Abstract
Many factors contribute to the creation and maintenance of a realistic environment for cell growth in vitro, e.g. the consistency of the growth medium, the addition of supplements, and the surface on which the cells grow. The nature of the surface on which cells are cultured plays an important role in their ability to attach, proliferate, migrate and function. Components of the extracellular matrix (ECM) are often used to coat glass or plastic surfaces to enhance cell attachment in vitro. Fragments of ECM molecules can be immobilised on surfaces in order to mimic the effects seen by whole molecules. In this study we evaluate the application of a novel technology for the immobilisation of functional domains of known ECM proteins in a controlled manner on a surface. By examining the adherence of cultured PC12 cells to alternative growth surfaces, we show that surfaces coated with motifs from collagen I, collagen IV, fibronectin and laminin can mimic surfaces coated with the corresponding whole molecules. Furthermore, we show that the adherence of cells can be controlled by modifying the hydropathic properties of the surface to either enhance or inhibit cell attachment. Collectively, these data demonstrate the application of a new technology to enable optimisation of cell growth in the tissue culture laboratory.
Keywords: Cell attachment, Cell culture surface, Collagen, Fibronectin, Laminin, PC12 cells
Introduction
Laboratory cultured cells are widely used in the field of biomedical science and provide scientists with model systems to investigate cell activity. The process of cell culture involves the removal of cells from their natural (in vivo) location and their subsequent growth in an artificial (in vitro) environment. Optimisation of the culture environment is critical to enable cells to grow, function and survive in a realistic and representative manner. There are many parameters that must be considered in this process including the constituents of the media, growth supplements, and the surface upon which cells grow. Often it is necessary to tailor the culture environment for optimal growth of a particular cell type. For example, some cell types do not attach to standard tissue culture plastic or glass unless it is coated with an appropriate growth molecule that more accurately represents the environment their native counterparts experience.
Within a tissue, cells are in contact with neighbouring cells and the extracellular matrix (ECM). The ECM is a complex milieu of molecules consisting of proteoglycans and insoluble fibrous proteins. Interactions between cells and ECM components are important in many biological processes such as cell growth and development (Adams and Watt 1993; Kleinman et al. 2003; Schwarz et al. 1990). In general cells utilise integrins, a family of transmembrane glycoproteins, to attach to specific cell binding sites that are displayed by ECM proteins such as collagen, fibronectin and laminin. The integrins are able to link the cell cytoskeleton to a material surface via these proteins present on the surface. Adsorption or immobilisation of specific ECM proteins can therefore be used to enhance cell attachment. In order to mimic the in vivo environment, purified components of the ECM are frequently used in the laboratory to coat cell culture plastic or glass to enhance cell adhesion (Kleinman et al. 1987). The biological effects elicited by key components of the ECM can be attributed to certain short peptide motifs within the whole molecule (Aumailley et al. 1987; Boateng et al. 2005; Goodman et al. 1987; Ranieri et al. 1995; Saneinejad and Shoichet 1998; Yamada and Kleinman 1992).
There are numerous methods by which such peptide motifs can be immobilised onto a surface (review: Love et al. 2005) e.g. direct adsorption of protein from solution (Fujii et al. 1982) or modifying the molecular characteristics of the surface using complex chemistry (Branch et al. 1998). However, these approaches have their limitations: the surface coating process is difficult to control, adsorption may lead to denaturation of the protein resulting in reduced functionality, the orientation and density of the protein are difficult to control leading to batch inconsistency and reduced reproducibility. Covalent attachment of protein by chemical coupling also results in substantial loss of protein activity owing to chemical modification of critical residues, denaturation and random orientation and inaccessibility (Lu et al. 1996). These limitations are problematic in cell culture. Accordingly, there is demand for improved surfaces for in vitro cell growth that possess known composition and more accurately represent a biologically relevant environment. Such surfaces must also be manufactured in a controlled fashion to reduce batch-to-batch variability and increase the consistency and robustness of the cell culture grown on such surfaces.
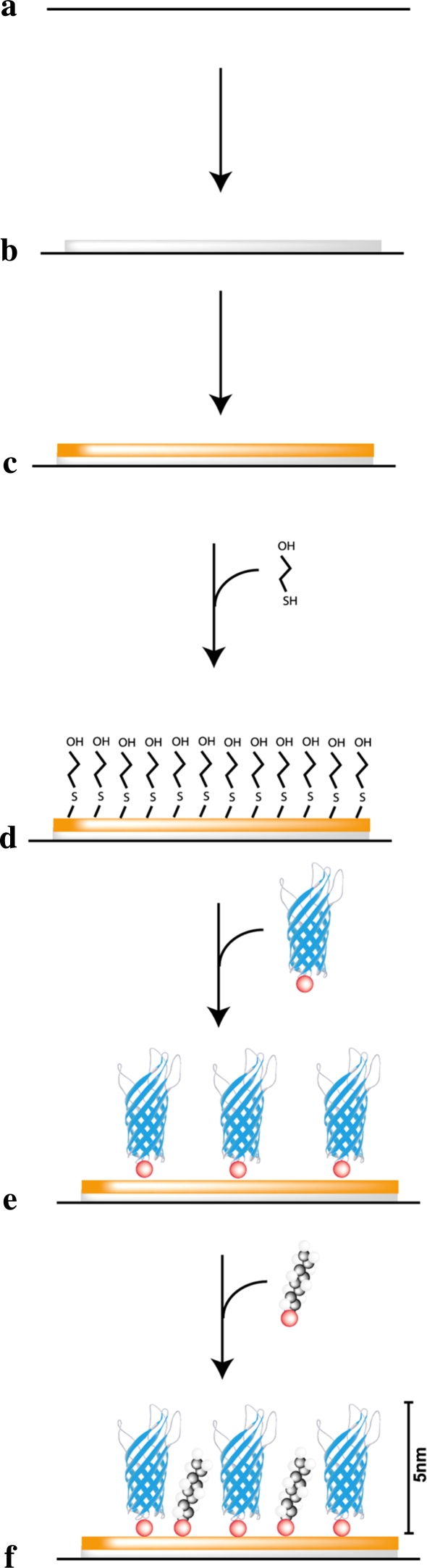
In this study, we have adapted an existing technology that enables the stable self-assembly of proteins on surfaces (Shah et al. 2007), to develop a novel tool for applications in cell culture (Fig. 1). The technology employs engineered variants of the N-terminal transmembrane (TM) domain from the Escherichia coli outer membrane protein, OmpA (Tamm et al. 2001). The motifs from ECM proteins are engineered into the flexible outer loops OmpA. The protein attaches to the cell culture surface via a single cysteine residue thereby forming an oriented monolayer. Gaps between protein molecules are filled in using thiolipids or thioalkanes so that only the surface loops and the motifs of interest are exposed. This method of protein immobilisation results in stable, oriented monolayers that display only the motifs of interest in a functional conformation (Tamm et al. 2001) and alleviates many of the problems associated with adsorption and chemical methods (Athey et al. 2005).
Fig. 1.
Schematic representation of the manufacture of self-assembled protein monolayers. (a) Borosilicate glass coverslips are first cleaned to remove any residues; (b) coverslips are coated with titanium to facilitate the attachment of gold; (c) gold is sputtered coated onto the surface; (d) the surface is passivated by the addition of β-mercaptoethanol to promote the attachment of OmpA protein; (e) OmpA molecules are allowed to self-assemble on the surface and attach via the unique cystein residue (red). OmpA proteins are orientated such that any motifs inserted in the β-barrel will be presented distal to the gold substratum; (f) thioalkane molecules self-assemble onto the gold surface filling gaps between the attached OmpA proteins forming an intact molecular monolayer
To evaluate the effectiveness of this technology in promoting the attachment of cultured cells to glass surfaces, we investigated the growth and adherence of the neuron-like rat pheocytochroma cell line, PC12, on OmpA surfaces presenting peptide motifs from ECM proteins. PC12 cells attach poorly to uncoated glass and in order for the cells to attach and grow as an adherent monolayer the culture surface must be coated with ECM protein (Schwarz et al. 1990; Tomaselli et al. 1987). Specifically, we tested the ability of ECM protein motifs derived from, collagen, fibronectin and laminin presented on OmpA in a protein monolayer to rescue the attachment of PC12 cells.
Materials and methods
Cell culture
Cultures of PC12 cells were purchased from American Type Culture Collection (ATCC; http://www.lgcpromochem-atcc.com) and were maintained according to a previously described method (Greene and Tischler 1976). In brief, cell stocks were routinely grown on cell culture plasticware (VRW; http://uk.vwr.com) which were pre-coated with a 0.1% solution of collagen IV from human placenta (Sigma-Aldrich; http://www.sigmaaldrich.com) in 0.1 M glacial acetic acid (Sigma-Aldrich) for 24 h at 37 °C. PC12 cells were grown in RPMI 1640 medium (Cambrex; http://www.cambrex.com) supplemented with 10% heat-inactivated foetal bovine serum (Invitrogen; http://www.invitrogen.com/), 2 mM l-glutamine (Cambrex), 20 units/mL of penicillin and 20 μg/mL streptomycin (Invitrogen). Cultures were maintained at 37 °C in a humidified 5% CO2 incubator. The medium was changed three times a week and cells were passaged as required using trypsin–EDTA solution as standard (Cambrex).
Coating glass coverslips with purified ECM proteins
Borosilicate glass coverslips were first sterilised by immersion in 70% ethanol for 15 min and then washed twice with sterile PBS (Cambrex). Coverslips were coated with solutions of either collagen I (Sigma-Aldrich) diluted in 0.1 M glacial acetic acid, collagen IV (as previously described herein); fibronectin (Sigma-Aldrich) diluted in sterile ddH2O; laminin (Sigma-Aldrich) diluted in sterile PBS (Cambrex). Collagen, laminin and fibronectin were coated at concentrations ranging from 1 ng/mL to 1 mg/mL. Collagen and laminin coatings were carried out at 37 °C while coating with fibronectin was conducted at room temperature. Coverslips were immersed in the coating solution for 24 h. Surfaces were washed twice with PBS to remove any traces of unadsorbed molecules and placed in fresh multi-well culture plate.
Protein engineering
The peptide motifs in ECM components most likely to enhance cell attachment were identified from literature (see Table 1). Oligonucleotides designed to encode the relevant motifs were synthesised (Sigma Genosys; http://www.sigmaaldrich.com/Brands/Sigma_Genosys.html). Annealed oligonucleotides were ligated into the gene encoding the modified OmpA TM domain scaffold such that the motif was within outer loop-1 of OmpA. Four different plasmids were constructed with motifs as shown in Table 1. The parental modified OmpA was used as a negative control. The DNA sequence of each construct was verified before use. All constructs thus possess a 6×His tag and a cysteine residue at the N-terminus.
Table 1.
ECM motifs fused to outer loop of OmpA
| ECM protein from which peptide motif derived | Peptide motif fused to OmpA | References |
|---|---|---|
| Collagen I | GTPGPQGIAGQRGVV | Nguyen et al. (2003), Thorwarth et al. (2005) |
| Collagen IV | MNYYSNS | Floquest et al. (2004), Han et al. (1997), Pedchenko et al. (2004) |
| Fibronectin | PHSRN | Aota et al. (1994), Dankers et al. (2005), Feng and Mrksich (2004), Mardilovich and Kokkoli (2004) |
| Laminin | YIGSR | Boateng et al. (2005), Ranieri et al. (1995), Saneinejad and Shoichet et al. (1998), Tong and Shoichet (1998) |
Table provides information about the motifs tested and citation of the appropriate literature for further information about the corresponding functional domains
Expression, purification and refolding
Exponential phase cultures of E. coli BL21 (Novagen; http://www.merckbiosciences.co.uk) containing expression plasmid were induced with isopropyl-β-d-thiogalactopyranoside and shaken for 6 h at 37 °C. After harvest and lysis, the insoluble fraction was enriched and solubilised in binding buffer (8 M Urea, 20 mM imidazole, 0.5 M NaCl, 20 mM sodium phosphate pH 7.4). The OmpA based proteins were purified by Ni-affinity using an AKTA Prime system (GE Healthcare; http://www.gehealthcare.com/worldwide.html) with a step elution at 250 mM imidazole. The fractions containing the desired proteins were further purified on a QFF anion exchange column (GE Healthcare) in 30 mM ethanolamine buffer at pH 9.5 with elution in the same buffer plus 80 mM NaCl. The proteins were shown to be >98% homogenous as judged by gel electrophoresis (data not shown).
The proteins were refolded by very slow dilution (1/40) into refold buffer (50 mM Tris–HCl, 0.1 mM EDTA, 1% n-octylglucopyranoside, 1 mM dithiothreitol pH 8.0). Correct refolding was indicated by bandshift on SDS polyacrylamide gel electrophoresis (Tamm et al. 2001) and correct formation of the β-barrel structure confirmed by circular dichroism spectroscopy (data not shown).
Preparation of culture surfaces
Borosilicate glass coverslips (VWR) were cleaned and sputter coated with a 10 nm layer of titanium followed by a 25 nm layer of gold. The protein–thioalkane monolayer was manufactured essentially as described (Shah et al. 2007), Fig. 1. Briefly, proteins were diluted to 2 mg mL−1 (330 ng/cm2) in refold buffer (see above) and hexadecanethiol (HDT) (Sigma) was diluted to 0.15 mg mL−1 (11μg/cm2) in ethanol. The gold surfaces were cleaned in 1% Hellmanex (Hellma; http://www.hellma.co.uk). The surfaces were passivated by incubation with 1% (v/v in ddH2O) 2-mercaptoethanol (Fisher; http://www.fisher.co.uk) and washed with ddH2O. Protein was applied to the surface in three 1 h incubations with 1% SDS and ddH2O washes in between each protein application. After the last protein application the gaps between the immobilised protein molecules were filled in by overnight incubation with HDT solution followed by washes with ethanol followed by 1% SDS and finally ddH2O. Surface plasmon resonance demonstrated that 1.5 ng protein had bound per mm2 (data not shown). The coverslips with fully assembled surfaces were dried under N2 and stored at 4 °C with desiccant until required for cell culture. Control surfaces with HDT only or untreated gold were also prepared and stored.
Prior to use, the coverslips were sterilised by immersion in 3 mL 70% ethanol for 15 min followed by two washes with 5 mL sterile phosphate buffered saline (PBS) (Cambrex). There is no evidence that the sterilisation process affects the activity of the culture surface.
Cell attachment assay
PC12 cells growing on cell culture plastic were removed by trypsinisation to produce a single cell suspension and the number of cells was determined using a haemocytometer. Cells were subsequently seeded at a density of 300,000 cells/well of a 6-well culture plate (VWR) containing coverslips presenting alternative growth surfaces. Cells were maintained at 37 °C in a humidified, 5% CO2 incubator for 24 h. Cultures were washed once with 2 mL PBS and fixed using 4% parafomaldehyde (Sigma-Aldrich) in PBS for 30 min, washed twice with PBS and mounted using 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen) in Vectashield (Vector Laboratories; http://www.vectorlabs.com). Cells were visualised using a Nikon Diaphot 300 fluorescence microscope and digital images recorded using a Nikon Digital Camera DXM1200. Nine random fields of view were sampled using the 10× objective lens for each surface tested. Experiments were repeated in triplicate.
Statistical analysis
For normally distributed data an ANOVA test was preformed. For data that were not normally distributed a Kruskal–Wallis test was conducted and subsequent post hoc analysis was carried out as described elsewhere (Zar 1996). P < 0.05 (*), P < 0.005 (**) and P < 0.001 (***) were used to indicate levels of statistical significance.
Results and discussion
The growth of cells outside the body in the laboratory requires the development of technologies to recreate the natural environment cells experience in living tissues for optimal cell survival, growth and function. In this study, we have used an existing technology to present known biologically active molecules to living cells in a highly controlled manner (Fig. 1). We propose that this culture surface can be used to present the active peptide domain of proteins to cells in an appropriate molecular configuration to promote the adherence of cultured cells to a glass surface.
Coating glass surfaces is necessary for the attachment of PC12 cells
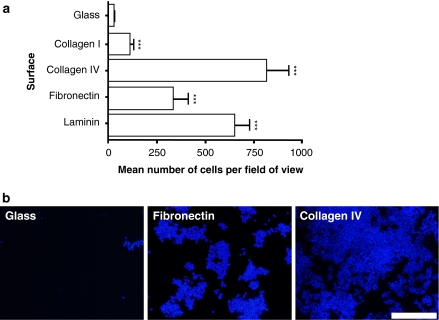
In agreement with earlier work (Schwarz et al. 1990; Tomaselli et al. 1987), our data showed that PC12 cells attached poorly to uncoated glass surfaces (Fig. 2). In contrast, coverslips with adsorbed collagen I, collagen IV, fibronectin or laminin significantly increased the adherence of cells (Fig. 2). Cell attachment was proportional to rising concentrations of adsorbed ECM protein up to a point after which no further increase in cell attachment was observed (data not shown). Effective initial coating concentrations for optimal cell attachment were achieved for collagen I (250 μg/mL), collagen IV (8 μg/mL), fibronectin (50 μg/mL) and laminin (10 μg/mL). Collagen IV and laminin were particularly effective at enabling the attachment of PC12 cells (Fig. 2). In addition, control glass surfaces prepared with acetic acid, ddH2O or PBS alone in the absence of ECM protein did not result in enhanced cell attachment (data not shown).
Fig. 2.
Coating glass surfaces affects the attachment of PC12 cells. (a) Bar chart showing the differential adhesion of cultured cells to alternative surfaces including: uncoated glass; glass coated with adsorbed ECM proteins collagen I (250 μg/mL), collagen IV (8 μg/mL), fibronectin (50 μg/mL) and laminin (10 μg/mL). Data represent mean number of cells per field of view (nine randomly selected fields were examined per surface) for three independent experiments (+SEM). Values for each condition were compared to the mean number of cells attached to uncoated glass surfaces. (b) Fluorescence micrographs of cultures grown on either glass surfaces coated with adsorbed fibronectin protein (50 μg/mL) or adsorbed collagen IV protein (8 μg/mL) (see text for further details). DAPI staining revealed large numbers of cells attached to fibronectin coated surfaces whilst there was little if any evidence of cells adhering to glass. Scale bar: 100 μm
Culture surfaces with ECM domains expressed in OmpA enhance cell attachment
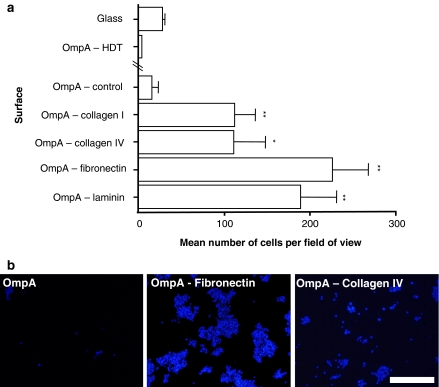
The level of cell attachment to culture surfaces consisting of the OmpA scaffold with or without the insertion of various ECM molecule motifs was examined (Fig. 3). Only a low level of cell attachment was observed on surfaces containing only OmpA filled in with HDT on gold (OmpA-control). The inclusion of sequences from the functional domains of the ECM proteins, collagen I, collagen IV, fibronectin or laminin, into OmpA significantly enhanced the attachment of PC12 cells compared to OmpA-control surfaces. We directly compared the level of cell attachment for known amounts of solubilised ECM protein absorbed to glass with the number of cells attached to OmpA surfaces expressing the corresponding ECM domain. Each ECM domain tested had levels of cell attachment equivalent to the following initial coating concentrations of their corresponding adsorbed protein: collagen I 129 μg/mL; collagen IV 0.1 μg/mL; fibronectin 18 μg/mL; laminin 2.0 μg/mL.
Fig. 3.
Surfaces displaying ECM motifs presented by the OmpA molecule enhance the attachment of cultured cells. (a) Bar chart showing the differential adhesion of PC12 cells to surfaces coated with self-assembled OmpA or OmpA-ECM motif fusion protein. The protein surfaces were all completed by filling in with HDT. Data represent mean number of cells per field of view (nine randomly selected fields were examined per surface) for three independent experiments (+SEM). Values for each OmpA-ECM motif condition were compared to the number of cells attached to surfaces coated with OmpA without a motif insertion (OmpA, control). (b) Fluorescence micrographs of cultures grown on surfaces consisting of either OmpA (OmpA-control, without any ECM motif insertion) or OmpA carrying a motif from the ECM protein, fibronectin (OmpA-fibronectin) or collagen IV (OmpA-collagen IV). Scale bar: 100 μm
Although some PC12 cells appeared to adhere to the OmpA-control surface, such binding was weak and cells were dislodged relatively easily unlike cultures grown on OmpA surfaces presenting an ECM domain. The gold-coated coverslips with only the HDT monolayer appeared to completely inhibit cell attachment (Fig. 3). HDT monolayers present a flat, very hydrophobic surface thereby creating an unfavourable environment for cell attachment. The presence of OmpA even without any ECM protein motifs appears to alleviate this effect slightly, but where an ECM motif is present this effect is almost completely overcome. Therefore, it is likely that there is some molecular recognition of the ECM components on the surface by the cells. In addition, the presence of protein within the HDT increases the topographical features on the surface that the cells may also recognise as favourable for attachment and growth (Karuri et al. 2004).
Interestingly, the distribution of cells over the culture surface was not homogeneous for each condition tested. For example, PC12 cells attached to glass surfaces coated with adsorbed fibronectin protein grew as aggregations of cells (Fig. 2b) whereas cells grown on collagen-coated surfaces were more evenly distributed. Similarly, cells grown on culture surfaces using OmpA to present the fibronectin domain formed clumps (Fig. 3b) whereas collagen motifs resulted in a more even cell distribution.
In contrast to the data from experiments coating glass with solubilised whole protein (Fig. 2), it was surfaces displaying the fibronectin motif, PHSRN, which enabled the greatest levels of cell attachment (Fig. 3). Each surface was manufactured such that the concentration of OmpA molecules presenting the different ECM domains was approximately equivalent. Thus it appears that displaying the PHSRN motif of fibronectin in this way has somehow enhanced its activity compared to the complete fibronectin molecule. In comparison to PHSRN in initial experiments, we found that the more widely investigated RGD motif was not as effective in our system when presented to PC12 cells combined with a HDT infill background. Many factors affect the efficacy of immobilised peptides on surfaces: e.g. the distance between the peptide and the substratum (Houseman and Mrksich 2001; Tong and Shoichet 2001); the immediate environment surrounding the functional domain (Dori et al. 2000); the orientation and configuration of the motif and its secondary structure (Ochsenhirt et al. 2006). These factors can affect the accessibility of the active part of the peptide to the cell, the activity of the peptide and the topography of the surface.
In summary, we have shown that self-assembled monolayers of OmpA molecules can be used as scaffolds to present the functional domain of ECM proteins to enhance the adherence of cultured cells to glass surfaces. This approach enables greater control over the molecular configuration of peptide sequences presented to cells. In addition, these surfaces possess a defined composition that can be finely controlled during manufacture. By altering the components of the surface, we can either inhibit or enhance cell attachment. An obvious advantage of this technology is that mixtures of peptide motifs from different source proteins can be displayed on the same surface.
Future experiments will demonstrate the versatility of this technology and will involve the inclusion of mixtures of different ECM functional domains at appropriate concentrations to more closely model the structure of the microenvironment found in tissues. Consequently, we propose that cells cultured on such surfaces will be exposed to an environment that more closely resembles that experienced by their native counterparts in vivo and thus function in a more realistic manner.
Acknowledgements
This work was supported by Orla Protein Technologies Ltd. (http://www.orlaproteins.com) and The Biotechnology and Biological Sciences Research Council (BBSRC, http://www.bbsrc.ac.uk).
References
- Adams JC, Watt FM (1993) Regulation of development and differentiation by the extracellular matrix. Development 117:1183–1198 [DOI] [PubMed]
- Aota S, Nomizu M, Yamada KM (1994) The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J Biol Chem 269:24756–24761 [PubMed]
- Athey D, Shah DHS, Phillips SR, Lakey JH (2005) A manufacturable surface-biology platform for nano applications; cell culture, analyte detection, diagnostics sensors. Ind Bioetchnol 1:185–189 [DOI]
- Aumailley M, Nurcombe V, Edgar D, Paulsson M, Timpl R (1987) The cellular interactions of laminin fragments. Cell adhesion correlates with two fragment-specific high affinity binding sites. J Biol Chem 262:11532–11538 [PubMed]
- Boateng SY, Lateef SS, Mosley W, Hartman TJ, Hanley L, Russell B (2005) RGD and YIGSR synthetic peptides facilitate cellular adhesion identical to that of laminin and fibronectin but alter the physiology of neonatal cardiac myocytes. Am J Physiol Cell Physiol 288:C30–C38 [DOI] [PubMed]
- Branch DW, Corey JM, Weyhenmeyer JA, Brewer GJ, Wheeler BC (1998) Microstamp patterns of biomolecules for high-resolution neuronal networks. Med Biol Eng Comput 36:135–141 [DOI] [PubMed]
- Dankers PY, Harmsen MC, Brouwer LA, van Luyn MJ, Meijer EW (2005) A modular and supramolecular approach to bioactive scaffolds for tissue engineering. Nat Mater 4:568–574 [DOI] [PubMed]
- Dori Y, Bianco-Peled H, Satija SK, Fields GB, McCarthy JB, Tirrell M (2000) Ligand accessibility as means to control cell response to bioactive bilayer membranes. J Biomed Mater Res 50:75–81 [DOI] [PubMed]
- Feng Y, Mrksich M (2004) The synergy peptide PHSRN and the adhesion peptide RGD mediate cell adhesion through a common mechanism. Biochemistry 43:15811–15821 [DOI] [PubMed]
- Floquet N, Pasco S, Ramont L, Derreumaux P, Laronze JY, Nuzillard JM, Maquart FX, Alix AJ, Monboisse JC (2004) The antitumor properties of the alpha3(IV)-(185–203) peptide from the NC1 domain of type IV collagen (tumstatin) are conformation-dependent. J Biol Chem 279:2091–2100 [DOI] [PubMed]
- Fujii DK, Massoglia SL, Savion N, Gospodarowicz D (1982) Neurite outgrowth and protein synthesis by PC12 cells as a function of substratum and nerve growth factor. J Neurosci 2:1157–1175 [DOI] [PMC free article] [PubMed]
- Goodman SL, Deutzmann R, von der Mark K (1987) Two distinct cell-binding domains in laminin can independently promote nonneuronal cell adhesion and spreading. J Cell Biol 105:589–598 [DOI] [PMC free article] [PubMed]
- Greene LA, Tischler AS (1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA 73:2424–2428 [DOI] [PMC free article] [PubMed]
- Han J, Ohno N, Pasco S, Monboisse JC, Borel JP, Kefalides NA (1997) A cell binding domain from the alpha3 chain of type IV collagen inhibits proliferation of melanoma cells. J Biol Chem 272:20395–20401 [DOI] [PubMed]
- Houseman BT, Mrksich M (2001) The microenvironment of immobilized Arg-Gly-Asp peptides is an important determinant of cell adhesion. Biomaterials 22:943–955 [DOI] [PubMed]
- Karuri NW, Liliensiek S, Teixeira AI, Abrams G, Campbell S, Nealey PF, Murphy CJ (2004) Biological length scale topography enhances cell-substratum adhesion of human corneal epithelial cells. J Cell Sci 117:3153–3164 [DOI] [PMC free article] [PubMed]
- Kleinman HK, Luckenbill-Edds L, Cannon FW, Sephel GC (1987) Use of extracellular matrix components for cell culture. Anal Biochem 166:1–13 [DOI] [PubMed]
- Kleinman HK, Philp D, Hoffman MP (2003) Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol 14:526–532 [DOI] [PubMed]
- Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM (2005) Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem Rev 105:1103–1169 [DOI] [PubMed]
- Lu B, Smyth MR, O’Kennedy R (1996) Oriented immobilization of antibodies and its applications in immunoassays and immunosensors. Analyst 121:29R–32R [DOI] [PubMed]
- Mardilovich A, Kokkoli E (2004) Biomimetic peptide-amphiphiles for functional biomaterials: the role of GRGDSP and PHSRN. Biomacromolecules 5:950–957 [DOI] [PubMed]
- Nguyen H, Qian JJ, Bhatnagar RS, Li S (2003) Enhanced cell attachment and osteoblastic activity by P-15 peptide-coated matrix in hydrogels. Biochem Biophys Res Commun 311:179–186 [DOI] [PubMed]
- Ochsenhirt SE, Kokkoli E, McCarthy JB, Tirrell M (2006) Effect of RGD secondary structure and the synergy site PHSRN on cell adhesion, spreading and specific integrin engagement. Biomaterials 27:3863–3874 [DOI] [PubMed]
- Pedchenko V, Zent R, Hudson BG (2004) Alpha(v)beta3 and alpha(v)beta5 integrins bind both the proximal RGD site and non-RGD motifs within noncollagenous (NC1) domain of the alpha3 chain of type IV collagen: implication for the mechanism of endothelia cell adhesion. J Biol Chem 279:2772–2780 [DOI] [PubMed]
- Ranieri JP, Bellamkonda R, Bekos EJ, Vargo TG, Gardella JA Jr, Aebischer P (1995) Neuronal cell attachment to fluorinated ethylene propylene films with covalently immobilized laminin oligopeptides YIGSR and IKVAV. II. J Biomed Mater Res 29:779–785 [DOI] [PubMed]
- Saneinejad S, Shoichet MS (1998) Patterned glass surfaces direct cell adhesion and process outgrowth of primary neurons of the central nervous system. J Biomed Mater Res 42:13–19 [DOI] [PubMed]
- Schwarz MA, Mitchell M, Emerson DL (1990) Reconstituted basement membrane enhances neurite outgrowth in PC12 cells induced by nerve growth factor. Cell Growth Differ 1:313–318 [PubMed]
- Shah DS, Thomas MB, Phillips S, Cisneros DA, Le Brun AP, Holt SA, Lakey JH (2007) Self-assembling layers created by membrane proteins on gold. Biochem Soc Trans 35:522–526 [DOI] [PubMed]
- Tamm LK, Arora A, Kleinschmidt JH (2001) Structure and assembly of beta-barrel membrane proteins. J Biol Chem 276:32399–32402 [DOI] [PubMed]
- Thorwarth M, Schultze-Mosgau S, Wehrhan F, Srour S, Wiltfang J, Neukam FW, Schlegel KA (2005) Enhanced bone regeneration with a synthetic cell-binding peptide—in vivo results. Biochem Biophys Res Commun 329:789–795 [DOI] [PubMed]
- Tomaselli KJ, Damsky CH, Reichardt LF (1987) Interactions of a neuronal cell line (PC12) with laminin, collagen IV, and fibronectin: identification of integrin-related glycoproteins involved in attachment and process outgrowth. J Cell Biol 105:2347–2358 [DOI] [PMC free article] [PubMed]
- Tong YW, Shoichet MS (1998) Peptide surface modification of poly(tetrafluoroethylene-co-hexafluoropropylene) enhances its interaction with central nervous system neurons. J Biomed Mater Res 42:85–95 [DOI] [PubMed]
- Tong YW, Shoichet MS (2001) Enhancing the neuronal interaction on fluoropolymer surfaces with mixed peptides or spacer group linkers. Biomaterials 22:1029–1034 [DOI] [PubMed]
- Yamada Y, Kleinman HK (1992) Functional domains of cell adhesion molecules. Curr Opin Cell Biol 4:819–23 [DOI] [PubMed]
- Zar JH (1996) Biostatistical analysis. Prentice Hall International Editions, pp 228–229