Abstract
Purpose
We have now applied our MORF/cMORF pre-targeting technology to the targeting of CWR22 prostate tumor in nude mice.
Methods
The antiTAG-72 antibody B72.3 was conjugated with an 18 mer MORF while the cMORF was radiolabeled with 99mTc. The specific binding of the antibody to the CWR22 cells was first confirmed in an assay placing the radiolabeled B72.3 antibody in competition with increasing concentrations of native B72.3. Thereafter, a group of four CWR22 tumored mice intravenously received the MORF-B72.3 and, 3 days later, the 99mTc-cMORF, and were killed at 3 h postradioactivity injection. The dosage of the labeled cMORF was selected on the basis of previous experience in LS174T tumored mice. As controls, four animals received only the radiolabeled cMORF and another four received only the 111In-B72.3. The maximum percent tumor accumulation (MPTA) of the labeled cMORF was subsequently determined by a dosage study of labeled cMORF. Both a multipinhole SPECT image and a planar gamma camera image were obtained of a representative mouse.
Results
The CWR22 tumor was confirmed to be TAG-72-positive. The MPTA of the labeled cMORF in the CWR22 tumor was 2.22%ID/g compared to only 0.12%ID/g in control mice without pretargeting. Both the planar and tomographic images confirmed the success of the CWR22 pretargeting.
Conclusions
The MORF/cMORF pretargeting approach has been successfully applied to tumor targeting of the prostate xenograft CWR22. However, the MPTA in this tumor model is lower than that in the LS174T tumor model investigated earlier, possibly due to a lower tumor blood supply.
Keywords: 99mTc, Monoclonal antibody, Biodistribution, Drug delivery, Molecular imaging
Introduction
Pretargeting is an improved approach to targeting solid tumor over the conventional approach using radiolabeled antibody [1–6]. Most tumor pretargeting studies employ one of two popular recognition systems, namely, bispecific antibody/hapten [7, 8] and (strept)avidin/biotin [9]. We have been investigating a relatively new system using a pair of mutually complementary phosphorodiamidate morpholino oligomers (MORF/cMORF) [10]. MORF/cMORF pretargeting involves a MORF conjugated antibody and a radio-labeled cMORF. Among the several advantages of pretargeting with oligomers such as MORF is the latitude in the selection of base sequences, although, up to now, only two complementary pairs of 18 mer base sequences have been investigated [11–13].
A characteristic of pretargeting is complexity. Even for conventional two-step pretargeting, after the tumor model, the pretargeting antibody, and the radiolabeled effector have been selected, there are still four variables, namely, the dosage of the pretargeting agent, the pretargeting interval, the dosage of effector, and the detection time. Earlier, we investigated and characterized the relationship in a mouse tumor model between the pretargeting agent and the effector [12, 13] to simplify the pretargeting optimization. It can be deduced from these previous studies that the antibody dosage is not critical as long as it is below that required to saturate the tumor antigenic sites; the pretargeting interval should be selected to provide the highest practical tumor/ normal tissue (T/NT) ratios of accessible antibody; the effector dosage should be that just sufficient to saturate the accessible MORF-antibody in tumor (the saturating dosage) such that the maximum percent tumor accumulation (MPTA) and the highest T/NT ratios of the effector can all be achieved; and, finally, the detection time should be selected to allow the free effector to clear to the lowest practical level.
Furthermore, the T/NT ratios of the effector can never exceed the T/NT ratios of the accessible antibody. If the effector dosage is below the saturating dosage, the absolute tumor accumulation at the detection time will be less than the maximum. Because the accessible antibody in normal tissue is usually saturated by the effector, the T/NT ratios of effector will then be lower than the T/NT ratios of the accessible antibody. Conversely, if the effector dosage is above the saturating dosage, the T/NT ratios of the effector will be equal to that of the accessible antibody, but the percent tumor accumulation will be lower than MPTA. The only exception is in kidneys, where the effector concentration is independent of the antibody concentration therein [12, 13]. However, the T/NT ratios of the accessible antibody and, therefore, of the effector can be improved by increasing the pretargeting interval or by using a clearing agent, but the MPTA is a value depending only on the tumor and the labeled effector. That the MPTA depends only on the tumor model and the properties of the effector is explained in the Appendix.
In this investigation, the MORF/cMORF pretargeting was applied to prostate cancer. Prostate cancer usually starts as an androgen-dependent tumor. However, about 2 years after androgen ablation, the tumor often becomes androgen-independent [14, 15]. An ability to target early-stage androgen-dependent prostate cancer would be useful because early detection can be critical to patient management. The CWR22 is an androgen-dependent tumor model and, therefore, is suitable for this investigation [16]. Because TAG-72 has been widely reported as being overexpressed in prostate cancers [17–22], most probably the CWR22 tumor is also TAG-72-positive. If so, antiTAG-72 antibodies such as B72.3 or CC49 may be used for our purpose. The cellular expression of TAG-72 is usually defined by the binding of antiTAG-72 antibody B72.3 to the cells [23–25]. In this paper, the CWR22 was first tested for its TAG-72 expression followed by the use of the B72.3 antibody in the pretargeting study. We now report on successful MORF/cMORF pretargeting of the CWR22 prostate xenograft mouse model.
Materials and methods
The sequences and molecular weights of the MORF and cMORF from Gene Tools (Philomath, OR, USA) were identical to those in our previous reports [11–13]. The B72.3 antiTAG-72 IgG antibody was a gift from the NCI BRB Preclinical Repository (Rockville, MD, USA). This antibody does not internalize and is suitable for pretargeting [26]. The diethylenetriamine pentaacetic acid (DTPA) cyclic anhydride was from Sigma-Aldrich (St. Louis, MO, USA), while the p-isothiocyanate (SCN)-benzyl-DTPA was from Macrocyclics (Dallas, TX, USA). The S-acetyl NHS-MAG3 was synthesized in house [27] and the structure was confirmed by elemental analysis, proton NMR, and mass spectroscopy. The Sephadex G-100 and medium P4 gel powder were from Pharmacia Biotech (Uppsala, Sweden) and Bio-Rad laboratories (Hercules, CA, USA) respectively. The 99Mo/99mTc generator and the 111InCl3 were from Perkin Elmer Life Science (Boston, MA, USA). All other chemicals were reagent grade. For tumor model development, both the 90-day release testosterone and the 10-gauge precision trochar to implant the testosterone pill were from Innovative Research of America (Sarasota, FL, USA). The 130-mL Bellco tissue sieve kit was from Bellco Glass (Vineland, NJ, USA). The BD Matrigel™ Matrix basement membrane was from BD Biosciences (Bedford, MA, USA).
Cells were washed using a Heraeus Biofuge microbiocentrifuge (Kendro Laboratory Products, Langenselbold, Germany) and a CR412 centrifuge (Jouan, Winchester, VA, USA). Radioactivity was measured on a Cobra II auto-gamma counter (Packard Instrument, Meriden, CT, USA). The optical density of the MORF solutions was determined on a U-2000 UV spectrophotometer (Hitachi Instrument, Dabury, CT, USA). The size exclusion HPLC system consisted of Waters 515 pumps (Milford, MA, USA), a Superose-12 size exclusion column (Amersham Pharmacia Biotech, Piscataway, NJ, USA), an in-line Waters 2487 dual wavelength UV detector, and an in-line radioactivity detector.
Preparation of 111In-DTPA-B72.3, 99mTc-cMORF, and MORF-B72.3
Conjugation of DTPA to B72.3 using the DTPA cyclic anhydride was as previously reported [12]. Briefly, the B72.3 was mixed with the anhydride in 0.15–0.25 M (final concentration), pH 8.0 bicarbonate buffer at a 50:1 DTPA/ B72.3 molar ratio. The reaction mixture was incubated at room temperature for 1 h and purified on a Sephadex G-100 column. Conjugation with p-SCN-benzyl-DTPA was achieved in the same bicarbonate buffer at the same DTPA/antibody molar ratio but at pH 9.3, and the mixture was allowed to react for 15–24 h at room temperature before purification. Labeling of both conjugates was achieved by mixing 0.5 M pH 6.0 NaOAc buffer with 111InCl3 in 50 mM HCl (v/v=1/1) and then adding to the purified DTPA conjugates of B72.3. Radiochemical purity was measured by size exclusion HPLC, in which recovery was routinely measured.
The labeling of cMORF with 99mTc was as previously described [28]. When stored at −20°C in pH 5.2 NH4OAc buffer, the MAG3–cMORF conjugate has been used for at least 3 years with labeling efficiency unchanged at more than 95%. Because pertechnetate is retained on the Superose-12 column, Whatman no. 1 paper was used with acetone as eluant to measure the percentage of pertechnetate (Rf=1.0). Radiochemical purity was therefore determined by both size exclusion HPLC and paper chromatography.
The MORF-B72.3 was prepared using the commercial Hydralink method [29]. The purity of the products in each step was monitored by size exclusion HPLC. The average number of MORFs per antibody (gpm) was determined by adding a known excess of 99mTc-labeled cMORF to a known amount of MORF-B72.3 as described previously [11].
The CWR22 tumor model
The CWR22 cell line was a gift from Dr. Thomas G. Pretlow (Case Western Reserve University School of Medicine, Cleveland, OH, USA). Subsequent generations were developed as follows: Briefly, a BALB/c mouse with a 1–3-g CWR22 tumor was killed under anesthesia and the tumor was excised under sterile conditions. In a Petri dish with 15 mL working media (RPMI 1640 with 1% fetal bovine serum), the tumor was cut into small pieces (2–3 mm), transferred to a small beaker, and washed three times with the working media. The tumor pieces were ground into fragments and sieved (80 mesh). Working media was periodically added. The slurry of cell fragments was collected into a 50-mL Corning centrifuge tube and washed with working media until no red blood cells were apparent. Throughout this process, the cell fragment suspension was kept at 4°C. The cell fragments were used for tumor inoculation and cell binding study or stored in a −80°C freezer for future use in mice for tumor development.
For tumor inoculation, cell fragments were washed with plain RPMI 1640 medium using a microcentrifuge at 900×g. The pellet was mixed with an equal volume of BD Matrigel™ Matrix basement membrane. Each BLAB/c mouse received subcutaneously 100 μL of fragment-Matrigel in the lower back using an ice-cold 1.0-mL syringe with a 22-gauge needle. Because the CWR22 tumor is androgen-dependent, half a 90-day-release testosterone pill (12.5 mg/pill) was subcutaneously implanted in one flank using a trochar 2 days before tumor implantation. About 30 days later, tumors became measurable and tumor growth was monitored by calipers to estimate tumor width, thickness, and length.
Throughout this investigation, the weights of all tumors were measured at death. As a result, it was possible to correlate tumor dimensions and weights for better control of tumor size for subsequent studies.
Confirmation of B72.3 binding to CWR22 cells
The radioactive complex 99mTc-cMORF/MORF-B72.3 was prepared by mixing MORF-B72.3 (gpm 0.5) with 99mTc-cMORF at a MORF/cMORF molar ratio of between 2 and 5 to assure no free labeled cMORF was present. The complex was purified over a Sephadex G-100 column (0.7×20 cm) and the peak fractions were pooled and adjusted to a concentration of 0.70 μg (2.5 μCi) B72.3 per 100 μL using native B72.3 and working media.
An aliquot containing 20 mg of CWR22 fragments was added to each of 30 microcentrifuge tubes. After centrifugation, the supernatant was discarded and 150 μL of working media containing 0.70 μg (2.5 μCi) of 99mTc-cMORF/MORF-B72.3 was added to 27 of the tubes. After dividing into nine groups of three, different dosages of native B72.3 between 0.0 and 300 μg was added to the tubes such that the concentration of B72.3 was between 0.0 and 12.11 pmol/μL. The remaining three tubes each received 150 μL working media containing only 2.5 μCi of 99mTc-cMORF as controls. All the tubes were incubated on ice for 50 min before being centrifuged at 900×g. The pellets were washed twice with 1 mL working media. The supernatants were collected and counted on an auto-gamma counter together with the pellets.
In vivo tumor pretargeting
Twelve BALB/c tumored mice were divided into three groups. Group 1 mice each received only the 99mTc-cMORF effector at 1.0 μg (90 μCi) and were killed at 3 h postradioactivity injection. Group 2 mice each received 30 μg of MORF-B72.3 (gpm 0.41) and, 72 h later, 1.0 μg (90 μCi) 99mTc-MAG3–cMORF and were killed at 3 h. Group 3 mice each received only 30 μg (25 μCi) 111In-DTPA-B72.3 and were killed at 72 h. All administrations were intravenous and death was achieved under anesthesia by heart puncture and exsanguination. All organs and tumors were collected and the radioactivity was counted in an auto-gamma counter along with an injectate standard. Blood and muscle were assumed to constitute 7 and 40% of the total body weight. The percent of the injected dosage in each organ (%ID) and per gram of organ (%ID/g) were calculated.
A separate study was performed to determine the MPTA by varying the dosage of the labeled cMORF. The experimental conditions were identical to group 2 above, except the dosage of cMORF was varied between 0 and 2.0 μg. Furthermore, tumor size influence on the tumor accumulation of labeled antibody was examined ranging 0.35–1.30 g after each mouse received 30 μg 111In-DTPA-benzyl-B72.3 under identical conditions to those of group 3.
MicroSPECT imaging
The microSPECT imaging system consisted of a three-head clinical gamma camera (PRISM 3000, Philips, Cleveland, OH, USA) fitted with three multipinhole tungsten apertures from Bioscan (Washington, DC). In this study, three nine-pinhole apertures were used and 10 acquisitions by each head were obtained within 20 min. Images were reconstructed using the HiSPECT program (Bioscan). One BALB/c mouse bearing a 1.02-g CWR22 tumor in one flank received 150 μg of MORF-B72.3 (gpm 0.53) via a tail vein. Three days later, 6 μg (7.5 mCi) of 99mTc-MAG3–cMORF was injected followed by imaging at 3 h under ketamine/xylazine anesthesia.
Results
The CWR22 tumor model
Correlation of tumor dimensions to tumor weight based on our data produced a slope of 0.53 for the plot of tumor weight vs the product of tumor width, thickness, and length. This result is identical to that from a recent report of 0.5234 for the same model [30]. Figure 1 shows the tumor growth over time in one batch of 20 BALB/C nude mice of this investigation, in which the tumor weights are estimated from the tumor dimensions.
Fig. 1.
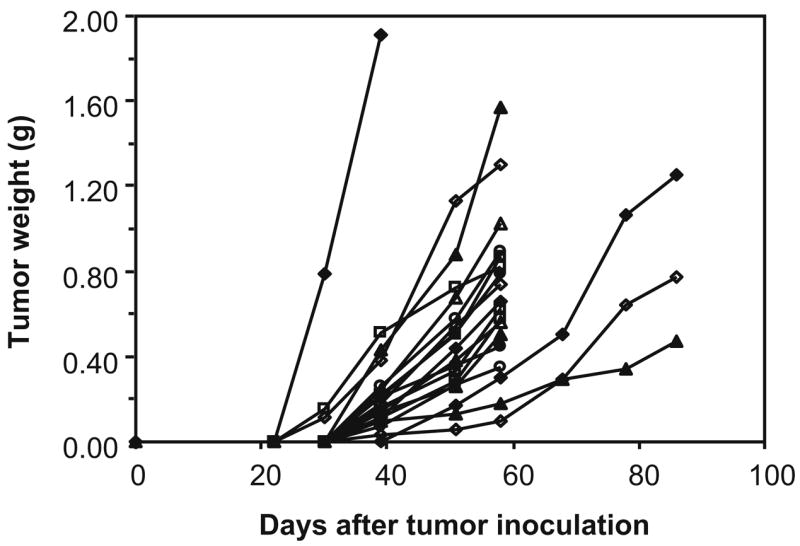
Estimated tumor weight vs days after tumor inoculation plotted individually for each animal
Confirmation of B72.3 binding to CWR22 cells
The decreasing percentage of 99mTc labeled B72.3 (99mTc-cMORF/MORF-B72.3) bound to the cell fragments with increasing concentrations of total B72.3 is shown in Fig. 2, indicating that the binding of antibody to tumor is specific and, therefore, that the CWR22 prostate xenograft expresses the TAG-72 antigen. As a control, the binding of 99mTc-cMORF to cells not pretargeted was also measured and found to be undetectable (data not shown).
Fig. 2.
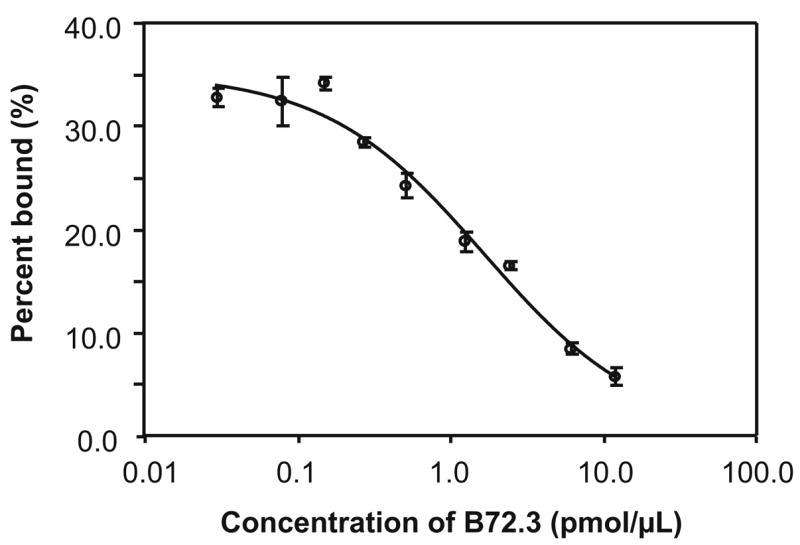
The percentage of 99mTc labeled B72.3 binding to CWR22 cell fragments with increasing concentrations of total B72.3. For each data point, N=3, with the error bar showing one standard deviation
In vivo tumor pretargeting
Table 1 presents the biodistribution of 99mTc-cMORF in CWR22 tumored BALB/c mice with and without the prior administration of 30 μg of MORF-B72.3 and, also, the biodistribution of 30 μg B72.3 radiolabeled with 111In by the cyclic anhydride of DTPA and by p-SCN-benzyl-DTPA. The tumor accumulation of the labeled cMORF in the pretargeted group is 15 times that without pretargeting (1.81 vs 0.12%ID/g), indicating pretargeting as the mechanism of tumor localization. The generally lower tissue accumulations of the effector alone contrast with the typically higher normal tissue levels observed in pretargeting studies without the benefit of a clearing agent. Because of the incomplete clearance of the MORF-B72.3 in the circulation at the time of effector administration, radioactivity levels in the pretargeted mice are elevated in circulation and in all normal organs except the kidney. As we have observed previously for the LS174T tumor model, the kidney accumulation in pretargeting studies is characteristic of the labeled cMORF alone [12, 13].
Table 1.
Biodistribution at 3 h of 1.0 μg of 99mTc-cMORF in CWR22 tumored BALB/c mice with and without the 72 h prior administration of 30 μg of MORF-B72.3, as well as the biodistribution at 72 h of 30 μg 111In labeled B72.3 radiolabeled by cDTPA and by p-SCN-benzyl-DTPA
| Organ | 99mTc-cMORF control (N=4) | 99mTc-cMORF/MORF- B72.3 pretargeted (N=4) | 111In-DTPA-B72.3 alone (N=4) | 111In-DTPA-benzyl- B72.3 alone (N=14) |
|---|---|---|---|---|
| %ID/g | ||||
| Liver | 0.20±0.03 | 0.76±0.16 | 15.72±2.38 | 6.25±1.16 |
| Heart | 0.04±0.01 | 0.56±0.13 | 1.72±0.35 | 2.74±0.21 |
| Kidney | 5.76±1.09 | 4.64±0.51 | 7.94±1.68 | 4.32±0.55 |
| Lung | 0.13±0.02 | 1.02±0.21 | 2.65±0.32 | 4.76±1.83 |
| Spleen | 0.07±0.01 | 0.42±0.04 | 6.42±0.60 | 3.57±0.36 |
| Muscle | 0.02±0.00 | 0.26±0.08 | 0.59±0.12 | 0.90±0.12 |
| Tumor | 0.12±0.02 | 1.81±0.18 | 4.28±0.56 | 4.71±0.73 |
| Salivary | 0.40±0.10 | 0.82±0.12 | 2.36±0.37 | 3.17±0.42 |
| Blood | 0.03±0.01 | 2.50±0.60 | 2.58±0.41 | 10.01±1.84 |
| %ID | ||||
| Liver | 0.28±0.04 | 1.27±0.20 | 21.51±2.21 | 9.61±1.81 |
| Heart | 0.01±0.00 | 0.09±0.02 | 0.24±0.02 | 0.37±0.05 |
| Kidney | 3.05±0.44 | 2.73±0.40 | 4.18±0.75 | 2.24±0.23 |
| Lung | 0.02±0.00 | 0.18±0.05 | 0.42±0.01 | 0.76±0.22 |
| Spleen | 0.01±0.00 | 0.07±0.01 | 0.86±0.28 | 0.52±0.10 |
| Stomach | 0.04±0.01 | 0.11±0.01 | 0.33±0.11 | 0.29±0.04 |
| Sm. Int. | 0.15±0.03 | 0.46±0.14 | 1.97±0.54 | 1.66±0.23 |
| Lg. Int. | 1.46±0.25 | 1.60±0.31 | 0.92±0.34 | 1.03±0.27 |
| Muscle | 0.19±0.03 | 2.56±0.76 | 5.51±1.03 | 8.67±1.11 |
| Tumor | 0.08±0.04 | 0.97±0.50 | 3.65±1.62 | 3.24±1.70 |
| Salivary | 0.08±0.03 | 0.17±0.04 | 0.44±0.06 | 0.53±0.09 |
| Blood | 0.05±0.01 | 4.39±1.02 | 4.18±0.55 | 16.91±2.96 |
| Weight (g) | ||||
| Tumor | 0.65±0.31 | 0.55±0.31 | 0.85±0.38 | 0.67±0.31 |
Results presented as %ID/g and %ID/organ as the mean ± one standard deviation
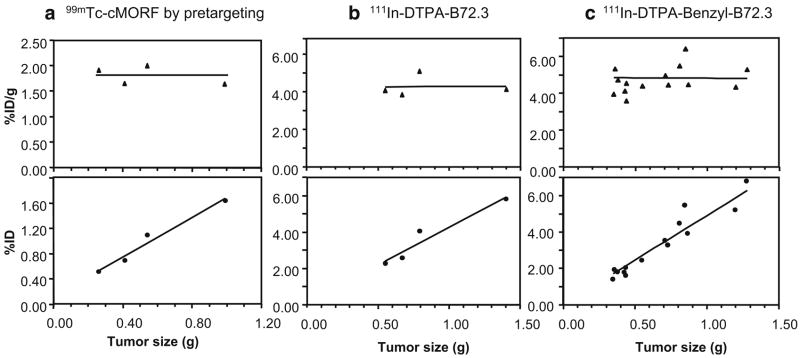
The individual tumor accumulations are plotted against tumor size in Fig. 3. As shown in panels a and b, the tumor accumulations of the effector and the 111In labeled antibody shows minimal variation among animals despite a range of tumor sizes from 0.2 to 1.0 g and from 0.4 to 1.4 g, respectively. This is in contrast with our previous experiences with the LS174 T tumor model [12, 13]. Therefore, a study was performed in which 30 μg of 111In-DTPA-benzyl-B72.3 was administered to each of 14 CWR22 tumored mice, and the tumor accumulations were then correlated with tumor size at death. As shown in panel c, the individual tumor accumulations of the antibody were confirmed as fairly independent of tumor size in the case of the CWR22 tumor.
Fig. 3.
Tumor accumulation vs tumor size of 99mTc-cMORF by pretargeting (a), of 111In-DTPA-B72.3 (b), and of 111In-DTPA-benzyl-B72.3 (c)
Table 1 also shows that the tumor accumulations in the pretargeted mice are 2.5 times lower than the tumor accumulations of 111In labeled B72.3 regardless of the chelators. However, the T/NT ratios in liver and spleen are higher in pretargeted animals as shown in Table 2. The T/ NT ratios in other normal organs except blood and kidney by pretargeting at 3 h postradioactivity administration are comparable to those for animals receiving 111In-DTPA-B72.3 and superior to those for animals receiving 111In-DTPA-benzyl-B72.3, both at 72 h postadministration. Since the tumor-to-blood ratio by pretargeting is theoretically equal to that of the accessible MORF-antibody as discussed above, it is not surprising that they are comparable to those of 111In labeled antibody.
Table 2.
The T/NT ratios derived from Table 1
| Organ | 99mTc-cMORF control (N=4) | 99mTc-cMORF/MORF- B72.3 pretargeted (N=4) | 111In-DTPA- B72.3 alone (N=4) | 111In-DTPA-benzyl- B72.3 alone (N=14) |
|---|---|---|---|---|
| Liver | 0.60 | 2.38 | 0.31 | 0.75 |
| Heart | 3.00 | 3.23 | 2.49 | 1.72 |
| Kidney | 0.02 | 0.39 | 0.54 | 1.09 |
| Lung | 0.92 | 1.77 | 1.62 | 0.99 |
| Spleen | 1.71 | 4.31 | 0.67 | 1.32 |
| Muscle | 6.00 | 6.96 | 7.25 | 5.23 |
| Salivary | 0.30 | 2.21 | 1.81 | 1.49 |
| Blood | 4.00 | 0.72 | 1.66 | 0.47 |
MPTA of the labeled cMORF
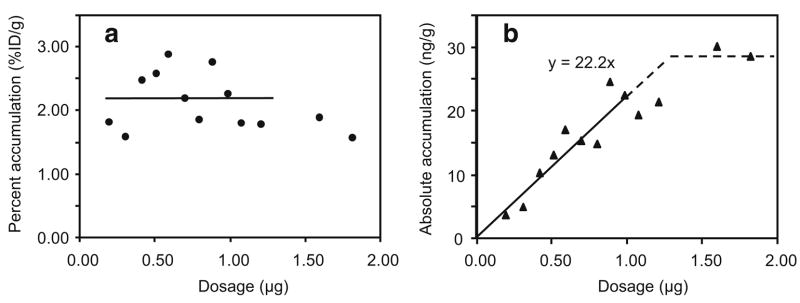
Figure 4 presents the percent and absolute tumor accumulations of labeled effector in pretargeted mice at 3 h as a function of effector dosage. As shown in panel b, the absolute tumor accumulation increases linearly to 29 ng/g and then levels off at an effector dosage of 1.25 μg, as the MORF-B72.3 in tumor becomes saturated. The MPTA, defined as the percent tumor accumulation of effector administered at a dosage below that necessary to saturate the accessible MORF-antibody in tumor, is calculated as 2.21±0.45%ID/g by averaging all the percent tumor accumulations below the saturation dosage of 1.25 μg. Alternatively, the MPTA is also calculated as 2.22%ID/g from the initial slope of the absolute tumor accumulation curve vs dosage. Both values are in agreement with the 1.81±0.18%ID/g listed in Table 1 for the earlier pretargeting study at a fixed effector dosage of 1.0 μg.
Fig. 4.
Percent (a) and absolute (b) accumulation vs dosage of 99mTc-cMORF per gram of tumor by pretargeting
MicroSPECT imaging
Figure 5 presents a planar scintigraphic image (b) of the pretargeted mouse and coronal (c) and sagittal (d) slices across the tumor of the 3-D HiSPECT image at 3 h postradioactivity injection, along with a photograph of the animal (a). As shown, radioactivity is much higher in the tumor than in normal organs except for the kidneys. Because the effector is cleared through this organ, radioactivity is usually apparent there.
Fig. 5.
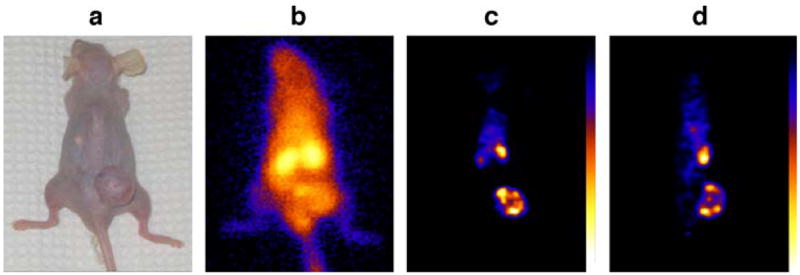
A dorsal whole-body gamma camera image (b) of one pretargeted mouse presented along with a photograph of the animal (a). Also presented are a coronal (c) and a sagittal SPECT section (d) crossing the tumor
Discussion
The CWR22 prostate cell line used in this investigation grew more rapidly compared to another androgen-dependent cell line LNCaP and provided a higher tumor take rate of over 90% in BALB/c nude mice compared to 75% for LNCaP. For that reason, the CWR22 was selected for this investigation, even though this cell line cannot be maintained in cell culture. The MPTA of the labeled cMORF of 2.22%ID/g for CWR22 tumors ranging in size from 0.4 to 1.8 g is lower than the previous MPTAs of 4.40, 6.25, and 8.89%ID/g for LS174T tumors of 1.0, 0.5, and 0.4 g respectively, using the same MORF/cMORF recognition pair ([13] our unpublished data). Much higher values have occasionally been reported from other laboratories for other effectors. For example, a tumor accumulation of 250%ID/g for the 111In-diDTPA hapten in a SK-RC-52 tumor mouse model pretargeted with a bispecific antibody has been reported [31]. As explained above, the MPTA of a labeled effector depends upon the tumor model and the labeled effector. In a different SK-RC-1 tumor mouse model, the same authors using the same pretargeting approach reported a much lower tumor accumulation of about 25%ID/g [32]. It is therefore incorrect to compare MPTAs among pretargeting approaches using different tumor models.
A more meaningful comparison is to compare in the same CWR22 tumor model the MPTA of the labeled cMORF effector in pretargeted mice with the tumor accumulations of other labeled agents by conventional direct targeting. The CWR22 tumor accumulation of deoxyglucose radiolabeled with 18F (18F-FDG) in BALB/c mice was reported to be about 2.5%ID/g at 1 h [33, 34] but with high and persistent normal tissue levels. In the same report [34], the tumor accumulation of 11C-acetate was reported to be lower but also with poor T/NT ratios. The CWR22 tumored mouse model was also used to test the 3'-deoxy-3'-18F-fluorothymidine (18F-FLT) accumulations [35]. Retention of 18F-FLT was poor with tumor accumulation of 0.69%ID/g at 2 h. Finally, the tumor accumulation of 64Cu labeled peptide bombesin in BALB/C mice with CWR22 tumors was about 1.75%ID/g at 0.5 h [36]. Thus, among these studies, MORF/cMORF pretargeting provided comparable or higher tumor accumulations and T/NT ratios superior to those from conventional tumor targeting. Finally, as already mentioned, the T/NT ratios by MORF/ cMORF pretargeting can be further improved by increasing the pretargeting interval and/or using a clearing agent.
The differences in biodistributions between the 111In-DTPA-benzyl-B72.3 and 111In-DTPA-B72.3 shown in Table 1 may raise questions about the accuracy of using 111In as an antibody tracer, as is routine in this laboratory and elsewhere [12, 13]. Fortunately, what is important in pretargeting is not the absolute antibody concentration, measured by a radioactive tracer such as 111In-antibody, but the accessible antibody concentration, measured by the retention of labeled effector.
Conclusion
Successful pretargeting of an androgen-dependent prostate cancer xenograft in mice was achieved with MORF-B72.3 and radiolabeled cMORF.
Acknowledgments
The authors are grateful to Dr. Pretlow (Case Western Reserve University School of Medicine, Cleveland, OH, USA) for the CWR22 tumor cells, to Dr. Aurigemma (NCI BRB Preclinical Repository, Rockville, MD, USA) for the B72.3 antibody, and to Dr. Shayne Squires for helping with the tumor model development. Financial support was provided in part by NIH (CA107360 and CA94994).
Financial support: CA107360 and CA94994.
Appendix
If we define a space containing the whole tumor, the amount of labeled effector dQ (ng) accumulated in tumor in an infinitely short period of time dt is:
where F is the cardiac output in grams of blood per second (g/s), f is the blood fraction flowing into tumor, E is the trapping efficiency, i.e., the fraction of the effector reaching the tumor that is retained, and C is the blood concentration of the effector in nanograms of effector per gram of blood (ng/g). Given that the F and f are constants for a given tumor model (i.e., animal, tumor type, location, and tumor size), the total tumor accumulation Q over the entire period from administration to the complete clearance of free effector will be:
Dividing both sides of the equation by the injected dosage of effector (ng) and the tumor weight W and multiplying by 100, the accumulation of effector now in percent of injected dosage per gram of tumor (%ID/g) becomes:
As described earlier and verified experimentally [12, 13], Q is a constant when the dosage of effector is below that required to saturate the MORF-antibody in the tumor. Because the product F×f×W−1 is also constant, the integral must also be a constant under these conditions. Furthermore, is a constant for the free labeled effector alone. In pretargeted mice, if the antibody concentration in normal tissues is sufficiently low, to a first approximation, the integral will be unchanged from the effector alone condition. Therefore, E must be a constant and can also be put outside of the integral. However, at dosages of effector greater than required to saturate the MORF antibody in tumor, E will become zero at some point when there is no longer MORF in tumor available to retain the effector, resulting in a smaller percent accumulation. Therefore, the accumulation before saturation of pretargeting agent in tumor will be the MPTA:
This equation shows that the MPTA is independent of the pretargeting agent and dependent only on the tumor and the effector.
References
- 1.Karacay H, Brard PY, Sharkey RM, Chang CH, Rossi EA, McBride WJ, et al. Therapeutic advantage of pretargeted radioimmunotherapy using a recombinant bispecific antibody in a human colon cancer xenograft. Clin Cancer Res. 2005;11:7879–85. doi: 10.1158/1078-0432.CCR-05-1246. [DOI] [PubMed] [Google Scholar]
- 2.Sharkey RM, Cardillo TM, Rossi EA, Chang CH, Karacay H, McBride WJ, et al. Signal amplification in molecular imaging by pretargeting a multivalent, bispecific antibody. Nat Med. 2005;11:1250–5. doi: 10.1038/nm1322. [DOI] [PubMed] [Google Scholar]
- 3.Pagel JM, Hedin N, Subbiah K, Meyer D, Mallet R, Axworthy D, et al. Comparison of anti-CD20 and anti-CD45 antibodies for conventional and pretargeted radioimmunotherapy of B-cell lymphomas. Blood. 2003;101:2340–8. doi: 10.1182/blood-2002-03-0874. [DOI] [PubMed] [Google Scholar]
- 4.Subbiah K, Hamlin DK, Pagel JM, Wilbur DS, Meyer DL, Axworthy DB, et al. Comparison of immunoscintigraphy, efficacy, and toxicity of conventional and pretargeted radioimmunotherapy in CD20-expressing human lymphoma xenografts. J Nucl Med. 2003;44:437–45. [PubMed] [Google Scholar]
- 5.Magnani P, Paganelli G, Modorati G, Zito F, Songini C, Sudati F, et al. Quantitative comparison of direct antibody labeling and tumor pretargeting in uveal melanoma. J Nucl Med. 1996;37:967–71. [PubMed] [Google Scholar]
- 6.Sung C, van Osdol WW. Pharmacokinetic comparison of direct antibody targeting with pretargeting protocols based on streptavidin-biotin binding. J Nucl Med. 1995;36:867–76. [PubMed] [Google Scholar]
- 7.Reardan DT, Meares CF, Goodwin DA, McTigue M, David GS, Stone MR, et al. Antibodies against metal chelates. Nature. 1985;316(6025):265–8. doi: 10.1038/316265a0. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin DA, Meares CF, MnTigue M, et al. Rapid localization of haptens in sites containing previously administrated antibody for immunoscintigraphy with short half-life tracers [abstract] J Nucl Med. 1986;27(suppl):959. [Google Scholar]
- 9.Hnatowich DJ, Virzi F, Rusckowski M. Investigations of avidin and biotin for imaging applications. J Nucl Med. 1987;28:1294–302. [PubMed] [Google Scholar]
- 10.Liu G, Mang’era K, Liu N, Gupta S, Rusckowski M, Hnatowich DJ. Tumor pretargeting in mice using 99mTc-labeled morpholino, a DNA analog. J Nucl Med. 2002;43:384–91. [PubMed] [Google Scholar]
- 11.Liu G, He J, Dou S, Gupta S, Vanderheyden JL, Rusckowski M, et al. Pretargeting in tumored mice with radiolabeled morpholino oligomer showing low kidney uptake. Eur J Nucl Med Mol Imaging. 2004;31:417–24. doi: 10.1007/s00259-003-1393-9. [DOI] [PubMed] [Google Scholar]
- 12.Liu G, He J, Dou S, Gupta S, Rusckowski M, Hnatowich DJ. Further investigations of morpholino pretargeting in mice—establishing quantitative relations in tumor. Eur J Nucl Med Mol Imaging. 2005;32:1115–23. doi: 10.1007/s00259-005-1853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu G, Dou S, He J, Liu X, Rusckowski M, Hnatowich DJ. Predicting the biodistribution of radiolabeled cMORF effector in MORF-pretargeted mice. Eur J Nucl Med Mol Imaging. 2007;34:237–46. doi: 10.1007/s00259-006-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scher HI, Buchanan G, Gerald W, Butler LM, Tilley WD. Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocr Relat Cancer. 2004;11:459–76. doi: 10.1677/erc.1.00525. [DOI] [PubMed] [Google Scholar]
- 15.Miyamoto H, Messing EM. Androgen deprivation therapy for prostate cancer: current status and future prospects. Prostate. 2004;61:332–53. doi: 10.1002/pros.20115. [DOI] [PubMed] [Google Scholar]
- 16.Nagabhushan M, Miller CM, Pretlow TP, Giaconia JM, Edgehouse NL, Schwartz S, et al. CWR22: the first human prostate cancer xenograft with strongly androgen-dependent and relapsed strains both in vivo and in soft agar. Cancer Res. 1996;56:3042–6. [PubMed] [Google Scholar]
- 17.Mazur MT, Shultz JJ. Prostatic adenocarcinoma. Evaluation of immunoreactivity to monoclonal antibody B72.3. Am J Clin Pathol. 1990;93:466–70. doi: 10.1093/ajcp/93.4.466. [DOI] [PubMed] [Google Scholar]
- 18.Badalament RA, Burgers JK, Petty LR, Mojzisik CM, Berens A, Marsh W, et al. Radioimmunoguided radical prostatectomy and lymphadenectomy. Cancer. 1993;71:2268–75. doi: 10.1002/1097-0142(19930401)71:7<2268::aid-cncr2820710717>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Myers RB, Meredith RF, Schlom J, LoBuglio AF, Bueschen AJ, Wheeler RH, et al. Tumor associated glycoprotein-72 is highly expressed in prostatic adenocarcinomas. J Urol. 1994;152:243–6. doi: 10.1016/s0022-5347(17)32870-7. [DOI] [PubMed] [Google Scholar]
- 20.Myers RB, Schlom J, Srivastava S, Grizzle WE. Expression of tumor-associated glycoprotein 72 in prostatic intraepithelial neo-plasia and prostatic adenocarcinoma. Mod Pathol. 1995;8:260–5. [PubMed] [Google Scholar]
- 21.Meredith RF, Bueschen AJ, Khazaeli MB, Plott WE, Grizzle WE, Wheeler RH, et al. Treatment of metastatic prostate carcinoma with radiolabeled antibody CC49. J Nucl Med. 1994;35:1017–22. [PubMed] [Google Scholar]
- 22.Brenner PC, Rettig WJ, Sanz-Moncasi MP, Reuter V, Aprikian A, Old LJ, et al. TAG-72 expression in primary, metastatic and hormonally treated prostate cancer as defined by monoclonal antibody CC49. J Urol. 1995;153:1575–9. [PubMed] [Google Scholar]
- 23.Thor A, Gorstein F, Ohuchi N, Szpak CA, Johnston WW, Schlom J. Tumor-associated glycoprotein (TAG-72) in ovarian carcinomas defined by monoclonal antibody B72.3. J Natl Cancer Inst. 1986;76:995–1006. [PubMed] [Google Scholar]
- 24.Thor A, Ohuchi N, Szpak CA, Johnston WW, Schlom J. Distribution of oncofetal antigen tumor-associated glycoprotein-72 defined by monoclonal antibody B72.3. Cancer Res. 1986;46:3118–24. [PubMed] [Google Scholar]
- 25.Loy TS, Nashelsky MB. Reactivity of B72.3 with adenocarcinomas. An immunohistochemical study of 476 cases. Cancer. 1993;72:2495–8. doi: 10.1002/1097-0142(19931015)72:8<2495::aid-cncr2820720830>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Starling JJ, Maciak RS, Law KL, Hinson NA, Briggs SL, Laguzza BC, et al. In vivo antitumor activity of a monoclonal antibody-Vinca alkaloid immunoconjugate directed against a solid tumor membrane antigen characterized by heterogeneous expression and noninternalization of antibody–antigen complexes. Cancer Res. 1991;51:2965–72. [PubMed] [Google Scholar]
- 27.Winnard P, Jr, Chang F, Rusckowski M, Mardirossian G, Hnatowich DJ. Preparation and use of NHS-MAG3 for technetium-99m labeling of DNA. Nucl Med Biol. 1997;24:425–32. doi: 10.1016/s0969-8051(97)00027-9. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Dou S, He J, Yin D, Gupta S, Zhang S, et al. Radiolabeling of MAG3-morpholino oligomers with 188Re at high labeling efficiency and specific radioactivity for tumor pretargeting. Appl Radiat Isot. 2006;64:971–8. doi: 10.1016/j.apradiso.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He J, Liu G, Dou S, Gupta S, Rusckowski M, Hnatowich DJ. An improved method for covalently conjugating morpholino oligomers to antitumor antibodies. Bioconjug Chem. 2007;18:983–8. doi: 10.1021/bc060208v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou JX, Zhong Z, Shi XB, Tepper CG, deVere White RW, Kung HJ, et al. ACTR/AIB1/SRC-3 and androgen receptor control prostate cancer cell proliferation and tumor growth through direct control of cell cycle genes. Prostate. 2006;66:1474–86. doi: 10.1002/pros.20477. [DOI] [PubMed] [Google Scholar]
- 31.van Schaijk FG, Oosterwijk E, Molkenboer-Kuenen JD, Soede AC, McBride BJ, Goldenberg DM, et al. Pretargeting with bispecific anti-renal cell carcinoma x anti-DTPA (In) antibody in 3 RCC models. J Nucl Med. 2005;46:495–501. [PubMed] [Google Scholar]
- 32.van Schaijk FG, Broekema M, Oosterwijk E, van Eerd JE, McBride BJ, Goldenberg DM, et al. Residualizing iodine markedly improved tumor targeting using bispecific antibody-based pretargeting. J Nucl Med. 2005;46:1016–22. [PubMed] [Google Scholar]
- 33.Agus DB, Golde DW, Sgouros G, Ballangrud A, Cordon-Cardo C, Scher HI. Positron emission tomography of a human prostate cancer xenograft: association of changes in deoxyglucose accumulation with other measures of outcome following androgen withdrawal. Cancer Res. 1998;58:3009–14. [PubMed] [Google Scholar]
- 34.Oyama N, Kim J, Jones LA, Mercer NM, Engelbach JA, Sharp TL, et al. MicroPET assessment of androgenic control of glucose and acetate uptake in the rat prostate and a prostate cancer tumor model. Nucl Med Biol. 2002;29:783–90. doi: 10.1016/s0969-8051(02)00346-3. [DOI] [PubMed] [Google Scholar]
- 35.Oyama N, Ponde DE, Dence C, Kim J, Tai YC, Welch MJ. Monitoring of therapy in androgen-dependent prostate tumor model by measuring tumor proliferation. J Nucl Med. 2004;45:519–25. [PubMed] [Google Scholar]
- 36.Chen X, Park R, Hou Y, Tohme M, Shahinian AH, Bading JR, et al. MicroPETand autoradiographic imaging of GRP receptor expression with 64Cu-DOTA-[Lys3]bombesin in human prostate adenocarcinoma xenografts. J Nucl Med. 2004;45:1390–7. [PubMed] [Google Scholar]