Abstract
Background and purpose:
Characterization of human embryonic stem cell-derived cardiomyocytes (hESC-CM) in relation to adult myocytes is essential for their future use in transplantation or as a model system. The β-adrenoceptor pathways, which are known to be effective early in hESC-CM development, are of major importance because of their control of rate and force of beating, arrhythmia generation and apoptosis/necrosis. We have therefore performed detailed pharmacological analysis of the β-adrenoceptor responses in developing hESC-CM.
Experimental approach:
hESC-CMs were differentiated from H7 ESCs and studied up to 79 days of differentiation. Rate of beating and time course of contraction and relaxation were measured in superfused preparations.
Key results:
Responses to the mixed β1- and β2-adrenoceptor agonist isoprenaline were evident from day 10 to day 79. Stability of the responses during an application, for repeated applications on the same experimental day and over the time of development, was determined. Concentrations for half-maximal response (12.9 nM) were similar to those from adult human heart, but closer to those obtained from failing rather than normal ventricle. Acceleration of both contraction and relaxation was quantitatively similar to that in adult ventricular myocytes, as was sensitivity to muscarinic inhibition. Use of specific antagonists showed that both β1- and β2-adrenoceptors contributed to contractile responses, as seen with adult myocytes.
Conclusions and implications:
These data show the compatibility of hESC-CM with adult human myocardium in terms of β-adrenoceptor response. The experiments described here also confirm the utility of the hESC-CM preparation for detailed pharmacological analysis.
Keywords: β-adrenoceptor, cardiomyocyte, embryonic stem cell, human, muscarinic, beating rate
Introduction
Human embryonic stem cell-derived cardiomyocytes (hESC-CM) are a potential source of functionally intact cells for the treatment of cardiovascular diseases. Although the ability of some adult stem cell types to transdifferentiate into cardiac muscle has been questioned (Reinecke et al., 2002; Balsam et al., 2004; Murry et al., 2004), there is extensive evidence that hESCs can differentiate into functional cardiomyocytes in vitro (Kehat et al., 2001; Mummery et al., 2003; Reppel et al., 2004) and successfully engraft into adult animal hearts (of various species) (Kehat et al., 2004; Laflamme et al., 2005; Xue et al., 2005). There is also great interest in hESC-CM as a model preparation for the cardiac scientist and as a potential high-throughput test system for pharmaceutical efficacy and toxicity (Chaudhary et al., 2005; Harding et al., 2007). Initial studies have shown that hESC-CM express cardiac markers, develop a complete range of cardiac phenotypes in terms of contractile activity and electrophysiological properties, and respond appropriately to inotropic agents (Kehat et al., 2001; He et al., 2003; Reppel et al., 2004; Wei et al., 2005).
To realise the potential of hESC-CM, it is now important to take this further and perform more detailed analyses of their functional properties and pharmacological responses and to define how these develop with time in culture. Comparison of these properties with those of adult ventricular myocytes, from both normal and failing hearts, is essential to predict the behaviour of the hESC-CM after implantation and to characterize their utility as a test system. The β-adrenoceptor system is a canonical signalling pathway for the cardiomyocyte and, as such, has been the first one investigated in studies of hESC-CM. Stimulation of β-adrenoceptors by noradrenaline and adrenaline produces increases in beating rate, force of contraction and velocity of contraction/relaxation in adult cardiomyocytes. The relative effects on these parameters in hESC-CM will depend on the maturity of the excitation–contraction system, with acceleration of relaxation predicted to develop later, compared to increases in force and rate, because of a greater dependence of relaxation on intracellular sarcoplasmic reticulum (SR) Ca2+ uptake.
Human adult ventricular myocytes, unlike those of other species, can be stimulated maximally through either the β1- or β2-adrenoceptor subtype (del Monte et al., 1993). Although the stimulatory effects of both β1- and β2-adrenoceptors are mediated through the stimulatory guanine nucleotide binding protein (Gs)/adenylyl cyclase/cAMP system, the β2-adrenoceptor can also act via inhibitory guanine nucleotide binding proteins(s) Gi/Go to activate protective and/or cardiodepressant pathways, and there is evidence that this occurs in human ventricle (Brown and Harding, 1992). The relative effect of β1- and β2-adrenoceptors assumes a particular importance in human heart failure, where prolonged sympathetic drive leads to desensitization of the β-adrenoceptor response, with selective loss of β1-adrenoceptor and upregulation of inhibitory guanine nucleotide binding protein (Gi) (Lohse et al., 2003). As hESC-CM implanted into failing heart will also be exposed to this high sympathetic drive, it is important to quantitate their relative responses through the two subtypes. Similarly, the ability of the muscarinic system to oppose the β-adrenoceptor-mediated response through Gi needs to be characterized.
In this study, we define more precisely the sensitivity to catecholamines, the subtypes mediating contraction and relaxation, and the sensitivity to inhibition by the muscarinic system in hESC-CM derived from the H7 line, up to 79 days after differentiation. We also show that hESC-CMs are a viable pharmacological preparation in terms of stability of basal contractility and reproducibility of chronotropic responses.
Materials and methods
Methods
ESC culture
Human ESCs (H7 line), supplied by Geron Corporation (Menlo Park, CA, USA), were maintained under feeder cell-free conditions on Matrigel-coated six-well plates in mouse embryonic fibroblast-conditioned medium, supplemented with 8 ng ml−1 recombinant basic human fibroblast growth factor. Cells were fed daily by complete media change. The procedures for the preparation of mouse embryonic fibroblasts, mouse embryonic fibroblast-conditioned medium, subculturing and differentiation of H7 cells were as described by Geron Corporation (http://www.geron.com/PDF/scprotocols.pdf), with the exception of mouse strain used (MF-1 mouse, instead of CF-1) and the method of mitotic inactivation of mouse embryonic fibroblasts (treatment with 0.01 mg ml−1 mitomycin C instead of irradiation). Briefly, after isolation and expansion in culture, mouse embryonic fibroblasts were mitotically inactivated at passage 3. After overnight attachment of 12 million inactive mouse embryonic fibroblasts onto pre-gelatinized T225 flasks in 10% fetal calf serum, medium was replaced by ‘human embryonic stem medium' containing 20% knockout serum replacement, 1 mM L-glutamine, 10 mM non-essential amino acids (1% of stock), 0.1 mM β-mercaptoethanol, antibiotics (50 U ml−1 penicillin 50 μg ml−1 streptomycin) and 4 ng ml−1 basic human fibroblast growth factor. Collections of 150 ml of mouse embryonic fibroblast-conditioned medium were made daily for a week. Undifferentiated H7 cells were fed each day with filtered mouse embryonic fibroblast-conditioned medium, which was further supplemented with 8 ng ml−1 basic human fibroblast growth factor. Before subculturing or induction of differentiation, the latter usually 1 week following subculturing, spontaneously differentiated fibroblast-like cells among the ESC colonies were removed by treatment with collagenase for 10 min at 37 °C. H7 embryoid bodies (EBs) were made by mechanically breaking the hESC colonies (scraping with the tip of a 5-ml pipette), followed by culturing for 4 days in suspension (in low-adherence six-well plates) in hESC differentiation medium (20% fetal calf serum without basic human fibroblast growth factor). The differentiation medium contained 80% knockout Dulbecco's modified Eagle's medium, 1 mM L-glutamine, 0.1 mM β-mercaptoethanol, 10 mM non-essential amino acids and 20% non-heat-inactivated fetal calf serum. After 4 days in suspension, 1–3 EBs were transferred onto gelatin-coated glass coverslips (10 mm in diameter) in the centre of 35 mm dishes (MatTek Corp., Ashland, MA, USA) and cultured for additional days as described in Results.
Immunostaining
Adherent EBs were rinsed with PBS and fixed with 4% paraformaldehyde for 10 min at room temperature. After three 5-min washes in PBS, cells were permeabilized with 0.02% Triton X-100 in ‘blocking' solution (1% BSA in PBS) for 10 min. The samples were incubated with the primary antibody (mouse monoclonal (3–48) cardiac myosin heavy-chain antibody (Ab15)) at 1:200 dilution in 1% BSA/PBS at room temperature for 2 h, washed (3 × in PBS as above), mounted and visualized using a confocal microscope (Leica systems) after incubating for 1 h at room temperature with FITC (fluorescein isothiocyanate)-conjugated secondary antibody (Mouse IgG antibody-FITC, Ab6785, 1:800 dilution in 1% BSA/PBS).
Measurement of contraction
Beating EBs in MatTek dishes were transferred to the stage on a Nikon TE2000 microscope and superfused with Krebs–Henseleit solution (in mM): NaCl 119, KCl 4.7, MgSO4 0.94, KH2PO4 1.2, NaHCO3 25, glucose 11.5, CaCl2 2, continuously bubbled with 5% CO2/ 95% O2, at a rate of 2 ml min−1. The perfusate was warmed to 32 °C by a heater placed next to the chamber inlet. hESC-CMs are tracked in the same way as adult cardiomyocytes, using a video edge detection system (SP143/4, spatial resolution 1:256, time resolution, 50 ms), with the amorphous clusters of beating cells seeded with 5–30 μm particles of inactivated charcoal to provide high-contrast points. Rate of beating and time-to-peak shortening (TTP) or time from peak to 50/90% relaxation (R50/90) were recorded. Measurements were made during spontaneous contractions, in the absence and presence of the β-adrenoceptor agonist isoprenaline (Sigma) and antagonists (CGP 20712A (0.3 μM; a β1-adrenoceptor antagonist) or ICI 118,551 (ICI) (50 nM; a β2-adrenoceptor antagonist)) or muscarinic the agonist carbachol (10 μM), as indicated. For concentration–response curves, applications of different concentrations were made non-cumulatively and the order of application was varied on a Latin square design. These concentrations have previously been determined as selective for the β1- and β2-adrenoceptor subtypes and the muscarinic receptor. During the frequency–response experiments, hESC-CMs were paced through platinum wires at 60, 120 or 180 b.p.m. (beats per min) with a biphasic pulse of 50 V.
Statistical methods
Results are expressed as mean±s.e.mean (or s.d., when n>30) for beating rate, and geometric mean with 95% confidence intervals for concentration to reach half-maximal effect (EC50). n values given are for number of beating foci; one beating focus per coverslip was studied. Differences between multiple responses were analysed using one-way analysis of variance with Tukey's post hoc test. Log concentration–response curves were fitted to a sigmoid curve using Graphpad Prism 4.
Materials
All tissue culture materials were purchased from Gibco/Invitrogen (Paisley, Scotland), unless otherwise stated. Nunc Petri dishes and tissue culture plasticware were from Fisher Scientific UK (Loughborough, UK). Antibodies were obtained from Abcam Ltd (Cambridge Science Park, Cambridge, UK).
Results
Cardiomyocyte differentiation in H7 cells
Spontaneous cardiac differentiation was initiated by inducing EB formation from undifferentiated hESC (Figures 1a and b). After 4 days of culturing in suspension, EBs were plated out onto 0.5% gelatin-coated glass coverslips. From days 9–12 (d9–d12) following induction of differentiation, spontaneously beating cardiomyocytes emerged as rhythmically contracting clusters in the outgrowths of differentiating EBs. Although only a very few (approximately 1–2%) EBs with beating areas were seen at d9, this increased dramatically (70–80%) by d15–d16. We found that contracting cells were present in long-term cultures up to 6 months following differentiation (the longest time which we have cultured so far). Work described in this report was carried out on cells from d10 to d79 of differentiation.
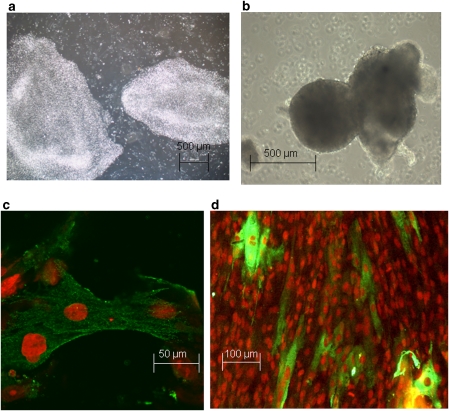
Figure 1.
(a) Two colonies of undifferentiated H7 hESC. (b) Embryoid bodies immediately after plating. (c, d) hESC-CM stained using propidium iodide for nuclei and an antibody against α- and β-cardiac-specific myosin heavy chains (MHC), followed by FITC (fluorescein isothiocyanate)-conjugated secondary antibody against mouse IgG. (c) Isolated hESC-CM from d14 cultures, confocal image. (d) Later cultures (d90), low-magnification view. hESC-CMs, human embryonic stem cell-derived cardiomyocytes.
The cardiac cell nature of the beating areas was further confirmed by immunostaining with an antibody that recognizes both α- and β-cardiac-specific myosin heavy chains. Figure 1c shows individual isolated hESC-CM from early (∼d14) cultures, whereas Figure 1d is a lower magnification view of later cultures (d90) demonstrating that hESC-CM are mixed with other cell types. The cardiac myosin heavy chain-positive cells corresponded well to the location of beating areas, which had been marked on the underside of the dish before fixation of cells for immunocytochemistry.
Pharmacological responses of hESC-CM
Positive chronotropic, inotropic and lusitropic responses to isoprenaline
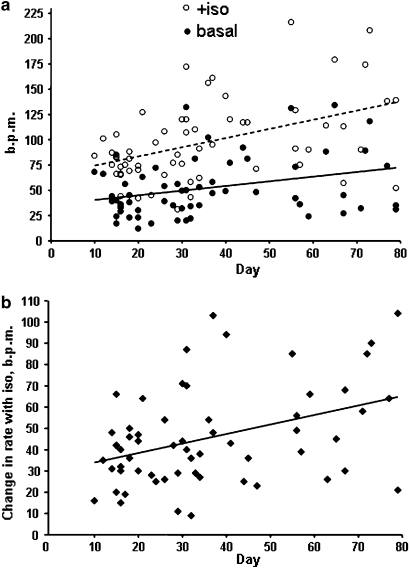
The average basal beating rate in hESC-CM over the whole study period from developmental day d10 to d79 was 52.1±29.8 b.p.m. (mean±s.d., n=60). There was a modest but statistically significant increase during this period (r2=0.097, P<0.02, n=60, Figure 2a, closed circles). Beating rate initially dropped significantly (P<0.02) over the first 30 min in the superfusion bath, from 49±7 to 38±7 b.p.m., but did not decline significantly between 30 min and 4 h (33±9 b.p.m.). All data were, therefore, taken during the steady period after 30 min equilibration.
Figure 2.
Variation of rate and responses with developmental day. (a) Increase in beating rate, beats per min (b.p.m.) with day of differentiation from d10 to d79 in hESC-CM under basal conditions (r2=0.097, n=60, P<0.02) or after stimulation with 100 nM isoprenaline (r2=0.20, n=60, P<0.001). (b) Change in beating rate in response to isoprenaline (stimulated basal, b.p.m.) (r2=0.15, P<0.002). hESC-CMs, human embryonic stem cell-derived cardiomyocytes.
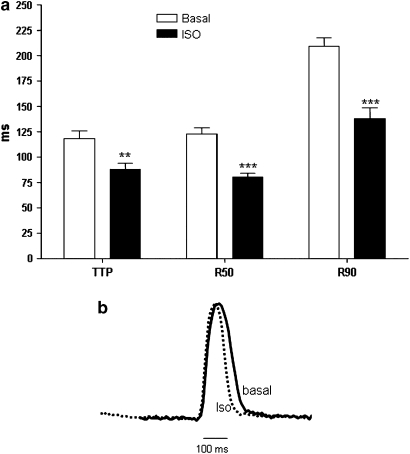
Isoprenaline (10−7 M) significantly (P<0.001) increased the rate to 97.8±40.4 b.p.m. (mean from d10 to d79; n=60); both rate (Figure 2a, open circles) and change in the rate from basal (response, Figure 2b) showed statistically significant increases with developmental day (rate: r2=0.20, P<0.001; response: r2=0.15, P<0.002). To determine the effect of isoprenaline on speed of contraction and relaxation, we used three parameters taken from averaged beats: time from baseline to peak (TTP), R50 and R90. Abbreviation of beat duration was observed in the presence of isoprenaline, with TTP, R50 and R90 significantly reduced (Figure 3). A sample beat is shown, normalized for contraction amplitude to show the more rapid relaxation in the presence of isoprenaline.
Figure 3.
Effect of isoprenaline on beat duration. (a) Time-to-peak contraction (TTP), time from peak to 50% relaxation (R50), time from peak to 90% relaxation (R90) in hESC-CM from nine experiments before (basal) and after (Iso) exposure to 10 μM isoprenaline (**P<0.01, ***P<0.001 vs basal). (b) Example trace of the contraction before and during exposure to isoprenaline. hESC-CMs, human embryonic stem cell-derived cardiomyocytes.
As increases in rate in adult myocytes will decrease beat duration per se, we compared the effect of pacing-induced increases in rate on hESC-CM. TTP values at 60, 90 and 180 b.p.m. were 109±16, 120±18 and 90±11 ms, respectively, n=8; these were significantly different by one-way ANOVA (P<0.001), but the only difference between 120 and 180 b.p.m. was statistically significant on the post-test (Tukey's, P<0.05). The corresponding R50 values were 130±13, 108±17 and 87±5 ms, and difference was significant (P<0.05) only between 60 and 180 b.p.m.
Desensitization of responses to isoprenaline
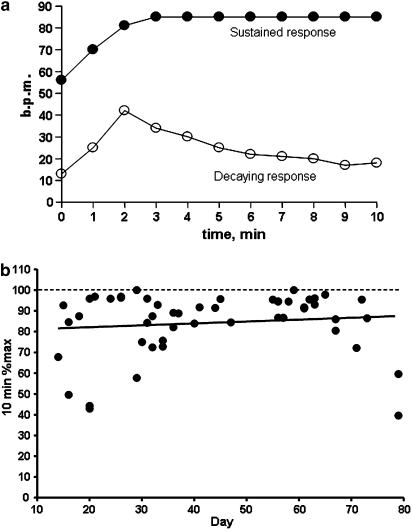
Previous results from our laboratory on mouse ESC-CM showed that β-adrenoceptor-dependent chronotropic effects were not always stable, with rate dropping back towards basal during continued application of isoprenaline (Ali et al., 2004). This was more evident at earlier days of differentiation, and indicated that a non-cumulative protocol for the construction of concentration–response curves was required. The results obtained for hESC-CMs were not so dramatic, with the response to isoprenaline relatively well maintained throughout the 10 min exposure. However, a number of preparations did show this type of desensitization, and this occurred with both early and later days of differentiation. Figure 4a shows examples of maintained and desensitizing responses, and Figure 4b shows the rate at 10 min after isoprenaline challenge relative to the maximum attained within the 10 min. Concentration–response curves to isoprenaline were therefore constructed in a non-cumulative manner with a Latin square design.
Figure 4.
(a) Time course of beating rate, b.p.m. (beats per min) for hESC-CM during 10 min of exposure to isoprenaline (Iso) on different days of development. Examples of sustained response in a d29 culture, and of decaying response in a d20 culture are shown. (b) Relation between desensitization and day of development (rate at 10 min expressed as a percentage of the maximum rate, r2=0.013, P=NS, n=51). hESC-CMs, human embryonic stem cell-derived cardiomyocytes; NS, nonsignificant.
Concentration–response curves to isoprenaline
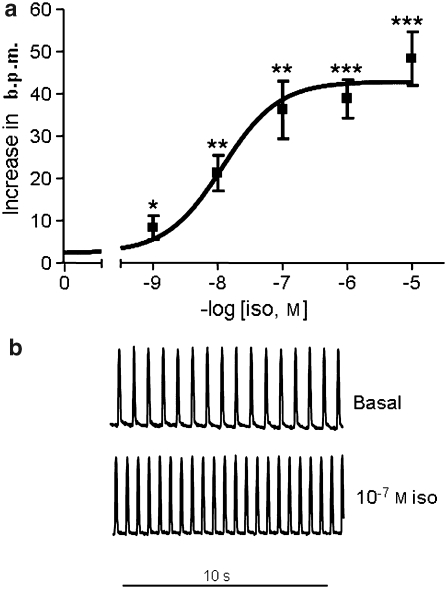
Human embryonic stem cell-derived cardiomyocytes were incubated with concentrations between 10−9 and 10−5 M isoprenaline, with washout between each application; increase in rate over preceding basal is shown in Figure 5. Although fit to a sigmoid curve with variable slope function gave a Hill coefficient of 0.5, this was not significantly (P>0.05) better fitting than a single sigmoid curve with Hill slope of 1. The concentration for half-maximal effect (EC50) was 12.9 nM (95% confidence intervals 3.9–35.8 nM, n=8) and mean rate increased significantly (P<0.001) by 144±42% over initial basal rate (P<0.001) at maximum.
Figure 5.
Concentration–response curve to isoprenaline in hESC-CM. (a) Increase in beating rate, beats per min (b.p.m.) at each concentration (mean±s.e.mean, n=7, *P=0.02, **P<0.01, **P<0.001 vs basal). (b) Example traces of hESC-CM contraction before and during exposure to 100 nM isoprenaline (iso). hESC-CMs, human embryonic stem cell-derived cardiomyocytes.
Subtype dependence of the β-adrenoceptor response
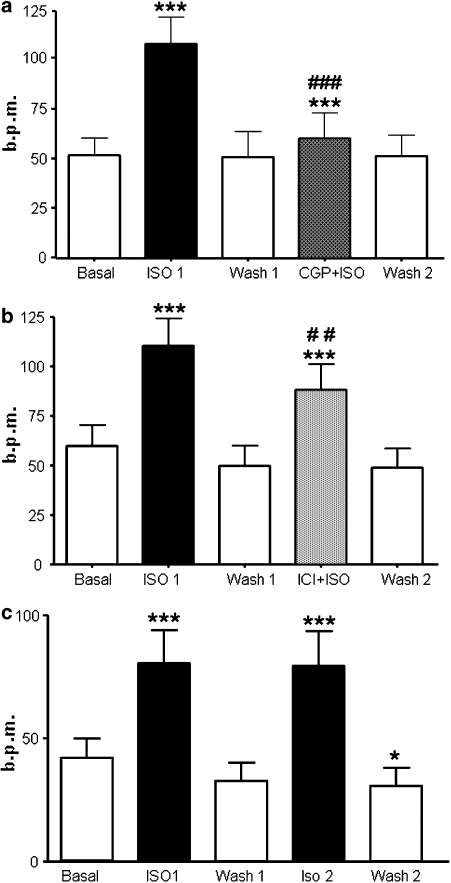
Responses of the spontaneous beating rates of ESC-CM to isoprenaline were measured first in the absence and then in the presence of subtype-selective antagonists (Figure 6), the β1-adrenoceptor blocker CGP 20712A (0.3 μM) or the β2-adrenoceptor blocker ICI (50 nM). The increase in beating rate with isoprenaline was significantly (n=11, P<0.001) reduced in the presence of CGP 20712A (Figure 6a), indicating a strong contribution of the β1-adrenoceptor. However, isoprenaline in the presence of CGP did still significantly (P<0.001) increase beating rate above the level of CGP alone (52±10 b.p.m.). The increase in beating rate with isoprenaline was significantly (n=13, P<0.001) reduced in the presence of ICI (Figure 6b). Taken together, these last two observations indicate a significant contribution of β2-adrenoceptor-dependent stimulation to isoprenaline-induced increase in beating rate. The contribution of the β1-adrenoceptor was again indicated by the ICI-insensitive increase in beating rate. Two consecutive challenges with isoprenaline separated by a similar wash interval without antagonists resulted in increases in rate that did not differ significantly (n=7, Figure 6c), showing the stability of the preparation. A third challenge after a further washout period produced a slightly, but not significantly, lower rate (71±16 b.p.m.). There was no difference in rate during wash periods and initial basal rate in the experiments using CGP or ICI (Figures 6a and b), but there was a significant (P<0.05) reduction in rate during the last wash period compared to initial basal in the experiments with isoprenaline alone (Figure 6c).
Figure 6.
Responses to isoprenaline following β1- or β2-adrenoceptor blockade. Consecutive applications of isoprenaline and/or isoprenaline plus blocker and washout periods (a). β1-Adrenoceptor-selective blockade with 0.3 μM CGP 20712A, n=11 (b). β2-Adrenoceptor-selective blockade with 50 nM ICI 118,551, n=13, (c). Two consecutive applications of isoprenaline in the absence of antagonist (***P<0.001 vs preceding basal/wash; ##P<0.01, ###P<0.001 vs Iso 1; *P<0.05 vs basal; all tests paired). For (a and b), there was no significant difference between Basal/Wash 1/Wash 2.
Interestingly, only stimulation through the β1-adrenoceptor produced significant acceleration of relaxation (R50: basal 112.3±16.5 ms, isoprenaline+ICI: 97.6±8.4 ms, NS (non-significant); R90: basal 192.8±23.6 ms, isoprenaline+ICI: 174.3±21.5 ms, n=7, P<0.05); there was no effect through stimulation of the β2-adrenoceptor (R50: basal 96.6±5.8 ms, isoprenaline+CGP: 104.0±10.1 ms, NS; R90: basal 186.4±14.3 ms, isoprenaline+CGP: 198.3±21.8 ms, NS, n=12). However, it is noticeable that even β1-adrenoceptor stimulation did not produce the same magnitude of acceleration as β1- and β2-adrenoceptor together (Figure 3). It may be that the decreased lusitropic effect is simply a result of the reduced cyclic AMP signal when either subtype is stimulated separately.
Effect of muscarinic stimulation
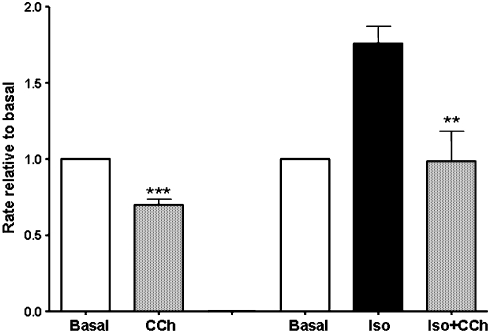
Human ESC-CMs between day 12 and 30 were challenged with the classical muscarinic agonist carbachol (10 μM) in the presence and absence of isoprenaline (Figure 7). With carbachol alone the beating rate dropped significantly (P<0.01) from 41.8±3.1 to 29±2.6 ms (n=12). In the presence of isoprenaline, the decrease was larger, with rate dropping significantly (P<0.01) from 63.7±4.5 to 38±5.9 ms (n=12). The final rate in the presence of both isoprenaline and carbachol was not significantly different from the initial basal rate. Within the time range studied (d12–d30), there was no relation between day of development and the magnitude of the decrease with carbachol.
Figure 7.
Muscarinic inhibition of beating frequency under basal and isoprenaline-stimulated conditions. Rate, beats per min (b.p.m.) relative to basal. Beating rate drops significantly after carbachol (CCh) application under basal conditions (10 μM, ***P<0.001, n=12) and in the presence of 100 nM isoprenaline (**P<0.02, n=9).
Discussion
This study significantly extends the potential of the hESC-CM as a preparation for the detailed investigation of cardiac pharmacology. We have defined the stability of beating rate within an experiment as well as over 79 days in culture. The response to catecholamines has been characterized in terms of desensitization within a 10 min exposure, stability over repeated exposures within an experiment and development from d10 to d79 in culture. This has given a basis for detailed analysis of β1- and β2-adrenoceptor subtype dependence of contraction and relaxation.
Cardiomyocyte differentiation into hESC-CM was readily obtained with this H7 line, as has been reported by other authors (Xu et al., 2002; He et al., 2003), with a high proportion of EBs showing contractile foci; the phenotype was confirmed by the presence of cardiac myosin heavy chain. Spontaneous beating rates of hESC-CM initially averaged approximately 50 b.p.m., which is again similar to previous reports (He et al., 2003; Mummery et al., 2003), although there was a wide range of values observed (17–85 b.p.m.). The identity as a cardiac myocyte phenotype was confirmed in each experiment by challenge with isoprenaline, as smooth and skeletal muscle can potentially show slow contractile activity, but will not respond to β-adrenoceptor stimulation with an increase in rate. With increasing time after differentiation, we observed a modest but statistically significant increase in beating rate, in contrast to other groups which report a decrease (He et al., 2003; Reppel et al., 2004). This may represent a difference in the proportion of cells in the ventricular phenotype, which have a lower spontaneous rate (He et al., 2003). Clusters that develop the ventricular phenotype may stop beating altogether; a decrease in percentage of active EBs was seen at later time intervals. As noted previously (Harding et al., 2007), transfer to physiological saline from serum-containing medium results in an initial drop in beating rate; occasionally there was complete loss of spontaneous activity. This is likely due to the perfusate, as addition of 10% culture medium restored contraction in those cases (results not shown). After a 30-min stabilization period, both rate (in spontaneous beating cells) and amplitude of contraction (in paced cells) were steady for 3–4 h (Harding et al., 2007).
Isoprenaline, a mixed β1- and β2-adrenoceptor agonist, increased frequency of beating at the earliest time points measured and produced an approximate doubling of rate. This is consistent with the reports of early development of the receptors and their downstream targets, especially the L-type Ca2+ channel (Mummery et al., 2003; Satin et al., 2004). There was a modest increase in β-adrenoceptor response with time in culture. Measurements of stability of isoprenaline responses over various experimental periods were made. The drop in rate during a 10-min application of isoprenaline was not as evident in hESC-CM as in mouse (Ali et al., 2004). Clearly, any mild desensitization was reversed on washout, as repeated applications of the same concentration of isoprenaline elicited similar responses. Concentration–response curves had an EC50 of 12.9 nM, which is closer to the values for failing than non-failing adult human heart, as described previously (Harding et al., 2007). As hESC-CMs, at least at early days of differentiation, have the characteristics of immature cardiomyocytes (Kehat et al., 2001), this may represent the reversion of the failing heart towards the fetal gene program. A concentration between EC75 and EC90 was chosen for subsequent subtype experiments.
A hallmark of β-adrenoceptor stimulation in adult ventricle is acceleration of relaxation, and this was also observed in the hESC-CM. Quantitative comparison with the same effect in adult shows a remarkable similarity between hESC-CM and failing human heart, with R50 reduced by 35 and 30%, respectively (Harding et al., 1996). This suggests that the main phosphorylation targets for cyclic AMP-dependent protein kinase-mediated lusitropic effects, phospholamban and troponin I, may be functional in hESC-CM. Phospholamban is the predominant modulator of relaxation through its effect on the sarcoplasmic reticulum Ca2+ uptake. A large effect of β-adrenoceptor stimulation of relaxation implies the involvement of phospholamban and, therefore, active contributions to Ca2+ movement into sarcoplasmic reticulum stores. We eliminated a possible rate-dependent effect on relaxation, which will occur due to earlier repolarization at higher rates, by showing that pacing at 120 vs 60 b.p.m. did not significantly affect R50. Interestingly, the hESC-CMs were once again closer to failing than non-failing human myocytes, which have a smaller lusitropic response. Cyclic AMP-dependent protein kinase targets in non-failing adult cells may already be highly phosphorylated (Harding et al., 1996).
Changes in rate with isoprenaline will change amplitude of contraction, and we showed previously that the frequency–amplitude curve when the hESC-CMs were externally paced was negative from 60 to 180 b.p.m. (Harding et al., 2007). We have therefore not reported amplitude of contraction in the present study, as increases in rate in the unpaced hESC-CM will offset any isoprenaline-induced increase in amplitude. Additionally, there can be technical difficulties in placing the charcoal tracking points in the correct locations on a cluster to give an optimal and stable measure of amplitude. The scanning ion conductance microscope method we described previously is likely to prove more reliable for this parameter (Gorelik et al., 2006).
Evidence of the contribution of both β1- and β2-adrenoceptors to increases in rate was obtained from the antagonist studies, where neither β1- nor β2-adrenoceptor blockade completely abolished the increase in rate with isoprenaline. The proportion of β2-adrenoceptor effect in hESC-CM was 17–37%, which is in the range for non-failing human ventricle (Brodde, 1991). Although the increase in beating rate primarily measures β1- and β2-adrenoceptor coupling to Gs, it is known that the β2-adrenoceptor can also couple to Gi (Kuschel et al., 1999). It will be important to establish the relative coupling of the β2-adrenoceptor to Gs and Gi in hESC-CM, as β2-adrenoceptor-Gi signalling gives protection against apoptosis, whereas β1/β2-adrenoceptors-Gs is proapoptotic (Chesley et al., 2000). Stimulation through the β1-adrenoceptor alone still produced significant (although reduced) lusitropic effects, whereas that through the β2-adrenoceptor did not. This difference in β-adrenoceptor subtypes has been reported (Xiao and Lakatta, 1993) (although challenged; Bartel et al., 2003) for rat heart but not for human heart (del Monte et al., 1993; Molenaar et al., 2001). However, the inability to detect the lusitropic effect in the present study may be more simply related to the small contribution of β2-adrenoceptor to contraction.
Muscarinic stimulation had only a small effect on basal rate, but abolished the response to isoprenaline. This suggests that the Gi-dependent anti-adrenergic pathway is highly active in hESC-CM (von Scheidt et al., 1992; Nagata et al., 2001) and that the phenotype of the cells is closer to the adult ventricular myocyte than to the nodal cells (where basal contraction is abolished through muscarinic receptors) (Hopwood et al., 1987). Importantly, this implies that parasympathetic activation in vivo would not completely abolish contractile activity and, therefore, that hESC-CM implanted within the ventricle would not produce an area of heart block but would behave in a manner similar to the surrounding ventricle.
This study goes some way towards predicting the behaviour of hESC-CM implanted into the diseased human ventricle in the dynamic hormonal milieu of the heart failure patient. Basal rate of spontaneous beating might initially be sufficiently slow to be suppressed by the natural pacemaker rhythm (as long as gap junctions to host myocardium become established), but may increase with development. If this occurs, then a secondary pacemaker site may be set up, with the potential to disrupt rhythm. In the context of the heart failure patient, where 50% of deaths are related to arrhythmias, this could represent a significant risk. Implanted hESC-CM would be predicted to respond to sympathetic and parasympathetic output in the same manner and concentration range as the host myocardium. Although this would mean that inotropic and lusitropic responses were well matched, the increase in spontaneous rate with β-adrenoceptor stimulation could be an extra risk for arrhythmias. This may be less of concern, however, now that β-blockers are standard treatment for heart failure patients. It might be envisaged that selective β1-adrenoceptor blockade could improve implantation of hESC-CM in failing human heart because of protection against catecholamine-mediated apoptosis.
In conclusion, we have provided a characterization of hESC-CM in relation to adult human myocytes. This has given information relevant to their potential use in cardiac repair and also to define their utility as a model system for the cardiac scientist. Given the paucity of cardiac cell lines with the appropriate phenotype, the hESC-CM has a real and important future role in the investigation of cardiac development, physiology, pharmacology and pathophysiology.
Acknowledgments
We are grateful for the continuing support of Geron. This work was supported by Fundação para a Ciência e a Tecnologia, Portugal (M Brito-Martins), the BBSRC and NC3Rs.
Abbreviations
- EB
embryoid body
- ESC
embryonic stem cells
- ESC-CM
embryonic stem cell-derived cardiomyocytes
- Gi
inhibitory guanine nucleotide binding protein
- Gs
stimulatory guanine nucleotide binding protein
- hESC
human embryonic stem cells
- hESC-CM
human embryonic stem cell-derived cardiomyocytes
- ICI
ICI 118,551
- PFA
paraformaldehyde
- R50
time from peak to 50% relaxation
- R90
time from peak to 90% relaxation
- TTP
time-to-peak shortening
Conflict of interest
The H7 line used in these studies was donated by Geron (Menlo Park, CA, USA) under a collaborative agreement without further financial benefit to the authors.
References
- Ali NN, Xu X, Brito-Martins M, Poole-Wilson PA, Harding SE, Fuller SJ. Beta-adrenoceptor subtype dependence of chronotropy in mouse embryonic stem cell-derived cardiomyocytes. Basic Res Cardiol. 2004;99:382–391. doi: 10.1007/s00395-004-0484-5. [DOI] [PubMed] [Google Scholar]
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- Bartel S, Krause EG, Wallukat G, Karczewski P. New insights into beta2-adrenoceptor signaling in the adult rat heart. Cardiovasc Res. 2003;57:694–703. doi: 10.1016/s0008-6363(02)00720-4. [DOI] [PubMed] [Google Scholar]
- Brodde OE. Beta 1- and beta 2-adrenoceptors in the human heart: properties, function, and alterations in chronic heart failure. Pharmacol Rev. 1991;43:203–242. [PubMed] [Google Scholar]
- Brown LA, Harding SE. The effect of pertussis toxin on β-adrenoceptor responses in isolated cardiac myocytes from noradrenaline-treated guinea-pigs and patients with cardiac failure. Br J Pharmacol. 1992;106:115–122. doi: 10.1111/j.1476-5381.1992.tb14302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary KW, Barrezueta NX, Bauchmann MB, Milici AJ, Beckius G, Stedman DB, et al. Embryonic stem cells in predictive cardiotoxicity: laser capture microscopy enables assay development. Toxicol Sci. 2005;90:149–158. doi: 10.1093/toxsci/kfj078. [DOI] [PubMed] [Google Scholar]
- Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, et al. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3′-kinase. Circ Res. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- del Monte F, Kaumann AJ, Poole-Wilson PA, Wynne DG, Harding SE. Coexistence of functioning β1- and β2-adrenoceptors in single myocytes from human ventricle. Circulation. 1993;88:854–863. doi: 10.1161/01.cir.88.3.854. [DOI] [PubMed] [Google Scholar]
- Gorelik J, Ali NN, Shevchuk A, Lab M, Williamson C, Harding SE, et al. Functional characterization of embryonic stem cell-derived cardiomyocytes using scanning ion conductance microscopy. Tissue Eng. 2006;12:657–664. doi: 10.1089/ten.2006.12.657. [DOI] [PubMed] [Google Scholar]
- Harding SE, Ali NN, Brito-Martins M, Gorelik J. The human embryonic stem cell-derived cardiomyocyte as a pharmacological model. Pharmacol Ther. 2007;113:341–353. doi: 10.1016/j.pharmthera.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Harding SE, Brown LA, del Monte F, Davies CH, O'Gara P, Vescovo G, et al. Acceleration of contraction by β-adrenoceptor stimulation is greater in ventricular myocytes from failing than non-failing human hearts. Basic Res Cardiol. 1996;91 Suppl 2:53–56. doi: 10.1007/BF00795363. [DOI] [PubMed] [Google Scholar]
- He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- Hopwood AM, Harding SE, Harris P. Pertussis toxin reduces the antiadrenergic effect of 2- chloroadenosine on papillary muscle and the direct negative inotropic effect of 2-chloroadenosine on atrium. Eur J Pharmacol. 1987;141:423–428. doi: 10.1016/0014-2999(87)90560-7. [DOI] [PubMed] [Google Scholar]
- Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- Kuschel M, Zhou YY, Cheng H, Zhang SJ, Chen Y, Lakatta EG, et al. G(i) protein-mediated functional compartmentalization of cardiac beta(2)-adrenergic signaling. J Biol Chem. 1999;274:22048–22052. doi: 10.1074/jbc.274.31.22048. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, et al. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005;167:663–671. doi: 10.1016/S0002-9440(10)62041-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure. Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- Molenaar P, Bartel S, Cochrane A, Vetter D, Jalali H, Pohlner P, et al. Both beta(2)- and beta(1)-adrenergic receptors mediate hastened relaxation and phosphorylation of phospholamban and troponin I in ventricular myocardium of Fallot infants, consistent with selective coupling of beta(2)-adrenergic receptors to G(s)-protein. Circulation. 2001;102:1814–1821. doi: 10.1161/01.cir.102.15.1814. [DOI] [PubMed] [Google Scholar]
- Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den BS, Hassink R, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- Nagata K, Ye C, Jain M, Milstone DS, Liao R, Mortensen RM. Galpha(i2) but not Galpha(i3) is required for muscarinic inhibition of contractility and calcium currents in adult cardiomyocytes. Circ Res. 2001;87:903–909. doi: 10.1161/01.res.87.10.903. [DOI] [PubMed] [Google Scholar]
- Reinecke H, Poppa V, Murry CE. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J Mol Cell Cardiol. 2002;34:241–249. doi: 10.1006/jmcc.2001.1507. [DOI] [PubMed] [Google Scholar]
- Reppel M, Boettinger C, Hescheler J. Beta-adrenergic and muscarinic modulation of human embryonic stem cell-derived cardiomyocytes. Cell Physiol Biochem. 2004;14:187–196. doi: 10.1159/000080326. [DOI] [PubMed] [Google Scholar]
- Satin J, Kehat I, Caspi O, Huber I, Arbel G, Itzhaki I, et al. Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. J Physiol (Lond) 2004;559:479–496. doi: 10.1113/jphysiol.2004.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Scheidt W, Bohm M, Stablein A, Autenrieth G, Erdmann E. Antiadrenergic effect of M-cholinoceptor stimulation on human ventricular contractility in vivo. Am J Physiol. 1992;263:H1927–H1931. doi: 10.1152/ajpheart.1992.263.6.H1927. [DOI] [PubMed] [Google Scholar]
- Wei H, Juhasz O, Li J, Tarasova YS, Boheler KR. Embryonic stem cells and cardiomyocyte differentiation: phenotypic and molecular analyses. J Cell Mol Med. 2005;9:804–817. doi: 10.1111/j.1582-4934.2005.tb00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R-P, Lakatta EG. 1-Adrenoceptor stimulation and β2-adrenoceptor stimulation differ in their effects on contraction, cytosolic Ca2+ and Ca2+ current in single rat ventricular cells. Circ Res. 1993;73:286–300. doi: 10.1161/01.res.73.2.286. [DOI] [PubMed] [Google Scholar]
- Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–508. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- Xue T, Cho HC, Akar FG, Tsang SY, Jones SP, Marban E, et al. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation. 2005;111:11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]