Abstract
The current study investigated whether human influenza viral infection in midpregnancy leads to alterations in proteins involved in brain development. Human influenza viral infection was administered to E9 pregnant Balb/c mice. Brains of control and virally exposed littermates were subjected to microarray analysis, SDS-PAGE and western blotting at three postnatal stages. Microarray analysis of virally-exposed mouse brains showed significant, two-fold change in expression of multiple genes in both neocortex and cerebellum when compared to sham-infected controls. Levels of mRNA and protein levels of four selected genes were examined in brains of exposed mice. Nucleolin mRNA was significantly decreased in day 0 and day 35 neocortex and significantly increased in day 35 cerebellum. Protein levels were significantly upregulated at days 35 and 56 in neocortex and at day 56 in cerebellum. Connexin 43 protein levels were significantly decreased at day 56 in neocortex. Aquaporin 4 mRNA was significantly decreased in day 0 neocortex. Aquaporin 4 protein levels decreased in neocortex significantly at day 35. Finally, microcephalin mRNA was significantly decreased in day 56 neocortex and protein levels were significantly decreased at 56 cerebellum. These data suggest that influenza viral infection in midpregnancy in mice leads to long term changes in brain markers for enhanced ribosome genesis (nucleolin), increased production of immature neurons (microcephalin), and abnormal glial-neuronal communication (connexin 43 and aquaporin 4).
Keywords: human influenza, mouse, brain, schizophrenia, autism, microarray
1. Introduction
Schizophrenia and autism are major neurodevelopmental disorders with prevalences of 1% and 16 per 10,000, respectively (American Psychiatric Association, 1994; Fombonne, 2003). Genetic and environmental etiologies have been cited as being responsible for the genesis of both disorders (Andreasen, 1999; Acosta and Pearl, 2003). While both disorders are neurodevelopmental, the behavioral phenotype varies between the two disorders. Schizophrenia manifests itself in adolescence with signs of thought disorganization and psychosis. In autism, overt behavioral symptoms peak at the thirtieth month postnatally and symptoms include regression in speech, social deficits, communication deficits, and an increase in stereotypic behavior. There is significant epidemiological support for a viral etiology of schizophrenia with some support for an infectious etiology of autism (Brown et al, 2004; Chess, 1971; Chess et al. 1978; Mednick et al. 1994; Stubbs et al. 1984; Susser et al. 1999). New serologic data provide evidence for an infectious origin of some forms of schizophrenia (Brown et al. 2004).
Recent emergence of experimental data show that administration of human influenza virus (H1N1) or a viral mimic – polyriboinosinic polyribocytidilic acid (PolyI:C), at critical stages during pregnancy in mice, can cause deleterious effects in brains of offspring (Fatemi et al. 1999; Fatemi et al. 2002a; Shi et al. 2003a). We have previously shown infection of day E9 pregnant mice by a neurotrophic strain of human (H1N1) influenza virus causes significant biochemical, behavioral, genetic, and structural brain disorders in the offspring (Fatemi et al. 1999; Fatemi et al. 2002a; Shi et al. 2003a). Recent reports by different groups have supported our published data showing that viral mimic PolyI:C at critical stages during pregnancy in rodents can cause similar deleterious effects in the brains of offspring (Shi et al. 2003b; Meyer et al. 2005; Meyer et al. 2006a; Meyer et al 2006b; Meyer et al. 2007a; Meyer et al. 2007b; Nyffeller et al. 2007). The major difference between the new supportive data and ours is that they posit that the intrauterine insult is due to maternal cytokine upregulation and not the direct effects of a viral antigen (Shi et al. 2003b, Meyer et al. 2006). We now show that there is evidence of a potential insult by the virus on fetal brains' neuronal and glial synthetic machinery leading to perturbed gene expression in the affected offspring (Aronson et al. 2001; Aronson et al. 2002; Fatemi et al. 2007a; Fatemi, 2007). Despite these data we cannot dismiss the idea that fetal or maternal cytokine production could not be responsible for the altered mRNA and protein levels.
A previous microarray study of virally-exposed mouse brains by our laboratory has shown significantly decreased expression of nucleolin and aquaporin 4 in the brains of Day 0 mice (Fatemi et al. 2005). Nucleolin, a major protein of the nucleolus, is involved in regulating the transcription of ribosomal RNA genes by RNA polymerase I, ribosome maturation and assembly, and nucleocytoplasmic transportation of ribosomal components (Srivastava et al. 1989) and may be involved in viral replication (Hiscox, 2002). Aquaporin 4 is localized to astrocytes and ependymal cells in brain and is involved with water transport (Papadopoulos et al. 2002; Verkman et al. 2006). Our laboratory has demonstrated that aquaporin 4 and another glial marker, connexin 43 (Cx43), are significantly altered in brains of autistic subjects (Fatemi et al. unpublished observations).
In the current communication we further investigate changes in mRNA and protein levels of nucleolin, aquaporin 4, and connexin 43 at P35 and P56 in offspring of mice infected with human influenza virus, integrating our data with a microarray study by our laboratory of cerebellum from the same mice at the same two postnatal dates (Fatemi et al, 2007b). Based on our microarray results we also added microcephalin as a previous report form our laboratory has demonstrated macrocephaly using this animal model (Fatemi et al. 2002a). We propose that viral infection in midpregnancy in mice leads to long-term changes in these four important brain markers.
2. Methods
2.1. Animals and Infection
Balb/c male and female 12-14 week old mice were used for the induction of pregnancy and all infection protocol using human influenza virus following previous protocol (Fatemi et al. 1999). Female and male 12-14 week old specific pathogen-free Balb/c mice were obtained from Simonsen laboratories (Gilroy, CA) and were used for breeding. For virus titration, the animals were quarantined 24 hours prior to use, and maintained on Wayne Lab Blox and tap water. After being infected, their drinking water contained 0.006% oxytetracycline (Pfizer, N.Y.) to control possible bacterial infections. Pregnant mice were infected intranasally on day 9 of pregnancy. Pups born to infected and sham infected mothers (n=10 each) were culled and used for blotting studies (n=3 per group) on postnatal days 0, 35 and 56.
The choice of E9 for our infection protocol was based on supportive literature showing that this day preceded the timetable for proliferation, differentiation and migration of neurons and glia in embryonic neocortex, hippocampus and cerebellum (Susser et al. 1999) and initiation of migration of neural crest derived cells to craniofacial structures (Yamagishi et al. 1999) and production of Cajal-Retzius cells (D'Arcangelo et al. 1997). The timetables for brain development vary based on various regions of the brain and are clearly based on species of interest. Thus, extrapolation from mouse to man may not be possible on specific days of pregnancy, but can be construed from prenatal and postnatal peaks of neurogenesis, gliogenesis and neuron/glia differentiation and migration (Susser et al. 1999). The choice of postnatal days 35 and 56 in our studies are based on a large group of supportive literature (Avishai-Eliner et al. 2002; Boksa et al. 2003; Kaufmann, 2000; Morgane et al. 2002; Romijn et al. 1991; Spear, 2000; Susser et al. 1999) denoting E0-E10 in rodents as equal to first trimester in humans, E11-E19 as equal to 2nd trimester, P0-P7 as equal to third trimester (Morgane et al. 2002; Stead et al. 2006), P35 as equal to adolescence (Spear, 2000) and P56 as equal to early adulthood (Spear, 2000).
The protocol for infection reported previously was used (Fatemi et al, 1999). All animal experiments were performed following IRB approval in accordance with institutional animal care and use committee guidelines at Utah State University and University of Minnesota. Briefly, day 9 pregnant Balb/c mice were exposed to influenza A/NWS/33 (H1N1) or vehicle, following determination of viral dosage, causing sublethal lung and upper respiratory infection. The virus was diluted to 10−5 CCID50/ml based on previous titrations using this species (Fatemi et al. 1999; Fatemi et al. 2005). Pregnant mice survived the infection protocol and were allowed to deliver pups. The day of delivery was considered day 0. Groups of exposed (n=3) and sham infected pups (n=3) were deeply anesthetized using ketamine (167mg/kg, intraperitoneally) and sacrificed on postnatal days 0, 35 or 56.
2.2. DNA microarray
DNA microarray was performed as described previously (Fatemi et al. 2005). Cerebellum and neocortex (whole brains minus brain stem and cerebellum) were homogenized by standard procedures in Trizol (Gibco BRL) for total RNA extraction. 10μg of total RNA from each sample was used to generate a high fidelity cDNA, which is modified at the 3' end to contain an initiation site for T7 RNA polymerase as per the manufacturer protocol (SuperChoice, Gibco BRL). Upon completion of cDNA synthesis, 1μg of product was used in an in vitro transcription (IVT) reaction that contained biotinylated UTP and CTP which was utilized for detection following hybridization to the microarray as per the manufacturer's protocol (ENZO). 20μg of full length IVT product was subsequently fragmented in 200mM Tris-acetate (pH 8.1), 500 mM KOAc and 150 mM MgOAc at 94°C for 35 minutes. Following fragmentation, all components generated throughout the processing procedure (cDNA, full-length cRNA, and fragmented cRNA) were analyzed by gel electrophoresis to assess the appropriate size distribution prior to microarray analysis. All samples generated were subjected to gene expression analysis by the Affymetrix murine 430 high-density oligonucleotide array set which contained sequences from approximately 20 000 known mouse genes and EST's at the University of Minnesota BioMedical Genomics Center. Each gene on the array was represented by 16-20 pairs of 25mer oligonucleotides that spanned the coding region for each gene represented. Each probe pair consisted of a perfect match (PM) sequence that was complementary to the cRNA target and a miss match (MM) sequence that had a single base pair mutation in a region critical for target hybridization; this sequence served as a control for non-specific hybridization. Hybridization, staining, and washing of all arrays were performed in the Affymetrix fluidics module as per the manufacturer's protocol. Streptavidin phycoerythrin stain (SAPE, Molecular Probes) was the florescent conjugate used to detect hybridized target sequences. The detection and quantitation of target hybridization was performed with a GeneArray Scanner (Hewlett Packard/Affymetrix) set to scan each array twice at a factory set PMT level and resolution. In addition, all arrays were scanned pre- and post- amplification to address potential issues with respect to the dynamic range of the scanner.
Multiple data analysis approaches were used to identify genes of interest. The Microarray Analysis Suite 5.0 (Affymetrix) was employed to generate one approach to comparative analysis presented in this study. Distinct algorithms were used to determine the detection signal, which distinguished the presence or absence of a transcript, the differential change in gene expression (increase (I), decrease (D), marginal increase (MI), marginal decrease (MD), and no change (NC)), and the magnitude of change, which is represented as fold change. The fold change of any transcript between the baseline and experimental is calculated following global scaling. All data represented from this first approach were from pairwise comparison analysis. The second approach to identifying differentially expressed genes utilized Li Wong which fits a model to the probe set data from multiple microarrays. This method excludes cross hybridizing probes, single outliers and arrays with image contaminations at certain probe sets (Harr and Schlötterer, 2006). The third method was Robust Multi-array Analysis (RMA) which provides probe specific background to correct for nonspecific binding, probe level multichip quantile normalization and robust probe set summary of the probe level data (Irizarry et al. 2003). (Please see Supplemental Tables 1 (P35) and 2 (P56) for the complete data set.) Following each normalization approach, all genes differentially expressed were clustered. Genes were assessed based on primary biological function and grouped accordingly.
2.3. Western blotting
SDS-PAGE and western blotting details followed previous protocol (Fatemi et al. 2006). Sixty μg of protein per lane was loaded onto the gel and electrophoresed. The immune complexes were visualized using the ECL Plus detection system (Amersham Pharmacia Biotech) and exposed to CL-Xposure film (Pierce). Sample densities were analyzed using a Bio-Rad densitometer and Quantity One software.
Connexin 43 and β-actin were analyzed using a modification of a previous protocol (9) using mouse β-actin (Sigma A5441, 1:5000) or Connexin 43 (Fred Hutchinson Cancer Research Center, 1:10 000). Aquaporin 4, Nucleolin and microcephalin were analyzed using the previous protocol, with the following changes: either an 8% (microcephalin or nucleolin) or a 12% (aquaporin 4) SDS-polyacrylamide gel was used; after transfer, the blot was blocked at RT for 1 hour, followed by overnight incubation with primary antibody (rabbit anti-aquaporin 4, Chemicon AB3594, 1:1000; rabbit anti-microcephalin, AbCam ab2612, 1:1000 or rabbit anti-nucleolin, NB 600-241, 1:4000) at 4°C. Secondary antibody was (goat-anti-rabbit IgG, HRP conjugated, 1:80 000, from Sigma) applied for 1 hour at RT, and all washes and visualization took place as described above.
The densities of approximately 34 kDa (aquaporin 4), 106 kDa (nucleolin), 105 kDa (microcephalin), 43 kDa (connexin 43), and 42 kDa (β-actin), immunoreactive bands were quantified with background subtraction. Results obtained are based on between two and eight independent experiments with n=3 mice per gel. For each experiment, control and infected samples were run on the same gel and processed simultaneously to avoid variability due to intragel differences.
2.4. Statistical analysis
All statistical analyses were performed using SPSS. Differences of the normalized mRNA expression levels of selected genes between virally-infected and sham-infected mice were assayed using student's t-test. Significant differences are defined as those with a p value < 0.05. For western blots, differences of the protein levels of selected genes between virally-infected and sham-infected mice were normalized against β-actin and assayed using a student's t-test. Significant differences are defined as those with a p value < 0.05.
3. Results
3.1. Microarray results
Microarray analysis of virally-exposed mouse brains showed significant (p<0.05) at least two-fold upregulation of 50 genes (Table 1) and downregulation of 21 genes (Table 2) in neocortex vs. control in day 35 mice. At day 56, microarray analysis showed significant (p<0.05) upregulation of 13 genes (Table 1) and downregulation of 11 genes (Table 2) in neocortex vs. control. A concurrent microarray study of cerebellum by our laboratory using the same virally-exposed mouse brains has shown significant (p<0.05) at least two-fold upregulation of 103 genes and downregulation of 102 genes in at P35 and at least two-fold upregulation of 27 genes and downregulation of 23 genes at P56 (Fatemi et al. 2007b). Previously, our laboratory has shown significantly decreased expression of nucleolin and aquaporin 4 in the brains of Day 0 mice (Fatemi et al. 2005). Interestingly we found that nucleolin expression at day 35 was significantly upregulated in cerebellum (Fatemi et al. 2007b) while it was significantly downregulated in neocortex (Table 2). Finally, our data set showed downregulation of microcephalin expression at day 56 in the neocortex (Table 2).
Table 1.
Neocortical genes upregulated by prenatal viral infection on postnatal days 35 and 56
| Day 35 | |||
|---|---|---|---|
| Function | Name | Fold Change | Unigene |
| Apoptosis | Suppression of tumorigenicity 18 | 2.18 | Mm.234612 |
|
Cell Communication; Signal Transduction |
cerebellin 1 precursor protein | 2.91 | Mm.4880 |
| chimerin (chimaerin) 2 | 2.27 | Mm.257073 | |
| frizzled homolog 7 (Drosophila) | 2.15 | Mm.297906 | |
| G substrate | 7.08 | Mm.42096 | |
| Glutamate receptor, ionotropic, delta 2 | 2.63 | Mm.321227 | |
| growth differentiation factor 10 | 3.06 | Mm.40323 | |
| nudix (nucleoside diphosphate linked moiety X)-type motif 11 |
2.11 |
Mm.41198 |
|
| Parvalbumin | 2.23 | Mm.422866 | |
| Cell Growth and/or Maintenance | tubulin, beta 2c | 2.13 | Mm.227260 |
| zinc finger protein 521 (AKA Evi3) | 2.07 | Mm.40325 | |
| Kinases and phosphatases | Mitogen activated protein kinase 12 | 2.31 | Mm.38343 |
| NIMA (never in mitosis gene a)-related expressed kinase 2 |
2.05 |
Mm.33773 |
|
| Metabolism; Energy Pathways | 4-hydroxyphenylpyruvic acid dioxygenase |
2.29 |
Mm.6584 |
| betaine-homocysteine methyltransferase | 2.00 | Mm.329582 | |
| carbamoyl-phosphate synthetase 1 | 2.13 | Mm.343942 | |
| Carbonic anhydrase 3 | 2.67 | Mm.300 | |
| carbonic anhydrase 8 | 10.49 | Mm.119320 | |
| esterase 31 | 2.00 | Mm.295534 | |
| malate dehydrogenase 2, NAD (mitochondrial) |
5.19 |
Mm.297096 |
|
| Protein Metabolism | aspartate-beta-hydroxylase | 2.06 | Mm.239247 |
| coagulation factor II | 2.85 | Mm.89048 | |
| fibrinogen, alpha polypeptide | 2.57 | Mm.88793 | |
| protein kinase inhibitor beta, cAMP dependent, testis specific |
2.23 |
Mm.262135 |
|
| WW domain containing E3 ubiquitin protein ligase 1 |
2.26 | Mm.78312 | |
|
Regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism |
atonal homolog 7 (Drosophila) (AKA Math5) |
2.13 | Mm.228661 |
| myelin transcription factor 1 | 2.30 | Mm.130005 | |
| Myocyte maintenance | 2.68 | NM_010134a | |
| nescient helix loop helix 2 | 2.31 | Mm.137286 | |
| neurogenic differentiation 1 | 4.56 | Mm.4636 | |
| RB1-inducible coiled-coil 1 (AKA Fip200) |
4.08 |
Mm.293811 |
|
| synaptonemal complex protein 1 | 2.55 | Mm.243849 | |
| zinc finger protein of the cerebellum 1 | 3.09 | Mm.335350 | |
| zinc finger, matrin-like | 2.11 | Mm.132392 | |
| Synthesis and Degradation | cytochrome P450, family 2, subfamily c, polypeptide 29 |
2.34 |
Mm.20764 |
| cytochrome P450, family 2, subfamily e, polypeptide 1 |
2.99 |
Mm.21758 |
|
| cytochrome P450, family 3, subfamily a, polypeptide 11 |
2.32 |
Mm.358586 |
|
| Transport | albumin 1 | 3.87 | Mm.1673 |
| apolipoprotein A-II | 2.10 | Mm.389209 | |
| aquaporin 6 | 3.41 | Mm.202309 | |
| ATPase, Ca++ transporting, ubiquitous | 2.39 | Mm.6306 | |
| fatty acid binding protein 1, liver | 3.64 | Mm.22126 | |
| potassium voltage-gated channel, subfamily G, member 4 |
2.11 | Mm.358699 | |
| transient receptor potential cation channel, subfamily M, member 4 |
2.22 |
Mm.349430 |
|
| Miscellaneous | ankyrin repeat domain 25 | 2.19 | Mm.257371 |
| serine (or cysteine) proteinase inhibitor, clade A, member 1a |
5.67 |
Mm.358636 |
|
| Serine (or cysteine) proteinase inhibitor, clade A, member 3m |
2.40 |
Mm.291569 |
|
| Serine (or cysteine) proteinase inhibitor, clade C (antithrombin), member 1 |
2.08 |
Mm.260770 |
|
| Tumor-suppressing subchromosomal transferable fragment 8 |
2.47 | BB233597a | |
| urate oxidase | 2.25 | Mm.10865 | |
| Day 56 | |||
| Cell Growth and/or Maintenance | Lipin 2 | 2.07 | Mm.227924 |
| Growth Factors and Hormones | insulin-like growth factor binding protein 5 |
2.15 |
Mm.309617 |
| Metabolism; Energy Pathways | mannosidase 2, alpha B2 | 2.05 | Mm.761 |
|
Regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism |
speckle-type POZ protein | 2.19 | Mm.285454 |
| Signaling Intermediates | peptidylglycine alpha-amidating monooxygenase |
2.98 |
Mm.5121 |
| Ribosomal protein L37a | 2.06 | Mm.379003 | |
| Transcription Regulators | SRY-box containing gene 8 | 2.09 | Mm.258220 |
| Transport and Trafficing | ATPase, H+ transporting, V1 subunit E isoform 1 |
3.13 |
Mm.29045 |
| D site albumin promoter binding protein | 2.02 | Mm.3459 | |
| Miscellaneous | deaminase domain containing 1 | 2.49 | Mm.331841 |
| metastasis associated lung | 2.24 | ||
| adenocarcinoma transcript 1 (non-coding RNA) |
Mm.298256 |
||
| NECAP endocytosis associated 1 | 2.00 | Mm.288114 | |
| Tumor-suppressing subchromosomal transferable fragment 8 |
2.22 |
BB233597a |
|
All accession numbers from Unigene except for Entrez[A].
Table 2.
Neocortical genes downregulated by prenatal viral infection on postnatal days 35 and 56
| Day 35 | |||
|---|---|---|---|
| Function | Name | Fold Change | Unigene |
| Apoptosis | nuclear receptor subfamily 4, group A, member 1 |
−2.34 | Mm.119 |
|
Cell Communication; Signal Transduction |
cyclin D2 | −2.06 | Mm.333406 |
| Discs, large homolog 2 (Drosophila) (AKA PSD93) |
−2.22 |
Mm.323861 |
|
| hematological and neurological expressed sequence 1 |
−2.40 |
Mm.1775 |
|
| Homer homolog 1 (Drosophila) | −2.51 | Mm.37533 | |
| suppression of tumorigenicity 13 | −2.03 | Mm.180337 | |
| Cell Growth and/or Maintenance | actin, beta, cytoplasmic | −2.22 | Mm.297 |
| Immune Response | sialophorin (AKA CD43) | −2.38 | Mm.283714 |
| Kinases and phosphatases | protein kinase C, zeta | −2.09 | Mm.28561 |
| Protein Metabolism | Heat shock protein 1A | −2.18 | Mm.6388 |
| peptidylglycine alpha-amidating monooxygenase |
−3.50 |
Mm.5121 |
|
|
Regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism |
CCAAT/enhancer binding protein (C/EBP), delta |
−2.79 |
Mm.347407 |
| early growth response 2 (AKA Krox20) | −2.92 | Mm.290421 | |
| FBJ osteosarcoma related oncogene | −2.66 | Mm.246513 | |
| fos-like antigen 2 (AKA Fra2) | −2.24 | Mm.24684 | |
| Nucleolin | −2.16 | Mm.154378 | |
| THO complex 1 | −2.18 | Mm.219648 | |
| Miscellaneous | mRNA fragment for heat shock cognate hsc73 |
−2.50 |
AK004608a |
| transmembrane and coiled-coil domains | −2.20 | Mm.131623 | |
| BAT2 domain containing 1 | −2.13 | Mm.245446 | |
| tubulin cofactor a | −2.37 | Mm.379025 | |
| Day 56 | |||
| Function | Name | Fold Change | Unigene |
|
Cell Communication; Signal Transduction |
Membrane-associated protein 17 | −2.02 | Mm.30181 |
| nudix (nucleoside diphosphate linked moiety X)-type motif 11 |
−2.07 |
Mm.41198 |
|
| Ras homolog enriched in brain like 1 | −2.15 | Mm.259708 | |
| Membrane receptors | chemokine (C-X-C motif) receptor 3 | −2.09 | Mm.12876 |
| Signaling Intermediates | Ribosomal protein S12 | −2.32 | Mm.353923 |
| Sjogren syndrome antigen B | −2.72 | Mm.10508 | |
| Transport and Trafficing | apolipoprotein A-I | −2.68 | Mm.26743 |
| transient receptor potential cation channel, subfamily M, member 4 |
−2.04 |
Mm.349430 |
|
| Miscellaneous | metastasis associated lung | −2.15 | Mm.298256 |
| adenocarcinoma transcript 1 (non-coding RNA) gene |
|||
| Microcephaly, primary autosomal recessive 1 |
−6.99 |
Mm.235296 |
|
| Serine (or cysteine) proteinase inhibitor, clade A, member 3M |
−2.11 | Mm.379018 | |
All accession numbers from Unigene except for Entrez[A].
3.2. Changes in Protein Levels at Day 35
Day 35 neocortex showed a 22% significant decrease (p<0.041) in AQP4/β-actin values (Table 3; Figure 1A). Nucleolin/β-actin values increased by 40% (p<0.032) in the neocortex of exposed mice (Table 3; Figure 2A). In cerebellar tissues, none of the protein values were significantly different between sham-infected and virally-exposed offspring.
Table 3.
Descriptive Data for Aquaporin 4, Connexin 43, Microcephalin, Nucleolin, and β-Actin Protein Levels
| Area | Protein Level | Control (n=3) | Infected (n=3) | % Δ | p Value |
|---|---|---|---|---|---|
| Neocortex | Aquaporin/β-Actin | 0.107 ± .089 | 0.198 ± .12 | 85% ↑ | p < 0.34 |
| Day 0 | Microcephalin/β-Actin | 0.29 ± .12 | 0.16 ± .03 | 45% ↓ | p < 0.15 |
| Nucleolin/β-Actin | 0.032 ± .021 | 0.049 ± .005 | 53% ↑ | p < 0.25 | |
| β-Actin | 10.13 ± 1.57 | 10.25 ± 0.88 | 1% ↑ | p < 0.92 | |
| Cerebellum | Aquaporin/β-Actin | 1.15 ± .42 | 0.64 ± .44 | 44% ↓ | p < 0.21 |
| Day 35 | Connexin 43/β-Actin | 1.92 ± .30 | 1.72 ± .09 | 10% ↓ | p < 0.33 |
| Microcephalin/β-Actin | 0.64 ± .038 | 0.49 ± .097 | 23% ↓ | p < 0.067 | |
| Nucleolin/β-Actin | 0.038 ± .014 | 0.062 ± .014 | 63% ↑ | p < 0.11 | |
| β-Actin | 10.11 ± .81 | 9.85 ± 1.53 | 3% ↓ | p < 0.86 | |
| Neocortex | Aquaporin/β-Actin | 1.20 ± .052 | 0.94 ± .15 | 22% ↓ | p < 0.041 |
| Day 35 | Connexin 43/β-Actin | 1.42 ± .06 | 1.72 ± .25 | 21% ↑ | p < 0.11 |
| Microcephalin/β-Actin | 0.56 ± .069 | 0.47 ± .052 | 16% ↓ | p < 0.18 | |
| Nucleolin/β-Actin | 0.025 ± .005 | 0.035 ± .001 | 40% ↑ | p < 0.032 | |
| β-Actin | 10.63 ± .91 | 11.82 ± .96 | 11% ↑ | p < 0.19 | |
| Cerebellum | Aquaporin/β-Actin | 0.35 ± .20 | 0.10 ± .02 | 71% ↓ | p < 0.085 |
| Day 56 | Connexin 43/β-Actin | 0.98 ± .26 | 1.44 ± .62 | 47% ↑ | p < 0.31 |
| Microcephalin/β-Actin | 0.28 ± .02 | 0.19 ± .035 | 32% ↓ | p < 0.016 | |
| Nucleolin/β-Actin | 0.034 ± .013 | 0.081 ± .021 | 138% ↑ | p < 0.031 | |
| β-Actin | 13.37 ± .17 | 13.62 ± .92 | 2% ↑ | p < 0.67 | |
| Neocortex | Aquaporin/β-Actin | 0.78 ± .087 | 0.73 ± .02 | 6% ↓ | p < 0.42 |
| Day 56 | Connexin 43/β-Actin | 1.64 ± .06 | 1.31 ± .15 | 20% ↓ | p < 0.025 |
| Microcephalin/β-Actin | 0.41 ± .09 | 0.44 ± .05 | 7% ↑ | p < 0.59 | |
| Nucleolin/β-Actin | 0.036 ± .007 | 0.055 ± .004 | 53% ↑ | p < 0.014 | |
| β-Actin | 13.25 ± .70 | 13.20 ± .49 | 0.4% ↓ | p < 0.91 |
Figure 1.
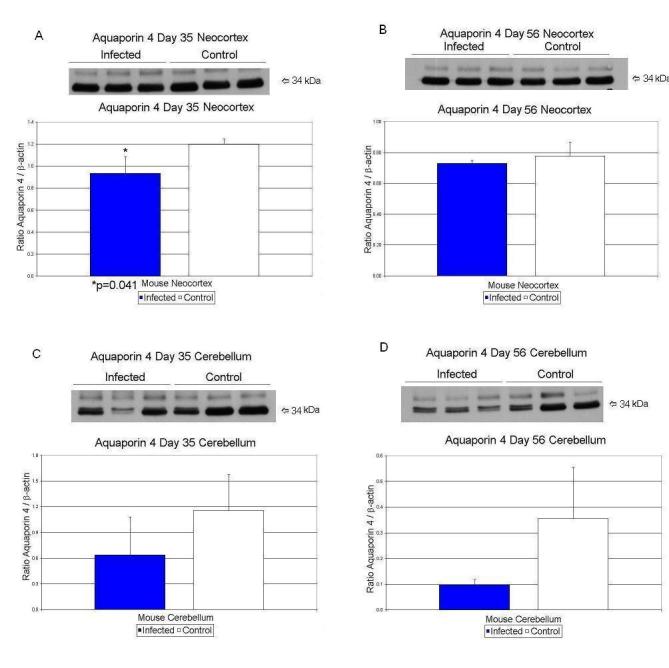
Effects of prenatal human influenza viral infection on expression of aquaporin 4 protein levels in neocortex (A, B) and cerebella (C, D) of mouse progeny on postnatal days 35 (A, C) and 56 (B, D).
Figure 2.
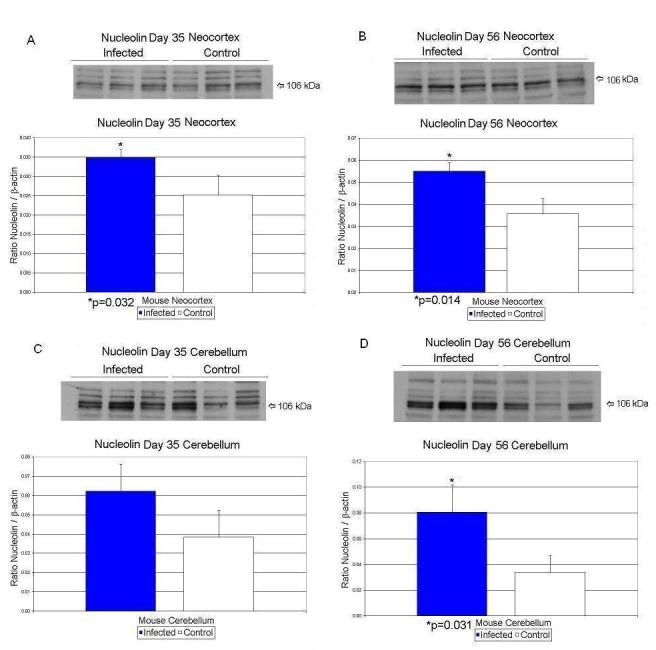
Effects of prenatal human influenza viral infection on expression of nucleolin protein levels in neocortex (A, B) and cerebella (C, D) of mouse progeny on postnatal days 35 (A, C) and 56 (B, D).
3.3. Changes in Protein Levels at Day 56
At day 56, CX43/β-actin values decreased significantly (p<0.025) by 20% in the exposed mice in neocortex (Table 3; Figure 3B). Nucleolin/β-actin values increased by 53% (p<0.014) in the neocortex of exposed mice (Table 3; Figure 2 B). At day 56, the exposed microcephalin/β-actin values decreased significantly (p<0.016) by 32% in cerebellar tissue when compared to controls (Table 3; Figure 4D). In cerebellar tissues, nucleolin/β-actin values rose by 138% (p<0.031) on day 56 in the exposed offspring (Table 3; Figure 2D).
Figure 3.
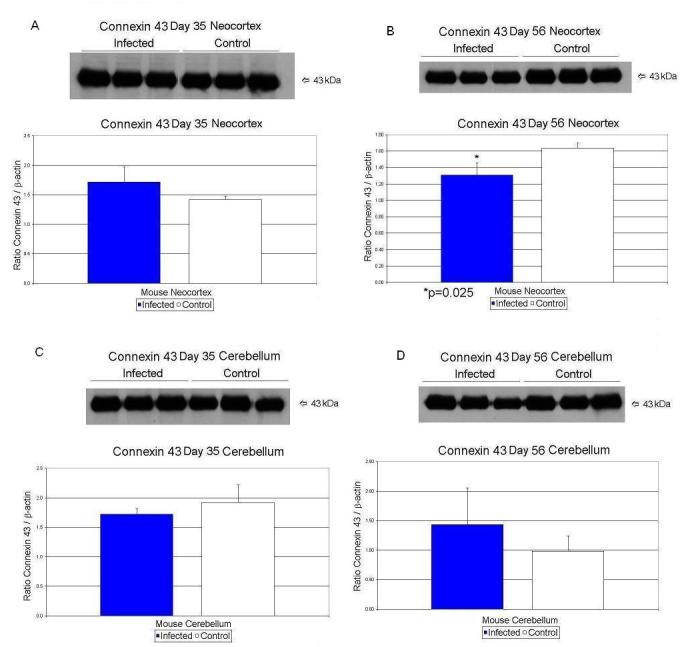
Effects of prenatal human influenza viral infection on expression of connexin 43 protein levels in neocortex (A, B) and cerebella (C, D) of mouse progeny on postnatal days 35 (A, C) and 56 (B, D).
Figure 4.
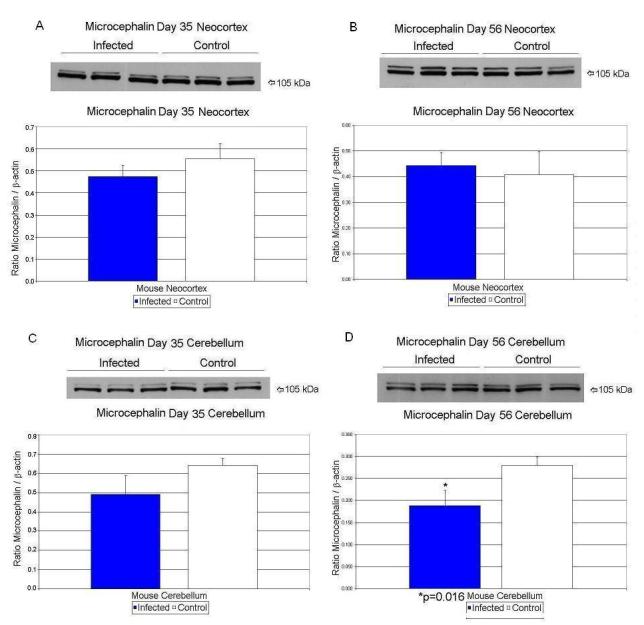
Effects of prenatal human influenza viral infection on expression of microcephalin protein levels in neocortex (A, B) and cerebella (C, D) of mouse progeny on postnatal days 35 (A, C) and 56 (B, D).
β-actin values did not differ significantly between exposed and control neocortex and cerebellar tissues (Table 1; Figure 5 A, B, C, D).
Figure 5.
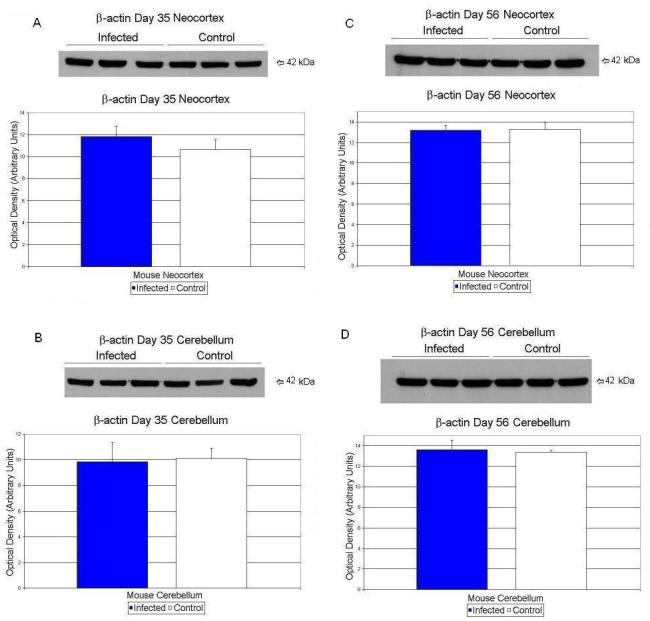
Effects of prenatal human influenza viral infection on expression of β-actin protein levels in neocortex (A, B) and cerebella (C, D) of mouse progeny on postnatal days 35 (A, C) and 56 (B, D).
4. Discussion
The current investigation provides further evidence that human influenza viral infection on day 9 of pregnancy in Balb/c mice causes significant alterations in gene expression and protein synthesis involving four major brain markers (nucleolin, microcephalin, aquaporin 4 and connexin 43) in the exposed offspring from postnatal day 0 through days 35 and 56. The presence of changes on days 35 and 56 suggest that long after the viral insult and long after any changes due to maternal-fetal cytokine production changes in brain gene expression persist. Absence of any significant change between β-actin protein product indicated the specificity of our protein levels of interest.
We present evidence that nucleolin protein levels are altered significantly at days 35 and 56 in neocortex of exposed animals. Nucleolin mRNA levels were significantly upregulated at day 35 in cerebellum while it was reduced at day 35 in neocortex. Additionally, there is also evidence that nucleolin levels continue to rise in the infected cerebellar tissues beyond day 35 with a significant increase at day 56, most likely due to the delay in cerebellar growth observed postnatally compared to the neocortex (Morgane et al. 1992).
Nucleolin is a major protein of nucleolus (Scheer and Hock, 1999) and is involved in ribosome biogenesis. It encompasses about 10% of the total nucleolar protein content and is highly phosphorylated, methylated and susceptible to ADP-ribosylation (Hiscox, 2002; Ginisty et al. 1999). Nucleolin may be involved in cleavage of rRNA, acts as a chaperone in correct folding of pre-rRNA processing, and in repression of transcription (Allain et al. 2000; Hiscox, 2002; Yang et al. 1994). The amount of nucleolin is stable in proliferative cells, but it undergoes self-cleavage in quiescent cells (Chen et al. 1991; Hiscox, 2002) and is also involved in process of cell growth and proliferation (Hiscox, 2002; Srivastava and Pollard, 1999).
Several viruses have been known to alter the distribution of nucleolin such as adenovirus, poliovirus, hepatitis delta virus, coxsackie B viruses, Maloney murine leukemia virus, and HIV (Hiscox, 2002). Occasionally, viral proteins may be anchored to nucleolin and the complex can be exchanged between nucleoplasm and nucleolus (Hiscox, 2002). Thus, interaction between viruses and nucleolin can lead to enhancement of viral replication via alterations in host cell transcription, translation, and cell cycle kinetics and contribute to autoimmune disease development (Hiscox, 2002). Increased expression of nucleolin in the brains of mice exposed to prenatal viral insult observed in the current report denotes a potential effect of the influenza virus on fetal brain cells or is due to maternal-fetal cytokine production.
Microcephalin protein levels were significantly reduced on day 56 in cerebellum while mRNA was reduced significantly at day 56 in neocortex. Microcephalin (MCPH1 or BRIT1) is a large, 835 amino acid protein which is expressed in the cerebral cortex of the fetal brain (Tang, 2006). Mutations of MCPH1 and/or related genes result in abnormalities in brain size, such as seen in primary microcephaly (Tang, 2006) where brain size is reduced to 1/3 of its normal size (Tang, 2006). Additionally, mental retardation is a major clinical finding in primary microcephaly (Tang, 2006). Thus, microcephalin has multiple important biological functions that implicate this protein in brain size regulation, cognition, and cancer (Bartek, 2006; Tang, 2006). Recent reports show that microcephalin is involved in regulation of unperturbed mitotic cell cycles such as proper execution of the intra-S phase and G2/M checkpoints in response to genotoxic stress such as ionizing radiation (Lin et al. 2005; Xu et al. 2004). Microcephalin-deficient cells exhibit abnormal G2-like lymphocytes that show elevated number of cells with prematurely-condensed chromosomes (Neitzel et al. 2002) which have not progressed normally into mitosis. Microcephalin has also recently been shown to be involved in centrosomal function potentially modulating the number of neurons generated by neuronal precursor cells (Zhong et al. 2006). Our current results show that human influenza viral infection either directly or indirectly caused a reduction of microcephalin mRNA in neocortex and protein in cerebellum on P56 which may lead to an increase in the number of highly immature neurons that might contribute to the macrocephaly observed in the adult virally exposed mouse offspring (Fatemi et al. 2002a) similar to same scenario in brains of subjects with autism (Casanova et al. 2002). Pyramidal cells in virally exposed adult brains exhibit cell atrophy and are increased numerically compared to sham-infected mice (Fatemi et al. 2002a), potentially due to reductions in microcephalin gene expression.
The association of macrocephaly with autism has been replicated in a number of studies, showing an average rate of macrocephaly of 20% (Fombonne et al. 1999). Recent data from 10 sites of the NICHD/NIDCD Collaborative Programs of Excellence in Autism showed a rate of absolute macrocephaly of 17.3% for autistics vs. 3% for normal controls (p<0.0004; Lainhart et al. 2006). In light of these findings along with our finding of macrocephaly in the offspring of prenatally infected mice (Fatemi et al. 2002a), the observed alterations in microcephalin expression have the potential to be important for future studies investigating microcephalin and autism.
AQP4 protein values showed a significant reduction at day 35. In the brain, AQP4 is strongly expressed in astrocytes and ependymal cells (Yang et al. 1995). Expression is strongest at sites of fluid transport including the pial and ependymal surfaces in contact with the cerebrospinal fluid (CSF), in the subarachnoid space, and the ventricular system (Rash et al. 1998) suggesting a role for AQP4 in movement of water between brain and CSF compartments (Verkman et al. 2006). Additionally, a role for AQP4 in the movement of water between blood and brain is suggested by polarized AQP4 expression found in astrocytic foot processes in direct contact with blood vessels (Verkman et al. 2006).
Increased AQP4 expression has been demonstrated in response to a number of pathological conditions marked by astrocytic activation and/or blood-brain barrier changes including gliomas (Papadopoulos et al. 2002), stroke (Papadopoulos et al. 2002), and HIV-related dementia (St Hilarie et al. 2005). Overexpression of AQP4 may cause an increased propensity for cerebral edema. Experiments using AQP4 knockout mice revealed that AQP4 deletion offered protection from cytotoxic brain edema (Papadopoulos et al. 2005) and vasogenic (non-cellular) brain edema (Papadopoulos et al. 2004). Our laboratory has observed a significant decrease in AQP4 in the cerebellum of patients with autism (Fatemi et al. unpublished observations).
We observed that AQP4 was initially increased at day 0 in neocortex but then decreased precipitously in postnatal days 35 and 56. Changes observed in AQP4 in the virally exposed animals may reflect brain edema causing alterations in cell morphology, and potential instability in F-actin molecules leading ultimately to downregulation in CX43 and cell-cell coupling (Nicchia et al. 2005).
CX43 protein levels in neocortex showed a significant drop in day 56 animals. Glial connexins have important functions in the brain (Theis et al. 2004), including long range signaling by Ca++ waves (Sohl et al. 2005), gap-junction-mediated intercellular communication between astrocytes, transport of nutrients from blood to neurons, K+ spatial buffering, glutamate uptake, and dissipation (Sohl et al. 2005; Theis et al. 2004). Additionally, connexins - especially CX43 - help in several other important functions, such as regulation of cell growth, interaction with β-catenin, localization to nucleus and affecting gene expression, and cell-cell adhesion (Theis et al. 2004). CX43 is widely present in the astrocytes of the adult brain (Sohl et al. 2005).
Connexin 43 upregulation has been identified in several neurological abnormalities, such as Parkinson's, Huntington's, Alzheimer's, seizure/epilepsy, transient brain ischemia, and facial nerve lesion (Rouach et al. 2002). Interestingly, our laboratory has observed a significant increase in CX43 levels in frontal cortex of autistic subjects (Fatemi et al. unpublished observations). In contrast, CX43 downregulation has been seen with severe brain ischemia, prolonged exposure to IL-1, mechanical lesions of the visual cortex, exposure to NMDA agonists, and gliomas (Rouach et al. 2002). Examination of the neocortex of CX43 null mutant mice showed delays in neuronal migration (Fushiki et al. 2003). CX43 expression was correlated inversely with cell proliferation activity during postnatal development (Miragall et al. 1996). Activated microglial cells release cytokines like IL-Iβ, and TNF-α and downregulate CX43 in cultures of astrocytes exposed to bacterial lipoplysaccarides (Meme et al. 2006). Finally, mice with astrocyte-directed inactivation of CX43 exhibit increased exploratory behavior, impaired motor capacities, and change in brain acetylcholine levels (Frisch et al. 2003). Our observed decrease in CX43 protein levels of exposed mice suggests increased cell proliferation and decreased cell-cell communication, and cell-cell coupling in the growing brains of the virally exposed mice.
Previous work in our laboratory (Fatemi et al. 1999; Fatemi et al. 2002a) has shown a deleterious viral effect during E9 which leads to abnormal corticogenesis and changes in levels of several neuroregulatory proteins in postnatal mice. E9 in the mouse coincides with the start of several important events in brain development, i.e., early neural crest cell migration to the presumptive craniofacial structures, onset of neurogenesis and neuronal migration, gliogenesis, and early synaptogenesis (Acuff-Smith and Vorhees, 1999). Moreover, E9 in mouse is equal to late 1st trimester in man (Rodier, 1980). Recent reports by Brown et al. (2004) and Buka et al. (2001) have demonstrated serological evidence that human influenza virus and herpes virus infection, respectively, during pregnancy are linked to the development of schizophrenia in offspring. Brown's study is particularly important as the infection occurred during the first trimester of pregnancy (similar to E9 in mouse) with no increased risk of schizophrenia with influenza during the second and third trimesters (Brown et al. 2004). More recently, Lloyd et al (2007) reported on minor physical abnormalities (MPAs) associated with individuals with first-episode psychosis (schizophrenia, affective psychosis, drug-induced psychosis). Lloyd found that overall facial asymmetry, asymmetry of the orbital landmarks, as well as other abnormalities distinguished patients with schizophrenia and affective psychosis from controls, suggesting an insult during organogenesis in the first trimester of pregnancy (Lloyd et al., 2007). Despite these findings most of the epidemiological evidence points to infection during the 2nd trimester to be responsible for the development of schizophrenia in offspring (Mednick et al. 1988; Barr et al. 1990; Mednick et al. 1994; Kungi et al. 1995).
It has previously been demonstrated in this model (Shi et al. 2003a) that prenatal viral infection on E9 leads to increased anxiety and deficits in PPI in the acoustic startle response similar to what has been observed in schizophrenics and autistic subjects (Geyer and Braff, 1982; Perry et al. 2007). Treatment of pregnant mice with a viral mimic – polyriboinosinic polyribocytidilic acid (PolyI:C), at critical stages during pregnancy in mice, can cause deleterious effects in brains of offspring and behavioral pathology (Meyer et al. 2005; Meyer et al. 2006a; Meyer et al 2006b; Meyer et al. 2007a; Meyer et al. 2007b) similar to what we have observed in our model. These investigators have argued that these results are due to maternal cytokine upregulation (Meyer et al. 2006; Shi et al. 2003b). We believe that the changes in nucleolin expression may indicate a potential direct viral effect on the exposed offspring. However, did not detect the presence of the pathogen in the offspring's brain and we do not exclude the possibility that it may be due to maternal-fetal cytokine production.
In conclusion, we have presented evidence that prenatal infection on day 9 of pregnancy (Figure 6) causes significant changes in four key proteins involved in cell-cell coupling, cell communication, and cell proliferation. Two of these proteins, AQP4 and CX43, are likewise altered in autistic brain (Fatemi et al. unpublished observations) and a third may be implicated in the macrocephaly, which is commonly observed in autistic subjects (Fombonne et al. 1999; Lainhart et al. 2006) as well as in our animal model (Fatemi et al. 2002a). Most importantly, the weight of accumulated evidence so far showing presence of gliosis (Fatemi et al. 2002b), pyramidal cell atrophy (Fatemi et al. 2002a), and upregulation of nucleolin, as well as previous supportive evidence (Aronsson et al. 2001; Aronsson et al. 2002), showing persistence of influenza viral RNA in brain implicate a potential viral effect on the developing fetal brain.
Figure 6.
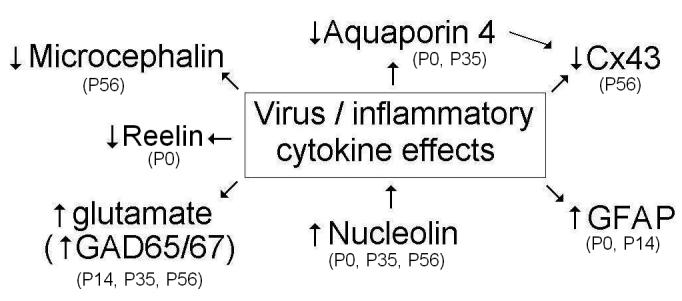
Prenatal viral infection on day 9 of pregnancy causes abnormal brain structure and function in adult Balb/c mice including: 1) apoptotic change in exposed mice as seen by increased pyramidal cell atrophy and increased neuronal density (Fatemi et al. 2002a); 2) migrational abnormalities as seen by increased GFAP, decreased Reelin early thinning and enlargement of brain; and 3) abnormal cell (neurons and astrocytes) proliferation as seen by decreased microcephalin and connexin 43 expression and by increased nucleolin and GFAP expression. Please note that postnatal dates denote significant changes in levels of various mRNAs or proteins.
Supplementary Material
Acknowledgments
Grant support by National Institute of Child Health and Human Development (#1R01 HD046589-01A2) to SHF and from the virology branch NIAID, NIH (N01-AI-15435) to RWS is gratefully acknowledged.
Role of Funding Source
Funding for this study was provided by NICHHD grant 1R01 HD046589-01A2 (SHF) and virology branch NIAID grant N01-AI-15435 (RWS). NICHHD and NIAID. Funding sources had no further role in study design; in the collection, analysis and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta MT, Pearl PL. The neurobiology of autism. New pieces of the puzzle. Curr. Neurol. Neurosci. Therp. 2003;3(2):149–156. doi: 10.1007/s11910-003-0067-0. [DOI] [PubMed] [Google Scholar]
- Acuff-Smith KD, Vorhees CV. Neurobehavioral teratology. In: Niesink RJM, Jaspers RMA, Kornet LMW, VanRee JM, Tilson HA, editors. Introduction to Neurobehavioral Toxicology, Food and Environment. CRC Press; Boca Raton, FL: 1999. pp. 26–69. [Google Scholar]
- Allain FH, Bouvet P, Dieckmann T, Feigon J. Molecular basis of sequence-specific recognition of pre-ribosomal RNA by nucleolin. EMBO J. 2000;19(24):6870–6881. doi: 10.1093/emboj/19.24.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) APA; Washington D.C.: 1994. [Google Scholar]
- Andreasen NC. A unitary model of schizophrenia. Bleuler's “Fragmented phrene” as schizencephaly. Arch. Gen. Psychiatry. 1999;56(9):781–793. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- Aronsson F, Karlsson H, Ljunggren HG, Kristensson K. Persistence of the influenca A/WSN/33 virus RNA at midbrain levels of immunodefective mice. J. Neurovirol. 2001;7(2):117–124. doi: 10.1080/13550280152058771. [DOI] [PubMed] [Google Scholar]
- Aronsson F, Lannebo C, Paucar M, Brask J, Kristensson K, Karlson H. Persistence of viral RNA in the brain of offspring to mice infected with influenza A/WSN/33 virus during pregnancy. J. Neurovirol. 2002;8(4):353–357. doi: 10.1080/13550280290100480. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends. Neurosci. 2002;25(10):518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CE, Mednick SA, Munk-Jorgensen P. Exposure to influenza epidemics during gestation and adult schizophrenia: a 40-year study. Arch. Gen. Psychiatry. 1990;47(9):869–874. doi: 10.1001/archpsyc.1990.01810210077012. [DOI] [PubMed] [Google Scholar]
- Bartek J. Microcephalin guards against small brains, genetic instability, and cancer. Cancer Cell. 2006;10(2):91–93. doi: 10.1016/j.ccr.2006.07.014. 2006. [DOI] [PubMed] [Google Scholar]
- Boksa P, Luheshi GN. On the use of animal modeling to study maternal infection during pregnancy and prenatal cytokine exposure as risk factors for schizophrenia. Clin. Neurosci. 2003;3:339–346. [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry. 2004;61(8):774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch. Gen. Psych. 2001;58(11):1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- Chen CM, Chang SY, Yeh NH. Increased stability of nucleolin in proliferating cells by inhibition of its self-cleaving activity. J. Biol. Chem. 1991;266(12):7754–7758. [PubMed] [Google Scholar]
- Chess S. Autism in children with congenital rubella. J. Autism Child. Schizophr. 1971;1(1):33–47. doi: 10.1007/BF01537741. [DOI] [PubMed] [Google Scholar]
- Chess S, Fernandez P, Dorn S. Behavioral consequences of congenital rubella. J. Pediatr. 1978;93(4):699–703. doi: 10.1016/s0022-3476(78)80921-4. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Curran T. Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J. Neurosci. 1997;17(1):23–31. doi: 10.1523/JNEUROSCI.17-01-00023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, Shier A, Sheikh S, Bailey K. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol. Psychiatry. 1999;4(2):145–154. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle J, Kanodia R, Kist D, Patterson P, Shi L, Sidwell RW. Prenatal viral infection causes macrocephaly and pyramidal cell atrophy in the developing mice. Cell. Mol. Neurobiol. 2002a;22(1):25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Sidwell RW, Kist DA, Stary JM, Earle JA, Thuras P. Human influenza viral infection in utero alters glial fibrillary acidic protein immunoreactivity in the developing brains of neonatal mice. Mol. Psychiatry. 2002b;7(6):633–640. doi: 10.1038/sj.mp.4001046. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Pearce DA, Brooks AI, Sidwell RW. Prenatal viral infection in mouse causes differential expression of genes in brains of mouse progeny: A potential animal model for schizophrenia and autism. Synapse. 2005;57(2):91–99. doi: 10.1002/syn.20162. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Bell C, Nos L, Fried P, Pearce DA, Singh S, Siderovski DS, Willard F, Fukuda M. Chronic olanzapine treatment causes differential expression of genes in frontal cortex of rats as revealed by DNA microarray technique. Neuropsychopharmacology. 2006;31(9):1888–1899. doi: 10.1038/sj.npp.1301002. [DOI] [PubMed] [Google Scholar]
- Fatemi SH. The role of neurodevelopmental genes in infectious etiology of autism. Am. J. Biochem. Biotech. 2007 In Press. [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Smee D, Sidwell RW, Pearce DA. Viral infection and abnormal brain development: a DNA microarray study. FASEB J. 2007a;21(5):622.12. [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Sidwell RW. The role of cerebellar genes in pathology of autism and schizophrenia. Cerebellum. 2007b doi: 10.1007/s12311-008-0017-0. In Press. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Roge B, Claverie J, Courty S, Fremolle J. Microcephaly and macrocephaly in autism. J. Autism Dev. Disord. 1999;29(2):113–119. doi: 10.1023/a:1023036509476. [DOI] [PubMed] [Google Scholar]
- Fombonne E. The prevalence of autism. JAMA. 2003;289(1):87–89. doi: 10.1001/jama.289.1.87. [DOI] [PubMed] [Google Scholar]
- Frisch C, Theis M, De Souza-Silva MA, Dere E, Sohl G, Teubner B, Namestova K, Willecke K, Huston JP. Mice with astrocyte-directed inactivation of connexin43 exhibit increased exploratory behaviour, impaired motor capacities, and changes in brain acetylcholine levels. Eur. J. Neurosci. 2003;18(8):2313–2318. doi: 10.1046/j.1460-9568.2003.02971.x. [DOI] [PubMed] [Google Scholar]
- Fushiki S, Perez-Velazquez JL, Zhang L, Bechberger JF, Carlen PL, Naus CC. Changes in neuronal migration in neocortex of connexin43 null mutant mice. J. Neuropathol. Exp. Neurol. 2003;62(3):304–314. doi: 10.1093/jnen/62.3.304. [DOI] [PubMed] [Google Scholar]
- Geyer M, Braff DL. Habituation of the blink reflex in normals and schizophrenic patients. Psychophysiol. 1982;19:1–16. doi: 10.1111/j.1469-8986.1982.tb02589.x. [DOI] [PubMed] [Google Scholar]
- Ginisty H, Sicard H, Roger B, Bouvet P. Structure and functions of nucleolin. J. Cell Sci. 1999;112(Pt 6):761–772. doi: 10.1242/jcs.112.6.761. [DOI] [PubMed] [Google Scholar]
- Harr B, Schlötterer C. Comparison of algorithms for the analysis of Affymetrix microarray data as evaluated by co-expression of genes in known operons. Nucleic Acids Res. 2006;23(2):e8. doi: 10.1093/nar/gnj010. 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscox JA. The nucleolus—a gateway to viral infection? Arch. Virol. 2002;147(6):1077–1089. doi: 10.1007/s00705-001-0792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. Developmental neurotoxicity. In: Krinke GJ, editor. The Laboratory Rat. Academic Press; New York: 2000. pp. 227–250. [Google Scholar]
- Kunugi H, Nanko S, Takei N, Saito K, Hayashi N, Kazamatsuri H. Schizophrenia following in utero exposure to the 1957 influenza epidemics in Japan. Am. J. Psychiatry. 1995;152(3):450–452. doi: 10.1176/ajp.152.3.450. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, Deutsch CK, Dunn M, Estes A, Tager-Flusberg H, Folstein S, Hepburn S, Hyman S, McMahon W, Minshew N, Munson J, Osann K, Ozonoff S, Rodier P, Rogers S, Sigman M, Spence MA, Stodgell CJ, Volkmar F. Head circumference and height in autism: a study by the Collaborative Program of Excellence in Autism. Am. J. Med. Genet. A. 2006;140(21):2257–2274. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Rai R, Li K, Xu ZX, Elledge SJ. BRIT1/MCPH1 is a DNA damage responsive protein that regulates the Brca1-Chk1 pathway, implicating checkpoint dysfunction in microcephaly. Proc. Natl. Acad. Sci. USA. 2005;102(42):15105–15109. doi: 10.1073/pnas.0507722102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd T, Dazzan P, Dean K, Park SB, Frearon P, Doody GA, Tarrant J, Morgan KD, Morgan C, Hutchinson G, Leff J, Harrison G, Murray RM, Jones PB. Minor physical anomalies in patients with first-episode psychosis: their frequency and diagnostic specificity. Psychol. Med. 2007 doi: 10.1017/S0033291707001158. 2007. In press. [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Pemberton M, Welham JL, Muray RM. Schizophrenia and the influenza epidemics of 1954, 1957 and 1959: a Southern hemisphere study. Schizphr. Res. 1994;14(1):1–8. doi: 10.1016/0920-9964(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch. Gen. Psychiatry. 1988;45(2):189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- Mednick SA, Huttunen MO, Machon RA. Prenatal influenza infections and adult schizophrenia. Schizophr. Bull. 1994;20(2):263–267. doi: 10.1093/schbul/20.2.263. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schwendener S, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci. Biobehav. Rev. 2005;29(6):913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J. Neurosci. 2006a;26(18):4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schwendener S, Yee BK. Immunological stress at the maternal-foetal interface: a link between neurodevelopment and adult psychopathology. Brain. Behav. Immun. 2006b;2006(4):378–388. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol. Psychiatry. 2007a doi: 10.1038/sj.mp.4002042. In Press. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J. Relative Prenatal and Postnatal Maternal Contributions to Schizophrenia-Related Neurochemical Dysfunction after In Utero Immune Challenge. Neuropsychopharmacology. 2007b doi: 10.1038/sj.npp.1301413. In Press. [DOI] [PubMed] [Google Scholar]
- Meme W, Calvo CF, Froger N, Ezan P, Amigou E, Koulakoff A, Giaume C. Proinflammatory cytokines released from microglia inhibit gap junctions in astrocytes: potentiation by beta-amyloid. FASEB J. 2006;20(3):494–496. doi: 10.1096/fj.05-4297fje. [DOI] [PubMed] [Google Scholar]
- Miragall F, Simburger E, Dermeitzel R. Mitral and tufted cells of the mouse olfactory bulb possess gap junctions and express connexin43 mRNA. Neurosci. Lett. 1996;216(3):199–202. doi: 10.1016/0304-3940(96)13042-1. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Austin-LaFrance RJ, Bronzino JD, Tonkiss J, Galler JR. Malnutrition and the developing central nervous system. In: Isaacson RL, Jensen RF, editors. The Vulnerable Brain and Environmental Risks, Volume 1: Malnutrition and Hazard Assessment. Plenum Press; New York: 1992. pp. 3–44. [Google Scholar]
- Morgane PJ, Mokler DJ, Galler JR. Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci. Biobehav. Rev. 2002;26(4):471–483. doi: 10.1016/s0149-7634(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Neitzel H, Neumann LM, Schindler D, Wirges A, Tonnies H, Trimborn M, Krebsova A, Richter R, Sperling K. Premature chromosome condensation in humans associated with microcephaly and mental retardation: a novel autosomal recessive condition. Am. J. Hum. Genet. 2002;70(4):1015–1022. doi: 10.1086/339518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchia GP, Srinivas M, Lei W, Brosnan CF, Frigeri A, Spray D,C. New possible roles for aquaporin-4 in astrocytes: cell cytoskeleton and functional relationship with connexin43. FASEB J. 2005;19(12):1674–1676. doi: 10.1096/fj.04-3281fje. [DOI] [PubMed] [Google Scholar]
- Nyffeler M, Meyer U, Yee BK, Feldon J, Knuesel I. Maternal immune activation during pregnancy increases limbic GABAA receptor immunoreactivity in the adult offspring: implications for schizophrenia. Neuroscience. 2006;143(1):51–62. doi: 10.1016/j.neuroscience.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Krishna S, Verkman AS. Aquaporin water channels and brain edema. Mt. Sinai. J. Med. 2002;69(4):242–248. [PubMed] [Google Scholar]
- Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18(11):1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Verkman AS. Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J. Biol. Chem. 2005;280(14):13906–13912. doi: 10.1074/jbc.M413627200. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biol. Psychiatry. 2007;61(4):482–486. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc. Natl. Acad. Sci. USA. 1998;95(20):11981–11986. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM. Chronology of neuron development: animal studies and their clinical implications. Dev. Med. Child Neurol. 1980;22(4):525–545. doi: 10.1111/j.1469-8749.1980.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum. Dev. 1991;26(1):61–67. doi: 10.1016/0378-3782(91)90044-4. [DOI] [PubMed] [Google Scholar]
- Rouach N, Avignone E, Meme W, Koulakoff A, Venance L, Blomstrand E, Giaume C. Gap junctions and connexin expression in the normal and pathological central nervous system. Biol. Cell. 2002;94(78):457–475. doi: 10.1016/s0248-4900(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Scheer U, Hock R. Structure and function of the nucleolus. Curr. Opin. Cell Biol. 1999;11(3):385–892. doi: 10.1016/S0955-0674(99)80054-4. [DOI] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci. 2003a;23(1):297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int. J. Dev. Neurosci. 2003b;23:299–305. doi: 10.1016/j.ijdevneu.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Sohl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat. Rev. Neurosci. 2005;6(3):191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Fleming PJ, Pollard HB, Burns AL. Cloning and sequencing of the human nucleolin cDNA. FEBS Lett. 1989;250(1):99–105. doi: 10.1016/0014-5793(89)80692-1. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Pollard HB. Molecular dissection of nucleolin's role in growth and cell proliferation: new insights. FASEB J. 1999;13(14):1911–1922. [PubMed] [Google Scholar]
- Hilarie C, Vargas D, Prado CA, Gincel D, Mann J, Rothstein JD, McArthur JC, Conant K. Aquaporin 4 is increased in association with human immunodeficiency virus dementia: implications for disease pathogenesis. J. Neurovirol. 2005;11(6):535–543. doi: 10.1080/13550280500385203. [DOI] [PubMed] [Google Scholar]
- Stead JD, Neal C, Meng F, Wang Y, Evans S, Vazquez DM, Watson SJ, Akil H. Transcriptional profiling of the developing rat brain reveals that the most dramatic regional differentiation in gene expression occurs postpartum. J. Neurosci. 2006;26(1):345–353. doi: 10.1523/JNEUROSCI.2755-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs EG, Ash E, Williams CPS. Autism and congenital cytomegalovirus. J. Autism Dev. Disord. 1984;14(2):183–189. doi: 10.1007/BF02409660. [DOI] [PubMed] [Google Scholar]
- Susser ES, Brown AS, Gorman AS. Prenatal Exposure in Schizophrenia. American Psychiatry Press; Washington DC: 1999. [Google Scholar]
- Tang BL. Molecular genetic determinants of human brain size. Biochem. Biophys. Res. Comm. 2006;345:911–916. doi: 10.1016/j.bbrc.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Theis M, Speidel S, Willecke K. Astrocyte cultures from conditional connexin43-deficient mice. Glia. 2004;46(2):130–141. doi: 10.1002/glia.10350. [DOI] [PubMed] [Google Scholar]
- Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim. Biophys. Acta. 2006;1758(8):1085–1093. doi: 10.1016/j.bbamem.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Xu X, Lee J, Stern DF. Microcephalin is a DNA damage response protein involved in regulation of CHK1 and BRCA1. J. Biol. Chem. 2004;279(33):34091–34094. doi: 10.1074/jbc.C400139200. [DOI] [PubMed] [Google Scholar]
- Yamagishi H, Garg V, Matsuoka R, Thomas T, Srivastava D. A molecular pathway revealing a genetic basis for human cardiac and craniofacial defects. Science. 1999;283(5405):1158–1161. doi: 10.1126/science.283.5405.1158. [DOI] [PubMed] [Google Scholar]
- Yang B, Ma T, Verkman AS. cDNA cloning, gene organization, and chromosomal localization of a human mercurial insensitive water channel. Evidence for distinct transcriptional units. J. Biol. Chem. 1995;270(39):22907–22913. doi: 10.1074/jbc.270.39.22907. [DOI] [PubMed] [Google Scholar]
- Yang TH, Tsai WH, Lee YM, Lei HY, Lai MY, Chen DS, Yeh NH, Lee SC. Purification and characterization of nucleolin and its identification as a transcription repressor. Mol. Cell. Biol. 1994;14(9):6068–6074. doi: 10.1128/mcb.14.9.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Pfeifer GP, Xu X. Microcephalin encodes a centrosomal protein. Cell Cycle. 2006;5(4):457–458. doi: 10.4161/cc.5.4.2481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


