Abstract
Background and purpose:
The most common preclinical models of neuropathic pain involve surgical ligation of sensory nerves, which is especially difficult in mice. Transient models of chemically sensitized allodynia are potentially useful for rapidly characterizing the analgesic profile of compounds and conducting mechanistic studies.
Experimental approach:
Increasing doses of NMDA, sulprostone (an EP1/EP3 prostaglandin receptor agonist) or phenylephrine (an α 1 adrenoceptor agonist) were injected intrathecally (i.t.) or i.p., and animals were subsequently assessed for allodynia. The effects of receptor antagonists and analgesic compounds on allodynia were also assessed.
Key results:
A comparison of total body doses that cause allodynia following spinal or systemic administration indicated that NMDA induces allodynia in the spinal cord while sulprostone and phenylephrine act through a peripheral mechanism. Inhibition of the allodynia with receptor antagonists indicated that each agent induces allodynia by a distinct mechanism. The three models were benchmarked using compounds known to be active in neuropathic pain patients and nerve injury animal models, including gabapentin, amitriptyline and clonidine.
Conclusions and implications:
These transient allodynia models are a useful addition to the toolbox of preclinical pain models. They are simple, rapid and reproducible, and will be especially useful for characterizing the pain phenotype of knockout mice.
Keywords: preclinical models, neuropathic pain, allodynia
Introduction
Chronic neuropathic pain, often characterized by a persistent aberrant responsiveness to both painful and non-painful stimuli, results from a variety of aetiologies and is largely untreated in the clinic. Symptoms can include cold and tactile allodynia, lancinating pain and burning sensations. The onset of neuropathic pain is secondary to conditions that result in peripheral nerve injury, such as diabetic neuropathy, viral neuralgia, some chemotherapy treatments, cancer and surgery.
Although most forms of neuropathic pain are not associated with traumatic nerve injury, the most common preclinical models of neuropathic pain involve ligation of sensory nerves. These models include loose ligation of the sciatic nerve (chronic constriction injury) (Bennett and Xie, 1988), tight ligation of two spinal nerves (spinal nerve ligation (SNL)) (Kim and Chung, 1992) and ligation of two branches of the sciatic nerve (spared nerve injury) (Decosterd and Woolf, 2000). The nerve injury models mimic some of the symptoms of human neuropathic pain including allodynia, the perception of normally non-noxious stimuli like light touch as painful. The limitations of these models include the association with a neuronal response to traumatic injury, the variability in symptoms, the difficulty of the surgical preparations and the long-term pain sensitivity of the animals.
Models of chemically induced neuronal sensitization are potentially useful for characterizing the analgesic profile of novel compounds in animals without nerve injury. The models can be used as rapid drug screens and to localize sites of action in the pain pathway. They are readily implemented in mice, requiring smaller drug quantities than in rats and enabling the use of knockout mice models. It has been demonstrated previously that spinal injection of various agents in mice results in a transient allodynia, as measured by scoring increased sensitivity to light stroking of the flank (Yaksh and Harty, 1988). Intrathecal (i.t.) injection of prostaglandin E2 caused allodynia over a broad dose-range (Minami et al., 1994c). It was subsequently shown using selective prostaglandin receptor ligands and prostaglandin receptor knockout mice that this allodynia was mediated by the prostaglandin receptor EP1 (Minami et al., 1994a, 2001b). Activation of the receptor is likely to enhance release of glutamate from the central terminals of C-nociceptive fibres, since the allodynia could be prevented with N-methyl-D-aspartate (NMDA) glutamate antagonists (Minami et al., 1994b) or prior treatment with dermal capsaicin, which selectively desensitizes C-fibres (Minami et al., 1999). Interestingly, spinal injection of NMDA to directly activate dorsal horn NMDA receptors also results in an allodynia that is longer lasting and may mimic central sensitization (Minami et al., 2001a).
We further characterized the chemical allodynia induced by NMDA and a prostaglandin receptor agonist selective for the EP1 and EP3 receptors, sulprostone. In addition, we tested allodynia caused by an α1 adrenoceptor agonist, phenylephrine, since altered pain sensation in nerve injury models is partly due to sympathetic nervous system activation of pain fibres (Sato and Perl, 1991; McLachlan et al., 1993; Chung et al., 1997). The three agents (NMDA, sulprostone and phenylephrine) were studied to determine whether the models are mechanistically distinct and whether neuropathic pain drugs are effective. We found that NMDA induces allodynia in the spinal cord, whereas sulprostone- and phenylephrine-induced allodynia was most likely to be mediated by activation of peripheral receptors. The sulprostone model was the best model for identifying clinically efficacious compounds, such as tricyclic antidepressants and gabapentin; this model appeared to be most pharmacologically similar to the rat SNL model, which was used as a model for comparison.
Methods
Animals
Male Black/6-C57 mice (336; Charles River, Wilmington, MA, USA) weighing approximately 25 g were used for the transient chemical allodynia studies. Male Sprague–Dawley rats (110; Charles River) weighing 100–120 g were used for the rat SNL model. All experimental animals were housed in standard plastic cages designed to allow easy access to food and water ad libitum. All animals were kept in controlled temperature chambers (24±1°C) on a 12:12 light–dark cycle (light on 0600–1800 hours). All experiments were in accordance with protocols approved by the Allergan Institutional Animal Care and Use Committee.
Experimental design
In the transient chemical allodynia studies, mice were given i.t. injections (made in a 5 μl volume; Hylden and Wilcox, 1982) and intraperitoneal (i.p.) injections (made in a 1 ml kg−1 volume) of increasing doses of NMDA, sulprostone or phenylephrine. To assess whether various antagonists could prevent the allodynia, antagonists were administered 15 min before the allodynic agent; the antagonists used were the NMDA receptor antagonist memantine (1 μg i.t.), the EP1 prostaglandin receptor antagonist ZM325802 (30 ng kg−1 i.p.), the α1 adrenoceptor antagonist 5-methylurapidil (30 μg kg−1 i.p.), the non-NMDA glutamate receptor antagonist cyanonitroquinoxaline (CNQX; 3 μg i.t.) and the γ-aminobutyric acid (GABA)-A antagonist bicuculline (5 μg i.t.). Doses of the antagonists that reversed the allodynia caused by their corresponding agonist to vehicle levels were selected for testing against the other agents; the doses of CNQX and bicuculline were based on the literature (Zou et al., 2001). To assess the utility of the models for identifying potential drug candidates for the treatment of clinical neuropathic pain, analgesic compounds (per os (p.o.) gabapentin 30 mg kg−1, i.t. amitriptyline 1 μg, i.t. clonidine 0.4 and 1 μg, i.p. morphine 15 mg kg−1) were administered 15 min before the allodynic agent. Selected doses were based on dose–response data from the rat SNL model. In the antagonist and analgesic studies, the allodynic agents were i.t. NMDA 100 ng, i.p. sulprostone 300 ng kg−1 or i.p. phenylephrine 30 ng kg−1.
For the rat SNL model, the L5 and L6 spinal nerves were tightly ligated with 6–0 silk thread as originally described by Kim and Chung (1992). Analgesic compounds were administered 30 min before allodynia testing. The agents assessed were gabapentin (p.o.; 0.3, 1, 3, 10 and 30 mg kg−1), amitriptyline (i.p.; 10, 30, 100 and 300 μg kg−1), clonidine (i.t.; 0.1, 0.3 and 1 μg) and morphine (i.p.; 1, 5 and 15 mg kg−1).
Assessments
For the chemical allodynia model, the mice were assessed for tactile sensitivity by light stroking of the rear flank with a small paintbrush at 5-min intervals from 15 to 50 min after injection of the allodynic agent. It was previously determined that any confounding hyperactivity owing to the injection subsides by 15 min and the allodynia response to all three agents remains at peak levels during this testing period. Mice were scored as 0 (no response), 1 (avoidance) or 2 (vigorous avoidance) and the scores were summed to yield a total pain score (maximum 16 for the sum of the scores at each of the eight time points) (Minami et al., 1994c).
For the rat SNL model, at least 1 week after surgery, allodynia was assessed by applying various calibrated von Frey filaments in an up-down manner to the affected surgical paw (Dixon, 1980) until the 50% withdrawal threshold was established. The von Frey hairs were applied vertically through a wire-mesh floor to the plantar surface of the surgical paw with just enough force to bend them. A positive response was recorded when the paw was rapidly withdrawn.
Data analysis and statistical procedures
Percent allodynia reversal was calculated using the following equation:
Data were compiled and analysed using Statistical Package for the Social Sciences (SPSS, Chicago), Microsoft Office Excel and/or KaleidaGraph. Data are expressed as mean±s.e.m. Comparisons between groups were made using a one-way analysis of variance analysis followed by a Dunnett's test. The significance value was set at P<0.05.
Drugs
Sulprostone (Cayman Chemical Company, Ann Arbor, MI, USA) and NMDA (Sigma Chemical Company, St Louis, MO, USA) were dissolved in 100% dimethyl sulphoxide (DMSO; Sigma). ZM325802 (6-((5-chloro-2-isobutoxybenzyl)(ethyl)amino)-N-(3,5-dimethylisoxazol-4-ylsulphonyl)pyridazine-3-carboxamide), bicuculline (Tocris, Ellisville, MO, USA) and CNQX (Research Biochemicals Inc., Natick, MA, USA) were formulated in 50% DMSO. Phenylephrine (Sigma), gabapentin (Victor Medical, Irvine, CA, USA), amitriptyline (Sigma), clonidine (Sigma), memantine (synthesized by Allergan) and 5-methyl urapidil (Sigma) were all dissolved in distilled water. Morphine (Baxter, Deerfield, IL, USA) is provided in saline. The vehicle was DMSO or distilled water.
Results
Induction of allodynia by NMDA
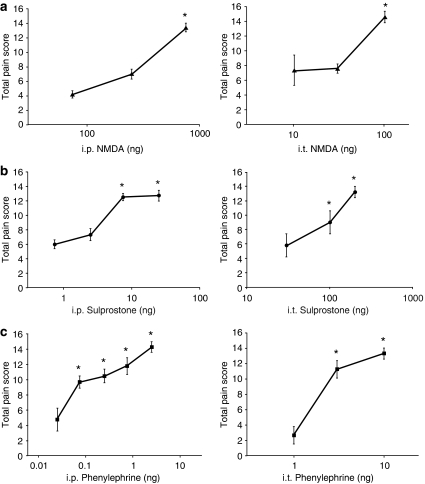
Mice were administered i.t. and i.p. injections of increasing doses of NMDA, sulprostone or phenylephrine to determine whether the sensitizing agents induce allodynia via a spinal or peripheral site. Consistent with previous reports of NMDA activating post-synaptic dorsal horn neurons, injection of increasing i.t. doses of NMDA resulted in a dose-dependent tactile allodynia with a maximal effect at a 100 ng dose (Figure 1a; Table 1). The allodynia lasted for approximately 60 min (data not shown). NMDA administered i.p. also induced allodynia, with a peak allodynic effect elicited by 30 μg kg−1 NMDA, or a total body dose of 750 ng for a 25 g mouse (Figure 1a; Table 1). The fact that a lower total body dose injected i.t. induces allodynia suggests that NMDA acts primarily through a spinal action to induce allodynia.
Figure 1.
A comparison of total body doses (i.p. ng kg−1 × 0.025 kg=ng total body dose) that induce tactile sensitivity following i.p. (left) or i.t. (right) injection. (a) NMDA (NMDA glutamate agonist); (b) Sulprostone (EP1/EP3 prostaglandin agonist); (c) Phenylephrine (α1 adrenoceptor agonist). The corresponding i.p. and i.t. vehicle values were (a, b) (DMSO): 5.8±0.40 and 3.7±1.20, respectively; (c) (distilled water): 4.7±0.56 and 4.5±0.99, respectively. All values are mean±s.e.m.; *P<0.05 versus vehicle; n=5–6 in all groups. DMSO, dimethyl sulphoxide.
Table 1.
Characteristics of allodynia induced by sensitizing agents
| Sensitizing agent |
Total body dose at peak allodynic effect |
Duration of allodynia |
||
|---|---|---|---|---|
| i.p. | i.t. | i.p. | i.t. | |
| NMDA | 750 ng | 100 ng | ND | 60 min |
| Sulprostone | 7.5 ng | 200 ng | 100 min | ND |
| Phenylephrine | 750 pg | 3 ng | 60 min | ND |
Abbreviations: i.p., intraperitoneal; i.t., intrathecal; ND, not determined; NMDA, N-methyl-D-aspartate.
Induction of allodynia by sulprostone
Administration by i.t. injection of the EP1/EP3 prostaglandin agonist sulprostone resulted in a peak allodynic effect at a 200 ng i.t. dose (Figure 1b; Table 1). In contrast, maximal allodynia was induced by i.p. injection of 300 ng kg−1 sulprostone, equivalent to a total body dose of approximately 7.5 ng for a 25 g mouse (Figure 1b; Table 1), indicating that, in addition to a proposed spinal EP1 receptor, there is also a peripheral site of action. The duration of allodynia following i.p. injection exceeded 100 min (data not shown).
Induction of allodynia by phenylephrine
The α1 adrenoceptor agonist phenylephrine was a potent inducer of allodynia, with significant dose-related responses observed at doses of 3 ng i.t. and 3 ng kg−1 i.p. (Figure 1c; Table 1). The i.p. total body dose equivalent of approximately 75 pg for a 25 g mouse, which is less than the i.t. dose, is indicative of primarily a peripheral site of action. The allodynia lasted approximately 60 min following peripheral administration (data not shown).
Ability of antagonists to prevent allodynia
The ability of a number of pharmacological agents to prevent the allodynia was tested to assess whether the three allodynic stimuli sensitize sensory pathways by different mechanisms (Table 2). Antagonists of the NMDA receptor (memantine), EP1 prostaglandin receptor (ZM325802) and α1 adrenoceptor (5-methylurapidil) only blocked the allodynia caused by their corresponding receptor agonist (NMDA, sulprostone and phenylephrine, respectively). Antagonists of receptors involved in pain processing in the spinal cord were also tested. The non-NMDA glutamate receptor antagonist CNQX and the GABA-A antagonist bicuculline blocked NMDA-induced allodynia, but had no effect on the allodynia caused by the peripheral sensitizing agents.
Table 2.
Characterization with receptor antagonists demonstrates distinct mechanisms of allodynia
| Model |
Antagonist |
||||
|---|---|---|---|---|---|
| Memantine | ZM325802 | 5-MU | CNQX | Bicuculline | |
| NMDA | + | − | − | + | + |
| Sulprostone | − | + | − | − | − |
| Phenylephrine | − | − | + | − | − |
Abbreviations: CNQX, cyanonitroquinoxaline; 5-MU, 5-methylurapidil; NMDA, N-methyl-D-aspartate.
+=antagonist reversed allodynia to vehicle levels; −=no effect on allodynia.
Effects of analgesic compounds
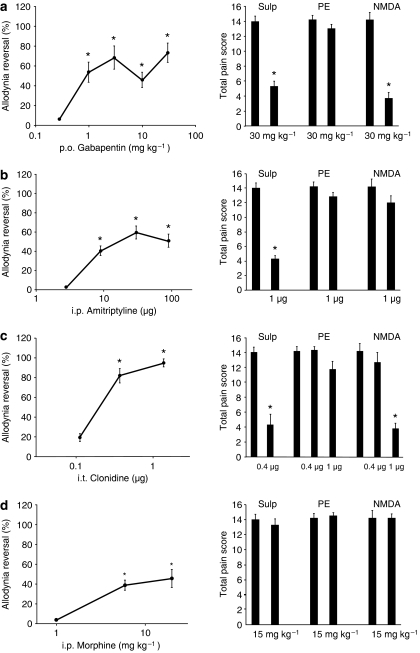
To compare the utility of these models for identifying potential drugs for neuropathic pain, gabapentin, amitriptyline, clonidine and morphine were tested in the mouse allodynia models and the rat SNL model. Gabapentin at a maximal dose of 30 mg kg−1 alleviated allodynia in the SNL model and inhibited allodynia in the mice when elicited by sulprostone or NMDA, but not by phenylephrine (Figure 2a). The tricyclic antidepressant amitriptyline also alleviated allodynia in the SNL model with a maximal effect at 0.1 mg kg−1 (Figure 2b). An i.t. injection (1 μg) in the mice alleviated allodynia caused by sulprostone, but not NMDA or phenylephrine (Figure 2b). A 0.3 and 1 μg i.t. injection of the α2-adrenergic agonist clonidine alleviated allodynia in SNL rats, while a 0.4 μg i.t. dose alleviated sulprostone-induced allodynia and a 1 μg i.t. dose alleviated NMDA-induced allodynia (Figure 2c). Neuropathic pain responds variably to opioid treatment; in this study, non-sedating doses of morphine (5 and 15 mg kg−1 i.p.) had an approximately 40% effect on the allodynia in the SNL rats and no effect in the mouse models (Figure 2d).
Figure 2.
A comparison of the effect of neuropathic pain drugs in the rat SNL model (left) and the three mouse allodynia models (right). (a) Gabapentin (anticonvulsant); (b) amitriptyline (tricyclic antidepressant); (c) clonidine (α2 adrenoceptor agonist); (d) morphine. The corresponding vehicle values for the rat model were (a) 1.56±1.39 (p.o. DMSO); (b) 0.94±0.62 (i.p. saline); (c) 0.56±2.07 (i.t. saline); (d) 1.68±1.55 (i.p. distilled water); and for the mouse models were 5.8±0.4 for i.p. DMSO (sulprostone vehicle); 3.7±1.2 for i.t. DMSO (NMDA vehicle); 4.7±0.6 for i.p. distilled water (phenylephrine vehicle). All values are mean±s.e.m.; *P<0.05 versus vehicle; n=5–6 in all groups. Abbreviations: AMTRP, amitriptyline; clon, clonidine; DMSO, dimethyl sulphoxide; GPT, gabapentin; mor, morphine; PE, phenylephrine; SNL, spinal nerve ligation; Sulp, sulprostone.
Discussion and conclusions
Most preclinical models of neuropathic pain require surgery that results in a permanent nerve injury state. These models have been benchmarked with clinically relevant compounds and have been useful for identifying novel clinical candidate compounds. Numerous studies have demonstrated that, following nerve injury, dorsal root ganglion neurons exhibit dramatic changes in gene expression patterns and electrophysiological properties. One potential drawback of these models is that the relevance of these changes to human pain is as yet unknown. Transient models of allodynia may be useful for identifying analgesic mechanisms and compounds that are independent of nerve injury and associated gene expression changes. To develop a transient, facile and consistent model of allodynic pain in mice, we characterized pharmacologically the effects of several agents that sensitize peripheral and spinal sensory nerves.
Spinal NMDA is known to elicit an allodynic response in mice, presumably by activation of post-synaptic dorsal horn NMDA receptors. The spinal action is consistent with our finding that a lower total body dose was needed following spinal, versus systemic, injection (Figure 1a). The allodynia was blocked with the NMDA antagonist memantine, but it was surprisingly also blocked by the non-NMDA antagonist CNQX. Bicuculline, a GABA-A receptor antagonist, also blocked the NMDA-induced allodynia (Table 2). Such a pharmacological profile has been reported in a mouse model of capsaicin-induced neurogenic inflammation, which has been proposed to involve spinal GABAergic interneurons and the activation of a dorsal root reflex that drives release of inflammatory mediators from primary afferent terminals (Lin et al., 1999; Zou et al., 2001).
The selective prostaglandin agonist sulprostone has also been reported as a spinal allodynic agent that activates EP1 prostaglandin receptor-mediated dorsal horn glutamate release from C-nociceptive fibres (Minami et al., 1994c, 1999). However, we observed that doses of i.p. sulprostone that are 25- to 30-fold lower than doses of i.t. sulprostone evoked an allodynia response, indicating that there is a peripheral site of action (Figure 1b). The selective EP1 prostaglandin receptor antagonist ZM325802 blocked the peripheral allodynia, confirming that the peripheral sulprostone target is an EP1 receptor (Table 2), perhaps in dorsal root ganglion neurons (Oida et al., 1995). The spinal antagonists, memantine, CNQX and bicuculline, did not block peripheral sulprostone allodynia (Table 2), so its mechanism of action was distinct from that of NMDA and spinal sulprostone.
Since the sympathetic nervous system can enhance some neuropathic and inflammatory pain states, we also tested the allodynic effect of peripheral activation of post-synaptic α1 adrenoceptors with phenylephrine. We confirmed that this agent acts peripherally, since a 40-fold lower dose was active following i.p. versus i.t. injection (Figure 1b). The α1 adrenoceptor antagonist 5-methyl urapidil blocked the phenylephrine allodynia, but did not block the sulprostone or NMDA allodynic effects. Likewise, the EP1 antagonist and the spinal antagonists did not block phenylephrine-induced allodynia (Table 2), consistent with a distinct mechanism of action.
The ability of clinical therapeutic drugs to alleviate each model of allodynia was benchmarked and compared with their effects in the standard rat SNL model. Gabapentin, an anticonvulsant, amitriptyline, a tricyclic antidepressant and spinal clonidine, an α2 adrenoceptor agonist, are all effective in the SNL model (Figures 2a–c). Likewise, peripheral sulprostone-induced allodynia was sensitive to all three therapeutic agents. Spinal NMDA-evoked allodynia was sensitive to gabapentin and a higher dose of clonidine, while phenylephrine-induced allodynia was only alleviated by gabapentin (Figures 2a–c). Morphine was weakly active in the SNL model and not active in any of the mouse models (Figure 2d), which is consistent with partial preclinical activity reported previously (Lemberg et al., 2006) and the variable clinical activity in neuropathic pain states. It should be noted that the i.p. rather than subcutaneous route of administration may have contributed to the lack of activity in the mouse models. Thus, the peripheral sulprostone model may be most similar to the SNL model in its pharmacology. While the relevance of EP1 receptor activation to neuropathic pain is unclear, this finding implies that the peripheral sensory nerves sensitized in the sulprostone and SNL models converge on a common pain pathway.
Since each transient allodynia model involves a distinct pathway, identifying compounds such as gabapentin that work broadly in many models may be a useful strategy for identifying therapeutic agents. These models will enable the identification of drugs whose anti-allodynic activity is independent of traumatic nerve injury. The use of these models, in combination with the standard rodent nerve injury models, can provide a broader view of a compound's analgesic potential in mechanistically distinct types of pain. The models can also be applied to pain studies in knockout mice.
Acknowledgments
This study was conducted by Allergan Inc., Irvine, CA, USA. We thank Tricia K Young for her technical assistance.
Abbreviations
- CNQX
cyanonitroquinoxaline
- DMSO
dimethyl sulphoxide
- SNL
spinal nerve ligation
Conflict of interest
Daniel W Gil, Cynthia V Cheevers, and John E Donello are employees of Allergan Inc.
References
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Chung K, Yoon YW, Chung JM. Sprouting sympathetic fibers form synaptic varicosities in the dorsal root ganglion of the rat with neuropathic injury. Brain Res. 1997;751:275–280. doi: 10.1016/s0006-8993(96)01408-4. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal opioids block a spinal action of substance P in mice: functional importance of both mu- and delta-receptors. Eur J Pharmacol. 1982;86:95–98. doi: 10.1016/0014-2999(82)90403-4. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Lemberg K, Kontinen VK, Viljakka K, Kylanlahti I, Yli-Kauhaluoma J, Kalso E. Morphine, oxycodone, methadone and its enantiomers in different models of nociception in the rat. Anesth Analg. 2006;102:1768–1774. doi: 10.1213/01.ane.0000205751.88422.41. [DOI] [PubMed] [Google Scholar]
- Lin Q, Wu J, Willis WD. Dorsal root reflexes and cutaneous neurogenic inflammation after intradermal injection of capsaicin in rats. J Neurophysiol. 1999;82:2602–2611. doi: 10.1152/jn.1999.82.5.2602. [DOI] [PubMed] [Google Scholar]
- McLachlan EM, Janig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363:543–546. doi: 10.1038/363543a0. [DOI] [PubMed] [Google Scholar]
- Minami T, Matsumura S, Okuda-Ashitaka E, Shimamoto K, Sakimura K, Mishina M, et al. Characterization of the glutamatergic system for induction and maintenance of allodynia. Brain Res. 2001a;895:178–185. doi: 10.1016/s0006-8993(01)02069-8. [DOI] [PubMed] [Google Scholar]
- Minami T, Nakano H, Kobayashi T, Sugimoto Y, Ushikubi F, Ichikawa A, et al. Characterization of EP receptor subtypes responsible for prostaglandin E2-induced pain responses by use of EP1 and EP3 receptor knockout mice. Br J Pharmacol. 2001b;133:438–444. doi: 10.1038/sj.bjp.0704092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami T, Nishihara I, Uda R, Ito S, Hyodo M, Hayaishi O. Characterization of EP-receptor subtypes involved in allodynia and hyperalgesia induced by intrathecal administration of prostaglandin E2 to mice. Br J Pharmacol. 1994a;112:735–740. doi: 10.1111/j.1476-5381.1994.tb13139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami T, Nishihara I, Uda R, Ito S, Hyodo M, Hayaishi O. Involvement of glutamate receptors in allodynia induced by prostaglandins E2 and F2 alpha injected into conscious mice. Pain. 1994b;57:225–231. doi: 10.1016/0304-3959(94)90227-5. [DOI] [PubMed] [Google Scholar]
- Minami T, Okuda-Ashitaka E, Hori Y, Sakuma S, Sugimoto T, Sakimura K, et al. Involvement of primary afferent C-fibres in touch-evoked pain (allodynia) induced by prostaglandin E2. Eur J Neurosci. 1999;11:1849–1856. doi: 10.1046/j.1460-9568.1999.00602.x. [DOI] [PubMed] [Google Scholar]
- Minami T, Uda R, Horiguchi S, Ito S, Hyodo M, Hayaishi O. Allodynia evoked by intrathecal administration of prostaglandin E2 to conscious mice. Pain. 1994c;57:217–223. doi: 10.1016/0304-3959(94)90226-7. [DOI] [PubMed] [Google Scholar]
- Oida H, Namba T, Sugimoto Y, Ushikubi F, Ohishi H, Ichikawa A, et al. In situ hybridization studies of prostacyclin receptor mRNA expression in various mouse organs. Br J Pharmacol. 1995;116:2828–2837. doi: 10.1111/j.1476-5381.1995.tb15933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991;251:1608–1610. doi: 10.1126/science.2011742. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Harty GJ. Pharmacology of the allodynia in rats evoked by high dose intrathecal morphine. J Pharmacol Exp Ther. 1988;244:501–507. [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. NMDA or non-NMDA receptor antagonists attenuate increased Fos expression in spinal dorsal horn GABAergic neurons after intradermal injection of capsaicin in rats. Neuroscience. 2001;106:171–182. doi: 10.1016/s0306-4522(01)00175-0. [DOI] [PubMed] [Google Scholar]