Abstract
Background and purpose:
Rifampicin has been extensively reported to exacerbate the hepatotoxicity of isoniazid in patients with tuberculosis. However, this was controversially claimed by previous reports using rat models. This study evaluated the effect of rifampicin on isoniazid-induced hepatocyte toxicity by using human and rat hepatocytes in tissue-like culture.
Experimental approach:
Hepatocytes in tissue-like gel entrapment were used to examine isoniazid toxicity, as shown by cell viability, intracellular glutathione content and albumin secretion. For demonstration of the differential effects of rifampicin on human and rat hepatocytes, induction by rifampicin of cytochrome P450 (CYP) 2E1, a major enzyme associated with isoniazid hepatotoxicity, was detected by 4-nitrocatechol formation and RT-PCR analysis.
Key results:
Rifampicin (12 μM) enhanced isoniazid-induced toxicity in human hepatocytes but not in rat hepatocytes. Enhanced CYP 2E1 enzymic activity and mRNA expression were similarly detected in human hepatocytes but not in rat hepatocytes. Both rat and human hepatocytes in gel entrapment were more sensitive to isoniazid treatment compared with the corresponding hepatocytes in a monolayer culture.
Conclusions and implications:
The difference in induction of CYP 2E1 by rifampicin between rat and human hepatocytes accounted for the difference in exacerbation of isoniazid hepatocyte toxicity by rifampicin, with more significant toxicity in gel entrapment than in monolayer cultures. Thus, human hepatocytes in tissue-like cultures (gel entrapment) could be an effective model for hepatotoxicity research in vitro, closer to the in vivo situation.
Keywords: tissue-like culture, human hepatocytes, rat hepatocytes, rifampicin, isoniazid, hepatotoxicity
Introduction
Isoniazid, used clinically since 1952 as a first-line antimicrobial for tuberculosis, is adversely associated with hepatotoxicity (Girling, 1978). The bioactive metabolites of isoniazid generated by the drug-metabolizing enzymes, in particular cytochrome P450 (CYP) 2E1, have been implicated in isoniazid-induced hepatotoxicity in humans (Huang et al., 2003), rats (Yue et al., 2004), rabbits (Sarich et al., 1999) and HepG2 (human hepatocellular liver carcinoma cell line) cells (Wu and Cederbaum, 1996). Rifampicin, normally given with isoniazid to patients, is said to enhance the hepatotoxicity of isoniazid, owing to its potent induction of CYP 450 enzymes (Yew, 2002; Yew and Leung, 2006).
Research on isoniazid-induced hepatotoxicity has been hampered by the lack of suitable animal models that closely resemble the toxicity in humans (Sarich et al., 1995). The closest animal model of isoniazid hepatotoxicity was established in rabbits, which showed susceptibility to isoniazid-induced hepatotoxicity, as in humans (Sarich et al., 1995, 1999). For convenience, rats have been extensively used to investigate the hepatotoxicity of isoniazid treatment together with rifampicin for screening antihepatotoxic drugs (Attri et al., 2000; Tasduq et al., 2005; Santhosh et al., 2006). In contrast to the synergistic hepatotoxicity seen in humans after co-administration of isoniazid and rifampicin (Steele et al., 1991; Yew, 2002), no such enhancement of isoniazid-induced hepatotoxicity by rifampicin was observed in rats (Thomas and Solomonraj, 1977; Yue et al., 2004). Thus, there is a clear species difference in the exacerbation of isoniazid hepatotoxicity by co-treatment with rifampicin.
Primary hepatocytes in vitro or cell lines are generally accepted as effective models for hepatotoxicity evaluation, and particularly primary human hepatocytes are considered to be the ‘gold standard' in vitro to avoid the possibility of species-related differences. However, no in vitro study on primary human hepatocytes has ever been reported for investigation of isoniazid hepatotoxicity with or without rifampicin. There are only a few in vitro studies with isoniazid treatment alone, for example, in cell lines (Schwab and Tuschl, 2003; Biagini et al., 2006) and rat hepatocytes (de Longueville et al., 2003; Xu and Purcell, 2006; Shen et al., 2006b). Unfortunately, the hepatotoxic concentrations of isoniazid in cell lines (such as HepG2 and WIF-B9 cells) were around 50 mM, which was much higher than the peak plasma concentrations (3–5.5 mg l−1, equivalent to 0.022–0.040 mM) in humans after oral administration of 300 mg isoniazid (Yew, 1998; Agrawal et al., 2004). This marked difference between toxic concentrations in vitro and the plasma concentrations in vivo was most likely to be due to the difference between the metabolic system of cell lines and that of hepatocytes in vivo. To eliminate this source of difference, intact primary hepatocytes were consequently used for studies on isoniazid hepatotoxicity because of the preservation of all the phase I and phase II enzymes in vivo. Nevertheless, even though primary hepatocytes in monolayer culture have gained widespread popularity over many years in hepatotoxicity studies in vitro, the toxic concentrations of isoniazid were over 10 mM in rat hepatocyte monolayers, by the methyl thiazolyl tetrazolium (MTT) assay (de Longueville et al., 2003; Shen et al., 2006b). This could be due to the rapid loss of liver-specific functions of the hepatocyte monolayers, especially of CYP 450 activity. Later, rat hepatocyte spheroids, which maintained CYP 450 activity for more than 1 week, were used to evaluate isoniazid hepatotoxicity, but here the toxic concentration of isoniazid was still around 5 mM, as indicated by pyruvate uptake and lactic acid release (Xu and Purcell, 2006). It seems that rat hepatocyte spheroids still lacked sensitivity to isoniazid exposure, similar to the insensitivity of rat hepatocyte spheroids to methotrexate treatment (Walker et al., 2000). This could be related to the delivery of test compounds to hepatocytes within the tight structure of hepatocyte spheroids due to the high diffusion barrier (Walker et al., 2000). Species difference, however, could also result in a different toxicity for isoniazid in rat and human hepatocytes in vivo.
Primary human hepatocytes in tissue-like gel entrapment culture were therefore proposed as a closer model of the liver in vivo. According to our previous studies, rat hepatocytes entrapped in collagen gel as well as cytocompatible hollow fibres maintained liver-specific functions at higher levels and for longer times (Qiu et al., 2006; Shen et al., 2006a, 2006b; Meng et al., 2007) and were more sensitive to isoniazid than the well-known hepatocyte monolayers (Shen et al., 2006b). In this study, both rat and human hepatocytes were used to evaluate the potential species difference on hepatotoxic responses to a low dose of isoniazid. Moreover, the synergistic effect of rifampicin on isoniazid hepatocyte toxicity was evaluated by measuring markers of hepatotoxicity, the metabolic activities and gene expression of CYP 2E1, in both rat and human hepatocytes.
Materials and methods
Hepatocyte cultures
All animal procedures were carried out in accordance with the Animal Welfare Act. Hepatocytes were isolated from male Sprague–Dawley rats (200–250 g) by the two-step collagenase perfusion method as described previously (Wu et al., 2005). Cell viability was assessed by Trypan blue exclusion and hepatocytes with a viability of greater than 85% were used.
Human hepatocytes were harvested from non-transplantable liver tissue from five patients, kindly offered by Zhejiang University Medical Center (Hangzhou, China). The study was approved by the Ethical and Research Committee of Zhejiang University, China, and written informed consent was obtained from each patient. Donor 1 was a 28-year-old yellow race Chinese male; donor 2 was a 37-year-old yellow race Chinese female; donor 3 was a 35-year-old yellow race Chinese male; donor 4 was a 74-year-old yellow race Chinese female; and donor 5 was a 59-year-old yellow race Chinese male. Donors 1–3 were used to show the effects of rifampicin on isoniazid hepatotoxicity and CYP 2E1 metabolic activity, Donors 3–5 were used for isoniazid (0.037–0.11 mM) hepatotoxicity and Donor 5 was used for gene expression assay. All donors were negative for HIV (human immunodeficiency virus), HBV (hepatitis virus B) and HCV (hepatitis virus C). Hepatocyte isolation was conducted by a two-step collagenase perfusion of the liver sample as described before (LeCluyse et al., 2005). Cell viability was assessed by Trypan blue exclusion, and hepatocytes with a viability of greater than 80% were used.
Hepatocytes were cultured in Williams' E medium supplemented with L-glutamine 2 mM, penicillin 100 U ml−1, streptomycin 100 μg ml−1, dexamethasone 1 μM, insulin 0.2 U ml−1, glucagon 4 ng ml−1 and 5% (v/v) fetal bovine serum.
For the monolayer culture, each well of 24-well plates was pre-coated with 0.16 mg l−1 of collagen (type I). Freshly isolated rat hepatocytes were seeded at a density of 2 × 105 cells per well, whereas human hepatocytes were seeded at a density of 3 × 105 cells per well. The cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2.
For the gel entrapment culture, freshly harvested hepatocytes were mixed with the collagen solution and loaded into hollow fibres by injection as described before (Wu et al., 2005). Briefly, hepatocytes were inoculated into a 3:1 (v/v) mixture of type I collagen (2.75 mg ml−1) and fourfold concentrated Williams' E medium at pH 7.4. Hepatocyte suspension at a density of 1 × 106 cells ml−1 was loaded into the lumen of hollow fibres and maintained in a 5% CO2 incubator for collagen gelation. Ten minutes later, hollow fibres were placed into 6 cm culture dishes full of 8 ml pre-warmed culture medium and placed into a 5% CO2 incubator for hepatocyte cultures.
Exposure of hepatocytes to isoniazid and rifampicin
After 4 h of culture, hepatocytes in monolayer or gel entrapment culture were washed with PBS and then treated with isoniazid or isoniazid+rifampicin for 48 or 72 h. To evaluate induction of CYP 2E1, hepatocytes in monolayer or gel entrapment culture were only treated with rifampicin for 48 h before the measurement of CYP 2E1. The culture medium was changed every 2 days before the assays were conducted.
Cell viability assay
The MTT assay was used to evaluate the viability of hepatocyte monolayer and gel-entrapped hepatocytes cultured in hollow fibres. Briefly, the gels entrapping hepatocytes were extruded from the hollow fibres with a 5 ml syringe and immersed in 0.65 ml of the MTT-PBS (1.15 mg ml−1) in 24-well plates followed by incubation at 37 °C for 3 h. After removing the MTT solution, 1.5 ml of isopropanol was added to the cells. After agitation for 1 h, the absorbance of the solution containing the extracts was read at 570 nm on a spectrophotometer as described previously (Wang et al., 1996). The same procedure was used to determine the viability of hepatocytes in monolayer cultures.
Glutathione assay
Hepatocytes in monolayer cultures were rinsed with PBS and scraped off from the wells with a rubber policeman. Gel-entrapped cells were washed with PBS, released from the hollow fibres as described above and then suspended in 100 μl of PBS. After collecting, the hepatocytes were sonicated at 40 kHz and 900 W for 10 s and centrifuged at 18 500 × g to precipitate cellular fragments. The glutathione (GSH) content in cell supernatants was determined by DTNB (5,5-dithiobis(2-nitrobenzoic acid)) assay as described previously (Nieusma et al., 1998).
Determination of albumin synthesis
Albumin secreted into the culture medium by hepatocytes in monolayer and gel entrapment cultures was determined by an immunometric method (ELISA) as described previously (Meng et al., 2006).
Measurement of CYP 2E1 activity
CYP 2E1 activities were measured by the formation of 4-nitrocatechol (4-NC) from 4-nitrophenol (Tassaneeyakul et al., 1993). The CYP 2E1 activities were assayed in both rat and human hepatocytes, with and without rifampicin. In detail, medium in the monolayer culture was replaced by 200 μl PBS containing 1 mM 4-nitrophenol after 48 h incubation. At the same time, gel-entrapped hepatocytes were extruded from the hollow fibre as described above and then immersed in 200 μl PBS containing 1 mM 4-nitrophenol in 24-well plates. After 3 h in the incubator at 37 °C, supernatants were taken and frozen immediately at −20 °C. The formation of 4-NC was analysed by HPLC using an Agilent 1100 Series HPLC (Palo Alto, CA, USA) equipped with an ODS Hypersil column (250 mm × 4.6 mm, 5 μm particle size). The mobile phase, running at a flow rate of 1 ml min−1, consisted of acetonitrile–glacial acetic acid–water (22:1:77) containing triethylamine (30 mM) with pH adjusted to 3.0 by phosphoric acid and the absorbance of isolated peaks was monitored at 250 nm.
Reverse transcription-PCR analysis
Total RNA was isolated from rat and human hepatocytes after 48 h of incubation with or without rifampicin (12 μM) using trizol reagent (Invitrogen, Karlsruhe, Germany). The quality of RNA was assessed by the absorbance of the samples at 260 and 280 nm. Synthesis of cDNA was performed according to the method described before (Wilkening and Bader, 2003) and amplified by PCR. Expression of the gene for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) in hepatocytes was used as a control. The primers for rat CYP 2E1 (accession number AF061442) and rat GADPH (accession number AB017801) used for the PCR were as follows: CYP 2E1, 5′-ATGTCATCCCCAAGGGTACA-3′ and 5′-CGGGGAATGACACAGAGTTT-3′; product size is 334 bp. GADPH, 5′-TCAAGGCTGAGAATGGGAAG-3′ and 5′-GGATGCAGGGATGATGTTCT-3′; product size is 452 bp. The primers for human CYP 2E1 (accession number AF084225.1) and human GADPH (accession number AB062273.1) used for the PCR were as follows: CYP 2E1, 5′-CCTACATGGATGCTGTGGTG-3′ and 5′-AGGGCTGAGGTCGATATCCT-3′; product size is 377 bp. GADPH, 5′-TCACCAGGGCTGCTTTTAAC-3′ and 5′-TGTGGTCATGAGTCCTTCCA-3′; product size is 479 bp.
Data analysis
All values are means±s.d. from three independent experiments. Comparisons between multiple groups were performed with one-way ANOVA, or results from two different treatments were tested for statistical significance with the unpaired student t-test. P-values less than 0.05 were considered statistically significant.
Chemicals
Rifampicin and isoniazid were gifts from Zhejiang Medicine Co. Ltd, Xinchang Pharmaceutical Factory (Shaoxing, China). Williams' E basal medium and L-glutamine were from Gibco (Gaithersburg, MD, USA) and Amresco Inc. (Solon, OH, USA), respectively. Fetal bovine serum was obtained from Hangzhou Sijiqing Biological Eng. Material Co. Ltd (Hangzhou, China). MTT was purchased from Amresco Inc. DTNB was purchased from Sigma (St Louis, MO, USA). The rat and human albumin ELISA quantitation kits were both purchased from BETHYL Laboratories Inc. (Montgomery, TX, USA). 4-NC (Fluka, Seelza, Germany) was kindly offered by Dr Qiu Xinhui (Institute of Zoology, Chinese Academy of Sciences, Beijing, China). The primers for rat CYP 2E1 and rat GAPDH were from Operon Biotechnologies GmbH (Cologne, Germany). The remaining chemicals were obtained from local chemical suppliers and were all of reagent grade.
Results
Toxicity of isoniazid in rat hepatocytes
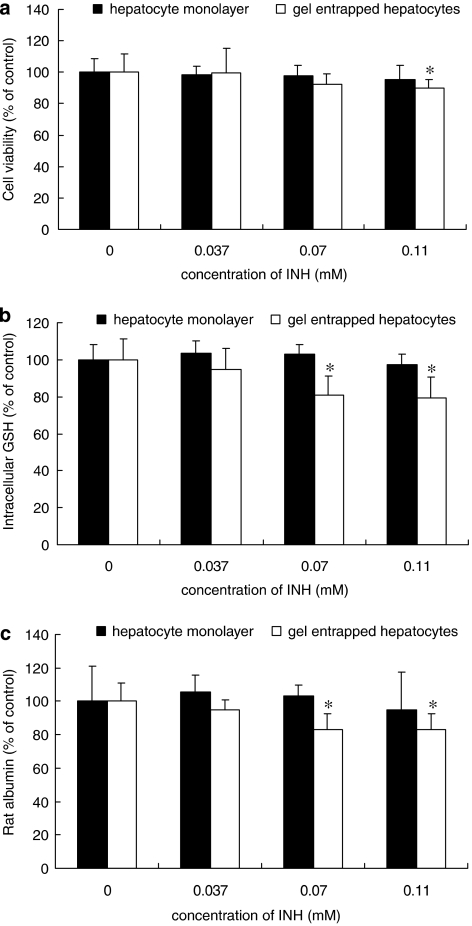
The toxicity of isoniazid at 0.037, 0.07 and 0.11 mM was determined in rat hepatocytes after 72 h incubation. As shown in Figure 1a, rat hepatocytes in monolayer were insensitive to isoniazid at all the concentrations tested, whereas rat hepatocytes in gel entrapment showed a decrease in cell viability after treatment with 0.11 mM isoniazid. Similarly, intracellular GSH and rat albumin in gel-entrapped rat hepatocytes were decreased at 0.07 mM isoniazid, a lower level than affected cell viability, as indicated in Figures 1b and c. Therefore, the lowest toxic concentration of isoniazid in rat hepatocytes was 0.07 mM for gel entrapment cultures, much lower than in monolayer cultures.
Figure 1.
Cell toxicity induced by isoniazid (INH) in rat hepatocytes in monolayer and gel entrapment culture. The hepatocytes were incubated with 0.037, 0.07 and 0.11 mM isoniazid for 72 h, whereas the groups without isoniazid treatment were used as control. The results were assessed by cell viability (a), intracellular glutathione (GSH) content (b) and rat albumin secretion (c). Data are given as mean±s.d. (n=3); *P<0.05 (compared to controls).
Effect of rifampicin on isoniazid-induced hepatotoxicity in rat hepatocytes
The effect of rifampicin on isoniazid-induced toxicity was first evaluated in rat hepatocytes. For the combined treatment (isoniazid+rifampicin), we used incubation times of 72 h, because no toxic effect of rifampicin given alone was observed over this period (data not shown). Even at a high concentration of isoniazid (1.1 mM), rat hepatocytes in monolayer cultures did not show any decrease in cell viability or decrease in GSH content or albumin secretion, when isoniazid was given either alone or in combination with rifampicin (Table 1). By contrast, rat hepatocytes in gel entrapment cultures did show toxicity to isoniazid at 0.11 mM and greater toxicity at 1.1 mM (Table 1); however, at both levels of isoniazid, there was no exacerbation of toxicity on addition of rifampicin, as indicated by decreases in MTT reduction, intracellular GSH content and rat albumin secretion (Table 1).
Table 1.
Cell toxicity in cultured rat hepatocytes exposed to INH with or without RFP
| Groups |
Cell viability (% of control) |
Intracellular glutathione content (μmol per 106 cells) |
Albumin secretion (μg per 106 cells) |
|||
|---|---|---|---|---|---|---|
| Culture time | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h |
| Monolayer culture | ||||||
| Control | 100.0±5.8 | 100.0±8.8 | 0.43±0.04 | 0.36±0.03 | 46.5±15.2 | 55.0±11.5 |
| INH 0.11 mM | 98.7±8.5 | 95.1±9.5 | 0.43±0.02 | 0.35±0.02 | 45.2±11.5 | 52.1±12.4 |
| INH 0.11 mM+RFP 12 μM | 97.1±4.2 | 96.3±8.4 | 0.41±0.05 | 0.35±0.05 | 42.1±8.6 | 57.5±7.6 |
| INH 1.1 mM | 94.2±7.2 | 95.5±5.6 | 0.41±0.02 | 0.37±0.02 | 43.0±9.5 | 51.2±8.5 |
| INH 1.1 mM+RFP 12 μM | 94.8±9.1 | 93.5±4.5 | 0.39±0.06 | 0.32±0.08 | 42.5±9.6 | 52.6±9.1 |
| Gel entrapment culture | ||||||
| Control | 100.0±7.1 | 100.0±11.7 | 0.57±0.04 | 0.53±0.06 | 52.0±8.5 | 77.6±8.6 |
| INH 0.11 mM | 93.8±8.9 | 89.7±5.7* | 0.47±0.04* | 0.42±0.06* | 48.5±7.2 | 64.5±7.2* |
| INH 0.11 mM+RFP 12 μM | 93.2±10.2 | 87.5±10.5* | 0.42±0.10* | 0.40±0.05* | 50.6±7.6 | 60.5±10.5* |
| INH 1.1 mM | 43.7±15.2** | 49.0±16.3** | 0.25±0.08** | 0.21±0.06** | 38.2±5.6* | 40.0±15.6** |
| INH 1.1 mM+RFP 12 μM | 40.1±9.5** | 45.2±6.4** | 0.21±0.10** | 0.19±0.09** | 35.2±9.5* | 34.2±11.0** |
Abbreviations: INH, isoniazid; RPF, rifampicin.
Data are given as mean±s.d. for each point of three separate experiments; *P<0.05 and **P<0.01 (compared to controls).
Toxicity of isoniazid in human hepatocytes
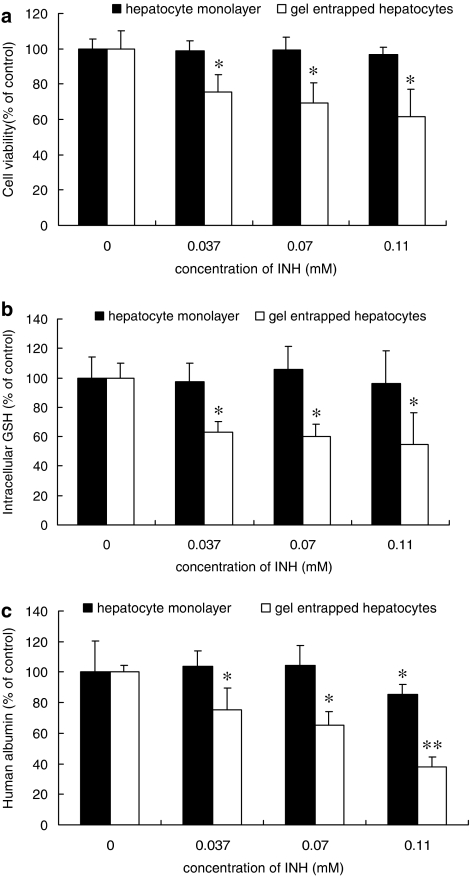
Toxicity of isoniazid (0.037, 0.07 and 0.11 mM) was determined in human hepatocytes after treatment for 72 h. As seen with rat hepatocytes (Figure 1), isoniazid treatment induced no significant decrease in cell viability (Figure 2a) or intracellular GSH (Figure 2b) in human hepatocyte monolayers. Only a slight reduction of human albumin secretion was noted at the highest concentration of isoniazid (0.11 mM), as shown in Figure 2c. However, gel-entrapped human hepatocytes were more sensitive to isoniazid, showing signs of toxicity even at the lowest concentration used, 0.037 mM, which is close to the peak plasma concentrations in humans. Thus, human hepatocytes were more sensitive to isoniazid toxicity in both monolayer and gel entrapment cultures than the corresponding rat hepatocytes (Figures 1 and 2).
Figure 2.
Cell toxicity induced by isoniazid (INH) on human hepatocytes in monolayer and gel entrapment cultures. The hepatocytes were incubated with 0.037, 0.07 and 0.11 mM isoniazid for 72 h, whereas the groups without isoniazid treatment were used as control. The results were assessed by cell viability (a), intracellular glutathione (GSH) content (b) and human albumin secretion (c). Data are given as mean±s.d. (n=3); *P<0.05, **P<0.01 (compared to controls).
Effect of rifampicin on isoniazid-induced hepatotoxicity in human hepatocytes
The effect of rifampicin on isoniazid toxicity was also evaluated in human hepatocyte cultures. These cells, in either form of culture, showed increased toxicity to isoniazid in combination with rifampicin, and this exacerbation was evident in all three measures, namely cell viability, intracellular GSH and human albumin secretion (Table 2).
Table 2.
Cell toxicity in cultured human hepatocytes exposed to INH with or without RFP
| Groups |
Cell viability (% of control) |
Intracellular glutathione content (μmol per 106 cells) |
Albumin secretion (μg per 106 cells) |
|||
|---|---|---|---|---|---|---|
| Culture time | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h |
| Monolayer culture | ||||||
| Control | 100.0±6.5 | 100.0±5.3 | 0.54±0.10 | 0.49±0.07 | 4.3±0.8 | 4.9±1.0 |
| INH 0.11 mM | ND | 96.7±2.4 | ND | 0.47±0.11 | 4.3±0.7 | 4.2±0.3* |
| INH 0.11 mM+RFP 12 μM | ND | 82.0±6.4*,# | ND | 0.36±0.04*,# | 3.4±0.5*,# | 3.1±0.5*,# |
| INH 1.1 mM | 67.7±3.4** | 58.2±10.3** | 0.33±0.07* | 0.27±0.08** | 3.3±0.6* | 3.4±0.7* |
| INH 1.1 mM+RFP 12 μM | 54.3±3.5**,# | 40.0±4.6**,# | 0.20±0.05**,# | 0.16±0.07**,# | 2.0±0.3**,# | 2.1±0.6*,# |
| Gel entrapment culture | ||||||
| Control | 100.0±11.8 | 100.0±10.3 | 0.55±0.06 | 0.51±0.05 | 12.4±6.5 | 19.1±0.8 |
| INH 0.11 mM | ND | 61.4±15.9* | ND | 0.28±0.11* | 6.5±3.2* | 7.3±1.2** |
| INH 0.11 mM+RFP 12 μM | ND | 27.6±13.3**,# | ND | 0.15±0.11**,# | 5.6±2.1**,# | 6.0±0.4**,# |
| INH 1.1 mM | 50.7±10.4** | 45.4±10.8** | 0.19±0.05** | 0.11±0.04** | 5.1±0.3** | 5.2±0.3** |
| INH 1.1 mM+RFP 12 μM | 20.6±7.6**,# | 20.9±6.7**,# | 0.09±0.06**,# | 0.07±0.03**,# | 2.5±2.1**,# | 2.1±0.3**,# |
Abbreviations: INH, isoniazid; ND, not detected; RPF, rifampicin.
Data are given as mean±s.d. for each point of three separate experiments; *P<0.05 and **P<0.01 (compared to controls), #P<0.05 (compared to isoniazid treatment alone).
Role of CYP 2E1 activity in isoniazid–rifampicin-induced hepatotoxicity
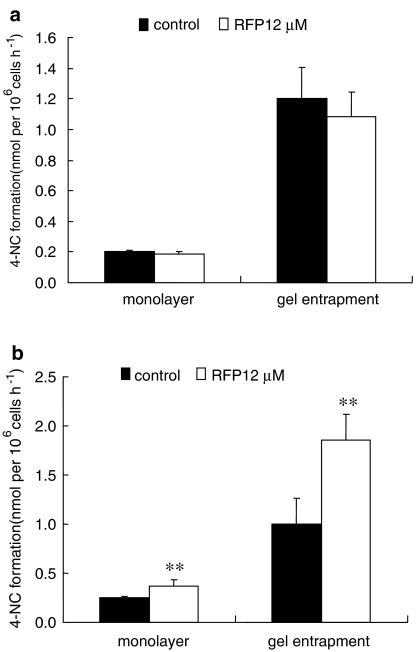
CYP 2E1 was previously suggested to be closely associated with isoniazid hepatotoxicity (Shen et al., 2006b). The induction by rifampicin of CYP 2E1 activity was first detected by 4-NC formation, using hepatocytes from both species. As shown in Figure 3, rifampicin did not increase CYP 2E1 activity in rat hepatocytes but produced a significant induction of CYP 2E1 in human hepatocytes, with an approximate increase of 1.5-fold for monolayer culture and 2.0-fold for gel entrapment culture. Because of the difference in basal activity of CYP 2E1 between the two forms of cell culture, the effect of rifampicin was that CYP 2E1 expression was around fivefold higher in gel entrapment than in the monolayer cultures.
Figure 3.
CYP 2E1 induction by rifampicin (RFP) in rat (a) and human (b) hepatocytes after 48 h treatment, as shown by 4-nitrocatechol (4-NC) formation from 4-nitrophenol. Data are given as mean±s.d. (n=3); **P<0.01 (compared to controls).
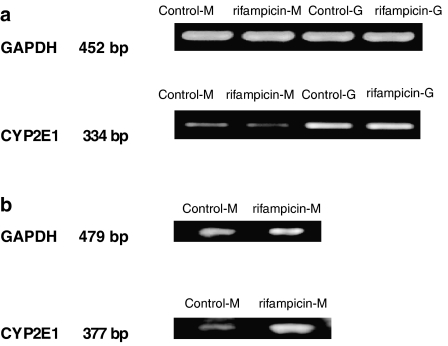
To confirm the effect of rifampicin on CYP 2E1 of rat and human hepatocytes, CYP 2E1 gene expression was then evaluated by reverse transcription-PCR analysis. In agreement with the results from CYP2E1 activity shown above, reverse transcription-PCR analysis showed that rifampicin did not induce CYP 2E1 mRNA in rat hepatocytes (Figure 4a) but elicited a significant induction in human hepatocytes (Figure 4b). Furthermore, CYP 2E1 mRNA was expressed at a higher level in gel-entrapped hepatocytes than in hepatocyte monolayers, corresponding well with the corresponding assays of enzymic activity.
Figure 4.
Reverse transcription-PCR analysis for the expression of CYP 2E1. Results by PCR were from rat hepatocytes (a) and human hepatocytes (b) of monolayer and gel entrapment after 48 h treatment by 12 μM rifampicin (RFP). Control-M and Control-G represent the control groups of hepatocyte monolayer and gel-entrapped hepatocytes, respectively; rifampicin-M and rifampicin-G represent the rifampicin-treated groups of hepatocyte monolayer and gel-entrapped hepatocytes, respectively.
Discussion
It is difficult to select a suitable animal model to provide reliable predictions of human responses in vivo, if only because of species differences. This problem still seems to be far from solution now for the complex interactions of hepatic phase I and phase II-metabolizing enzymes, because even a high degree of sequence identity of CYP 450 proteins does not mean similar catalytic specificity (O'Brien et al., 2004). Although humanized mice have been generated for that purpose, they can only model a part of liver-specific functions in humans. Actually, there are no ‘perfect' animal models that will accurately predict all of the metabolism of any drug in humans. Therefore, an appropriate human hepatocyte model in vitro could be an alternative system, to predict drug disposition in vivo.
Our present work has shown an important species difference in isoniazid toxicity in rat and human hepatocytes. Firstly, a species difference in toxicity of isoniazid treatment alone was suggested by the results that human hepatocytes were more sensitive to isoniazid toxicity than the corresponding rat hepatocytes, as indicated by the greater decreases in cell viability, GSH content and albumin secretion in human hepatocytes (Figures 1 and 2). The findings in vitro corresponded well with the previous observations in vivo that rats did not show the same susceptibility to isoniazid-induced hepatotoxicity as observed in humans (Wright et al., 1986; Sarich et al., 1999), because the major metabolites of isoniazid were less hepatotoxic in rats than in humans (Wright et al., 1986). Secondly, the differential effect of rifampicin on isoniazid-induced hepatotoxicity was clearly demonstrated by the results that rifampicin enhanced isoniazid-induced cell damage, assessed by cell viability, GSH content and albumin secretion in human hepatocytes but not in rat hepatocytes (Tables 1 and 2). Finally, combining our results (Figures 3 and 4) with previous findings that rifampicin induced CYP 2E1 gene expression in human hepatocytes in vitro (Hesse et al., 2003; Madan et al., 2003), treatment with rifampicin enhanced gene expression and enzymic activity of CYP 2E1 in human hepatocytes but not in rat hepatocytes. Our in vitro studies on the effects of rifampicin on rat hepatocytes (Table 1) corresponded well with the lack of induction by rifampicin of CYP 2E1 activity in rats (Yue et al., 2004). As CYP 2E1 is a major drug-metabolizing enzyme associated with isoniazid-induced hepatotoxicity (Huang et al., 2003), we have concluded that rifampicin elicited species-selective effects on isoniazid-induced hepatotoxicity because of two findings: a greater induction of CYP 2E1 and a greater exacerbation of toxicity in human hepatocytes than in rat hepatocytes after rifampicin treatment. Taken together, the species difference in CYP 2E1 reflected not only the differential synergistic effect of rifampicin on isoniazid toxicity but also the differential hepatotoxicity by isoniazid treatment alone between rat and human hepatocytes.
It is well known that there are species-selective differences in induction of CYP 450s. Lu and Li (2001) reported that rifampicin and omeprazole are potent inducers for CYP3A and CYP1A, respectively, in human hepatocytes but not in rat hepatocytes. Therefore, the species difference observed in both previous and our studies suggest that human hepatocytes represent the most appropriate preclinical experimental system in vitro for the evaluation of CYP 450 induction in humans.
Although primary human hepatocytes in monolayers or in suspension are routinely used as an experimental model to evaluate drug hepatotoxicity and drug–drug interactions in humans, via the induction of proteins, it is also widely understood that primary hepatocytes lose expression of liver-specific functions including CYP 450s within a few days of culture. Thus, tissue-like cultures of human hepatocytes, which maintain liver-specific functions for 1–2 weeks, were studied here. From a comparison of gel-entrapped rat hepatocytes with rat hepatocyte monolayers in our previous work, we recognized that gel-entrapped hepatocytes not only expressed higher activity of CYP 450 isoforms (CYP 1A2, CYP 2E1 and CYP 3A) as well as some phase II enzymes (UDP-glucuronosyltransferase and sulfotransferase), but also were more susceptible to treatment with isoniazid, paracetamol (acetaminophen) and tacrine (Qiu et al., 2006; Shen et al., 2006a, 2006b; Meng et al., 2007). In fact, good preservation of metabolic enzyme activities in gel-entrapped hepatocytes has been regarded as a major factor for the higher sensitivity to drug toxicity (Meng et al., 2006, 2007). The present results using human hepatocytes have highlighted again the higher sensitivity to isoniazid exposure in gel entrapment, than monolayer cultures. The hepatotoxic concentration of isoniazid in humans, hepatocytes and cell lines are summarized in Table 3, which indicates how close the isoniazid toxicity in gel-entrapped human hepatocytes was to that observed in humans in vivo. It was noteworthy that gel-entrapped human hepatocytes possessed higher levels of drug-metabolizing enzymes than human hepatocyte monolayers and cell lines. These higher levels probably explain why the toxic limit of isoniazid was as low as 0.037 mM (which was comparable to peak plasma concentrations in humans) in gel entrapment cultures. These findings demonstrate that primary human hepatocytes in gel entrapment culture would be a more effective model for prediction of drug hepatotoxicity including drug–drug interactions, by establishing a good correlation between in vitro and in vivo test systems.
Table 3.
Lower limits of toxic concentrations of isoniazid in human liver in vivo, primary hepatocytes and cell lines
| Various conditions or culture models | Lower limits of toxic concentration of isoniazida | References |
|---|---|---|
| Human hepatotoxicity | 0.022–0.04 mM | Yew (1998); Agrawal et al. (2004) |
| Gel-entrapped human hepatocytes | About 0.037 mM | This paper, Figure 2 |
| Gel-entrapped rat hepatocytes | About 0.07 mM | This paper, Figure 1 |
| Human hepatocyte monolayer | About 0.11 mM | This paper, Figure 2 |
| Rat hepatocyte monolayer | About 10 mM | de Longueville et al. (2003); Shen et al. (2006b) |
| HepG2 and WIF-B9 cells | About 50 mM | Schwab and Tuschl (2003); Biagini et al. (2006) |
Abbreviation: MTT, methyl thiazolyl tetrazolium.
Cell toxicity of isoniazid in vitro was measured by MTT reduction assay except for the toxicity in HepG2 cells that was measured by flow cytometric methods.
The concentrations of isoniazid in human hepatotoxicity represent the peak plasma concentrations in vivo.
In conclusion, the enhanced isoniazid toxicity observed by co-treatment with rifampicin in patients could be reproduced in primary human hepatocytes, whereas the corresponding in vitro results using rat hepatocytes also reflected the facts that rifampicin co-treatment with isoniazid did not exacerbate isoniazid hepatotoxicity in rats. Moreover, the difference in gene expression and metabolic activity of CYP 2E1 after rifampicin treatment could account for the different toxic responses to isoniazid+rifampicin treatment between human and rat hepatocytes. Thus, we would recommend the inclusion of human hepatocytes in drug hepatotoxicity studies, especially when this hepatotoxicity is mediated by drug-metabolizing enzymes. Meanwhile, hepatocytes in tissue-like gel entrapment culture were more sensitive to drug toxicity and would appear to be a more relevant model system for evaluating the hepatotoxic effects of metabolized compounds. This factor, taken together with the avoidance of species differences (Plant, 2004), leads us to conclude that, human hepatocytes in tissue-like culture in three-dimensional gels represents a highly relevant model system for hepatotoxicity studies in vivo.
Acknowledgments
This research was supported by grants (No. 20576119) from NSFC (National Natural Science Foundation of China) and the PhD Programs Foundation of Ministry of Education of China (No. 20060335083).
Abbreviations
- CYP 450
cytochrome P450
- GSH
glutathione
- MTT
methyl thiazolyl tetrazolium
- 4-NC
4-nitrocatechol
Conflict of interest
The authors state no conflict of interest.
References
- Agrawal S, Singh I, Kaur KJ, Bhade SR, Kaul CL, Panchagnula R. Comparative bioavailability of rifampicin, isoniazid and pyrazinamide from a four drug fixed dose combination with separate formulations at the same dose levels. Int J Pharm. 2004;276:41–49. doi: 10.1016/j.ijpharm.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Attri S, Rana SV, Vaiphei K, Sodhi CP, Katyal R, Goel RC, et al. Isoniazid- and rifampicin-induced oxidative hepatic injury—protection by N-acetylcysteine. Hum Exp Toxicol. 2000;19:517–522. doi: 10.1191/096032700674230830. [DOI] [PubMed] [Google Scholar]
- Biagini CP, Boissel E, Borde F, Bender VE, Bouskila M, Blazy F, et al. Investigation of the hepatotoxicity profile of chemical entities using Liverbeads and WIF-B9 in vitro models. Toxicol In Vitro. 2006;20:1051–1059. doi: 10.1016/j.tiv.2006.01.013. [DOI] [PubMed] [Google Scholar]
- de Longueville F, Atienzar FA, Marcq L, Dufrane S, Evrard S, Wouters L, et al. Use of a low-density microarray for studying gene expression patterns induced by hepatotoxicants on primary cultures of rat hepatocytes. Toxicol Sci. 2003;75:378–392. doi: 10.1093/toxsci/kfg196. [DOI] [PubMed] [Google Scholar]
- Girling DJ. The hepatic toxicity of antituberculosis regimens containing isoniazid, rifampicin and pyrazinamide. Tuberclosis. 1978;59:13–32. doi: 10.1016/0041-3879(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Hesse LM, Sakai Y, Vishnuvardhan D, Li AP, von Moltke LL, Greenblatt DJ. Effect of bupropion on CYP2B6 and CYP3A4 catalytic activity, immunoreactive protein and mRNA levels in primary human hepatocytes: comparison with rifampicin. J Pharm Pharmacol. 2003;55:1229–1239. doi: 10.1211/0022357021657. [DOI] [PubMed] [Google Scholar]
- Huang YS, Chern HD, Su WJ, Wu JC, Chang SC, Chiang CH, et al. Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology. 2003;37:924–930. doi: 10.1053/jhep.2003.50144. [DOI] [PubMed] [Google Scholar]
- LeCluyse EL, Alexandre E, Hamilton GA, Viollon-Abadie C, Coon DJ, Jolley S, et al. Isolation and culture of primary human hepatocytes. Methods Mol Biol. 2005;290:207–229. doi: 10.1385/1-59259-838-2:207. [DOI] [PubMed] [Google Scholar]
- Lu C, Li AP. Species comparison in P450 induction: effects of dexamethasone, omeprazole, and rifampin on P450 isoforms 1A and 3A in primary cultured hepatocytes from man, Sprague–Dawley rat, minipig, and beagle dog. Chem Biol Interact. 2001;134:271–281. doi: 10.1016/s0009-2797(01)00162-4. [DOI] [PubMed] [Google Scholar]
- Madan A, Graham RA, Carroll KM, Mudra DR, Burton LA, Krueger LA, et al. Effects of prototypical microsomal enzyme inducers on cytochrome P450 expression in cultured human hepatocytes. Drug Metab Dispos. 2003;31:421–431. doi: 10.1124/dmd.31.4.421. [DOI] [PubMed] [Google Scholar]
- Meng Q, Ru J, Zhang G, Shen C, Schmitmeier S, Bader A. Re-evaluation of tacrine hepatotoxicity using gel entrapped hepatocytes. Toxicol Lett. 2007;168:140–147. doi: 10.1016/j.toxlet.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Meng Q, Zhang G, Shen C, Qiu H. Sensitivities of gel entrapped hepatocytes in hollow fibers to hepatotoxic drug. Toxicol Lett. 2006;166:19–26. doi: 10.1016/j.toxlet.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Nieusma JL, Claffey DJ, Ruth JA, Ross D. Stereochemical aspects of the conjugation of epoxide metabolites of butadiene with glutathione in rat liver cytosol and freshly isolated rat hepatocytes. Toxicol Sci. 1998;43:102–109. doi: 10.1006/toxs.1998.2461. [DOI] [PubMed] [Google Scholar]
- O'Brien PJ, Chan K, Silber PM. Human and animal hepatocytes in vitro with extrapolation in vivo. Chem Biol Interact. 2004;150:97–114. doi: 10.1016/j.cbi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Plant N. Strategies for using in vitro screens in drug metabolism. Drug Discov Today. 2004;9:328–336. doi: 10.1016/s1359-6446(03)03019-8. [DOI] [PubMed] [Google Scholar]
- Qiu HX, Su GG, Tang X, Meng Q. Tissue-like cultures of rat hepatocytes in study of phase I and phase II drug metabolism. J Zhejiang Univ Med Sci. 2006;35:541–546. doi: 10.3785/j.issn.1008-9292.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Santhosh S, Sini TK, Anandan R, Mathew PT. Effect of chitosan supplementation on antitubercular drugs-induced hepatotoxicity in rats. Toxicology. 2006;219:53–59. doi: 10.1016/j.tox.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Sarich TC, Adams SP, Petricca G, Wright JM. Isoniazidibition of isoniazid-induced hepatotoxicity in rabbits by pretreatment with an amidase isoniazidibitor. J Pharmacol Exp Ther. 1999;289:695–702. [PubMed] [Google Scholar]
- Sarich TC, Zhou T, Adams SP, Bain AI, Wall RA, Wright JM. A model of isoniazid-induced hepatotoxicity in rabbits. J Pharmacol Toxicol Methods. 1995;34:109–116. doi: 10.1016/1056-8719(95)00044-i. [DOI] [PubMed] [Google Scholar]
- Schwab CE, Tuschl H. In vitro studies on the toxicity of isoniazid in different cell lines. Hum Exp Toxicol. 2003;22:607–615. doi: 10.1191/0960327103ht401oa. [DOI] [PubMed] [Google Scholar]
- Shen C, Zhang G, Qiu H, Meng Q. Acetaminophen-induced hepatotoxicity of gel entrapped rat hepatocytes in hollow fibers. Chem Biol Interact. 2006a;162:53–61. doi: 10.1016/j.cbi.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Shen C, Zhang H, Zhang G, Meng Q. Isoniazid-induced hepatotoxicity in rat hepatocytes of gel entrapment culture. Toxicol Lett. 2006b;167:66–74. doi: 10.1016/j.toxlet.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Steele MA, Burk RF, DesPrez RM. Toxic hepatitis with isoniazid and rifampin. A meta-analysis. Chest. 1991;99:465–471. doi: 10.1378/chest.99.2.465. [DOI] [PubMed] [Google Scholar]
- Tasduq SA, Kaisar P, Gupta DK, Kapahi BK, Maheshwari HS, Jyotsna S, et al. Protective effect of a 50% hydroalcoholic fruit extract of Emblica officinalis against anti-tuberculosis drugs induced liver toxicity. Phytother Res. 2005;19:193–197. doi: 10.1002/ptr.1631. [DOI] [PubMed] [Google Scholar]
- Tassaneeyakul W, Veronese ME, Birkett DJ, Miners JO. High-performance liquid chromatographic assay for 4-nitrophenol hydroxylation, a putative cytochrome P-4502E1 activity, in human liver microsomes. J Chromatogr. 1993;616:73–78. doi: 10.1016/0378-4347(93)80473-h. [DOI] [PubMed] [Google Scholar]
- Thomas BH, Solomonraj G. Drug interactions with isoniazid metabolism in rats. J Pharm Sci. 1977;66:1322–1326. doi: 10.1002/jps.2600660930. [DOI] [PubMed] [Google Scholar]
- Walker TM, Rhodes PC, Westmoreland C. The differential cytotoxicity of methotrexate in rat hepatocyte monolayer and spheroid cultures. Toxicol In Vitro. 2000;14:475–485. doi: 10.1016/s0887-2333(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Wang HZ, Chang CH, Lin CP, Tsai MC. Using MTT viability assay to test the cytotoxicity of antibiotics and steroid to cultured porcine corneal endothelial cells. J Ocul Pharmacol Ther. 1996;12:35–43. doi: 10.1089/jop.1996.12.35. [DOI] [PubMed] [Google Scholar]
- Wilkening S, Bader A. Influence of culture time on the expression of drug-metabolizing enzymes in primary human hepatocytes and hepatoma cell line HepG2. J Biochem Mol Toxicol. 2003;17:207–213. doi: 10.1002/jbt.10085. [DOI] [PubMed] [Google Scholar]
- Wright JM, Ngai H, Adams S, Behm A, Wall RA. Lack of hepatotoxicity of acetylhydrazine in rodents. Acta Pharmacol Toxicol. 1986;59 Suppl 5:221. [Google Scholar]
- Wu D, Cederbaum AI. Ethanol cytotoxicity to a transfected HepG2 cell line expressing human cytochrome P4502E1. J Biol Chem. 1996;271:23914–23919. doi: 10.1074/jbc.271.39.23914. [DOI] [PubMed] [Google Scholar]
- Wu DQ, Zhang GL, Shen C, Zhao Q, Li H, Meng Q. Evaluation of diffusion in gel entrapment cell culture within hollow fibers. World J Gastroenterol. 2005;11:1599–1604. doi: 10.3748/wjg.v11.i11.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Purcell WM. Energy metabolism and biotransformation as endpoints to pre-screen hepatotoxicity using a liver spheroid model. Toxicol Appl Pharmacol. 2006;216:293–302. doi: 10.1016/j.taap.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Yew WW. Therapeutic drug monitoring in antituberculosis chemotherapy. Ther Drug Monit. 1998;20:469–472. doi: 10.1097/00007691-199810000-00004. [DOI] [PubMed] [Google Scholar]
- Yew WW. Clinically significant interactions with drugs used in the treatment of tuberculosis. Drug Saf. 2002;25:111–133. doi: 10.2165/00002018-200225020-00005. [DOI] [PubMed] [Google Scholar]
- Yew WW, Leung CC. Antituberculosis drugs and hepatotoxicity. Respirology. 2006;11:699–707. doi: 10.1111/j.1440-1843.2006.00941.x. [DOI] [PubMed] [Google Scholar]
- Yue J, Peng RX, Yang J, Kong R, Liu J. CYP2E1 mediated isoniazid-induced hepatotoxicity in rats. Acta Pharmacol Sin. 2004;25:699–704. [PubMed] [Google Scholar]