Abstract
Single-gene germline mutations conferring a high lifetime risk of CRC account for up to 6% of all CRC cases. The most widely studied monogenic colorectal cancer syndromes include Familial Adenomatous Polyposis (FAP) and Lynch syndrome. However, additional syndromes continue to be defined and new predisposition genes are continuing to be identified.
Most recently, MYH associated polyposis (MAP) and an “Atypical Lynch syndrome” related to the presence of MSH6 mutations have been linked to an increased risk of CRC. In this review, we summarize basic information related to these newly recognized gene mutations, including the accumulating data on the prevalence and penetrance of deleterious mutations, as well as the management options for identified carriers and their families.
Recognizing these heritable syndromes is essential and predictive genetic testing will continue to transform the field of cancer risk assessment by offering the opportunity to focus on more precise risk management and cancer prevention.
Colorectal cancer (CRC) is a common malignancy affecting over 148,000 new patients each year in the United States and is estimated to have caused 55,170 deaths in the year 2006.2 Multiple risk factors have been studied, and one of the most sophisticated advances made to date has been related to the use of molecular genetics in identifying heritable colorectal cancer syndromes.
Although the majority of patients with CRC have sporadic disease without evidence of an inherited disorder, up to 30% have a familial component and a potentially definable genetic basis.2,3 Over the last decade single-gene germline mutations conferring high lifetime risk of CRC have been identified and account for 5–6% of all colorectal cancer cases.4–6 Other genes with more subtle or modifying effects continue to be characterized.
Readily identifying populations with hereditary CRC syndromes, such as Familial Adenomatous Polyposis (FAP) and Lynch syndrome (also known as Hereditary Nonpolyposis Colorectal Cancer or HNPCC), is of great importance given the known benefit of intensive endoscopic surveillance and prophylactic surgery on morbidity and mortality. Recognizing these syndromes also has an impact on referral for predictive genetic testing where identifying gene carriers improves the efficiency of cancer surveillance and helps identify which other family members require intense management versus those who can receive the standard of care.7–9
Genetic information has the potential not only to inform but also transform cancer risk identification, risk reduction, and treatment practices. The incorporation of careful genetic testing into oncology practice is an important step toward more precise risk management and prevention.
Familial Adenomatous Polyposis: Classic and Attenuated
FAP is the most easily identified form of hereditary colorectal cancer. It is an autosomal dominant condition with a prevalence of one in 8000 individuals. It is classically characterized by multiple (>100) adenomatous polyps in the colon and rectum that develop after the first decade of life. The age of onset of colonic adenomas is variable; by age 10 approximately 15% of patients manifest adenomas, by age 20 the probability increases to 75%, and by age 30, 90% will have presented with FAP.10 Without intervention, FAP confers a risk of colorectal cancer of nearly 100% in affected individuals at a mean age of 39 years.
Although total colectomy can drastically reduce colorectal cancer risk in patients with FAP, they remain at risk for other manifestations of the disorder that require frequent medical follow-up and have the potential for considerable morbidity. These associated conditions include polyps in the upper gastrointestinal tract, extraintestinal manifestations such as desmoid tumors, osteomas, epidermoid cysts, congenital hypertrophy of retinal pigment epithelium, and other malignant lesions such as thyroid tumors, small bowel adenocarcinoma, central nervous system tumors, and in children, hepatoblastoma.
Most cases of FAP are due to mutations of the APC gene on chromosome 5q21. APC gene testing has been commercially available since 1994 and has led to changes in management guidelines. Genetic evaluation for individuals fulfilling clinical criteria for FAP is recommended, and presymptomatic genetic testing removes the necessity of annual endoscopic screening of family members who do not inherit the gene mutation present in the family.
While patients affected with classic FAP can be easily identified, a subset of patients has a less obvious phenotype. Patients with 10 or more cumulative colorectal adenomas but less than 100 are at risk for attenuated FAP (AFAP), which arises from APC mutations at the 5′ or 3′ ends of the gene or in certain areas of exon 9.11,12 With this phenotype, adenomas and CRC occur later in life than in classic FAP, extraintestinal manifestations are uncommon, and cases may appear to be sporadic.
MYH-Associated FAP
Inherited mutations in the recently described human analogue of the Escherichia coli muty gene, MYH, have been shown to predispose individuals to multiple colorectal adenomas and carcinoma. Patients with MYH-associated polyposis (MAP) present with clinical features similar to FAP or AFAP but in the absence of a strong multigenerational family history of polyposis. Patients typically present between the ages of 40–60 years with a variable number of colorectal adenomatous polyps.13 However, MYH mutation carriers do not usually present with multiple polyps before the age of 30 years.14 There are limited reports on the prevalence of extracolonic tumors associated with MAP. In one study, where 16 MAP patients underwent upper gastrointestinal endoscopy, duodenal adenomas or gastric fundic gland polyps were found in 31%.15
The Gene
MYH is a base excision repair (BER) gene located on chromosome 1p35 and is involved in repairing DNA that is damaged by reactive oxygen species generated during aerobic metabolism as well as by exogenous stimuli such as various chemical oxidants and ionizing radiation. Loss of MYH activity leads to an unusually high frequency of G:C to T:A transversions resulting in nonsense or splicesite mutations in APC. This suggests faulty repair of 7,8-dihydro-8-oxo-2′deoxyguanosine (8-oxoG), one of the most deleterious products of oxidative DNA damage, where 8-oxoG readily mispairs with adenine residues leading to transversion mutations in the daughter strand. When effective, MYH glycosylase provides defense against such damage by scanning the daughter strand after replication and removing the mispaired nucleoside.
Estimated CRC risk
Biallelic Mutation Carriers
The contribution of MYH to the multiple adenoma phenotype was first described in a Welsh kinship with multiple colorectal adenomatous polyps and carcinoma but no inherited APC mutation or mismatch repair gene.16 In the mutational analysis of MYH in this family, all three affected siblings were noted to be compound heterozygotes for the missense mutations, Y165C and G382D. Unaffected family members were either homozygous wildtype or heterozygous for one of the mutations, and this finding suggested an autosomal recessive pattern of inheritance. Subsequent studies have revealed that this family was not an isolated instance and further demonstrated the prevalence of biallelic MYH mutations in populations with multiple colorectal adenomas. Sieber et al17 noted that in 259 APC mutation negative British patients with five to hundreds of adenomas, 5.4% (14/259) were found to be mutation carriers, and the two original mutations described, Y165C and G382D, accounted for the majority (82%) of mutant alleles. Overall, 30% of patients with 15–100 adenomas were biallelic mutation carriers. In another similar British cohort of 111 probands with more than 10 adenomas, Sampson et al18 found that 23% were biallelic MYH carriers. In these studies the mean age of MAP presentation was about 50 years, and almost all patients had multiple adenomas (5–100) or a mild classical FAP phenotype with fewer than 1000 polyps. CRC was present in about 50% of patients at the time of diagnoses.
These studies have assessed the contribution of MYH to colorectal carcinoma in patients selected for multiple colorectal adenomas and do not account for its role in cases not associated with multiple polyps. To gain insight into the contribution of MYH to the overall CRC burden on the population level, Enholm et al19 conducted a case-control study of 1042 Finnish CRC patients, regardless of their polyp phenotype, and 424 cancer-free controls and found that MYH-associated CRC appeared as common as CRC associated with FAP. Fleischmann et al20 appreciated similar findings when examining the gene in a total of 358 affected individuals with CRC diagnosed before age 55 years and 354 controls. These data20 suggest that up to 2.8 % (upper limit of 95% CI) of early onset CRC could be ascribed to biallelic MYH mutations. Therefore, biallelic mutation carriers not only have some clinical features similar to those with polyposis resulting from APC mutations, but MYH confers a near equal contribution to early onset CRC comparable to that of germline APC mutations.
The role of MYH in early onset CRC has been confirmed in multiple larger case-control studies.21,22 The following studies assessed the magnitude of the increased risk attributable to MYH regardless of polyp phenotype. In the largest cohort studied to date, Farrington et al22 screened a series of 2239 CRC cases and 1845 controls for germline variants of MYH mutations and found that biallelic MYH defects impart a 93-fold excess risk of CRC, which accounts for 0.8% of cases greater than 55 years and 0.54% of the entire cohort. Additionally, penetrance for homozygous carriers was almost complete by age 60 years.22 In a recent meta-analysis pooling the data of all published case-control studies assessing CRC risk in biallelic MYH carriers, Tenesa et al23 validated these findings showing an estimated genotype relative risk (GRR) of 117 (95% CI: 74–184). Jenkins et al24 reported that biallelic carriers were 53 times more likely to be diagnosed with CRC compared with the general population with a cumulative risk of 80% by age 70 years (Table 1).
Table 1.
Risk of Colorectal Cancer in MYH carriers
| Carrier | Lifetime Risk* |
|---|---|
| Monoallelic | 8% (4–19%) |
| Biallelic | 80% (35–100%) |
Cumulative risk by age 70 years 24
Monoallelic Mutation Carriers
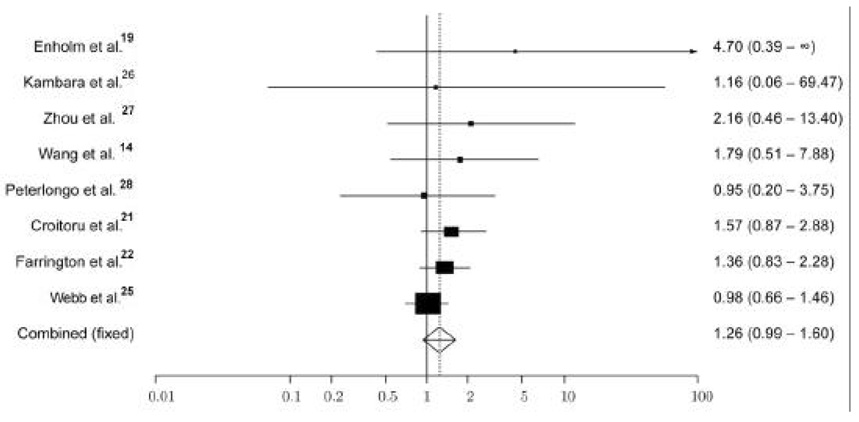
While the risk of developing CRC is appreciated in biallelic mutation carriers, the question of whether individuals known to carry monoallelic MYH mutations are at increased risk of CRC remains greatly debated and unanswered. The studies to date suggest that monoallelic carriers have a slight increase in CRC risk. Jenkins et al24 reported that monoallelic carriers were on average 2.9 times more likely to be diagnosed with CRC compared to the general population and that there is an 8% cumulative risk to age 70 years. In two recent meta-analyses examining the contribution of monoallelic carriers on CRC risk, the pooled estimated odds ratio has been noted to be 1.2623,25 (Figure 1). However, the available data,14,19,21,22,26–28 even when combined, are underpowered to detect an association. This problem is in part due to the low allele frequency of the MYH mutations in all populations studied thus far.
Figure 1. CRC Risk associated with monoallelic MYH variants*.
*Funnel plot of OR of CRC risk associated with monoallelic Y165C and G382D MYH variants, under a fixed-effects model. Studies are plotted in order of decreasing variance of the log (Odds Ratio). Horizontal lines represent 95% Confidence Intervals (CI). Each box represents the Odds Ratio (OR) point estimate, and its area is proportional to the weight of the study. The diamond and broken line represent the overall summary estimate, with the 95% CI given by the width of the diamond. The unbroken vertical line is at the null value (OR 1.0). Reprinted with permission.25
Genetic Testing
Appropriate candidates for MYH mutational analysis testing include individuals who exhibit features of classic or attenuated FAP in the absence of a germline APC mutation and those who have a family history of adenomas or CRC compatible with a recessive pattern of inheritance. Routine screening techniques fail to identify pathogenic APC mutations in an estimated 10–30% of classic FAP cases and in up to 70–90% of AFAP patients, and this situation makes it necessary to consider the possibility of an existing MYH mutation.29–32 Additionally, MYH testing should be considered in patients with young onset of CRC whose tumors do not exhibit defective DNA MMR14,33 (Table 2).
Table 2.
Indications for MYH gene testing
| Recommended: | |
| • | APC gene negative individuals with features of classic FAP/AFAP |
| • | Autosomal recessive inheritance of CRC or multiple adenomas |
| • | Siblings of known MYH mutation carriers* |
| Suggested: | |
| • | Young onset CRC with negative MMR gene mutation14 |
| • | Partners of known biallelic MYH mutation carriers** |
Abbreviations: APC=Adenomatous Polyposis Coli, FAP=Familial Adenomatous Polyposis, AFAP=Attenuated Familial Adenomatous Polyposis, CRC=colorectal cancer, MMR=mismatch repair.
Siblings have highest risk of carrying biallelic mutations (25%) amongst all first-degree relatives
Biallelic mutation carriers produce affected offspring if partner is monoallelic mutation carrier
Genetic testing for MYH mutations is done first by mutation specific testing since two of the most common MYH mutations, Y165C and G382D, account for approximately 85% of MAP cases.34 If one of these mutations is present, then sequencing is done to identify an inactivating mutation on the opposite allele, because if both alleles are mutated to inactivate the gene, disease ensues and CRC risk increases. If neither of the two most common mutations is found and a MAP etiology is strongly suspected, then primary sequencing can be done to detect other less common mutations. Screening of APC and MYH may be considered and performed in parallel in some patients, such as those with isolated cased of multiple adenomas.
Whether it is worthwhile to undertake genetic testing in the partners of patients with biallelic MYH mutations is uncertain, but it is important to consider because a biallelic carrier will produce affected children with a partner who carriers a single mutation. In such cases the disease will appear to be inherited in an autosomal dominant manner.
Clinical Management
To date, no specific screening guidelines for MAP have been established. However, given the established increased CRC risk associated with biallelic mutation carriers, these individuals should be managed similarly to those persons with classic FAP due to APC gene mutations.14,17 This includes screening endoscopies of the upper and lower GI tracts, with consideration for colectomy if the polyp burden makes endoscopic surveillance improbable. Since most data support a later age of polyposis onset than that seen in FAP, suggestions have been made to initiate surveillance at age 25 years.
Baseline endoscopic screening is also recommended for first-degree relatives of biallelic mutation carriers, particularly in siblings, since they carry the highest risk (25%) of also harboring biallelic mutations. Parents and children of biallelic mutation carriers are obligate carriers of at least one of the mutations, and the risk in monoallelic carriers to date is not fully defined. However, given the slight increase in adenoma and CRC likelihood in monoallelic mutation carriers, baseline colonoscopy is suggested, and intense lifetime surveillance should be pursued if colorectal adenomas are noted. In the absence of adenomatous polyps on initial colonoscopy, repeat screening is recommended every 3–5 years14 (Table 3).
Table 3.
| Mutation | Type of Intervention | Recommendation |
|---|---|---|
| MYH | ||
| Biallelic | Colonoscopy/EGD | Start at age 25 years; perform every 1–2 years |
| Colectomy (total or subtotal based on presentation) | For CRC diagnosis or if endoscopic surveillance improbable due to polyp burden | |
| Monoallelic | Colonoscopy | Start at age 25 years; If adenomatous polyps present: perform every 1–2 years; otherwise perform every 3–5 years 14 |
| MSH6* | Colonoscopy | Start at age 30 years (or 10 years earlier than youngest age of CRC diagnosis in family); perform every 1–2 years63 |
| Endometrial sampling/transvaginal ultrasound | Start at age 30–35 years; perform annually | |
| Colectomy (subtotal vs segmental resection) | For CRC diagnosis or if polyp nonresectable by colonoscopy** | |
| Prophylactic colectomy not recommended*** | ||
| Hysterectomy + bilateral salpingo-oophorectomy | For endometrial or ovarian cancer or abnormal endometrial sampling/transvaginal ultrasound | |
| Option of prophylactic surgery should be discussed after childbearing completed64 |
Abbreviations: EGD=esophagogastroduodenoscopy, CRC=colorectal cancer.
Consider cancer specific screening for affected individuals/family members if presence of overrepresented non-endometrial Lynch syndrome-associated cancers
Subtotal colectomy preferred over segmental resection. Segmental resection requires annual colonoscopy given increased risk of metachronous cancers
Prophylactic colectomy can be discussed as alternative to annual colonoscopy in noncompliant patients
Lynch Syndrome
In 1966 Dr Henry Lynch and colleagues35 described an aggregation of colorectal and endometrial cancers in two large midwestern kindreds, inherited in an autosomal dominant manner. Subsequently, investigators have termed this inherited condition “Lynch syndrome” and expanded the constellation of associated malignancies. Lynch syndrome is estimated to account for about 3–5% of all CRC and is caused predominantly by mutations in one of four of the DNA mismatch repair (MMR) genes: MSH2, MLH1, MSH6, and PMS2. Mutation carriers have an increased risk of CRC (60–80%), as well as an increased risk for a wide variety of extracolonic malignancies, most notably endometrial cancer (40–60%).4–6
There are several classification systems for the clinical diagnosis of Lynch syndrome, ranging from the most restrictive Amsterdam criteria36,37 to the most inclusive revised Bethesda Guidelines,38,39 all relying on personal and/or family medical histories. In families that do fulfill the Amsterdam criteria, the chance of identifying a germline mutation in MLH1 and MSH2 ranges from 45–85%.40–43 However, when families with multiple colon cancers do not fulfill the Amsterdam criteria but do fulfill the less stringent Bethesda Criteria, a mutation is detected in approximately 15–30%.41–43
MSH6-Associated “Atypical Lynch Syndrome”
In 1996 it was discovered that germline mutations in MSH6 cause an “atypical” form of Lynch syndrome.44,45 MSH6 defects account for about 7–15% of all Lynch syndrome-associated mutations, but the majority of families with MSH6 mutations do not meet Amsterdam criteria.45–47 Although the link between Lynch syndrome and MSH2 and MLH1 has been firmly established, our understanding of the exact role of MSH6 in inherited cancer susceptibility continues to evolve. MSH6-related Lynch syndrome has a reduced occurrence of CRC that tends to occur at a later age compared to MSH2 and MLH1 carriers as well as an increased prevalence of endometrial cancer.45,48–51 The reported mean age of onset of CRC and endometrial cancer in MSH6 mutation-positive families was 50 years (compared to 44 years in MSH2 and 41 in MLH1) and 53 years (compared to 49 in MSH2 and 48 in MLH1), respectively.45,47 The apparently later age of onset has been confirmed by a more recent study of 20 families (146 carriers) with a truncating mutation in MSH6.52 However, despite the delay in the age of CRC onset, MSH6 mutation carriers are at the same high lifetime risk of cancer (up to 70%) as those individuals with MLH1 and MSH2 mutations (Table 4).
Table 4.
| Colorectal Cancer | Endometrial Cancer | |
|---|---|---|
| Age at diagnosis (years) | ||
| Mean | 53.1 | 53.5 |
| Range | 32–84 | 43–65 |
| Lifetime Risk* | 60–70% | 52–71% |
Cumulative risk by age 80 years
The Gene
The general finding of an older age at diagnosis in MSH6 mutation carriers when compared with carriers of a mutation in MSH2 or MLH1 may be explained from the functional level of the MMR proteins. MLH1 and MSH2 proteins are involved in MMR of both single nucleotide mismatches and insertion-deletion loops, and repair is impaired in the absence of either MLH1 or MSH2. Likewise, the MSH6 protein is involved in the repair of both single-base mismatches and insertion-deletion loops, but it is not absolutely required for DNA MMR activity. In the absence of MSH6, MSH3 protein (the product of a closely related gene) can partially replace MSH6 repair function and may represent a protecting factor against accumulation of DNA damage.53–55
Genetic Testing
The use of microsatellite instability (MSI) testing in the evaluation of Lynch syndrome improves the cost-effectiveness of the molecular strategy for diagnosing these families. MSI analysis gives an indication of abnormal MMR in general, irrespective of the MMR gene and type of mutation involved. It detects length variations of simple, repetitive sequences (microsatellites) distributed throughout the genome as a consequence of faulty MMR. A set of five markers has been developed to test for MSI and includes the markers D2S123, D5S346, D17S250, BAT25, and BAT26. When tumors score MSI-high, there are at least 30% of markers that show instability. This phenotype is reported in 85–92% of CRC associated with Lynch syndrome and in 10–15% of sporadic CRC cases.41,56,57 A distinguishing feature of MSH6-associated Lynch syndrome is that many cancers arising in mutation carriers exhibit an MSI-low or microsatellite stable (MSS) tumor phenotype.49,52,58 This feature may be due in part to the limited number of markers used by most investigators. It is proposed that a more extensive panel of markers be utilized in order to improve the sensitivity of the test.59,60
Given this limitation and the broad variability regarding penetrance associated with MSH6 germline mutations, multiple studies have emphasized the concomitant use of immunohistochemistry (IHC) with MSI analysis as a preselection tool for MSH6 DNA analysis.61,62 IHC offers additional information on which MMR gene should be selected for DNA analysis. Hendricks et al52 have shown that applying IHC to CRC tumors increases the sensitivity in predicting MSH2 mutations to 92% and in MSH6 to 90%. For MLH1, IHC has a sensitivity of 48% when using MLH1 specific antiserum but increases to 71% when PMS2 staining is also applied.52 Since the mismatch repair proteins form heterodimeric complexes, distinct IHC patterns can be expected and patients can thereafter be selected for DNA mutation analysis. If a tumor is found to exhibit MSI with loss of specific protein expression, the targeted germline testing of an affected patient for MLH1, MSH2, or MSH6 mutations can be offered.
It is important to emphasize that IHC is not always diagnostic. It is most useful in identifying particular protein expression loss when MMR mutations result in protein truncation (which includes nonsense, frameshift, and splicesite mutations) and large genomic rearrangements. In cases of missense mutations, IHC can show expression of a protein despite its being functionally abnormal. For individuals with a family history suggestive of atypical Lynch syndrome, but with tumors that are MSS or MSI-L and inconclusive IHC testing, MSH6 germline mutation analysis should be pursued. In addition, screening for MSH6 mutations is recommended for kindreds with suspected Lynch syndrome who are negative for MSH2 and MLH1 germline mutations.4
Clinical Management
Individuals with Lynch syndrome who have a known or a suspected MLH1 or MSH2 mutation, as well as family members who are at increased risk based on an identified mutation in their family, should undergo colonoscopy every 1 to 2 years starting at age 25 years (Table 3). Given the later onset for CRC in MSH6 mutation carriers, it is recommended that colonoscopic screening be initiated at age 30 years and continued every 1 to 2 years thereafter.63 Prophylactic colectomy for at-risk individuals without a history of CRC, as an alternative to screening colonoscopy, is generally not recommended given the insufficient evidence to assess the impact on health outcomes.
With respect to endometrial cancer screening in women with Lynch syndrome, future studies are necessary to determine the most appropriate and effective screening modalities and intervals. At present, it is recommended that women with a known or suspected MMR mutation, or who are at increased risk due to a documented mutation in the family, should undergo annual endometrial biopsy and transvaginal ultrasound beginning at 30 to 35 years. Transvaginal ultrasound is used to assess for ovarian abnormalities but is believed not to have a role in endometrial cancer screening in premenopausal women. Although a general recommendation has not been made regarding prophylactic hysterectomy and oophrectomy, this option should be discussed with postmenopausal women and women who have completed childbearing.64
Cancers other than those of the colon and endometrium account for about 30% of cancers in MLH1 and MSH2 carriers, and perhaps up to 50% of cancers seen in MSH6 carriers.46 However, no standard screening recommendations have been created and to date, there are no studies to support additional surveillance. An empiric approach has been to offer site-specific screening for non-endometrial cancers that appear to be overrepresented in families with Lynch syndrome.
For individuals with a diagnosed CRC or colorectal polyp not amenable to endoscopic resection, a subtotal colectomy should be favored but has not been proven to be superior to annual colonoscopic surveillance. Issues that should be taken into consideration in such patients when recommending subtotal colectomy versus segmental resection include compliance with endoscopic surveillance and personal preference.65
Considerable advances have been made over the last decade in the understanding of genetic predispositions to colorectal cancer. Translating new findings made in molecular genetics has been challenging, and many questions still remain unanswered. However, the potential beneficial impact on the clinical care and well-being of affected patients and their susceptible families is well recognized, and future research is underway to better define associated CRC risk and optimal screening and surveillance strategies. Determining the appropriate candidates for predictive genetic testing and managing cancer risk after testing are critical functions for healthcare providers as exciting and new opportunities emerge in cancer risk and prevention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Burt RW. Colon cancer screening. Gastroenterology. 2000;119:837–853. doi: 10.1053/gast.2000.16508. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 4.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 5.Grady WM. Genetic testing for high-risk colon cancer patients. Gastroenterology. 2003;124:1574–1594. doi: 10.1016/s0016-5085(03)00376-7. [DOI] [PubMed] [Google Scholar]
- 6.Chung DC, Rustgi AK. The hereditary nonpolyposis colorectal cancer syndrome: genetic and clinical implications. Ann Intern Med. 2003;138:560–570. doi: 10.7326/0003-4819-138-7-200304010-00012. [DOI] [PubMed] [Google Scholar]
- 7.Jarvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 8.de Jong AE, Hendiks YM, Kleibeuker JH, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology. 2006;130:665–671. doi: 10.1053/j.gastro.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Heiskanen I, Luostarinen T, Jarvinen HJ. Impact of screening examinations on survival in familial adenomatous polyposis. Scand J Gastroenterol. 2000;35:1284–1287. doi: 10.1080/003655200453638. [DOI] [PubMed] [Google Scholar]
- 10.Petersen GM, Slack J, Nakamura Screening guidelines and premorbid diagnosis of familial adenomatous polyposis. Gastroenterology. 1991;100:1658–1671. doi: 10.1016/0016-5085(91)90666-9. [DOI] [PubMed] [Google Scholar]
- 11.Burt RW, Jacoby RF. Polyposis Syndromes. In: Yamada T, editor. Textbook of Gastroenterology. 4th ed. Volume 2. New York: Lippincott Williams & Wilkins; 2003. pp. 1914–1939. [Google Scholar]
- 12.Knudsen AL, Bisgaard ML, Burlow S. Attenuated familial adenomatous polyposis (AFAP). A review of the literature. Fam Cancer. 2003;2:43–55. doi: 10.1023/a:1023286520725. [DOI] [PubMed] [Google Scholar]
- 13.Doxey BW, Kuwada SK, Burt RW. Inherited polyposis syndromes: Molecular mechanisms, clinicopathology, and genetic testing. Clin Gatroenterol Hepatol. 2005;3:633–641. doi: 10.1016/s1542-3565(05)00370-8. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Baudhuin LM, Boardman LA, et al. MYH mutations in patients with attenuated and classical polyposis and with young-onset colorectal cancer without polyps. Gastroenterology. 2004;127:9–16. doi: 10.1053/j.gastro.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen M, Franken PF, Reinards TM, et al. Multiplicity in polyp count and extracolonic manifestations in 40 Dutch patients with MYH associated polyposis coli (MAP) J Med Genet. 2005;42:e54. doi: 10.1136/jmg.2005.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Tassan N, Chmiel NH, Maynard J, et al. Inherited variants of MYH associated with G:C->T:A mutations in colorectal tumors. Nat Genet. 2002;30:227–232. doi: 10.1038/ng828. [DOI] [PubMed] [Google Scholar]
- 17.Sieber O, Lipton L, Crabtree M, et al. Multiple colorectal adenomas, classic adenomatous polyposis, and germline mutations in MYH. N Engl J Med. 2004;348:791–799. doi: 10.1056/NEJMoa025283. [DOI] [PubMed] [Google Scholar]
- 18.Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362:39–41. doi: 10.1016/S0140-6736(03)13805-6. [DOI] [PubMed] [Google Scholar]
- 19.Enholm S, Hienonen T, Soumanlainen A, et al. Proportion and phenotype of MYH-associated colorectal neoplasia in a population-based series of Finnish colorectal cancer patients. Am J Path. 2003;163:827–832. doi: 10.1016/S0002-9440(10)63443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleischmann C, Peto J, Chradle J, et al. Comprehensive analysis of the contribution of germline MYH variation to early-onset colorectal cancer. Int J Cancer. 2004;109:554–558. doi: 10.1002/ijc.20020. [DOI] [PubMed] [Google Scholar]
- 21.Croitoru ME, Cleary SP, DiNicola N, et al. Association between biallelic and monoallelic germline MYH gene mutations and colorectal cancer risk. J Natl Cancer Inst. 2004;96:1631–1634. doi: 10.1093/jnci/djh288. [DOI] [PubMed] [Google Scholar]
- 22.Farrington SM, Tenesa A, Barnetson R, et al. Germline susceptibility to colorectal cancer due to base-excision repair gene defects. Am J Hum Genet. 2005;77:112–119. doi: 10.1086/431213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenesa A, Campbell H, Barnetson R, et al. Association of MUTYH and colorectal cancer. Br J Cancer. 2006;95:239–242. doi: 10.1038/sj.bjc.6603239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins MA, Croitoru ME, Monga N, et al. Risk of Colorectal Cancer in Monoallelic and Biallelic Carriers of MYH Mutations: A Population-Based Case-Family Study. Cancer Epidemiol Biopmarkers Prev. 2006;15:312–314. doi: 10.1158/1055-9965.EPI-05-0793. [DOI] [PubMed] [Google Scholar]
- 25.Webb EL, Rudd MF, Houlston RS. Colorectal cancer risk in monoallelic carriers of MYH variants. Am J Hum Genet. 2006;79:768–771. doi: 10.1086/507912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kambara T, Whitehall VL, Spring KJ, et al. Role of inherited defects of MYH in the development of sporadic colorectal cancer. Genes Chromosomes Cancer. 2004;40:1–9. doi: 10.1002/gcc.20011. [DOI] [PubMed] [Google Scholar]
- 27.Zhou XL, Djureinovic T, Werelius B, et al. Germline mutations in the MYH gene in Swedish familial and sporadic colorectal cancer. Genet Test. 2005;9:147–151. doi: 10.1089/gte.2005.9.147. [DOI] [PubMed] [Google Scholar]
- 28.Peterlongo P, Mitra N, Chuai S, et al. Colorectal cancer risk in individuals with biallelic or monoallelic mutations of MYH. Int J Cancer. 2005;114:505–507. doi: 10.1002/ijc.20767. [DOI] [PubMed] [Google Scholar]
- 29.Renkonen ET, Nieminen P, Abdel-Rahman WM, et al. Adenomatous polyposis families that screen APC mutation-negative by conventional methods are genetically heterogeneous. J Clin Oncol. 2005;23:5651–5659. doi: 10.1200/JCO.2005.14.712. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong JG, Davies DR, Guy SP, et al. APC mutations in familial adenomatous polyposis families in the Northwest of England. Hum Mutat. 1997;10:376–380. doi: 10.1002/(SICI)1098-1004(1997)10:5<376::AID-HUMU7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 31.Russell AM, Zhang J, Luz J, et al. Prevalence of MYH germline mutations in Swiss APC mutation-negative polyposis patients. Int J Cancer. 2006;118:1937–1940. doi: 10.1002/ijc.21470. [DOI] [PubMed] [Google Scholar]
- 32.Aretz S, Uhlhaas S, Goergens H, et al. MUTYH-associated polyposis: 70 of 71 patients with biallelic mutations present with attenuated or atypical phenotype. Int J Cancer. 2006;119:807–814. doi: 10.1002/ijc.21905. [DOI] [PubMed] [Google Scholar]
- 33.Jo WS, Bandipalliam P, Shannon KM, et al. Correlation of polyp number and family history of colon cancer with germline MYH mutations. Clin Gastroenterol Hepatol. 2005;10:1022–1028. doi: 10.1016/s1542-3565(05)00411-8. [DOI] [PubMed] [Google Scholar]
- 34.Jones S, Emmerson P, Maynaard J, et al. Biallelic germline mutations in MYH predispose to multiple colorectal adenoma and somatic G:C-> T:A mutations. Hum Mol Genet. 2002;11:2961–2967. doi: 10.1093/hmg/11.23.2961. [DOI] [PubMed] [Google Scholar]
- 35.Lynch HT, Shaw MW, Magnuson CW, et al. Hereditary factors in cancer. Study of two large midwestern kindreds. Arch Intern Med. 1966;117:106–112. [PubMed] [Google Scholar]
- 36.Vasen HFA, Mecklin JP, Meera Khan P, Lynch HT. The international collaborative group on HNPCC. Dis Colon Rectum. 1991;34:424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 37.Vasen HFA, Watson P, Mecklin JP, Lynch HT. New criteria for HNPCC proposed by the ICG-HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 38.Rodriquez-Bigas MA, Boland CR, Hamilton SR, et al. An NCI workshop on HNPCC: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89:1758–1762. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 39.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nyström-Lahti M, Wu Y, Moisio AL, et al. DNA mismatch repair gene mutations in 55 kindreds with verified or putative hereditary nonpolyposis colorectal cancer. Hum Mol Genet. 1996;5:763–769. doi: 10.1093/hmg/5.6.763. [DOI] [PubMed] [Google Scholar]
- 41.Moslein G, Tester DJ, Lindor NM, et al. Microsatellite instability and mutation analysis of hMSH2 and hMLH1 in patients with sporadic, familial and hereditary colorectal cancer. Hum Mol Genet. 1996;5:1245–1252. doi: 10.1093/hmg/5.9.1245. [DOI] [PubMed] [Google Scholar]
- 42.Wijnen J, Khan PM, Vasen H, et al. Hereditary nonpolyposis colorectal cancer families not complying with the Amsterdam criteria show extremely low frequency of mismatch-repair-gene mutations. Am J Hum Genet. 1997;61:329–335. doi: 10.1086/514847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lynch HT, de la Chapelle A. Genetic susceptibility to non-polyposis colorectal cancer. J Med Genet. 1999;36:801–818. [PMC free article] [PubMed] [Google Scholar]
- 44.Peterlongo P, Nafa K, Lerman GS, et al. MSH6 germline mutations are rare in colorectal cancer families. Int J Cancer. 2003;107:571–579. doi: 10.1002/ijc.11415. [DOI] [PubMed] [Google Scholar]
- 45.Wijnen J, De Leeuw W, Vasen H, et al. Familial endometrial cancer in female carriers of MSH6 germline mutations. Nat Genet. 1999;23:142–144. doi: 10.1038/13773. [DOI] [PubMed] [Google Scholar]
- 46.Plaschke J, Engel C, Kruger S, et al. Lower incidence of colorectal cancer and later age of disease onset in 27 families with pathogenic MSH6 germline mutations compared to families with MLH1 or MSH2 mutations: the German Hereditary Non-Polyposis Colon Cancer Consortium. J Clin Oncol. 2004;22:4486–4494. doi: 10.1200/JCO.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 47.Miyaki M, Konishi M, Tanaka K, et al. Germline mutation of MSH6 as the cause of hereditary Nonpolyposis colorectal cancer. Nat Genet. 1997;17:271–272. doi: 10.1038/ng1197-271. [DOI] [PubMed] [Google Scholar]
- 48.Kolodner RD, Tytell JD, Schmeits Jl, et al. Germline MSH6 mutations in colorectal cancer families. Cancer Res. 1999;59:5068–5074. [PubMed] [Google Scholar]
- 49.Berends MJ, Wu Y, Sijmons RH, et al. Molecular and clinical characteristics of MSH6 variants: an analysis of 25 index carriers of a germline variant. Am J Hum Genet. 2002;70:26–37. doi: 10.1086/337944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buttin BM, Powell MA, Mutch DG, et al. Penetrance and expressivity of MSH6 germline mutations in seven kindreds not ascertained by family history. Am J Hum Genet. 2004;74:1262–1269. doi: 10.1086/421332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cederquist K, Emanuelsson M, Wiklund F, et al. Two Swedish founder MSH6 mutations, one nonsense and one missense, conferring high cumulative risk of Lynch syndrome. Clin Genet. 2005;68:533–541. doi: 10.1111/j.1399-0004.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- 52.Hendriks YMC, Wagner A, Morreau H, et al. Cancer risk in hereditary nonpolyposis colorectal cancer due to MSH6 mutations: impact on counseling and surveillance. Gastroenterology. 2004;127:17–25. doi: 10.1053/j.gastro.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 53.Fishel R. Signaling mismatch repair in cancer. Nat Med. 1999;5:1239–1241. doi: 10.1038/15191. [DOI] [PubMed] [Google Scholar]
- 54.Jiricny J. Mediating mismatch repair. Nat Genet. 2000;24:6–8. doi: 10.1038/71698. [DOI] [PubMed] [Google Scholar]
- 55.Lipkin SM, Wang J, Jacoby R, et al. MLH3: a DNA mismatch repair gene associated with mammalian microsatellite instability. Nat Genet. 2000;24:27–35. doi: 10.1038/71643. [DOI] [PubMed] [Google Scholar]
- 56.Lothe RA, Peltomaki P, Meling GI, et al. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993;53:5849–5852. [PubMed] [Google Scholar]
- 57.Aaltonen LA, Peltomaki P, Mecklin JP, et al. Replication errors in benign and malignant tumors from hereditary nonpolyposis colorectal cancer patients. Cancer Res. 54:1645–1648. [PubMed] [Google Scholar]
- 58.Wu Y, Berends MJ, Mensink RG, et al. Association of hereditary nonpolyposis colorectal cancer-related tumors displaying low microsatellite instability with MSH6 germline mutations. Am J Hum Genet. 1999;65:1291–1298. doi: 10.1086/302612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 60.Hendriks YMC, Franken P, Dierssen JW, et al. Conventional and tissue microarray immunohistochemical expression analysis of mismatch repair in hereditary colorectal tumors. Am J Pathol. 2003;162:469–477. doi: 10.1016/S0002-9440(10)63841-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plaschke J, Kruger S, Dietmaier W, et al. Eight novel MSH6 germline mutations in patients with familial and nonfamilial colorectal cancer selected by loss if protein expression in tumor tissue. Hum Mutat. 2004;23:285. doi: 10.1002/humu.9217. [DOI] [PubMed] [Google Scholar]
- 62.Wagner A, Hendriks YMC, Meijers-Heijboer EJ, et al. Atypical HNPCC owing to MSH6 germline mutations: analysis of a large Dutch pedigree. J Med Genet. 2001;38:318–322. doi: 10.1136/jmg.38.5.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA. 2006;296:1507–1517. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- 64.Schmeler KM, Lynch HT, Chen LM, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261–296. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- 65.Syngal S, Weeks JC, Schrag D, et al. Benefits of colonoscopic surveillance and prophylactic colectomy in patients with hereditary nonpolyposis colorectal cancer mutations. Ann Intern Med. 1998;129:787–796. doi: 10.7326/0003-4819-129-10-199811150-00007. [DOI] [PubMed] [Google Scholar]