Abstract
Background and purpose:
Testosterone alleviates symptoms in patients with ischaemic heart disease. Androgen receptors are present in the heart, and testosterone upregulates gene expression of cardiac β1-adrenoceptors. We hypothesize that testosterone may confer cardioprotection by interacting with adrenoceptors.
Experimental approach:
In isolated perfused hearts and ventricular myocytes from orchidectomized rats without or with testosterone (200 μg/100 g) replacement, we first determined the effect of ischaemia/reperfusion in the presence of noradrenaline (10−7 M). Then we determined the contribution of interactions between testosterone and α1- or β1-adrenoceptors in cardiac injury/protection (infarct size, release of lactate dehydrogenase, viability of myocytes, recovery of contractile function and incidence of arrhythmias) upon ischaemia/reperfusion by pharmacological manipulation using selective adrenoceptor agonists (α1-adrenoceptor agonist: phenylephrine 10−6 M; non-selective β-adrenoceptor agonist: isoprenaline 10−7 M) and antagonists (α1: prazosin or benoxathian 10−6 M; β1: CGP 20712A 5 × 10−7 M). We also determined the expression of α1 and β1-adrenoceptor in the hearts from rats with and without testosterone.
Key results:
Testosterone reduced injury induced by ischaemia/reperfusion and noradrenaline. This was achieved by enhancing the beneficial effect of α1-adrenoceptor stimulation, which was greater than the deleterious effect of β1-adrenoceptor stimulation (also enhanced by testosterone). The effects of testosterone were abolished or attenuated by blockade of androgen receptors. Testosterone also enhanced the expression of α1A and β1-adrenoceptor.
Conclusions and implications:
Testosterone conferred cardioprotection by upregulating the cardiac α1-adrenoceptor and enhancing the effects of stimulation of this adrenoceptor. The effect of testosterone was at least partly mediated by androgen receptors.
Keywords: testosterone, ischaemia–reperfusion, α/β-adrenoceptors
Introduction
Men with heart disease or ischaemic heart disease (IHD) have lower levels of androgens than men with normal coronary angiograms (English et al., 2000a) and testosterone, the predominant androgen, improves ischaemic threshold and quality of life in hypogonadal men with angina (English et al., 2000b; Malkin et al., 2004). These observations suggest that androgens may confer cardioprotection against ischaemic insults. Androgen receptors are present in the heart (McGill et al., 1980) and mediate hypertrophy in cardiac myocytes (Marsh et al., 1998). More importantly, testosterone replacement in orchidectomized (ORX) rats improves recovery of contractile function and ischaemia/reperfusion-induced injury, and this is associated with attenuation of intracellular Ca2+ ([Ca2+]i) overload (Callies et al., 2003). These findings prompted us to postulate that androgen may confer cardioprotection by direct action on the myocardium.
The sympathetic nervous system is one of the most important extrinsic mechanisms regulating cardiac function. Noradrenaline released from the nerve terminals of the sympathetic nervous system activates both α- and β-adrenoceptors. Activation of α1- (Iwai-Kanai et al., 1999), and β2- (Communal et al., 1999; Patterson et al., 2004) adrenoceptors reduces apoptosis, whereas activation of β1-adrenoceptors is proapoptotic (Communal et al., 1998, 1999; Iwai-Kanai et al., 1999; Zhu et al., 2003). During myocardial ischaemia, there is a marked increase of noradrenaline discharge, which contributes significantly to cardiac injury (Waldenstrom et al., 1978; Schomig and Richardt, 1990; Schomig, 1990). It is now known that oestrogen, the female sex hormone, suppresses the expression of the β1-adrenoceptor (Kam et al., 2004) and protein kinase A (Kam et al., 2005), thus, in turn, reducing cardiac responses to β1-adrenoceptor stimulation. Similarly, there is evidence that testosterone increases the expression of the β1-adrenoceptor (Golden et al., 2004), suggesting that like oestrogen, testosterone may also interact with the β1-adrenoceptor and its signalling pathway.
We therefore hypothesized that testosterone interacts (by cross-talk) with adrenoceptors during myocardial ischaemia. Cross-talk between testosterone and adrenoceptors that are antiapoptotic may result in cardioprotection against cardiac injury in response to ischaemic insults, whereas cross-talk between testosterone and adrenoceptors that are proapoptotic may lead to increased injury. If the effect of the former is greater than that of the latter, the overall result is cardioprotection. To test the hypothesis that the male sex hormone confers cardioprotection against ischaemic insult by direct action on the myocardium, we determined the effects of ischaemic insults and reperfusion on isolated perfused hearts and isolated ventricular myocytes from sham control, orchidectomized rats without (ORX) or with testosterone replacement (ORX+T).
To mimic the increased sympathetic activation during myocardial ischaemia, 10−7 M noradrenaline was administered during ischaemic insults. This concentration was chosen based on the fact that the circulating level of noradrenaline is 10−9–10−10 M (Engelhard et al., 2002) and there may be an up to 1000-fold increase during myocardial ischaemia (Schomig and Richardt, 1990; Schomig, 1990). Secondly, we determined the effects of testosterone on ventricular myocytes subjected to simulated ischaemia with activation of either α1- or β1-adrenoceptors. In the third series of experiments, we determined the injury/survival induced by simulated ischaemia and reperfusion in ventricular myocytes exposed to noradrenaline, with blockade of the β2-adrenoceptor and either the α1- or β1-adrenoceptors, which allowed us to compare the contributions of cross-talk between testosterone and either α1- or β1-adrenoceptors. We also determined the expression of adrenoceptor subtypes in the three groups of rats hoping to obtain useful information on signalling mechanism at receptor level. The results showed that testosterone conferred cardioprotection by enhancing the beneficial action of α1-adrenoceptor stimulation. Preliminary results have been communicated previously (Tsang et al., 2005, 2007).
Methods
Animal model
The study was approved by the Committee on the Use of Live Animals in Teaching and Research of The University of Hong Kong. Male Sprague–Dawley rats weighing 300–350 g were purchased from Charles River Breeding Laboratories (Wilmington, MA, USA) and randomly divided into two groups. One group was sham operated and served as normal control (sham control). The other group underwent bilateral orchidectomy (ORX) and was divided into two subgroups. One week after ORX, one subgroup was supplemented with a physiological dose of testosterone (200 μg per 100 g, s.c.) daily for 8 weeks (ORX+T) according to a previous study (Banu et al., 2001), and another subgroup was treated with vehicle. All surgical procedures were performed under anaesthesia with sodium pentobarbital (60 mg kg−1, i.p.; Abbott Laboratory, Chicago, IL, USA).
Rat isolated heart preparation
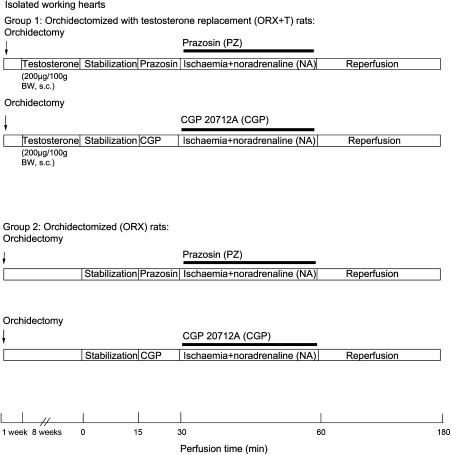
Nine weeks after orchidectomy, rats were anaesthetized with sodium pentobarbital (60 mg kg−1, i.p.) and given heparin (200 IU, i.v.) before decapitation. Hearts were excised immediately and, placed in ice-cold Krebs–Henseleit perfusion buffer before mounting on the Langendorff apparatus for perfusion. Isolated hearts were perfused retrogradely with Krebs–Henseleit buffer (in mM: 118 NaCl, 5 KCl, 1.2 MgSO4, 1.2 KH2PO4, 1.25 CaCl2, 25 NaHCO3 and 11 glucose) equilibrated with 95% O2+5% CO2 at a constant pressure of 80 cm H2O (Waldenstrom et al., 1978) and a temperature of 37°C. The hearts were allowed to stabilize for 15 min and then subjected to 30 min regional ischaemia with modified Krebs–Henseleit buffer supplemented with 10−7 M noradrenaline and 120 min reperfusion as described previously (Wang et al., 2001). Hearts exhibiting arrhythmias during stabilization were discarded. Regional ischaemia was achieved by ligation of the left anterior descending coronary artery as described previously (Wang et al., 2001). Briefly, a fine silk thread was passed below the left anterior descending coronary artery. The ends of the thread were passed through a propylene tube to form a snare. Pulling the snare produced ischaemia. Release of the ligature allowed reperfusion. The effectiveness of ischaemia was confirmed by regional cyanosis and a substantial decrease in coronary flow. In the series of experiments that determined the effect of α- and β-adrenoceptors, 10−6 M prazosin (PZ) a selective α1-adrenoceptor antagonist, shown to effectively block the receptor (Gallego et al., 2005) or 5 × 10−7 M CGP 20712A (CGP), a selective β1-adrenoceptor antagonist, known to block the β1-adrenoceptor (Zhang et al., 2000), and a selective β2-adrenoceptor antagonist, ICI 118, 551 (ICI) at 5 × 10−7 M (Communal et al., 1999; Zhang et al., 2000), were administered.
Measurement of infarct size
At the end of reperfusion, the coronary artery was re-occluded, and the hearts were perfused with Evans blue (10%). The ischaemic risk zone and the infarct size were determined as described previously (Wang et al., 2001). The areas of infarct (unstained by 2,3,5-triphenyl-tetrazolium chloride) and the risk area (unstained by Evans blue) were determined by a computerized planimetric technique (ImageJ, NIH). The severity of infarct was expressed as the percentage of infarct size over risk area.
LV pressure recordings
Cardiac variables of left ventricular developed pressure (LVDP), velocity of contraction and relaxation (±dP/dtmax), and heart rate were monitored continuously by a PowerLab/4SD analogue-to-digital converter (AD instruments, Castle Hill, Australia). A latex balloon inserted through left atrium into left ventricle was adjusted to a mean left ventricular end-diastolic pressure (LVEDP) to 6–10 mm Hg. The hearts were paced at 5 Hz (300 beats per minute). After stabilization of mechanical function, regional ischaemia was initiated. Pacing was discontinued 5 min after the initiation of ischaemia. Hearts were reperfused after 30 min of ischaemia. Transient ventricular fibrillation occurred in all groups. Pacing was reinitiated 5 min after reperfusion.
Arrhythmia analysis
With the use of a lead II ECG tracing, the episodes of premature ventricular beats (PVB), ventricular tachycardia (VT) and ventricular fibrillation were recorded and monitored during the 30 min of ischaemia and 2 h of reperfusion. The signal electrode was superficially connected to the left ventricular wall and the reference electrode to the aorta. Arrhythmias were defined according to the Lambeth Conventions (Walker et al., 1988). PVBs were defined as discrete and identifiable premature QRS complexes, whereas VT was defined as a run of four or more consecutive ventricular premature beats (VPBs). An episode of ventricular fibrillation was defined as a signal where individual QRS deflections could not easily be distinguished from each other and where rate could no longer be measured (Walker et al., 1988).
Preparation of isolated ventricular myocytes
Ventricular myocytes were isolated from the hearts of sham control, ORX and ORX+T rats, using the collagenase perfusion method as described previously (Wu et al., 1999; Wang et al., 2001). Preliminary experiments in our lab showed that there was no difference in the viability of isolated myocytes between all the groups immediately after isolation, indicating that testosterone deficiency as a result of removal of the testes did not affect the viability of heart muscle cells. After isolation, they were allowed to stabilize for at least 30 min before experiments. The yield of myocytes was determined microscopically using a haemocytometer. Myocyte viability was assessed by both the Cell Titer Blue (CTB) reagent (Promega, Madison, WI, USA) and Trypan blue exclusion (Wu et al., 1999; Pei et al., 2003). Preparations were considered satisfactory only if rods accounted for >80% of the counted cells at the beginning of each experiment. Myocytes were re-suspended in minimal essential medium containing 1.25 mM Ca2+, 5% fetal bovine serum, 5 μM insulin, 5 μM apo-transferrin, 100 U ml−1 penicillin G and 100 μM streptomycin and seeded at a density of 3 × 105 cells per well on laminin-coated (1 μM, Sigma, St Louis, MO, USA) six-well plates. To mimic ischaemia, ventricular myocytes were incubated with a modified glucose-free Krebs solution supplemented with 10 mM 2-deoxy-D-glucose (2-DOG), an inhibitor of glycolysis, and 10 mM sodium dithionite, an oxygen scavenger, that induce metabolic inhibition and anoxia (MIA). The cells were subjected to MIA for 10 min with or without adrenoceptor agonists at 37 °C under a 5% CO2 atmosphere and then transferred back to normal Krebs buffer for another 10 min to simulate reperfusion (MIA/R). In the series of experiments that determined and compared the effect of α- and β-adrenoceptors, the same batch of myocytes isolated from ORX rats incubated without or with a physiological concentration of testosterone at 10−8 M (Phillippe et al., 1991; Weidemann and Hanke, 2002) for 24 h were used. In pilot studies, we showed consistent results with myocytes obtained from ORX+T rats (200 μg per 100 g, s.c.) and from ORX rats incubated with 10−8 M testosterone for 24 h.
Lactate dehydrogenase assay
The lactate dehydrogenase (LDH) release assay was performed using a cytotoxicity detection kit (Roche, Indianapolis, IN, USA) according to the manufacturer's instructions. Release of the enzyme, which indicates cytotoxicity, was measured after MIA/R. Background release was assessed by measuring LDH release from untreated cells and was subtracted from the experimental value. Maximum release of LDH was obtained by adding 2% Triton X-100 to untreated cells. For measurements, each sample was transferred to a 96-well microtitre plate. LDH reagent was added to each well and incubated for 30 min at room temperature in the dark. The absorbance of samples was then measured at 490 nm. No significant LDH release was detected in the tissue culture medium alone or in drug-containing incubation medium.
Viability assay
Cell viability was determined using Cell Titer Blue reagent (Promega) according to the manufacturer's instructions. The Cell Titer Blue assay is based on determining the number of viable cells in culture, using an indicator dye, resazurin (dark blue). Viable cells retain the ability to reduce resazurin into resorufin, which is pink and highly fluorescent. Nonviable cells, which rapidly lose metabolic capacity, do not reduce the indicator dye. Cells were transferred to a 96-well microtitre plate with Cell Titer Blue reagent. The absorbance of samples was measured at 570 nm using 600 nm as a reference wavelength. No change in absorbance was detected in tissue culture medium alone or in drug-containing medium.
Immunoassays
Nine weeks after orchidectomy, rats were anaesthetized with sodium pentobarbital (60 mg kg−1, i.p.) before decapitation. Blood samples were allowed to clot and sera were separated by centrifugation and stored at −80 °C until assayed. Total serum testosterone was determined by a commercially available RIA (Diagnostic Products Corp., Los Angeles, CA, USA; sensitivity 0.4 nM) according to the manufacturer's instructions.
Protein extraction and western blot
Membrane protein from ventricular myocardium was extracted as described previously (Shen et al., 2000; Pei et al., 2003). Briefly, left ventricular tissue was homogenized followed by centrifugation at 10 000 g for 10 min. The supernatant was centrifuged again at 100 000 g for 1 h at 4 °C, and the pellet was collected as the membrane fraction. Protein concentration was determined with a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA). Membrane proteins (60 μg) were loaded onto 10% sodium dodecyl sulphate-polyacrylamide gels. Separated proteins were transferred to polyvinylidine difluoride membrane, blocked and probed with goat polyclonal antibodies SC-568 (V-19) for the β1-adrenoceptor and SC-28982 (H-136) for the α1A-adrenoceptor (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) (O'Connell et al., 2006). Monoclonal antibody against β-actin (Sigma) was chosen as an internal control (supplementary information). Bands were detected by enhanced chemiluminescence reagent (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Membranes were stripped and reblotted (Re-Blot Plus solution, Chemicon, Temecula, CA, USA) with anti-β-tubulin monoclonal antibody (Sigma) to ensure equal amount of protein loading.
Statistical analysis
Data were expressed as mean±s.e.mean. Between-group comparisons were performed using ANOVA. The nonparametric Kruskal–Wallis test was used to analyse drug effects. A difference of P<0.05 was considered statistically significant. χ2 or Fisher exact test was used for percentages of VPBs and VT.
Drugs and chemicals
All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA) except benoxathian HCl (Research Biochemical International, USA). Drugs were dissolved in double-distilled H2O or Krebs buffer unless otherwise stated. Stock solutions of prazosin and propranolol were dissolved in methanol and ethanol, respectively. Testosterone was dissolved in ethanol/DMSO. The final concentration of methanol/ethanol was <0.01% (vol/vol), which itself had no effect on the heart.
Results
Animal characteristics and hormone determination
The total serum testosterone level in the sham control group was 5.22 ng ml−1 (18.1 nmol l−1). Nine weeks after orchidectomy (ORX), the level fell below the detection limit of 0.4 ng ml−1 (Table 1). The decrease in testosterone level was accompanied by significant reductions in body and heart weights, and heart weight/body weight ratio in the ORX rats.
Table 1.
Animal characteristics and hormone determination
| Total serum testosterone (ng ml−1) | Body weight (g) | Heart weight (g) | Heart/body weight (%) | |
|---|---|---|---|---|
| Sham control (12) | 5.22±0.96 | 542±13.7 | 2.14±0.05 | 0.39±0.01 |
| ORX (14) | <0.4***, ### | 489±6.65* | 1.73±0.04**, # | 0.35±0.01* |
| ORX+T (14) | 5.78±1.49 | 516±15.2 | 1.94±0.06* | 0.37±0.02 |
Each value represents the mean±s.e.mean. The figures in parentheses indicate the number of animals.
*P<0.05, **P<0.01, ***P<0.001 vs sham control; #P<0.05, ###P<0.001 vs ORX with testosterone replacement (ORX+T) rats.
Effects of adrenergic stimulation and ischaemia/reperfusion on injury/viability in the isolated perfused heart and ventricular myocytes from sham control, ORX and ORX+T rats
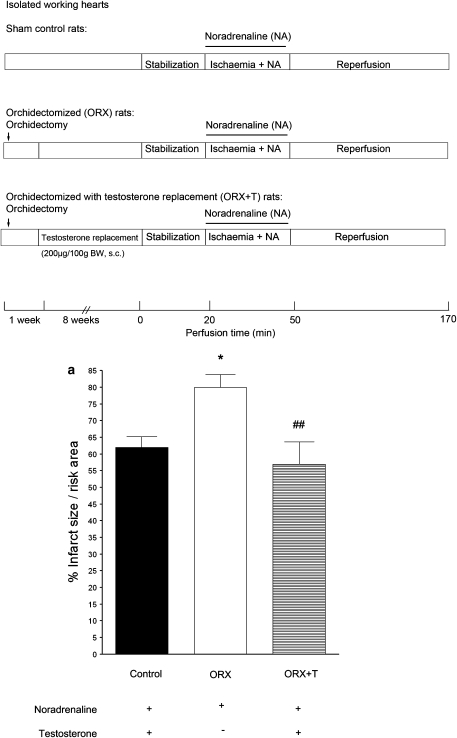
When isolated perfused rat hearts were subjected to regional ischaemia/reperfusion in the presence of 10−7 M of noradrenaline to mimic sympathetic over-activity during ischaemia, myocardial infarction occurred. The infarct size was significantly greater in the ORX group, and testosterone replacement (ORX+T: 200 μg per 100 g, s.c.) restored the value to that of the sham control (Figure 1a).
Figure 1.
Effects of ischaemic insults and administration of noradrenaline (NA) on (a) infarct size in isolated hearts, (b) LDH release and (c) viability of isolated ventricular myocytes from sham, ORX and ORX+T rats. Isolated perfused hearts were subjected to regional ischaemia by occlusion of the coronary artery for 30 min followed by release of the occlusion for 2 h. This produced reperfusion shown in the protocol (a). Infarct size was determined at the end of reperfusion. Noradrenaline (NA; 10−7 M) was administered during ischaemia. As shown in (b) and (c), left ventricular myocytes were superfused for 10 min with a medium containing 10 mM 2-deoxy-D-glucose, an inhibitor of glycolysis, to induce metabolic inhibition, and 10 mM sodium dithionite, an oxygen scavenger, to produce anoxia. This simulated ischaemic insult and was followed by superfusion with the normal Krebs buffer for 10 min to simulate reperfusion. Values are mean±s.e.mean from 12 hearts in each group. *P<0.05, **P<0.01, ***P<0.001 vs sham control rats; # P<0.05, ## P<0.01, ### P<0.001 vs ORX rats. ORX, orchidectomized male rats; ORX+T, orchidectomized male rats with testosterone replacement (200 μg per 100 g).
In ventricular myocytes subjected to MIA/R in the presence of 10−7 M noradrenaline, the release of LDH increased, whereas the viability decreased (Figures 1b and c). The LDH release from isolated cardiomyocytes (Figure 1b) was significantly greater, whereas the viability (Figure 1c) was significantly lower, in the ORX group than the sham control group. Testosterone replacement (ORX+T) restored the values to those of the sham control.
Effects of adrenergic stimulation and ischaemia/reperfusion on cardiac variables and reperfusion arrhythmias in the isolated heart from sham control, ORX and ORX+T rats
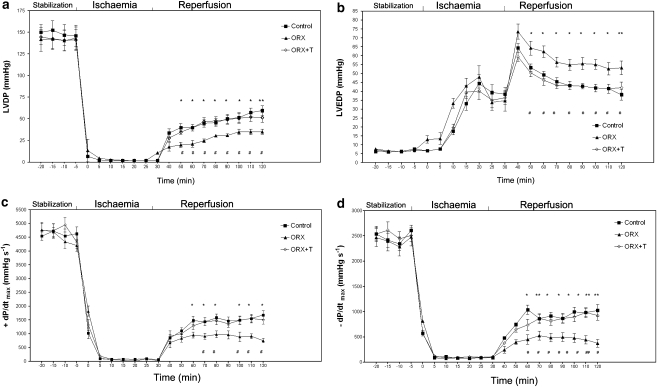
There were no significant differences in contractile function up to the end of ischaemia between sham control, ORX and ORX+T. During ischaemia, the LVDP and ±dP/dtmax were markedly reduced, whereas the LVEDP was elevated. Upon reperfusion, the LVDP and ±dP/dtmax were partially restored towards the base-line level, whereas the LVEDP was further increased. The post-ischaemic LVDP and ±dP/dtmax were higher, whereas LVEDP was lower, at the end of reperfusion in sham control and ORX+T rats than in ORX rats (Figure 2). PVB and VT were observed during reperfusion in all groups. The incidence of both PVB and VT was higher in the hearts of the ORX rats (Table 2). Testosterone replacement restored the incidence of PVB to that of the sham control (Table 2).
Figure 2.
Effects of ischaemic insults and administration of noradrenaline (NA) on contractile variables in isolated hearts (a–d) from sham, ORX and ORX+T rats. (a) LVDP, (b) LVEDP, (c) +dP/dtmax and (d) –dP/dtmax obtained from perfused hearts in pre-ischaemic conditions (stabilization), during ischaemia and then during reperfusion. Values are mean±s.e.mean from 12 hearts from sham control, 12 hearts from ORX and 9 hearts from ORX+T group. # P<0.05, ## P<0.01 vs sham control rats; *P<0.05, **P<0.01 vs ORX rats. ORX, orchidectomized male rats; ORX+T, orchidectomized male rats with testosterone replacement (200 μg per 100 g).
Table 2.
Incidence of premature ventricular beats (PVB) and ventricular tachycardia (VT) in perfused hearts from sham control, ORX and ORX+T rats over first 30 min of reperfusion period after 30 min of regional ischaemia
| PVB (%) | VT (%) | |
|---|---|---|
| Sham control (n=12) | 50 | 25 |
| ORX (n=12) | 100* | 50 |
| ORX+T (n=9) | 56# | 44 |
Values shown are the percentage of rats exhibiting PVBs and VT (mean±s.e.mean rounded to the nearest whole number) over the first 30 min reperfusion period.
*P<0.05 relative to sham control; #P<0.05 relative to ORX by χ2 test for percentages.
Effects of MIA/R and stimulation of α1- or β1-adrenoceptors on injury/viability of isolated ventricular myocytes from sham control, ORX and ORX+T rats
To determine the roles of cross-talk between testosterone and adrenoceptor subtypes, an agonist of α1- or β1-adrenoceptors was administered to ventricular myocytes, which had been isolated from ORX and ORX+T rats, and subjected to MIA/R. Phenylephrine (10−6 M), an α1-adrenoceptor agonist, with a selective β-adrenoceptor antagonist, propranolol significantly decreased LDH release (Figure 3a) and increased viability (Figure 3b) of myocytes from control, ORX and ORX+T rats. These effects were abolished by 10−6 M prazosin, a selective α1-adrenoceptor antagonist. The reduction in LDH release and increase in viability were significantly greater in the control and ORX+T rats than the corresponding values in the ORX rats (Figures 3a and b). On the contrary, the non-selective β-adrenoceptor agonist isoprenaline at 10−7 M increased LDH release (Figure 3c) and decreased viability (Figure 3d) upon blockade of α- and β2-adrenoceptors in all three groups. The effects were abolished by 5 × 10−7 M CGP, a selective β1-adrenoceptor antagonist. Similar to α1-adrenoceptor stimulation, the changes in LDH release and viability were also significantly greater in the control and ORX+T groups than in the ORX group (Figures 3c and d). The enhancing effects of testosterone on adrenoceptor stimulation were abolished by 10−6 M cyproterone acetate, an androgen receptor antagonist (Figure 3).
Figure 3.
Effects of MIA/R in the presence of α1 (a, b) or β1-adrenoceptors (c, d) stimulation on LDH release and viability of left ventricular myocytes from sham, ORX and ORX+T groups. For stimulation of α1-adrenoceptors, 10−6 M phenylephrine (PE) in the presence of 10−6 M propranolol, a β-adrenoceptor blocker, and 5 × 10−7 M yohimbine, an α2-adrenoceptor blocker, was administered during MIA. Cyproterone acetate (Cyp), 10−6 M, prazosin (PZ), 10−6 M, was used for blockade of the androgen receptor and α1, adrenoceptor, respectively. (a) % LDH release resulting from stimulation of α1-adrenoceptors. (b) Viability resulting from stimulation of α1-adrenoceptors. Values represent mean±s.e.mean of triplicate determinations from six hearts in each group. *, **, *** P<0.05, 0.01, 0.001 vs corresponding sham control, respectively. #, ##, ### P<0.05, 0.01, 0.001 vs ORX rats, respectively. +, ++, +++ P<0.05, 0.01, 0.001 vs ORX+T group with phenylephrine, respectively. For stimulation of β1-adrenoceptors, 10−7 M isoprenaline (ISO) in the presence of 10−6 M phentolamine, an α-adrenoceptor antagonist, and 5 × 10−7 M ICI, a β2-adrenoceptor antagonist, were administered during MIA. (c) % LDH release resulting from stimulation of β1-adrenoceptors. (d) Viability resulting from stimulation of β1-adrenoceptors. Values are mean±s.e.mean of triplicate determinations from six hearts in each group, respectively. *, **, *** P<0.05, 0.01, 0.001 vs corresponding sham control, respectively.#, ##, ### P<0.05, 0.01, 0.001 vs ORX group, respectively. +, ++, +++ P<0.05, 0.01, 0.001 vs ORX+T group with isoprenaline, respectively. ICI, ICI 118, 551; MIA/R, metabolic inhibition and anoxia/reperfusion; ORX, orchidectomized male rats; ORX+T, orchidectomized male rats with testosterone replacement (200 μg per 100 g).
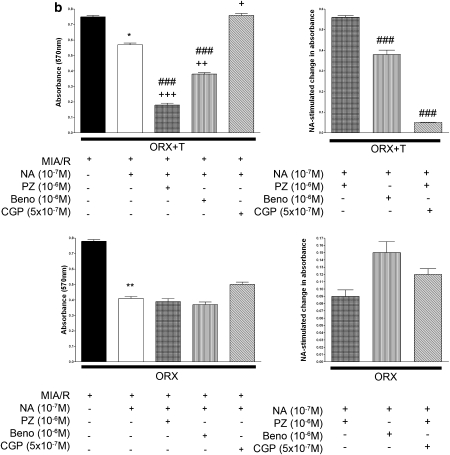
Effects of MIA/R and adrenergic stimulation on injury/viability of ventricular myocytes from ORX rats with or without testosterone replacement in the presence of either α1- and β2-adrenoceptor or β1- and β2-adrenoceptor blockades
To compare the beneficial effects of cross-talk between testosterone and the α1-adrenoceptor and the harmful effects of cross-talk between testosterone and the β1-adrenoceptor, we determined the effects of blocking β1- and β2-adrenoceptors, and effects of blocking α1- and β2-adrenoceptors in ventricular myocytes subjected to MIA/R in the presence of 10−7 M noradrenaline. We eliminated the effects of the β2-adrenoceptor (which may also be beneficial) based on the marked effect of testosterone on α1-adrenoceptor stimulation (Figures 3a and b) by using a selective β2-adrenoceptor antagonist, ICI at 5 × 10−7 M. Noradrenaline significantly increased the LDH release (Figure 4a) and reduced the viability (Figure 4b) induced by MIA/R in ventricular myocytes exposed to testosterone at a physiological concentration 10−8 M (ORX+T) for 24 h. The LDH release (Figure 4a) was enhanced and viability (Figure 4b) was attenuated upon blockade of α1- and β2-adrenoceptors. On the contrary, LDH release (Figure 4a) was attenuated and the viability (Figure 4b) was enhanced upon blockade of β1- and β2-adrenoceptors. The injury caused by blockade of the α1-adrenoceptor was markedly greater than the beneficial effect of blockade of the β1-adrenoceptor (Figures 4a and b). This was in contrast to the findings in myocytes not incubated with testosterone: a much smaller difference in LDH release between α1-adrenoceptor blockade in the presence of benoxathian and blockade of β1-adrenoceptors with CGP (Figure 4c), and no significant difference in viability between α1- and β1-adrenoceptor blockades (Figure 4d).
Figure 4.
Effects of MIA/R in the presence of noradrenaline (NA) on the release of LDH and viability of left ventricular myocytes from ORX rats upon blockade of either α1- and β2-adrenoceptors or β1- and β2-adrenoceptors. For blockade of α1-adrenoceptors, two selective α1-adrenoceptor antagonists, 10−6 M prazosin (PZ) or 2 × 10−6 M benoxathian (Beno), were administered. The β1-adrenoceptor-selective antagonist CGP at 5 × 10−7 M in the presence of ICI at 5 × 10−7 M was used to block both β-adrenoceptor subtypes. NA (10−7 M) was administered. (a) LDH release from myocytes with or without testosterone replacement. Left panels indicate % release after different treatments and right panels indicate net changes in LDH release after blockade of either α1- and β2-adrenoceptors or β1- and β2-adrenoceptors. (b) Viability of myocytes with or without testosterone replacement. Left panels indicate viability after different treatments and right panels indicate net changes in viability after blockade of either α1- and β2-adrenoceptors or β1- and β2-adrenoceptors. The results are expressed as percentage of noradrenaline and antagonist-stimulated change in LDH release/viability. Where appropriate, data are expressed as percentage of baseline (i.e., 100% × induced value/value before stimulation. Data represent mean±s.e.mean of triplicate determinations in eight independent experiments using cell preparations from different rats. Differences between multiple groups were determined by one-way ANOVA followed by post hoc Neuman–Keuls multiple comparison tests. *P<0.05, **P<0.01 vs MIA/R. +, ++, +++ P<0.05, 0.01, 0.001 vs MIA/R with NA, respectively.### P<0.001 vs CGP. CGP, CGP 20712A; ICI, ICI 118, 551; MIA/R, metabolic inhibition and anoxia/reperfusion; ORX, orchidectomized male rats.
Effects of adrenergic stimulation and ischaemia/reperfusion on cardiac variables and reperfusion arrhythmias in the isolated heart from sham control, ORX and ORX+T rats in the presence of either α1- or β1-adrenoceptor blockade
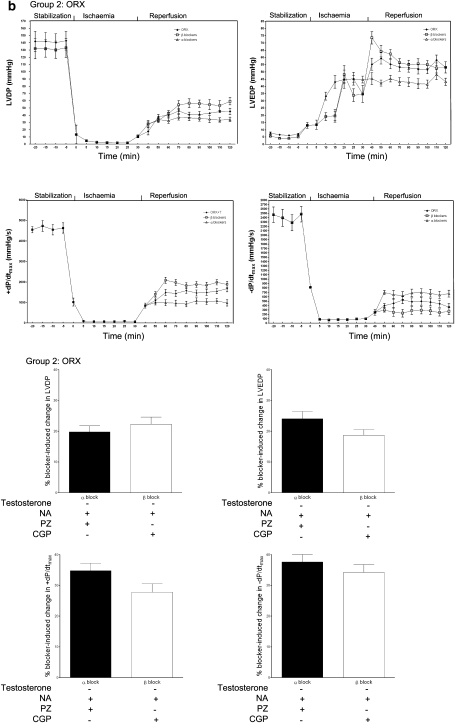
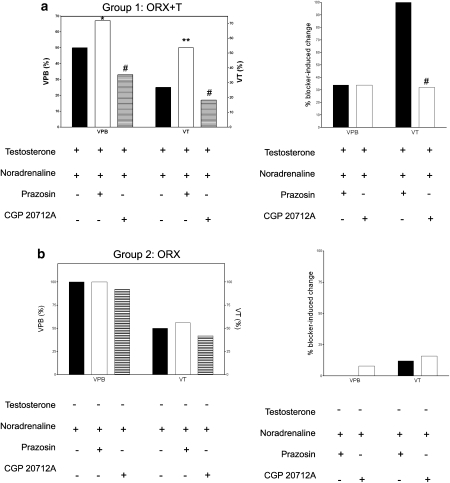
To substantiate the beneficial effects of cross-talk between testosterone and the α1-adrenoceptor and the harmful effects of cross-talk between testosterone and the β1-adrenoceptor, we compared the post-ischaemic recovery of contractile functions and arrhythmias in presence of β1- and β2-adrenoceptor blockers, and effects of blocking α1- and β2-adrenoceptors in the isolated hearts. The post-ischaemic recovery of LVDP and ±dP/dtmax was attenuated, whereas that of the LVEDP was enhanced upon blockade of α1- and β2-adrenoceptors (Figure 5a upper panel). On the contrary, the post-ischaemic recovery of LVDP and ±dP/dtmax was enhanced, whereas that of the LVEDP was attenuated upon blockade of β1- and β2-adrenoceptors (Figure 5a upper panel). The changes in LVDP and +dP/dtmax were greater, whereas the change in LVEDP was smaller after α1-adrenoceptor blockade than after β1-adrenoceptor blockade (Figure 5a lower panel). This was in contrast to the isolated hearts not incubated with testosterone: smaller and insignificant differences in post-ischaemic recovery of LVDP, ±dP/dtmax and LVEDP upon blockade of either α1- or β1-adrenoceptors (Figure 5b). There was a slight, but significant, greater change in +dP/dtmax upon α1-adrenoceptor blockade than β1-adrenoceptor blockade (Figure 5b lower panel). As seen with injury and contractile recovery, arrhythmias during reperfusion were significantly enhanced upon blockade of α1-adrenoceptor blockade but significantly increased upon β1-adrenoceptor blockade (Figure 6a). More importantly, the increase in incidence of VT following α1-adrenoceptor blockade was 23%, which was significantly greater than that following β1-adrenoceptor blockade (Figure 6a). In the absence of testosterone, blockade of either adrenoceptor did not lead to any significant difference in arrhythmias (Figure 6b).
Figure 5.
Effects of ischaemic insult in the presence of noradrenaline (NA) on cardiac contractile variables in the isolated hearts from ORX and ORX+T rats upon blockade of either α1- and β2-adrenoceptors or β1- and β2-adrenoceptors. For blockade of α1-adrenoceptors, the selective α1-adrenoceptor antagonist 10−6 M prazosin (PZ) was administered. The β1-adrenoceptor-selective antagonist CGP at 5 × 10−7 M in the presence of ICI at 5 × 10−7 M was used to block both β-adrenoceptor subtypes. Noradrenaline (10−7 M) was administered. Analyses were made during stabilization (baseline), ischaemia and reperfusion in (a) ORX+T and (b) ORX rats. LVDP, left ventricular developed pressure; LVEDP, left ventricular end-diastolic pressure; ±dP/dtmax, velocity of contraction and relaxation, respectively. Lower panel: net changes in contractile variables after blockade of either α1- and β2-adrenoceptors or β1- and β2-adrenoceptors. Values are mean±s.e.mean of 9–12 rats. *P<0.05, **P<0.01 vs ORX+T; # P<0.05, ## P<0.01, ### P<0.001 vs CGP. CGP, CGP 20712A; ICI, ICI 118, 551; ORX, orchidectomized male rats; ORX+T, orchidectomized male rats with testosterone replacement (200 μg per 100 g).
Figure 6.
Effects of ischaemic insult in the presence of noradrenaline on reperfusion arrhythmias in the isolated hearts from ORX and ORX+T rats upon blockade of either α1- and β2-adrenoceptors or β1- and β2-adrenoceptors. The protocol shown in Figure 5 was adopted. Values shown are the percentage of hearts exhibiting VPBs and VT in (a) ORX+T and (b) ORX rats over the first 30 min reperfusion period. Values are mean±s.e.mean rounded to the nearest whole number. Right panels: net changes in VPBs and VT after blockade of either α1- and β2-adrenoceptors or β1- and β2-adrenoceptors. Values are mean±s.e.mean of 9–12 rats. *P<0.05, ** P<0.01 relative to vehicle control and#P<0.05 relative to prazosin by χ2 test for percentages. ORX, orchidectomized male rats; ORX+T, orchidectomized male rats with testosterone replacement (200 μg per 100 g); VPBs, ventricular premature beats; VT, ventricular tachycardia.
Expression of α1- and β1-adrenoceptors in the isolated heart from sham control, ORX and ORX+T rats
The expression of α1A- and β1-adrenoceptors was lower in hearts from ORX rats than sham control (Figure 7), whereas that of α1B and α1D-adrenoceptors was unchanged (data not shown). Testosterone replacement (ORX+T) restored the expression of α1A- and β1-adrenoceptors (Figure 7).
Figure 7.
Effect of orchidectomy (ORX) on the expression of α1A- and β1-adrenoceptors in the rat heart. The expression of subtypes of α1- and β1-adrenoceptors was evaluated by western blotting from sham control, ORX and ORX+T as described in Methods. The relative arbitrary unit for sham control group was assigned as 1. Data are normalized to average value of sham control obtained from the same blot. Values are mean±s.e.mean of 5–6 rats. *P<0.05 vs control; **P<0.01 vs control; #P<0.05 vs ORX. ORX, orchidectomized male rats; ORX+T, orchidectomized male rats with testosterone replacement (200 μg per 100 g).
Discussion
The first important finding of the present study was that testosterone reduced injury and arrhythmias and improved contractile recovery during ischaemia and reperfusion and upon increased sympathetic stimulation in vitro, a situation that mimics myocardial ischaemia in vivo, when there is an increased sympathetic discharge to the heart. The observation indicates that testosterone confers cardioprotection against ischaemic insult. The protective effects were observed in both the isolated perfused heart, a model that is close to the in vivo situation, and cardiomyocytes, showing that the effects were directly on the myocardium. This finding is also in agreement with a previous observation that testosterone is associated with a reduced susceptibility to myocardial ischaemia (Callies et al., 2003). The advance in the present study is that we showed that the protective effect of testosterone is due to a direct action on the myocardium in addition to the beneficial effects of androgen, such as dilation of the coronary artery (Weidemann and Hanke, 2002). The cardioprotective action of testosterone observed previously (Callies et al., 2003) and in the present study provides an explanation of the observation that administration of testosterone improves the angina threshold in men (English et al., 2000b; Malkin et al., 2004).
Another important finding was that testosterone enhanced the effects of stimulation of both α1- and β1-adrenoceptors, thus enhancing both cardioprotection and injury, respectively. The effects of testosterone were abolished or attenuated upon androgen receptor blockade, indicating that the effects were mainly androgen receptor mediated. Interestingly, the enhanced cardioprotective effects (reduced injury and arrhythmias, and improved contractile recovery) of cross-talk between testosterone and the α1-adrenoceptor were greater than the deleterious effect of cross-talk between testosterone and the β1-adrenoceptor, and therefore the overall effects were beneficial. The β1-adrenoceptor has long been known to be the predominant receptor in the heart, mediating the sympathetic stimulation of cardiac contraction. This is in agreement with recent observations that myocytes from male mice with double knockout of the α1A- and α1B-adrenoceptor subtypes are more susceptible to apoptosis after oxidative and β-adrenoceptor stimulation, and survival of these mice in response to pressure overload by transverse aortic constriction is significantly reduced (O'Connell et al., 2006). Taken together, the previous findings and the present study provide evidence that the α1-adrenoceptor may play an important role in cardioprotection, particularly in the male.
There are three α1-adrenoceptor subtypes, namely, α1A, α1B and α1D, having different actions. In the present study, we found that testosterone upregulated the expression of the α1A subtype, suggesting that α1A-adrenoceptor may be the main receptor subtype cross-talking with testosterone. In support of this, studies from transgenic mice showed that overexpression of the α1A-adrenoceptor improved outcome after myocardial infarction (Rorabaugh et al., 2005; Du et al., 2006), enhanced cardiac contractility (Lin et al., 2001) and reduced arrhythmias (Woodcock, 2007), which are in agreement with our findings from experiments with selective agonists and antagonists that activation of the α1-adrenoceptor led to reduced cardiac injury, increased contractile recovery and reduced arrhythmias in response to ischaemic insult, whereas the opposite was observed with blockade of the receptor. In addition, a previous study showed that the α1-adrenoceptor is antiapoptotic (Iwai-Kanai et al., 1999). Further study is urgently needed to determine the signalling mechanisms of the α1-adrenoceptor subtypes in cardioprotection conferred by testosterone.
In the present study, our focus on α1- and β1-adrenoceptors was based on the following considerations. Firstly, we believed that α1- and β1-adrenoceptors were mainly responsible for cardioprotection and injury, respectively. Secondly, α2-adrenoceptors are not present in the heart (Porter et al., 2003) and the β3-adrenoceptor is not expressed in the rat heart (Evans et al., 1996; Gauthier et al., 1999). It should, however, be noted that the expression of the β2-adrenoceptor, which is also antiapoptotic (Communal et al., 1999; Patterson et al., 2004), was downregulated in rats without testosterone (data not shown), indicating that testosterone may also interact with this receptor subtype. In the present study, the β2-adrenoceptor was blocked, thus allowing us to compare the contribution of the α1-adrenoceptor, which was beneficial, and the β1-adrenoceptor, which was deleterious. Interestingly, even without β2-adrenoceptors, the beneficial effect of testosterone on cardioprotection was already greater than the deleterious effect, suggesting that testosterone may be even more beneficial than demonstrated in the experimental conditions of the present study.
We found that the enhancing effects of testosterone on α1-adrenoceptor stimulation were completely abolished by overnight incubation with cyproterone acetate, indicating that these were genomic, androgen receptor-mediated events. On the other hand, the enhancing effect of testosterone on α1-adrenoceptor action on cell viability was only attenuated by the androgen receptor antagonist, indicating that testosterone may have non-genomic actions. It has been shown that endogenous testosterone induces cytoprotection via activating cardiac mitochondrial ATP-sensitive K+ channels, an effect not mediated by the androgen receptor (Er et al., 2004). These effects are acute, suggesting that they are non-genomic actions and independent of the androgen receptor. This may explain, at least in part, why blockade of its receptor did not abolish the effect of testosterone on cell survival (Er et al., 2004).
Two recent studies have shown that chronic administration of testosterone at physiological concentrations enhances Ca2+ influx via the L-type Ca2+ channel in neonatal (Michels et al., 2006) and adult (Er et al., 2007) rat ventricular myocytes. In the adult ventricular myocyte, testosterone also increases the Ca2+ spark, indicating an increase in Ca2+ release from the sarcoplasmic reticulum. These effects are abolished upon androgen receptor blockade, indicating receptor-mediated events. It has also been shown that testosterone increases the Na+–Ca2+ exchange mRNA level in the heart (Golden et al., 2004), suggesting increased activity of the exchanger, which is responsible for Ca2+ removal. So testosterone may alter Ca2+ homeostasis, thus attenuating the [Ca2+]i overload in response to ischaemic insult and conferring cardioprotection.
It is believed that conversion of testosterone to oestrogen by aromatization may also be responsible for the protective action of testosterone. In the present study, the effects of testosterone were attenuated by androgen receptor blockade, indicating that its actions are mainly mediated via its receptor. So it is unlikely that oestrogen contributes significantly to the protective action of testosterone.
We found that the heart weights differed among groups, which may reflect differences in many parameters such as wall thickness and ventricular volume. So, the cardiac responses to ischaemic insult may not have been due to the presence or absence of testosterone only, but rather the differences in heart weight. However, the cardiac responses to ischaemic insult were similar in the sham and ORX+T groups, which had different heart weights, indicating that the responses to ischaemic insult were not directly correlated with heart size. In support of this, we found in a previous study that after ovariectomy, the heart weight was the same in the sham and ovariectomy groups, but the cardiac responses to ischaemic insult were significantly different (Kam et al., 2004, 2005). So, it is unlikely that the different responses to ischaemic insults in different groups were due to heart weight.
IHD is a leading cause of mortality, particularly in the aged, who have low testosterone levels. That testosterone enhances the cardioprotective effect of α-adrenoceptor stimulation makes testosterone particularly useful for the treatment of IHD in patients with low testosterone, particularly aged patients.
In conclusion, the present study has provided the first evidence that testosterone upregulated the expression of α1-adrenoceptors and enhanced the cardiac responses to their stimulation, thus reducing cardiac injury. Testosterone also improved contractile recovery and reduced arrhythmia upon ischaemia and reperfusion. The effects of testosterone were mediated by androgen receptors. Further studies are warranted to delineate the signalling mechanisms and to explore the possibility of using testosterone and α1-adrenoceptor activators in the aging male population with IHDs.
External data objects
Acknowledgments
We thank Professor IC Bruce for his comments on the paper, particularly on the use of English, and Professors P Vanhoutte, Y Huang and Q Xia, and Dr G Leung for advice. We also thank Mr CP Mok for assistance. This work was supported by the Strategic Theme on Healthy Aging, a grant from the Committee for Research and Conference Grants, HKU, and the Cardiovascular Research Fund donated by LCST (Holdings) Ltd.
Abbreviations
- CGP
CGP 20712A
- Cyp
cyproterone acetate
- ICI
ICI 118, 551
- IHD
ischaemic heart disease
- LDH
lactate dehydrogenase
- MIA/R
metabolic inhibition, anoxia and reperfusion
- ORX
orchidectomized male rats
- ORX+T
orchidectomized male rats with testosterone replacement (200 μg per 100 g)
- PVB
premature ventricular beats
- VT
ventricular tachycardia
Conflict of interest
The authors state no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Banu KS, Govindarajulu P, Aruldhas MM. Testosterone and estradiol modulate TSH-binding in the thyrocytes of Wistar rats: influence of age and sex. J Steroid Biochem Mol Biol. 2001;78:329–342. doi: 10.1016/s0960-0760(01)00107-8. [DOI] [PubMed] [Google Scholar]
- Callies F, Stromer H, Schwinger RH, Bolck B, Hu K, Frantz S, et al. Administration of testosterone is associated with a reduced susceptibility to myocardial ischemia. Endocrinology. 2003;144:4478–4483. doi: 10.1210/en.2003-0058. [DOI] [PubMed] [Google Scholar]
- Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the β-adrenergic pathway. Circulation. 1998;98:1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of β1- and β2-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- Du XJ, Gao XM, Kiriazis H, Moore XL, Ming Z, Su Y, et al. Transgenic α1A-adrenergic activation limits post-infarct ventricular remodeling and dysfunction and improves survival. Cardiovasc Res. 2006;71:735–743. doi: 10.1016/j.cardiores.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Engelhard K, Werner C, Kaspar S, Mollenberg O, Blobner M, Bachl M, et al. Effect of the α2-agonist dexmedetomidine on cerebral neurotransmitter concentrations during cerebral ischemia in rats. Anesthesiology. 2002;96:450–457. doi: 10.1097/00000542-200202000-00034. [DOI] [PubMed] [Google Scholar]
- English KM, Mandour O, Steeds RP, Diver MJ, Jones TH, Channer KS. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur Heart J. 2000a;21:890–894. doi: 10.1053/euhj.1999.1873. [DOI] [PubMed] [Google Scholar]
- English KM, Steeds RP, Jones TH, Diver MJ, Channer KS. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled study. Circulation. 2000b;102:1906–1911. doi: 10.1161/01.cir.102.16.1906. [DOI] [PubMed] [Google Scholar]
- Er F, Michels G, Brandt MC, Khan I, Haase H, Eicks M, et al. Impact of testosterone on cardiac L-type calcium channels and Ca2+ sparks: acute actions antagonize chronic effects. Cell Calcium. 2007;41:467–477. doi: 10.1016/j.ceca.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Er F, Michels G, Gassanov N, Rivero F, Hoppe UC. Testosterone induces cytoprotection by activating ATP-sensitive K+ channels in the cardiac mitochondrial inner membrane. Circulation. 2004;110:3100–3107. doi: 10.1161/01.CIR.0000146900.84943.E0. [DOI] [PubMed] [Google Scholar]
- Evans BA, Papaioannou M, Bonazzi VR, Summers RJ. Expression of β3-adrenoceptor mRNA in rat tissues. Br J Pharmacol. 1996;117:210–216. doi: 10.1111/j.1476-5381.1996.tb15176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego M, Setien R, Puebla L, Boyano-Adanez Mdel C, Arilla E, Casis O. α1-Adrenoceptors stimulate a Gαs protein and reduce the transient outward K+ current via a cAMP/PKA-mediated pathway in the rat heart. Am J Physiol Cell Physiol. 2005;288:C577–C585. doi: 10.1152/ajpcell.00124.2004. [DOI] [PubMed] [Google Scholar]
- Gauthier C, Tavernier G, Trochu JN, Leblais V, Laurent K, Langin D, et al. Interspecies differences in the cardiac negative inotropic effects of β3-adrenoceptor agonists. J Pharmacol Exp Ther. 1999;290:687–693. [PubMed] [Google Scholar]
- Golden KL, Marsh JD, Jiang Y. Testosterone regulates mRNA levels of calcium regulatory proteins in cardiac myocytes. Horm Metab Res. 2004;36:197–202. doi: 10.1055/s-2004-814445. [DOI] [PubMed] [Google Scholar]
- Iwai-Kanai E, Hasegawa K, Araki M, Kakita T, Morimoto T, Sasayama S. α- and β-adrenergic pathways differentially regulate cell type-specific apoptosis in rat cardiac myocytes. Circulation. 1999;100:305–311. doi: 10.1161/01.cir.100.3.305. [DOI] [PubMed] [Google Scholar]
- Kam KW, Kravtsov GM, Liu J, Wong TM. Increased PKA activity and its influence on isoprenaline-stimulated L-type Ca2+ channels in the heart from ovariectomized rats. Br J Pharmacol. 2005;144:972–981. doi: 10.1038/sj.bjp.0706123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam KW, Qi JS, Chen M, Wong TM. Estrogen reduces cardiac injury and expression of β1-adrenoceptor upon ischemic insult in the rat heart. J Pharmacol Exp Ther. 2004;309:8–15. doi: 10.1124/jpet.103.058339. [DOI] [PubMed] [Google Scholar]
- Lin F, Owens WA, Chen S, Stevens ME, Kesteven S, Arthur JF, et al. Targeted α1A-adrenergic receptor overexpression induces enhanced cardiac contractility but not hypertrophy. Circ Res. 2001;89:343–350. doi: 10.1161/hh1601.095912. [DOI] [PubMed] [Google Scholar]
- Malkin CJ, Pugh PJ, Morris PD, Kerry KE, Jones RD, Jones TH, et al. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90:871–876. doi: 10.1136/hrt.2003.021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JD, Lehmann MH, Ritchie RH, Gwathmey JK, Green GE, Schiebinger RJ. Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation. 1998;98:256–261. doi: 10.1161/01.cir.98.3.256. [DOI] [PubMed] [Google Scholar]
- McGill HC, Jr, Anselmo VC, Buchanan JM, Sheridan PJ. The heart is a target organ for androgen. Science. 1980;207:775–777. doi: 10.1126/science.6766222. [DOI] [PubMed] [Google Scholar]
- Michels G, Er F, Eicks M, Herzig S, Hoppe UC. Long-term and immediate effect of testosterone on single T-type calcium channel in neonatal rat cardiomyocytes. Endocrinology. 2006;147:5160–5169. doi: 10.1210/en.2006-0186. [DOI] [PubMed] [Google Scholar]
- O'Connell TD, Swigart PM, Rodrigo MC, Ishizaka S, Joho S, Turnbull L, et al. α1-adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J Clin Invest. 2006;116:1005–1015. doi: 10.1172/JCI22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AJ, Zhu W, Chow A, Agrawal R, Kosek J, Xiao RP, et al. Protecting the myocardium: a role for the β2-adrenergic receptor in the heart. Crit Care Med. 2004;32:1041–1048. doi: 10.1097/01.ccm.0000120049.43113.90. [DOI] [PubMed] [Google Scholar]
- Pei JM, Kravtsov GM, Wu S, Das R, Fung ML, Wong TM. Calcium homeostasis in rat cardiomyocytes during chronic hypoxia: a time course study. Am J Physiol Cell Physiol. 2003;285:C1420–C1428. doi: 10.1152/ajpcell.00534.2002. [DOI] [PubMed] [Google Scholar]
- Phillippe M, Saunders T, Bangalore S. A mechanism for testosterone modulation of α1-adrenergic receptor expression in the DDT1 MF-2 smooth muscle myocyte. Mol Cell Biochem. 1991;100:79–90. doi: 10.1007/BF00230812. [DOI] [PubMed] [Google Scholar]
- Porter AC, Svensson SP, Stamer WD, Bahl JJ, Richman JG, Regan JW. α2-adrenergic receptors stimulate actin organization in developing fetal rat cardiac myocytes. Life Sci. 2003;72:1455–1466. doi: 10.1016/s0024-3205(02)02381-0. [DOI] [PubMed] [Google Scholar]
- Rorabaugh BR, Ross SA, Gaivin RJ, Papay RS, McCune DF, Simpson PC, et al. α1A- but not α1B-adrenergic receptors precondition the ischemic heart by a staurosporine-sensitive, chelerythrine-insensitive mechanism. Cardiovasc Res. 2005;65:436–445. doi: 10.1016/j.cardiores.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Schomig A. Catecholamines in myocardial ischemia. Systemic and cardiac release. Circulation. 1990;82 Suppl 3:II13–II22. [PubMed] [Google Scholar]
- Schomig A, Richardt G. The role of catecholamines in ischemia. J Cardiovasc Pharmacol. 1990;16 Suppl 5:S105–S112. [PubMed] [Google Scholar]
- Shen H, Peri KG, Deng XF, Chemtob S, Varma DR. Distribution of α1-adrenoceptor subtype proteins in different tissues of neonatal and adult rats. Can J Physiol Pharmacol. 2000;78:237–243. [PubMed] [Google Scholar]
- Tsang S, Liu J, Wong TM. Testosterone and cardioprotection against myocardial ischemia. Cardiovasc Hematol Disord Drug Targets. 2007;7:119–125. doi: 10.2174/187152907780830888. [DOI] [PubMed] [Google Scholar]
- Tsang S, Wu S, Das R, Wong TM. Testosterone confers cardioprotection by up-regulating α1- adrenoceptors. [Abstract] J Mol Cell Cardiol. 2005;39:563. [Google Scholar]
- Waldenstrom AP, Hjalmarson AC, Thornell L. A possible role of noradrenaline in the development of myocardial infarction: an experimental study in the isolated rat heart. Am Heart J. 1978;95:43–51. doi: 10.1016/0002-8703(78)90395-2. [DOI] [PubMed] [Google Scholar]
- Walker MJ, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, et al. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- Wang GY, Wu S, Pei JM, Yu XC, Wong TM. κ- but not δ-opioid receptors mediate effects of ischemic preconditioning on both infarct and arrhythmia in rats. Am J Physiol Heart Circ Physiol. 2001;280:H384–H391. doi: 10.1152/ajpheart.2001.280.1.H384. [DOI] [PubMed] [Google Scholar]
- Weidemann W, Hanke H. Cardiovascular effects of androgens. Cardiovasc Drug Rev. 2002;20:175–198. doi: 10.1111/j.1527-3466.2002.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Woodcock EA. Roles of α1A- and α1B-adrenoceptors in heart: insights from studies of genetically modified mice. Clin Exp Pharmacol Physiol. 2007;34:884–888. doi: 10.1111/j.1440-1681.2007.04707.x. [DOI] [PubMed] [Google Scholar]
- Wu S, Li HY, Wong TM. Cardioprotection of preconditioning by metabolic inhibition in the rat ventricular myocyte. Involvement of κ-opioid receptor. Circ Res. 1999;84:1388–1395. doi: 10.1161/01.res.84.12.1388. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Cheng H, Zhou YY, Wang DJ, Zhu W, Ziman B, et al. Inhibition of spontaneous β2-adrenergic activation rescues β1-adrenergic contractile response in cardiomyocytes overexpressing β2-adrenoceptor. J Biol Chem. 2000;275:21773–21779. doi: 10.1074/jbc.M909484199. [DOI] [PubMed] [Google Scholar]
- Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, et al. Linkage of β1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.