Abstract
Over recent years the role of biomarkers in anticancer drug development has expanded across a spectrum of applications ranging from research tool during early discovery to surrogate endpoint in the clinic. However, in Europe when biomarker measurements are performed on samples collected from subjects entered into clinical trials of new investigational agents, laboratories conducting these analyses become subject to the Clinical Trials Regulations. While these regulations are not specific in their requirements of research laboratories, quality assurance and in particular assay validation are essential. This review, therefore, focuses on a discussion of current thinking in biomarker assay validation. Five categories define the majority of biomarker assays from ‘absolute quantitation' to ‘categorical'. Validation must therefore take account of both the position of the biomarker in the spectrum towards clinical end point and the level of quantitation inherent in the methodology. Biomarker assay validation should be performed ideally in stages on ‘a fit for purpose' basis avoiding unnecessarily dogmatic adherence to rigid guidelines but with careful monitoring of progress at the end of each stage. These principles are illustrated with two specific examples: (a) absolute quantitation of protein biomarkers by mass spectrometry and (b) the M30 and M65 ELISA assays as surrogate end points of cell death.
Keywords: biomarker, method validation, clinical trials, anticancer drugs, mass spectrometry, M30 and M65 ELISAs
Importance of biomarkers in anticancer drug development
The importance of biomarkers in anticancer drug development today cannot be overemphasized (Lesko and Atkinson, 2001; Wagner, 2002; Verma and Srivastava, 2003; Kelloff et al., 2004). As an extension of our increased understanding of the molecular basis of cancer, anticancer drug development now focuses on targeting specific molecular alterations present in cancer cells (Collins and Workman, 2006; Kummar et al., 2006). In the clinic, biomarkers may thus enable selection of patients most likely to respond to molecularly targeted drugs, allow real-time monitoring of treatment efficacy or identify early signs of toxicity (Ludwig and Weinstein, 2005; Maruvada and Srivastava, 2006). Throughout the long and costly cycle of anticancer drug development, biomarkers are also seen as facilitating go/no go decision making during early discovery up to the point of preclinical evaluation (Kelloff and Sigman, 2005; Collins and Workman, 2006; Sarker and Workman, 2007).
Although the number of publications on experimental biomarkers in cancer extends into the several thousand each year, only a small number of these will ever achieve regulatory (US Food and Drug Administration (FDA)) approval as surrogate clinical end points (Ludwig and Weinstein, 2005). Method validation constitutes a critical component in biomarkers research, and it is acknowledged that a biomarker can sometimes fail in the clinic not because of the underlying scientific rationale but rather from poor assay choice and lack of robust validation (Pepe et al., 2001; Barker, 2003; Bast et al., 2005; Wagner et al., 2007). This review therefore seeks to focus on the issue of biomarker assay validation during anticancer drug development.
Biomarker definitions
The US National Institute of Health (NIH) sponsored Biomarkers Definitions working group has published the following definitions: ‘Biological Marker—Biomarker—a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacologic responses to a therapeutic agent' (NIH, 2001). Whereas, a Clinical End point was defined as ‘a characteristic or variable that reflects how a patient feels, functions, or survives' while a Surrogate End point was defined as ‘a biomarker that is intended to substitute for a clinical end point'. The evidentiary process of proving a linkage between the biomarker and a clinical end point was termed ‘evaluation' in preference to validation (NIH, 2001). More recently, evaluation has been replaced with Qualification, which has become accepted terminology (Wagner, 2002).
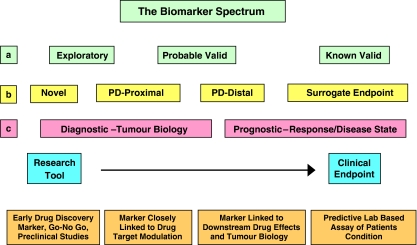
Biomarker definitions have evolved to encompass a spectrum of activities depending on the perspective of the stakeholder (Figure 1). Within the pharmaceutical industry, ‘novel' biomarkers are envisaged as playing a vital role in candidate selection during drug discovery (including preclinical studies), whereas pharmacodynamic (PD) biomarkers are applied in early clinical trials to evaluate target modulation, either proximal or distal to the proposed drug target, but fall short of being surrogate end points (Figure 1; Kelloff and Sigman, 2005; Lee et al., 2005). In cancer medicine, biomarkers may be seen as being either diagnostic of tumour biology or prognostic of disease or therapeutic outcome (see Figure 1; Ludwig and Weinstein, 2005; Basil et al., 2006). Finally in the regulatory compliance spectrum, a biomarker is envisaged as passing through three evidentiary stages towards full acceptance: exploratory, probable valid and known valid (Goodsaid and Frueh, 2007). The importance of all these above definitions, from a method validation perspective, is that the further along the spectrum towards a clinical end point the biomarker is positioned the greater the degree of thoroughness necessary to validate the biomarker assay (Lee et al., 2007).
Figure 1.
The Biomarkers spectrum from the perspective of different stakeholders. (a) The US Food and Drug Administration envisages three evidentiary stages towards biomarker approval (Goodsaid and Frueh, 2007). (b) Within the pharmaceutical industry biomarkers are utilized throughout the whole drug discovery process from compound selection to clinical trials (Lee et al., 2005). (c) In cancer medicine, biomarkers may be seen as being either diagnostic of tumour biology, or prognostic of disease or therapeutic outcome (Ludwig and Weinstein, 2005).
Current regulatory requirements for biomarker assay validation
Extensive supporting evidence to qualify a biomarker and validate the biomarker assay are not normally required during early phase drug discovery and preclinical drug evaluation (Lee et al., 2006, 2007; Workman, 2006). Nonetheless, since the ultimate aim of most biomarker research is to transfer the assay into the clinic, it is advisable to plan ahead as early as possible during method development. Thus, attempts should be made to assemble all the key components required in a validated method such as a consistent supply of reagents, certificated standard of the target molecule to use as a calibration standard and a control matrix free from the target molecule that replicates closely the clinical specimens under investigation (Miller et al., 2001). A lack of such resources can result in a compromised analytical technique, and even lead to failure in biomarker qualification (Bast et al., 2005). However, in Europe when biomarker measurements are performed on samples collected from subjects entered into clinical trials of new investigational agents, laboratories conducting these analyses are subject to the Clinical Trials Regulations (Fontaine and Rosengren, 2001; HMSO, 2004, 2006). Though these regulations are focused on the implementation of Good Clinical Practice (GCP) during the clinical governance of a trial, it is clear that they extend to the research laboratory. Thus, the European directive (2001/20/EC) defines a ‘clinical trial' as: ‘any investigation in human subjects intended to discover or verify the clinical, pharmacological and/or other PD effects of one or more investigational medicinal product(s)' (Fontaine and Rosengren, 2001). The directive also highlights the need to subject all trial-related information and documents, including those generated by the research laboratory, to inspection to confirm their accuracy. Implementation of the EC directive in the United Kingdom occurred on May 2004 as UK Statutory Instruments (SI) 1031, and identified the Medicines and Healthcare Regulatory Agency as the competent authority responsible for carrying out inspections (HMSO, 2004). Failure to comply with these regulations is an offence. Despite this, the clinical trials regulations make few specific references to laboratories and are somewhat vague in terms of what is expected, although it is clear that quality assurance (QA) is a requirement.
As a response to the clear lack of direction in this field, the British Association for Research into Quality Assurance (BARQA) published a detailed quality system termed Good Clinical Laboratory Practice (GCLP) just prior to publication of the UK SI 1031 (Stiles et al., 2003). Their hopes were that this QA system would become widely adopted by relevant laboratories (Stiles, 2004). To a large extent this has been the case within the UK translational cancer research community, while Cancer Research UK (CR-UK) adopts GCLP as one of its benchmark standards, when conducting QA audits of translational cancer science laboratories. In essence GCLP is very closely related to Good Laboratory Practice (GLP), but takes accounts of the context and terminology employed in clinical trials. GCLP covers every aspect of trial sample analysis from a formalized contractual agreement with the study sponsor(s), through staff training, facilities being fit for purpose, apparatus qualification, certificated reagents, method validation, study plan preparation, sample tracking, conduct of work, data capture and storage, report writing and finally archiving of study documents. All stages of the system are governed by standard operating procedures (SOPs) and all aspects of the system are subject to QA audits.
While there appears to be no requirement for laboratories conducting biomarker analyses to comply to a full QA system like GCLP in the United States, several key publications have recommended adopting significant elements of a quality system, such as patient sample tracking, SOPs, analytical plans, facilities fit for purpose, study reports, compliance with FDA regulations on electronic records and signatures (FDA, 1997) and certificates of analysis for standards (James and Hill, 2007; Lee et al., 2007).
Established method validation guidance
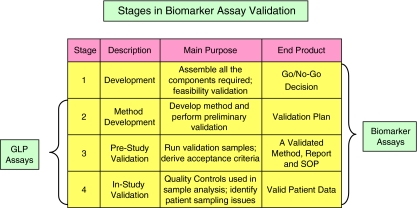
Bioanalytical method validation involves a systemic evaluation of all the processes required to demonstrate that a particular technique is reliable for its intended purpose (Shah, 2007). Ideally, validation is conducted in three phases each with its own predefined goals (Figure 2). The first stage is method development, where the aim is to perform feasibility studies, assess reagent availability and so on, resulting in the construction of the validation plan. This phase is then followed by pre-study method validation, where the validation plan is put into effect and is conducted normally utilizing validation samples (VS, but referred to as quality controls (QCs) during patient sample analysis). VS and QC are samples employing a test matrix, either patient-derived or a suitable surrogate, containing a known concentration (nominal) of analyte that are treated as unknowns in an assay. The threefold goal of this phase is to (1) produce a body of data that proves that the method meets acceptable standards of performance, (2) formalize these data into an analytical report and (3) draft a method SOP that is then taken forward to patient sample analysis. Finally, during patient sample analysis, QCs are incorporated to confirm that the method continues to perform consistently within specifications, thus allowing patient-derived data to be confidently accepted as valid. Performance parameters that are studied during the second stage of validation of bioanalytical methods would normally include: selectivity, sensitivity, calibration response, choice of QC samples, analyte recovery, precision, accuracy and reproducibility (Shah et al., 1991, 2000). For a fuller definition of these terms see Appendix I. Bioanalytical method validation also requires a systematic study of analyte stability in calibration standards, QCs and study samples (Nowatzke and Wood, 2007).
Figure 2.
The stages of biomarker assay validation. Three stages are proposed for bioanalytical techniques referred to as Good Laboratory Practice (GLP) assays, each with a predefined purpose and goal. Biomarker assays require an additional phase where feasibility studies are conducted to determine whether or not to proceed with the particular technique.
Internationally recognized standards for the various different parameters of bioanalytical method performance have been established (Shah et al., 1991, 2000; Peters et al., 2007). These were devised primarily by the pharmaceutical industry for small molecule analysis using the techniques of liquid chromatography (LC) or LC–mass spectrometry (LC–MS), but have nevertheless been endorsed by several regulatory authorities and national agencies (FDA, 2001a). Selectivity (lack of interference) is normally demonstrated in six independent specimens of drug free matrix. A calibration curve is acceptable if 75% or a minimum of six different standard concentrations fall within ±15% of their nominal values except at the lower limit of quantitation (LLOQ) when ±20% is acceptable. Precision (coefficient of variation (CV)) and accuracy (% bias from nominal concentration) in repeat analyses of VS are expected to vary by less than ±15%. During in-study patient sample analysis QCs are employed at three different concentrations in duplicate. The analytical run is accepted as valid when at least 67% (4/6) of the QCs fall within 15% of their nominal values (the 4:6:15 rule; Shah et al., 1991, 2000; Bansal and DeStefano, 2007).
These stringent standards have also being applied to pharmacokinetic (PK) studies of macromolecules, particularly involving the technique of ELISA (Findlay et al., 2000; DeSilva et al., 2003; Smolec et al., 2005). Here, greater leeway is granted within the 4–6 acceptance rule at either 25 or 30%, acknowledging that the bioanalysis of macromolecules carries a degree greater inherent variability compared to the bioanalysis of small molecules (Miller et al., 2001; DeSilva et al., 2003; Smolec et al., 2005).
Critical issues in biomarker assay validation
The principles of bioanalytical method validation developed for drug analysis during toxicokinetic studies using LC or LC–MS have been referred to by others as GLP method validation (Korfmacher, 2005; Lee et al., 2005, 2006). This is to distinguish such validation from that applied in routine clinical chemistry and diagnostic testing laboratories (FDA, 2001b), where emphasis is placed on QC and the adoption of global standards for assay acceptance (Westgard et al., 1981).
In October 2003, a workshop was held in Salt Lake City, Utah, which was cosponsored by the American Association of Pharmaceutical Scientists (AAPS) and the US Clinical Ligand Society (CLAS) to address the unresolved issue of validation of biomarker assays in support of drug development (Lee et al., 2005). At this meeting it was concluded that biomarker methods should not be classified as ‘GLP' assays, nor should they be validated by the same guiding principles developed for drug analysis by LC–MS. Table 1 summarizes many of the reasons for this decision. Biomarker assays span diverse methodologies, ranging from the relatively low technology end such as immunohistochemistry (IHC), through electrophoresis, reverse transcription–PCR to ELISA, to the high technology end including platforms for genomics (gene chip), proteomics (SELDI TOF) and multiplex ligand-binding assays (Jones et al., 2003; Johann et al., 2004; Seligson, 2005; Scholler et al., 2006).
Table 1.
Comparison between GLP and biomarker assay validation parameters
| Parameter | GLP assay | Biomarker assay |
|---|---|---|
| Specificity/selectivity | Drugs as xenobiotics are not present in study sample matrix and samples are almost always subjected to clean-up and analyte recovery | Biomarkers as endogenous macromolecules are present in sample matrix. Samples not subject to clean up; thus detection occurs in a complex matrix resulting often in specificity issues |
| Sensitivity | LC–MS highly sensitive | Many biomarker assays lack sensitivity |
| Calibration standard | Certified chemical standards readily available | Often composition of the analyte not known or fully characterized. Thus certified standard not available |
| Calibration model | Mostly linear, extending over several orders of magnitude | Many biomarker assays have limited dynamic range and baseline patient values not known |
| Quality controls | Certified standard and blank patient sample matrix available | Adequate supplies of blank matrix and certified standard problematic—surrogate matrices utilized |
| Precision/accuracy/reproducibility | Robust technology subjected to internationally recognized acceptance criteria | Inherently variable because of methodological and biological issues. No recognized ‘acceptance criteria' |
| Stability | Drug standards, QCs and sample analyte stability often very good | Stability of biological standards and biological matrices analyte often very poor |
| Parallelism | Studied due to of use of surrogate standards and matrices for calibration purposes | |
| Dilution linearity | Studied due to complex analyte and matrix | |
| Reagents | Method dependent on the performance of reagents that are themselves derived from biologic sources where supply, quality and stability issues become important |
Abbreviations: GLP, Good Laboratory Practice; LC–MS, liquid chromatography–mass spectrometry.
When surveying the differences between a typical GLP assay and a typical biomarker assay, certain critical issues emerge in biomarker method validation (Table 1). As biomarkers are invariably endogenous substances, an analyte-free matrix either to perform specificity studies on or to be used as a resource to construct a calibration curve is often not available. Perhaps, more common is the lack of availability of the target biomarker molecule to act as a certified calibration standard (Miller et al., 2001). Biomarker analytical methods frequently lack sensitivity and dynamic range, are labour-intensive and prone to variability. However, they are expected to detect unambiguously the target molecule in a complex biologic milieu, often without sample preparation. Biomarker analysis is also highly dependent on the integrity of reagents such as antibodies, which are themselves derived from biologic sources and thus are subject to their own problems of supply, QC and stability. In many biomarker assay scenarios, researchers have to resort to the use of a noncertified standard, perhaps a recombinant protein, and a variety of surrogate matrices, in order to construct a calibration curve. Thus, parallelism studies need to be performed where the response of the assay to a range of calibration standard concentrations made up in the surrogate matrix is compared to that of a series of dilutions of patient samples (Smolec et al., 2005). Dilution linearity can also be problematic, as antibody and ligand-binding affinities can vary significantly in different media.
The goal of biomarker assay development and validation thus becomes to ‘develop a valid assay' rather than to ‘validate a developed method', that is that the biomarker assay should be demonstrably ‘fit for purpose' (Smith and Sittampalam, 1998). In many respects the key to successful biomarker method validation resides in making the right choice of assay and posing the right questions at the onset. What is the nature of the biologic end point under investigation: is it target detection; proof of principle of drug action or PD effects such as enzyme inhibition or subtle changes in protein phosphorylation state? Is the biomarker assay that is chosen realistically and reliably capable of measuring the above biologic end point?
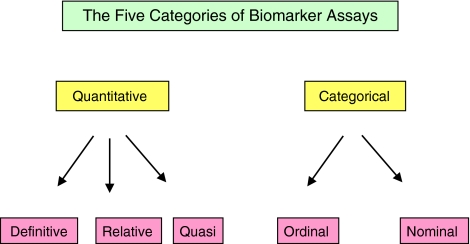
Biomarker assay validation, like bioanalytical method validation, should be conducted ideally in several stages (Lee et al., 2005, 2006), but with the addition of a new phase entitled ‘development' (Figure 2). During this preliminary stage issues such as adequate supply of reagents; existence of a certified reference standard for the biomarker and the availability of an analyte test matrix should be fully addressed prior to taking the assay forward to formal validation experiments. To aid in the formulation of a method validation plan, a biomarker assay can normally be placed into one of the five functional categories (see Figure 3), each requiring a distinct level of validation (see Table 2). The following definitions of the five classes of biomarker assays have been summarized from those proposed at the AAPS/CLAS workshop (Lee et al., 2005). A definitive quantitative assay makes uses of calibrators and a regression model to calculate absolute quantitative values for unknowns. The reference standard is well defined and representative of the biomarker. A relative quantitative assay uses a response–concentration calibration with reference standards that are not fully representative of the biomarker. Precision can be validated but accuracy only estimated. A quasi-quantitative assay (possesses certain attributes) does not employ a calibration standard, but has a continuous response that is expressed in terms of a characteristic of the test sample. Precision can be validated, but not accuracy. Together these three categories constitute the bulk of quantitative techniques. Two categories of qualitative (categorical) assays were described: ordinal refers to an assay that relies on discrete scoring scales like those often used for IHC while nominal pertains to a yes/no situation; for example the presence or absence of a gene product (Lee et al., 2005, 2006). In addition to assay functionality, it is also important to ensure that the degree of validation performed reflects the level of importance of the biomarker itself. In the United Kingdom, the PD/PK Technologies Advisory Committee of CR-UK adopts a policy of requesting validation of PD assays based on the priority given to the biomarker in terms of its proximity to the trial end point, either having primary, secondary or tertiary importance (Cummings et al., 2005).
Figure 3.
The five categories of Biomarker assays. For a more comprehensive definition of each category, see main body of text.
Table 2.
Recommended biomarker assay validation parameters
| Performance characteristic | Definitive quantitative | Relative quantitative | Quasi-quantitative | Qualitative |
|---|---|---|---|---|
| Accuracy | ✓ | ✓ | ||
| Precision | ✓ | ✓ | ✓ | |
| Sensitivity | ✓ | ✓ | ✓ | ✓ |
| LLOQ | LLOQ | |||
| Specificity | ✓ | ✓ | ✓ | ✓ |
| Dilution linearity | ✓ | ✓ | ||
| Parallelism | ✓ | ✓ | ||
| Assay range | ✓ | ✓ | ✓ | |
| LLOQ-ULOQ | LLOQ-ULOQ | |||
| Reagent stability | ✓ | ✓ | ||
| Sample stability | ✓ | ✓ | ✓ | ✓ |
Table 2 summarizes the AAPS/CLAS consensus opinion on which validation parameters should apply to each category of biomarker assay. Qualitative assays only require demonstration that they are sufficiently sensitive and specific to detect the target analyte. In addition to sensitivity and specificity, precision and reproducibility should be investigated with quasi-quantitative assays. Relative and definitive quantitative methods additionally require an evaluation of accuracy, and especially in the case of relative quantitation where the calibration curve may utilize either a noncertified standard or surrogate matrix or both, studies on parallelism and dilution linearity become priorities. During pre-study validation of these two latter classes of assays at least five different concentrations of VS should be analysed in duplicate on at least six different runs. Although, this is greater than the three VS concentrations required in GLP assays (Shah et al., 1991, 2000), because quantitative biomarker assays often exhibit nonlinear calibration curves more VS are required (Smolec et al., 2005; Lee et al., 2006). Acceptance criteria for precision and accuracy should not be dogmatically set at a fixed value, as in GLP assays, but evaluated on a case per case basis, with ±25% acting as default value (±30% at the LLOQ). Likewise, a similar attitude should be adopted in determining acceptance limits for QCs during patient sample analysis, either in terms of a 4–6-X rule or through adoption of confidence intervals. Since the target molecule is often present in pre-dose samples or in the QC matrix, limitations are often placed on LLOQ. Also, as part of early stage biomarker assay validation/qualification, it would be beneficial to acquire samples from cancer patients to derive target concentration ranges and characterize normal biologic variations in the marker prior to commencement of clinical investigations (Cummings et al., 2006). Sample integrity should be carefully assessed during method validation, including studies on patient specimen stability during collection, storage and analysis.
Specific examples of biomarker assay validation
While this review has concentrated on the general principles of validation of biomarker methods, to illustrate some of these points and issues in more detail two specific examples have been chosen. Mass spectrometry has been chosen as an example of a definitive quantitative method. As an example of a relative quantitative technique ELISA will be discussed and here the focus will be on two specific assays that have been extensively studied in our laboratory as PD biomarkers of drug induced cell death, the M30 and the M65 ELISAs (Cummings et al., 2005, 2006, 2007).
Mass spectrometry as an absolute quantitative technique in biomarker research
MS it now well established as the technology platform of choice in the field of proteomics (Zhang et al., 2007). Such studies, although immensely complex, tend to operate in the realm of ‘browsing mode' yielding data described as ‘survey' by nature (Aebersold and Goodlett, 2001; Petricoin et al., 2002; Simpkins et al., 2005; Engwegen et al., 2006). In this mode, a number of different strategies have been employed to introduce a quantitative element into proteomics such as metabolic pre-labelling (Gu et al., 2002), isotope-coded affinity tags (iCATs; Gygi et al., 1999), incorporation of 18O into tryptic digests (Yao et al., 2003) and isobaric tags for relative and absolute quantitation (iTRAQ; Ross et al., 2004). MS determination of the proteome can span the entire range of biomarker assay definitions from a yes/no nominal result through to absolute quantitation. However, many manifestations of the technique cannot be applied directly to the analysis of samples collected from patients, while often relative quantitation actually means a comparison between a control and a test sample (Bronstrup, 2004).
At least six different MS techniques have been categorized as quantitative in proteomics (Unwin et al., 2006). Perhaps, one of the most relevant and promising to the field of biomarker research and anticancer drug development is the internal standard technique. This relies on adding to samples an isotopically labelled but otherwise identical peptide to achieve absolute quantitation of a target peptide/protein by a method known as AQUA (Stemmann et al., 2001). The advantage of adding a defined amount of an internal standard over pre-labelling of proteins is that it is applicable in clinical investigations, whereas pre-labelling is restricted to cells in culture (Bronstrup, 2004). Post-labelling of samples with iCAT or iTRAQ can also be problematic, since it depends on efficient and reproducible chemical derivatization for accurate quantitation (Unwin et al., 2005). However, a limitation with the AQUA approach is that the internal standard peptide is not normally added until samples have undergone clean up, fractionation and proteolytic digestion and it may not control for losses that occur prior to the production of peptides (Fenselau, 2007). Using an isotopically labelled protein internal standard is one way round such a problem. AQUA also has an important role in quantitation of post-translational modifications—here in vitro studies have shown that the technique can detect quantitative changes in the phosphorylation status of proteins during different phases of the cell cycle (Gerber et al., 2003; Kirkpatrick et al., 2005).
The critical importance of sample preparation in biomarker analysis by AQUA and other quantitative MS approaches should not be underestimated. This is exemplified in the study of Petricoin and co-workers, where SELDI-TOF was utilized to identify novel biomarkers in ovarian cancer (Petricoin et al., 2002). However, others failed to confirm these findings, and reanalysis of the original data set suggested that sample handling and processing variables may have been responsible for the differences observed between diseased and healthy individuals (Baggerly et al., 2004, 2005). Since this study, many laboratories have concentrated on methods for sample handling and data analysis to allow for the generation of reproducible and reliable profiling (Poon, 2007).
Serum concentrations of proteins can vary by a factor of 108 to 1010 between high abundance proteins such as albumin and classic tumour markers such as prostate-specific antigen (PSA) and carcinoembryonic antigen (CEA), and perhaps sensitivity remains one of the greatest challenges open to absolute quantitation of protein biomarkers by MS (van der Merwe et al., 2007). Nonetheless, in a study using a visible iCAT method, where a 14C-label was incorporated into the linker, a detection limit of 66 fmol of group V phospholipase A2 per 100 μg of cell lysate was reported, which was one order of magnitude superior to that achieved by western blot analysis (Bottari et al., 2004). By adopting an LC/MS/ion trap technique, four intermediate abundance serum proteins have been quantitated by AQUA: coagulation factor V, adiponectin, C-reactive protein and thyroxin-binding globulin at levels of 9.2, 110, 120 and 246 pmol ml−1 (approximately 0.5–10 μg ml−1) with CVs for 12 repeat measurements of 17, 25, 24 and 14% respectively (Lin et al., 2006).
In a seminal study to determine the performance characteristics of AQUA in the quantitation of PSA, method validation was conducted on a fit for purpose basis (Barnidge et al., 2004). Serum was spiked with five different concentrations of a PSA protein standard ranging from 70 pmol l−1 to 6.5 μmol l−1 (2–184 μg ml−1) to construct a calibration curve. The internal standard consisted of IVGGWECEK, a sequence identical to the N-terminal tryptic fragment of PSA but with each glycine containing two 13C atoms and one 15N atom. After digestion and AQUA, the values determined for the five different concentrations of PSA yielded a calibration curve with a regression correlation coefficient (r2) of 0.971. Recovery of the calibrators ranged from 70 to 85% with a mean run-to-run CV of 13% and a mean within-run CV of 5.7%. However, at the lowest standard concentration, 2 μg ml−1, recovery become more variable at >25%. These results demonstrated that many of the key parameters of a quantitative assay can be met by AQUA and the internal standard method, although in a control situation where plasma samples were spiked with high levels of the target protein. However, to put these results in context, the endogenous level of PSA is reported to be in the region of 1 ng ml−1, 3–4 orders of magnitude below that achieved by AQUA (Bronstrup, 2004), and it is conceded by many workers in the field of proteomics that immunoassays such as ELISA, still offer far greater sensitivity, reproducibility and dynamic range (Barnidge et al., 2004; van der Merwe et al., 2007).
M30 and M65 ELISA assays as surrogate serum/plasma biomarkers of cell death
Although considered the archetypical quantitative biomarker assay, offering the analyst a range of concentration options, spanning a full repertoire of 10 orders of magnitude (van der Merwe et al., 2007), many commercially available, single analyte, sandwich, ELISA assays fall into the category of relative quantitation. This is because many are calibrated with recombinant proteins or peptide standards which are reconstituted in a surrogate matrix, as is the case with many cytokine assays (Lee et al., 2005).
M30 and M65 are (relatively) newly described sandwich ELISA assays that determine in either plasma or serum different circulating forms of the protein cytokeratin 18 (CK18) and are proposed to be surrogate biomarkers of different mechanisms of cell death (Biven et al., 2003; Kramer et al., 2004). Under normal physiological conditions cytokeratins are complexed in intermediate filaments of epithelial cells and remain insoluble (Fuchs and Weber, 1994). Proliferating cancer cells also contain a substantial pool of soluble cytokeratins (CK 8, 18 and 19), which can increase in response to stress (Ditzel et al., 2002; Schutte et al., 2004). Keratins have been recognized as tumour markers in the diagnosis of cancer for over 20 years (Weber et al., 1984; Sundstrom and Stigbrand, 1994). CK18 is closely associated with the tumour marker tissue polypeptide-specific antigen (Einarsson and Barak, 1997; Barak et al., 2004). During necrosis, mobilization of CK18 into the soluble pool occurs through remodelling of the intermediate filaments (Strnad et al., 2002), whereas during apoptosis intermediate filament proteins (including CK18) are targeted for rapid breakdown by activated caspases 3, 7 and 9 to facilitate the formation of apoptotic bodies (Kramer et al., 2004). Nonetheless, the fragments of CK18 produced by proteolysis are stable and persist as large aggregates eventually appearing in the circulation of cancer patients (Ku et al., 1997). The mechanisms by which intact CK18 and its caspase-cleaved fragments are released into the circulation remain poorly understood (Linder et al., 2004).
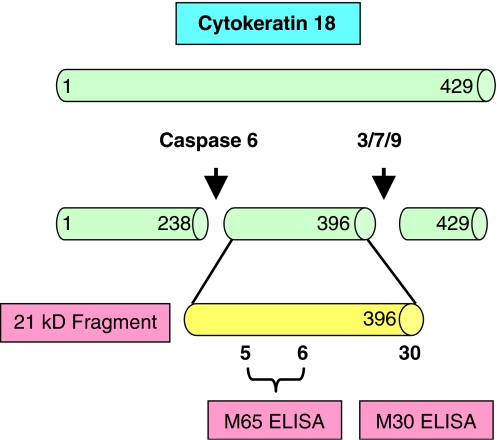
The M30 ELISA assay utilizes the M5 antibody as a catcher and the M30 antibody to detect CK18 fragments that contain a neo-epitope (NE) at positions 387–396 generated by the action of caspases 3, 7 and 9 activated during the early stages of apoptosis (Figure 4; Leers et al., 1999; Schutte et al., 2004). Thus, the M30 ELISA is proposed as a specific assay of apoptosis and immunological staining with the M30 (Cytodeath) antibody has been shown to correlate with other apoptosis assays such as TUNEL, ISEL (Carr, 2000) and active caspase 3 (Duan et al., 2003). M65 also detects cleaved fragments (Kramer et al., 2004), however, it uses a different detection antibody from M30 (namely M5) that does not distinguish between the full-length protein and its fragments (Figure 4). Thus, M65 theoretically measures both caspase cleavage (apoptosis) and cellular release of intact CK18 (necrosis).
Figure 4.
Schematic representation of the cytokeratin 18 (CK18) epitope map targeted by the antibodies used in the M30 and M65 sandwich ELISA assays. In the case of M65 ELISA the M6 antibody acts as the catcher and M5 as detection antibody. For the M30 ELISA assay M5 is the catcher and HRP conjugated M30 the detection antibody. Proposed caspase cleavage sites of CK18 are also indicated.
Both M30 and M65 assays have now been applied extensively in clinical trials as PD biomarkers of cell death induced by a variety of different cancer chemotherapeutic agents in a spectrum of different disease types (Biven et al., 2003; Ueno et al., 2003; Kramer et al., 2004, 2006; Demiray et al., 2006; Ulukaya et al., 2007). In some reports, the M30 assay has been claimed to be both predictive of drug response (Demiray et al., 2006) and prognostic of survival (Ulukaya et al., 2007). These latter studies were, however, only conducted in small populations of patients and can only be considered as preliminary observations. The two ELISAs have also been utilized as markers of host tissue toxicity in a number of different clinical conditions including trauma, sepsis (Roth et al., 2004); chronic liver disease (Yagmur et al., 2007); hepatitis C (Bantel et al., 2004; Kronenberger et al., 2005) and in response to liver transplantation (Baskin-Bey et al., 2007).
Our laboratory initially focused on method validation and early stage biomarker qualification prior to embarking on large-scale clinical trials (Cummings et al., 2005, 2006, 2007). Parameters studied included calibration response, within-day and between-day precision and accuracy, kit-to-kit QC and sample stability. A pivotal element in these studies was the use of in-house generated QCs that were independent of the kit reagents (Cummings et al., 2005, 2006).
Using a series of nine different dilutions of the independent QC, the calibration curve for the M30 ELISA assay was demonstrated to follow a sigmoid curve with a value of r2 equalling 0.997 and a plateau phase at antigen concentrations starting at 1000 U l−1, technically the upper limit of the calibration curve supplied by the manufacturers. Typical within-day precision using eight replicate independent QCs was 3.6%. Analyses performed on eight separate days over a 3-month period yielded a between-day precision of 2.4%. These data are in addition to values for both precision and accuracy generated using the supplied in kit QCs (Cummings et al., 2005). Kit-to-kit variations in the concentration of antigen determined in independent QCs ranged between 1.5 and 4.3%, well within the manufacturer's specifications of 10% (www.peviva.com).
By contrast, the M65 assay produced a linear calibration curve over the concentration range of 125–2000 U l−1, with a mean r2 value of 0.996±0.003 (n=27; Cummings et al., 2006). The linear response is believed to be due to the high-affinity constants (10−13 M) of the two mouse monoclonal antibodies utilized in this assay (M6 and M5; Kramer et al., 2004) and the subsequent faster reaction kinetics (personal communication, Peter Björklund, PEVIVA). Again utilizing in-house derived independent QCs, mean kit-to-kit variation ranged from 4.8 to 5.3%. M65 ELISA also proved to be highly reproducible with precision and accuracy normally varying by <5% when evaluated using the in kit supplied QCs.
By strict definition, the M30 and M65 ELISAs only provide relative quantitation (Lee et al., 2005, 2006), despite the fact that the kits include a seven-point calibration curve and QCs at two different concentrations (www.peviva.com). This is because both assays are calibrated with a 21-amino-acid fragment of CK18, (Kramer et al., 2004) prepared in a surrogate matrix. Although evidence has been presented that the M30 assay does indeed detect the 21-kDa-caspase-cleaved fragment of CK18 in the serum of patients with liver injury (Figure 4; Bantel et al., 2004), recent studies have shown that the natural antigen for these assays is probably a more complex heteromeric cytokeratin complex rather than a single peptide/protein moiety. Harnessing a combination of fractionation of breast cancer patient serum by gel permeation column chromatography and analysis of fractions with several different cytokeratin antibodies, a significant portion of antigenicity was shown to reside in a 50–100 kDa fraction, which also appeared to contain additional cytokeratins including CK7, CK8 and CK19 (Olofsson et al., 2007). This was in contrast to the release of antigen from cancer cells induced into apoptosis in culture, where the predominant species was a 13 kDa fragment. The high molecular weight heteromeric complexes were demonstrated to be resistant to further caspase cleavage and were concluded to be stable in serum. Cytokeratins are well known to favour heteromeric complexes to enhance their stability (Yamada et al., 2002).
The specificity of the M30 antibody has been validated primarily by IHC using the M30 Cytodeath assay, where it has been claimed to be a more reliable indicator of apoptosis than TUNEL and ISEL (Carr, 2000). Again in the IHC format M30 has been extensively applied during many clinical studies, where it has proved successful in the detection of apoptosis in human tumours (Kadyrov et al., 2001; Leers et al., 2002; Rupa et al., 2003). However, few definitive studies have been performed on the specificity of the ELISA assays to detect their respective antigens, and this is especially important in the light of the complex nature of the antigen, which is likely to vary considerably from patient to patient. We have observed that dilution of patient samples, whether plasma or serum with a variety of diluents, results in nonlinearity and artefactually high values (unpublished observations). Dilutions of samples in antigen-free control serum or plasma is now recommended by the manufacturers to preserve linearity.
The dynamic range of the calibration curve for most ELISAs is normally limited to less than two orders of magnitude and the M30 and M65 are no exceptions (Cummings et al., 2005). As biomarkers of cell death, the M30 and M65 assays appear perfectly capable of detecting in plasma or serum drug-induced cell death emanating from the tumour, as numerous clinical studies have now demonstrated (Biven et al., 2003; Ueno et al., 2003; Kramer et al., 2004, 2006; Demiray et al., 2006; Ulukaya et al., 2007). It has been estimated that apoptosis in 108 cancer cells in patients would be more than sufficient to result in a doubling in the circulating signal above normal baseline levels (Biven et al., 2003). By comparison, to detect the caspase-cleaved 21 kDa fragment of CK18 in patient serum by western blot after immunoprecipitation, a 3-ml sample was required whereas the ELISA assays require only 25 μl of sample (Bantel et al., 2004).
Conclusion
This review has focused on the general principles of method validation for assays utilised as PD biomarkers in cancer research. While, the discussion has tended to focus on established technologies (such as ELISA and MS), it is our belief that these principles can and must be applied to new and emerging technologies, such as multiplex ELISA platforms, regardless of the format (Liu et al., 2005; Lee et al., 2006).
Acknowledgments
We thank Professor Andrew Hughes, School of Cancer and Imaging Sciences, University of Manchester, UK, for critical reading of this article.
Abbreviations
- AAPS
American Association of Pharmaceutical Scientists
- BARQA
British Association for Research into Quality Assurance
- CK18
cytokeratin 18
- CLAS
US Clinical Ligand Society
- CR-UK
Cancer Research UK
- CV
coefficient of variation
- FDA
US Food and Drug Administration
- GCLP
Good Clinical Laboratory Practice
- GCP
Good Clinical Practice
- GLP
Good Laboratory Practice
- iCAT
isotope-coded affinity tag
- ICH
International Conference on Harmonisation
- IHC
immunohistochemistry
- iTRAQ
isobaric tags for relative and absolute quantitation
- LC
liquid chromatography
- LC–MS
liquid chromatography–mass spectrometry
- LLOQ
lower limit of quantitation
- NIH
US National Institute of Health
- PD
pharmacodynamic
- PK
pharmacokinetic
- PSA
prostate-specific antigen
- QA
quality assurance
- QC
quality control
- SI
UK Statutory Instruments
- SOP
standard operating procedure
- ULOQ
upper limit of quantitation
- VS
validation standard
Appendix I
Glossary of terms used in analytical method validation
Selectivity: The extent to which a bioanalytical method can measure particular analyte(s) in a complex mixture without interference from other components of the mixture.
Specificity: The ability to unequivocally measure the analyte in the presence of other components that may be expected to be present in the biological specimen, including impurities, metabolites and endogenous matrix components.
Limit of detection: The lowest concentration of analyte for which the response can be reliably distinguished from background noise.
Lower limit of quantitation (International Conference on Harmonisation (ICH)): The lowest concentration (amount) of analyte in a test sample that can be determined quantitatively with suitable accuracy (mean bias) and precision.
Calibration curve: A functional relationship between the analyte concentration in the standards (calibrators) and the measured response. The calibration curve is used to estimate the analyte concentration in test samples by dose interpolation.
Calibration standards: Samples having a known concentration of analyte that are used in an assay to gauge the relationship between system responses (for example, absorbance units) concentrations of an analyte.
Quality control (QC) samples: Pre-study validation and in-study samples having a known concentration (nominal) of analyte that are treated as unknowns in an assay. During pre-study validation, QC samples are used to generate information to demonstrate the method is suitable for its intended purpose. During in-study runs, QC values are used as the basis for accepting and rejecting bioanalytical method batches.
Accuracy (ICH): The closeness of agreement between the value that is accepted either as a conventional true value or an accepted reference value and the value found experimentally. This is sometimes termed trueness.
Precision (ICH): The closeness of agreement (degree of scatter) between a series of measurements obtained from multiple sampling of the same homogeneous sample under the prescribed conditions. Precision may be considered at three levels: repeatability, intermediate precision and reproducibility.
Intermediate precision (ICH): Precision of repeated measurements within-laboratories taking into account all relevant sources of variation affecting the results (for example, day, analyst or batch). Intermediate precision is also referred to as inter-batch, inter-assay and inter-run precision.
Repeatability (ICH): The precision under the same operating conditions over a short interval of time. Repeatability is also termed as intra-batch or intra-run precision.
Reproducibility (ICH): Precision of repeated measurements between laboratories and is termed inter-laboratory precision. Usually applies to collaborative studies that involve the standardization of a bioanalytical method across multiple laboratories.
Dilution linearity: A test to demonstrate that the analyte of interest, when present in concentrations above the range of quantification, can be diluted to bring the analyte concentrations into the validated range for analysis by the method. Samples used for this test are, in general, the ones containing high concentrations of spiked analyte, not endogenous analyte.
Parallelism: Relative accuracy from recovery tests on the biological matrix, incurred study samples, or diluted matrix against the calibrator in a substitute matrix. It is commonly assessed with multiple dilutions of actual study samples or samples that represent the same matrix and analyte combination of the study samples.
Conflict of interest
The authors state no conflict of interest.
References
- Aebersold R, Goodlett DR. Mass spectrometry in proteomics. Chem Rev. 2001;101:269–295. doi: 10.1021/cr990076h. [DOI] [PubMed] [Google Scholar]
- Baggerly KA, Morris JS, Coombes KR. Reproducibility of SELDI-TOF protein patterns in serum: comparing datasets from different experiments. Bioinformatics. 2004;20:777–785. doi: 10.1093/bioinformatics/btg484. [DOI] [PubMed] [Google Scholar]
- Baggerly KA, Morris JS, Edmonson SR, Coombes KR. Signal in noise: evaluating reported reproducibility of serum proteomic tests for ovarian cancer. J Natl Cancer Inst. 2005;97:307–309. doi: 10.1093/jnci/dji008. [DOI] [PubMed] [Google Scholar]
- Bansal S, DeStefano A. Key elements of bioanalytical method validation for small molecules. AAPS J. 2007;9:E109–E114. doi: 10.1208/aapsj0901011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantel H, Lugering A, Heidemann J, Volkmann X, Poremba C, Strassburg CP, et al. Detection of apoptotic caspase activation in sera from patients with chronic HCV infection is associated with fibrotic liver injury. Hepatology. 2004;40:1078–1087. doi: 10.1002/hep.20411. [DOI] [PubMed] [Google Scholar]
- Barak V, Goike H, Panaretakis KW, Einarsson R. Clinical utility of cytokeratins as tumor markers. Clin Biochem. 2004;37:529–540. doi: 10.1016/j.clinbiochem.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Barker PE. Cancer biomarker validation: standards and process: roles for the National Institute of Standards and Technology (NIST) Ann NY Acad Sci. 2003;983:142–150. doi: 10.1111/j.1749-6632.2003.tb05969.x. [DOI] [PubMed] [Google Scholar]
- Barnidge DR, Goodmanson MK, Klee GG, Muddiman DC. Absolute quantification of the model biomarker prostate-specific antigen in serum by LC–MS/MS using protein cleavage and isotope dilution mass spectrometry. J Proteome Res. 2004;3:644–652. doi: 10.1021/pr049963d. [DOI] [PubMed] [Google Scholar]
- Basil CF, Zhao Y, Zavaglia K, Jin P, Panelli MC, Voiculescu S, et al. Common cancer biomarkers. Cancer Res. 2006;66:2953–2961. doi: 10.1158/0008-5472.CAN-05-3433. [DOI] [PubMed] [Google Scholar]
- Baskin-Bey ES, Washburn K, Feng S, Oltersdorf T, Shapiro D, Huyghe M, et al. Clinical trial of the pan-caspase inhibitor, IDN-6556, in human liver preservation injury. Am J Transplant. 2007;7:218–225. doi: 10.1111/j.1600-6143.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- Bast RC, Jr, Lilja H, Urban N, Rimm DL, Fritsche H, Gray J, et al. Translational crossroads for biomarkers. Clin Cancer Res. 2005;11:6103–6108. doi: 10.1158/1078-0432.CCR-04-2213. [DOI] [PubMed] [Google Scholar]
- Biven K, Erdal H, Hagg M, Ueno T, Zhou R, Lynch M, et al. A novel assay for discovery and characterization of pro-apoptotic drugs and for monitoring apoptosis in patient sera. Apoptosis. 2003;8:263–268. doi: 10.1023/a:1023672805949. [DOI] [PubMed] [Google Scholar]
- Bottari P, Aebersold R, Turecek F, Gelb MH. Design and synthesis of visible isotope-coded affinity tags for the absolute quantification of specific proteins in complex mixtures. Bioconjug Chem. 2004;15:380–388. doi: 10.1021/bc034174s. [DOI] [PubMed] [Google Scholar]
- Bronstrup M. Absolute quantification strategies in proteomics based on mass spectrometry. Expert Rev Proteomics. 2004;1:503–512. doi: 10.1586/14789450.1.4.503. [DOI] [PubMed] [Google Scholar]
- Carr NJ. M30 expression demonstrates apoptotic cells, correlates with in situ end-labeling, and is associated with Ki-67 expression in large intestinal neoplasms. Arch Pathol Lab Med. 2000;124:1768–1772. doi: 10.5858/2000-124-1768-MEDACC. [DOI] [PubMed] [Google Scholar]
- Collins I, Workman P. New approaches to molecular cancer therapeutics. Nat Chem Biol. 2006;2:689–700. doi: 10.1038/nchembio840. [DOI] [PubMed] [Google Scholar]
- Cummings J, Ranson M, Butt F, Moore D, Dive C.Qualification of M30 and M65 ELISAs as surrogate biomarkers of cell death: long term antigen stability in cancer patient plasma Cancer Chemother Pharmacol 2007. Online first [DOI] [PubMed]
- Cummings J, Ranson M, Lacasse E, Ganganagari JR, St-Jean M, Jayson G, et al. Method validation and preliminary qualification of pharmacodynamic biomarkers employed to evaluate the clinical efficacy of an antisense compound ( AEG35156) targeted to the X-linked inhibitor of apoptosis protein XIAP. Br J Cancer. 2006;95:42–48. doi: 10.1038/sj.bjc.6603220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J, Ward TH, Lacasse E, Lefebvre C, St-Jean M, Durkin J, et al. Validation of pharmacodynamic assays to evaluate the clinical efficacy of an antisense compound (AEG 35156) targeted to the X-linked inhibitor of apoptosis protein XIAP. Br J Cancer. 2005;92:532–538. doi: 10.1038/sj.bjc.6602363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demiray M, Ulukaya EE, Arslan M, Gokgoz S, Saraydaroglu O, Ercan I, et al. Response to neoadjuvant chemotherapy in breast cancer could be predictable by measuring a novel serum apoptosis product, caspase-cleaved cytokeratin 18: a prospective pilot study. Cancer Invest. 2006;24:669–676. doi: 10.1080/07357900600981307. [DOI] [PubMed] [Google Scholar]
- DeSilva B, Smith W, Weiner R, Kelley M, Smolec J, Lee B, et al. Recommendations for the bioanalytical method validation of ligand-binding assays to support pharmacokinetic assessments of macromolecules. Pharm Res. 2003;20:1885–1900. doi: 10.1023/b:pham.0000003390.51761.3d. [DOI] [PubMed] [Google Scholar]
- Ditzel HJ, Strik MC, Larsen MK, Willis AC, Waseem A, Kejling K, et al. Cancer-associated cleavage of cytokeratin 8/18 heterotypic complexes exposes a neoepitope in human adenocarcinomas. J Biol Chem. 2002;277:21712–21722. doi: 10.1074/jbc.M202140200. [DOI] [PubMed] [Google Scholar]
- Duan WR, Garner DS, Williams SD, Funckes-Shippy CL, Spath IS, Blomme EA. Comparison of immunohistochemistry for activated caspase-3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC-3 subcutaneous xenografts. J Pathol. 2003;199:221–228. doi: 10.1002/path.1289. [DOI] [PubMed] [Google Scholar]
- Einarsson R, Barak V. TPS: a cytokeratin serum tumour marker for effective therapy control of cancer patient with focus on breast cancer. J Clin Ligand Assay. 1997;22:348–351. [Google Scholar]
- Engwegen JY, Gast MC, Schellens JH, Beijnen JH. Clinical proteomics: searching for better tumour markers with SELDI-TOF mass spectrometry. Trends Pharmacol Sci. 2006;27:251–259. doi: 10.1016/j.tips.2006.03.003. [DOI] [PubMed] [Google Scholar]
- FDA Code of Federal Regulation Title 21, Part 11. Electronic Records, Electronic Signatures. 1997.
- FDA Guidance for Industry: Bioanalytical Method Validation. 2001a.
- FDA Code of Federal Regulation Title 42, Vol 3. Clinical Laboratory Improvement Amendment. 2001b.
- Fenselau C. A review of quantitiative methods for proteomic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;855:14–20. doi: 10.1016/j.jchromb.2006.10.071. [DOI] [PubMed] [Google Scholar]
- Findlay JW, Smith WC, Lee JW, Nordblom GD, Das I, DeSilva BS, et al. Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J Pharm Biomed Anal. 2000;21:1249–1273. doi: 10.1016/s0731-7085(99)00244-7. [DOI] [PubMed] [Google Scholar]
- Fontaine N, Rosengren B. Directive 2001/20/EC of the European Parliament and of the Council: on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. OJEC. 2001;L121:34–44. [PubMed] [Google Scholar]
- Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Gerber SA, Rush J, Stemman O, Kirschner MW, Gygi SP. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc Natl Acad Sci USA. 2003;100:6940–6945. doi: 10.1073/pnas.0832254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodsaid F, Frueh F. Biomarker qualification pilot process at the US Food and Drug Administration. AAPS J. 2007;9:E105–E108. doi: 10.1208/aapsj0901010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Pan S, Bradbury EM, Chen X. Use of deuterium-labeled lysine for efficient protein identification and peptide de novo sequencing. Anal Chem. 2002;74:5774–5785. doi: 10.1021/ac0204350. [DOI] [PubMed] [Google Scholar]
- Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotechnol. 1999;17:994–999. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- HMSO UK Statutory Instrument 2004 No. 1031. The Medicines for Human Use (Clinical Trials) Regulations 2004. 2004.
- HMSO UK Statutory Instrument 2006 No. 1928. The Medicines for Human Use (Clinical Trials) Amendment Regulations 2006. 2006.
- James CA, Hill HM. Procedural elements involved in maintaining bioanalytical data integrity for good laboratory practices and regulated clinical studies. AAPS J. 2007;9:E123–E127. doi: 10.1208/aapsj0902014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johann DJ, Jr, McGuigan MD, Patel AR, Tomov S, Ross S, Conrads TP, et al. Clinical proteomics and biomarker discovery. Ann NY Acad Sci. 2004;1022:295–305. doi: 10.1196/annals.1318.045. [DOI] [PubMed] [Google Scholar]
- Jones CD, Yeung C, Zehnder JL. Comprehensive validation of a real-time quantitative bcr-abl assay for clinical laboratory use. Am J Clin Pathol. 2003;120:42–48. doi: 10.1309/60A9-C8WG-EGHR-NXEE. [DOI] [PubMed] [Google Scholar]
- Kadyrov M, Kaufmann P, Huppertz B. Expression of a cytokeratin 18 neo-epitope is a specific marker for trophoblast apoptosis in human placenta. Placenta. 2001;22:44–48. doi: 10.1053/plac.2000.0616. [DOI] [PubMed] [Google Scholar]
- Kelloff GJ, Bast RC, Jr, Coffey DS, D'Amico AV, Kerbel RS, Park JW, et al. Biomarkers, surrogate end points, and the acceleration of drug development for cancer prevention and treatment: an update prologue. Clin Cancer Res. 2004;10:3881–3884. doi: 10.1158/1078-0432.CCR-03-0783. [DOI] [PubMed] [Google Scholar]
- Kelloff GJ, Sigman CC. New science-based endpoints to accelerate oncology drug development. Eur J Cancer. 2005;41:491–501. doi: 10.1016/j.ejca.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick DS, Gerber SA, Gygi SP. The absolute quantification strategy: a general procedure for the quantification of proteins and post-translational modifications. Methods. 2005;35:265–273. doi: 10.1016/j.ymeth.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Korfmacher WA. Principles and applications of LC–MS in new drug discovery. Drug Discov Today. 2005;10:1357–1367. doi: 10.1016/S1359-6446(05)03620-2. [DOI] [PubMed] [Google Scholar]
- Kramer G, Erdal H, Mertens HJ, Nap M, Mauermann J, Steiner G, et al. Differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin 18. Cancer Res. 2004;64:1751–1756. doi: 10.1158/0008-5472.can-03-2455. [DOI] [PubMed] [Google Scholar]
- Kramer G, Schwarz S, Hagg M, Havelka AM, Linder S. Docetaxel induces apoptosis in hormone refractory prostate carcinomas during multiple treatment cycles. Br J Cancer. 2006;94:1592–1598. doi: 10.1038/sj.bjc.6603129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberger B, Wagner M, Herrmann E, Mihm U, Piiper A, Sarrazin C, et al. Apoptotic cytokeratin 18 neoepitopes in serum of patients with chronic hepatitis C. J Viral Hepat. 2005;12:307–314. doi: 10.1111/j.1365-2893.2005.00594.x. [DOI] [PubMed] [Google Scholar]
- Ku NO, Liao J, Omary MB. Apoptosis generates stable fragments of human type I keratins. J Biol Chem. 1997;272:33197–33203. doi: 10.1074/jbc.272.52.33197. [DOI] [PubMed] [Google Scholar]
- Kummar S, Gutierrez M, Doroshow JH, Murgo AJ. Drug development in oncology: classical cytotoxics and molecularly targeted agents. Br J Clin Pharmacol. 2006;62:15–26. doi: 10.1111/j.1365-2125.2006.02713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Devanarayan V, Barrett YC, Weiner R, Allinson J, Fountain S, et al. Fit-for-purpose method development and validation for successful biomarker measurement. Pharm Res. 2006;23:312–328. doi: 10.1007/s11095-005-9045-3. [DOI] [PubMed] [Google Scholar]
- Lee JW, Figeys D, Vasilescu J. Biomarker assay translation from discovery to clinical studies in cancer drug development: quantification of emerging protein biomarkers. Adv Cancer Res. 2007;96:269–298. doi: 10.1016/S0065-230X(06)96010-2. [DOI] [PubMed] [Google Scholar]
- Lee JW, Weiner RS, Sailstad JM, Bowsher RR, Knuth DW, O'Brien PJ, et al. Method validation and measurement of biomarkers in nonclinical and clinical samples in drug development: a conference report. Pharm Res. 2005;22:499–511. doi: 10.1007/s11095-005-2495-9. [DOI] [PubMed] [Google Scholar]
- Leers MP, Bjorklund V, Bjorklund B, Jornvall H, Nap M. An immunohistochemical study of the clearance of apoptotic cellular fragments. Cell Mol Life Sci. 2002;59:1358–1365. doi: 10.1007/s00018-002-8513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leers MP, Kolgen W, Bjorklund V, Bergman T, Tribbick G, Persson B, et al. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999;187:567–572. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Lesko LJ, Atkinson AJ., Jr Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: criteria, validation, strategies. Annu Rev Pharmacol Toxicol. 2001;41:347–366. doi: 10.1146/annurev.pharmtox.41.1.347. [DOI] [PubMed] [Google Scholar]
- Lin S, Shaler TA, Becker CH. Quantification of intermediate-abundance proteins in serum by multiple reaction monitoring mass spectrometry in a single-quadrupole ion trap. Anal Chem. 2006;78:5762–5767. doi: 10.1021/ac060613f. [DOI] [PubMed] [Google Scholar]
- Linder S, Havelka AM, Ueno T, Shoshan MC. Determining tumor apoptosis and necrosis in patient serum using cytokeratin 18 as a biomarker. Cancer Lett. 2004;214:1–9. doi: 10.1016/j.canlet.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Liu MY, Xydakis AM, Hoogeveen RC, Jones PH, Smith EO, Nelson KW, et al. Multiplexed analysis of biomarkers related to obesity and the metabolic syndrome in human plasma, using the Luminex-100 system. Clin Chem. 2005;51:1102–1109. doi: 10.1373/clinchem.2004.047084. [DOI] [PubMed] [Google Scholar]
- Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;5:845–856. doi: 10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- Maruvada P, Srivastava S. Joint National Cancer Institute-Food and Drug Administration workshop on research strategies, study designs, and statistical approaches to biomarker validation for cancer diagnosis and detection. Cancer Epidemiol Biomarkers Prev. 2006;15:1078–1082. doi: 10.1158/1055-9965.EPI-05-0432. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Bowsher RR, Celniker A, Gibbons J, Gupta S, Lee JW, et al. Workshop on bioanalytical methods validation for macromolecules: summary report. Pharm Res. 2001;18:1373–1383. doi: 10.1023/a:1013062600566. [DOI] [PubMed] [Google Scholar]
- NIH Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Nowatzke W, Wood E. Best practices during bioanalytical method validation for the characterisation of assay reagents and the evaluation of analyte stability in assay standards, quality controls and study samples. AAPS J. 2007;9:E117–E122. doi: 10.1208/aapsj0902013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson MH, Ueno T, Pan Y, Xu R, Cai F, van der Kuip H, et al. Cytokeratin-18 is a useful serum biomarker for early determination of response of breast carcinomas to chemotherapy. Clin Cancer Res. 2007;13:3198–3206. doi: 10.1158/1078-0432.CCR-07-0009. [DOI] [PubMed] [Google Scholar]
- Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- Peters FT, Drummer OH, Musshoff F. Validation of new methods. Forensic Sci Int. 2007;165:216–224. doi: 10.1016/j.forsciint.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Petricoin EF, Ardekani AM, Hitt BA, Levine PJ, Fusaro VA, Steinberg SM, et al. Use of proteomic patterns in serum to identify ovarian cancer. Lancet. 2002;359:572–577. doi: 10.1016/S0140-6736(02)07746-2. [DOI] [PubMed] [Google Scholar]
- Poon TC. Opportunities and limitations of SELDI-TOF-MS in biomedical research: practical advices. Expert Rev Proteomics. 2007;4:51–65. doi: 10.1586/14789450.4.1.51. [DOI] [PubMed] [Google Scholar]
- Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- Roth GA, Krenn C, Brunner M, Moser B, Ploder M, Spittler A, et al. Elevated serum levels of epithelial cell apoptosis-specific cytokeratin 18 neoepitope M30 in critically ill patients. Shock. 2004;22:218–220. doi: 10.1097/01.shk.0000136098.49672.0e. [DOI] [PubMed] [Google Scholar]
- Rupa JD, de Bruine AP, Gerbers AJ, Leers MP, Nap M, Kessels AG, et al. Simultaneous detection of apoptosis and proliferation in colorectal carcinoma by multiparameter flow cytometry allows separation of high and low-turnover tumors with distinct clinical outcome. Cancer. 2003;97:2404–2411. doi: 10.1002/cncr.11366. [DOI] [PubMed] [Google Scholar]
- Sarker D, Workman P. Pharmacodynamic biomarkers for molecular cancer therapeutics. Adv Cancer Res. 2007;96:213–268. doi: 10.1016/S0065-230X(06)96008-4. [DOI] [PubMed] [Google Scholar]
- Scholler N, Crawford M, Sato A, Drescher CW, O'Briant KC, Kiviat N, et al. Bead-based ELISA for validation of ovarian cancer early detection markers. Clin Cancer Res. 2006;12:2117–2124. doi: 10.1158/1078-0432.CCR-05-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte B, Henfling M, Kolgen W, Bouman M, Meex S, Leers MP, et al. Keratin 8/18 breakdown and reorganization during apoptosis. Exp Cell Res. 2004;297:11–26. doi: 10.1016/j.yexcr.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Seligson DB. The tissue micro-array as a translational research tool for biomarker profiling and validation. Biomarkers. 2005;10 Suppl 1:S77–S82. doi: 10.1080/13547500500214418. [DOI] [PubMed] [Google Scholar]
- Shah VP. The history of bioanalytical method validation and regulation: evolution of a guidance document on bioanalytical methods validation. AAPS J. 2007;9:E43–E47. [Google Scholar]
- Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A, et al. Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies. Conference report. Eur J Drug Metab Pharmacokinet. 1991;16:249–255. doi: 10.1007/BF03189968. [DOI] [PubMed] [Google Scholar]
- Shah VP, Midha KK, Findlay JW, Hill HM, Hulse JD, McGilveray IJ, et al. Bioanalytical method validation—a revisit with a decade of progress. Pharm Res. 2000;17:1551–1557. doi: 10.1023/a:1007669411738. [DOI] [PubMed] [Google Scholar]
- Simpkins F, Czechowicz JA, Liotta L, Kohn EC. SELDI-TOF mass spectrometry for cancer biomarker discovery and serum proteomic diagnostics. Pharmacogenomics. 2005;6:647–653. doi: 10.2217/14622416.6.6.647. [DOI] [PubMed] [Google Scholar]
- Smith WC, Sittampalam GS. Conceptual and statistical issues in the validation of analytic dilution assays for pharmaceutical applications. J Biopharm Stat. 1998;8:509–532. doi: 10.1080/10543409808835257. [DOI] [PubMed] [Google Scholar]
- Smolec J, DeSilva B, Smith W, Weiner R, Kelly M, Lee B, et al. Bioanalytical method validation for macromolecules in support of pharmacokinetic studies. Pharm Res. 2005;22:1425–1431. doi: 10.1007/s11095-005-5917-9. [DOI] [PubMed] [Google Scholar]
- Stemmann O, Zou H, Gerber SA, Gygi SP, Kirschner MW. Dual inhibition of sister chromatid separation at metaphase. Cell. 2001;107:715–726. doi: 10.1016/s0092-8674(01)00603-1. [DOI] [PubMed] [Google Scholar]
- Stiles T. Good Clinical Laboratory Practice Accreditation Scheme. 2004.
- Stiles T, Grant V, Mawbery N. Good Clinical Laboratory Practice (GCLP): BARQA. 2003.
- Strnad P, Windoffer R, Leube RE. Induction of rapid and reversible cytokeratin filament network remodeling by inhibition of tyrosine phosphatases. J Cell Sci. 2002;115:4133–4148. doi: 10.1242/jcs.00096. [DOI] [PubMed] [Google Scholar]
- Sundstrom BE, Stigbrand TI. Cytokeratins and tissue polypeptide antigen. Int J Biol Markers. 1994;9:102–108. doi: 10.1177/172460089400900207. [DOI] [PubMed] [Google Scholar]
- Ueno T, Toi M, Biven K, Bando H, Ogawa T, Linder S. Measurement of an apoptotic product in the sera of breast cancer patients. Eur J Cancer. 2003;39:769–774. doi: 10.1016/s0959-8049(02)00865-1. [DOI] [PubMed] [Google Scholar]
- Ulukaya E, Yilmaztepe A, Akgoz S, Linder S, Karadag M. The levels of caspase-cleaved cytokeratin 18 are elevated in serum from patients with lung cancer and helpful to predict the survival. Lung Cancer. 2007;40:651–655. doi: 10.1016/j.lungcan.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Unwin RD, Evans CA, Whetton AD. Relative quantification in proteomics: new approaches for biochemistry. Trends Biochem Sci. 2006;31:473–484. doi: 10.1016/j.tibs.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Unwin RD, Pierce A, Watson RB, Sternberg DW, Whetton AD. Quantitative proteomic analysis using isobaric protein tags enables rapid comparison of changes in transcript and protein levels in transformed cells. Mol Cell Proteomics. 2005;4:924–935. doi: 10.1074/mcp.M400193-MCP200. [DOI] [PubMed] [Google Scholar]
- van der Merwe DE, Oikonomopoulou K, Marshall J, Diamandis EP. Mass spectrometry: uncovering the cancer proteome for diagnostics. Adv Cancer Res. 2007;96:23–50. doi: 10.1016/S0065-230X(06)96002-3. [DOI] [PubMed] [Google Scholar]
- Verma M, Srivastava S.New cancer biomarkers deriving from NCI early detection research Recent Results Cancer Res 200316372–84.discussion 264–266 [DOI] [PubMed] [Google Scholar]
- Wagner JA. Overview of biomarkers and surrogate endpoints in drug development. Dis Markers. 2002;18:41–46. doi: 10.1155/2002/929274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JA, Williams SA, Webster CJ. Biomarkers and surrogate end points for fit-for-purpose development and regulatory evaluation of new drugs. Clin Pharmacol Ther. 2007;81:104–107. doi: 10.1038/sj.clpt.6100017. [DOI] [PubMed] [Google Scholar]
- Weber K, Osborn M, Moll R, Wiklund B, Luning B. Tissue polypeptide antigen (TPA) is related to the non-epidermal keratins 8, 18 and 19 typical of simple and non-squamous epithelia: re-evaluation of a human tumor marker. EMBO J. 1984;3:2707–2714. doi: 10.1002/j.1460-2075.1984.tb02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westgard JO, Barry PL, Hunt MR, Groth T. A multi-rule Shewhart chart for quality control in clinical chemistry. Clin Chem. 1981;27:493–501. [PubMed] [Google Scholar]
- Workman P. Using biomarkers in drug development. Clin Adv Hematol Oncol. 2006;4:736–739. [PubMed] [Google Scholar]
- Yagmur E, Trautwein C, Leers MP, Gressner AM, Tacke F. Elevated apoptosis-associated cytokeratin 18 fragments (CK18Asp386) in serum of patients with chronic liver diseases indicate hepatic and biliary inflammation. Clin Biochem. 2007. [DOI] [PubMed]
- Yamada S, Wirtz D, Coulombe PA. Pairwise assembly determines the intrinsic potential for self organization and mechanical properties of keratin filament. Mol Biol Cell. 2002;13:382–391. doi: 10.1091/mbc.01-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Afonso C, Fenselau C. Dissection of proteolytic 18O labeling: endoprotease-catalyzed 16O-to-18O exchange of truncated peptide substrates. J Proteome Res. 2003;2:147–152. doi: 10.1021/pr025572s. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wei D, Yap Y, Li L, Guo S, Chen F. Mass spectrometry-based ‘omics' technologies in cancer diagnostics. Mass Spectrom Rev. 2007;26:403–431. doi: 10.1002/mas.20132. [DOI] [PubMed] [Google Scholar]