (St Louis, MO, USA). The following reagents were purchased as indicated: human VEGF165 from Calbiochem (Darmstadt, Germany); oxytocin, TG-oxytocin, Pertussis toxin (PTx), U73122 and its inactive analogue U73343, LY294002, Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), tyrphostin AG1478 and SU5416 from Sigma Chemicals; collagen type IV and Matrigel from BD Bioscience (Bedford, MA, USA). The human vasopressin receptor antagonist SR49059 [(2S)1-[(2R3S)-5-chloro-3-(2-chlorophenyl)-1-(3,4-dimethoxybenzene-sulphonyl)-3-hydroxy-2,3-dihydro-1H-indole-2-carbonyl]-pyrrolidine-2-carboxamide] (Serradeil-Le Gal et al., 1993) and the oxytocin receptor antagonist OTA [d(CH2)5[Tyr(Me)2,Thr4,Tyr-NH29]OVT] (Elands et al., 1988) were gifts, respectively, of Claudine Serradeil-Le Gal, Sanofi-Aventis Recherche and Développement, Toulouse, France, and of Maurice Manning, College of Medicine, Toledo, OH, USA.
Antibodies used were rabbit polyclonals anti-phospho eNOS (Ser 1177) (Cell Signalling, Beverly, MA, USA), anti-eNOS (Cayman Chemical, Ann Arbor, MI, USA), anti-phospho AKT (Ser 473) (Biosource International, Camarillo, CA, USA) and a mouse monoclonal anti-AKT (Upstate Biotechnology, Lake Placid, NY, USA).
Results
Oxytocin and the selective analogue TG-oxytocin stimulate HUVEC migration and invasion
We first investigated the ability of oxytocin to stimulate HUVEC motility and invasion by the means of chemotaxis and chemoinvasion experiments. As shown in Figure 1a, oxytocin induced HUVEC migration in a concentration-dependent manner, showing the bell-shaped response curve that is typical of the most chemotactic substances and probably due to the gradient rupture that occurs at the highest concentrations. As the maximal stimulation of cell motility was observed at a concentration of 1 nM, this concentration was chosen for all of the subsequent experiments.
To distinguish true chemotaxis (that is, a directed locomotory response following a chemical gradient) from chemokinesis (that is, random movement activation due to the presence of a chemical), we undertook a checkboard analysis by placing 1 nM of oxytocin in the upper and/or the lower compartments of a Boyden chamber. Figure 1b shows that HUVECs only migrated in response to a positive chemical gradient (that is, when the oxytocin was placed in the lower compartment of the chamber), thus indicating that oxytocin has true chemotactic activity.
To support the involvement of specific oxytocin receptors in the promigratory action of oxytocin and to exclude that this effect could be due to the possible interaction of the hormone with vasopressin receptors (Gimpl and Fahrenholz, 2001), we performed the following experiments. We first checked for the promigratory effect of an oxytocin-derived agonist, TG-oxytocin, which binds to human oxytocin receptors and vasopressin 1a receptors (V1a) with the same affinities as oxytocin, but it is characterized by a much lower affinity for V1b and V2 receptor subtypes (Chini and Manning, 2007). As shown in Figure 1c, the chemotactic index (that is, the fold stimulation induced by a chemoattractant over baseline values) of 1 nM oxytocin was 2.75±0.41 (n=10), and the same concentration of TG-oxytocin had an identical index of 2.72±0.16 (n=15). Oxytocin and its TG-oxytocin analogue were therefore equally potent and effective in stimulating HUVEC motility. Second, the specific V1a receptor inhibitor SR49059 (10 nM) (Serradeil-Le Gal et al., 1993) affected neither basal nor TG-oxytocin-stimulated response, thus excluding a V1a receptor involvement (Figure 1d). These data are in agreement with previously published reverse transcription-PCR experiments in which no DNA amplification with primers specific for V1, V2 and V3 vasopressin receptors was found in HUVECs (Thibonnier et al., 1999). Finally, the selective oxytocin receptor antagonist OTA (Elands et al., 1988) was used. As can be seen in Figure 1d, OTA (10 nM) was devoid of any agonist effect, although abolishing the TG-oxytocin-induced HUVEC migration. All the above experiments strongly suggest the involvement of specific oxytocin receptors in TG-oxytocin-induced migration.
We also compared the promigratory effects of oxytocin and TG-oxytocin with that of VEGF (the most potent and specific promigratory endothelial factor currently known) (Shibuya and Claesson-Welsh, 2006) and found that the chemotactic index of VEGF (Figure 1c) was 3.36±0.23 (n=23) thus indicating that 1 nM of oxytocin and TG-oxytocin were almost as effective as VEGF (25 ng ml−1) in inducing HUVEC migration (76 and 75% as effective). On the basis of the above-described results, TG-oxytocin was used as an oxytocin receptor-selective chemotactic agent in the subsequent experiments.
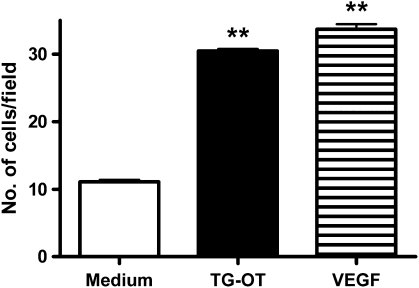
The chemoattractant ability of TG-oxytocin was tested in an invasion assay in which the use of Matrigel as a three-dimensional matrix provided information not only about cell motility, but also about the ability of the cells to cross tissue barriers (Albini et al., 2004). As shown in Figure 2, 1 nm TG-oxytocin not only had a promigratory effect, but also induced the same level of HUVEC invasion through a layer of Matrigel as that induced by VEGF.
Figure 2.
TG-oxytocin (TG-OT) induces HUVEC invasion through Matrigel. The chemoinvasion experiments were performed as described in the Methods section using Matrigel to coat the filters. TG-OT (1 nM) or VEGF (25 ng ml−1) were used as attractants in the lower compartment of a Boyden chamber. The results are expressed as the number of migrating cells per field and are the mean values±s.e.mean of 15 counts from a representative experiment, repeated once with similar results. **P<0.001 compared to basal invasion (medium alone). HUVEC, human umbilical vein endothelial cell; VEGF, vascular endothelial growth factor.
OTR/Gi coupling is not involved in promoting HUVEC migration
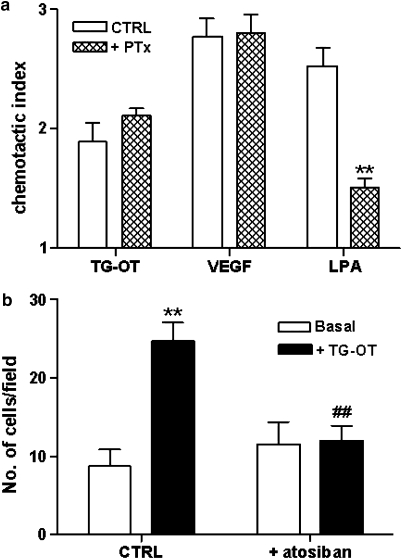
In peripheral tissues, oxytocin receptors are functionally coupled to various G proteins (including Gq−11, Gi and Gh), and are thus considered ‘promiscuous' (Phaneuf et al., 1996; Strakova and Soloff, 1997; Sanborn, 2001; Rimoldi et al., 2003). As the G proteins involved in chemotactic factor response are more often PTx sensitive (Thelen, 2001), we first analysed the role of oxytocin receptor coupling to Gi in promoting cell migration by performing chemotaxis experiments with cells pretreated overnight with PTx (final concentration of 15 ng ml−1), with TG-oxytocin (1 nM), VEGF (25 ng ml−1) and lysophosphatidic acid (5 μM) as chemoattractants (Moolenaar, 1999). As shown in Figure 3a, PTx pretreatment did not interfere with the chemotactic effect of TG-oxytocin, as its chemotactic index was the same in the PTx-treated and -untreated cells. As expected, the VEGF response, which is mediated by a classical tyrosine kinase receptor, was not affected by the toxin, whereas the stimulatory effect of lysophosphatidic acid, which is mediated by both PTx-sensitive and PTx-insensitive G proteins (Moolenaar et al., 2004), was partially inhibited. These findings indicate that the promigratory effect of TG-oxytocin acting on oxytocin receptors does not depend on receptor coupling to Gi.
Figure 3.
Oxytocin receptor/Gi coupling is not involved in the promigratory effect of TG-oxytocin (TG-OT). (a) HUVECs were pretreated overnight with Pertussis toxin (PTx, 15 ng ml−1) before chemotaxis in the presence of TG-OT (1 nM), VEGF (25 ng ml−1) or LPA (5 μM) as attractants. The results are expressed as chemotactic index (see Figure 1c). Mean values±s.e.mean of three independent experiments. **P<0.01 compared to LPA in the absence of PTx pretreatment (Student's t-tests). (b) HUVECs were treated for 5 min with atosiban (100 nM) before chemotaxis in the presence of TG-OT (1 nM) as attractant. The results are expressed as the number of migrating cells per field. Mean values±s.e.mean of six independent experiments. **P<0.001 compared to basal migration in the absence of the drug (control cells, CTRL); ##P<0.001 compared to TG-OT in the absence of atosiban; no significant differences between basal values (two-way ANOVA with Tukey's test). HUVEC, human umbilical vein endothelial cell; LPA, .lysophosphatidic acid; VEGF, vascular endothelial growth factor.
To confirm the role of PTx-insensitive G proteins of the Gq family in the promigratory effect of oxytocin, we performed chemotaxis experiments in the presence of a coupling-specific ligand of the human oxytocin receptor. Coupling-specific ligands are analogues that may only activate a selective downstream signalling pathway in a promiscuous receptor (Urban et al., 2007). It has been recently shown that atosiban, a peptidic oxytocin derivative, acts as an agonist at oxytocin receptor/Gi and as an antagonist at oxytocin receptor/Gq−11 coupling, and is thus one of the first pharmacologically characterized ‘coupling-specific agonists' (Reversi et al., 2005). When used alone, atosiban did not significantly affect HUVEC migration, but when used in combination with TG-oxytocin, it completely inhibited TG-oxytocin-stimulated HUVEC motility (n=6, Figure 3b). This result confirm that the promigratory effect of TG-oxytocin does not depend on receptor coupling to Gi and strongly supports the involvement of receptor coupling to Gq−11.
Signals involved in oxytocin-stimulated HUVEC migration
Phospholipase C pathway
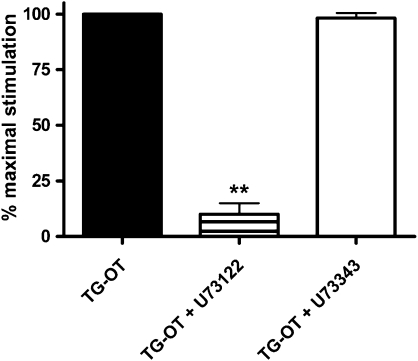
The results described above suggest that the effects of oxytocin receptor on HUVEC migration may be due to Gq coupling. As the main effector of Gq is phospholipase Cβ (PLCβ), we performed chemotaxis experiments in the presence of U73122, a widely used inhibitor of PLC-linked events. As shown in Figure 4, U73122 (5 μM) inhibited TG-oxytocin-stimulated cell motility in HUVECs by 90±5.0%, whereas the same concentration of the inactive U73343 analogue had no effect. Neither drug affected basal HUVEC motility (8.3±2.6 cells per field for U73122 and 8.9±2.3 cells per field for U73343, n=3) in comparison with control cells (9.5±2.3 cells per field, n=3). These results indicate the strong involvement of the PLC pathway in oxytocin receptor-mediated promigratory action in HUVECs.
Figure 4.
Effect of the phospholipase C inhibitor U73122 on migration induced by TG-oxytocin (TG-OT). HUVECs were pretreated for 30 min with U73122 (5 μM) or U73343 (5 μM) before chemotaxis in the presence of TG-OT (1 nM) as attractant. The results are expressed as the percentage of the maximal migration induced by TG-OT in the absence of drugs (100%). Mean values±s.e.mean of three independent experiments. **P<0.001 compared to TG-OT. HUVEC, human umbilical vein endothelial cell.
Endothelial NOS pathway
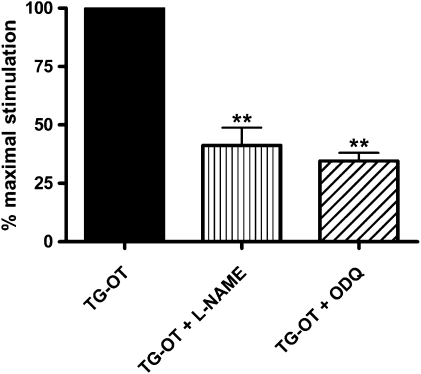
The PLC/calcium/calmodulin pathway in HUVECs is particularly important because it can induce the activation of endothelial nitric oxide synthase (eNOS), leading to nitric oxide (NO) formation, the activation of the soluble guanylate cyclase (sGC) and an increase in cGMP production (Dudzinski et al., 2006). NO plays a key role in endothelial cell migration (Dimmeler et al., 2000) and angiogenesis (Fukumura et al., 2001; Morbidelli et al., 2003), and its formation has been described as being induced by oxytocin in HUVECs (Thibonnier et al., 1999). For these reasons, we decided to test whether NO production may be involved in the promigratory action mediated by oxytocin receptors. As shown in Figure 5, the NOS inhibitor L-NAME (100 μM) and the sGC inhibitor ODQ (10 μM) (Garthwaite et al., 1995) reduced TG-oxytocin-activated cell motility by, respectively, 58.7±7.5 and 65.5±3.5%, but neither affected basal HUVEC motility (13.9±4.8 cells per field for L-NAME and 14.2±6.5 cells per field for ODQ, n=3) in comparison with control cells (12.4±3.9 cells per field, n=3). These results indicate that the NO/sGC pathway is partially involved in the endothelial cell migration initiated by oxytocin receptors.
Figure 5.
Effect of L-NAME and ODQ on migration induced by TG-oxytocin (TG-OT). HUVECs were pretreated for 30 min with the NOS inhibitor L-NAME (100 μM) or the soluble guanylate cyclase inhibitor ODQ (10 μM) before chemotaxis in the presence of TG-OT (1 nM) as attractant. The results are expressed as the percentage of the maximal migration induced by TG-OT in the absence of inhibitors (100%). Mean values±s.e.mean of three independent experiments. **P<0.001 compared to TG-OT. HUVEC, human umbilical vein endothelial cell; L-NAME, Nω-nitro-L-arginine methyl ester hydrochloride; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one.
PI-3-K/AKT pathway
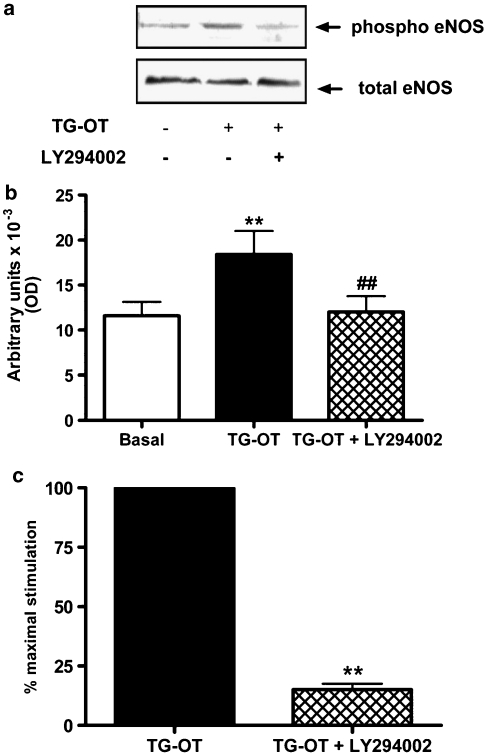
eNOS is generally activated through a PLC/calcium/calmodulin pathway, but eNOS activation may also occur via the phosphatidylinositol-3-kinase (PI-3-K)/AKT pathway, in which it is activated by AKT-mediated phosphorylation in a specific serine residue, at position 1177 in the human sequence (Dudzinski et al., 2006). As shown in Figure 6a, TG-oxytocin phosphorylated eNOS in position 1177, and this phosphorylation is inhibited by the PI-3-K inhibitor LY294002 (20 μM). A densitometric analysis performed on four independent experiments is shown in Figure 6b. AKT was phosphorylated after TG-oxytocin treatment (see Figure 8) and, as expected, this phosphorylation was also blunted by LY294002 (data not shown). These data indicate that, in addition to the PLC/calcium pathway, oxytocin receptors also activate the PI-3-K/AKT pathway and phosphorylate eNOS. The inhibition of PI-3-K activity by LY294002 reduced TG-oxytocin-induced chemotaxis in HUVECs by 85.0±2.5% (Figure 6c) without affecting basal motility (11.4±1.3 cells per field in the absence, and 12.9±2.3 cells per field in the presence of LY294002). These results suggest that the PI-3-K pathway is crucial for the promigratory effect of TG-oxytocin. Furthermore, the PI-3-K/AKT pathway is upstream of eNOS because, as shown above, its inhibition by LY294002 abolished the phosphorylation of eNOS induced by TG-oxytocin.
Figure 6.
Involvement of the PI-3-K/AKT pathway in migration induced by TG-oxytocin (TG-OT). (a) HUVECs were preincubated for 30 min with LY294002 (20 μM) before being stimulated with TG-OT (10 nM) for 10 min. Aliquots of cell lysates (30 μg protein per lane) were separated by 7.5% SDS-PAGE and immunoblotted with the indicated antibodies. The experiment was repeated four times with similar results. (b) Densitometric analysis of four independent experiments performed as described in (a). Each bar represents the mean value±s.e.mean. **P<0.001 compared to basal; ##P<0.001 compared to TG-OT. (c) In parallel experiments, HUVECs were pretreated with LY294002 as described in (a) before chemotaxis in the presence of TG-OT (1 nM) as attractant. The results are expressed as the percentage of the maximal migration induced by TG-OT in the absence of LY294002 (100%). Mean values±s.e.mean of four independent experiments. **P<0.001 compared to TG-OT. EGF, epidermal growth factor; HUVECs, human umbilical vein endothelial cells; PI-3-K, phosphatidylinositol-3-kinase; SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis.
Transactivation experiments
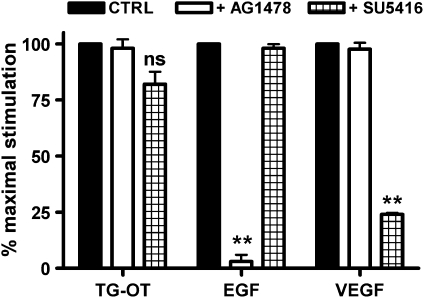
In an attempt to elucidate the mechanism(s) by which oxytocin receptors (which belong to the GPCR family) can activate the PI-3-K signalling and consequently, migratory behaviour in HUVECs, we investigated the possibility of an oxytocin receptor-mediated transactivation of epidermal growth factor receptor and/or VEGF receptor. To this end, HUVECs were pretreated for 30 min with the epidermal growth factor receptor-specific antagonist tyrphostin AG1478 (3 μM) or the specific VEGF receptor inhibitor SU5416 (5 μM), and then challenged with 1 nM TG-oxytocin as chemoattractant. As shown in Figure 7, AG1478 had no effect on TG-oxytocin-stimulated cell migration, and SU5416 induced only a small and not significant decrease (18±5.5%, n=3), although they were both very effective inhibitors of HUVEC motility when used against their related specific growth factor. These data indicate that oxytocin receptor-induced HUVEC migration does not depend on epidermal growth factor receptor or VEGF receptor transactivation.
Figure 7.
Effect of AG1478 and SU5416 on migration induced by TG-oxytocin (TG-OT). HUVECs were pretreated for 30 min with the EGFR inhibitor AG1478 (3 μM) or the VEGF receptor inhibitor SU5416 (5 μM) before chemotaxis in the presence of TG-OT (1 nM), EGF (50 ng ml−1) or VEGF (25 ng ml−1) as attractants. The results are expressed as the percentage of the maximal migration induced by TG-OT, EGF or VEGF in the absence of inhibitors (100%). Mean values±s.e.mean of three independent experiments. NS compared to TG-OT; **P<0.001 compared to EGF or VEGF in the absence of drugs. EGF, epidermal growth factor; HUVECs, human umbilical vein endothelial cells; VEGF, Vascular endothelial growth factor.
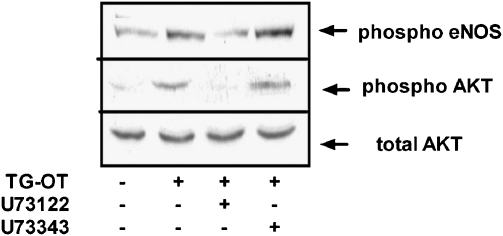
Relationship between PLC and PI-3-K activation
Although our results indicated the strong involvement of the PLC and the PI-3-K pathways in the promigratory effect of TG-oxytocin (see Figures 4 and 6c), we tried to elucidate the relationship between oxytocin receptor-induced PLC and PI-3-K activation in HUVECs. As shown in Figure 8, at a concentration of 5 μM, the PLC-linked events inhibitor U73122 abolished TG-oxytocin-induced AKT phosphorylation, thus indicating that PLC plays a crucial role in AKT activation. It also inhibited the phosphorylation of eNOS induced by TG-oxytocin. In the same experiments, a 5 μM concentration of the inactive compound U73343 had no effect on AKT or eNOS phosphorylation.
Figure 8.
Effect of the PLC inhibitor U73122 on the eNOS and AKT phosphorylation induced by TG-oxytocin (TG-OT). HUVECs were preincubated for 30 min with U73122 (5 μM) or U73343 (5 μM) before being stimulated with TG-OT (10 nM) for 10 min. Aliquots of cell lysates (30 μg protein per lane) were separated by 7.5% SDS-PAGE and immunoblotted with the indicated antibodies. The experiment was repeated three times with similar results. eNOS, endothelial nitric oxide synthase; HUVEC, human umbilical vein endothelial cell; PLC, phospholipase C; SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis.
Discussion
The results of this study demonstrated that, at nanomolar concentrations, oxytocin and its analogue, TG-oxytocin, stimulated HUVEC migration to almost the same extent as VEGF, the most potent proangiogenic factor acting in vivo on endothelial cells (Shibuya and Claesson-Welsh, 2006). We also found that TG-oxytocin could induce HUVECs to cross a three-dimensional protein matrix in an invasion assay, once again similar to VEGF. This contrasts with the lack of effect on HUVEC migration found by Cassoni et al. (2002) but, together with experimental variability in HUVEC preparation and/or maintenance in culture, this may be explained by the fact that oxytocin was used at a concentration of 100 nM, which is already in the descending part of the bell-shaped dose–response curve (Figure 1a).
As previously observed in its opposing effects on cell proliferation (Bussolati and Cassoni, 2001; Guzzi et al., 2002; Rimoldi et al., 2003), oxytocin may either inhibit or stimulate cell migration. It has been found to inhibit the migration of ovarian cancer cells (Morita et al., 2004), but it promotes the migration of HUVECs (this study), immortalized human dermal endothelial cells and breast-derived endothelial cells (Cassoni et al., 2006). As the opposing effects of oxytocin on cell proliferation may originate from the different activation of specific oxytocin-mediated signalling pathways, even in a single cell type (Rimoldi et al., 2003), elucidating the signalling events through which oxytocin regulates cell migration is a fundamental step in understanding its role in those pathophysiological conditions in which oxytocin-mediated cell migration may be involved.
The biological effects of oxytocin are mediated by its binding to a specific oxytocin receptor belonging to the class of GPCRs. In the human genome, oxytocin receptors are encoded by a single gene located on chromosome 3 and it is generally assumed that the majority of its actions are due to the activation of this unique receptor subtype (Gimpl and Fahrenholz, 2001). As has been demonstrated for a number of GPCRs, oxytocin receptors are promiscuous receptors that can couple to both Gq and Gi by activating multiple intracellular responses (Phaneuf et al., 1996; Strakova and Soloff, 1997; Sanborn, 2001; Rimoldi et al., 2003). Notably, the signalling pathways activated by oxytocin receptors may act synergistically, as in the case of the contraction induced in myometrial cells by oxytocin receptor coupling to Gq and the small G proteins of the Rho family (Sanborn, 2001), but they may also have opposing effects on the same cell function. In HEK293 cells stably transfected with human oxytocin receptors, receptor coupling to Gi is responsible for inhibiting cell growth, whereas receptor coupling to a different G protein (possibly Gq) is associated with the stimulation of cell growth (Guzzi et al., 2002; Rimoldi et al., 2003). We have now shown that the promigratory effect of oxytocin in HUVECs is PTx insensitive (that is not dependent on Gi coupling) and inhibited by the specific oxytocin receptor antagonist OTA (Elands et al., 1988) and also by atosiban, a peptidic compound endowed with biased agonist properties that acts as an antagonist at the Gq pathway and as an agonist at the Gi pathway (Reversi et al., 2005). These results suggest that Gq is the primary G-protein coupling oxytocin receptors to the promigratory effect of oxytocin in HUVECs. The suggestion that oxytocin receptors act via a Gq/PLC-dependent pathway is further strengthened by the ability of the PLC-linked event inhibitor U73122 to suppress the HUVEC motility stimulated by TG-oxytocin by more than 90%.
To elucidate the sequence of intracellular signals originating from PLC and involved in oxytocin-stimulated endothelial cell migration, we first examined the role of NO. As a result of eNOS activation, NO production plays an important role in the control of endothelial cell motility and, more generally, in the control of angiogenesis, and it has been shown that the messenger is generated in a calcium-dependent manner by oxytocin in HUVECs (Thibonnier et al., 1999). We found that the pharmacological inhibition of eNOS by L-NAME and of sGC by ODQ induces a partial inhibition of oxytocin-stimulated HUVEC migration, thus indicating that NO production and the consequent sGC activation are involved in the migratory response. However, as the PLC inhibitor U73122 almost completely abolished the action of oxytocin, the presence of additional signalling components depending on PLC activation is required to stimulate migration.
The GPCR-mediated activation of eNOS is a quite complex process that involves both calcium-dependent and calcium-independent mechanisms (Dudzinski et al., 2006). Receptors coupled to Gq proteins activate PLCβ, mobilize intracellular calcium and promote rapid eNOS activation via a Ca2+/calmodulin-dependent mechanism. However, ligands such as lysophosphatidic acid and sphingosine 1-phosphate can stimulate eNOS via a Gi, PTx-sensitive pathway, which involves a PI-3-K/AKT cascade leading to eNOS phosphorylation at serine 1177 (in the human sequence). In HUVECs, we found that TG-oxytocin phosphorylates eNOS at serine 1177, and the dependence of this phosphorylation on the PI-3-K/AKT pathway was confirmed by its inhibition in the presence of the PI-3-K inhibitor LY294002. Furthermore, TG-oxytocin was able to induce AKT phosphorylation, which was blunted by LY294002. These results indicate that oxytocin receptor activation by TG-oxytocin in HUVECs stimulates the PI-3-K/AKT pathway, which in turn is responsible for eNOS phosphorylation Furthermore, the PI-3-K inhibitor LY294002 almost completely suppressed the oxytocin receptor-induced migration, thus suggesting that the PI-3-K/AKT pathway plays a central role in controlling the endothelial cell motility stimulated by oxytocin receptors. However, the ability of oxytocin receptors to phosphorylate AKT and eNOS and activate motility is not PTx dependent but Gq/PLC dependent, as the PLC inhibitor U73122 abolished all of these oxytocin receptor-stimulated events. These findings suggest a new intracellular pathway (currently under investigation) in which the PI-3-K/AKT pathway is activated by PLC, and which plays a major role in controlling the oxytocin receptor-induced migration of HUVECs (Figure 9).
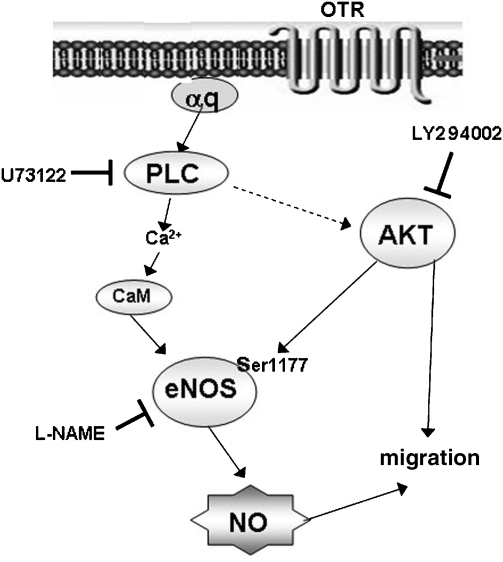
Figure 9.
Proposed model for the mechanism(s) underlying the promigratory effect of oxytocin. By acting on its GPCRs coupled to Gq, oxytocin activates PLC, which leads to an increase in intracellular Ca2+. Ca2+ binds to calmodulin, and the Ca2+/calmodulin complex (CaM) stimulates eNOS, leading to NO production and guanylate cyclase activation. The relevance of this pathway to the action of oxytocin is demonstrated by the lack of PTx dependence, and the inhibitory effects of U73122, L-NAME and ODQ. But oxytocin also induces AKT phosphorylation (via an unknown PLC-dependent mechanism), which phosphorylates eNOS at the Ser1177 residue. This phosphorylation is blunted by the PI-3-kinase inhibitor LY294002, as is the promigratory effect of oxytocin. eNOS, endothelial nitric oxide synthase; L-NAME, Nω-nitro-L-arginine methyl ester hydrochloride; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; PLC, phospholipase C.
In conclusion, the promigratory and proinvasive effects of oxytocin shown here in HUVECs, together with the previously reported stimulatory effect on proliferation (Thibonnier et al., 1999), suggest that this hormone may contribute in vivo to the onset of angiogenesis, a process that requires the degradation of the extracellular matrix and the proliferation and migration of endothelial cells. Experiments specifically addressed to test other oxytocin angiogenic activities are underway.
Acknowledgments
We thank M Isipato, F Palazzotto and M Pozzi for their technical assistance. This study was supported by research grants from the Italian Association for Cancer Research (AIRC 2006) to BC, Fondazione Cariplo GRANT 2004/1419 to LV and BC, and Ministero Università e Ricerca (PRIN 2006) to LV.
Abbreviations
- eNOS
endothelial NOS
- GPCR
G-protein-coupled receptor
- HUVEC
human umbilical vein endothelial cell
- PI-3-K
phosphatidylinositol-3-kinase
- PLC
phospholipase C
- PTx
Pertussis toxin
- sGC
soluble guanylate cyclase
- TG-oxytocin
Thr4Gly7-oxytocin
- VEGF
vascular endothelial growth factor
Conflict of interest
The authors state no conflict of interest.
References
- Albini A, Benelli R, Noonan DM, Brigati C. The ‘chemoinvasion assay': a tool to study tumor and endothelial cell invasion of basement membranes. Int J Dev Biol. 2004;48:563–571. doi: 10.1387/ijdb.041822aa. [DOI] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Bussolati G, Cassoni P. The oxytocin/oxytocin receptor system: expect the unexpected. Endocrinology. 2001;142:1130–1136. doi: 10.1210/endo.142.4.8147. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Marrocco T, Bussolati B, Allia E, Munaron I, Sapino A, et al. Oxytocin induces proliferation and migration in immortalized human dermal microvascular endothelial cells and human breast tumor-derived endothelial cells. Mol Cancer Res. 2006;4:351–359. doi: 10.1158/1541-7786.MCR-06-0024. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Sapino A, Deaglio S, Bussolati B, Volante M, Munaron L, et al. Oxytocin is a growth factor for Kaposi's sarcoma cells: evidence of endocrine-immunological cross-talk. Cancer Res. 2002;62:2406–2413. [PubMed] [Google Scholar]
- Cassoni P, Sapino A, Marrocco T, Chini B, Bussolati G. Oxytocin and oxytocin receptors in cancer cells and proliferation. J Neuroendocrinol. 2004;16:362–364. doi: 10.1111/j.0953-8194.2004.01165.x. [DOI] [PubMed] [Google Scholar]
- Cattaneo MG, Pola S, Francescato P, Chillemi F, Vicentini LM. Human endostatin-derived synthetic peptides possess potent antiangiogenic properties in vitro and in vivo. Exp Cell Res. 2003;283:230–236. doi: 10.1016/s0014-4827(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Chini B, Manning M. Agonist selectivity in the oxytocin/vasopressin receptor family: new insights and challanges. Biochem Soc Trans. 2007;35:737–741. doi: 10.1042/BST0350737. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Dernbach E, Zeiher AM. Phosphorylation of the endothelial nitric oxide synthase at ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett. 2000;477:258–262. doi: 10.1016/s0014-5793(00)01657-4. [DOI] [PubMed] [Google Scholar]
- Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- Elands J, Barberis C, Jard S, Tribollet E, Dreifuss JJ, Bankowski K, et al. I-Labelled d(CH2)5[Tyr(Me)2, Thr4, Tyr-NH2(9)]OVT: a selective oxytocin receptor ligand. Eur J Pharmacol. 1988;147:197–207. doi: 10.1016/0014-2999(88)90778-9. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Guzzi F, Zanchetta D, Cassoni P, Guzzi V, Francolini M, Parenti M, et al. Localisation of the human oxytocin receptor in caveolin-1 enriched domains turns the receptor-mediated inhibition of cell growth into a proliferative response. Oncogene. 2002;21:1658–1667. doi: 10.1038/sj.onc.1205219. [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyendecker G, Kunz G, Noe M, Herbertz M, Mall G. Endometriosis: a dysfunction and disease of the archimetra. Hum Reprod Update. 1998;4:752–762. doi: 10.1093/humupd/4.5.752. [DOI] [PubMed] [Google Scholar]
- Moolenaar WH. Bioactive lysophospholipids and their G protein-coupled receptors. Exp Cell Res. 1999;253:230–238. doi: 10.1006/excr.1999.4702. [DOI] [PubMed] [Google Scholar]
- Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26:870–881. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- Morbidelli L, Donnini S, Ziche M. Role of nitric oxide in the modulation of angiogenesis. Curr Pharm Des. 2003;9:521–530. doi: 10.2174/1381612033391405. [DOI] [PubMed] [Google Scholar]
- Morita T, Shibata K, Kikkawa F, Kajiyama H, Ino K, Mizutani S. Oxytocin inhibits the progression of human ovarian carcinoma cells in vitro and in vivo. Int J Cancer. 2004;109:525–532. doi: 10.1002/ijc.20017. [DOI] [PubMed] [Google Scholar]
- Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- Phaneuf S, Carrasco MP, Europe-Finner GN, Hamilton CH, Lopez Bernal A. Multiple G proteins and phospholipase C isoforms in human myometrial cells: implication for oxytocin action. J Clin Endocrinol Metab. 1996;81:2098–2103. doi: 10.1210/jcem.81.6.8964834. [DOI] [PubMed] [Google Scholar]
- Reversi A, Rimoldi V, Marrocco T, Cassoni P, Bussolati G, Parenti M, et al. The oxytocin receptor antagonist atosiban inhibits cell growth via a ‘biased agonist' mechanism. J Biol Chem. 2005;280:16311–16318. doi: 10.1074/jbc.M409945200. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Grazul-Bilska AT, Redmer DA. Angiogenesis in the female reproductive organs: pathological implications. Int J Exp Pathol. 2002;83:151–163. doi: 10.1046/j.1365-2613.2002.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi V, Reversi A, Taverna E, Rosa P, Francolini M, Cassoni P, et al. Oxytocin receptor elicits different EGFR/MAPK activation patterns depending on its localization in caveolin-1 enriched domains. Oncogene. 2003;22:6054–6060. doi: 10.1038/sj.onc.1206612. [DOI] [PubMed] [Google Scholar]
- Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- Sanborn BM. Hormones and calcium: mechanisms controlling uterine smooth muscle contractile activity. The Litchfield Lecture. Exp Physiol. 2001;86:223–237. doi: 10.1113/eph8602179. [DOI] [PubMed] [Google Scholar]
- Serradeil-Le Gal C, Wagnon J, Garcia C, Lacour C, Gulraudou P, Christophe B, et al. Biochemical and pharmacological properties of SR49059, a new, potent, non peptide antagonist of rat and human vasopressin V1a receptors. J Clin Invest. 1993;92:224–231. doi: 10.1172/JCI116554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Steinwall M, Hansson S, Bossmar T, Larsson I, Pilka R, Akerlund M. Oxytocin mRNA content in the endometrium of non-pregnant women. BJOG. 2004;111:266–270. doi: 10.1111/j.1471-0528.2004.00049.x. [DOI] [PubMed] [Google Scholar]
- Strakova Z, Soloff MS. Coupling of oxytocin receptor to G proteins in rat myometrium during labor: Gi receptor interaction. Am J Physiol. 1997;272:E870–E876. doi: 10.1152/ajpendo.1997.272.5.E870. [DOI] [PubMed] [Google Scholar]
- Thelen M. Dancing to the tune of chemokines. Nat Immunol. 2001;2:129–134. doi: 10.1038/84224. [DOI] [PubMed] [Google Scholar]
- Thibonnier M, Conarty DM, Preston JA, Plesnicher CL, Dweik RA, Erzurum SC. Human vascular endothelial cells express oxytocin receptors. Endocrinology. 1999;140:1301–1309. doi: 10.1210/endo.140.3.6546. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Van Haastert PJ, Devreotes PN. Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol. 2004;5:626–634. doi: 10.1038/nrm1435. [DOI] [PubMed] [Google Scholar]