Abstract
The efficacy of vaccine adjuvants can be influenced by the immunological environment of the host, depending on the mechanism(s) by which they exert their immunopotentiating activities. Interleukin-6 is a pleiotropic cytokine that has a broad range of biological activities on immune and non-immune cells. We investigated the role of IL-6 on the ability of nine adjuvant formulations to induce antibody responses to the P. falciparum MSP1-19 malaria vaccine, using IL-6 -/- (KO) mice. Results showed that some adjuvants, ie. MPL-SE, CFA/IFA, ISA720/QS21/MPL, depended on IL-6 for their efficacy, while others exhibited increased potency in its absence. The efficacy of adjuvants in the IL-6 KO environment cannot be solely attributed to their ability to stimulate antigen-specific cellular responses, suggesting that other biological activities of IL-6 are also important. The results further suggest that two adjuvants utilized dissimilar pathways to potentiate the same type of immune response.
INTRODUCTION
It is widely recognized that immunological adjuvants will play a crucial role on the efficacy of many subunit vaccines against infectious diseases. Adjuvants have known to affect vaccine-induced immune responses in terms of magnitude; types of immunity, eg. TH1/TH2, CTL, antibodies (reviewed in [1-3]; as well as the specificity of epitope recognition [4-6]. On the other hand, the immunological background of the host may have reciprocal effects on the activities of vaccine adjuvants, which in turn may influence efficacy. There are many circumstances in which the host’s immunological environment is altered. These may include various forms of inherent immunodeficiencies; altered immune status due to aging [7-14], as well as results of prior and/or concurrent infections [15-22]. Previously, we have shown that a number of adjuvant formulations differ in their ability to potentiate the immunogenicity of a malaria vaccine under the altered immunological environment created by Interferon-γ and Interleukin-4 knockouts [23]. While these studies focused on the development of TH1/TH2 type responses, there are other key immune mediators that have been shown to have broad ranges of effects on the development of immunity. The cytokine, Interleukin 6, has been extensively studied and shown to have pleiotropic activity on a broad range of immune and hematopoietic cells (reviewed in [24, 25]. These include activity on B cell stimulation and the development of primary antibody responses; T cell activation, growth and differentiation; hematopoiesis including the growth and differentiation of bone marrow cells such as macrophages, dendritic cells, and megakaryocytes; as well as a critical role in the regulatory function of T(reg) cells [26]. Since adjuvant formulations have differential effects on immune cells leading to various unique immunological cascades, and since IL-6 plays a central role in many immunological processes; we hypothesize that the efficacy of adjuvants may be dependent on IL-6 mediated immuno-biological pathways. In the present study, we investigated the effects of IL-6 knockout (KO) on the ability of nine different adjuvant formulations to induce antibody and cellular responses to a blood-stage malaria vaccine based on the P. falciparum Merozoite Surface Protein 1, MSP1-19. Results showed that some adjuvant formulations were dependent on IL-6 to exert their full potency; whereas other formulations were more active in the IL-6 KO environment. Specific constituents in the adjuvant formulations were shown to have an effect on IL-6 dependency in the development of antibody responses to MSP1-19. Our data further showed that the requirement of IL-6 for adjuvanticity is not strictly attributable to the induction of antigen-specific cellular responses. The preliminary studies detailed here represent the first of a series of investigations into the role of IL-6 on adjuvant efficacy.
MATERIALS AND METHODS
Malaria vaccine antigen
The C-terminal 19 kDa fragment of Plasmodium falciparum Merozoite Surface Protein 1, MSP1-19 was used as the immunogen. The recombinant protein was expressed in Pichia pastoris as a fusion protein with the P30 and P2 universal T epitopes [27]. The inclusion of the universal T helper epitopes was to insure that any observed differences in immunogenicity is not due to preferential recognition of T epitopes regulated by immune response (IR) genes [28]. The purification of P30P2MSP1-19 was described previously [27], and this antigen was a kind gift from Dr. Anthony Stowers.
Adjuvant formulations
The following adjuvants were used. Montanide ISA720, a metabolizable oil adjuvant (Seppic Inc. Fairfield, NJ)[29]; MF59, squalene/oil emulsion (Chiron Corp. Emeryville, CA)[30]; QS21, a saponin derivative (Antigenics Inc. Lexington, MA)[31]; MPL (from E. coli F583 Rd mutant, Sigma-Aldrich, St Louis, MO); MPL-AF, monophosphoryl lipid A in aqueous formulation (Corixa Inc. Seattle, WA)[32]; MPL-SE, monophosphoryl lipid A in squalene emulsion (Corixa Inc. Seattle WA)[33]; and Freund’s Adjuvants, CFA/IFA (Gibco, Grand Island, NY). Additional formulations comprised of combinations of above adjuvants were also used, ISA720/MPL, ISA720/QS21, ISA720/QS21/MPL. This is based on combining the carrier-type adjuvant (ISA720) with the immunomodulators. Due to stringent MTA (Material Transfer Agreement) restrictions, we were not able to study the combined formulations of QS21 with MPL-AF or with MPL-SE, or ISA720 with MPL-AF; instead, commercially available MPL (from E. coli F583 Rd mutant, Sigma-Aldrich, St Louis, MO) was used in the combined formulations. Similarly, since MTA prohibits combining QS21 with MF59, only the ISA720 was used.
Formulation of antigen and adjuvants, and dosing
Each dose of P30P2MSP1-19 (MSP1-19) is 10 ug. The antigen was diluted in PBS (pH 7.0 or pH 6.8 for QS21 preparations). For MSP1-19/QS21, 10 ug of antigen was diluted in PBS (pH 6.8) and reconstituted with 5 ug QS21 to 100 ul. For MSP1-19/MF59, 10 ug MSP1-19 was vortexed with MF59 at a volume ratio of 1:1 in a final volume of 100 ul. For MSP1-19/MPL-SE, 10 ug of MSP1-19 in 150 ul PBS was vortexed with 50 ul MPL-SE (MPL content at 1000ug/ml). For MSP1-19/MPL-AF, 10 ug of MSP1-19 was mixed with 50 ug MPL-AF to a final volume of 100 ul. For MSP1-19/ISA720, 10 ug MSP1-19 was emulsified with ISA720 at a volume ratio of 7:3 (oil:water) in a total volume of 100 ul. Formulations of ISA720/MPL, ISA720/QS21, ISA720/QS21/MPL were similarly emulsified with MSP1-19 except that 50 ug of MPL and/or 5 ug of QS21 was reconstituted with the antigen before mixing with ISA720. For CFA/IFA, 10 ug of MSP1-19 was emulsified with an equal volume of CFA or IFA to give a final volume of 100 ul per dose.
Immunization regimen
Mice (5 per group) were immunized intraperitoneally with MSP1-19 (P30P3MSP1-19) in different adjuvant formulations as described above. A total of three immunizations were given at 4 weeks interval. Mice were bled one week before the first and three weeks after the last immunization.
Mice
IL-6 deficient mice (IL-6 KO, B6 background, female, 8-12 weeks old) were purchased from Jackson Lab. (Bar Harbor, Maine). C57Bl6 mice were used as Wild-Type (WT) controls.
Serum antibody assays
Antibody responses to MSP1-19 in the immunized mice were evaluated by ELISAs as described [5, 23]. Briefly, ELISA plates (Costar/Corning, Acton, MA) were coated with MSP1-19 [34] at 0.4ug/mL and blocked with 1% Bovine Serum Albumin (BSA) in Borate Buffered Saline (BBS). Test sera were serially diluted in 1% BSA/0.5% yeast extract (DIFCO/BD Biosciences, San Jose, CA) in BBS. Diluted sera were incubated in MSP1-19 coated wells for 60 min., washed with BBS, and then incubated for 60 min with horseradish peroxidase (HRP) conjugated, goat anti-mouse antibodies at a 1/1000 dilution (H & L chain specific, Kirkgaard and Perry Laboratories). Peroxidase substrates, H202 and 2,2’azinobis (3-ethylbenzthiazolinesulfonic acid)/ABTS (Kirkgaard and Perry Laboratories, Gaithersburg, MD). The optical density (O.D.) was determined at 410nm. End-point titers were calculated as the reciprocal serum dilutions giving an O.D. of 0.2, which is 4 fold greater than the mean O.D. value of baseline sera. The isotypes of the anti-MSP1-19 antibodies were determined by ELISA as described [23]. Mouse sera were diluted to 1/500, and HRP-conjugated, goat anti-mouse IgG1, IgG2a, IgG3, and IgM (SouthernBiotech Inc., Birmingham, AL) at a 1/4000 dilution were used as secondary antibodies.
Antigen stimulated ELISPOT assays
IFN-γ and IL-4 ELISPOT assays were performed using splenocytes from immunized mice as described [35-38]. Briefly, 96 wells multiscreen filter plates (Milipore Inc., Bedford, MA) were coated with monoclonal antibodies to mouse IFN-γ (R4-6A2, 10ug/ml) and IL-4 (11B11, 5 ug/l) (BD Biosciences, San Diego, CA) overnight at room temp. Plates were washed and further incubated with DMEM/10% Fetal Bovine Serum at 37°C for 1 hr. Spleens from immunized mice were removed 5 days after the last immunization and single cell suspensions were prepared. Triplicate wells were plated with 2.5 × 105 splenocytes/well and P30P2MSP1-19 was added at a final concentration of 2 ug/ml. This antigen concentration was previously determined to be optimal from a dose range of 1 ug to10 ug. Positive Control wells were incubated with phorbol myristate acetate (PMA, 5 ng/ml) and ionomycin (1 ng/ml). Negative Control wells were incubated with growth medium alone. After a 48 hr. incubation at 37°C in 5% CO2, wells were washed, incubated with biotinylated monoclonal anti- IFN-γ (XMG1.2, 2 ug/ml) and anti-IL-4 (BVD6-24G2, 1 ug/ml) antibodies (BD Biosciences, San Diego, CA). Wells were then washed, incubated in a 1/800 dillution of peroxidase labeled strepstravidin (Kirkgaard and Perry Laboratories, Gaithersburg, MD), and spots were developed in a solution of 3’3’-diaminobenzidine tetrahydrochloride, DAB and 30% H2O2 (Sigma-Aldrich, St. Louis, MO). Spots were enumerated microscopically.
RESULTS
Immunogenicity of P30P2MSP1-19 potentiated by different adjuvant formulations in the IL-6 deficient (KO) environment
Figure 1 shows the ELISA antibody titers to MSP1-19 of sera from IL-6 KO and Wild-Type (WT) mice immunized with various adjuvant formulations. MSP1-19 specific antibody titers of IL-6 KO mice receiving MPL-SE and ISA720/QS21/MPL were significantly lower than the respective WT controls. In contrast, IL-6 KO mice receiving MF59 and ISA720 had antibody titers significantly higher than WT mice. Of note is that these two adjuvant formulations do not contain immunomodulators. There were no significant differences in the antibody titers between the IL-6 KO and WT mouse groups for the remaining five adjuvant formulations.
Figure 1.
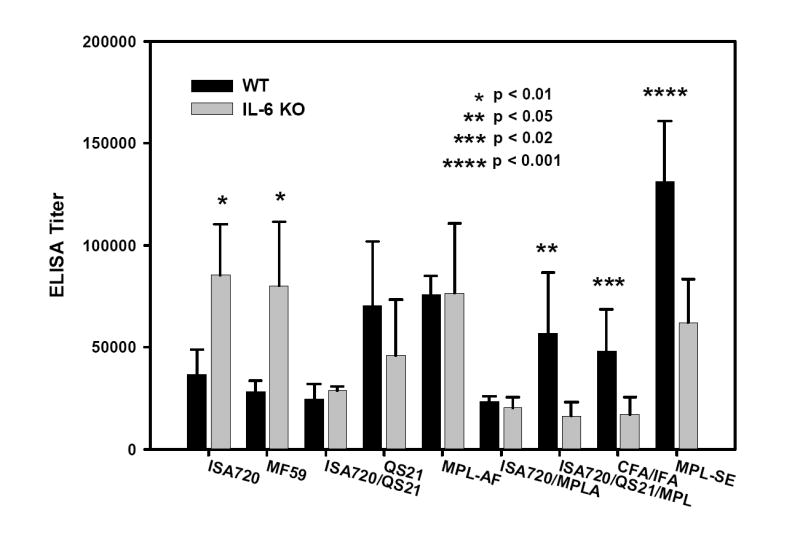
ELISA antibody titers to MSP1-19 of mice, IL-6 KO and WT, immunized with P30P2MSP1-19 in different adjuvant formulations. Mouse sera were obtained at 21 days after tertiary immunizations. Symbols: Black bars: WT mice; Grey bars: IL-6 KO mice. Significant differences (Student t test) between WT and IL-6 KO mice of an adjuvant group are indicated by asterisks.
Dependency of IL-6 for full adjuvanticity is not strictly related to the ability of adjuvants to stimulate antigen-specific IFN-γ and IL-4 responses
Figure 2 shows the IFN-γ and IL-4 ELISPOT of P30P2MSP1-19 stimulated splenocytes from IL-6 KO and WT mice receiving the MPL-SE and ISA720/QS21/MPL adjuvants. There were significant reduction in the frequency of both IFN-γ and IL-4 producing cells in IL-6 KO mice immunized with P30P2MSP1-19/MPL-SE, as compared with WT mice. In contrast, no significant difference in the IFN-γ and IL-4 production was observed between IL-6 KO and WT mice receiving ISA720/QS21/MPL. In the IL-6 KO mouse groups that showed increases in anti-MSP1-19 antibody titers, ie. MF59 and ISA720 adjuvant groups, there were also no significant differences in IFN-γ and IL-4 production as compared with WT controls (data not shown).
Figure 2.
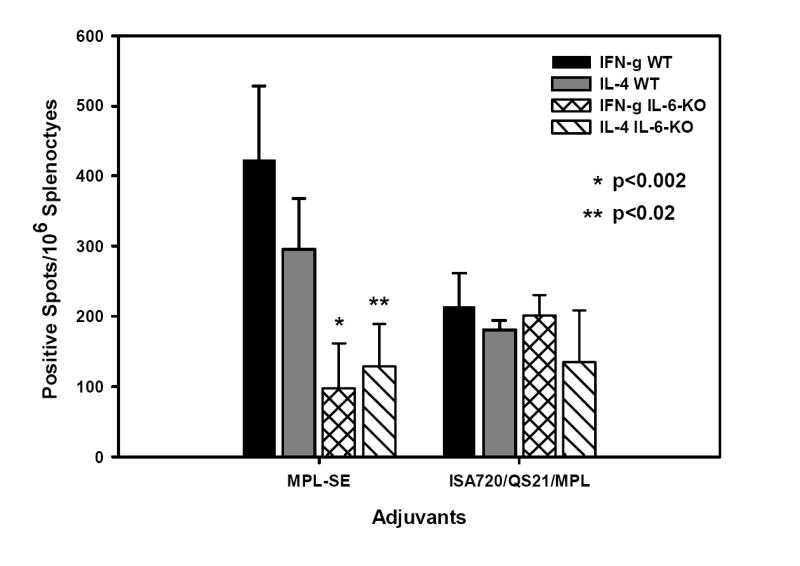
ELISPOT (IFN-γ and IL-4) of antigen stimulated splenocytes from WT and IL-6 KO mice immunized with P30P2MSP1-19 in MPL-SE, and ISA720/QS21/MPL formulations. Symbols: Black bars: IFN-γ, WT mice; grey bars: IL-4, WT mice; cross-hatched bars: IFN-γ, IL-6 KO mice; hatched bars: IL-4, IL-6 KO mice. Significant differences (Student t test) between WT and KO mice of an adjuvant group are indicated by asterisks.
Effects of IL-6 KO on the induction of immunoglobulin isotypes of the anti-MSP1-19 antibodies
The IgG1 and IgG2a profiles of the anti-MSP1-19 antibodies in IL-6 KO and WT mice receiving ISA720, MF59, and CFA/IFA are shown in Figure 3A. Depletion of IL-6 caused significant reductions in the IgG2a responses in all adjuvant groups. In fact, the IgG2a responses were barely above detectable levels, despite the fact that two of the adjuvant groups, ie. ISA720 and MF59, showed increases in total antibody responses in IL-6 KO environment (Figure 1).
Figure 3.
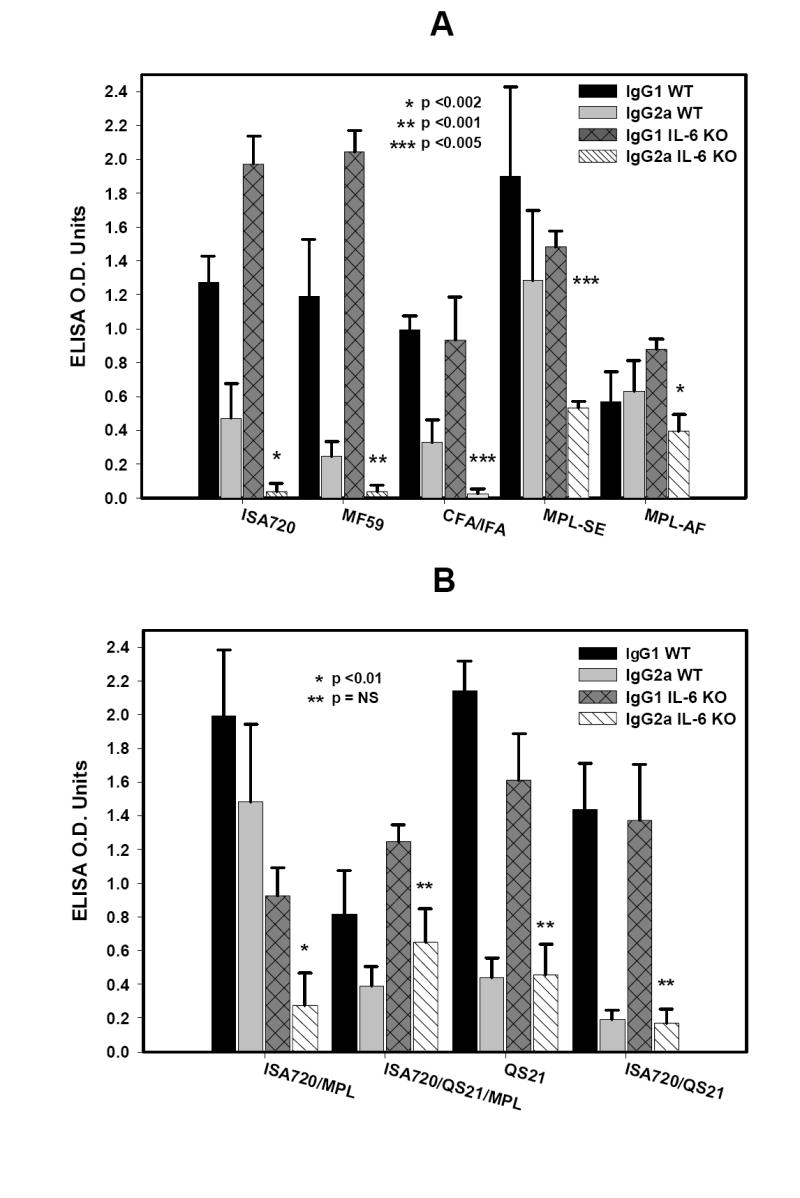
Immunoglobulin isotypes profiles, IgG1 and IgG2a, of anti-MSP1-19 antibodies from IL-6 KO and WT mice immunized with P30P2MSP1-19 in different adjuvant formulations. Panel A, formulations, ISA720; MF59; CFA/IFA; MPL-SE; and MPL-AF. Panel B, formulations ISA720/MPL; ISA720/QS21/MPL; QS21; and ISA720/QS21. Symbols: Black bars, WT, IgG1; grey bars, WT, IgG2a; dark grey cross-hatched bars, IL-6 KO, IgG1; hatched bars, IL-6 KO, IgG2a. Significant differences (Student t test) in the IgG2a levels between WT and IL-6 KO mice of an adjuvant group are indicated by asterisks.
MPL-type adjuvants, when formulated alone in saline as in the MPL-AF, or in two different emulsions, ie. MPL-SE and ISA720/MPL, (see Figure 3A and 3B) showed significant reduction of IgG2a in IL-6 KO mice as compared with WT controls. However, the addition of QS21 into the later formulation, ie ISA720/QS21/MPL, could restore the ability to produce IgG2a in IL-6 KO mice to comparable levels seen in WT mice. Furthermore, it is noteworthy that the remaining two adjuvant formulations that contain the QS21 component, ie. ISA720/QS21 and QS21, were also able to induce IgG2a responses in IL-6 KO mice similar to WT controls.
DISCUSSION
While some adjuvants such as the TLR ligands, CpG ODNs, and MPL have specific modes of activation on immune cells (reviewed in [39], other adjuvants such as Alum, oil-based and other “depot-type” adjuvants have less well defined immunological activities. Even for adjuvants with known specific targets, the ensuing immunological cascades often have broad and differential effects on immune as well as non-immune cell types, each may exert unique influence on the outcome of the adjuvant-potentiated immunological responses. Monophosphoryl lipid A derivatives is one such example [40-42]. It will be important to assess which key immunological mediators driven by adjuvants are influential in determining their efficacy, as perturbation of these mediators in the host due to a variety of intrinsic and extrinsic factors can limit potency/efficacy. A recent study demonstrating reduced efficacy of a malaria vaccine in Titermax® adjuvant under a skewed TH2 environment clearly demonstrates this phenomenon [43].
IL-6 is a pleiotropic cytokine produced by lymphoid and non-lymphoid cells [24]. Its affects on B and T cells, and APCs have been demonstrated, in addition to induction of inflammatory cascade[24]. Thus, the importance of IL-6 on adjuvants’ efficacy is of particular interest. Our present study with the nine adjuvant formulations showed distinctive adjuvanticity in the IL-6 deficient environment. While some adjuvants, ie. MPL-SE, ISA720/QS21/MPL and CFA/IFA, showed dependency of IL-6 for full adjuvanticity, our data suggested key differences in the nature of this dependency. In the MPL-SE group, the decrease in antibody responses in IL-6 KO mice was associated with decreases in both IFN-γ and IL-4 producing splenocytes upon antigen stimulation. Thus, for the MPL-SE adjuvant-assisted immunization, the IL-6 dependency may lie with the cytokine’s effects on lymphocyte functions. On the other hand, although the adjuvant formulation, ISA720/QS21/MPL required IL-6 for full adjuvanticity in inducing antibody responses to MSP1-19, this dependency was not at the level of antigen-specific T cell responses, since there were no differences in the ELISPOT results as compared with WT mice. Since IL-6 is known for its ability to enhance B cell growth and differentiation [24], it is possible that potentiation of antibody responses by ISA720/QS21/MPL requires IL-6 for optimal B cell function.
Another interesting result is the enhanced antibody responses in IL-6 KO mice immunized with P30P2MSP1-19 formulated with MF59 or ISA720, as compared with WT. These adjuvants do not contain immunomodulators. Studies have shown that IL-6 may skew development of monocytes toward macrophage lineage and away from DC [44]. Immunization with adjuvant formulations containing potent immunomodulators (eg. MPL) may mask this effect between WT and IL-6 KO mice by driving DC differentiation/activation. For MF59 and ISA720, IL-6 KO may provide a sufficient increase in DC differentiation that may result in an observable enhancement of immunogenicity. A parallel study using the same panel of adjuvant formulations in ICAM-1 KO mice (Hui et al, in preparation) showed that formulations without immunomodulators (ie. MF59, ISA720) have reduced efficacy in potentiating anti-MSP1-19 antibodies with concomitant decreases in antigen-stimulated IFN-γ production. Thus, these two adjuvants may be more sensitive to changes in the DC population than adjuvant formulations containing immunomodulators.
There has not been consensus in the literature regarding the role of IL-6 in the polarization of T cell responses. A dual role of IL-6 in TH1/TH2 differentiation has also been proposed [45]. Our ELISPOT results did not show a preferential induction of TH1 or TH2 responses in the IL-6 KO mice. However, in most IL-6 KO mouse groups, there were significant decreases in the IgG2a responses as compared with WT mice. Since there were no accompanied reductions of IFN-γ responses, the effect may be a result of the lack of direct interaction of IL-6 on B cells [24, 46], and/or via the activities of DCs [47]. Of further interest is the lack of reduction of IgG2a responses in IL-6 KO mice receiving adjuvant formulations containing QS21, ie. ISA720/QS21/MPL, ISA720/QS21, and QS21 alone. Thus, this may be the dominant feature of QS21, even in the presence of MPL in the formulation. It has been established that QS21 enhances IgG2a production [31], and our data suggested that the adjuvant can mediate IgG2a responses in the absence of IL-6 and without a compensatory increase in IFN-γ response. A direct stimulatory effect of QS21 on B cells remains to be established. Along the same lines, it is known that MPL adjuvants stimulate TH1 responses and IgG2a production [42, 48-50]. Our data show that all adjuvant formulations containing MPL required IL-6 to sustain production of IgG2a; in spite of the ability of MPLs to directly interact with B cells via TLR ligation. These results suggest that the mechanisms by which QS21 and MPL promote IgG2a responses are distinct.
The present study, though it is limited in scope and should be interpreted with caution, provides preliminary evidence of a role of IL-6 on the efficacy of adjuvants; and more importantly its effects varied with the particular adjuvant formulation. This should not be surprising knowing the pleiotropic nature of this cytokine. An adjuvant may rely on one of the biological activities of IL-6 more than another formulation, depending on their ability to drive compensatory pathways. Our data provide a starting point from which further studies will focus on how each biological activity of the pleiotropic IL-6 can uniquely mediate the activities of a particular adjuvant formulation.
Acknowledgments
We thank Antigenics Inc., Chiron Corp., and Corixa Inc. for providing the adjuvants for this study. This work was supported by a grant from NIAID/NIH (RO1AI45768) to G.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.O’Hagan DT, Valiante NM. Recent advances in the discovery and delivery of vaccine adjuvants. Nat Rev Drug Discov. 2003;2(9):727–35. doi: 10.1038/nrd1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82(5):488–96. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 3.Singh M, O’Hagan DT. Recent advances in vaccine adjuvants. Pharm Res. 2002;19(6):715–28. doi: 10.1023/a:1016104910582. [DOI] [PubMed] [Google Scholar]
- 4.Chang SP, Nikaido CM, Hashimoto AC, Hashiro CQ, Yokota BT, Hui GS. Regulation of antibody specificity to Plasmodium falciparum merozoite surface protein-1 by adjuvant and MHC haplotype. J Immunol. 1994;152(7):3483–90. [PubMed] [Google Scholar]
- 5.Hui GS, Chang SP, Gibson H, Hashimoto A, Hashiro C, Barr PJ, et al. Influence of adjuvants on the antibody specificity to the Plasmodium falciparum major merozoite surface protein, gp195. J Immunol. 1991;147(11):3935–41. [PubMed] [Google Scholar]
- 6.Rawlings DJ, Kaslow DC. Adjuvant-dependent immune response to malarial transmission-blocking vaccine candidate antigens. J Exp Med. 1992;176(5):1483–7. doi: 10.1084/jem.176.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns EA. Effects of aging on immune function. J Nutr Health Aging. 2004;8(1):9–18. [PubMed] [Google Scholar]
- 8.Frasca D, Riley RL, Blomberg BB. Effect of age on the immunoglobulin class switch. Crit Rev Immunol. 2004;24(5):297–320. doi: 10.1615/critrevimmunol.v24.i5.10. [DOI] [PubMed] [Google Scholar]
- 9.Ginaldi L, De Martinis M, D’Ostilio A, Marini L, Loreto MF, Quaglino D. The immune system in the elderly: III. Innate immunity. Immunol Res. 1999;20(2):117–26. doi: 10.1007/BF02786468. [DOI] [PubMed] [Google Scholar]
- 10.Ginaldi L, De Martinis M, D’Ostilio A, Marini L, Loreto MF, Martorelli V, et al. The immune system in the elderly: II. Specific cellular immunity. Immunol Res. 1999;20(2):109–15. doi: 10.1007/BF02786467. [DOI] [PubMed] [Google Scholar]
- 11.Ginaldi L, De Martinis M, D’Ostilio A, Marini L, Loreto MF, Corsi MP, et al. The immune system in the elderly: I. Specific humoral immunity. Immunol Res. 1999;20(2):101–8. doi: 10.1007/BF02786466. [DOI] [PubMed] [Google Scholar]
- 12.Haynes L. The effect of aging on cognate function and development of immune memory. Curr Opin Immunol. 2005;17(5):476–9. doi: 10.1016/j.coi.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawelec G, Barnett Y, Forsey R, Frasca D, Globerson A, McLeod J, et al. T cells and aging, January 2002 update. Front Biosci. 2002;7:d1056–183. doi: 10.2741/a831. [DOI] [PubMed] [Google Scholar]
- 14.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3(4):161–7. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 15.Araujo MI, Bliss SK, Suzuki Y, Alcaraz A, Denkers EY, Pearce EJ. Interleukin-12 promotes pathologic liver changes and death in mice coinfected with Schistosoma mansoni and Toxoplasma gondii. Infect Immun. 2001;69(3):1454–62. doi: 10.1128/IAI.69.3.1454-1462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bermudez LE, Covaro G, Remington J. Infection of murine macrophages with Toxoplasma gondii is associated with release of transforming growth factor beta and downregulation of expression of tumor necrosis factor receptors. Infect Immun. 1993;61(10):4126–30. doi: 10.1128/iai.61.10.4126-4130.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bermudez LE. Production of transforming growth factor-beta by Mycobacterium avium-infected human macrophages is associated with unresponsiveness to IFN-gamma. J Immunol. 1993;150(5):1838–45. [PubMed] [Google Scholar]
- 18.Guo JT, Hayashi J, Seeger C. West Nile virus inhibits the signal transduction pathway of alpha interferon. J Virol. 2005;79(3):1343–50. doi: 10.1128/JVI.79.3.1343-1350.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haga IR, Bowie AG. Evasion of innate immunity by vaccinia virus. Parasitology. 2005;130(Suppl):S11–S25. doi: 10.1017/S0031182005008127. [DOI] [PubMed] [Google Scholar]
- 20.Jankovic D, Steinfelder S, Kullberg MC, Sher A. Mechanisms underlying helminth- induced Th2 polarization: default, negative or positive pathways? Chem Immunol Allergy. 2006;90:65–81. doi: 10.1159/000088881. [DOI] [PubMed] [Google Scholar]
- 21.Lin RJ, Liao CL, Lin E, Lin YL. Blocking of the alpha interferon-induced Jak-Stat signaling pathway by Japanese encephalitis virus infection. J Virol. 2004;78(17):9285–94. doi: 10.1128/JVI.78.17.9285-9294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munoz-Jordan JL, Sanchez-Burgos GG, Laurent-Rolle M, Garcia-Sastre A. Inhibition of interferon signaling by dengue virus. Proc Natl Acad Sci USA. 2003;100(24):14333–8. doi: 10.1073/pnas.2335168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hui GS, Hashimoto CN. Pathways for potentiation of immunogenicity during adjuvant-assisted immunizations with Plasmodium falciparum major merozoite surface protein 1. Infect Immun. 1998;66(11):5329–36. doi: 10.1128/iai.66.11.5329-5336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kishimoto T, Thomson AW, Lotze MT, editors. The Cytokine Handbook. 4. San Diego, CA: Academic Press; 2003. [Google Scholar]; Interleukin-6 Family. pp. 281–304. [Google Scholar]
- 25.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175(6):3463–8. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 26.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 27.Brady CP, Shimp RL, Miles AP, Whitmore M, Stowers AW. High-level production and purification of P30P2MSP1(19), an important vaccine antigen for malaria, expressed in the methylotropic yeast Pichia pastoris. Protein Expr Purif. 2001;23(3):468–75. doi: 10.1006/prep.2001.1526. [DOI] [PubMed] [Google Scholar]
- 28.Chang SP, Hui GS, Kato A, Siddiqui WA. Generalized immunological recognition of the major merozoite surface antigen (gp195) of Plasmodium falciparum. Proc Natl Acad Sci USA. 1989;86(16):6343–7. doi: 10.1073/pnas.86.16.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawrence GW, Saul A, Giddy AJ, Kemp R, Pye D. Phase I trial in humans of an oil-based adjuvant SEPPIC MONTANIDE ISA 720. Vaccine. 1997;15(2):176–8. doi: 10.1016/s0264-410x(96)00150-8. [DOI] [PubMed] [Google Scholar]
- 30.Ott G, Barchfeld GL, Chernoff D, Radhakrishnan R, van Hoogevest P, Van Nest G. MF59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm Biotechnol. 1995;6:277–96. doi: 10.1007/978-1-4615-1823-5_10. [DOI] [PubMed] [Google Scholar]
- 31.Kensil CR, Wu JY, Soltysik S. Structural and immunological characterization of the vaccine adjuvant QS-21. Pharm Biotechnol. 1995;6:525–41. doi: 10.1007/978-1-4615-1823-5_22. [DOI] [PubMed] [Google Scholar]
- 32.Childers NK, Miller KL, Tong G, Llarena JC, Greenway T, Ulrich JT, et al. Adjuvant activity of monophosphoryl lipid A for nasal and oral immunization with soluble or liposome-associated antigen. Infect Immun. 2000;68(10):5509–16. doi: 10.1128/iai.68.10.5509-5516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skeiky YA, Coler RN, Brannon M, Stromberg E, Greeson K, Crane RT, et al. Protective efficacy of a tandemly linked, multi-subunit recombinant leishmanial vaccine (Leish-111f) formulated in MPL adjuvant. Vaccine. 2002;20(2728):3292–303. doi: 10.1016/s0264-410x(02)00302-x. [DOI] [PubMed] [Google Scholar]
- 34.Kaslow DC, Hui G, Kumar S. Expression and antigenicity of Plasmodium falciparum major merozoite surface protein (MSP1(19)) variants secreted from Saccharomyces cerevisiae. Mol Biochem Parasitol. 1994;63(2):283–9. doi: 10.1016/0166-6851(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 35.Favre N, Bordmann G, Rudin W. Comparison of cytokine measurements using ELISA, ELISPOT and semi-quantitative RT-PCR. J Immunol Methods. 1997;204(1):57–66. doi: 10.1016/s0022-1759(97)00033-1. [DOI] [PubMed] [Google Scholar]
- 36.Nagabhushanam V, Cheers C. Non-major histocompatibility complex control of antibody isotype and Th1 versus Th2 cytokines during experimental infection of mice with Mycobacterium avium. Infect Immun. 2001;69(3):1708–13. doi: 10.1128/IAI.69.3.1708-1713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su HC, Cousens LP, Fast LD, Slifka MK, Bungiro RD, Ahmed R, et al. CD4+ and CD8+ T cell interactions in IFN-gamma and IL-4 responses to viral infections: requirements for IL-2. J Immunol. 1998;160(10):5007–17. [PubMed] [Google Scholar]
- 38.Miyahira Y, Murata K, Rodriguez D, Rodriguez JR, Esteban M, Rodrigues MM, et al. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181(1):45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 39.Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11:s63–s68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 40.De Becker G, Moulin V, Pajak B, Bruck C, Francotte M, Thiriart C, et al. The adjuvant monophosphoryl lipid A increases the function of antigen-presenting cells. Int Immunol. 2000;12(6):807–15. doi: 10.1093/intimm/12.6.807. [DOI] [PubMed] [Google Scholar]
- 41.Ismaili J, Rennesson J, Aksoy E, Vekemans J, Vincart B, Amraoui Z, et al. Monophosphoryl lipid A activates both human dendritic cells and T cells. J Immunol. 2002;168(2):926–32. doi: 10.4049/jimmunol.168.2.926. [DOI] [PubMed] [Google Scholar]
- 42.Ulrich JT, Myers KR. Monophosphoryl lipid A as an adjuvant. Past experiences and new directions. Pharm Biotechnol. 1995;6:495–524. [PubMed] [Google Scholar]
- 43.Su Z, Segura M, Stevenson MM. Reduced Protective Efficacy of a Blood-Stage Malaria Vaccine by Concurrent Nematode Infection. Infect Immun. 2006;74(4):2138–44. doi: 10.1128/IAI.74.4.2138-2144.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1(6):510–4. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 45.Diehl S, Rincon M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002;39(9):531–6. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 46.Muraguchi A, Hirano T, Tang B, Matsuda T, Horii Y, Nakajima K, et al. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988;167(2):332–44. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jego G, Pascual V, Palucka AK, Banchereau J. Dendritic cells control B cell growth and differentiation. Curr Dir Autoimmun. 2005;8:124–39. doi: 10.1159/000082101. [DOI] [PubMed] [Google Scholar]
- 48.Coler RN, Skeiky YA, Bernards K, Greeson K, Carter D, Cornellison CD, et al. Immunization with a polyprotein vaccine consisting of the T-Cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis. Infect Immun. 2002;70(8):4215–25. doi: 10.1128/IAI.70.8.4215-4225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujiwara RT, Vale AM, Franca da Silva JC, da Costa RT, Quetz JS, Martins Filho OA, et al. Immunogenicity in dogs of three recombinant antigens (TSA, LeIF and LmSTI1) potential vaccine candidates for canine visceral leishmaniasis. Vet Res. 2005;36(56):827–38. doi: 10.1051/vetres:2005033. [DOI] [PubMed] [Google Scholar]
- 50.Wheeler AW, Marshall JS, Ulrich JT. A Th1-inducing adjuvant, MPL, enhances antibody profiles in experimental animals suggesting it has the potential to improve the efficacy of allergy vaccines. Int Arch Allergy Immunol. 2001;126(2):135–9. doi: 10.1159/000049504. [DOI] [PubMed] [Google Scholar]


