Abstract
We report two infants with severe bronchopulmonary dysplasia (BPD) in whom left ventricular diastolic dysfunction (LVDD) contributed to clinical abnormalities, including pulmonary hypertension and recurrent pulmonary edema. We speculate that close monitoring for LVDD may assist with clinical management and improve outcomes of infants with severe BPD.
Keywords: Bronchopulmonary dysplasia, left ventricular dysfunction, pulmonary hypertension, cardiac catheterization, echocardiography
Bronchopulmonary dysplasia (BPD) is the chronic lung disease that follows ventilator and oxygen therapy for neonatal respiratory distress shortly after birth. Although pulmonary hypertension (PH) and right ventricular dysfunction are well-recognized cardiovascular complications of BPD, less is known about left ventricular disease in BPD. Left ventricular hypertrophy (LVH) was reported in association with systemic hypertension and steroid use, and may be associated with higher mortality (1, 2). However, the actual contribution of altered left ventricular (LV) function to the clinical pathophysiology of BPD is unknown.
Recent studies of children with diverse cardiac diseases have demonstrated that in addition to abnormalities of LV systolic function, diastolic dysfunction can also contribute to disease (3). Left ventricular diastolic dysfunction (LVDD) that contributes to the pathophysiology of severe BPD has not been previously reported. We present two representative cases from our recent experience in which potentially treatable LVDD played a significant role in disease morbidity associated with severe BPD, including an unrecognized and significant component of marked PH and persistent diuretic-dependent pulmonary edema.
CASE HISTORIES
Case 1
A boy twin B born at 28 weeks’ gestation weighing 829 g was delivered via C-section due to chorioamnionitis. Oligohydramnios complicated the pregnancy. He was intubated and treated with surfactant at birth. Due to poor oxygenation with PH, he was treated with high frequency oscillatory ventilation and inhaled nitric oxide (iNO). He was extubated to nasal continuous positive airway pressure after 5 weeks, and discharged home at 4 months on 0.5 lpm oxygen. Echocardiogram at discharge was normal except for mild ventricular septal flattening when agitated.
He was rehospitalized 1 month after NICU discharge with severe respiratory distress that required mechanical ventilation. Echocardiogram revealed severe PH, which was estimated at 3/4 systemic level and mild biventricular hypertrophy, but with good systolic function (Table). INO therapy (20 ppm) was initiated, but despite aggressive diuretic use, serial chest radiographs showed worsening pulmonary edema (Figure). Doppler tissue imaging by echocardiogram was consistent with diastolic dysfunction (Table), and he underwent cardiac catheterization to better define the severity of his PH and its treatment. Pulmonary artery pressure was almost 2/3 systemic pressure, and an elevated pulmonary capillary wedge pressure (PCWP) suggested LVDD (Table). Milrinone (0.75 mcg/kg/min) was added to reduce LV afterload, reduce pulmonary edema and improve cardiac output. Progressive improvement in respiratory status led to subsequent extubation. Serial echocardiograms demonstrated improved PH to less than 1/3 systemic level. He was transitioned from iNO and milrinone to sildenafil and enalapril, and diuretic therapy was reduced. He was discharged from the hospital after 5 weeks on 0.25 lpm oxygen, enalapril, sildenafil, diuretics, and inhaled steroids. Subsequent outpatient follow-up visits through 10 months of age demonstrate continued improvement in cardiopulmonary status, including normal echocardiograms, and he has not required further hospitalization.
Table.
Hemodynamic data
| Patient 1 | Patient 2 | |
|---|---|---|
| Age at cath (mos) | 5.7 | 11 |
| Echocardiogram | ||
| sPAP (mm Hg) | 80 | N/A |
| Sys sBP (mm Hg) | 87 | 112 |
| pctFS (%) | 46.15 | 31.7 |
| LVEF | 0.84 | 0.68 |
| Transmitral E (cm/s) | 86 | 103 |
| Transmitral A (cm/s) | 83 | 108 |
| Transmitral E/A | 1.04 | 0.95 |
| LV Tei | 0.48 | 0.39 |
| Lateral Ea (cm/s) | 10 | N/A |
| E/Ea | 8.6 | N/A |
| Lateral Aa (cm/s) | 10 | N/A |
| Qualitative findings | Left deviation of atrial septum | Normal structure and function |
| Biventricular hypertrophy | ||
| Ventricular septal flattening | ||
| Cardiac Catheterization | ||
| RAP (mm Hg) | 16 | 6 |
| sPAP (mm Hg) | 56 | 40 |
| mPAP (mm Hg) | 41 | 24 |
| PCWP (mm Hg) | 17 | 12 |
| mean AoP (mm Hg) | 68 | 58 |
| PVR/SVR | 0.48 | 0.27 |
| CI (L/min/M²) | 5 | 3.5 |
Abbreviations: sPAP = systolic pulmonary artery pressure, Sys sBP = systemic systolic artery pressure, pctFS = percent fractional shortening, LVEF = left ventricular ejection fraction, E = early diastolic Doppler peak inflow velocity, A = atrial contraction Doppler peak inflow velocity, LV Tei = left vetricular isovolumic contraction time plus isovolumic relaxation time divided by ejection time, Lateral Ea = early diastolic lateral mitral annular tissue Doppler myocardial velocity, lateral Aa = atrial contraction lateral mitral annular tissue Doppler myocardial velocity, RAP = right atrial pressure, mPAP = mean pulmonary artery pressure, PCWP = pulmonary artery wedge pressure, AoP = aortic pressure, PVR = pulmonary vascular resistance, SVR = systemic vascular resistance, CI = cardiac index, N/A = not available.
Figure.
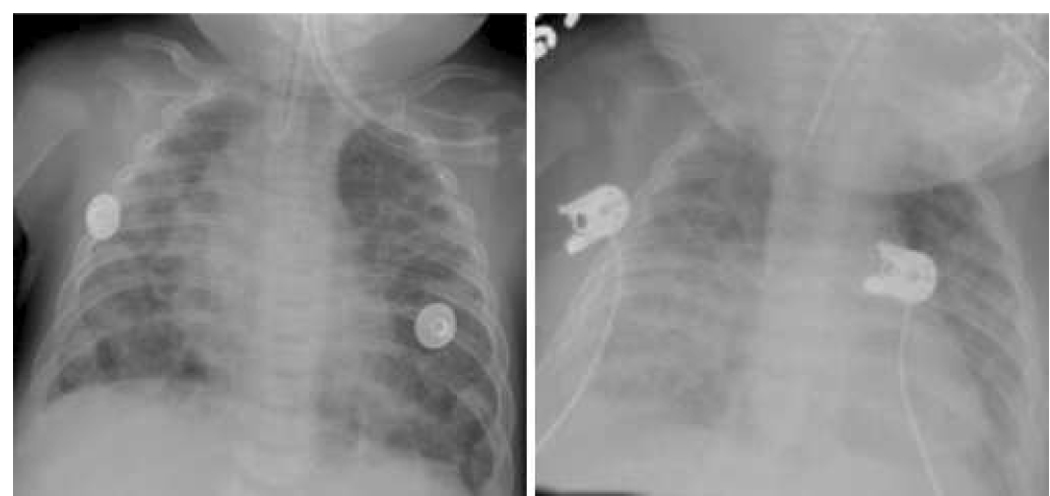
Chest x-rays from Case 1, illustrating progressive increase in pulmonary edema during inhaled NO therapy (left panel, baseline; right panel, during iNO therapy).
Case 2
This male patient was born at 24 weeks’ gestation weighing 723 g. Rupture of membranes occurred at 19 weeks’ gestation. He was intubated and treated with surfactant at birth, and required mechanical ventilation for 63 days. After 5 months, he was discharged to home at relatively high altitude (2500 m) on supplemental oxygen (0.5 lpm by nasal cannula), diuretics, and an inhaled steroid. He was readmitted to the NICU on the day after discharge with respiratory syncytial virus bronchiolitis. Due to concerns of the potential impact of exposure to higher altitude, he was subsequently discharged but remained at lower altitude (1600 m). Despite frequent courses of antibiotics, intermittent systemic steroids, and high dose diuretics, he required 5 hospitalizations over the next 6 months due to respiratory decompensation with worsening pulmonary edema. His chronic oxygen requirement progressively increased to 2 lpm and persistent pulmonary edema was noted on chest X-ray. Electrocardiogram showed right ventricular hypertrophy without LVH, and serial echocardiograms showed good systolic function without PH.
At 10 months of age, cardiac catheterization was performed to define his cardiac anatomy and hemodynamics. No evidence of PH or cardiac shunt lesions were identified, but left atrial pressure (LAP) was mildly elevated (Table) despite aggressive diuretic use, suggesting LVDD. Captopril (starting at 0.5 mg/kg/dose TID and increased to 2 mg/kg/dose TID) was added for LVDD treatment. After one month, his pulmonary edema improved, and the need for oxygen, diuretic, and steroid use was decreased. He did not require further hospitalizations and moved to his home at higher altitude without difficulty.
DISCUSSION
We report two patients with severe BPD whose clinical course was complicated by LVDD. Both patients had delayed recognition of LVDD. Although there were subtle findings of LVDD by echocardiogram, cardiac catheterization was required to make the diagnosis in both patients. Both experienced significant hemodynamic and clinical improvement with afterload reduction therapy.
These findings are interesting because they bring to light a potentially treatable cardiac complication of late BPD. Pulmonary edema and PH are known complications of severe BPD attributed to chronic inflammation and impaired pulmonary alveolar and vascular development. Thus, hemodynamic assessment of patients with BPD has focused on evaluation for PH and right sided function. However, left ventricular abnormalities have been associated with death in severe BPD (1, 4, 5). Persistent late pulmonary edema, despite aggressive diuretic use, or worsening pulmonary edema in response to PH treatment with vasodilators such as iNO or sildenafil (Figure) are unexpected responses that may require cardiac catheterization to rule out left sided cardiac dysfunction or anatomic abnormalities, such as pulmonary venous obstruction. LVDD was found in our patients, and treatment with afterload reduction agents (although angiotensin-converting enzyme inhibitors likely have additional mechanisms of action) was associated with improved pulmonary status and subsequent weaning of ventilator support, oxygen, diuretics, and steroids.
LVDD is characterized by increased stiffness and abnormal relaxation of the ventricle, which leads to elevated LV filling pressures and abnormal early diastolic filling (6). LVDD is difficult to diagnose because it produces signs and symptoms often attributed to the lung disease of BPD, and patients are not routinely screened for this complication. The diagnosis of LVDD in our patients was based on elevated LAP (or its surrogate, PCWP) in the absence of volume overload or evidence of LV systolic dysfunction. Volume overload is an unlikely cause for increased PCWP because both patients were aggressively managed to achieve an even or negative fluid balance around the time of hemodynamic assessment. Thus, although a PCWP of 12 mm Hg measured in patient 2 can be interpreted as being only mildly elevated, it likely represents a significant elevation given the aggressive fluid management. Even though elevated LAP can be due to either LV systolic or diastolic dysfunction or both, measures of systolic function by echocardiogram (percent fractional shortening and LV ejection fraction) appeared normal, suggesting LVDD as the predominant reason for elevated PCWP. A retrospective review of BPD patients undergoing cardiac catheterization at our institution (including the patients reported here) revealed a PCWP ≥ 12 mm Hg in 8 of 33 studies (26%) and ≥ 16 mm Hg in 4 of 33 (12%), suggesting that LVDD may not be uncommon in patients with severe BPD.
Although there are echocardiographic techniques, including Doppler tissue imaging (DTI), that may provide noninvasive assessments of diastolic dysfunction (3), these techniques have not been well defined in infants and are not generally part of routine echocardiogram studies in patients with BPD. The profile of usual diastolic dysfunction by Doppler echocardiogram is characterized by a higher peak A wave (transmitral flow peak velocity during atrial contraction) than peak E wave (transmitral flow peak velocity of early diastole). Although this profile is considered normal in neonates, with advancing age, the ratio of peak E to peak A wave (E/A) progressively increases, which is interpreted as demonstrating the age-related maturation of myocardial compliance toward an adult pattern. Although pre-term infants may have delayed maturation (3), prior Doppler studies in both patients had shown this normal progression (data not shown), but at the time of clinical deterioration and cardiac catheterization, the ratio had decreased, consistent with worsening diastolic function. Recently, DTI of the myocardium was used to assess regional myocardial function. The ratio of E to the early diastolic mitral annular tissue Doppler myocardial velocity (Ea), termed E/Ea, correlates with PCWP (7). Although no specific cutoff values have been described in this population, the measurements performed in Case 1 appear consistent with diastolic dysfunction.
Mechanisms responsible for LVDD and physiological impact on BPD remain unclear, but LVH has been associated with dexamethasone treatment for the prevention of BPD in premature infants (2). In these patients, LVH completely resolved after steroid discontinuation, but LV chamber size remained low in some infants, suggesting the possibility of persistent subclinical LVDD. Both patients reported here were exposed to systemic steroids, but only the patient in Case 2 was exposed to prolonged and repeated courses.
In summary, these cases suggest that LVDD can be an important complication of severe BPD, especially in patients who fail to respond to traditional therapies. High diagnostic suspicion is required for diagnosis, but treatment of LVDD may improve outcome for selected patients.
Acknowledgments
Funding sources: This publication was made possible by the Thrasher Foundation and grant number 5 K23 RR021021, National Center for Research Resources (NCRR), a component of the National Institutes of health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH. Funding sources had no such involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Abbreviations
- BPD
Bronchopulmonary dysplasia
- DTI
Doppler tissue imaging
- iNO
inhaled nitric oxide
- LAP
left atrial pressure
- LVH
left ventricular hypertrophy
- LVDD
left ventricular diastolic dysfunction
- PH
pulmonary hypertension
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Melnick G, Pickoff AS, Ferrer PL, Peyser J, Bancalari E, Gelband H. Normal pulmonary vascular resistance and left ventricular hypertrophy in young infants with bronchopulmonary dysplasia: an echocardiographic and pathologic study. Pediatrics. 1980;66:589–596. [PubMed] [Google Scholar]
- 2.Zecca E, Papacci P, Maggio L, Gallini F, Elia S, De Rosa G, et al. Cardiac adverse effects of early dexamethasone treatment in preterm infants: a randomized clinical trial. J Clin Pharmacol. 2001;41:1075–1081. doi: 10.1177/00912700122012670. [DOI] [PubMed] [Google Scholar]
- 3.Frommelt PC. Echocardiographic measures of diastolic function in pediatric heart disease. Curr Opin Cardiol. 2006;21:194–199. doi: 10.1097/01.hco.0000221580.63996.93. [DOI] [PubMed] [Google Scholar]
- 4.Abman SH, Burchell MF, Schaffer MS, Rosenberg AA. Late sudden unexpected deaths in hospitalized infants with bronchopulmonary dysplasia. Am J Dis Child. 1989;143:815–819. doi: 10.1001/archpedi.1989.02150190065022. [DOI] [PubMed] [Google Scholar]
- 5.McConnell ME, Daniels SR, Donovan EF, Meyer RA. Echocardiographic correlates of survival in severe bronchopulmonary dysplasia. J Perinatol. 1990;10:386–389. [PubMed] [Google Scholar]
- 6.Chinnaiyan KM, Alexander D, Maddens M, McCullough PA. Curriculum in cardiology: integrated diagnosis and management of diastolic heart failure. Am Heart J. 2007;153:189–200. doi: 10.1016/j.ahj.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]


