Abstract
BACKGROUND AND AIM
Crohn’s disease (CD) is a heterogeneous disorder characterized by diverse clinical phenotypes. Childhood-onset CD has been described as a more aggressive phenotype. Genetic and immune factors may influence disease phenotype and clinical course. We examined the association of immune responses to microbial antigens with disease behavior and prospectively determined the influence of immune reactivity on disease progression in pediatric CD patients.
METHODS
Sera were collected from 196 pediatric CD cases and tested for immune responses: anti-I2, anti-outer membrane protein C (anti-OmpC), anti-CBir1 flagellin (anti-CBir1), and anti-Saccharomyces-cerevisiae (ASCA) using ELISA. Associations between immune responses and clinical phenotype were evaluated.
RESULTS
Fifty-eight patients (28%) developed internal penetrating and/or stricturing (IP/S) disease after a median follow-up of 18 months. Both anti-OmpC (p < 0.0006) and anti-I2 (p < 0.003) were associated with IP/S disease. The frequency of IP/S disease increased with increasing number of immune responses (p trend = 0.002). The odds of developing IP/S disease were highest in patients positive for all four immune responses (OR (95% CI): 11 (1.5–80.4); p = 0.03). Pediatric CD patients positive for ≥1 immune response progressed to IP/S disease sooner after diagnosis as compared to those negative for all immune responses (p < 0.03).
CONCLUSIONS
The presence and magnitude of immune responses to microbial antigens are significantly associated with more aggressive disease phenotypes among children with CD. This is the first study to prospectively demonstrate that the time to develop a disease complication in children is significantly faster in the presence of immune reactivity, thereby predicting disease progression to more aggressive disease phenotypes among pediatric CD patients.
INTRODUCTION
The currently accepted etiopathogenic hypothesis for inflammatory bowel disease (IBD) proposes the role of genetics, altered immune responses, and environmental factors for disease susceptibility and development. These factors and their interactions may also be important determinants of disease phenotype and disease progression. Genetic factors do play a role in determining disease phenotype, given the high concordance rate for disease location and disease behaviors within affected families and the recently identified link between NOD2/CARD15 genotype and Crohn’s disease (CD) phenotypes (1–7). Although not uniformly consistent, retrospective genotype-phenotype correlation studies among adult CD cohorts have suggested an association between NOD2 and earlier age of diagnosis, ileal disease location, and stricturing disease behavior (4–7). A pediatric study reported an association between NOD2 variants and stricturing disease and earlier time to surgery in children with CD (8).
As seen with the genetic heterogeneity of CD, studies have shown that immune response (immunotype) heterogeneity exists among CD patients. The results of a recent study suggest that there are subsets of patients with differing immune responses to microbial antigens; antibodies to the E. coli outer-membrane porin C (OmpC), the pseudomonas fluorescens CD-related protein (I2), as well as Saccharomyces cerevisiae (ASCA) and autoantigens; perinuclear anti-neutrophil antibody (pANCA) (9). Cluster analysis yielded four immune response groups: ASCA, anti-OmpC/anti-I2, pANCA, and a no response group. Initial immunotype-phenotype studies demonstrated that although pANCA has been established as an ulcerative colitis (UC)-specific marker, approximately 25% of all CD patients also express pANCA. These CD patients are described as “UC-like” and tend to have an uncomplicated disease course (10,11). In contrast, higher ASCA levels were shown to be associated with earlier age of disease onset, both stricturing and internal penetrating (IP) disease behaviors and need for small bowel surgery (11). ASCA has also been shown to be associated with a more aggressive disease course among a cohort of pediatric CD patients (12). More recently, anti-ompC and anti-I2 have also been shown, like ASCA, to be associated with complicated disease behaviors among adult CD patients (13). Anti-I2 was independently associated with stricturing disease and small bowel surgery, and anti-OmpC was independently associated with IP disease. This study also demonstrated that both the number of immune responses to the different microbial antigens expressed by a given individual as well as the magnitude (titer level) of these immune responses correlated most significantly with the presence of complicated CD phenotypes. Of great interest is the finding that immune responses to microbial antigens had a stronger association with the presence of complicated disease behaviors than known susceptibility genotypes, such that NOD2 was independently associated with small bowel disease location only and not fibrostenosing disease behavior as previously reported. This is subsequent to the multiple genotype-phenotype correlation studies that most consistently reported a significant association between NOD2 variants and fibrostenosing disease behavior (4–8). None of these earlier phenotype correlation studies, however, took into account the assessment of multiple immune responses. A novel immune response, anti-CBiR1 (antiflagellin) has recently been shown to be independently associated with small bowel disease, IP and fibrostenosing disease in an adult CD cohort (14). It is unknown whether these phenotype associations also exist among a pediatric CD cohort.
Childhood-onset IBD, CD in particular, may be an indicator of increased genetic predisposition leading to a higher penetrance and is often described as having a more severe disease phenotype; i.e., an aggressive or complicated clinical course (15). At the same time, immune responses to microbial antigens may be a more direct determinant or indicator of disease progression. We hypothesize that the presence and magnitude of immune responses to microbial antigens are subclinical markers of more aggressive clinical phenotypes and these immune responses are the most proximal predictors of disease progression among pediatric CD patients. In this study, we set out to examine associations between serological immune responses to microbial antigens with disease behaviors and disease progression, prospectively, in pediatric CD patients. The results of this study demonstrate that immune responses to certain microbial antigens are associated with complicating disease behaviors in pediatric CD patients. Moreover, our study is the first to prospectively demonstrate that immune responses are present prior to the onset of disease complication and that the time to develop a disease complication is significantly faster in the presence of immune reactivity, thereby predicting disease progression to more aggressive disease phenotypes among pediatric CD patients.
MATERIALS AND METHODS
Patient Population
Pediatric CD patients were enrolled from participating sites of the Western Regional Pediatric IBD Research Alliance. In order to be eligible, all CD patients must have undergone complete colonoscopy with ileal intubation or complete colonoscopy and small bowel follow through. A diagnosis of CD for this study required at least two of the following: (1) history of abdominal pain, weight loss, short stature, malaise, rectal bleeding, or diarrhea; (2) characteristic endoscopic findings of discontinuous ulcerations, cobblestoning, fistula, or severe perianal disease; (3) radiologic features of stricture, fistula, or evidence of cobblestoning, or ulceration of the mucosa; (4) macroscopic appearance at laparotomy of typical bowel wall induration, mesenteric lymphadenopathy, or serosal involvement showing creeping fat, or other inflammatory changes; (5) histopathology showing transmural inflammatory cell infiltrate or epithelial granulomas and absence of identifiable infectious agents (16). Blood for serological analysis was drawn at each of the participating sites and sent via overnight FedEx to the Genotyping Core Facility of the Medical Genetics Institute/GCRC and the Immunobiology Institute at Cedars-Sinai Medical Center (CSMC). This study was approved by the Ethics Review Board at each participating site.
Data Collection
Subjects and their families completed patient demographic forms at the time of blood draw and physicians completed clinical information forms in reference to both date of diagnosis and date of last follow-up. Once collected, all data were then transferred and stored in a secure relational (Oracle) database for analysis. For the purpose of this study, key variables included date of diagnosis, age at diagnosis, date of last follow-up and duration of disease as of last follow-up, ethnicity, family history, disease location (see definitions below), disease behavior (see definition below), granulomas, and surgical procedures.
Phenotype Definitions
All phenotype assessments were performed by clinical investigators blinded to genetic and immune response analysis and based on the following uniform definitions:
DISEASE LOCATION
Disease location at diagnosis was defined by the extent of the disease involvement at the time of initial presentation. Disease extent was based on endoscopic, histologic, and radiographic evidence of inflammation.
Disease location as of last follow-up was defined by the maximal extent of the disease involvement at the point of last follow-up or before a patient underwent first resection. Other than anal/perianal disease, location change was documented when clinically indicated investigations were performed anytime from diagnosis until the date of last follow-up. For the purpose of analysis, disease location as of last follow-up was used for all genotype/immune response-phenotype associations.
There were five disease locations that patients were categorized into (1) small bowel only: disease of the small bowel proximal to the cecum and distal to the ligament of treitz; (2) large bowel only: any colonic location between the cecum and rectum with no small bowel disease; (3) small and large bowel: disease of the small bowel and any location between the cecum and rectum; (4) upper digestive tract: disease involving at least one of the following sites: esophagus, stomach, and duodenum; (5) anal: perianal and anal lesions including skin tags and anal ulcers. Patients could have been in more than one category such that patients with small and/or large bowel disease may also have concomitant upper tract and/or anal disease.
DISEASE BEHAVIOR
Disease behavior at diagnosis was defined by the behavior of the disease at presentation.
Disease behavior as of last follow-up was defined by the disease behavior observed as of last follow-up. At both time points, data may have been obtained after a patient underwent a surgical resection, as reliable data are often obtained at the time of surgery for defining complicated disease behaviors.
Disease behavior was divided into two broad categories: noncomplicating and complicating disease behaviors (7, 17). Noncomplicating behavior referred to uncomplicated inflammatory disease without evidence of stricturing or penetrating disease behaviors (nonpenetrating nonstricturing [NPNS]). Complicating behaviors referred to penetrating and stricturing disease. (1) Stricturing disease (S) was defined as the occurrence of constant luminal narrowing demonstrated by radiologic, endoscopic, or surgical examination combined with pre-stenotic dilatation and/or obstructive signs or symptoms. (2) Penetrating disease was defined as either IP if patients had evidence of entero-enteric or entero-vesicular fistulae, intra-abdominal abscesses, or intestinal perforation, or perianal penetrating (PP) if patients developed either perianal fistulae or abscesses or recto-vaginal or ano-vaginal fistulae.
For the purpose of analysis, stricturing and IP complications were grouped into one outcome. PP and patients without complications (NPNS) comprised the other two comparison groups.
Immune Responses
All blood samples were taken at the time of consent and enrollment. Sera were analyzed for expression of ASCA, anti-OmpC, anti-I2, and anti-CBir1 antibodies in a blinded fashion by ELISA as previously described (13, 14). Analysis and IgG and IgA ASCA were performed at Cedars-Sinai Medical Center or Prometheus Laboratories using the same technology. All assays for anti-OmpC, anti-I2, and anti-CBir1 were performed at Cedars-Sinai. Antibody levels were determined and results expressed as ELISA units (EU/mL), which are relative to a Cedars-Sinai Laboratory (IgA-I2, IgA-OmpC, and IgG CBir1) or a Prometheus Laboratory Standard (IgA and IgG ASCA), which is derived from a pool of patient sera with well-characterized disease found to have reactivity to this antigen.
Statistical Analysis
To determine the associations between disease phenotype characteristics and antibody responses toward microbial antigens, univariate analyses using χ2 tests were performed. Odds ratios (OR) and 95% confidence intervals were calculated to compare the odds of positive serum reactivity toward the microbial antigens (CBir1, I2, OmpC, and ASCA) in the group of patients with a certain disease characteristic with the group of patients without such a characteristic. Quantitative comparison of immune response levels between groups (IP/S + vs IP/S−) for each antibody was performed using nonparametric Wilcoxin rank test. Multivariate analysis with logistic regression modeling was also performed to determine the primary associations among qualitative serological responses with disease phenotypes. To compare the length of time to the development of a disease complication between groups, Kaplan-Meier estimator of survival probability was calculated to construct survival curves. The log-rank test was used to test if the survival curves were significantly different between subgroups of patients. All analyses were performed by using Statistical Analysis Software (Version 8.02; SAS Institute, Inc., Cary, NC).
RESULTS
Patient Population
A total of 196 pediatric CD patients were eligible for analysis. Eighty-five percent (168/196) were Caucasians and 28% were of Jewish background. The median age at diagnosis was 12 yr (1–18) and the median age at study was 13 yr (4–19). The cohort comprised 47% males and 53% females. A positive family history of IBD was reported in 29% of patients.
Clinical Phenotypes
A total of 38 (19%) patients had either a stricturing and/or penetrating complication at the time of diagnosis. After a median follow-up time (median disease duration as of last follow-up) of 18 months (1–200), the total number of pediatric CD patients who experienced a disease complication increased to 58 (30%). Table 1 details the clinical phenotypes of the pediatric CD cohort. Of the 35 patients with internal penetrating and/or stricturing (IP/S) disease, 18 had isolated stricturing disease, 11 had IP, and 6 had both complications. Thirty-two of the 58 patients (55%) underwent a combined total of 53 surgeries related to disease complications, 38 (72%) of which were small bowel surgeries for IP/S disease complications. The remaining surgeries were for perianal perforating diseases. All but two patients (15/17) with IP disease and 45% of patients with isolated stricturing disease underwent small bowel surgery as of last follow-up.
Table 1.
Clinical Phenotypes in Pediatric CD Cohort
| Clinical Phenotype | N (%) |
|---|---|
| Disease location | |
| Small bowel only | 24 (12.2) |
| Large bowel only | 51 (26.0) |
| Small and large bowel | 120 (61.2) |
| and/or upper tract | 78 (39.8) |
| and/or anal disease | 39 (19.9) |
| Disease behavior at diagnosis | |
| Non-penetrating non-stricturing | 158 (80.6) |
| Internal penetrating and/or stricturing | 21 (10.7) |
| Perianal penetrating only | 17 (8.7) |
| Disease behavior as of last follow up | |
| Non-penetrating non-stricturing | 138 (70.4) |
| Internal penetrating and/or stricturing | 35 (17.9) |
| Perianal penetrating only | 23 (11.7) |
Immune Responses
Serum was collected at a median of 9.4 months (0–211.7) after diagnosis, 18% of patients (35/196) had serum collected at the time of diagnosis or within 1 month of diagnosis and 33% (64/196) within 3 months of diagnosis. A total of 77.0% of patients were positive for at least one immune response, 23.7% of which were positive for a combination of any two immune responses, 16.4% of patients were positive for all three responses, and 3.4% were positive for all four responses. ASCA, anti-I2, anti-OmpC, and anti-CBir1 were present in 43%, 26%, 22%, and 53%, respectively.
Immune Responses and CD Phenotypes
PRESENCE AND MAGNITUDE OF IMMUNE RESPONSES INFLUENCE DISEASE BEHAVIOR
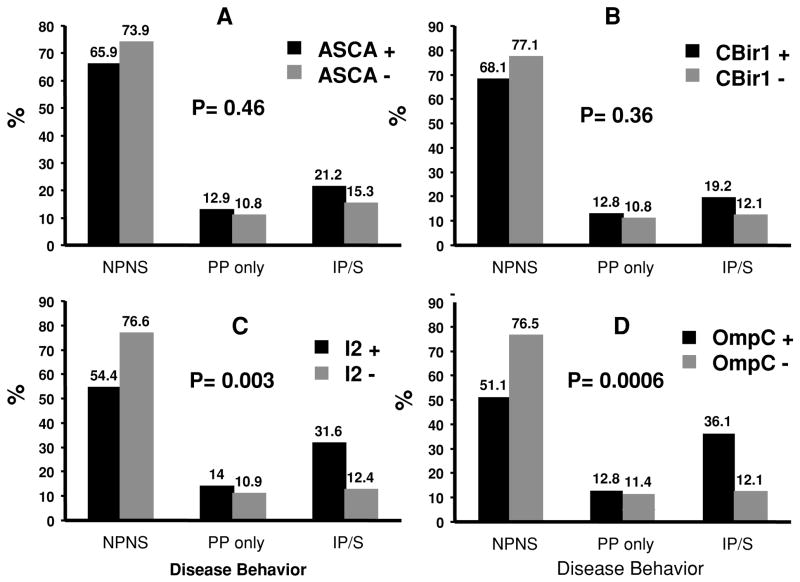
A statistically significant association was not found for any of the immune responses with family history, ethnicity, or the presence of granulomas. ASCA was the only antibody significantly associated with small bowel disease location; yet was not associated with disease behavior. Both anti-I2 (p = 0.0034) and anti-OmpC (p = 0.0006) were associated with complicating disease behaviors, more specifically IP/S disease (Fig. 1). The frequency of isolated perianal perforating disease was similar between immune response groups (±) for all four antibodies. In addition to the qualitative associations observed for anti-OmpC and anti-I2, the magnitude of the immune response to OmpC and I2 also had an association with internal perforation and/or stricturing disease (p = 0.008 and p = 0.002 for anti-OmpC and anti-I2, respectively). The anti-OmpC association continued to be significant in the multivariate logistic regression, which showed that anti-OmpC (p < 0.02) was independently associated with IP/S disease. ASCA, anti-I2, and anti-CBir1 did not show any independent association with disease behavior.
Figure 1.
Graphs A–D illustrate the percentage for the different disease behaviors among those seropositive and seronegative for the specific immune response. There are no differences in the distribution of disease behaviors among ASCA (A) and CBir1 (B) positive or negative patients. There are significant increases in frequency of IP/S in patients positive for anti-I2 (C) and anti-OmpC (D). NPNS = nonpenetrating nonstricturing; PP = perianal perforating only; IP/S = internal penetrating and/or stricturing.
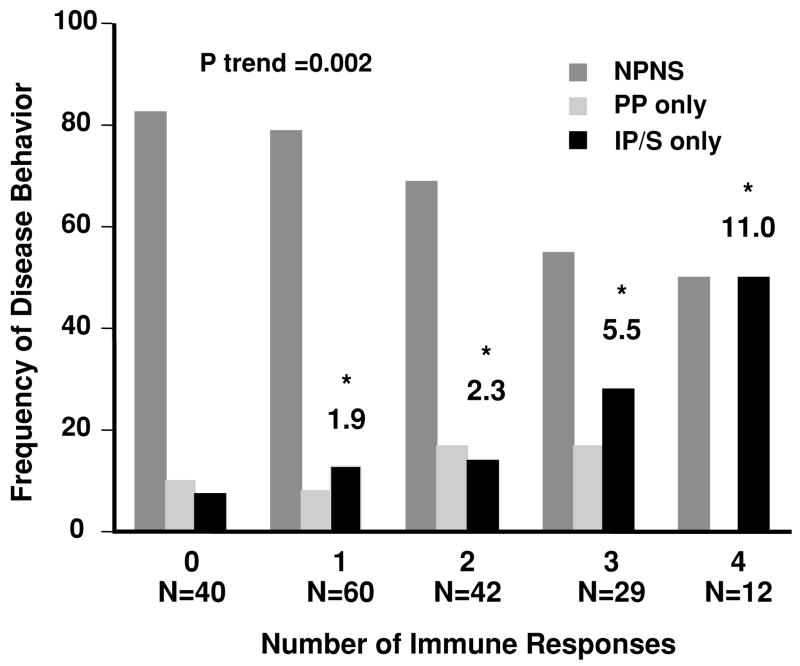
CUMULATIVE INFLUENCE OF IMMUNE RESPONSES ON DISEASE BEHAVIOR
Individually there is a clear association with individual immune responses I2 and OmpC with IP/S. We then examined whether there was a cumulative influence of immune responses on disease behavior and determined if the odds of having complicating IP/S disease were greater in the presence of multiple immune responses. As demonstrated in Figure 2, the frequency of IP/S disease significantly increased (p trend = 0.002) as the number of immune responses increased. The OR depicted in Figure 2 demonstrate that the odds of having IP/S disease was significantly increased in children positive for a combination of any three immune responses (OR [95% CI]; OR = 5.5 [1.3–23.6]; p = 0.02) and even more so in children positive for all four immune responses (OR [95% CI]; OR = 11.0 [1.5–80.4]; p = 0.03) as compared to those patients negative for all immune responses (baseline group).
Figure 2.
The frequency of disease behavior (
 nonpenetrating, nonstricturing disease, NPNS;
nonpenetrating, nonstricturing disease, NPNS;
 perianal perforating, PP only; and ■ internal penetrating and/or stricturing, IP/S disease) based on the number of immune responses. The test for trend demonstrated a positive linear trend in the frequency of patients with IP/S disease as the number of positive immune responses toward I2, OmpC, ASCA, and CBir1 increased (p = 0.002). The odds ratios (OR) reflect the odds of having IP/S disease when positive for any one, combination of two, three, or all four immune responses, as compared to those patients negative for all immune responses (baseline group).
perianal perforating, PP only; and ■ internal penetrating and/or stricturing, IP/S disease) based on the number of immune responses. The test for trend demonstrated a positive linear trend in the frequency of patients with IP/S disease as the number of positive immune responses toward I2, OmpC, ASCA, and CBir1 increased (p = 0.002). The odds ratios (OR) reflect the odds of having IP/S disease when positive for any one, combination of two, three, or all four immune responses, as compared to those patients negative for all immune responses (baseline group).
Disease Progression
Based on our cross-sectional data, immune responses are associated with the presence of disease complications. For the second aim of our study, we set out to examine whether seropositive patients (≥1 immune response) have a greater risk to progress to IP/S as compared to seronegative patients (0 immune responses). We used a longitudinal study to answer this question which included only those patients who did not have IP/S at diagnosis (NPNS ± PP) and continued to be uncomplicated (NPNS ± PP) at the time the serum was collected for immune response measurement so that we could be certain that when clinically recognizable IP/S occurred it was after the sera were collected for antibody measurement. The median time from diagnosis to serum draw was 9.2 months (0–142.3). Among those who developed IP/S (10/167) during the follow-up, the median time from diagnosis to the onset of IP/S was 48 months. As of last follow-up, 8.2% (8/97) of the seropositive group had IP/S versus only 2.9% (2/70) in the seronegative group. Because longer disease duration increases the chance of developing IP/S and not all patients are followed for the same amount of time, we performed survival analysis to take the length of follow-up into consideration (Fig. 3). We first evaluated survival with OmpC, I2, and ASCA. Given the same length of follow-up, among those patients positive for at least one serology, more progressed to IP/S than those negative for the three serologies (p = 0.03). Saying it differently, those patients positive for at least one serology progressed to IP/S faster than those negative for all three serologies. We then examined whether the addition of Cbir1 changed the survival outcome. Of significance is that the two patients who developed IP/S in the presumptive seronegative group, when measuring I2, OmpC, and ASCA only, were actually CBir1 positive. We have fewer patients followed out long enough in those who had all four antibodies measured. Thus, when we have adequate such numbers these anti-CBIR positive patients would be reclassified to the seropositive group. As of last follow-up, all seronegative patients remained complication free.
Figure 3.
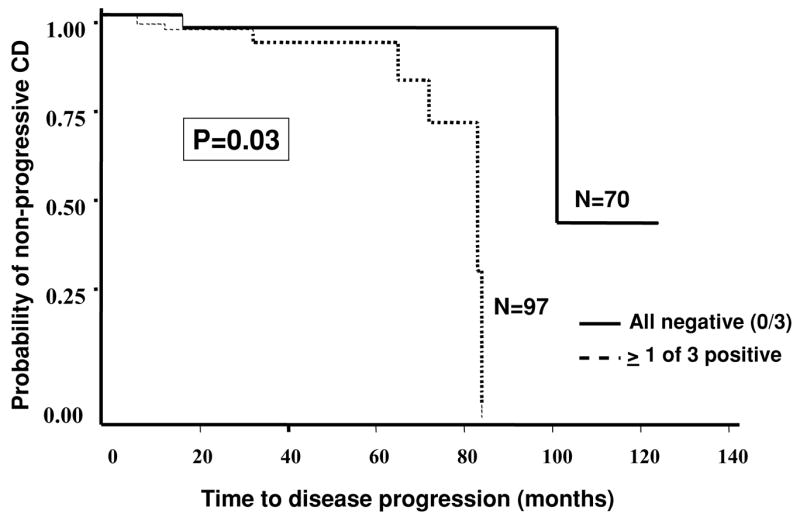
Kaplan-Meier survival analysis. Comparison of time to progression from noncomplicating to complicating disease behaviors between patients positive for ≥1 immune response to ASCA, I2, and OmpC (n = 97) (------) and those negative for all three (n = 70) (——).
DISCUSSION
This is the first study to report on the potential disease modifying role of immune responses in children with CD. In this study, we have demonstrated that immune reactivity to specific microbial antigens is associated with complicating disease behaviors. Moreover, disease progression to a more aggressive disease phenotype in children is accelerated in the presence of immune reactivity.
Disease phenotype is not always a static phenomenon. Retrospective studies have examined the stability of disease phenotypes over the course of disease from time of diagnosis until the point of last follow-up. It appears that disease location essentially remains stable over time, yet disease behavior evolves, such that after 20 years of follow-up, at least 80% of patients with originally noncomplicating disease progress to complication, either penetrating or stricturing in nature (18, 19). These findings suggest that inflammatory or noncomplicating disease behavior may not be a stable phenotype, but just a temporary state that evolves in to one of the two complicating disease states over time. Disease progression to IP/S was the primary outcome in this study. These disease behaviors were established after a change in symptoms prompted follow-up diagnostic evaluations or surgery. The goal of the study was not to try and differentiate between these two disease behaviors, but more so to define and classify these complications as progression to a more aggressive disease phenotype. Determining the factors that can predict the progression from uncomplicated to complicated disease states may stratify patients into at-risk populations and impact the ultimate therapeutic management of patients with the goal of halting or more importantly preventing progression to complicated behaviors. Serological immune responses may be important subclinical markers of a more aggressive clinical phenotype.
The prospective acquisition and determination of immune reactivity prior to the development of a disease complication is important to be able to truly evaluate the potential predictive value of immune responses. It is generally agreed upon that the presence and level of immune responses do not change in a given CD patient (12, 13). The presence of immune responses to bacteria as a result of perforating disease, however, could not be ruled out unless measured prior to the onset of the IP complication. Our study demonstrated that immune responses are present prior to the progression of symptomatic IP disease. A potential limitation to the interpretation of the prospective data is that it remains unknown what proportion, if any, of the asymptomatic patients had already developed an IP/S prior to immune response testing but went undetected in the absence of symptom driven diagnostic evaluation.
The currently accepted etiopathogenic hypothesis suggests that the chronic intestinal inflammation and related systemic manifestations characteristic of IBD are due to an overly aggressive or pathologic immune response to resident luminal bacterial constituents. Predisposing factors are genetic dys-regulation of mucosal immune responses and/or barrier function, with onset triggered by environmental stimuli. These factors and their interactions may also be important determinants of disease phenotype and disease progression. The interaction between genetic susceptibility and luminal bacteria in particular deserves particular attention. The results from animal studies indicate that not all commensal bacterial species are identical in their abilities to induce disease in the face of a common genetic defect. For instance, HLA B27 microglobulin transgenic rats raised under sterile conditions do not develop colitis. However, when colonized with normal specific pathogen-free commensal cecal bacteria, they develop aggressive disease involving the colon and gastro-duodenal area within 1 month (20). On the other hand, when colonized with a single bacterial strain, Bacteroides vulgatus, these same genetically susceptible rats develop moderate colitis, but no gastro-duodenal disease. However, when selectively colonized with E. coli, they exhibit neither disease not exhibit neither disease nor T-cell activation (21) This data suggest that not all commensal bacterial strains trigger an abnormal immune response for a given genetic susceptibility. The same non-pathogenic bacteria, however, may induce inflammation in a different genetically susceptible host. More specifically further research from the same group demonstrated that E. coli induced only mild cecal inflammation after 3 wk of monoassociation in interleukin 10−/− mice. In contrast, Enterococcus faecalis-monoassociated interleukin 10−/− mice developed distal colitis at 10–12 wk that was progressively more severe and associated with duodenal inflammation and obstruction by 30 wk. Their results suggest that different commensal bacterial species selectively initiate immune-mediated intestinal inflammation with distinctly different kinetics and anatomic distribution in the same host (22). These findings highlight the fact that both genetic susceptibility and luminal antigenic drive are important determinants of disease susceptibility and modification. There also appears to be a cumulative influence of multiple immune responses on the frequency and time to develop complicating disease behaviors. In our study, we found that the frequency of disease complications does increase significantly as the number of immune responses to individual microbial antigens is increased, and the odds of developing an IP/S complication during the course of disease are significantly higher in children positive for at least two of a combination of anti-I2, anti-OmpC, ASCA, or CBir1 antibodies. Additionally, the time to progress to a disease complication is accelerated in patients who express immune reactivity.
The results of our study confirm that the magnitude or degree of immune reactivity is also an important determinant of disease behavior. In theory, higher levels of immune reactivity may reflect the degree of loss of tolerance to specific microbial antigens. This may translate clinically to the magnitude of effect that specific bacteria have on the degree and extent inflammation resulting in more aggressive disease behaviors. Thus, as seen in both the pediatric cohort studied here and in previous adult CD cohorts (13, 14, 23), the frequency of patients with complicating disease behaviors increased as the level of antibody response toward I2 and OmpC increased. However, what appears to differentiate pediatric CD patients from the previous adult cohorts is that the presence and/or magnitude of ASCA and CBir1 responses among children do not significantly influence disease behavior. These distinct differences in immune reactivity continue to be an active area of investigation.
In summary, the results of this study demonstrate that immune responses to an increasing number of microbial antigens are associated with complicating IP/S disease behaviors in pediatric CD patients. Moreover, serum immune responses predict a more rapid disease progression from uncomplicated to complicated disease. There are, however, differences in the type of immune reactivity that most influences disease progression among children as compared to adults. Larger prospective studies are currently underway to validate these findings and further examine the predictive value of immune responses and susceptibility genes on disease progression among children with CD. Additionally, further studies in large independent cohorts will be important so as to validate the clinical applicability of these findings. Defining the natural history of childhood-onset CD and further delineation of the potential determinants of disease progression will lead to long-term efforts to develop intervention studies to prevent progression of clinical disease to clinical complications. With this knowledge, clinicians will be able to create and implement appropriate and timely therapeutic management regimes based on the aggressiveness of the IBD subtype in order to alter and thus improve the long-term prognosis.
STUDY HIGHLIGHTS.
What Is Current Knowledge
A relationship between Crohn’s disease phenotype and multiple immune responses to microbial antigens in children may be important.
What Is New Here
This study evaluated the influence of microbial immune responses in the serum in a prospective manner.
There is a subgroup of children who are predisposed to a more complicated disease course.
The risk of developing penetrating and/or stricturing Crohn’s disease was 11-fold increased in those with immune responses to all antigens (anti-12, anti-OmpC, anti-CBirl and ASCA).
Specific immune risk factors may identify those patients requiring more aggressive therapies.
Acknowledgments
The authors would like to acknowledge the research support of the Blinder Research Foundation for Crohn’s disease and the Treuman Katz Family Endowment for Crohn’s disease.
Supported by National Institute of Health K23 DK066248 (M.D.), and The Investigator Sponsored Trial Program of Astra Zeneca, IBD Program Project Grant DK 46763 (S.R.T.), The Cedars-Sinai Board of Governors’ Chair in Medical Genetics (J.I.R.), and General Clinical Research Center, Grant #MO1-RR00425 (K.D.T.).
Footnotes
Conflict of Interest Statement: Dr. Targan is a shareholder and co-founder of Prometheus Labs.
Dr. Dubinsky serves as a consultant for Prometheus Labs.
Carol Landers is a share holder for Prometheus Labs.
References
- 1.Colombel JF, Grandbastien B, Gower-Rousseau C, et al. Clinical characteristics of Crohn’s disease in 72 families. Gastroenterology. 1996;111:604–7. doi: 10.1053/gast.1996.v111.pm8780563. [DOI] [PubMed] [Google Scholar]
- 2.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 3.Ogura Y, Bonen D, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 4.Lesage S, Zouali H, Cezard JP, et al. EPWG-IBD Group; EPIMAD Group; GETAID Group. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am J Hum Genet. 2002;70:845–57. doi: 10.1086/339432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad T, Armuzzi A, Bunce M, et al. The molecular classification of the clinical manifestations of Crohn’s disease. Gastroenterology. 2002;122:854–66. doi: 10.1053/gast.2002.32413. [DOI] [PubMed] [Google Scholar]
- 6.Cuthbert AP, Fisher SA, Mirza MM, et al. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology. 2002;122:867–74. doi: 10.1053/gast.2002.32415. [DOI] [PubMed] [Google Scholar]
- 7.Abreu MT, Taylor KD, Lin YC, et al. Mutations in NOD2 are associated with fibrostenosing disease in patients with Crohn’s disease. Gastroenterology. 2002;123:679–88. doi: 10.1053/gast.2002.35393. [DOI] [PubMed] [Google Scholar]
- 8.Kugathasan S, Collins N, Maresso K, et al. CARD15 gene mutations and risk for early surgery in pediatric-onset Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2:1003–9. doi: 10.1016/s1542-3565(04)00452-5. [DOI] [PubMed] [Google Scholar]
- 9.Landers CJ, Cohavy O, Misra R, et al. Selected loss of tolerance evidenced by Crohn’s disease-associated immune responses to auto- and microbial antigens. Gastroenterology. 2002;123:689–99. doi: 10.1053/gast.2002.35379. [DOI] [PubMed] [Google Scholar]
- 10.Vasiliauskas EA, Plevy SE, Landers CJ, et al. Perinuclear antineutrophil cytoplasmic antibodies in patients with Crohn’s disease define a clinical subgroup. Gastroenterology. 1996;110:1810–9. doi: 10.1053/gast.1996.v110.pm8964407. [DOI] [PubMed] [Google Scholar]
- 11.Vasiliauskas EA, Kam LY, Karp LC, et al. Marker antibody expression stratifies Crohn’s disease into immunologically homogeneous subgroups with distinct clinical characteristics. Gut. 2000;47:487–96. doi: 10.1136/gut.47.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desir B, Amre DK, Lu SE, et al. Utility of serum antibodies in determining clinical course in pediatric Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2:139–46. doi: 10.1016/s1542-3565(03)00321-5. [DOI] [PubMed] [Google Scholar]
- 13.Mow WS, Vasiliauskas EA, Lin YC, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterology. 2004;126:414–24. doi: 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Targan SR, Landers CJ, Yang H, et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128(7):2020–8. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 15.Polito JM, Childs B, Mellits ED, et al. Crohn’s disease: Influence of age at diagnosis on site and clinical type of disease. Gastroenterology. 1996;111:580–6. doi: 10.1053/gast.1996.v111.pm8780560. [DOI] [PubMed] [Google Scholar]
- 16.Loftus EV, Silverstein MD, Sandborn WJ, et al. Crohn’s disease in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gastroenterology. 1998;114:1161–8. doi: 10.1016/s0016-5085(98)70421-4. [DOI] [PubMed] [Google Scholar]
- 17.Gasche C, Scholmerich J, Brynskov J, et al. A simple classification of Crohn’s disease: report of the working party for the World congress of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8–15. doi: 10.1097/00054725-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Louis E, Collard A, Oger AF, et al. Behaviour of Crohn’s disease according to the Vienna classification: Changing pattern over the course of the disease (comment) Gut. 2001;49:777–82. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–50. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Rath HC, Ikeda JS, Linde HJ, et al. Varying cecal bacterial loads influences colitis and gastritis in HLA-B27 transgenic rats. Gastroenterology. 1999;116:310–9. doi: 10.1016/s0016-5085(99)70127-7. [DOI] [PubMed] [Google Scholar]
- 21.Rath HC, Wilson KH, Sartor RB. Differential induction of colitis and gastritis in HLA-B27 transgenic rats selectively colonized with Bacteroides vulgatus or Escherichia coli. Infect Immun. 1999;67:2969–74. doi: 10.1128/iai.67.6.2969-2974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SC, Tonkonogy SL, Albright CA, et al. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005;128:891–906. doi: 10.1053/j.gastro.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Arnott ID, Landers CJ, Nimmo ER, et al. Sero-reactivity to microbial components in Crohn’s disease is associated with disease severity and progression, but not NOD2/CARD15 genotype. Am J Gastroenterol. 2004;99:2376–84. doi: 10.1111/j.1572-0241.2004.40417.x. [DOI] [PubMed] [Google Scholar]