Abstract
Transgenesis is a valuable methodology for studying gene expression patterns and gene function. It has recently become available for research on some parasitic nematodes, including Strongyloides stercoralis. Previously, we described a vector construct, comprising the promoter and 3′ UTR of the S. stercoralis gene Ss era-1 that gives expression of GFP in intestinal cells of developing F1 progeny. In the present study, we identified three new S. stercoralis promoters, which, in combination with the Ss era-1 3′ UTR, can drive expression of GFP or the red fluorescent protein, mRFPmars, in tissue-specific fashion. These include Ss act-2, which drives expression in body wall muscle cells, Ss gpa-3, which drives expression in amphidial and phasmidial neurons and Ss rps-21, which drives ubiquitous expression in F1 transformants and in the gonads of microinjected P0 female worms. Concomitant microinjection of vectors containing GFP and mRFPmars gave dually transformed F1 progeny, suggesting that these constructs could be used as co-injection markers for other transgenes of interest. We have developed a vector “toolkit” for S. stercoralis including constructs with the Ss era-1 3′ UTR and each of the promoters described above.
Index descriptors and abbreviations: Nematode, Strongyloides stercoralis, Transgenesis, Vector, Expression, Green Fluorescent Protein (GFP), Red Fluorescent Protein (RFP), Neuron, Muscle, 3′ Untranslated Region (3′ UTR)
1. Introduction
Transgenesis is an essential tool in modern molecular and cellular biology. In experimental models amenable to classical genetic study, this approach can facilitate cloning and characterization of novel genes by genetic complementation and in less tractable subjects can be used to infer gene function by assessing phenotypes resulting from over-expression or from expression of dominant loss-of-function constructs. Expression of transgenes carrying specific, targeted mutations allows structural/functional study of regulatory and other functional domains in DNA, RNA and protein (Evans 2006). Transgenesis in the model organism Caenorhabditis elegans was pioneered in the mid 1980s (Fire, 1986; Fire and Waterston, 1989; Fire, et al., 1990; Fire, et al., 1990), and its application to the study of parasitic nematodes began approximately a decade later. As recounted in a recent comprehensive review (Kalinna and Brindley, 2007), initial attempts at transgenesis in parasitic nematodes, which resulted in transient transformation, involved biolistics as a means of gene transfer into relatively large organisms such as adult filariae (Davis, et al., 1999; Jackstadt, et al., 1999; Higazi, et al., 2002) or stages such as the egg of Ascaris suum, which can be obtained in large quantities (Davis, et al., 1999). This approach has been used to study mRNA processing and translation in Ascaris suum (Cohen, et al., 2004; Lall, et al., 2004; Cheng, et al., 2007) and for structural and functional analysis of an HSP70 promoter and of message transplicing in Brugia malayi (Shu, et al., 2003; Higazi and Unnasch, 2004).
The ovaries of free-living females of the nematode superfamily Strongyloidoidea (De Ley and Blaxter, 2001; Dorris, et al., 2002) are very similar in structure to the gonads of C. elegans hermaphrodites. This has made it possible to directly apply standard methods for gonadal microinjection of C. elegans (Evans 2006) to Strongyloides spp. and Parastrongyloides spp. (Lok and Massey, 2002; Grant, et al., 2006; Li, et al., 2006). Using this approach we have developed a reliable method for gene delivery in S. stercoralis (Lok and Massey, 2002; Li, et al., 2006), and we have observed tissue-specific expression of a gfp-based reporter construct in viable F1 transformant larvae (Li, et al., 2006). Such regulated transgene expression requires both 5′ and 3′ flanking regulatory sequences from S. stercoralis. By contrast, gfp-based reporter constructs incorporating regulatory elements from C. elegans are not expressed in the first-through third-stage larval progeny of microinjected free-living female S. stercoralis but only in dysregulated fashion in degenerating embryos (Li, et al., 2006). We have previously detected DNA of the C. elegans-derived plasmid construct pTG96_2 (Gu, et al., 1998) in transgenic S. stercoralis up to the F5 generation (Li, et al., 2006), but we have not yet succeeded in deriving a stable transgene expressing line; that is, one that transmits an expressed transgene to its progeny at a constant frequency through successive generations. Grant et al. (2006) have achieved heritable transformation of P. trichosuri with β-gal-based reporter constructs, and have succeeded in maintaining transformant lines through multiple in vitro and in vivo passages, providing proof of principal for our studies in S. stercoralis.
The purpose of the present study was to determine whether the Ss era-1 3′ UTR can act as a multi-purpose terminator, allowing regulated expression of reporter gene constructs incorporating other tissue-specific promoters. We also evaluated an alternate in vivo reporter gene for S. stercoralis, mRFPmars, and used this to ascertain whether co-injected transgenes are incorporated together in transformant worms. We undertook in vivo experiments to determine whether S. stercoralis L3i expressing transgene constructs could infect the host, establish as parasitic adults in the gut and transmit the transgenes to their progeny. Finally, we sought to develop and make available for distribution a vector “tookit” for S. stercoralis, similar in concept to that developed and distributed for C. elegans by A. Fire and colleagues (Stanford University) (Fire, et al., 1990). We envisioned such a “toolkit” as comprising a series of modular vectors incorporating the Ss era-1 3′ UTR either alone or in combination with various reporter genes and tissue- and cell-specific promoters.
2. Materials and Methods
2.1. Parasite maintenance and culture
As in previous studies on transgenesis (Lok and Massey, 2002; Li, et al., 2006), all experiments were conducted with the UPD strain of S. stercoralis. General methods for maintenance of S. stercoralis in dogs, for experimental infections in gerbils and for derivation of free-living adults, L1 (both heterogonic and homogonic) and L3i from charcoal coproculture or from cultures on NGM agar plates were as described previously (Lok, 2007).
2.2 Genomic DNA preparation
Genomic DNA (gDNA) was prepared from L3i isolated by the Baermann technique from charcoal coprocultures incubated for seven days at 22° C and purified by percoll density gradient centrifugation as described (Lok, 2007). Purified worms were washed with 10 mL TEN solution (50 mM Tris, 20mM EDTA, 100mM NaCl, pH 7.5), centrifuged for 3 minutes at 107 X g and the supernatants discarded. Worm pellets were resuspended in 2mL TEN with 200ug/mL Proteinase K, 1% SDS (w/v) and 1% 2-mercaptoethanol (v/v) and incubated at 65° C for 30 min with periodic agitation until the solutions became viscous. Using standard techniques, DNA was extracted with phenol/chloroform and precipitated in sodium acetate and ethanol. DNA precipitates were wound out using sealed Pasteur pipets. DNA was dissolved in 0.4 mL TE buffer and the samples digested for 30 minutes at 37° C with 20ug/mL RNAseA. The phenol-chloroform extractions and ethanol precipitations were repeated, and resulting DNA samples were microdialysed against water for 30 min using MF-Millipore membrane filters (pore size = 0.025 μM). DNA was recovered as the filter retentate and stored at 4° C.
2.3. Preparation of regulatory sequences from S. stercoralis
Elements referred to as “promoters” in this section and throughout the paper are, in fact putative promoters comprising at least 1000 bp of 5′ sequence flanking the coding region of the specified gene. No attempt has been made to date to define a minimal promoter for S. stercoralis. The Ss act-2 promoter, comprising 1183 bp of 5′ flanking sequence was amplified by inverse PCR (iPCR) from a circularized Bgl II digest of S. stercoralis DNA as described (Lok and Massey, 2002). The Ss gpa-3 promoter, comprising approximately 3323 bp of 5′ flanking sequence was amplified by iPCR from a circularized Bgl II digest of S. stercoralis gDNA as described (Massey, et al., 2001). The promoter for Ss rps-21, a gene that encodes ribosomal small subunit protein S21, comprised 3301 bp of 5′ flanking sequence amplified by iPCR from a circularized Swa 1 digest of S. stercoralis gDNA using primers Ssrps21-hind3F TCTTCTAAGCTTAGCTCAACGTGACGGATTG and Ssrps21-age1R AGTTCAACCGGTTCTCCTTTGTCGTTTTGCATGA. These primers contained Hind III and AgeI sites (underscored), respectively, for cloning. The iPCR protocol used to amplify the Ss rps-21 promoter was as described for Ss gpa-3 (Massey, et al., 2001) except that gDNA was microdialyzed using a MF-Millipore Membrane Filter (pore size = 0.025 μM) for 30 min against nuclease free water prior to use and that 800 U T4 DNA ligase (New England BioLabs, Ipswich, Massachusetts, USA) were used in the circularization reaction. Also, PfuTurbo® Hotstart DNA Polymerase (Stratagene, La Jolla, California, USA) was used in a slightly modified protocol for long range PCR in which 25 μL reactions (manufacturer’s protocol) contained 3 μL circularized template and were subjected to cycling regimes of 94 °C for 1.5 min for initial denaturation, followed by 35 cycles of denaturation at 94 °C for 45 sec, primer annealing at 62 °C for 1 min, extension at 68 °C for not less than 3 min/1000bp expected fragment length, followed by final extension at 68 °C for 10 min. The 593 bp Ss era-1 3′ UTR was prepared previously and cloned into transformation vector pPV230.13 (Li, et al., 2006). The UTR was cut from pPV230.13 with restriction enzymes appropriate for cloning into new transformation vectors as described below.
2.4. Construction of the promoterless gfp-expression vectors
Starting materials for promoterless gfp expression vectors were one of the following standard C. elegans vectors pPD95.75, pPD95.77, pPD95.79 and pPD95.81 from the kit provided as a gift from Andrew Fire (Stanford University) and currently distributed on a nonprofit basis (Addgene, Cambridge, Massachusetts, USA, http://www.addgene.org). The GFP variant S65C encoded in these and all vectors described subsequently in this paper contains a serine-to-cysteine mutation at position 65, enhancing its fluorescence intensity (Reichel, et al., 1996). Fire vectors were digested with the restriction enzymes EcoR I and Eag I to remove the Ce unc-54 3′UTR, and the Ss era-1 3′UTR was cut from the vector pPV230.13 (Li, et al., 2006) with the same restriction enzymes and ligated to the above Fire vectors to make pAJ01, pAJ02, pAJ03 and pAJ04, respectively (Table 1). Modified versions of the pAJ constructs containing a new multi-cloning site were made by cutting with EcoR I and ligating with an excess of the short DNA sequence StuAvrMLuF/R containing three novel restriction sites. This sequence was made by annealing the following complementary oligonucleotides: StuAvrMLuF (5′-AATTCAGGCCTAGGACGCGT-3′) and StuAvrMLuR (5′-AATTACGCGTCCTAGGCCTG-3′). Restriction sites thus introduced were Mlu I, Avr II, and Stu I, and the resulting constructs were designated pAJ12, pAJ13, pAJ14 and pAJ15, respectively (Table 1).
Table 1.
Composition and derivation of plasmid vectors.
| Vector | Parent vector | Promoter | Reading frame** | marker | Stuffer | 3′ UTR |
|---|---|---|---|---|---|---|
| pAJ01 | pPD95.75* | None | 0 | GFP(S65C) | − | Ss era-1 |
| pAJ02 | pPD95.77* | None | 2 | GFP(S65C) | − | Ss era-1 |
| pAJ03 | pPD95.79* | none | 1 | GFP(S65C) | − | Ss era-1 |
| pAJ04 | pPD95.81* | none | S | GFP(S65C) | − | Ss era-1 |
| pAJ08 | pAJ04 | Ss act-2 | S | GFP(S65C) | − | Ss era-1 |
| pAJ09 | pAJ01 | Ss gpa-3 | 0 | GFP(S65C) | − | Ss era-1 |
| pAJ12 | pAJ01 | none | 0 | GFP(S65C) | + | Ss era-1 |
| pAJ13 | pAJ02 | none | 2 | GFP(S65C) | + | Ss era-1 |
| pAJ14 | pAJ03 | none | 1 | GFP(S65C) | + | Ss era-1 |
| pAJ15 | pAJ04 | none | S | GFP(S65C) | + | Ss era-1 |
| PAJ20 | pAJ04 | Ss rps-21 | S | GFP(S65C) | + | Ss era-1 |
| pAJ50 | pAJ08 | Ss act-2 | S | mRFPmars | − | Ss era-1 |
See 1995 Fire kit documentation http://www.addgene.org/ for plasmid details.
Frames 0, 1 and 2 are the possible reading frames relative to GFP. Frame S is identical to frame 1 except for the removal of the TAG codon, restoring Hind III, Sph I, Pst I and Sal I fusion junctions.
2.5. Synthesis of reporter DNA constructs
The Ss act-2 promoter was transferred as a Hind III-Pst I fragment from vector pPV101.1 (Lok and Massey, 2002) to pAJ04 to make pAJ08 (Table 1). The Ss gpa-3 promoter was transferred as a Hind III-Sal I fragment from the PCR-Script™ (Stratagene) clone (Massey, et al., 2001) to pAJ01 to make pAJ09 (Table 1). The Ss rps-21 promoter was transferred as a Hind III-Age I fragment (detailed in section 2.3.) to pAJ04 to make pAJ20 (Table 1). We constructed an expression vector containing the alternate reporter mRFPmars (Fischer, et al., 2004) as follows. The mRFPmars coding sequence (AY679163) was amplified from pBsrH, kindly provided in by Dr. Jakob Franke at the Dicty Stock Center, Columbia University (http://dictybase.org/stockcenter/stockcenter.htmL), using primers mRFPmarsAgeF AGTACCGGTAAAAATGGCATCATCAGAAGATG and mRFPmarAvEcR TTGGAATTCAGGCCTAGGAGATCCTGCACCTGTTGAATG, tagged with restriction sites Age I and Avr II (underscored), respectively. The resulting PCR product was transferred as an Age I-Avr II fragment to pAJ08 digested with the same enzymes to replace gfp and make pAJ50 (Table 1).
2.6. Gonadal microinjection of DNA constructs into S. stercoralis and C. elegans
Gonadal microinjections into S. stercoralis free-living females and C. elegans hermaphrodites were carried out as described (Mello and Fire, 1995, Evans 2006). Injection mixes for S. stercoralis consisted of expression construct alone, or with pBlueScript II SK(+) (Stratagene) as carrier DNA, in distilled water with a total combined plasmid concentration of 100 ng/μL. For promoter analysis the construct was injected into P0 worms at 100 ng/μL. For gerbil and dog infection trials 20 ng construct and 80 ng carrier DNA were injected, and for co-injection experiments 20 ng primary construct, 20 ng co-injection marker construct and 60ng carrier DNA were injected. Injection mixes for C. elegans consisted of 20 ng/μL of primary construct and 80 ng/μL empty vector plasmid SK+. Microinjected C. elegans hermaphrodites were plated singly on NGM agar plates with lawns of Escherichia coli OP50 (NGM/OP50) and cultured at 20° C. Beginning 48 hours after microinjection C. elegans broods were observed with an Olympus SZX12 stereomicroscope with coaxial epifluorescence and gfp-expressing transformants were picked from among the progeny of P0 hermaphrodites and replated. Microinjected S. stercoralis females were plated in pairs along with four or five males. At intervals of 22 and 48 h following injection, parental (P0) females and their broods were observed with the epifluorescence stereomicroscope and both total and GFP-positive or mRFPmars-positive F1 larvae were counted. P0 females and males were transferred to fresh plates following each daily count.
2.7. Co-transformation with two reporter constructs
The availability of mRFPmars as an alternative reporter gene to gfp allowed us to examine whether co-injected transgene constructs are inherited together or independently in F1 transformant S. stercoralis. Plasmids pAJ08 and pAJ50, and in another experiment plasmids pAJ09 and pAJ50, were combined at concentrations of 20 ng/μL each, together with 60 ng/μL SK+ plasmid, and injected into the gonads of S. stercoralis free-living females. These female worms were reared at 20° C with 3 male worms each and their progeny checked for both gfp and mRFPmars expression over the next 72 hours. Each co-injection experiment was repeated once.
2.8. Culture and passage of transgenic worms in gerbils and dogs
Host passage of transgenic worms was carried out by standard methods in Mongolian gerbils, with or without immunosuppressive prednisolone treatment (Nolan, et al., 1993), or in an immunosuppressed dog (Schad, et al., 1984). In gerbil infection trials 1–3, 7 and 8, transgenic L3i designated for host passage were hand selected based on gfp fluorescence using the epifluorescence stereomicroscope. In gerbil infection trials 4–6, an unselected population of transformed and untransformed larvae was inoculated, and the number of transgenic L3i delivered in each case was estimated by averaging counts of GFP positive individuals in 3–5 measured aliquots. In the case of a single canine infection (Trial 9, Table 4) L3i were automatically sorted from a mixed population using a COPAS BioSorter® (Union Biometrica, Holliston, Massachusetts)
Table 4.
Frequencies of reporter transgene expression and co-expression in F1 progeny of S. stercoralis free-living females co-injected with vector constructs
| Constructs co-injecteda | Replicate | No. P0 Female worms injected | No. F1 larvae obs. | No. (%) of all F1 larvae expressing GFP | No. (%) of all F1 larvae expressing RFP | No. (%) F1 larvae expressing GFP only | No. (%) F1 larvae expressing RFP only | No. (%) F1 larvae expressing GFP and RFP b |
|---|---|---|---|---|---|---|---|---|
| Ss act-2::gfp + Ss act- 2::mRFPmars | 1 | 20 | 480 | 51 (10.6) | 38 (7.9) | 13 (2.7) | 0 (0) | 38 (7.9) |
| (pAJ08:pAJ50:SK+) | 2 | 16 | 162 | 36 (22.2) | 31 (19.1) | 5 (3) | 0 (0) | 31 (19.1) |
| Ss gpa-3::gfp + Ss act- 2::mRFPmars | 1 | 21 | 397 | 12 (3.0) | 19 (4.8) | 0 (0) | 7 (1.7) | 12 (3.0) |
| (pAJ09:pAJ50:SK+) | 2 | 18 | 231 | 12 (5.2) | 17 (7.4) | 0 (0) | 5 (2.2) | 12 (5.2) |
Respective concentrations in ng/μL of construct components were 20:20:80 in all cases. SK+ denotes pBluescript II SK+.
Denotes F1 larvae co-expressing both reporter constructs.
2.9. Detection of transgene DNA and transgene expression in the F1 generation and beyond
Initial screening of F1 larvae for transgene expression was by epifluorescence stereomicroscopy. Detailed imaging of transgenic larvae was by Nomarski Differential Interference Contrast (DIC) microscopy or by epifluorescence using an Olympus BX60 compound microscope. Filter cubes used to detect GFP and mRFPmars, respectively, were Chroma Technology Corporation (Rockingham, VT 05101 USA) 41001 (510–560 nm emission band width) and 41002 (578–642 nm emission band width). Imaging was done with the BX60 microscope using a Spot RT Color® digital camera and the Spot Advanced® image analysis software package (Diagnostic Instruments, Inc., Sterling Heights, Michigan, USA).
Larvae from the F2 generation following transformation were isolated from the feces of infected hosts by the Baermann funnel technique (Lok, 2007) and screened for the presence of transgene DNA via PCR. All constructs used in host passage attempts included the coding region of gfp, a gene that does not occur in C. elegans nor, presumably, in S. stercoralis. Therefore, transgene DNA was detected in worms from the F2 generation using primers specific for the gfp coding region. These primers, CeGFP-476F CCCTTGTTAATAGAATCGAGTT and CeGFPnoTerR TTTGTATAGTTCATCCATGCC, yielded a 413 bp product. All PCR screening of F2 progeny from host passages included a negative control reaction conducted on pools of non-transformed S. stercoralis larvae, a positive control reaction conducted on purified vector plasmid and a loading control reaction using primers that amplify a 316 bp fragment of the gene Ss rps-21, which encodes the ribosomal small subunit protein in S. stercoralis (Li, et al., 2006).
3. Results
3. 1. Cell- and tissue-specific expression of reporter transgenes in S. stercoralis
Having found that the Ss era-1 3′ UTR could give regulated expression of reporter transgenes under the control of its cognate promoter, we sought to determine whether this element could also function in combination with other promoters. To this end, we made a series of reporter constructs (pAJ08, pAJ09, pAJ20 and pAJ50) by cloning 5′ flanking sequence from various S. stercoralis genes upstream of the gfp coding sequence in either of the promoterless vectors pAJ01 or pAJ04 (Table 1). Both of these promoterless vectors contain the Ss era-1 3′ UTR fused downstream of gfp. After microinjection of the promoter-containing reporter constructs into free-living females, >5% of F1 progeny showed tissue-specific GFP expression patterns (Tables 2 and 3). These expression patterns remained consistent from the L1 to the L3i stage.
Table 2.
Summary of reporter transgene expression frequencies among F1 progeny resulting from gonadal microinjection of vector constructs into free-living S. stercoralis females.
| Constructa | Replicate | No. P0 Female worms injected | No. F1 larvae obs. | No. (%) F1 larvae GFP + |
|---|---|---|---|---|
| pAJ08 | 1 | 18 | 445 | 51 (11.5) |
| (Ss act-2::gfp) | 2 | 20 | 349 | 59 (16.9) |
|
| ||||
| pAJ09:SK+b | 1 | 16 | 137 | 8 (5.8) |
| (Ss gpa-3::gfp) | 2 | 15 | 176 | 10 (5.7) |
|
| ||||
| pAJ50 | 1 | 16 | 560 | 55 (9.8) |
| (Ss act-2::mRFPmars) | 2 | 21 | 435 | 66 (15) |
|
| ||||
| pAJ20 | 1 | 21 | 280 | 98 (35.0) |
| (Ss rps-21::gfp) | 2 | 21 | 194 | 83 (42.8) |
All constructs injected at a concentration of 100 ng/μL.
Vector pAJ09 was injected in combination with pBluescript II SK+ as carrier DNA.
Table 3.
Expression patterns of GFP reporter constructs incorporating S. stercoralis promoters compared to those of GFP reporters incorporating orthologous promoters from C. elegans and to those seen following heterologous transfer of the parasite-based constructs into C. elegans.
| GFP reporter expression pattern
|
||
|---|---|---|
| Orthologous promoter pair* | S. stercoralis | C. elegans |
| Ss era-1 | Intestine | Not expressed |
|
|
||
| Ce cdc-48.2 | ND | Intestine |
|
| ||
| Ss gpa-3 | Amphidial, phasmidial neurons | Not expressed |
|
|
||
| Ce gpa-3 | ND** | Amphidial, phasmidial neurons |
|
| ||
| Ss act-2 | Body muscle | Pharynx (59)***, pharynx + body muscle (39), body muscle alone (2) in F1 transformants; pharyngeal muscle pm6 + body muscle (90) in stable line |
|
|
||
| Ce act-1 | ND | Body muscle |
|
| ||
| Ss rps-21 | Ubiquitous | A few neurons in stable line |
|
|
||
| Ce rps-21 | ND | Ubiquitous |
Designated S. stercoralis promoters (Ss) were cloned upstream of gfp in either pAJ01 or pAJ04 (Table 1) to derive the reporter constructs giving the expression patterns described.
ND = Not done
Numbers in parentheses indicate the percentages of 175 worms that exhibited the specified expression pattern.
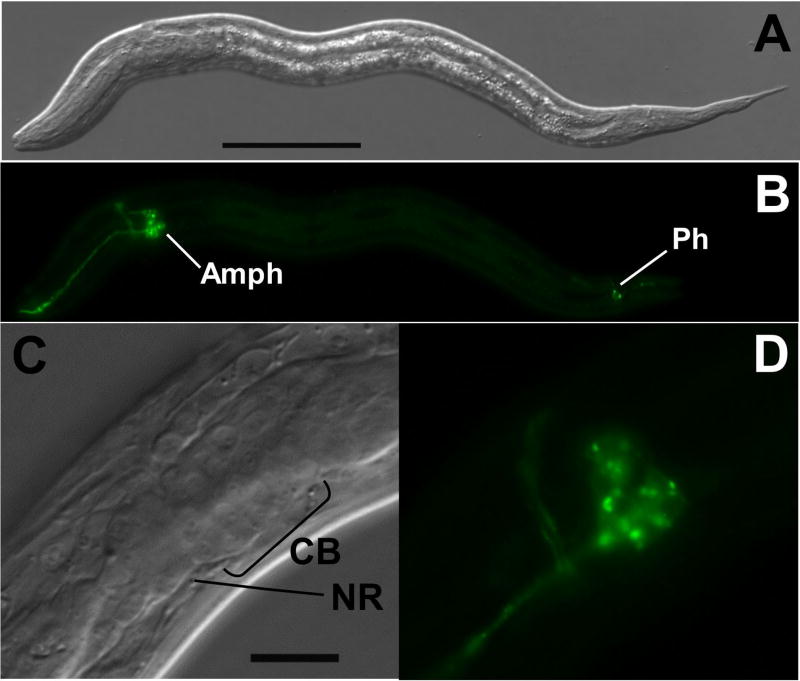
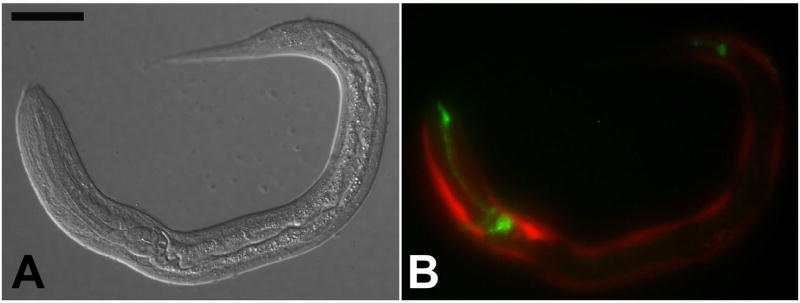
The reporter construct designated pAJ09 (Fig. 1, Table 1) contains 5′ flanking sequence from Ss gpa-3, the structural ortholog of C. elegans gpa-3 (Massey, et al., 2001), which encodes the α subunit of a G protein necessary for transduction of the pheromone signal triggering dauer development in C. elegans (Zwaal, et al., 1997; Massey, et al., 2001). Approximately 5.8% of F1 progeny of S. stercoralis free-living females microinjected with pAJ09 expressed GFP in a neuron-specific pattern (Tables 2 and 3). All such F1 first-stage larvae exhibited GFP expression in a cluster of amphidial neurons and in the area of the nerve ring (Fig. 1A, B). Dendritic processes of GFP expressing amphidial neurons could be traced from their origins in the lateral ganglion forward to the amphidial channnel. Some transformant larvae also showed expression in at least one and frequently two pairs of phasmidial neurons (Fig. 1A, B). High-magnification images (Fig. 1C, D) appear to show expression in eight amphidial cell bodies.
Fig. 1.
Neuron-specific expression of gfp under the Ss gpa-3 promoter in S. stercoralis transformed with construct pAJ09 (Table 1). A, B. DIC and fluorescence images, respectively, of a transformant L1 showing GFP localization in amphidial cell bodies (Amph), in their dendritic processes leading into the amphidial channel and in phasmidial neurons (Ph), C, D. high-magnification DIC and fluorescence images, respectively, of specimen in panels A–B showing GFP localization in nerve ring (NR) and 8 amphidial cell bodies (CB). Scale bars: A = 50 μM; C = 20 μM.
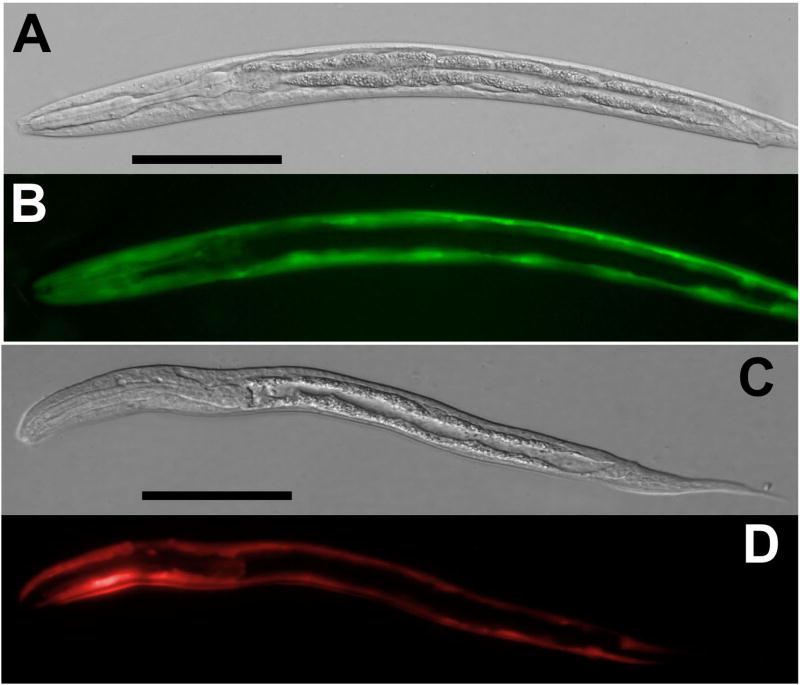
The Ss act-2 promoter drove gfp expression almost exclusively in body wall muscle of F1 larvae transformed with construct pAJ08 (Table 1, Table 3, Fig. 2A, B). This same promoter drove mRFPmars expression in an identical anatomical pattern in F1 larvae transformed with construct pAJ50 (Table 1, Fig. 2C, D). The frequencies of gfp expression among F1 progeny of worms microinjected with the two constructs containing the Ss act-2 promoter were similar: 13.9% on average for pAJ08 and 12.2% for pAJ50 (Table 2).
Fig. 2.
Body wall-specific expression of gfp and mRFPmars under the Ss act-2 promoter in S. stercoralis transformed with construct pAJ08 and pAJ50, respectively (Table 1). A, B. DIC and fluorescence images, respectively, showing GFP localization in F1 transformant first-stage larvae (L1) transformed with the reporter construct pAJ08. C, D. DIC and fluorescence images, respectively, showing mRFPmars localization in F1 transgenic L1 transformed with the reporter construct pAJ50. All scale bars = 50 μM.
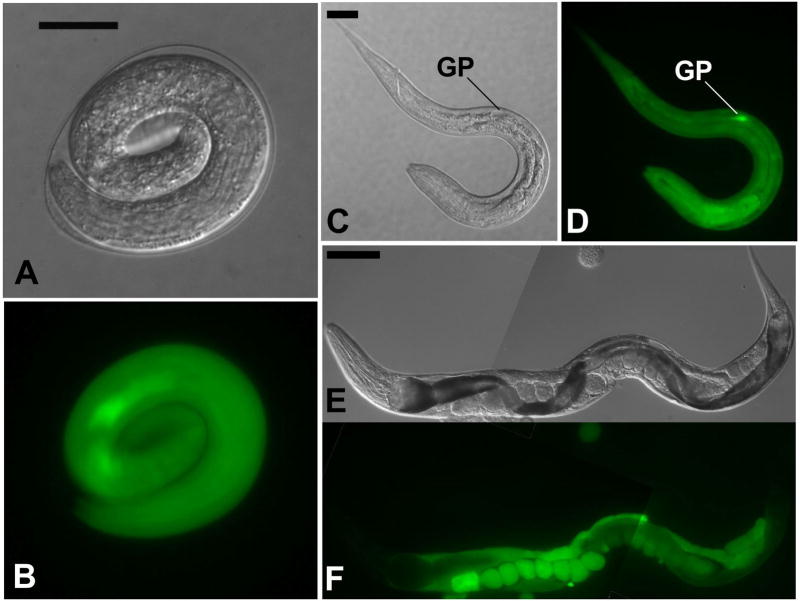
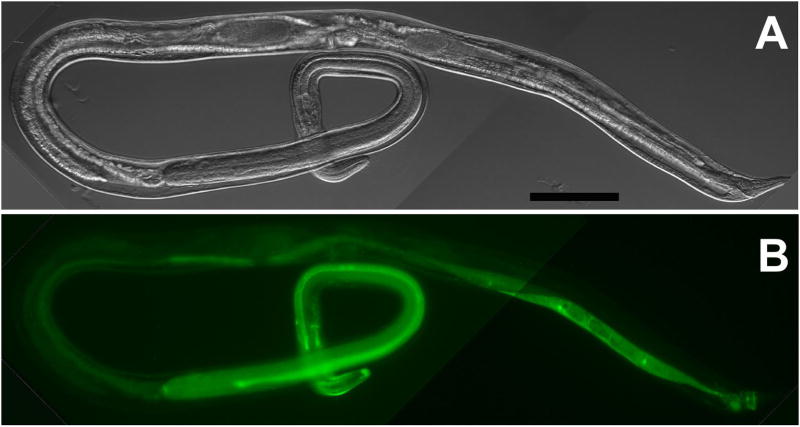
The reporter construct pAJ20 contains 5′ flanking sequence from the Ss rps-21 gene, which encodes a ribosomal protein. F1 pAJ20 transformant S. stercoralis expressed gfp beginning early in embryogenesis (Fig. 3A, B) and continuing through larval development. Expression of pAJ20 occurred in virtually all tissues of S. stercoralis L1 and was particularly intense in the genital primordium (Table 3, Fig. 3C, D). The mean frequency of gfp expression among F1 progeny of worms microinjected with pAJ20 was 38.2%, the highest of all constructs tested (Table 2). Unlike the other constructs examined in this study, pAJ20 was also highly expressed in the gonads and eggs of microinjected P0 free-living female worms (Fig. 3E, F).
Fig. 3.
Ubiquitous expression of gfp under the Ss rps-21 promoter in S. stercoralis transformed with construct pAJ20 (Table 1). A, B. DIC and fluorescence images, respectively showing ubiquitous distribution of GFP in a vermiform embryo transformed with pAJ20. C, D. a pAJ20 transformant first-stage larva showing gfp expression in virtually all body tissues with the highest level of expression occurring in the genital primordium (GP). E, F. Composite DIC and fluorescence images, respectively, of a P0 free-living female microinjected with pAJ20. Note the high level of gfp-expression in the gonad and eggs. Scale bars in A and C = 20 μM; E = 100 μM.
In summary, the promoters described here function with the Ss era-1 3′ UTR to drive sensory neuron-specific, muscle-specific or ubiquitous expression of fluorescent markers. Given their modular structure, these constructs could easily be modified to drive expression of any gene sequence of interest.
3.2. Expression of S. stercoralis-based constructs in C. elegans
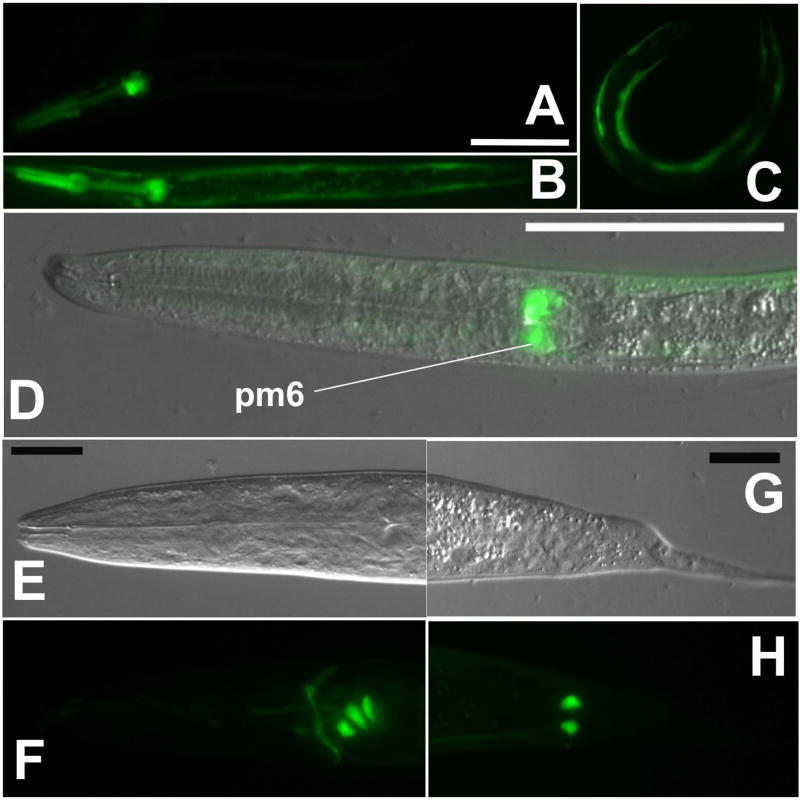
Reporter constructs containing S. stercoralis promoters showed expression patterns similar to those of their orthologs in C. elegans. However, these Strongyloides-based constructs either exhibited markedly different patterns of expression or were not expressed at all when used to transform C. elegans (Table 3). The construct pAJ09, which contains the Ss gpa-3 promoter, failed to express in C. elegans altogether. C. elegans transiently transformed with pAJ08, containing the Ss act-2 promoter, expressed gfp but did so in a pattern different from that seen in S. stercoralis. In the majority of F1 C. elegans transformants, the Ss act-2 promoter drove gfp expression in the pharynx, and in the pharynx and body wall (Table 3, Fig. 4A, B). Only a small fraction (2%) of C. elegans F1 transformed with pAJ08 expressed gfp exclusively in the body wall (Table 3, Fig. 4C) while all such S. stercoralis transformants observed to date have exhibited this expression pattern. C. elegans offers the advantage of easy establishment of stable transgenic lines, that is lines of germline transformants that transmit the transgene through many generations with a consistent frequency. In a stable line of C. elegans transformed with pAJ08, expression predominated in the sixth pharyngeal muscle and body wall (Table 3, Fig. 4D). S. stercoralis transformed with pAJ20, which contains the Ss rps-21 promoter, show ubiquitous expression of gfp (Table 3, Fig. 3). In stark contrast, C. elegans transformed with this construct expressed gfp in a grouping of three neuronal cell bodies near the pharyngeal bulb (Table 3, Fig. 4E, F) and in a pair of phasmidial cells (Table 3, Fig. 4G, H). This pattern was consistent among F1 transformants and in a stable line derived from them. We conclude that a transgene’s expression pattern in C. elegans is not a reliable indicator of its likely expression pattern in the S. stercoralis.
Fig. 4.
Expression of S. stercoralis-based reporter constructs in C. elegans. A–D. Variable expression patterns of gfp expression under the Ss act-2 promoter in C. elegans transiently transformed with construct pAJ08 (Table 1). D. DIC and fluorescence overlay image showing GFP localization in the sixth pharyngeal muscle ring (pm6). Scale bar for A–D = 50 μM. E–H. Images of gfp expression under the Ss rps-21 promoter in C. elegans transformed with construct pAJ20 (Table 1). Images show GFP localization in glial cells in the amphidial (E, F) and phasmidial (G, H) complexes. Scale bars in E and G = 20μM.
3.3. Co-transformation with two reporter constructs
Dually transformed F1 progeny were produced in both the replicates of the each plasmid combination (Table 4, Fig. 5). In these dual transformations, the relative expression frequencies of the vector constructs, as approximated by fluorescence of the reporter gene products GFP or mRFPmars, were consistent with those seen in singly transformed worms (Tables 2 and 4). The frequencies of expression of the constructs used in the co-transformation experiments were such that pAJ08 > pAJ50 > pAJ09 (χ2 = 12.53; P < 0.005). In each pairing of vector constructs, the construct with the lowest frequency of expression was always seen in combination with the construct having the highest frequency and never by itself. For example, in both replicates of the pairing of pAJ09 with pAJ50, pAJ09 (giving GFP expression) was always seen in combination with pAJ50 (giving mRFPmars expression) and never alone. A small proportion of transgenic worms in this experiment (2.8%), equal to the difference in the expression frequencies of the two constructs, expressed pAJ08 alone (Table 4). These data are consistent with the hypothesis that transgene constructs co-injected into S. stercoralis are incorporated together in F1 transgenic progeny. From a practical standpoint, the data suggest that the fluorescent reporters described here could be used as co-injection markers for other non-fluorescent transgenes.
Fig 5.
Co-transformation of S. stercoralis with dual reporter constructs, pAJ09 and pAJ50 (Table 1). A, B. DIC and merged red and green fluorescence images, respectively, of an F1 transgenic S. stercoralis L1 expressing gfp under the Ss gpa-3 promoter (pAJ09) in amphidial neurons and nerve ring and mRFPmars under the Ss act-2 promoter (pAJ50) in body wall muscles. Scale bar = 30 μM.
3.4. Host passage of transgenic S. stercoralis
We attempted to establish a stable transgene-expressing line of the parasite by passage through susceptible mammalian hosts. Because of the intensity of its expression in L3i we chose pAJ08 (Table 1) for these experiments. An estimated total of 1847 GFP-positive L3i were injected into gerbils over the course of 8 independent experiments (Table 5, trials 1–8). In addition, 1286 GFP-positive L3i were injected into a single dog (Table 5, trial 9). We observed continued expression of pAJ08 in 14 out of 35 F1 parasitic females recovered at necropsy from gerbils infected with pure populations of transgenic L3i (Table 5, trials 1–3 and 7; Fig. 6). Three of the gerbil infections (trials 3–5) yielded pools of F2 larvae in which transgene construct DNA could be detected by PCR. In trial 3, two PCR positive pools were detected from a total of 13 pools (2–90 larvae per pool) examined. In trial 4, two positive pools were detected among nine examined (125–1000 larvae per pool), and in trial 5 a single positive pool was observed among 8 examined (60–11,000 larvae per pool). The dog infection (trial 9) also yielded F2 progeny carrying the transgene construct (one of four pools positive; pool size ranging from 10–150 larvae per pool). Two of the necropsied gerbils (trials 3 and 4) found to harbor a total of 13 GFP-positive parasitic females yielded PCR-positive F2 larval pools. The yield of PCR-positive F2 larval pools indicates that a minimum of two or 15.4% of the F1 transgenics in these infections transmitted the transgene to their progeny. However, none of the 25,741 F2 larvae examined in all the host passage attempts expressed gfp. Control reactions with gfp-specific primers gave negative results with all pools of non-transformed larvae and positive results in every case with purified vector plasmid.
Table 5.
Summary of host passage attempts using F1 pAJ08 transformant S. stercoralis L3i.
| Parasitic females recovered at necropsy | F2 progeny | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trial | Host Species | Carrier DNA | Concentration (ng/μL; vector:carrier) | No. L3i inoculated | No. L3i GFP+ | Total (% total L3i inoculated) | GFP+ (% GFP+ L3i inoculated) | L1 Screened | No. L1 GFP+ | L1 pools PCR+ |
| 1 | Gerbila | SK+b | 20:80 | 130 | 130 | 4 (3.1) | 3 (2.3) | 23 | 0 | − |
| 2 | Gerbila | SK+ | 20:80 | 125 | 125 | 1 (0.8) | 1 (0.8) | 15 | 0 | − |
| 3 | Gerbila | SK+ | 20:80 | 105 | 105 | 29 (27.6) | 10 (9.5) | 330 | 0 | + |
| 4 | Gerbil | SK+ | 20:80 | 9000c | 360d | 500 (5.6) | 3 (0.8) | 4485 | 0 | + |
| 5 | Gerbil | SK+ | 2:98 | 3200 | 160d | ND | ND | 6130 | 0 | + |
| 6 | Gerbil | None | 200 | 2350 | 550d | 4 (0.2) | 3 (0.5) | 2900 | 0 | − |
| 7 | Gerbil | gDNA | 20:80 | 180 | 180 | 1 (0.6) | 0 | 101 | 0 | − |
| 8 | Gerbil | pTG96_2 | 20:80 | 237 | 237 | ND | ND | 77 | 0 | − |
| 9 | Dog | SK+ | 20:80 | 1286 | 1286 | ND | ND | 11,680 | 0 | + |
| Total | 16613 | 3133 | 539 | 20 | 25,741 | 0 | ||||
ND: Not determined
In these trials gerbils were immunosuppressed by treatment with methylprednisolone acetate as described (Nolan et al., 1993).
Denotes pBluescript II SK+
Consists of three gerbils inoculated with 3000 non-selected L3i each
Estimates derived by counting GFP+ individuals in 3–5 measured aliquots of an unselected population of transgenic and nontransgenic L3i
Fig. 6.
Photomontage depicting expression of gfp under the Ss act-2 promoter in an F1 parasitic female transformed with construct pAJ08 (Table 1) and recovered at necropsy from an experimentally infected gerbil. A, B. Composite DIC and fluorescence images, respectively. Scale bar = 100 μM.
Discussion
The ability to generate F1 transgenic S. stercoralis will allow many studies of gene expression and gene function in this parasite. We have developed a vector “toolkit” to facilitate such transgenic studies. We showed that the Ss era-1 3′ UTR can act as a multipurpose terminator for other reporter transgenes. This is analogous to the function of the relatively small collection of C. elegans 3′ UTRs (eg. unc-54 and let 858) used in the collection of modular vectors developed by A. Fire and colleagues (Fire, et al., 1990). By the same token, the fact that several different C. elegans 3′ UTRs can act as multipurpose terminators in vector constructs suggests that the Ss era-1 3′ UTR is probably not unique in this capability in S. stercoralis and that as 3′ flanking sequences are captured from more genes in this nematode, other 3′ regulatory elements will be obtained. Finally, we used these modular vectors to characterize three new S. stercoralis gene promoters that drive tissue-specific (gpa-3, act-2) or ubiquitous (rps-21) patterns of reporter expression in the parasite. These constructs could easily be modified to drive expression of any gene sequence of interest. All of the promoterless and promoter-containing vectors described in this and our previous paper (Li, et al., 2006), have been organized into a documented vector collection that is available to the research community through AddGene, Inc. (www.addgene.org).
Like that of its C. elegans ortholog, gpa-3 (Jansen, et al., 1999), the Ss gpa-3 promoter drives gfp expression predominantly in a cluster of amphidial neurons and in two pairs of phasmidial neurons in L1 (Fig. 1A–D). C. elegans gpa-3 is expressed in eight pairs of chemosensory neurons in the amphidial complex (ADF, ADL, ASE, ASG, ASH, ASI, ASJ and ASK), two pairs of sensory neurons in the phasmids (PHA and PHB) and two other non-sensory cells (Jansen, et al., 1999). The amphidial neurons of S. stercoralis have been described and their homologies with amphidial neurons of C. elegans have been proposed based on morphology, position of cell bodies within the lateral ganglion and, in several cases, function inferred from microlaser ablation studies (Ashton, et al., 1995; Ashton, et al., 1998; Lopez, et al., 2000; Forbes, et al., 2004; Nolan, et al., 2004; Ashton, et al., 2007). We have not yet determined the specific identities of the GFP-expressing neurons in S. stercoralis transformed with pAJ09. However our imaging thus far (Fig. 1C, D) indicates that the transgene is expressed in eight amphidial neuron pairs along with two phasmidial neuron pairs in some specimens. Thus, Ss gpa-3 expression appears to be localized in a pattern that is strikingly similar to that of its ortholog in the distantly related C. elegans. The expression of reporter constructs like pAJ09 in neurons may provide a fourth criterion, in addition to morphology, position and function, for assessing homologies between amphidial and other neurons of S. stercoralis and C. elegans (Ashton, et al., 1995). In addition, transformation constructs that are expressed in such small, defined groupings of cells in S. stercoralis may provide the basis for genetically targeted cell ablation using modified channel forming proteins as described for C. elegans (Driscoll and Chalfie, 1991; Harbinder, et al., 1997). In some instances such an approach could provide an alternative to microlaser surgical techniques currently in use for S. stercoralis (Lopez, et al., 2000; Forbes, et al., 2004; Nolan, et al., 2004; Ashton, et al., 2007).
The Ss act-2 promoter drives expression of both gfp and mRFPmars such that the fluorescent reporters are localized in the body muscle of S. stercoralis L1 (Fig. 2). The expression pattern indicated by this finding is consistent with the natural expression pattern of act-1, the presumed ortholog of Ss act-2, in C. elegans (Seydoux and Fire, 1994). Because of its relatively high frequency of F1 transformation, pAJ08 was selected as the construct for use in attempts to establish a stable transgenic line by host passage. A high level of gfp expression persisted in L3i transformed with pAJ08, facilitating both manual recovery of tranformants and automated selection with the COPAS BioSorter. Automated sorting with this instrument dramatically increased the number of transgenic worms that could be recovered for inoculation into the host, due to both an increase in the number of F1 progeny analyzed and an increase in the number of weaker GFP expressing individuals recovered, which are not routinely isolated using manual sorting. Contrasting with the body wall-specific pattern of reporter expression under the Ss act-2 promoter in L1 (Fig. 2) and L3i, the localization of GFP in the pharynges and intestines of parasitic females transformed with pAJ08 (Table 1; Fig. 6) more closely resembles the pharyngeal expression pattern of this construct in the majority of C. elegans larvae (Fig 4A, B, D).
As expected of an element driving expression of a gene encoding a protein required for basic cellular function, the promoter for the ribosomal small subunit protein gene Ss rps-21 drives gfp expression in virtually all body tissues of transformant S. stercoralis larvae. This finding is consistent with the broad pattern of C. elegans rps-21 expression (unpublished, WormBase Release WS177 http://www.wormbase.org, expression pattern ID Expr5980) and makes available a promoter to drive ubiquitous transgene expression in S. stercoralis. One potential application for such a promoter would be the in situ expression of inhibitory RNA hairpins in S. stercoralis as described recently as a high throughput RNAi screening system for C. elegans (Johnson, et al., 2005).
Confirmation that the mRFPmars coding sequence can give expression of red fluorescent protein in S. stercoralis makes an alternate in vivo reporter transgene available for this parasite. This will be important for future studies requiring expression of multiple reporters such as experiments designed to examine the relative localization patterns of two molecules at the tissue, cellular or sub-cellular levels. Results of the present study in which we co-transformed S. stercoralis with constructs gfp and mRFPmars and observed simultaneous expression of both reporters in individual F1 transformants (Fig. 5) demonstrate the feasibility of such an approach. Moreover, the expression frequencies of the two reporters in co-transformed S. stercoralis (Table 4) suggest that transgene sequences delivered to this nematode on separate plasmid constructs are incorporated together into transgenic F1 progeny. This finding is consistent with the hypothesis that in S. stercoralis microinjected transgene sequences are assembled in tandem extrachromosomal arrays as they are in C. elegans (Stinchcomb, et al., 1985; Mello and Fire, 1995). Our results with co-expression of transgenes in S. stercoralis also suggest that fluorescent reporters such as gfp or mRFPmars could be used as visual co-transformation markers in the same way that rol-6 is in C. elegans (Kramer, et al., 1990; Mello, et al., 1991).
In general, the promoters used in this and our previous study (Li, et al., 2006) drove reporter gene expression in an anatomical pattern approximating that of their orthologs in C. elegans. However, when expressed in C. elegans, constructs incorporating some of these S. stercoralis promoters gave unexpected and inconsistent patterns of expression. In previous studies where reporter constructs incorporating promoters from genes in parasitic nematodes were expressed in C. elegans, observed spatial expression patterns have, with a single exception, been consistent with putative gene function, with expression patterns of orthologous genes in C. elegans and, where available, with immunolocalization data from the parasite (Qin, et al., 1998; Britton, et al., 1999; Winter, et al., 2003). Each of these studies has concluded that C. elegans represents a reliable surrogate system in which to study temporal and spatial expression patterns of genes from parasitic nematodes. By contrast however, a construct fusing the promoter for Ov-GST1a, which encodes a glutathione S transferase in Onchocerca volvulus, is expressed in both the pharynx and hypodermis of C. elegans while immunolocalization studies indicate that the gene product is present exclusively in the hypodermis of the parasite (Wildenburg, et al., 1998). This discrepancy is strikingly similar to the present observation of differential expression patterns of the construct pAJ08 in C. elegans and S. stercoralis. Along with the highly disparate expression patterns of the Ss rps-21 promoter in transgenic S. stercoralis and C. elegans, these results argue for caution in interpreting spatial expression data obtained from heterologous expression of parasite regulatory sequences in C. elegans. Such caution in interpreting data on reporter construct expression patterns is standard in C. elegans science even where homologous promoters are used. Mello and Fire (1995), for example, stress that although such data may be useful they must be confirmed by more definitive studies involving in situ hybridization or immunohistochemistry using antibody probes specific for the gene product in question. We considered the possibility that the disparity between transgene expression patterns in S. stercoralis and C. elegans could be due to the pairing of non-cognate promoters and 3′UTRs. However, we now consider this unlikely, having observed that the gfp reporter construct pPV230.3, which incorporates both 5′ and 3′ regulatory sequences from the same gene, Ss era-1, is expressed efficiently in intestinal cells of S. stercoralis (Li, et al., 2006) but not at all in C. elegans (data not shown).
Because it can undergo only one generation of free-living development, all progeny of free-living males and females are fated to become parasitic L3i. Thus, establishment of continuous transgenic lines of S. stercoralis from F1 transformants will require passage through a susceptible host. Recovery of parasitic females expressing pAJ08 from experimentally infected gerbils is significant because it confirms that transgenic S. stercoralis L3i expressing the gfp marker on a strong promoter are viable and can undergo the extensive tissue migration necessary to establish infection in the host intestine. Our current estimate that approximately 15% of F1 S. stercoralis transformants transmit transgenes to their progeny compares favorably with the frequency of stable transformation with extrachromosomal transgene arrays following microinjection in C. elegans (Mello, et al., 1991). However, none of the F2 S. stercoralis transformants to date has been observed to express gfp, leaving open the possibility that active silencing of transgenes in their present configuration is occurring. Evidence of such transgene silencing has recently been presented for Parastrongyloides trichosuri. In this case, when F2 transgenics derived by in vitro passage (possible in P. trichosuri, which undergoes multiple free-living generations) were subjected to a single host passage, only 10–20% of the resulting F3 worms shown by PCR to harbor the transgene expressed the β galactosidase reporter (Grant, et al., 2006). We are currently investigating modifications to our basic vector backbone that may prevent apparent silencing in S stercoralis.
Although stable transgenesis in S. stercoralis remains a challenge, transgenic F1s are readily obtainable and can be used to address many biological questions relating to this parasite’s free-living and infectious larval stages in the environment and, most significantly, to the parasitic larval and adult stages in the tissues and gasterointestinal tract of the host. Many of these have been discussed at length in our previous paper (Li et al., 2006). It is hoped that the system of modular vectors described here will facilitate these and other investigations in the future.
Acknowledgments
This work was supported by grants from The Ellison Medical Foundation (Grant No. ID-IA-0037-02 to J.B. Lok) and the National Institutes of Health (AI-50688 to J.B. Lok, AI-22662 to G.A. Schad and RR02512 to M. Haskins). We thank Qiukan Chen, Mario Brenes and Michelle Castelletto for technical assistance and gratefully acknowledge Dr. Edward Pearce and three anonymous reviewers for comments on the manuscript.
Footnotes
Note: GenBank accession numbers for the Ss act-2, Ss gpa-3 and Ss rps-21 promoter sequences are EF587761, AF292562, EF589665, respectively. The GenBank accession number for the Ss era-1 3′ UTR is DQ333398. Plasmid distribution for all constructs described is available at http://www.addgene.org/James_Lok
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashton FT, Bhopale VM, Fine AE, Schad GA. Sensory neuroanatomy of a skin-penetrating nematode parasite: Strongyloides stercoralis. I. Amphidial neurons. J Comp Neurol. 1995;357:281–95. doi: 10.1002/cne.903570208. [DOI] [PubMed] [Google Scholar]
- Ashton FT, Bhopale VM, Holt D, Smith G, Schad GA. Developmental switching in the parasitic nematode Strongyloides stercoralis is controlled by the ASF and ASI amphidial neurons. Journal of Parasitology. 1998;84:691–695. [PubMed] [Google Scholar]
- Ashton FT, Zhu X, Boston R, Lok JB, Schad GA. Strongyloides stercoralis: Amphidial neuron pair ASJ triggers significant resumption of development by infective larvae under host-mimicking in vitro conditions. Exp Parasitol. 2007;115:92–7. doi: 10.1016/j.exppara.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton C, Redmond DL, Knox DP, McKerrow JH, Barry JD. Identification of promoter elements of parasite nematode genes in transgenic Caenorhabditis elegans. Molecular and Biochemical Parasitology. 1999;103:171–81. doi: 10.1016/s0166-6851(99)00121-8. [DOI] [PubMed] [Google Scholar]
- Cheng G, Cohen LS, Mikhli C, Jankowska-Anyszka M, Stepinski J, Darzynkiewicz E, Davis RE. In vivo translation and stability of trans-spliced mRNAs in nematode embryos. Molecular and Biochemical Parasitology. 2007;153:95–106. doi: 10.1016/j.molbiopara.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LS, Mikhli C, Friedman C, Jankowska-Anyszka M, Stepinski J, Darzynkiewicz E, Davis RE. Nematode m7GpppG and m3(2,2,7)GpppG decapping: activities in Ascaris embryos and characterization of C. elegans scavenger DcpS. RNA. 2004;10:1609–24. doi: 10.1261/rna.7690504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Parra A, LoVerde PT, Ribeiro E, Glorioso G, Hodgson S. Transient expression of DNA and RNA in parasitic helminths by using particle bombardment. Proc Natl Acad Sci U S A. 1999;96:8687–92. doi: 10.1073/pnas.96.15.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ley P, Blaxter M. Systematic position and phylogeny. In: Lee DL, editor. Biology of Nematodes. Harwood Academic Publishers; London: 2001. [Google Scholar]
- Dorris M, Viney ME, Blaxter ML. Molecular phylogenetic analysis of the genus Strongyloides and related nematodes. Int J Parasitol. 2002;32:1507–17. doi: 10.1016/s0020-7519(02)00156-x. [DOI] [PubMed] [Google Scholar]
- Driscoll M, Chalfie M. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature. 1991;349:588–93. doi: 10.1038/349588a0. [DOI] [PubMed] [Google Scholar]
- Fire A. Integrative transformation of Caenorhabditis elegans. The EMBO Journal. 1986;5:2673–2680. doi: 10.1002/j.1460-2075.1986.tb04550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TC. Transformation and microinjection (April 6, 2006) WormBook, ed. The C. elegans Research Community, WormBook. doi: 10.1895/wormbook.1.108.1. http://www.wormbook.org. [DOI]
- Fire A, Harrison SW, Dixon D. A modular set of lacZ fusion vectors for studying gene expression in Caenorhabditis elegans. Gene. 1990;93:189–98. doi: 10.1016/0378-1119(90)90224-f. [DOI] [PubMed] [Google Scholar]
- Fire A, Kondo K, Waterston R. Vectors for low copy transformation of C. elegans. Nucleic Acids Reseach. 1990;18:4269–70. doi: 10.1093/nar/18.14.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Waterston RH. Proper expression of myosin genes in transgenic nematodes. The EMBO Journal. 1989;8:3419–28. doi: 10.1002/j.1460-2075.1989.tb08506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Haase I, Simmeth E, Gerisch G, Muller-Taubenberger A. A brilliant monomeric red fluorescent protein to visualize cytoskeleton dynamics in Dictyostelium. FEBS Letters. 2004;577:227–32. doi: 10.1016/j.febslet.2004.09.084. [DOI] [PubMed] [Google Scholar]
- Forbes WM, Ashton FT, Boston R, Zhu X, Schad GA. Chemoattraction and chemorepulsion of Strongyloides stercoralis infective larvae on a sodium chloride gradient is mediated by amphidial neuron pairs ASE and ASH, respectively. Vet Parasitol. 2004;120:189–98. doi: 10.1016/j.vetpar.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Grant WN, Skinner SJM, Howes JN, Grant K, Shuttle worth G, Heath DD, Shoemaker CB. Heritable transgenesis of Parastrongyloides trichosuri: a nematode parasite of mammals. Int J Parasitol. 2006;36:475–483. doi: 10.1016/j.ijpara.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Gu T, Orita S, Han M. Caenorhabditis elegans SUR-5, a novel but conserved protein, negatively regulates LET-60 Ras activity during vulval induction. Mol Cell Biol. 1998;18:4556–64. doi: 10.1128/mcb.18.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbinder S, Tavernarakis N, Herndon LA, Kinnell M, Xu SQ, Fire A, Driscoll M. Genetically targeted cell disruption in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1997;94:13128–33. doi: 10.1073/pnas.94.24.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higazi TB, Merriweather A, Shu L, Davis R, Unnasch TR. Brugia malayi: transient transfection by microinjection and particle bombardment. Exp Parasitol. 2002;100:95–102. doi: 10.1016/S0014-4894(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Higazi TB, Unnasch TR. Intron encoded sequences necessary for trans splicing in transiently transfected Brugia malayi. Mol Biochem Parasitol. 2004;137:181–4. doi: 10.1016/j.molbiopara.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Jackstadt P, Wilm TP, Zahner H, Hobom G. Transformation of nematodes via ballistic DNA transfer. Molecular and Biochemical Parasitology. 1999;103:261–266. doi: 10.1016/s0166-6851(99)00089-4. [DOI] [PubMed] [Google Scholar]
- Jansen G, Thijssen KL, Werner P, van der Horst M, Hazendonk E, Plasterk RH. The complete family of genes encoding G proteins of Caenorhabditis elegans. Nature Genetics. 1999;21:414–9. doi: 10.1038/7753. [DOI] [PubMed] [Google Scholar]
- Johnson NM, Behm CA, Trowell SC. Heritable and inducible gene knockdown in C. elegans using Wormgate and the ORFeome. Gene. 2005;359:26–34. doi: 10.1016/j.gene.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Kalinna BH, Brindley PJ. Manipulating the manipulators: advances in parasitic helminth transgenesis and RNAi. Trends Parasitol. 2007;23:197–204. doi: 10.1016/j.pt.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Knox DP, Geldhof P, Visser A, Britton C. RNA interference in parasitic nematodes of animals: a reality check? Trends in Parasitology. 2007;23:105–7. doi: 10.1016/j.pt.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Kramer JM, French RP, Park EC, Johnson JJ. The Caenorhabditis elegans rol-6 gene, which interacts with the sqt-1 collagen gene to determine organismal morphology, encodes a collagen. Mol Cell Biol. 1990;10:2081–9. doi: 10.1128/mcb.10.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lall S, Friedman CC, Jankowska-Anyszka M, Stepinski J, Darzynkiewicz E, Davis RE. Contribution of trans-splicing, 5′-leader length, cap-poly(A) synergism, and initiation factors to nematode translation in an Ascaris suum embryo cell-free system. J Biol Chem. 2004;279:45573–85. doi: 10.1074/jbc.M407475200. [DOI] [PubMed] [Google Scholar]
- Li X, Massey HC, Nolan TJ, Schad GA, Kraus K, Sundaram M, Lok JB. Successful transgenesis of the parasitic nematode Strongyloides stercoralis requires endogenous non-coding control elements. Int J Parasitol. 2006;36:671–679. doi: 10.1016/j.ijpara.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Lok JB. Strongyloides stercoralis: a model for translational research on parasitic nematode biology (February 17, 2007) WormBook, ed. The C. elegans Research Community, WormBook. doi: 10.1895/wormbook.1.134.1. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Lok JB, Massey HC., Jr Transgene expression in Strongyloides stercoralis following gonadal microinjection of DNA constructs. Molecular and Biochemical Parasitology. 2002;119:279–84. doi: 10.1016/s0166-6851(01)00414-5. [DOI] [PubMed] [Google Scholar]
- Lopez PM, Boston R, Ashton FT, Schad GA. The neurons of class ALD mediate thermotaxis in the parasitic nematode, Strongyloides stercoralis. Int J Parasitol. 2000;30:1115–21. doi: 10.1016/s0020-7519(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Massey HC, Jr, Ball CC, Lok JB. PCR amplification of putative gpa-2 and gpa-3 orthologs from the (A+T)-rich genome of Strongyloides stercoralis. Int J Parasitol. 2001;31:377–83. doi: 10.1016/s0020-7519(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Mello C, Fire A. DNA transformation. Methods Cell Biol. 1995;48:451–82. [PubMed] [Google Scholar]
- Mello C, Kramer JM, Stinchcomb DT, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. The EMBO Journal. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan TJ, Brenes M, Ashton FT, Zhu X, Forbes WM, Boston R, Schad GA. The amphidial neuron pair ALD controls the temperature-sensitive choice of alternative developmental pathways in the parasitic nematode, Strongyloides stercoralis. Parasitology. 2004;129:753–9. doi: 10.1017/s0031182004006092. [DOI] [PubMed] [Google Scholar]
- Nolan TJ, Megyeri Z, Bhopale VM, Schad GA. Strongyloides stercoralis: the first rodent model for uncomplicated and hyperinfective strongyloidiasis, the Mongolian gerbil (Meriones unguiculatus) Journal of Infectious Diseases. 1993;168:1479–84. doi: 10.1093/infdis/168.6.1479. [DOI] [PubMed] [Google Scholar]
- Pepper M, Dzierszinski F, Crawford A, Hunter CA, Roos D. Development of a system to study CD4+-T-cell responses to transgenic ovalbumin-expressing Toxoplasma gondii during toxoplasmosis. Infection and Immunity. 2004;72:7240–6. doi: 10.1128/IAI.72.12.7240-7246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Smant G, Stokkermans J, Bakker J, Schots A, Helder J. Cloning of a trans- spliced glyceraldehyde-3-phosphate-dehydrogenase gene from the potato cyst nematode Globodera rostochiensis and expression of its putative promoter region in Caenorhabditis elegans. Molecular and Biochemical Parasitology. 1998;96:59–67. doi: 10.1016/s0166-6851(98)00108-x. [DOI] [PubMed] [Google Scholar]
- Reichel C, Mathur J, Eckes P, Langenkemper K, Koncz C, Schell J, Reiss B, Maas C. Enhanced green fluorescence by the expression of an Aequorea victoria green fluorescent protein mutant in mono- and dicotyledonous plant cells. Proc Natl Acad Sci U S A. 1996;93:5888–93. doi: 10.1073/pnas.93.12.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schad GA, Hellman ME, Muncey DW. Strongyloides stercoralis: hyperinfection in immunosuppressed dogs. Exp Parasitol. 1984;57:287–296. doi: 10.1016/0014-4894(84)90103-6. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Fire A. Soma-germline asymmetry in the distributions of embryonic RNAs in Caenorhabditis elegans. Development. 1994;120:2823–34. doi: 10.1242/dev.120.10.2823. [DOI] [PubMed] [Google Scholar]
- Shu L, Katholi CR, Higazi T, Unnasch TR. Analysis of the Brugia malayi HSP70 promoter using a homologous transient transfection system. Molecular and Biochemical Parasitology. 2003;128:67–75. doi: 10.1016/s0166-6851(03)00052-5. [DOI] [PubMed] [Google Scholar]
- Stinchcomb DT, Shaw JE, Carr SH, Hirsh D. Extrachromosomal DNA transformation of Caenorhabditis elegans. Mol Cell Biol. 1985;5:3484–96. doi: 10.1128/mcb.5.12.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenburg G, Liebau E, Henkle-Duhrsen K. Onchocerca volvulus: ultrastructural localization of two glutathione S-transferases. Exp Parasitol. 1998;88:34–42. doi: 10.1006/expr.1998.4189. [DOI] [PubMed] [Google Scholar]
- Winter AD, Myllyharju J, Page AP. A hypodermally expressed prolyl 4-hydroxylase from the filarial nematode Brugia malayi is soluble and active in the absence of protein disulfide isomerase. J Biol Chem. 2003;278:2554–62. doi: 10.1074/jbc.M210381200. [DOI] [PubMed] [Google Scholar]
- Zwaal RR, Mendel JE, Sternberg PW, Plasterk RH. Two neuronal G proteins are involved in chemosensation of the Caenorhabditis elegans dauer-inducing pheromone. Genetics. 1997;145:715–27. doi: 10.1093/genetics/145.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]