Abstract
Background and purpose:
The analgesics, paracetamol and dipyrone are weak inhibitors of the cyclooxygenase isoforms 1 or 2 (COX-1, COX-2) but more potent on COX-3. Both are also weak anti-inflammatory agents, relative to their analgesic and antipyretic activities. In a model of inflammatory pain mediated by prostaglandins, both compounds were analgesic. We have analysed this shared effect further in order to elucidate the underlying mechanisms.
Experimental approach:
Inflammation was induced in one hind paw of rats by intraplantar injection of 250 μg λ-carrageenan (CG) and the contralateral paw injected with saline. Nociceptive thresholds to mechanical stimulation were measured immediately before and for 6 h after, injection of CG. The analgesics were s.c. or locally (intraplantar) injected either 30 min before or 2 h after CG. In some groups, naltrexone was injected (s.c. or intraplantar), 1 h before CG.
Key results:
Pretreatment with paracetamol or dipyrone (60–360 mg kg−1) reversed hyperalgesia induced by CG and increased nociceptive threshold in the inflamed paw above the basal level (hypoalgesia). Paracetamol, but not dipyrone, also raised nociceptive thresholds in the non-inflamed paw. Subcutaneous, but not local, administration of naltrexone, a specific opioid antagonist, reversed the hypoalgesia induced by paracetamol, but similar naltrexone treatment had no effect on dipyrone-induced analgesia.
Conclusions and implications:
Although both paracetamol and dipyrone are inhibitors of COX isoforms and thus of prostaglandin biosynthesis and were analgesic in our model, their analgesic actions were functionally and mechanistically different. Satisfactory mechanisms of action for these analgesics still remain to be established.
Keywords: paracetamol, dipyrone, hypoalgesia, endogenous opioids
Introduction
Paracetamol and dipyrone are among the most commonly used analgesic and antipyretic drugs worldwide, either on prescription or over-the-counter. They are often, but anomalously, classified as non-steroidal anti-inflammatory drugs (NSAIDs) in textbooks of pharmacology (Brunton et al., 2005; Rang et al., 2006). Like their archetype, aspirin, most NSAIDs exhibit a triad of therapeutic activities—anti-inflammatory, antipyretic and analgesic. The NSAIDs also decrease prostaglandin (PG) biosynthesis by inhibiting the cyclooxygenase (COX) enzymes, either COX-1 or COX-2 or both, and this has been accepted as their mode of action for more than 30 years (Vane, 1971; Vane et al., 1998). The anomaly of paracetamol and dipyrone is that both are, relative to their antipyretic and analgesic activities, weak anti-inflammatory agents and weak inhibitors of either COX-1 or COX-2 (Botting, 2000). Thus, these two compounds have, for many years, presented a challenge in terms of a satisfactory explanation of their well-established actions.
Several possible mechanisms have been proposed, including the involvement of 5-HT (Alloui et al., 2002), of endogenous opioids (Pini et al., 1997; Vaughan et al., 1997; Vanegas and Tortorici, 2002) and of the arginine–nitric oxide–cGMP pathway (Duarte et al., 1992), effective at a variety of levels in the nociceptive pathway from the periaqueductal grey to the periphery. There are also suggestions that COX-2 is indeed the target (Kis et al., 2005) but through its peroxidase function (Graham and Scott, 2005; Aronoff et al., 2006), or that another variant of COX-1, located in the CNS, is the crucial enzyme inhibited by paracetamol and dipyrone (Chandrasekharan et al., 2002; Botting and Ayoub, 2005). As these last suggestions imputed the same mechanism of action for paracetamol and dipyrone, we compared the analgesic effects of these two compounds in a model of inflammatory pain, hyperalgesia, induced by carrageenan injected into rat hind paws. This model is long-established (Di Rosa, 1972) and is known to be mediated by PGs (Vinegar et al., 1987), and inhibitors of COX-1 or COX-2 are known to be analgesic in this model (Vane et al., 1998).
We have, therefore, carried out a further analysis of the antinociceptive effects of paracetamol and dipyrone in this model, particularly comparing the effects of systemic and local administration and assessing the relevance of the endogenous opioids to the antinociceptive action with an opioid antagonist, naltrexone, injected both systemically and locally in the paws of the rats. Our results showed important and unexpected differences in the functional expression of analgesia and in response to naltrexone, between these two analgesic drugs.
Methods
All animal procedures were adhered to the guidelines of the committee for Research and Ethical Issues of IASP (Zimmermann, 1983) and were approved by the local Ethics Committee for Animal Experimentation. Male Holtzman or Wistar rats weighing 140–170 g supplied by the Bioterism Center of UFMG were used in these experiments. Animals were housed at a maximal number of six per cage with food and water ad libitum, with light/dark cycles of 12/12 h, starting at 0700 hours. No animals were re-used in any further experiment, as the experimental animals were killed humanely at the end of the 6 h observation period (see below).
Induction of paw inflammation
Inflammation was induced in one hind paw (right) using carrageenan λ (250 μg 10 ml−1) in sterile physiological saline (NaCl, 0.9%) as the proinflammatory stimulus at time zero. The contralateral paw was injected with the same volume of physiological saline. This dose of carrageenan is known to induce paw hyperalgesia from previous studies (Francischi et al., 2002; Pereira et al., 2003).
Measurement of paw hyperalgesia (nociception)
Assessment of nociception consisted of measurement of the threshold stimulus for nociceptive reaction (paw withdrawal) using a weight (maximum limit of 500 g) applied to the pads of hind paws by an experimenter using a Ugo Basile apparatus; this is essentially the Randall–Selitto method (Randall and Selitto, 1957). The threshold for eliciting a nociceptive response was measured before (time zero) and 0.5, 1, 2, 3, 4 and 6 h after the intraplantar injection of carrageenan. Results are presented either as the difference in nociceptive thresholds between the inflamed paw and the contralateral, control (saline-injected) paw at each time (Figures 1a and d) or as the actual values of nociceptive threshold in each paw obtained at the time points indicated above (remaining figures). The term ‘hypoalgesia' is used to describe the condition when the nociceptive threshold was raised above the basal level, that is, the level at time zero before any treatment of the animal. ‘Anti-hyperalgesia' in pretreated animals is defined as any reversal of hyperalgesia, up to the basal nociceptive threshold.
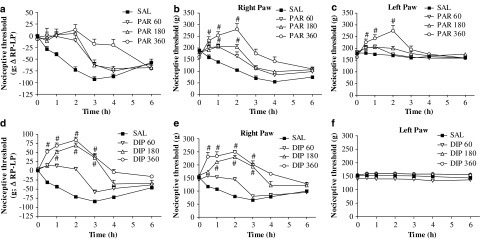
Figure 1.
Effect of paracetamol or dipyrone on reduced nociceptive threshold induced by carrageenan in rat paws. All rats were injected with carrageenan in the right paw and saline in the left paw. They were pretreated (s.c. injection) with saline (SAL), paracetamol (PAR) or dipyrone (DIP; 60, 180 or 360 mg kg−1) 30 min before carrageenan. (a) The nociceptive condition is expressed as the difference in nociceptive thresholds between the inflamed and non-inflamed paws. Reduced nociceptive threshold appears as a negative difference and paracetamol prevented the development of that hyperalgesia for most of the 6 h observation period. (b, c) The thresholds in the right (b) and left (c) paws are shown separately. For the first 2 h after carrageenan, the highest dose of paracetamol (360 mg kg−1) raised thresholds above the basal level (hypoalgesia) in the right paw (b). Note that nociceptive thresholds were raised also in the left non-inflamed paw (c) to the same levels as in the right paw. (d–f) Effects of dipyrone on the carrageenan-induced hyperalgesia are shown, as for paracetamol. Note in (e) that dipyrone (60, 180 or 360 mg kg−1) was as effective as paracetamol, with the two higher doses (180 or 360 mg kg−1) inducing hypoalgesia, for the first 3 h after carrageenan. The nociceptive thresholds in the left, non-inflamed, paw (f) were not affected by carrageenan or dipyrone treatment. Values shown are the mean (±s.e.mean) results from 4 to 6 animals at each time point. *Significantly different from values without analgesic treatment. #Significantly above basal threshold: ANOVA, P<0.05.
Drug preparation and administration
Paracetamol and dipyrone (200 and 500 mg ml−1, respectively) were diluted with physiological saline to obtain the doses required for s.c. injection, in a volume of 0.1 ml per 100 g of animal. Local, that is, intraplantar injections of paracetamol or dipyrone were made, with appropriate dilutions, in a volume of 0.1 ml per paw. These drugs were injected s.c. 30 min before or 2 h after the injection of carrageenan. In addition, a group of animals were injected into the paw with 3.6 mg per paw of either paracetamol or dipyrone, 30 min before carrageenan, and hyperalgesia measurements were carried out as described above. This dose for intraplantar injections was chosen from pilot experiments to determine the minimum effective analgesic dose of dipyrone. Control animals for these experiments received carrageenan in the right paw and saline in the contralateral paw, and were pretreated with vehicle s.c. at 30 min before or 2 h after carrageenan or locally in the paw.
The opioid antagonist naltrexone was dissolved and diluted further in physiological saline and injected s.c. (3 mg kg−1), in a volume of 0.1 ml 100 g−1, 1 h before carrageenan. In another series of experiments, naltrexone was injected intraplantarly (100 μg in 0.1 ml per paw), also 1 h before carrageenan. This dose of naltrexone was derived from pilot experiments with COX-2 inhibitors and from the work of Rodrigues and Duarte (2000). Control animals for these experiments were injected with the same volume of saline, at the same time. In a separate group of Wistar rats, paracetamol was given s.c. or orally by gavage in the same dose range and time as used for Holtzman rats, before carrageenan.
Statistical analysis
Results are presented as the mean values (±s.e.mean) from groups of at least four and usually six animals for each condition. In general, two sets of each condition were assessed on different days, and a group of animals receiving carrageenan only or treated with saline/vehicle were included in each set. Differences between mean values were considered statistically significant when comparisons between control (carrageenan+vehicle) and treated (carrageenan+analgesic) animals, using one-way ANOVA, gave P-values less than 0.05.
Materials
Paracetamol (Tylenol, J&J, São Paulo, Brazil), dipyrone (Novalgina, Aventis, São Paulo, Brazil), naltrexone hydrochloride (Sigma, Steinheim, Germany) and λ-carrageenan (Sigma, St Louis, MO, USA) were used.
Results
Effects of systemic paracetamol on hyperalgesia induced by carrageenan in rat paws
In Figure 1a, the effects of carrageenan alone (control: saline, SAL) and of pretreatment with paracetamol, over a range of doses (60–360 mg kg−1), are expressed as the difference in nociceptive thresholds between the inflamed (right) paw and the non-inflamed (left) paw. Carrageenan alone induced a fall in the threshold of the right paw, leading to the negative difference shown and demonstrating the hyperalgesia characteristic of this model. Pretreatment with increasing doses of paracetamol prevented this fall in threshold values to about zero, that is, at the levels observed at the start of the experiments. However, these difference values mask changes in the thresholds of the non-inflamed (left) paw.
We have, therefore, presented the threshold values in right and left paws for the same set of animals as in Figure 1a, separately in Figures 1b and c. Now it is clear that the hyperalgesia induced by carrageenan alone was unilateral, whereas in animals pretreated with paracetamol, changes in nociceptive thresholds were observed in both inflamed and non-inflamed paws. For the highest dose of paracetamol (360 mg kg−1), the threshold was increased to values well above that in basal condition and, thus, for up to 2 h after carrageenan, the rats were in a condition of hypoalgesia, as defined earlier (see Methods).
In another strain of rats, the Wistar strain, the same protocol of carrageenan inflammation and pretreatment with paracetamol or dipyrone at 360 mg kg−1, given s.c. 30 min before carrageenan, produced anti-hyperalgesic/-hypoalgesic effects comparable to those shown in Figure 1 for Holtzman rats (Table 1).
Table 1.
Nociceptive thresholds in carrageenan-injected rats of different strains before and after paracetamol or dipyrone treatment
| Treatment (mg kg−1) |
Nociceptive threshold (g) |
||||||
|---|---|---|---|---|---|---|---|
| 0 h | ½ h | 1 h | 2 h | 3 h | 4 h | 6 h | |
| C | |||||||
| RP H | 168±5.8 | 142±8.0 | 102±6.6 | 74±5.1 | 60±8.4 | 60±5.5 | 100±6.3 |
| LP | 180±0.0 | 180±10.0 | 175±5.0 | 170±0.0 | 160±0.0 | 165±5.0 | 160±0.0 |
| PAR 360 | |||||||
| RP H | 154±2.5 | 204±4.0* | 242±18.8* | 262±20.0* | 150±18.2* | 128±18.8* | 122±9.7 |
| LP | 156±4.0 | 202±8.6* | 232±17.7* | 256±18.9* | 172±12.7* | 172±5.8 | 158±3.7 |
| DIP 360 | |||||||
| RP H | 156±2.5 | 214±15.7* | 234±16.3* | 250±6.3* | 202±13.6* | 166±6.0* | 128±5.8* |
| LP | 154±4.0 | 160±6.3 | 162±7.5 | 156±5.1 | 154±4.0 | 150±3.2 | 146±2.5 |
| C | |||||||
| RP W | 164±11.7 | 142±15.9 | 94±8.1 | 58±3.7 | 48±3.7 | 56±5.1 | 88±10.2 |
| LP | 162±9.7 | 160±7.7 | 148±5.8 | 148±5.8 | 148±3.7 | 146±4.0 | 132±3.7 |
| PAR 360 | |||||||
| RP W | 162.5±6.3 | 227.5±4.8* | 257.5±2.5* | 245±22.2* | 180±7* | 117.5±35.9* | 122.5±14.4* |
| LP | 167.5±8.5 | 232.5±9.5* | 260±4.1* | 237.5±18.9* | 187.5±4.8* | 167.5±6.3 | 147.5±7.5 |
| DIP 360 | |||||||
| RP W | 158.3±4.8 | 211.7±11.4* | 258.3±19.2* | 245±30.3* | 191.7±12.2* | 133.3±13.3* | 131.7±10.8* |
| LP | 161.7±4.8 | 170±8.6 | 178.3±10.1 | 173.3±10.2 | 163.3±5.6 | 153.3±5.6 | 155±8.5 |
Abbreviations: C, control; DIP, dipyrone; H, Holtzman rats; LP, left paw; PAR, paracetamol; RP, right paw; W, Wistar rats.
Data shown are mean values±s.e.mean. *P<0.05, one-way ANOVA.
Effects of systemic dipyrone on hyperalgesia induced by carrageenan in rat paws
Dipyrone, given as a pretreatment over a similar dose range (60–360 mg kg−1), also reversed carrageenan-induced hyperalgesia, when expressed as the difference between thresholds in the right, inflamed and the left, non-inflamed paws (Figure 1d). In Figure 1, for the sake of clarity, only those values for nociceptive thresholds that are significantly above basal (time zero) levels have been marked with symbol (#) to show the development of the hypoalgesic state. However, nociceptive thresholds in rats pretreated with dipyrone were significantly greater than those in animals receiving carrageenan alone. These anti-hyperalgesic effects were maintained for 6 h, longer than with paracetamol. When the results for the right and left paws are shown separately (Figures 1e and f), they show marked hypoalgesia for the first 3 h in the right paws, but thresholds in the left paws did not change over the entire 6 h and were not different from those in the left paws of animals receiving carrageenan alone.
Effects of treatment with paracetamol or dipyrone after the onset of hyperalgesia induced by carrageenan
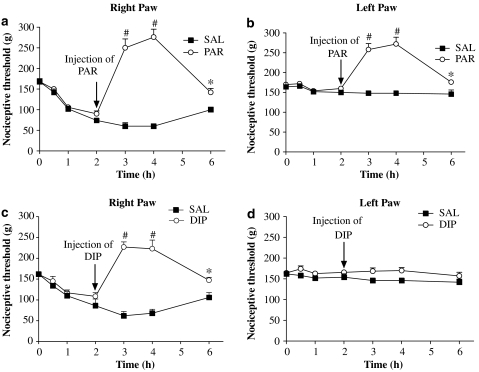
In another group of rats, the two analgesics were given 2 h after carrageenan, when the hyperalgesia was well developed. As illustrated in Figures 2a–d, paracetamol and dipyrone were as effective in inducing hypoalgesia, given after the stimulus, as they were when given as a pretreatment. Note also that the later treatment with paracetamol still affected both inflamed and non-inflamed paws, whereas dipyrone still affected only the inflamed paw.
Figure 2.
Effects of paracetamol or dipyrone administered 2 h following induction of inflammation on nociceptive thresholds of rat paws. All rats were injected with carrageenan in the right paw and saline in the left paw at time zero. They were then treated (s.c. injection) with saline (SAL), paracetamol (PAR, 360 mg kg −1) or dipyrone (DIP, 360 mg kg−1) 2 h after carrageenan. The thresholds of the right (a, c) and left (b, d) paws are shown separately. Treatment with paracetamol when hyperalgesia was well developed still raised the thresholds of both paws, the inflamed and non-inflamed paws. The late treatment with dipyrone raised the thresholds of only the inflamed paws. Values shown are the mean (±s.e.mean) results from 4 to 6 animals at each time point. *Significantly different from values without analgesic treatment. #Significantly above basal threshold: ANOVA, P<0.05.
Effects of paracetamol or dipyrone given locally on hyperalgesia induced by carrageenan
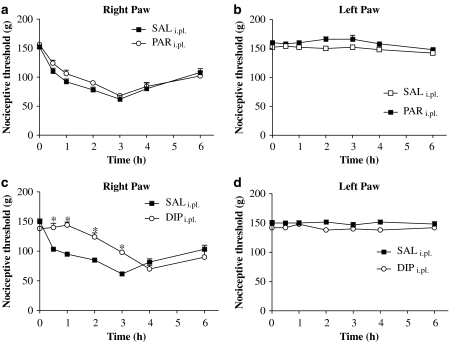
In these experiments, the analgesics were injected into the footpad of the right hind paw, 30 min before carrageenan was injected into the same paw. Given locally at a dose of 3.6 mg per paw, paracetamol showed no anti-hyperalgesic effects in the right paw (Figure 3a) and did not affect nociceptive thresholds in the left paw (Figure 3b). However, dipyrone at the same dose (3.6 mg per paw) did show anti-hyperalgesia for at least 3 h after carrageenan injection (Figure 3c), also without affecting the left paw (Figure 3d).
Figure 3.
Effects of local (intraplantar) injection of paracetamol or dipyrone on reduced nociceptive threshold induced by carrageenan in rat paws. Paracetamol (PARi.pl; 3.6 mg per paw), dipyrone (DIPi.pl; 3.6 mg per paw) or 0.1 ml saline (control paws: saline, SAL) was administered 30 min before carrageenan. Nociceptive thresholds are shown for right (inflamed; a, c) and left (non-inflamed; b, d) paws. For local paracetamol, no significant differences between groups were observed (a, b). For dipyrone (c, d), local injection significantly raised reduced nociceptive thresholds only in the inflamed paws. Values shown are the mean (±s.e.mean) results from 4 to 6 animals at each time point. *Significantly different from values without dipyrone treatment: ANOVA, P<0.05.
Effects of naltrexone, an opioid antagonist, on the actions of paracetamol or dipyrone in hyperalgesia induced by carrageenan
To assess the possible contribution of endogenous opioids to the hypoalgesic effects shown by paracetamol and dipyrone, naltrexone was injected s.c. 30 min before these analgesics. As shown in Figure 4a, for the right, inflamed paw, hypoalgesia induced by paracetamol, over the first 2 h, was abolished by pretreatment with naltrexone. In the left paw (Figure 4b), similar results were observed—loss of hypoalgesia in the first 2 h, with no change at the remaining time points.
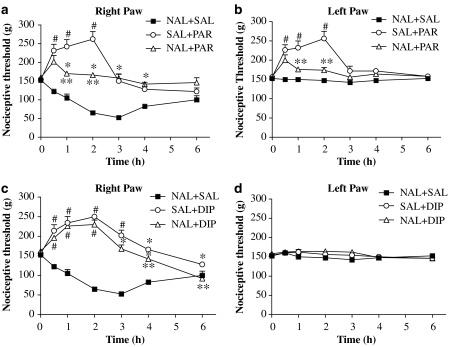
Figure 4.
Responses to naltrexone (NAL), given s.c., in rat paws inflamed by carrageenan and treated with paracetamol or dipyrone. Nociceptive thresholds for right, inflamed and left, non-inflamed paws are shown separately in (a), (c) and (b), (d), respectively. Note naltrexone alone (NAL+SAL; NAL, 3 mg kg−1) did not modify the hyperalgesia induced by carrageenan in the right paw but the hypoalgesia induced by paracetamol (360 mg kg−1; SAL+PAR) was markedly inhibited by naltrexone (NAL+PAR). A similar loss of hypoalgesia was also recorded for the left, non-inflamed paw (b). For dipyrone (c, d), hypoalgesia was largely unaffected by naltrexone. From 3 to 6 h, minor reversals of dipyrone-induced effects were observed. Values shown are the mean (±s.e.mean) results from 4 to 6 animals at each time point. *Significantly different from NAL+SAL. **Significantly different from SAL+PAR or SAL+DIP values. #Significantly above basal threshold: ANOVA, P<0.05.
Dipyrone induced hypoalgesia over the first 3 h, and these effects were largely unchanged by treatment with naltrexone (Figure 4c). After 3 h, naltrexone induced a minor reversal of the analgesic effects of dipyrone. Note that in the left paw, where no analgesic effects were seen (Figure 4d), naltrexone pretreatment did not induce any change in the nociceptive thresholds.
We then measured the effect of naltrexone, given locally into the footpad, on the analgesic actions of systemic paracetamol or dipyrone. Naltrexone was given only into the right paw, 1 h before the carrageenan and 30 min before the analgesics were given systemically. We administered 100 μg naltrexone per paw, which would be equivalent to a systemic dose of 0.67 mg kg−1 in our animals. Given locally, naltrexone did not affect either the hypoalgesia induced by paracetamol in the inflamed paw (Figure 5a) or the responses to systemic paracetamol in the left, non-inflamed paws of these animals (Figure 5b). There were late but minor reversals of paracetamol-induced analgesia, with intraplantar naltrexone. For dipyrone, local naltrexone did not affect any of its analgesic actions in the inflamed paw or the thresholds in the left paw (Figures 5c and d).
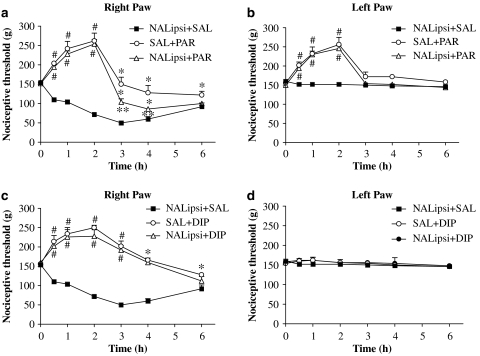
Figure 5.
Responses to local (intraplantar) naltrexone administration before systemic treatment with paracetamol or dipyrone. Naltrexone (NAL; 100 μg per paw) diluted in 0.1 ml physiological saline was given locally to right paws (ipsi), 30 min before carrageenan (ipsi). Paracetamol (SAL+PAR; 360 mg kg−1), dipyrone (SAL+DIP; 360 mg kg−1) or physiological saline (NALipsi+SAL) was injected s.c., 30 min before intraplantar carrageenan administration (time zero). Values for the inflamed (a, c) and non-inflamed (b, d) paws are shown. Minor reversal of paracetamol-induced analgesia by local naltrexone was seen only from 3 to 6 h with no effects in the left paw. However, for dipyrone, no reversal of analgesia was observed in naltrexone (NALipsi+DIP)-treated animals. Data are the mean (±s.e.mean) results from 4 to 6 animals at each time point. *Significantly different from NALipsi+SAL. **Significantly different from SAL+PAR or SAL+DIP values. #Significantly above basal threshold: ANOVA, P<0.05.
Discussion
Our experiments were designed to find out, for the two analgesic compounds studied, paracetamol and dipyrone, whether their analgesic effects are derived from a common causal mechanism, as implied by their shared profile of pharmacological activity and their shared inhibition of COX-3 (Chandrasekharan et al., 2002; Ayoub et al., 2006). In our model, at a similar range of doses, both compounds induced anti-hyperalgesia and hypoalgesia to a similar extent. The term hypoalgesia, as used here, describes a nociceptive state in which the threshold was raised above the normal, non-inflamed level (Francischi et al., 2002). In this model of inflammatory pain, where PGs are known to mediate the hyperalgesia, ‘classical' NSAIDs, such as indomethacin and piroxicam, induce anti-hyperalgesia but not hypoalgesia, implying a non-PG mediation of the hypoalgesic state. As paracetamol or dipyrone is not ‘classical' but, as previously mentioned, anomalous NSAIDs, non-classical effects were not unexpected. However, we did assume, given that these two compounds are inhibitors of COX-3 (Chandrasekharan et al., 2002), that their analgesic effects would be similarly shared. In the event, although they both induced hypoalgesia, in most of our other assessments, the two compounds exhibited important differences.
The most striking feature of the action of paracetamol was that it affected the nociceptive thresholds in both inflamed and non-inflamed paws equally. This bilateral analgesic effect was also observed in the same experimental model of carrageenan-induced hyperalgesia in a different strain of rats (Wistar), demonstrating that our observations were not strain-specific. Although bilateral hypoalgesia after paracetamol had been noted earlier (Alloui et al., 2002), it contrasted sharply with the unilateral (only in the inflamed paw) analgesia induced by systemically administered inhibitors of PG biosynthesis (catalysed by both COX-1 or COX-2) in our model (Francischi et al., 2002; França et al., 2006). The bilateral effect of paracetamol was expressed not only in its intensity but also in its time course, hypoalgesia—threshold above normal—in the early hours after carrageenan injection, fading to anti-hyperalgesia—threshold about normal—by 4–6 h, when administered either before or after the development of inflammation. However, there was no analgesic effect following local intraplantar injection of a dose of paracetamol (3.6 mg per paw equivalent to a systemic dose of 20 mg kg−1). Moreover, the actions of paracetamol in both paws were equally affected by pretreatment with naltrexone when given systemically and both paws were equally resistant to local naltrexone. The simplest explanation of these results is to postulate a ‘systemic' mechanism of analgesia, unrelated to the presence of inflammation and consequently unrelated to a direct effect on PG biosynthesis. In view of the sensitivity of paracetamol to naltrexone, this systemic mechanism should involve the release of endogenous opioids by paracetamol, but this release would neither be a local, peripheral release nor would the actions of the opioids be dependent on the presence of inflammation (Zöllner et al., 2003). Such a release could be in the brain or in the spinal cord where inputs from, or outputs to, both hind paws would need to be equally modified.
There are already reports of the central actions of paracetamol in a variety of pain models (Pini et al., 1997; Bonnefont et al., 2003; Graham and Scott, 2005) or of its actions at a spinal level (Alloui et al., 2002; Raffa et al., 2004; Bonnefont et al., 2005). These reports have also linked the actions of paracetamol to a descending 5-HT pathway (Alloui et al., 2002; Bonnefont et al., 2003, 2005; Sandrini et al., 2003) and/or to an endogenous opioid pathway (Pini et al., 1997; Raffa et al., 2004). Our results, thus, would be compatible with a mechanistic scheme, which involved a central site of action of paracetamol, with analgesia being mediated by endogenous opioids. If inhibition of PG biosynthesis is involved, either by a centrally located COX-3 (Ayoub et al., 2006) or COX-2 (Graham and Scott, 2005), in the analgesic effects of paracetamol, then the lack of PGs must stimulate, directly or indirectly, the release of endogenous opioids within the CNS.
The most important aspect of our comparison of paracetamol and dipyrone was that the similar analgesic effects were very differently expressed. These differences were unexpected, as dipyrone often shares, with paracetamol, the classification as an anomalous NSAID. Also, they were both shown to be reasonably potent inhibitors of COX-3 in the CNS (Chandrasekharan et al., 2002; Botting and Ayoub, 2005). Another common feature is an antioxidant, rather than antisubstrate, effect on COX (Aronoff et al., 2006; Pierre et al., 2007). Moreover, in our present work, paracetamol and dipyrone shared the ability to induce hypoalgesia. It was, therefore, surprising that dipyrone also was not bilateral in its analgesic effects, was not affected by naltrexone, systemically or locally administered, and was still effective when given locally. These findings characterized dipyrone as peripherally, not centrally, acting and with a mode of action independent of the endogenous opioid system.
One local mechanism proposed for analgesic effects of dipyrone is activation of ATP-gated K+ channels (Alves and Duarte, 2002; Sachs et al., 2004). However, activation of these channels also involves opioids, in that morphine is another activator (Rodrigues and Duarte, 2000) and the effects of other K+ channel openers are antagonized by opioid antagonists (Campbell and Welch, 2001).
There are other results showing endogenous opioid mediation of the antinociceptive effects of dipyrone in other pain models and using injections into the CNS (see Vasquez et al., 2005). However, our experiments showed the analgesic effects of dipyrone to be mostly unaffected by naltrexone given systemically or locally, suggesting strongly that endogenous opioids were not involved in our model. The late reversal (3–6 h; Figure 4c) by naltrexone is an intriguing finding and might reflect a correspondingly late involvement of opioids. One speculative explanation would be to postulate two separate mechanisms for hypoalgesia and for anti-hyperalgesia, with only the latter effect being opioid-mediated for dipyrone.
Although our present results do not lead to clear proposals for a mechanism of action for dipyrone, this profile of analgesic activity of dipyrone was closer to that of a ‘classical' NSAID, such as indomethacin, than to the profile of paracetamol. More significantly, these differences between paracetamol and dipyrone are difficult to reconcile on the basis of a common mechanism of action. Our results should not be taken as evidence refuting the ability of these compounds to inhibit COX-3 or any other COX isoform by any mechanism, but as a property common to both. Our experiments were not designed to test that proposition. However, inhibition of PG biosynthesis alone did not appear to be the direct cause of the analgesic actions observed in vivo with either compound. Nevertheless, what is clear is that, in our model, at least three mechanistically significant features of antinociception exhibited by dipyrone and paracetamol were different, the bilateral vs unilateral effects, the response to naltrexone pretreatment and the local anti-hyperalgesic effect.
Limitations of the study
The results with local injections (into the paws), although clear, are limited by the technical problems of intraplantar injection. Such injections are restricted in volume, which is critical for relatively insoluble agents such as paracetamol. Another inherent difficulty is the possibility of locally injected materials ‘leaking' into the systemic circulation and inducing systemic, rather than local, effects. Because of these limitations, we sought in pilot experiments to find the minimum effective dose of dipyrone given locally and then used the same dose of paracetamol, as these two agents were equiactive on s.c. administration. Higher doses of paracetamol required higher volumes, which induced noticeable oedema by themselves. As the doses used of paracetamol and dipyrone were well below the lowest dose given s.c., we assumed that the effects of possible leakages into the systemic circulation would be negligible. For naltrexone, we used a single, fixed dose, which was derived from pilot experiments with COX-2 inhibitors (Francischi et al., 2002; França et al., 2006) and from the work of Rodrigues and Duarte (2000) to provide a minimum effective dose. Again the systemic dose derived from the local administration of naltrexone was well below that resulting from s.c. injection, minimizing the contribution of ‘non-local' sites of action. However, such leakage of local naltrexone into the systemic circulation might explain the late reversal (3–6 h) of analgesic effects of paracetamol, illustrated in Figure 5a.
There are two other potential confounding factors, both related to the transformation of paracetamol or dipyrone to other active compounds. Paracetamol, after deacetylation, is converted to an amide of arachidonic acid, AM404, via the fatty acid hydrolase pathway, in brain and spinal cord (Hogestatt et al., 2005). AM404 has many actions but, particularly in this context, it prevented pain behaviour in rats after thermal or mechanical stimuli in a rat model of neuropathic pain (Costa et al., 2006). Further, cannabinoid type 1 receptor antagonists prevented the analgesic action of paracetamol in the formalin model in rats (Ottani et al., 2006). How far such conversion of paracetamol to AM404 is crucial to its activity in our model is unclear, but this possibility does provide a non-COX-mediated path to pain relief for paracetamol. For dipyrone, this compound is rapidly hydrolysed to the pharmacologically active 4-methyl amino antipyrine, which is an ‘anti-peroxide' inhibitor of COX (Pierre et al., 2007), as mentioned above. However, 4-methyl amino antipyrine is a tertiary amine and as such is unlikely to form an amide with arachidonic acid, corresponding to AM404. This may contribute to the difference between paracetamol and dipyrone that we have observed, but direct evidence for this point has yet to be provided.
In summary, our results show that, although both paracetamol and dipyrone exhibited very similar hypoalgesia in our model, in terms of intensity and duration, the underlying mechanisms were very different. We had expected to find a common functional analgesic effect based on a common mechanism of action. In the event, we have clearly demonstrated the important differences between these analgesics. Whereas hypoalgesic actions of paracetamol could represent a centrally located, opioid-dependent mechanism, for dipyrone, the mechanistic scheme would need to be peripheral and independent of opioids. None of the presently proposed mechanisms of action for these compounds provide satisfactory explanations of our observations in vivo and we still need an adequate explanation of the effects of these extensively used and effective analgesics.
Acknowledgments
The Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) supported this research.
Abbreviations
- AM404
amide of arachidonic acid
- DIP
dipyrone
- NAL
naltrexone
- NSAIDs
non-steroidal anti-inflammatory drugs
- PAR
paracetamol
- PG
prostaglandin
Conflict of interest
The authors state no conflict of interest.
References
- Alloui A, Chassaing C, Schimidt J, Ardidi D, Dubray C, Cloarec A, et al. Paracetamol exerts a spinal, tropisetron-reversible, antinociceptive effect in an inflammatory pain model in rats. Eur J Pharmacol. 2002;443:71–77. doi: 10.1016/s0014-2999(02)01578-9. [DOI] [PubMed] [Google Scholar]
- Alves D, Duarte IDG. Involvement of ATP-sensitive potassium channels in the peripheral antinociceptive effect induced by dipyrone. Eur J Pharmacol. 2002;444:47–52. doi: 10.1016/s0014-2999(02)01412-7. [DOI] [PubMed] [Google Scholar]
- Aronoff DM, Oates JA, Boutaud O. New insights into the mechanism of action of acetaminophen: its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin H2 synthases. Clin Pharmacol Ther. 2006;79:9–19. doi: 10.1016/j.clpt.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Ayoub SS, Colville-Nash PR, Willoughby DA, Botting RM. The involvement of a cyclooxygenase 1 gene-derived protein in the antinociceptive action of paracetamol in mice. Eur J Pharmacol. 2006;538:57–65. doi: 10.1016/j.ejphar.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Bonnefont J, Alloui A, Chapuy E, Clottes E, Eschalier A. Orally administered paracetamol does not act locally in the rat formalin test: evidence for a supraspinal, serotonin-dependent antinociceptive mechanism. Anesthesiology. 2003;99:976–981. doi: 10.1097/00000542-200310000-00034. [DOI] [PubMed] [Google Scholar]
- Bonnefont J, Chapuy E, Clottes E, Alloui A, Eschalier A. Spinal 5-HT1A receptors differentially influence nociceptive processing according to the nature of the noxious stimulus in rats: effect of WAY-100635 on the antinociceptive activities of paracetamol, venlafaxine and 5-HT. Pain. 2005;114:482–490. doi: 10.1016/j.pain.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Botting R, Ayoub SS. COX-3 and the mechanism of action of acetaminophen/paracetamol. Prostaglandins Leukot Essent Fatty Acids. 2005;72:85–87. doi: 10.1016/j.plefa.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Botting RM. Mechanism of action of paracetamol: is there a cyclooxygenase 3. Clin Infect Dis Suppl. 2000;5:S202–S210. doi: 10.1086/317520. [DOI] [PubMed] [Google Scholar]
- Brunton LL, Lazo JS, Parker KL, Buxton ILO, Blumenthal D.Analgesic-antipyretic and antiinflammatory agents: pharmacotherapy of gout Goodman & Gilman: The Pharmacological Basis of Therapeutics 2005Mac Graw-Hill: New York, USA; In: Hardman JG, Limbrid LE (eds).11th edn. [Google Scholar]
- Campbell VC, Welch SP. The role of minoxidil on endogenous opioid peptides in the spinal cord: a putative co-agonist relationship between K+-ATP openers and opioids. Eur J Pharmacol. 2001;417:91–98. doi: 10.1016/s0014-2999(01)00885-8. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan NV, Dai H, Roos KLT, Evanson NK, Tomsik J, Elton TS, et al. COX-3, a cyclooxygenase-1 variant inhibited by paracetamol and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Nat Acad Sci USA. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Siniscalco D, Trovato AE, Comelli F, Sotgiu ML, Colleoni M, et al. AM404, an inhibitor of anandamide uptake, prevents pain behaviour and modulates cytokine and apoptotic pathways in a rat model of neuropathic pain. Br J Pharmacol. 2006;148:1022–1032. doi: 10.1038/sj.bjp.0706798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosa M. Biological properties of the carrageenan. J Pharm Pharmacol. 1972;24:89–102. doi: 10.1111/j.2042-7158.1972.tb08940.x. [DOI] [PubMed] [Google Scholar]
- Duarte ID, Dos Santos IR, Lorenzetti B, Ferreira SH. Analgesia by direct antagonism of nociceptor sensitization involves the arginine–nitric oxide–cGMP pathway. Eur J Pharmacol. 1992;217:225–227. doi: 10.1016/0014-2999(92)90881-4. [DOI] [PubMed] [Google Scholar]
- França DS, Ferreira-Alves DL, Duarte ID, Ribeiro MC, Rezende RM, Bakhle YS, et al. Endogenous opioids mediate the hypoalgesia induced by inhibitors of cyclooxygenase-2 in rat paws treated with carrageenan. Neuropharmacology. 2006;51:37–43. doi: 10.1016/j.neuropharm.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Francischi JN, Chaves CT, Moura ACL, Lima AS, Rocha OA, Ferreira-Alves DL, et al. Selective inhibitors of cyclo-oxygenase-2 (COX-2) induce hypoalgesia in a rat paw model of inflammation. Br J Pharmacol. 2002;137:837–844. doi: 10.1038/sj.bjp.0704937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham GG, Scott KF. Mechanism of action of paracetamol. Am J Ther. 2005;12:46–55. doi: 10.1097/00045391-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Hogestatt ED, Jonsson BA, Ermund A, Andersson DA, Bjork H, Alexander JP, et al. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J Biol Chem. 2005;280:31405–31412. doi: 10.1074/jbc.M501489200. [DOI] [PubMed] [Google Scholar]
- Kis B, Snipes JA, Busija DW. Paracetamol and the cyclooxygenase-3 puzzle: sorting out facts, fictions, and uncertainties. J Pharm Exp Ther. 2005;315:1–7. doi: 10.1124/jpet.105.085431. [DOI] [PubMed] [Google Scholar]
- Ottani A, Leone S, Sandrini M, Ferrari A, Bertolini A. The analgesic activity of paracetamol is prevented by the blockade of cannabinoid CB1 receptors. Eur J Pharmacol. 2006;531:280–281. doi: 10.1016/j.ejphar.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Pereira LS, Ferreira-Alves DL, Resende MA, Romualdo MA, Dos Reis WGP, Lopes MT, et al. Reduced production of hyperalgesic substances by mononuclear cells from aged rats incubated with carrageenan: role of interleukin-2 and prostaglandins. Inflamm Res. 2003;52:119–125. doi: 10.1007/s000110300024. [DOI] [PubMed] [Google Scholar]
- Pierre SC, Schmidt R, Brenneis C, Michaelis M, Geisslinger G, Scholich K. Inhibition of cyclooxygenases by dipyrone. Br J Pharmacol. 2007;151:494–503. doi: 10.1038/sj.bjp.0707239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini LA, Vitale G, Ottani A, Sandrini M. Naloxone-reversible antinociception by paracetamol in the rat. J Pharmacol Exp Ther. 1997;280:934–940. [PubMed] [Google Scholar]
- Raffa RB, Walker EA, Sterious SN. Opioid receptors and paracetamol (acetaminophen) Eur J Pharmacol. 2004;503:209–210. doi: 10.1016/j.ejphar.2004.08.055. [DOI] [PubMed] [Google Scholar]
- Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissues. Arch Int Pharmacodyn Ther. 1957;111:409–419. [PubMed] [Google Scholar]
- Rang HP, Dale MM, Ritter JM, Moore P. Pharmacology 2006Churchill Livingstone: London; 6th edn. [Google Scholar]
- Rodrigues AR, Duarte ID. The peripheral antinociceptive effect induced by morphine is associated with ATP-sensitive K+ channels. Br J Pharmacol. 2000;129:110–114. doi: 10.1038/sj.bjp.0703038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs D, Cunha FQ, Ferreira SH. Peripheral analgesic blockade of hypernociception: activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proc Nat Acad Sci USA. 2004;101:3680–3685. doi: 10.1073/pnas.0308382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini M, Pini LA, Vitale G. Differential involvement of central 5-HT1B and 5-HT3 receptor subtypes in the antinociceptive effect of paracetamol. Inflamm Res. 2003;52:347–352. doi: 10.1007/s00011-003-1185-5. [DOI] [PubMed] [Google Scholar]
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenase-1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Vanegas H, Tortorici V. Opioidergic effects of nonopioid analgesics on the central nervous system. Cell Mol Neurobiol. 2002;22:655–661. doi: 10.1023/a:1021896622089. [DOI] [PubMed] [Google Scholar]
- Vasquez E, Hernandez N, Escobar W, Vanegas H. Antinociception induced by intravenous dipyrone (metamizol) upon dorsal horn neurons: involvement of endogenous opioids at the periaqueductal gray matter, the nucleus raphe magnus, and the spinal cord in rats. Brain Res. 2005;1048:211–217. doi: 10.1016/j.brainres.2005.04.083. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- Vinegar R, Truax JF, Selph JL, Johnston PR, Venable AL, McKenzie KK. Pathway to carrageenan-induced inflammation in the hind limb or the rat. Federation Proc. 1987;46:118–126. [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;6:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Zöllner C, Shaqura MA, Bopaiah CP, Mousa S, Stein C, Schäfer M. Painful inflammation-induced increase in μ-opioid receptor binding and G-protein coupling in primary afferent neurons. Mol Pharmacol. 2003;64:202–210. doi: 10.1124/mol.64.2.202. [DOI] [PubMed] [Google Scholar]