INTRODUCTION
Osteoarthritis (OA) of the knee is a leading cause of mobility limitations in late life and is often accompanied by quadriceps muscle weakness1, 2. Although quadriceps weakness appears to be both a consequence3–5 and a risk factor for knee OA6, muscle strengthening has not been proven to protect individuals from OA7. Moreover, recent evidence suggests that greater knee extensor strength increases risk of OA progression in knees that are malaligned8, 9. Thus, the role quadriceps muscle strength plays in the natural history of knee OA appears to be complex and worthy of further investigation.
Electromyography (EMG) measures the electrical activity of contracting skeletal muscle and can provide a unique physiologic assessment of the motor unit– the functional unit of skeletal muscle comprised of a single lower motor neuron and all the muscle fibers it innervates. The force generated during a muscle contraction is determined by the number of and size of active motor units, as well as the rate and timing of their discharge. Since all fibers in a given motor unit contract synchronously in an all or none fashion, the precision with which muscles are engaged in action is largely dependent on motor unit size, firing rate and activation strategy used to generate force. According to Henneman’s principle10, motor units are recruited into action beginning with smaller units and adding progressively larger units as force increases. In large muscles, such as those engaged during knee extension, force increases are achieved by progressive recruitment of motor units. Additional force is generated at higher levels by increasing the rate at which the motor units fire. In contrast, the strategy employed by small muscles, e.g. intrinsic hand or ocular muscles, firing rate increases prior to recruitment of additional units. Thus, the motor unit activation pattern and strategy employed by large muscles during knee extension allows for exertion of greater force, but with less precise control than that which is achievable by smaller muscles, e.g. intrinsic hand or ocular muscles.
EMG techniques have been developed to study motor unit activity during muscle activation that indicate the size and number of active motor units and the rate at which they discharge11, 12. Neuromuscular alterations, and specifically changes in the peripheral nerve, motor unit, and muscle composition, are likely contributors to age-associated sarcopenia13, and are also plausible contributors to the impact of the quadriceps on OA – an age-associated disease. We have previously demonstrated age-associated motor unit recruitment patterns during progressive, submaximal muscle activation of the vastus medialis.13–16. Since the size of active units is directly related to muscle force generated, alterations in the motor unit might at least in part explain the relationship of muscle weakness with OA. Thus far, EMG techniques used to study OA have been limited to the evaluation of whole muscle activation patterns assessed by surface EMG during mobility task performance17. Although informative with regards to the timing and magnitude of whole muscle contraction, surface EMG cannot distinguish between neural and muscle contributions to force generation. At this time, little is known regarding the pattern of motor unit activation during force generation in the presence of knee OA.
In this study, we employed a technique developed to sample active motor units during knee extension at different force levels using EMG signals simultaneously acquired from surface EMG (S-EMG) and an intramuscular needle. We used this method to assess motor unit activation patterns, specifically motor unit size and firing rate, in adults with symptomatic knee OA across the spectrum of radiographic severity and healthy control volunteers of comparable age.
METHODS
Study Design
We analyzed EMG data from participants in a case-control observational study that were pooled with pre-intervention data from an electrical stimulation treatment study for osteoarthritis of the knee18. This research was approved by the Institutional Review Boards of the Johns Hopkins Bayview Medical Center, MedStar Research Institute and the Johns Hopkins University Joint Committee on Clinical Investigation.
Participants
Cases from both studies comprised adults with symptomatic, radiographically confirmed osteoarthritis of the knee who were recruited from the Baltimore-Washington metropolitan area by newspaper and internet advertisements, and clinic posters. Healthy controls were recruited by the same means, and data were pooled with data from healthy adult participants of the Baltimore Longitudinal Study of Aging who denied knee symptoms or radiographic signs of osteoarthritis and who underwent the same EMG evaluation. The analysis sample was comprised of 21 participants with osteoarthritis of the knee and 18 controls.
Measures
Evaluation of osteoarthritis
Knee symptoms were assessed by asking, “During the past year, have you had pain, aching or discomfort in your knees on most days for at least one month?” In addition, weight-bearing AP radiographs were independently assigned a Kellgren and Lawrence (KL) grade (0–4) by 2 rheumatologists.19 Discordant readings were adjudicated by discussion and consensus between the two reviewers.
Strength Testing
All strength measurements and testing were performed on a Kin-Com 125E dynamometer (Chattecx, Chattanooga, TN) following the same standardized protocol20–22. Maximal voluntary isometric force in Newtons (N) was measured at a knee angle of 60° of flexion. The average of the two best trials out of three was used as maximal voluntary isometric contraction (MVIC).
Electromyographic Data Collection
These data were collected using previously published methods and analyzed by decomposition-enhanced spike-triggered averaging (DE-STA)15,16,20 as described below. The basic approach is to collect surface and intramuscular needle electrode EMG while subjects generate a fixed isometric force on the KinCom that represents a given percentages of their MVIC. The needle signal is used to identify individual motor units, while the surface EMG reflects the total muscle activity, and is decomposed to identify the surface representation of the individual motor units.
Electrical activity of the muscle was recorded using an active electrode over the vastus medialis (VM) muscle as the femoral nerve was electrically stimulated with a Teca bipolar stimulator. The inactive recording electrode was placed on the patellar tendon and ground electrode positioned between the stimulating and recording sites. The active and inactive recording electrodes were Teca 32mm diameter discs. The recording electrode was moved several times to assure that recording was over the innervation zone and that supra-maximal stimulation resulted in a compound muscle action potential (CMAP) of maximum amplitude and minimal rise time.
After determining MVIC, the concentric needle electrode was positioned in the VM directly beneath the surface electrode configuration. Since most physical activities are achieved at relatively low force levels, rather than at MVIC, data were collected during knee extension with subjects sustaining consistent knee extension contraction for a period of 20–30 seconds at intensities of 10, 20, 30 and 50 percent (%) effort relative to MVIC. EMG signals were amplified, filtered and acquired using a Clarke Davis Advantage Medical A100 EMG system (London, ON). Simultaneously detected intramuscular and surface detected EMG signals were acquired using band pass filtering from 10 Hz to 10 kHz and 5 Hz to 1000 Hz, and sampling rates of 25 kHz and 2.5 kHz, respectively. Repeated efforts were made at each force level with intramuscular electrode repositioning until 15–20 motor unit trains were sampled at each force level (coefficient of variation of approximately 10%)13. Motor unit (MU) trains and surface-represented motor unit action potentials (S-MUAP) were reviewed visually to verify accurate placement of onset and peak markers and S-MUAP peak-to-peak to baseline ratio of ≥ 10:1. Trains that contained fewer than 50 detected potentials or demonstrated inconsistent or non-physiologic firing properties were excluded from the analysis23.
To examine the extent of muscle activation during contractions, the surface EMG (S-EMG) was rectified, and the root mean square value of the EMG signal obtained over the 30-second contraction was calculated23, 24. S-EMG amplitude represents the average electrical activity of the muscle measurable by the electrode during the specific contraction. The DE-STA program allows a complex interference pattern to be broken down into single MU action potentials, relative to the measured S-EMG amplitude15, 16, 20, 23,25. S-MUAPs were analyzed and used to obtain estimates of mean MU firing rates (mFR) – the rate at which MUs discharge during the measured part of the contraction. S-MUAP area, although highly correlated with S-MUAP amplitude (r=0.97)16, 20, 25, was the preferred indicator of MU size for calculating the ratios described below.
Using the three primary measurements obtained from this method, S-MUAP area, mFR and S-EMG, several other properties were considered. Since S-EMG represents the total activity of the muscle, and the product of S-MUAP area and mFR represents the surface contribution to S-EMG of a single unit, the motor unit recruitment index (MURI) was calculated by dividing the S-EMG by the product of the S-MUAP area and mFR as an estimate of the number of active motor units at the site of EMG recording in the VM during each contraction16,20. Note that MURI is an indicator of the number of active unit assuming that factors that could potentially impact S-EMG are constant across subjects. The ratio of the estimated motor unit size relative to the force generated was calculated (S-MUAP area/force). The ratio of the number of active MUs relative to the force produced by the muscle contraction (MURI/force) was also calculated.
Co-variate information
Body mass index (BMI) was computed as weight (kg)/height (m²). Since body fatness could potentially influence the EMG data, body composition was assessed using a Lunar DPA densitometer (Lunar Corporation, Madison, WI).
Physical activity was assessed in a subset of participants using a pedometer (New Lifestyles Digi-walker SW-200, Yamax, Tokyo, Japan) that recorded the average number of steps taken over 3 days18, 26. The participants were asked to maintain their usual activity level and to wear the pedometer on a belt at hip level continuously throughout the day, except when swimming or bathing. Participants were instructed to keep a log of the number of steps walked each day, and also indicate when they wore and removed the pedometer. Limitations of the Digiwalker pedometer is the underestimating of counting steps at slower walking speeds27 and in overweight and obese individuals28, 29. Also, it does not capture all activity such as skiing, weight lifting, and bicycling.
Data Analysis
Analyses were completed in SPLUS 6.2 or 7.0 (In Sightful, Seattle, WA). Chi-square and t-tests were used to compare subject characteristics. All dependent variables were examined for deviation from normality. Terms that included S-MUAP were skewed and therefore log transformed for the analysis. Mixed effects models were used to examine the relationships between motor unit variables (S-MUAP area, mFR and MURI) OA grade and % effort. The mixed effects model included a random effect for subject. The model allowed for the study of all the motor units collected from each subject at each percent effort, while also accounting for the correlation between repeated measures. All models (except for S-EMG) had the following form:
Yij = (β0+bj0) + β1*OA gradej+β2*percentij+ β3*OA gradej*percentij
Where Yij represents the motor unit property for the ith motor unit and jth subject and bj0 being the random effect for subject j. S-EMG analysis used similar models except that an average value was calculated for each percent effort within a subject. The last term OA grade*%effort was included when examining the interaction between OA grade and %effort (force level). Fat mass and steps per day were added to models but were not significant and therefore excluded from the final models. We assumed that S-MUAP variance would increase as force increased, as larger units are sequentially activated with increasing variance, and modeled variance as a power function in relationship to the percent of effort. This assumption was found not to influence the reported findings. Statistical significance was accepted at P < 0.05.
RESULTS
Participants with OA included 11 women and 10 men with symptomatic, radiographically confirmed disease (4 each with KL grades = 1, 2 and 4, and 9 with grade 3) and analyzed together with 18 control participants (6 women and 12 men with KL= 0). As shown in Table 1, participants with knee OA exhibited lower isometric knee extensor strength (MVIC) than controls (P < 0.05), and also a trend towards lower mean S-EMG amplitude across all effort levels than controls (P = 0.095). Participants with OA also were heavier (30.5 vs 25.3; P = 0.022) and less active (2157 vs 8467 steps per day; P=0.0002).
Table 1.
Characteristics of Participants With and Without Knee Osteoarthritis
| Control (N=18) | Knee OA (N=21) | P- value | |
|---|---|---|---|
| Kellgren & Lawrence grade | |||
| 0 | 18 | 0 | |
| 1 | 4 | ||
| 2 | 4 | ||
| 3 | 9 | ||
| 4 | 4 | ||
| Age (years) | 66.14±2.3 | 65.49±2.8 | 0.861 |
| Female (N) | 6 | 11 | 0.423 |
| Maximal voluntary contraction | 461.33±41.14 | 344.12±24.29 | 0.016 |
| (MVIC, Newtons) | |||
| Surface EMG Amplitude (µV) | 104.64±11.55 | 79.689±9.168 | 0.095 |
| Body mass index (kg/m²) | 25.3±3.7 | 30.5±4.9 | 0.022 |
| Steps per day | 8467±2154 | 2157±1794 | 0.0002 |
Table 2 summarizes the knee extensor strength and S-EMG amplitude of the VM muscle by radiographic severity (KL grade). Participants with higher KL grades exhibited significantly lower knee extensor strength (MVIC) than participants with normal x-rays (KL grade=0; P=0.05). Participants with higher KL grades also exhibited significantly less effort-related increases in S-EMG amplitude than participants with lower KL grades (KL grade*%effort interaction P<0.0001). S-EMG amplitude was significantly lower in participants with higher KL grades at 30 and 50% MVIC (P<0.05).
Table 2.
Surface EMG Amplitude and Force by Radiographic Severity and Effort
| Kellgren & Lawrence Grade | 0 (N=18) | I (N=4) | II (N=4) | III (N=9) | IV (N=4) | P-Value | P-Value Interaction |
|---|---|---|---|---|---|---|---|
| MVIC (N) | 461.3±174.6 | 364.9±438.6 | 438.6±148.9 | 337.9±58.6 | 242.9±112.4 | 0.050 | |
| Surface-EMG Amplitude (µV*) | |||||||
| 10% | 53.6±26.1 | 81.3±16.2 | 76.3±40.8 | 46.6±21.4 | 43.3±11.1 | 0.800 | <.0001† |
| 20% | 84.5±37.0 | 120.7±18.61 | 110.7±54.3 | 66.0±31.2 | 48.8 | 0.255 | |
| 30% | 105.6±40.0 | 134.6±22.04 | 152.3±70.2 | 85.34±35.0 | 74.9±24.04 | 0.031 | |
| 50% | 173.22±71.8 | 194.3±47.6 | 236.8±146.9 | 123.0±39.8 | 92.7±26.3 | 0.031 | |
| MUs Detected of 2312 Total | 280 | 216 | 230 | 486 | 208 | ||
| S-MUAP Area (µV*msec) | |||||||
| 10% | 323.9±204.0 | 399.5±160.9 | 407.4±235.8 | 265.3±145.3 | 265.4±119.7 | 0.47 | <.0001† |
| 20% | 523.7±338.7 | 586.1±240.2 | 550.0±271.4 | 382.8±250.3 | 267.6±143.3 | 0.42 | |
| 30% | 650.6±398.6 | 714.9±281.3 | 731.3±367.9 | 508.0±316.0 | 416.6±198.1 | 0.18 | |
| 50% | 1078.9±818.3 | 1065.2±537.7 | 1035.8±563.6 | 791.4±441.0 | 500.1±251.4 | 0.06 | |
P-values from interactions using mixed effects model (OA by % effort).
We then examined the relationship between radiographic severity and MU activation characteristics (S-MUAP area, MURI, mFR) by comparing these characteristics by KL grade at each effort level. As shown in Figure 1A, S-MUAP area changed differently by KL grade as %effort increased (P<0.0001). In contrast, KL grade was not consistently associated with mean differences in firing rate (Figure 1B; P > 0.05); nor with the estimated number of active motor units (MURI) at any effort level (Figure 1C; p > 0.05).
Figure 1.
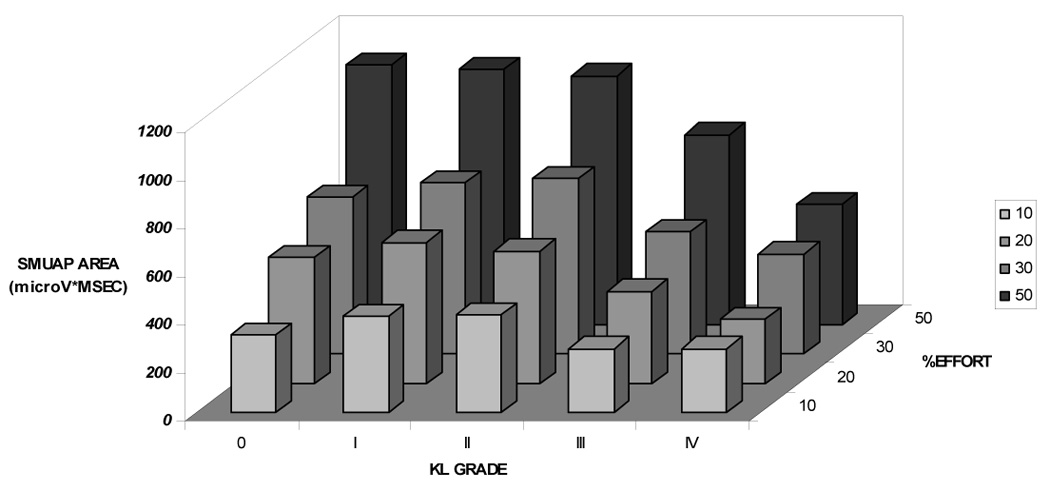
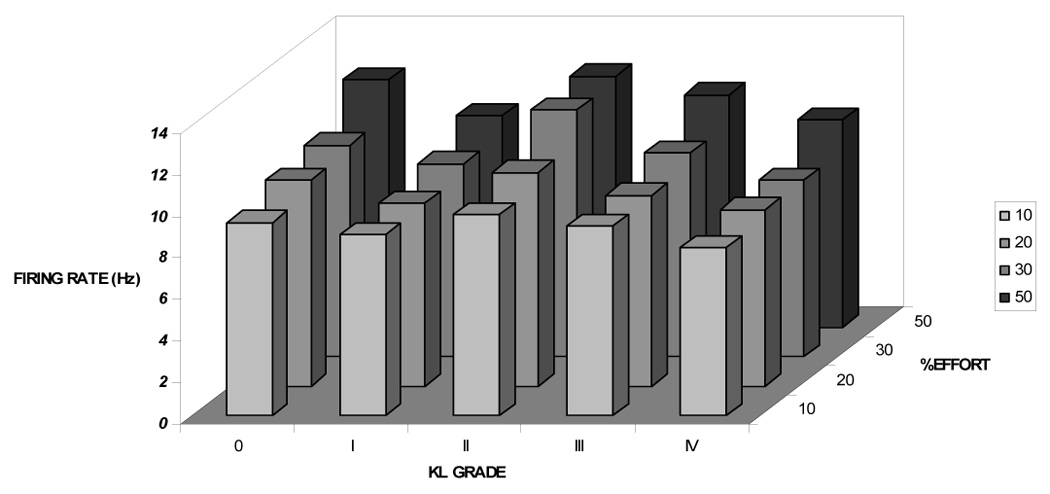

Figure 1A: Surface Representation of the Motor Unit Action Potential (S-MUAP) Area by Kellgren & Lawrence Grade and %Effort More advanced radiographic OA was associated with activation of smaller MUs (S-MUAP area) at lower effort levels that increased less as % effort increased compared to lower KL grades (P<0.0001), taking into account the observed effort-related increase in S-MUAP area.
Figure 1B: Firing rate (mFR) by KL Grade and %Effort mFR increased as %effort increased (P< 0.001), but comparably across all KL grades (interactive effect P > 0.05).
Figure 1C: Motor unit recruitment index (MURI) by KL grade and %Effort The estimated number of active motor units (MURI) also increased progressively as %effort increased (P<0.001), but did so comparably across all KL Grades (interactive effect P > 0.05).
Lastly, we assessed motor unit characteristics relative to the force achieved using ratios of S-MUAP area, MURI and mFR to force (MVIC*%effort). These models included KL grade, %effort and KL grade by %effort interaction terms to examine the extent to which these patterns changed differently by OA grade as effort increased. Figure 2A displays the size of motor units (S-MUAP area) activated per unit force by %effort for each KL grade. S-MUAP area per unit force changed differently by KL grade as %effort increased (Figure 2A; P <0.0001). Taking into account the main effects of %effort (p < 0.0001) and KL grade (KL grade 1; P =0.07), at 50% MVIC S-MUAP area per unit force was higher in participants with KL grade 1 (P=0.0545) and grade 4 (P=0.0004) compared to controls (Table 3). The index of the number of active units (MURI) per unit force at a given %effort also changed differently by OA grade, particularly with KL grade 4 (Figure 2B; P =0.0002). As shown in Table 4, MURI per unit force was higher in participants with KL grades 3 and 4 compared to controls at all effort levels (P<0.05), taking into account the main effects of %effort (P< 0.0001) and KL grade (KL grade 4; P=0.0001). Taken together, participants with more severe radiographic OA activated a greater number of larger motor units to achieve a given force level. In contrast, participants with less severe radiographic OA activated motor units which were larger, but not more numerous than controls.
Figure 2.
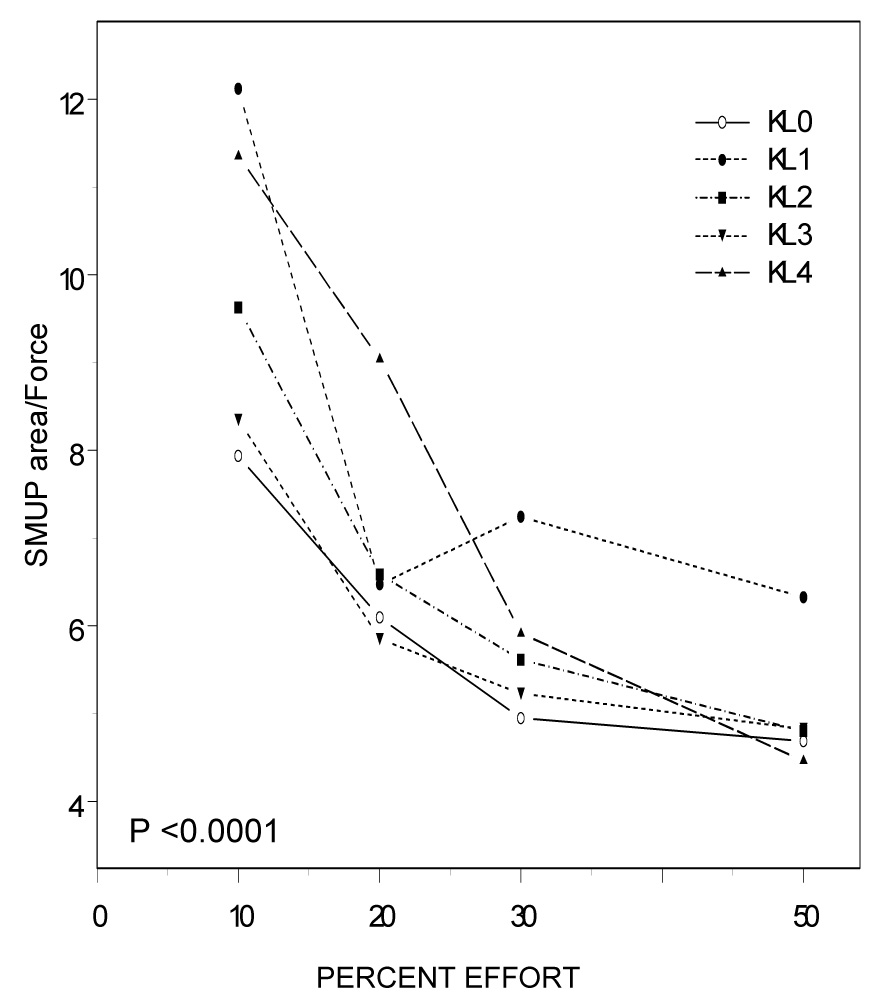
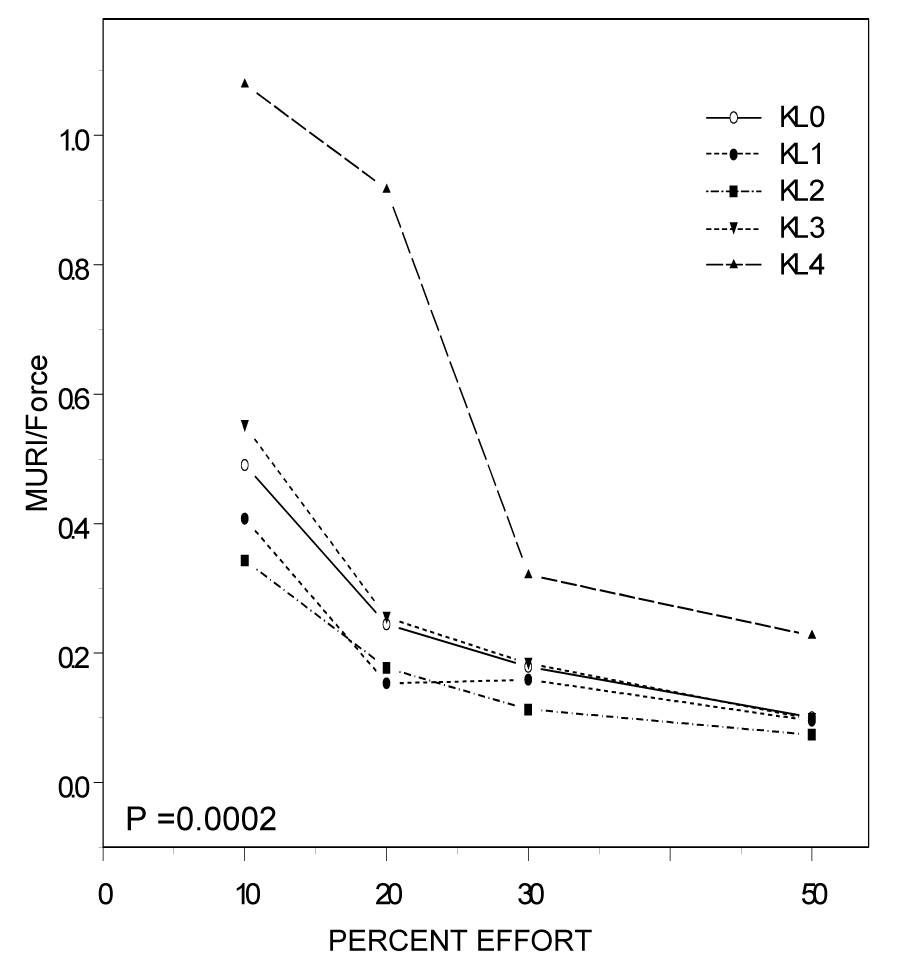
Figure 2A: Surface Detected Motor Unit Action Potential (S-MUAP) per Force (Uv*MSEC/N) by %Effort and Kellgren & Lawrence (KL) Grade Effort-related changes in S-MUAP area per unit force differed significantly by OA grade (P<0.0001). Specifically, OA group differences in S-MUAP area per unit force were most prominent at lower effort levels.
Figure 2B: Motor Unit Recruitment Index (MURI; 1/N) by %Effort and Kellgren & Lawrence (KL) Grade MURI per unit force changed differently as %effort increased by OA grade (P=0.0002). Interestingly, at lower effort levels MURI per unit force was significantly higher in participants with more severe radiographic OA and lower in participants with less severe disease.
TABLE 3.
Smuap area per force models
| B-Coefficient |
Std. Error |
P-Value |
|
|---|---|---|---|
| (Intercept) | 1.731 | 0.074 | <.0001 |
| Kellgren & Lawrence Grade Main Effect | |||
| Grade 1 | 0.199 | 0.107 | 0.0718 |
| Grade 2 | −0.015 | 0.073 | 0.8321 |
| Grade 3 | −0.037 | 0.040 | 0.3632 |
| Grade 4 | 0.021 | 0.042 | 0.6098 |
| Percent (%) MVIC Main Effect | |||
| 20% | −0.162 | 0.022 | <.0001 |
| 30% | −0.111 | 0.010 | <.0001 |
| 50% | −0.089 | 0.006 | <.0001 |
| Interactive Effects Between KL Grade and % MVIC | |||
| KL Grade 1 | |||
| 20% | 0.003 | 0.033 | 0.9123 |
| 30% | −0.011 | 0.151 | 0.4531 |
| 50% | −0.018 | 0.009 | 0.0545 |
| KL Grade 2 | |||
| 20% | −0.012 | 0.018 | 0.5178 |
| 30% | 0.005 | 0.009 | 0.5423 |
| 50% | −0.000 | 0.006 | 0.9715 |
| KL Grade 3 | |||
| 20% | 0.000 | 0.110 | 0.9466 |
| 30% | 0.000 | 0.005 | 0.9429 |
| 50% | 0.005 | 0.003 | 0.1365 |
| KL Grade 4 | |||
| 20% | −0.017 | 0.015 | 0.2585 |
| 30% | −0.006 | 0.006 | 0.3568 |
| 50% | −0.014 | 0.004 | 0.0004 |
TABLE 4.
Muri per force models
| B-Coefficient |
Std. Error |
P-Value |
|
|---|---|---|---|
| (Intercept) | 0.294 | 0.025 | <.0001 |
| Kellgren & Lawrence Grade Main Effect | |||
| Grade 1 | −0.019 | 0.036 | 0.5914 |
| Grade 2 | −0.018 | 0.024 | 0.4593 |
| Grade 3 | 0.012 | 0.013 | 0.3793 |
| Grade 4 | 0.066 | 0.014 | 0.0001 |
| Percent (%) MVIC Main Effect | |||
| 20% | −0.142 | 0.011 | <.0001 |
| 30% | −0.080 | 0.005 | <.0001 |
| 50% | −0.059 | 0.003 | <.0001 |
| Interactive Effects Between KL Grade and % MVIC | |||
| KL Grade 1 | |||
| * 20% | 0.005 | 0.016 | 0.7177 |
| * 30% | 0.007 | 0.007 | 0.3379 |
| * 50% | 0.004 | 0.004 | 0.3370 |
| KL Grade 2 | |||
| * 20% | 0.008 | 0.009 | 0.3234 |
| * 30% | 0.001 | 0.004 | 0.6818 |
| * 50% | 0.002 | 0.003 | 0.3832 |
| KL Grade 3 | |||
| * 20% | −0.011 | 0.005 | 0.0287 |
| * 30% | −0.004 | 0.002 | 0.0824 |
| * 50% | −0.004 | 0.001 | 0.0074 |
| KL Grade 4 | |||
| * 20% | −0.029 | 0.007 | <.0001 |
| * 30% | −0.022 | 0.003 | <.0001 |
| * 50% | 0.013 | 0.001 | <.0001 |
DISCUSSION
Weakness of the knee extensor muscles is associated with OA of the knee and contributes substantially to disease-related mobility limitations1,2,5. This study assessed the activation characteristics of functional motor units in the VM muscle in symptomatic knee OA across the spectrum of radiographic severity and in healthy control volunteers of comparable age by simultaneously examining EMG signals acquired through surface and intramuscular needle electrodes. The results of this study suggest that participants with knee OA exhibit less whole muscle activity by surface EMG at higher effort levels during knee extension. In addition, the surface represented motor unit area, changed differently as relative effort levels with less increase observed in subjects with higher compared to lower KL grades. Furthermore, participants with OA achieved a given force level by activating larger MUs–a difference of greatest magnitude at lower effort levels. Finally, the estimated number of units activated to achieve a given force level changed differently by KL grade as % effort increased. Taken together, these observations suggest that activation strategies differ between OA subjects and controls and also between KL grades.
The pattern of larger and more MUs activated per unit force achieved in subjects with higher KL grades suggest poorer muscle quality in subjects with more advanced disease. In contrast, the pattern of larger but fewer motor units per unit force that was observed in participants with lower grade OA would not be explained by poor muscle quality. The activation of larger but fewer MU’s to achieve a given force level observed in subjects with less severe OA may be related to the lower physical activity levels (fewer number of steps taken) exhibited by OA subjects, and is consistent with limited reports of increased MU amplitudes in association with knee immobilization for ligament or meniscus injury30. This pattern has also been described with re-innervation following nerve injury31 whereby orphaned muscle fibers are adopted by surviving adjacent lower motor neurons and re-organized into larger motor units. Additional explanations are that the observed MU size differences may reflect differences in muscle composition of a larger proportion of fast-twitch fibers or that thigh size differences between cases and controls may have resulted in differences in sampling depth32. Finally, simultaneously firing motor units (synchronization) would be detected as S-MUAP area of larger size and might also explain the larger MU’s observed with OA.
We can speculate that larger but fewer motor units would result in less neuromuscular control. These results complement reports of reduced force accuracy and steadiness in participants with knee OA who would typically overshoot when attempting to match 50N and 100N force targets33, and parallel observations of poor quadriceps muscle control and inappropriate quadriceps muscle activation in patients with anterior cruciate ligament deficiency34 – a known risk factor for OA development. We theorize that force exerted with less control could theoretically accelerate rather than prevent joint damage, particularly with coexisting proprioceptive deficits known to accompany OA35–37. This idea is consistent with Sharma and colleagues’ recent report of more rapid progression of knee OA in stronger subjects with malaligned knees compared to weaker subjects8, 9.
The observations in this study should be regarded as preliminary given the following limitations. Although the study sample included subjects with OA across the severity spectrum, this report was based upon a small sample in which the OA evaluation was limited to the tibiofemoral joint of the knee. Hence we cannot exclude the possibility that the observed changes might be influenced by patellofemoral OA, or might be attributable to OA in other locations such as the hip or spine. We also acknowledge that differences in physical activity levels and exercise habits between OA and control groups may potentially influence muscle composition and motor unit behavior, and were not adequately assessed in this study. We also acknowledge that the data were acquired during isometric knee extension testing at effort levels proportionate to each participant’s maximum voluntary contraction force. Although sub-maximal effort could explain the lower S-EMG values observed – particularly in participants with advanced OA, this would not explain the effort-related observations since the percentage effort examined was obtained relative to each individual’s maximum effort. Finally, data were analyzed using the spiked triggered averaging and the decomposition program (DE-STA); that although previously shown to reliably estimate motor unit numbers, may exhibit bias towards preferential sampling of larger motor units12,21. However, at low force levels this does not appear to be a problem. This method also provides mean values of firing rates across the motor units sampled that although consistent with the estimates observed in older adults previously published using different techniques38,39, do not yield information on firing rate variability between individual motor units15.
This study employs a unique approach to the problem of OA associated muscle weakness. This approach evaluates properties of the motor unit – the most discrete functional component of the muscle that imparts control of muscle activation, relative to force achieved by the whole muscle. This study uniquely examines MU activation patterns in the context of changes in knee extensor muscle strength across a spectrum of radiographic severity. Although not definitive, the results of this study suggest that muscle activation changes at the level of the motor unit in the context of symptomatic knee OA, and that this change is influenced by radiographic severity. Whether these changes are causal or in response to the development of OA will require further studies.
ACKNOWLEDGEMENTS
We thank Elizabeth M. Dredge and Laura M. Gabby for their assistance preparing this manuscript.
Funding Source: This research study was supported by the Intramural Research Program of the National Institutes of Health-National Institute on Aging, the National Institute of Neurological Disorders and Stroke, and the fund for Geriatric Medicine and Nursing at the Johns Hopkins University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer for publication: The views expressed are those of the author and do not reflect the official policy or position of USUHS, the Department of Defense, or the United States Government.
REFERENCES
- 1.Ling SM, Fried LP, Garrett ES, Fan MY, Rantanen T, Bathon JM. Knee osteoarthritis compromises early mobility function: The Women's Health and Aging Study II. J Rheumatol. 2003 Jan;30(1):114–120. [PubMed] [Google Scholar]
- 2.Ling SM, Xue QL, Simonsick EM, Tian J, Bandeen-Roche K, Fried LP, Bathon JM. Transitions to mobility difficulty associated with lower extremity osteoarthritis in high functioning older women: longitudinal data from the Women's Health and Aging Study II. Arthritis Rheum. 2006 Apr 15;55(2):256–263. doi: 10.1002/art.21858. [DOI] [PubMed] [Google Scholar]
- 3.Bennell KL, Hinman RS, Metcalf BR. Association of sensorimotor function with knee joint kinematics during locomotion in knee osteoarthritis. Am J Phys Med Rehabil. 2004 Jun;83(6):455–463. doi: 10.1097/00002060-200406000-00008. quiz 464–456, 491. [DOI] [PubMed] [Google Scholar]
- 4.Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004 Jan;22(1):110–115. doi: 10.1016/S0736-0266(03)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Baar ME, Dekker J, Lemmens JA, Oostendorp RA, Bijlsma JW. Pain and disability in patients with osteoarthritis of hip or knee: the relationship with articular, kinesiological, and psychological characteristics. J Rheumatol. 1998 Jan;25(1):125–133. [PubMed] [Google Scholar]
- 6.Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, Wolinski FD. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997 Jul 15;127(2):97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 7.Brandt KD, Heilman DK, Slemenda C, Katz BP, Mazzuca SA, Braunstein EM, Byrd D. Quadriceps strength in women with radiographically progressive osteoarthritis of the knee and those with stable radiographic changes. J Rheumatol. 1999 Nov;26(11):2431–2437. [PubMed] [Google Scholar]
- 8.Sharma L, Dunlop DD, Cahue S, Song J, Hayes KW. Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med. 2003 Apr 15;138(8):613–619. doi: 10.7326/0003-4819-138-8-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 9.Sharma L, Dunlop DD, Hayes KW. Is a strong quadriceps muscle bad for a patient with knee osteoarthritis? Ann Intern Med. 2004 Jan 20;140(2):150. doi: 10.7326/0003-4819-140-2-200401200-00030. [DOI] [PubMed] [Google Scholar]
- 10.Henneman E, Olson CB. Relations Between Structure And Function In The Design Of Skeletal Muscles. J Neurophysiol. 1965 May;28:581–598. doi: 10.1152/jn.1965.28.3.581. [DOI] [PubMed] [Google Scholar]
- 11.Doherty T, Simmons Z, O'Connell B, Felice KJ, Conwit R, Chan KM, et al. Methods for estimating the numbers of motor units in human muscles. J Clin Neurophysiol. 1995 Nov;12(6):565–584. doi: 10.1097/00004691-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 12.McComas AJ. Motor unit estimation: anxieties and achievements. Muscle Nerve. 1995 Apr;18(4):369–379. doi: 10.1002/mus.880180402. [DOI] [PubMed] [Google Scholar]
- 13.Conwit RA, Stashuk D, Tracy B, McHugh M, Brown WF, Metter EJ. The relationship of motor unit size, firing rate and force. Clin Neurophysiol. 1999 Jul;110(7):1270–1275. doi: 10.1016/s1388-2457(99)00054-1. [DOI] [PubMed] [Google Scholar]
- 14.Conwit RA, Stashuk D, Suzuki H, Lynch N, Schrager M, Metter EJ. Fatigue effects on motor unit activity during submaximal contractions. Arch Phys Med Rehabil. 2000 Sep;81(9):1211–1216. doi: 10.1053/apmr.2000.6975. [DOI] [PubMed] [Google Scholar]
- 15.Conwit RA, Tracy B, Cowl A, McHugh M, Stashuk D, Brown WF, Metter EJ. Firing rate analysis using decompostion-enhanced spike triggered averaging in the quadriceps femoris. Muscle Nerve. 1998 Oct;21(10):1338–1340. doi: 10.1002/(sici)1097-4598(199810)21:10<1338::aid-mus17>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 16.Conwit RA, Tracy B, Jamison C, McHugh M, Stashuk D, Brown WF, Metter EJ. Decomposition-enhanced spike-triggered averaging: contraction level effects. Muscle Nerve. 1997 Aug;20(8):976–982. doi: 10.1002/(sici)1097-4598(199708)20:8<976::aid-mus7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Lewek MD, Rudolph KS, Snyder-Mackler L. Control of frontal plane knee laxity during gait in patients with medial compartment knee osteoarthritis. Osteoarthritis Cartilage. 2004 Sep;12(9):745–751. doi: 10.1016/j.joca.2004.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talbot LA, Gaines JM, Ling SM, Metter EJ. A home-based protocol of electrical muscle stimulation for quadriceps muscle strength in older adults with osteoarthritis of the knee. J Rheumatol. 2003 Jul;30(7):1571–1578. [PubMed] [Google Scholar]
- 19.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957 Dec;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conwit RA, Ling S, Roth S, Stashuk D, Hurley B, Ferrell R, Metter EJ. The relationship between ciliary neurotrophic factor (CNTF) genotype and motor unit physiology: preliminary studies. BMC Physiol. 2005 Sep 23;5:15. doi: 10.1186/1472-6793-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol. 1997 Nov;83(5):1581–1587. doi: 10.1152/jappl.1997.83.5.1581. [DOI] [PubMed] [Google Scholar]
- 22.Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin J, Roy TA, et al. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol. 1999 Jan;86(1):188–194. doi: 10.1152/jappl.1999.86.1.188. [DOI] [PubMed] [Google Scholar]
- 23.Boe SG, Stashuk DW, Doherty TJ. Motor unit number estimation by decomposition-enhanced spike-triggered averaging: control data, test-retest reliability, and contractile level effects. Muscle Nerve. 2004 May;29(5):693–699. doi: 10.1002/mus.20031. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H, Conwit RA, Stashuk D, Santarsiero L, Metter EJ. Relationships between surface-detected EMG signals and motor unit activation. Med Sci Sports Exerc. 2002 Sep;34(9):1509–1517. doi: 10.1097/00005768-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Stashuk DW. Decomposition and quantitative analysis of clinical electromyographic signals. Med Eng Phys. 1999 Jul-Sep;21(6–7):389–404. doi: 10.1016/s1350-4533(99)00064-8. [DOI] [PubMed] [Google Scholar]
- 26.Talbot LA, Gaines JM, Huynh TN, Metter EJ. A home-based pedometer-driven walking program to increase physical activity in older adults with osteoarthritis of the knee: a preliminary study. J Am Geriatr Soc. 2003 Mar;51(3):387–392. doi: 10.1046/j.1532-5415.2003.51113.x. [DOI] [PubMed] [Google Scholar]
- 27.Bassett DR, Jr, Ainsworth BE, Leggett SR, Mathien CA, Main JA, Hunter DC, Duncan GF. Accuracy of five electronic pedometers for measuring distance walked. Med Sci Sports Exerc. 1996 Aug;28(8):1071–1077. doi: 10.1097/00005768-199608000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Crouter SE, Schneider PL, Bassett DR., Jr Spring-levered versus piezo-electric pedometer accuracy in overweight and obese adults. Med Sci Sports Exerc. 2005 Oct;37(10):1673–1679. doi: 10.1249/01.mss.0000181677.36658.a8. [DOI] [PubMed] [Google Scholar]
- 29.Melanson EL, Knoll JR, Bell ML, Donahoo WT, Hill JO, Nysse LJ, et al. Commercially available pedometers: considerations for accurate step counting. Prev Med. 2004 Aug;39(2):361–368. doi: 10.1016/j.ypmed.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 30.Hoyer A, Eickhoff W, Rumberger E. Alterations in electromyograms due to inactivity-induced atrophy of the human muscle. Electromyogr Clin Neurophysiol. 2000 Jul-Aug;40(5):267–274. [PubMed] [Google Scholar]
- 31.Andersen H, Stalberg E, Gjerstad MD, Jakobsen J. Association of muscle strength and electrophysiological measures of reinnervation in diabetic neuropathy. Muscle Nerve. 1998 Dec;21(12):1647–1654. doi: 10.1002/(sici)1097-4598(199812)21:12<1647::aid-mus4>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 32.Knight CA, Kamen G. Superficial motor units are larger than deeper motor units in human vastus lateralis muscle. Muscle Nerve. 2005 Apr;31(4):475–480. doi: 10.1002/mus.20265. [DOI] [PubMed] [Google Scholar]
- 33.Hortobagyi T, Garry J, Holbert D, Devita P. Aberrations in the control of quadriceps muscle force in patients with knee osteoarthritis. Arthritis Rheum. 2004 Aug 15;51(4):562–569. doi: 10.1002/art.20545. [DOI] [PubMed] [Google Scholar]
- 34.Williams GN, Barrance PJ, Snyder-Mackler L, Buchanan TS. Altered quadriceps control in people with anterior cruciate ligament deficiency. Med Sci Sports Exerc. 2004 Jul;36(7):1089–1097. doi: 10.1249/01.mss.0000131959.20666.11. [DOI] [PubMed] [Google Scholar]
- 35.Barrett DS, Cobb AG, Bentley G. Joint proprioception in normal, osteoarthritic and replaced knees. J Bone Joint Surg Br. 1991 Jan;73(1):53–56. doi: 10.1302/0301-620X.73B1.1991775. [DOI] [PubMed] [Google Scholar]
- 36.Sharma L. Proprioceptive impairment in knee osteoarthritis. Rheum Dis Clin North Am. 1999 May;25(2):299–314. doi: 10.1016/s0889-857x(05)70069-7. vi. [DOI] [PubMed] [Google Scholar]
- 37.Sharma L, Pai YC. Impaired proprioception and osteoarthritis. Curr Opin Rheumatol. 1997 May;9(3):253–258. doi: 10.1097/00002281-199705000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Kamen G. Aging, resistance training, and motor unit discharge behavior. Can J Appl Physiol. 2005 Jun;30(3):341–351. doi: 10.1139/h05-126. [DOI] [PubMed] [Google Scholar]
- 39.Kamen G, Knight CA. Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci. 2004 Dec;59(12):1334–1338. doi: 10.1093/gerona/59.12.1334. [DOI] [PubMed] [Google Scholar]


