Abstract
Background and purpose:
Interleukin (IL)-1 is a key mediator of inflammatory and host defence responses and its effects in the brain are mediated primarily via effects on glia. IL-1 induces release of inflammatory mediators such as IL-6 from glia via the type-1 receptor (IL-1R1) and established signalling mechanisms including mitogen-activated protein kinases and nuclear factor kappa-B. IL-1 also modifies physiological functions via actions on neurones, through activation of the neutral sphingomyelinase (nSMase)/Src kinase signalling pathway, although the mechanism of IL-1-induced IL-6 synthesis in neurones remains unknown.
Experimental approach:
Primary mouse neuronal cell cultures, ELISA, Western blot and immunocytochemistry techniques were used.
Key results:
We show here that IL-1β induces the synthesis of IL-6 in primary mouse neuronal cultures, and this is dependent on the activation of IL-1R1, nSMase and Src kinase. We demonstrate that IL-1β-induced Src kinase activation triggers the phosphorylation of the NMDA receptor NR2B subunit, leading to activation of Ca2+/calmodulin-dependent protein kinase II (CamKII) and the nuclear transcription factor CREB. We also show that NR2B, CamKII and CREB are essential signalling elements involved in IL-1β-induced IL-6 synthesis in neurones.
Conclusions and implications:
These results demonstrate that IL-1 interacts with the same receptors on neurones and glia to elicit IL-6 release, but does so via distinct signalling pathways. The mechanism by which IL-1β induces IL-6 synthesis in neurones could be critical in both physiological and pathophysiological actions of IL-1β, and may provide a new therapeutic target for the treatment of acute CNS injury.
Keywords: interleukin-1, neurones, signalling, Src kinase, CREB
Introduction
Interleukin (IL)-1 is a prototypic proinflammatory cytokine involved in the immune and host defence response to infection and injury, and is a primary mediator of inflammatory responses to acute and chronic CNS disorders. Brain IL-1 expression is dramatically increased in experimental and clinical CNS injury, whereas inhibiting its actions improves neurological outcome (see Allan et al., 1998; Allan and Rothwell 2003; Rothwell 2003 for review). Some central actions of IL-1 may contribute to the regulation of physiological functions such as sleep, memory and long-term potentiation (Schneider et al., 1998; Rachal et al., 2001; Ross et al., 2003), as well as host defence responses, such as fever and sickness behaviour (Takahashi et al., 1996; Dantzer et al., 1998; Schneider et al., 1998; Rachal et al., 2001; Watkins et al., 2001; Ross et al., 2003; Bilbo et al., 2005).
IL-1 is produced in the brain primarily by microglia and has pleiotropic actions on all resident cell types of the CNS, including astrocytes, microglia, oligodendrocytes, endothelial cells and neurones (Giulian et al., 1988; Mason et al., 2001; Vela et al., 2002; Basu et al., 2002, 2004; John et al., 2004; Konsman et al., 2007). In glial cells, IL-1 triggers cell proliferation and activation (that is astrogliosis), and the production of key inflammatory mediators, such as IL-6, nitric oxide, prostaglandin E2 and chemokines (Laflamme et al., 1999; Basu et al., 2004; John et al., 2005). These effects are all dependent on activation of the classical IL-1 signalling pathways, including the mitogen-activated protein kinases (MAPKs) and nuclear factor-κB pathways (Parker et al., 2002).
In neuronal cells, IL-1 triggers cellular responses that are associated with physiological functions, including changes in the electrophysiological state and excitability of neurones (for example GABAergic inhibition, cell depolarization and rapid changes in membrane ion currents) (Katsuki et al., 1990; Zeise et al., 1992; Murray et al., 1997; Schneider et al., 1998; Kelly et al., 2001; Desson and Ferguson 2003; Viviani et al., 2003; Shu et al., 2007). IL-1 induces changes in neuronal function via distinct pathways, including sphingomyelinase/ceramide and Src kinase activation, which can activate NMDA receptors via phosphorylation of the NMDA receptor 2A/NMDA receptor 2B (NR2A/NR2B) subunits, thus increasing receptor currents and Ca2+ influx (Salter and Kalia 2004; Viviani et al., 2006). Src kinase-induced NMDA receptor activation is believed to be involved in physiological functions, such as plasticity and gene expression, and ischaemic neuronal injury (Salter and Kalia 2004).
Previous studies have shown that IL-1 also induces the production of IL-6 in neurones (Murphy et al., 1995; Ringheim et al., 1995), but the signalling mechanisms of IL-1-induced IL-6 synthesis in neurones remain unknown. We report for the first time that the neutral sphingomyelinase (nSMase)/Src kinase signalling pathway mediates the synthesis of IL-6 in neurones, and we further demonstrate that IL-1β-induced IL-6 synthesis in neurones is dependent on the activation of the NR2B subunit, the Ca2+/calmodulin-dependent protein kinase II (CamKII) (and possibly CamKIV) and cAMP response element-binding protein (CREB). Thus, IL-1 induces the key inflammatory mediator, IL-6, by totally distinct and separate pathways in neurones and glia.
Methods
Animals
Animal care and experimental procedures were conducted in accordance with the guidelines set by the European Council directives (86/609/EEC) and the UK Home Office, Animals (Scientific Procedures) Act 1986. All studies were performed using cell cultures prepared from wild-type mice (C57/BL6x129sv, Charles River, UK).
Reagents and drugs
General laboratory reagents were obtained from Sigma (Poole, UK) or BDH Ltd (Lutherworth, UK). Tissue culture reagents were supplied by Invitrogen. Rat recombinant IL-1β and human recombinant IL-1RA were provided by Dr Steve Poole from the National Institute of Biological Standards and Controls (NIBSC, Potters Bar, UK).
Primary cortical neuronal cell culture and treatments
Primary, neuronal cell cultures were prepared from embryonic (day 16) mice as described previously (Moore et al., 2002). The cultures were used on days 12–14. Cell-specific immunostaining revealed that all cultures were highly enriched in neurones with no glial contamination (data not shown).
To assess the effect of IL-1β on the synthesis of IL-6 in neurones, primary cultures were treated with vehicle (0.1%, low-endotoxin, BSA, in sterile 0.9% NaCl) or with rat recombinant IL-1β (from 0.03 to 30 U ml−1 diluted in vehicle) for 7 h (optimum time of treatment for IL-6 synthesis (Tsakiri et al., 2007)). To investigate the effect of IL-1β on the activation of MAPKs (extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38), Src kinase, CamKII and CREB, on the phosphorylation of NR2B, and on the synthesis of IL-6, primary cultures were incubated with vehicle or IL-1β (0.3 U ml−1), in the absence or presence of the Src kinase inhibitor PP2 (10 μM, Calbiochem, Nottingham, UK), the nSMase inhibitor 3-O-methyl-sphingomyelin (3OMS) (15, 25 μM, Biomol, USA), the NMDA receptor inhibitors MK-801 (20 μM), AP5 (50 μM) or ifenprodil (20 μM) (Tocris, UK), the Ca2+/CamKII/IV inhibitor KN-62 (10 μM) (Calbiochem, UK) or interleukin-1 receptor antagonist (IL-1RA) (50 ng ml−1) at various time points from 5 min to 7 h.
Immunocytochemistry
Cultures grown on poly-D-lysine-treated glass coverslips were washed twice with ice-cold phosphate-buffered saline, and then fixed in 4% paraformaldehyde and 4% sucrose for 20 min at room temperature. The cells were then permeabilized in 0.2% Triton X-100 for 15 min, and nonspecific sites were blocked by incubation in 10% foetal calf serum for 1 h at room temperature. The cells were then incubated for 1 h at room temperature with primary antibodies diluted in 0.3% BSA: rabbit anti-phosphorylated-CREB (1:100; Cell Signalling, Danvers, USA) and mouse anti-neuronal nuclei (1:400; Chemicon, Chandlers Ford, UK). The cells were then washed three times with 0.1% BSA and incubated for 1 h with appropriate secondary antibodies diluted in 3% BSA: Texas-red horse anti-mouse (1:600; Vector Laboratories, Burlingame, UK) and Alexa Fluor 488 goat anti-rabbit (1:2000; Invitrogen, Paisley, UK). Finally, the cells were washed three times with phosphate-buffered saline and mounted on glass slides with Prolong gold antifade reagent with 4,6-diamidino-2-phenylindole (Invitrogen, UK). Negative control staining was performed with an unrelated primary antibody (rabbit anti-GFAP). Images were obtained using an Olympus fluorescent microscope attached to a digital camera with MetaVue software.
IL-6 synthesis and release
Immunoreactive IL-6 in neuronal cell culture supernatant and cell lysates was assayed using a validated mouse-specific ELISA (DuoSet) purchased from R&D Systems (Abingdon, UK). IL-6 standards were assayed in triplicate and samples (100 μl) in duplicate. The absorbance was then measured using a plate reader (MRX, Dynatech, UK), and results were calculated from the standard curve. The detection limit of the assay was 3 pg ml−1.
Western blot analysis
Western blot analysis for Src kinase, CamKII and CREB activation was carried out as described previously (Parker et al., 2002), using primary antibodies diluted in phosphate-buffered saline containing 0.1% Tween-20 and 0.1% BSA; anti-phosphorylated-ERK, anti-phosphorylated-JNK, anti-phosphorylated-p38, anti-total-p38 anti-phosphorylated-Src, anti-total-Src, anti-phosphorylated-CREB, anti-total-CREB, anti-phosphorylated-CamKII, anti-total-CamKII (all at 1:1000 dilution; Cell Signalling); anti-phospho-NR2B or anti-total-NR2B (1:1000; R&D Systems); anti-total-ERK or anti-total JNK (both at 1:5000 dilution; SantaCruz Biotech., Calne, UK); and subsequent incubation with horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:2000 dilution in 5% milk/phosphate-buffered saline/0.1% Tween-20) (Dako, Ely, UK). The secondary antibody was detected using enhanced chemiluminescence using western blotting detection reagent (Amersham, Little Chalfont, UK), and films were analysed densitometrically with Northern Eclipse software.
Data analysis
Data were analysed with GraphPad Prism 4.0 software and expressed as the mean of at least three independent experiments ±s.d. Differences between treated and control groups were analysed by one-way ANOVA with Dunnett's multiple comparisons post hoc test. Differences between IL-1β alone and IL-1β in the presence of inhibitors were analysed by one-way ANOVA with Bonferroni's post hoc test, and differences between cell lysates and supernatants of the same treatment were analysed by unpaired Student's t-test. For all analyses, a value of P<0.05 was considered statistically significant.
Results
IL-1β-induced IL-6 production in primary neurones is dependent on Src kinase and nSMase activation
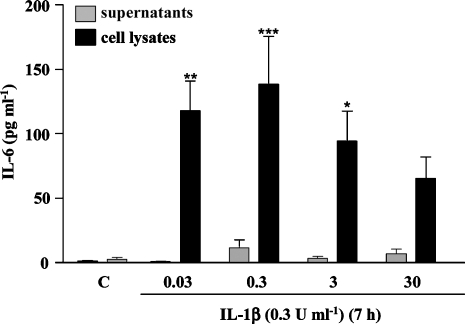
IL-1β (0.03, 0.3 and 3 U ml−1) induced significant synthesis of IL-6 protein in primary neurones, which was mainly detected in the intracellular compartment (Figure 1). The dose that optimally induced the production of IL-6 was 0.3 U ml−1 and was therefore selected for further studies.
Figure 1.
IL-1β-induced IL-6 synthesis in primary cortical neurones. Cultures were treated with vehicle (C) or IL-1β (0.03–30 U ml−1) for 7 h, and cell lysates and supernatants were collected and assayed for IL-6 using a mouse-specific ELISA. Data are presented as the mean±s.d. of seven independent experiments *P<0.05, **P<0.01, ***P<0.001, IL-1β vs control. IL, interleukin.
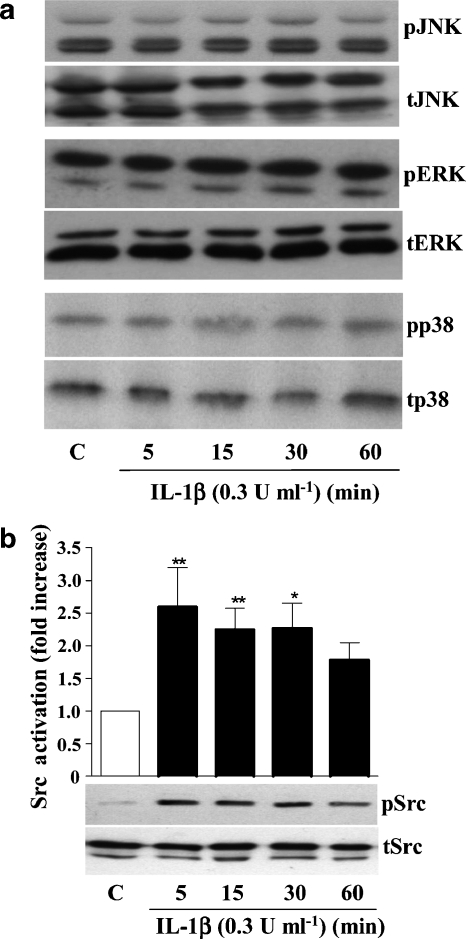
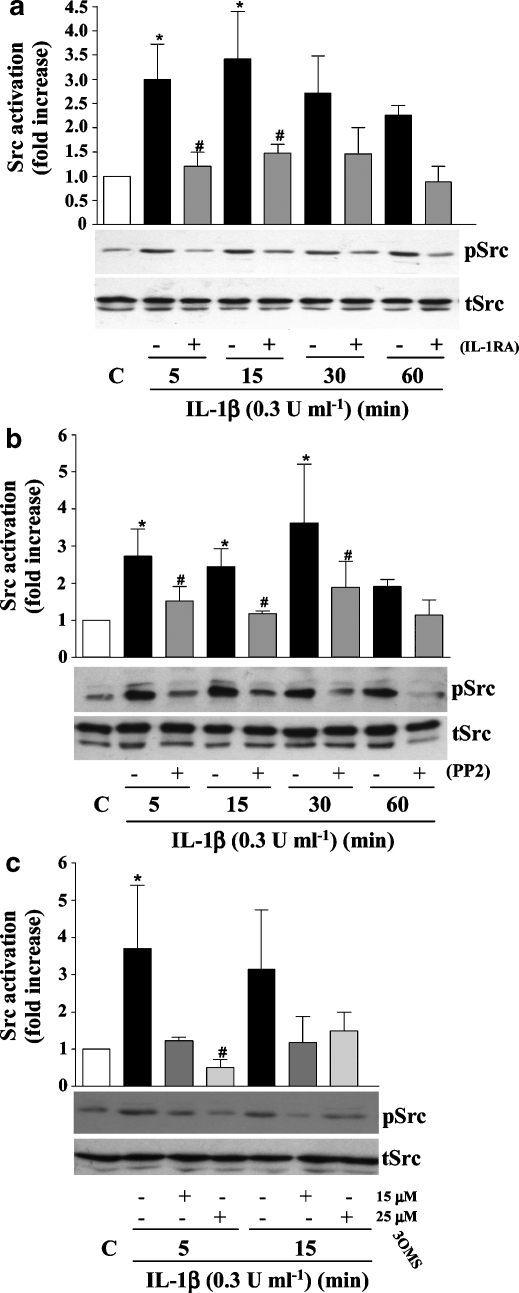
To identify the signalling pathways through which IL-1β induces IL-6 expression, cell lysates of IL-1β-treated neurones (0.3 U ml−1 for 5, 15, 30 or 60 min) were assayed for ERK, JNK and p38 activation by western blot analysis. These MAPKs mediate IL-1β-induced IL-6 synthesis in glial cells (Parker et al., 2002). IL-1β failed to activate any of these MAPKs at any time point tested (Figure 2a). In contrast, IL-1β induced a significant 2.5-fold activation of Src kinase after 5 and 15 min of treatment (Figure 2b). IL-1β-induced Src kinase activation was significantly reduced in the presence of excess IL-1RA (Figure 3a), the specific Src kinase inhibitor PP2 (Figure 3b) or the specific nSMase inhibitor 3OMS (Figure 3c), whereas the inhibitors alone had no effects (data not shown).
Figure 2.
Effect of IL-1β on MAPKs and Src kinase activity in primary cortical neurones. Cultures were treated with vehicle (C) or IL-1β (0.3 U ml−1) for 5, 15, 30 or 60 min, and cell lysates were assayed by western blot analysis for (a) ERK, JNK or p38 activation, or (b) Src kinase activation, using specific antibodies against total (t) or phosphorylated (p) isoforms of ERK, JNK, p38 or Src kinase. Images of blots in (a) are from a single experiment representative of three independent experiments carried out on separate cultures. Data in (b) are presented as the mean±s.d. of three independent experiments carried out on separate cultures. *P<0.05, **P<0.01, IL-1β vs control. ERK, extracellular signal-regulated kinase; IL, interleukin; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase.
Figure 3.
Effect of IL-1RA, PP2 or 3OMS on IL-1β-induced Src kinase in primary cortical neurones. Cultures were treated with vehicle (C) or IL-1β (0.3 U ml−1) for 5, 15, 30 or 60 min, in the absence or presence of (a) IL-1RA (50 ng ml−1), (b) PP2 (10 μM) or (c) 3OMS (15, 25 μM). Cell lysates were assayed by western blot analysis for Src kinase activation using specific antibodies against total or phosphorylated isoforms of Src kinase. Data are presented as the mean±s.d. of three independent experiments carried out on separate cultures. *P<0.05, IL-1β vs control, #P<0.05, IL-1β+IL-1RA/PP2/3OMS vs IL-1β alone. IL, interleukin; 3OMS, 3-O-methyl-sphingomyelin.
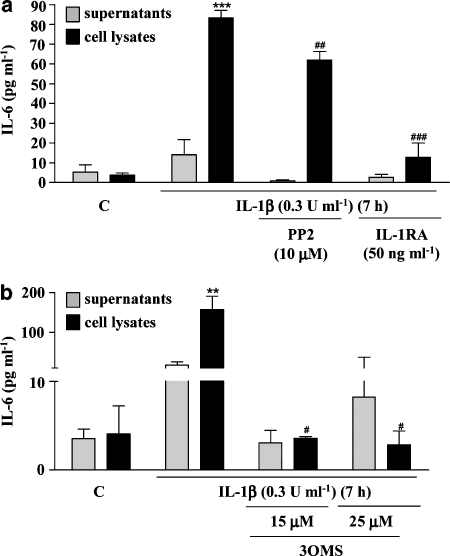
To investigate whether Src kinase and nSMase are involved in IL-1β-induced IL-6 synthesis in neurones, supernatants and cell lysates from cultures treated with IL-1β (0.3 U ml−1) for 7 h, in the presence or absence of PP2, IL-1RA or 3OMS, were assayed for IL-6 by ELISA. The levels of IL-6 detected in cell lysates in response to IL-1β were significantly decreased by pretreatment with PP2 (30%) or IL-1RA (80%) and were comparable to control levels in the presence of 3OMS (25 μM) (Figures 4a and b). The inhibitors alone had no significant effect on IL-6 levels (data not shown).
Figure 4.
Effect of IL-1RA, PP2 or 3OMS on IL-1β-induced IL-6 synthesis in primary cortical neurones. Cultures were treated with IL-1β (0.3 U ml−1) for 7 h in the absence or presence of (a) PP2 (10 μM) or IL-1RA (50 ng ml−1), or (b) 3OMS (15, 25 μM). Cell lysates and supernatants were assayed for IL-6 using a specific mouse ELISA. Data are presented as the mean±s.d. of three different experiments carried out on separate cultures. **P<0.01, ***P<0.001, IL-1β vs control, #P<0.05, ##P<0.01, ###P<0.001, IL-1β+PP2/IL-1RA/3OMS vs IL-1β alone. IL, interleukin; 3OMS, 3-O-methyl-sphingomyelin.
IL-1β-induced IL-6 production in primary neurones is dependent on NMDA receptor activation
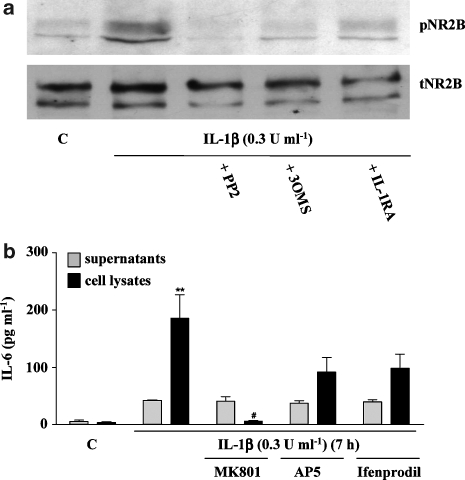
Activation of Src kinase is linked to NMDA receptor activation, mainly through phosphorylation of NR2 subunits (Salter and Kalia 2004). To test the hypothesis that NMDA receptor activation is involved in the signalling mechanisms of IL-1β-induced IL-6 synthesis, cytoplasmic membrane preparations from primary neuronal cultures treated with IL-1β (0.3 U ml−1) for 10 min, in the absence or presence of PP2, 3OMS or IL-1RA, were assessed for NR2B phosphorylation by western blot. IL-1β induced the phosphorylation of NR2B, which was prevented by co-incubation with PP2, 3OMS or IL-1RA (Figure 5a). Furthermore, three NMDA receptor inhibitors, MK-801, AP5 and ifenprodil, reduced IL-1β-induced IL-6 synthesis, but only MK-801 induced a statistically significant decrease (95%) (Figure 5b), suggesting that the effects of IL-1β on IL-6 production are dependent on NMDA receptor activation.
Figure 5.
IL-1β-induced NR2B phosphorylation and effect of NMDA inhibitors on IL-1β-induced IL-6 synthesis in primary cortical neurones. (a) Cultures were treated with vehicle (C) or IL-1β (0.3 U ml−1) for 10 min, in the absence or presence of the Src kinase inhibitor PP2 (10 μM), 3OMS (25 μM) or IL-1RA (50 ng ml−1). Cytoplasmic membrane extracts were assayed for NR2B phosphorylation by western blot analysis using specific antibodies against total (t) or phosphorylated (p) isoforms of the NR2B subunit of the receptor. (b) Cultures were treated with vehicle (C) or IL-1β (0.3 U ml−1) for 7 h in the absence or presence of the NMDA receptor blocker MK-801 (20 μM), the competitive inhibitor AP5 (50 μM), and the specific inhibitor of NR2B-containing NMDA receptors, ifenprodil (20 μM). Cell lysates and supernatants were assayed for IL-6 using a specific mouse ELISA. Data are presented as the mean±s.d. of three independent experiments carried out on separate cultures. **P<0.05, IL-1β vs control, #P<0.05, IL-1β+MK-801 vs IL-1β alone. IL, interleukin; 3OMS, 3-O-methyl-sphingomyelin.
IL-6 synthesis induced by IL-1β in primary neurones is mediated by CamKII and CREB
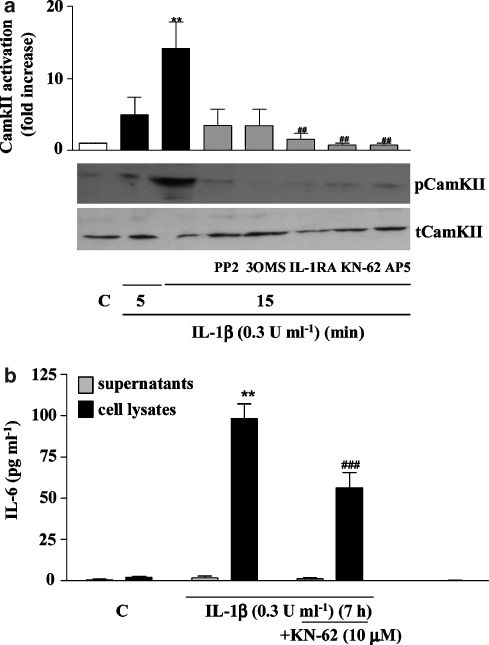
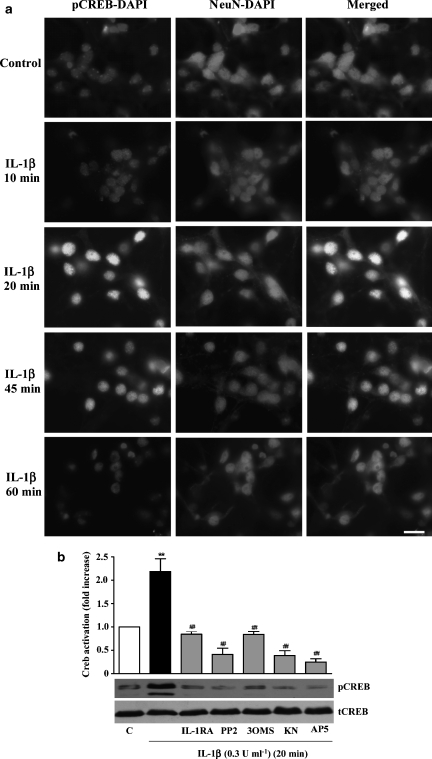
As activation of NMDA receptors leads to Ca2+ influx, we next investigated the effect of IL-1β on Ca2+-dependent CamKII activation. Neurones treated with IL-1β (0.3 U ml−1) in the absence or presence of the selective inhibitor of Ca2+/CamKII/IV, KN-62 or inhibitors of identified upstream signalling elements (PP2, 3OMS, IL-1RA or AP5) were assayed for CamKII activity by western blot. IL-1β induced significant activation of CamKII (Figure 6a), which was completely blocked by all the inhibitors tested, with significant inhibition produced by IL-1RA, AP-5 and KN-62. In addition, KN-62 significantly reduced (58%) IL-1β-induced IL-6 synthesis (Figure 6b), further implicating CamKII in the signalling mechanism of IL-1β actions in neurones. We also found that IL-1β induced activation of the Ca2+-dependent transcription factor CREB, after 20 and 45 min of treatment (Figure 7a). Western blot analysis of the nuclear extracts of neurones treated with IL-1β for 20 min confirmed strong activation of CREB, which was totally abolished by IL-1RA, PP2, 3OMS, KN-62 or AP5 (Figure 7b).
Figure 6.
IL-1β-induced CamKII activation and effect of KN-62 on IL-1β-induced IL-6 synthesis in primary cortical neurones. (a) Western blot analysis of CamKII activation in total cell extracts of cultures treated with vehicle (C) or IL-1β (0.3 U ml−1) for 5 or 15 min, in the absence or presence of IL-1RA (50 ng ml−1), 3OMS (25 μM), PP2 (10 μM), KN-62 (CamkII/IV inhibitor, 10 μM) or AP5 (50 μM). Data are presented as the mean±s.d. of five different experiments in separate cultures, **P<0.01, IL-1β vs control, ##P<0.01, IL-1β+inhibitor vs IL-1β alone. (b) Cultures were treated with IL-1β (0.3 U ml−1) for 7 h in the absence or presence of KN-62 (10 μM). Cell lysates and supernatants were assayed for IL-6 using a specific mouse ELISA. Data are presented as the mean±s.d. of six different experiments in separate cultures. **P<0.01 IL-1β vs control, ###P<0.001, IL-1β+KN-62 vs IL-1β alone. IL, interleukin; 3OMS, 3-O-methyl-sphingomyelin.
Figure 7.
Effect of IL-1β on CREB activation and effect of IL-1RA, PP2, 3OMS, KN-62 and AP5 on IL-1β-induced CREB activation in primary cortical neurones. (a) Cultures were treated with vehicle (C) or IL-1β (0.3 U ml−1) for 10, 20, 45 or 60 min, fixed and stained for pCREB, NeuN (neuronal nuclei) and DAPI (all nuclei). Scale bar 5 μm. (b) Primary neurones were treated with vehicle (C) or IL-1β (0.3 U ml−1) for 20 min, in the absence or presence of PP2 (10 μM), 3OMS (25 μM), IL-1RA (50 ng ml−1), KN-62 (10 μM) or AP5 (50 μM). Nuclear extracts were assayed for CREB activity by western blot analysis using specific antibodies against total (tCREB) or phosphorylated CREB (pCREB). Data are presented as the mean±s.d. of four independent experiments carried out on separate cultures. **P<0.01, IL-1β vs control, ##P<0.01 IL-1β+inhibitor vs IL-1β alone. CREB, cAMP response element-binding protein; DAPI, 4,6-diamidino-2-phenylindole; IL, interleukin; NeuN, anti-neuronal nuclei; 3OMS, 3-O-methyl-sphingomyelin.
Discussion and conclusions
Proinflammatory actions of IL-1β are mediated in the CNS primarily by glial cells, but direct actions of this cytokine on neurones have been identified and mediate important physiological functions via fast activation of the nSMase/Src kinase pathway (Sanchez-Alavez et al., 2006). IL-1β also induces IL-6 expression in neurones (Murphy et al., 1995; Ringheim et al., 1995), but the signalling mechanisms that mediate this response were unknown. In the present study, we identified signalling pathways involved in the mechanisms of IL-6 synthesis induced by IL-1β in primary cortical neurones and show that IL-1 induces IL-6 synthesis by totally distinct signalling pathways in glia and in neurones.
Firstly, we demonstrated that IL-1β does not activate the classical IL-1 signalling pathways (that is MAPKs) in cortical neurones, but activates nSMase and Src kinase in an IL-1R1-dependent manner, which confirms previous findings obtained on hypothalamic and hippocampal neurones (Viviani et al., 2003; Davis et al., 2006). In our study, IL-1β-induced Src kinase activation was blocked by the nSMase-specific inhibitor 3OMS, providing evidence that nSMase activity is upstream of Src kinase in this pathway, which also confirms the findings of Viviani et al. (2003) and Davis et al. (2006). We also showed that IL-1β induces the phosphorylation of the NMDA receptor NR2B subunit, which in turn leads to the activation of CamKII and CREB, and we demonstrated, using pharmacological approaches, that this series of signalling events occurs downstream of nSMase/Src kinase activation, and are all IL-1R1 dependent. This neuronal-specific IL-1 signalling pathway (nSMase/Src/NR2B/CamKII/CREB), which is known to mediate the fast electrophysiological actions of IL-1 on neurones, also causes IL-6 protein synthesis in these cells, as IL-1RA, 3OMS, PP2, KN-62 and MK-801 all significantly inhibited IL-1β-induced IL-6 synthesis in primary neurones. In contrast, we found that IL-1β failed to activate Src kinase activation in glia (data not shown), whereas IL-1β-induced IL-6 synthesis in glia is dependent on the activation of the classical IL-1 signalling pathway (that is MAPKs and nuclear factor-κB) (Parker et al., 2002).
These observations demonstrate that IL-1 can trigger, in two different brain cell types, the synthesis of the same inflammatory mediator (that is IL-6) via two different cell-specific signalling mechanisms. In addition, IL-1β induces optimal IL-6 expression at a much lower concentration (0.3 U ml−1) in neurones than those required for optimal actions on glia (30 U ml−1). These discoveries suggest that the IL-1 receptor complexes may differ between glia and neurones and suggest new targets to differentiate cell specific effects of IL-1.
In the normal brain, IL-1 and IL-6 are known to modulate neuronal functions and physiological events, such as long-term potentiation, sleep, memory, pain and fever (Takahashi et al., 1996; Schneider et al., 1998; Rachal et al., 2001; Watkins et al., 2001; Ross et al., 2003; Bilbo et al., 2005). Src kinase and its ability to modify NMDA receptors has also been linked with several physiological functions, such as memory, long-term potentiation and pain (Salter and Kalia 2004). Further in vitro studies showed that NMDA receptor activation triggered by cell depolarization leads to IL-6 expression in neurones (Ali et al., 2000; Sallmann et al., 2000). These observations combined with our data suggest that the mechanism of IL-1β-induced IL-6 synthesis that is mediated by the nSMase/Src kinase pathway could play a very important role in the physiological actions of these two cytokines in the brain. IL-1 and IL-6 are also key mediators of neuroinflammation (Gallo et al., 1989; Yamasaki et al., 1995; Bagetta et al., 1999; Vezzani et al., 2000; Allan and Rothwell 2001) and the effect of IL-1β on neuronal cells in CNS injury could profoundly influence the inflammatory response and cell death. Indeed, it has been shown that Src kinase activity is increased after experimentally induced cerebral ischaemia, whereas its inhibition improves neurological outcome (Pei et al., 2000; Paul et al., 2001; Ardizzone et al., 2007). Furthermore, NMDA receptor activation induces cytokine expression after ischaemia (Acarin et al., 2000), whereas IL-6 expression is suppressed by inhibition of Ca2+ influx in cerebral ischaemia (Suzuki et al., 2000). We have shown that IL-1β induces NR2B subunit phosphorylation and this could be a critical control mechanism to modulate the susceptibility of neurones to injury.
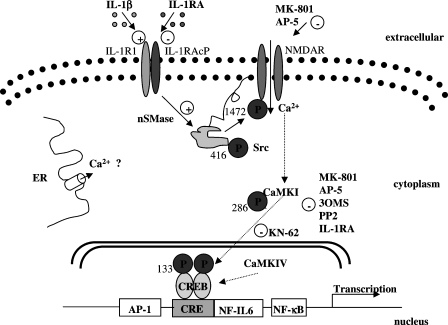
In summary, we have elucidated a major signalling pathway through which IL-1β acts on cortical neurones to induce the synthesis of IL-6, and these signalling mechanisms are summarized in Figure 8. In cortical neurones, IL-1β action through nSMase/Src kinase/NMDA receptor activation triggers the synthesis of an inflammatory mediator (IL-6) via CamKII/CREB activation, which links neuronal activity with gene expression. This mechanism could be critical in both physiological and pathological actions of IL-1β, and could be a new therapeutic target for the treatment of acute CNS injury.
Figure 8.
Schematic diagram of proposed mechanism of the IL-1β-induced signalling pathway involved in the synthesis of IL-6 in primary cortical neurones. IL-1β binds to IL-1R1 in primary neurones to triggers downstream activation of nSMase and Src kinase, which in turn activates NMDA receptors. This leads to an increase in Ca2+ influx in the cells, which triggers activation of CamKII and CREB, leading to the ultimate expression of IL-6 gene and synthesis of the protein. CREB, cAMP response element-binding protein; IL, interleukin.
Acknowledgments
This work was supported by Syngenta and the Medical Research Council. We thank Dr Steve Poole from NIBSC for kindly providing rat recombinant IL-1β and human recombinant IL-1RA, and Dr Deborah Bentley for critically reviewing this paper.
Abbreviations
- 3OMS
3-O-methyl-sphingomyelin
- CamKII
calmodulin-dependent protein kinase II
- CREB
cAMP response element-binding protein
- ERK
extracellular signal-regulated kinase
- IL
interleukin
- IL-1RA
interleukin-1 receptor antagonist
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- nSMase
neutral sphingomyelinase
Conflict of interest
The authors state no conflict of interest.
References
- Acarin L, Gonzalez B, Castellano B. Neuronal, astroglial and microglial cytokine expression after an excitotoxic lesion in the immature rat brain. Eur J Neurosci. 2000;12:3505–3520. doi: 10.1046/j.1460-9568.2000.00226.x. [DOI] [PubMed] [Google Scholar]
- Ali C, Nicole O, Docagne F, Lesne S, MacKenzie ET, Nouvelot A, et al. Ischemia-induced interleukin-6 as a potential endogenous neuroprotective cytokine against NMDA receptor-mediated excitotoxicity in the brain. J Cereb Blood Flow Metab. 2000;20:956–966. doi: 10.1097/00004647-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Allan SM, Lawrence CB, Grundy RP, Stroemer RP, Rothwell NJ.Sites and mechanisms of interleukin-1 action Pharmacology of Cerebral Ischemia 1998Medpharm Scientific Publishers: Stuttgart; 395–399.In: Krieglstein J and Oberpichler-Schwenk HWV (eds) [Google Scholar]
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Inflammation in central nervous system injury. Philos Trans R Soc Lond B Biol Sci. 2003;358:1669–1677. doi: 10.1098/rstb.2003.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardizzone TD, Zhan X, Ander BP, Sharp FR. SRC kinase inhibition improves acute outcomes after experimental intracerebral hemorrhage. Stroke. 2007;38:1621–1625. doi: 10.1161/STROKEAHA.106.478966. [DOI] [PubMed] [Google Scholar]
- Bagetta G, Corasaniti MT, Berliocchi L, Nistico R, Giammarioli AM, Malorni W, et al. Involvement of interleukin-1beta in the mechanism of human immunodeficiency virus type 1 (HIV-1) recombinant protein gp120-induced apoptosis in the neocortex of rat. Neuroscience. 1999;89:1051–1066. doi: 10.1016/s0306-4522(98)00363-7. [DOI] [PubMed] [Google Scholar]
- Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78:151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- Basu A, Krady JK, O'Malley M, Styren SD, DeKosky ST, Levison SW. The type 1 interleukin-1 receptor is essential for the efficient activation of microglia and the induction of multiple proinflammatory mediators in response to brain injury. J Neurosci. 2002;22:6071–6082. doi: 10.1523/JNEUROSCI.22-14-06071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Gheusi G, Cremona S, Laye S, Parnet P, et al. Molecular basis of sickness behavior. Ann N Y Acad Sci. 1998;856:132–138. doi: 10.1111/j.1749-6632.1998.tb08321.x. [DOI] [PubMed] [Google Scholar]
- Davis CN, Tabarean I, Gaidarova S, Behrens MM, Bartfai T. IL-1beta induces a MyD88-dependent and ceramide-mediated activation of Src in anterior hypothalamic neurons. J Neurochem. 2006;98:1379–1389. doi: 10.1111/j.1471-4159.2006.03951.x. [DOI] [PubMed] [Google Scholar]
- Desson SE, Ferguson AV. Interleukin 1beta modulates rat subfornical organ neurons as a result of activation of a non-selective cationic conductance. J Physiol. 2003;550:113–122. doi: 10.1113/jphysiol.2003.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo P, Frei K, Rordorf C, Lazdins J, Tavolato B, Fontana A. Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system: an evaluation of cytokines in cerebrospinal fluid. J Neuroimmunol. 1989;23:109–116. doi: 10.1016/0165-5728(89)90029-5. [DOI] [PubMed] [Google Scholar]
- Giulian D, Young DG, Woodward J, Brown DC, Lachman LB. Interleukin-1 is an astroglial growth factor in the developing brain. J Neurosci. 1988;8:709–714. doi: 10.1523/JNEUROSCI.08-02-00709.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GR, Chen L, Rivieccio MA, Melendez-Vasquez CV, Hartley A, Brosnan CF. Interleukin-1beta induces a reactive astroglial phenotype via deactivation of the Rho GTPase-Rock axis. J Neurosci. 2004;24:2837–2845. doi: 10.1523/JNEUROSCI.4789-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GR, Lee SC, Song X, Rivieccio M, Brosnan CF. IL-1-regulated responses in astrocytes: relevance to injury and recovery. Glia. 2005;49:161–176. doi: 10.1002/glia.20109. [DOI] [PubMed] [Google Scholar]
- Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M. Interleukin-1 beta inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. Eur J Pharmacol. 1990;181:323–326. doi: 10.1016/0014-2999(90)90099-r. [DOI] [PubMed] [Google Scholar]
- Kelly A, Lynch A, Vereker E, Nolan Y, Queenan P, Whittaker E, et al. The anti-inflammatory cytokine, interleukin (IL)-10, blocks the inhibitory effect of IL-1 beta on long term Potentiation. A role for JNK. J Biol Chem. 2001;276:45564–45572. doi: 10.1074/jbc.M108757200. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Drukarch B, Van Dam AM. (Peri)vascular production and action of pro-inflammatory cytokines in brain pathology. Clin Sci (London) 2007;112:1–25. doi: 10.1042/CS20060043. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Lacroix S, Rivest S. An essential role of interleukin-1beta in mediating NF-kappaB activity and COX-2 transcription in cells of the blood–brain barrier in response to a systemic and localized inflammation but not during endotoxemia. J Neurosci. 1999;19:10923–10930. doi: 10.1523/JNEUROSCI.19-24-10923.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1beta promotes repair of the CNS. J Neurosci. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JD, Rothwell NJ, Gibson RM. Involvement of caspases and calpains in cerebrocortical neuronal cell death is stimulus-dependent. Br J Pharmacol. 2002;135:1069–1077. doi: 10.1038/sj.bjp.0704538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PG, Grondin J, Altares M, Richardson PM. Induction of interleukin-6 in axotomized sensory neurons. J Neurosci. 1995;15:5130–5138. doi: 10.1523/JNEUROSCI.15-07-05130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CA, McGahon B, McBennett S, Lynch MA. Interleukin-1 beta inhibits glutamate release in hippocampus of young, but not aged, rats. Neurobiol Aging. 1997;18:343–348. doi: 10.1016/s0197-4580(97)80317-x. [DOI] [PubMed] [Google Scholar]
- Parker LC, Luheshi GN, Rothwell NJ, Pinteaux E. IL-1beta signalling in glial cells in wildtype and IL-1RI deficient mice. Br J Pharmacol. 2002;136:312–320. doi: 10.1038/sj.bjp.0704715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RL, et al. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat Med. 2001;7:222–227. doi: 10.1038/84675. [DOI] [PubMed] [Google Scholar]
- Pei L, Li Y, Zhang GY, Cui ZC, Zhu ZM. Mechanisms of regulation of tyrosine phosphorylation of NMDA receptor subunit 2B after cerebral ischemia/reperfusion. Acta Pharmacol Sin. 2000;21:695–700. [PubMed] [Google Scholar]
- Rachal PC, Fleshner M, Watkins LR, Maier SF, Rudy JW. The immune system and memory consolidation: a role for the cytokine IL-1beta. Neurosci Biobehav Rev. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Ringheim GE, Burgher KL, Heroux JA. Interleukin-6 mRNA expression by cortical neurons in culture: evidence for neuronal sources of interleukin-6 production in the brain. J Neuroimmunol. 1995;63:113–123. doi: 10.1016/0165-5728(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Ross FM, Allan SM, Rothwell NJ, Verkhratsky A. A dual role for interleukin-1 in LTP in mouse hippocampal slices. J Neuroimmunol. 2003;144:61–67. doi: 10.1016/j.jneuroim.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Rothwell N. Interleukin-1 and neuronal injury: mechanisms, modification, and therapeutic potential. Brain Behav Immun. 2003;17:152–157. doi: 10.1016/s0889-1591(02)00098-3. [DOI] [PubMed] [Google Scholar]
- Sallmann S, Juttler E, Prinz S, Petersen N, Knopf U, Weiser T, et al. Induction of interleukin-6 by depolarization of neurons. J Neurosci. 2000;20:8637–8642. doi: 10.1523/JNEUROSCI.20-23-08637.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Tabarean IV, Behrens MM, Bartfai T. Ceramide mediates the rapid phase of febrile response to IL-1beta. Proc Natl Acad Sci USA. 2006;103:2904–2908. doi: 10.1073/pnas.0510960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci USA. 1998;95:7778–7783. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu HF, Wang BR, Bi H, Pei JM, Wang X, Fan J, et al. PC12 cells express IL-1 receptor type I and response to IL-1beta stimulation. Respir Physiol Neurobiol. 2007;157:187–195. doi: 10.1016/j.resp.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Tanaka K, Nogawa S, Dembo T, Kosakai A, Fukuuchi Y. Expression of interleukin-6 is suppressed by inhibition of voltage-sensitive Na+/Ca2+ channels after cerebral ischemia. Neuroreport. 2000;11:2565–2569. doi: 10.1097/00001756-200008030-00043. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kapas L, Fang J, Seyer JM, Wang Y, Krueger JM. An interleukin-1 receptor fragment inhibits spontaneous sleep and muramyl dipeptide-induced sleep in rabbits. Am J Physiol. 1996;271:R101–R108. doi: 10.1152/ajpregu.1996.271.1.R101. [DOI] [PubMed] [Google Scholar]
- Tsakiri N, Kimber I, Rothwell N, Pinteaux E.Mechanisms of interleukin-6 synthesis and release induced by interleukin-1 and cell depolarisation in neurones Mol Cell Neurosci 2007. doi:10.1016/j.mcn.2007.09.001 [DOI] [PubMed]
- Vela JM, Molina-Holgado E, Arevalo-Martin A, Almazan G, Guaza C. Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol Cell Neurosci. 2002;20:489–502. doi: 10.1006/mcne.2002.1127. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Moneta D, Conti M, Richichi C, Ravizza T, De Luigi A, et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci USA. 2000;97:11534–11539. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B, Gardoni F, Bartesaghi S, Corsini E, Facchi A, Galli CL, et al. Interleukin-1 beta released by gp120 drives neural death through tyrosine phosphorylation and trafficking of NMDA receptors. J Biol Chem. 2006;281:30212–30222. doi: 10.1074/jbc.M602156200. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–680. doi: 10.1161/01.str.26.4.676. [DOI] [PubMed] [Google Scholar]
- Zeise ML, Madamba S, Siggins GR. Interleukin-1 beta increases synaptic inhibition in rat hippocampal pyramidal neurons in vitro. Regul Pept. 1992;39:1–7. doi: 10.1016/0167-0115(92)90002-c. [DOI] [PubMed] [Google Scholar]