Abstract
The anion activates sperm motility, an important early step in capacitation, by increasing flagellar beat frequency through a pathway that requires the atypical adenylyl cyclase SACY and the sperm-specific Cα2 catalytic subunit of PKA. Here we show that the accelerating action of also requires the continued presence of external Ca2+ (EC50 ~0.5 mM), and find that Ca2+ can be replaced by Sr2+ but not by Mn2+. Ca2+ is required for to elevate cAMP, but not for cAMP-AM to increase beat frequency, indicating that external Ca2+ acts before rather than after stimulation of SACY by . With external Ca2+ present, does not alter cytosolic or near-membrane [Ca2+]. Removal of external Ca2+ initiates a slow decline in intracellular [Ca2+] and rapid block of the -evoked acceleration that is not relieved upon increasing internal [Ca2+] by rapid photolysis of caged Ca2+. We also find that the rapid (t1/2 ~10 s) accelerating action of is slowed more than three-fold by the carbonic anhydrase inhibitor acetazolamide. It is unaltered by the broad spectrum anion transport inhibitor SITS, and is not accompanied by detectable changes in intracellular pH. We propose that external Ca2+ binds an unidentified extracellular protein that is required for to engage cAMP-mediated activation of motility.
Keywords: NP-EGTA, cyclic AMP, capacitation, CA-II, CAH2, sAC, uncaging
Introduction
A very early stage of capacitation involves the initiation and activation of sperm motility. Activation specifically refers to the more vigorous movement that occurs when sperm come into contact with the contained in male and/or female reproductive fluids (Boatman and Robbins, 1991; David et al., 1969; David et al., 1973; Hamner and Williams, 1965; Okamura et al., 1985) and for reviews see (Bedford and Yanagimachi, 1992; Yanagimachi, 1994). Freshly ejaculated sperm need to travel a large distance from the site of sperm deposition (typically the vagina or uterus) to the site of fertilization (oviducts). Vigorous swimming in relatively linear trajectories may aid sperm in achieving this goal. In physiological salt solutions in vitro, is sufficient to produce this activation of vigorous motility.
Mammalian male germ cells contain an atypical adenylyl cyclase (Braun et al., 1977) now identified as SACY (formerly known as sAC for “soluble Adenylyl Cyclase”; (Buck et al., 1999)). Demonstration that SACY is present in mature sperm and that recombinant SACY is stimulated by (Chen et al., 2000; Jaiswal and Conti, 2001; Sinclair et al., 2000) supports the hypothesis that cAMP is a mediator of sperm activation (Okamura et al., 1985). Further, demonstration that SACY-null males are infertile (Esposito et al., 2004; Hess et al., 2005), and that their sperm do not activate motility when presented with (Xie et al., 2006) indicates that SACY translates the signal for activation in a pathway that is essential for fertilization.
The cAMP produced when stimulates SACY liberates the sperm-specific catalytic subunit Cα2 from the PKA holoenzyme. Cα2-null mutant male mice are infertile and their sperm also do not activate in response to (Nolan et al., 2004). Although the required involvement of SACY and PKA is established, the remainder of the pathway whereby activates sperm motility is uncharted. Here we examine the upstream portion of this signaling pathway that precedes stimulation of SACY by .
Materials and methods
Chemicals
Pluronic F127 and the acetoxymethyl (AM) esters of fura-2, of 2’,7’-bis(carboxyethyl)-5,6-carboxyfluorescein (BCECF), and of o-nitrophenyl-EGTA (NP-EGTA) were from Molecular Probes (Eugene, OR), and FFP18-AM ester was from TEF Labs (Austin, TX). Sp-5,6-dichloro-1-β-D-ribofuranosylbenzimidazole-3’,5’-monophosphorothioate (cBiMPS) was from Axxora (San Diego, CA), cGMP-AM from BioMol (Hamburg, Germany) and H89 from Calbiochem (San Diego, CA). Benzolamide was the generous gift of Dr. Erik Swenson (University of Washington). The cAMP enzyme immunoassay kit was from Assay Designs (Ann Arbor, MI). All other chemicals were from Sigma (St. Louis, MO).
Animals and cell preparation
As in prior work (Wennemuth et al., 2003; Xie et al., 2006), sperm were obtained from male Swiss-Webster retired-breeder mice using accepted standards of humane animal care, approved by the Animal Care and Use Committee at the University of Washington. Following CO2 asphyxiation, caudae epididymides and vasa deferentia were excised and rinsed in medium HS (also known as medium Na7.4; in mM): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgSO4, 20 HEPES, 5 glucose, 10 lactic acid, 1 pyruvic acid, adjusted to pH 7.4 with NaOH. Semen was allowed to exude from several small incisions, into 1 ml ‘swimout/capacitation’ medium (medium HS with 5 mg BSA/ml and 15 mM NaHCO3) for 15 min at 37 °C, in 5% CO2. All subsequent operations were at room temperature (22-25 °C) in medium HS, unless noted otherwise. Sperm were washed twice, then dispersed and stored at 1-2 × 107 cells ml-1. A modified medium HS with no added CaCl2 was used to examine dependency of cellular responses on external Ca2+. Experiments monitoring pHi used a modified medium HS prepared with no added lactic or pyruvic acids and with 10 mM glucose. Depolarization-evoked responses were produced with medium K8.6 (in mM): 135 KCl, 5 NaCl, 2 CaCl2, 1 MgSO4, 30 TAPS [N-tris(hydroxymethyl)-methyl-3-aminopropane sulfonic acid], 10 glucose, 10 lactic acid, 1 pyruvic acid, adjusted to pH 8.6 with NaOH.
All waveform analyses and photometric experiments were performed in glass-bottomed 35 mm incubation chambers. Sperm suspension (5-10 μl) was applied directly onto a smaller (~5 mm square) coverslip and allowed to settle for ~60 s. Those sperm selected for study were loosely attached to the covership at the base of the head, with a freely-beating flagellum. Test solutions were applied either in the bathing medium or by a solenoid-controlled, gravity-fed, multi-barreled local perfusion device with an estimated exchange time of <0.5 s.
Waveform analysis
The flagellar waveform was analyzed as previously described (Wennemuth et al., 2003). Briefly, stroboscopic stop-motion images were captured at 30 Hz by a CoolSnap HQ cooled CCD camera (Photometrics, Tuscon AZ), under the direction of Metamorph (Universal Imaging; West Chester PA). Semi-automated analysis software written in Igor (Wavemetrics, Lake Oswego OR) allowed tracing of the flagellum and determination of various waveform parameters including flagellar beat frequency. Results are presented as mean ± SEM, except where noted.
Dye loading and photometry
Calcium and pH photometry
Fura-2-AM or FFP18-AM was dispensed from 2 mM and BCECF-AM from 0.05 mM stocks in DMSO, dispersed in 10-15% Pluronic F127, diluted to 20 μM for fura-2-AM or FFP18-AM (0.5 μM for BCECF-AM) in 0.5 ml sperm suspension in medium HS. After 30-40 min, sperm were diluted, sedimented, resuspended in fresh medium, and incubated 45 min or more before use. Excitation light of 340 and 380 nm (460 and 490 nm for BCECF) was provided from a computer-controlled galvanometric monochromator (T.I.L.L., Gräfelfing Germany), and >450 nm (>500 nm for BCECF) emitted light was collected by a photodiode detector from an adjustable viewfinder that selected a rectangular region containing a small cluster (3-5 cells) of loosely-tethered sperm. Only sperm with beating flagella were selected for study. The raw photometric signals were corrected for the cell-free background collected prior to each series of measurements.
For the Ca2+ probes, the ratio of the corrected signals was calibrated with the following equation (Grynkiewicz et al., 1985): [Ca2+] = B × ((R - RFREE)/(RMAX − R)). The constants, B= 1228, RFREE = 0.3795, and RMAX = 1.792, were obtained from cells equilibrated in solutions fortified with ionomycin (10 μM) and containing (in mM): 20 EGTA, 15 CaCl2, or 20 EGTA with 15 CaCl2 (calculated free Ca2+ concentration of 226 nM, estimated with Winmaxc32 v2.50; Patton et al., 2004). The pH signal was calibrated with the equation: [H+] = KA × ((R-RA)/(RB-R)) × FA(λ2)/FB(λ2). The constants RA = 0.996, RB = 2.433, and KA = 1.26 × 10-7 M were obtained by the nigericin method (Thomas et al., 1979) from cells equilibrated in solutions fortified with 10 μM nigericin and containing (in mM): 120 KCl, 10 NaCl, and 1 MgSO4, with 10 MES (2-[N-morpholino] ethanesulfonic acid) and pH 5, or 10 HEPES and pH 7, or 10 CHES (2-[N-cyclohexylamino] ethanesulfonic acid) and pH 9. The calibrated signals report spatially-averaged internal [Ca2+] and pH from the head and proximal flagellum of several sperm. Further analyses were performed in Igor. Statistical analyses were performed in Excel (Microsoft, Redmond, WA).
Ester loading of cyclic nucleotides and chelators and photolytic uncaging of Ca2+
The cAMP-AM or cGMP-AM was dispensed from a 20 mM stock in DMSO, dispersed in 10-15% Pluronic F127, then diluted with sperm suspension in medium HS to a final concentration of 60 μM. After 30 min, an aliquot was added to the imaging chamber containing medium HS. Data were collected within 5 min to preclude loss of signal due to the declining cyclic nucleotide content that follows dilution of external cAMP-AM or cGMP-AM. NP-EGTA-AM (20 μM) was co-loaded with fura-2-AM as described above. Photolysis of NP-EGTA (to “uncage” Ca2+) was produced by 3 s of continuous exposure to 365 nm light provided by the T.I.L.L monochromator.
Cyclic-AMP measurements
Sperm was pooled from 3-6 animals for each experimental trial. Samples destined for 0 mM Ca2+ treatment were washed in modified HS with no added CaCl2. A 100 μl aliquot of sperm suspension (1.5-4.5 × 106 sperm) was added to an equal volume of medium with 0 or 30 mM NaHCO3. Treatments continued for 30 s at room temperature. Reactions were stopped by lysis with 1 ml of ice-cold acidified ethanol (ethanol: 1M HCl; 100:1, v/v%). The lysates were cooled on ice for at least 30 min, then NaHCO3 and CaCl2 were added as needed to ensure identical ionic composition of the samples for assay. The cAMP content was measured using an enzyme-linked immunoassay kit (Assay Designs, Ann Arbor MI). We made one modification to the instructions from the kit’s manufacturer; all samples and standards were prepared in medium of the same ionic composition to control for ion-induced alterations in cAMP-antibody binding and sensitivity. Samples were acetylated to increase assay sensitivity. All samples were run in triplicate and each experiment was repeated on three separate occasions.
Results
action requires extracellular Ca2+
Fig. 1A confirms our past work (Carlson et al., 2003), reporting that external Ca2+ is required for to speed the flagellar beat. When bathed for 1-5 min in medium containing both and Ca2+, sperm beat on average ~3-fold faster than when bathed with medium containing Ca2+ but not (7.6 ± 0.3 vs 2.7 ± 0.1 Hz). However, the mean beat frequency for sperm bathed with in the nominal absence of external Ca2+ (3.4 ± 0.3 Hz) was only slightly faster than the 2.7 ± 0.1 Hz beat observed for sperm examined in media that lacked or that lacked both added and Ca2+. In Fig. 1B, sperm were bathed in media with 15 mM NaHCO3 and various extracellular Ca2+ concentrations. About 0.5 mM Ca2+ was required for to produce a half-maximal increase in beat frequency. The experiments of Fig. 1C examined responses to in media that contained the divalent cations Mn2+ or Sr2+ rather than Ca2+. We find that Sr2+ effectively replaced Ca2+, whereas Mn2+ did not.
Fig. 1. action requires external Ca2+.

A, C: Averaged beat frequencies of sperm bathed in medium HS with 0 or 2 mM Ca2+, Sr2+, or Mn2+, and 0 or 15 mM NaHCO3 as indicated (mean ± sem, N=26-37 cells in 3 independent experiments). B: Concentration-dependence of the accelerating action of upon extracellular [Ca2+]. Sperm bathed in media with 15 mM NaHCO3 and indicated Ca2+ concentrations; motility assessed at 1-10 min (mean ± sem, N=12-14 cells in 3 independent experiments). The best-fit Hill equation (black line) had the following parameters: minimum= 2.2, maximum = 8.6, n= 3.03, and EC50 = 499 μM. D: Averaged beat frequencies (mean ± sem, N=4 cells in 2 independent experiments) of single sperm sequentially perfused with HS containing 2 or 0 mM Ca2+ and 0 or 15 mM NaHCO3 as indicated.
In Fig. 1D, individual sperm were monitored while the composition of the medium was rapidly altered by local perfusion. As above (Fig. 1A, 1C) sperm beat at the same 2.5-3 Hz frequency during the initial perfusion with medium HS. When the medium was changed to a NaHCO3-fortified, but nominally Ca2+-free version of HS, the beat frequency was not increased during 60 s of application. Thus, removal of external Ca2+ totally blocked of the accelerating action of . When Ca2+ was restored to the NaHCO3-fortified perfusing medium, the beat frequency increased nearly 3-fold within 30 s. Thus, the blockade imposed by removal of external Ca2+ was fully and rapidly reversible. When sperm were again perfused with the NaHCO3-fortified, but Ca2+-deficient version of HS, the beat gradually (t1/2 ~5 min) returned to the basal frequency observed initially for sperm exposed only to HS. Extracellular Ca2+ therefore was required to maintain the elevated beat evoked by simultaneous application of Ca2+ and . The recovery observed here upon removal of external Ca2+ is much slower than the t1/2 of ~10 s observed for the acceleration of the beat in the presence of external Ca2+ (Wennemuth et al., 2003). To explain the initial, complete suppression of acceleration by that was seen in Fig. 1D, the blockade imposed by removal of external Ca2+ must have a very rapid onset.
Intracellular Ca2+ declines slowly after removal of external Ca2+
Is extracellular Ca2+ required simply to maintain a threshold internal [Ca2+] necessary for action? We monitored intracellular [Ca2+] before, during, and after exposure to the Ca2+-deficient version of medium HS that blocks from speeding the flagellar beat. Upon removal of external Ca2+, the intracellular [Ca2+] declined slowly over tens-of-seconds (Fig. 2A). This slow decline contrasts sharply with the very rapid blockade of actions on beat frequency seen above (Fig. 1D). Therefore, it is unlikely that a fall in intracellular [Ca2+] produces the blockade imposed by removal of external Ca2+.
Fig. 2. does not evoke Ca2+ entry.
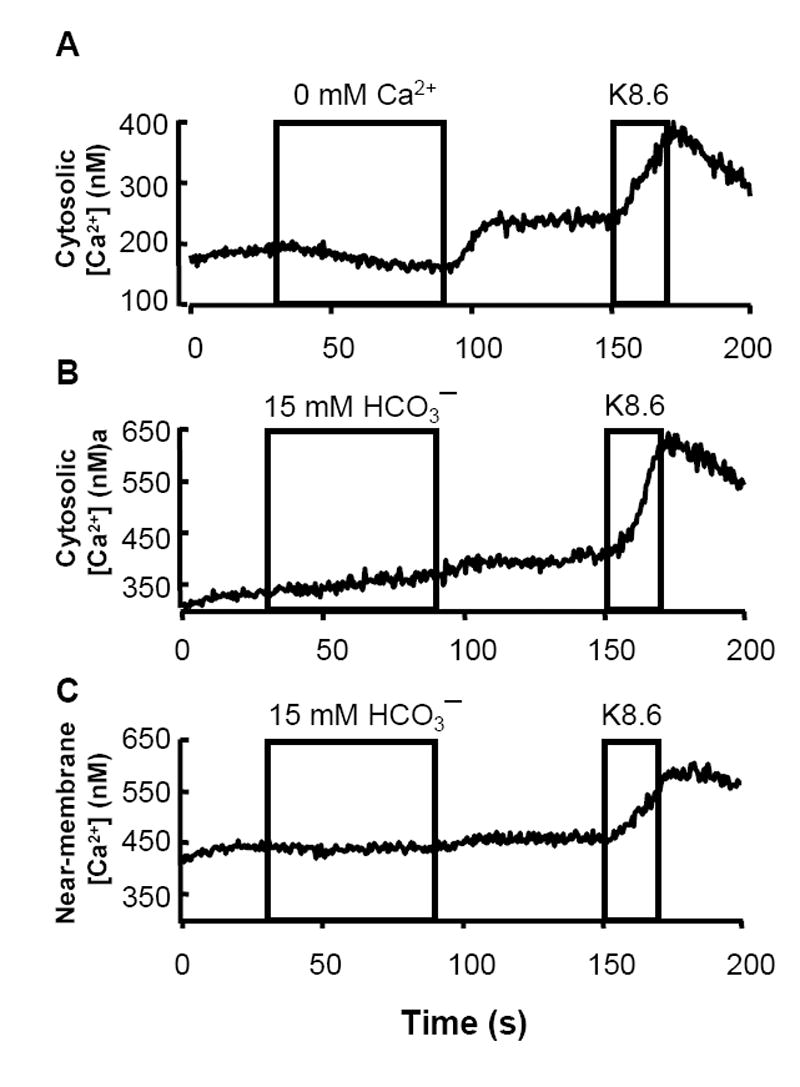
Averaged intracellular [Ca2+ ] of small groups of 3-5 sperm loaded with the AM ester of a ratiometric indicator dye. A, B: fura-2 (N=24 trials in 2 independent experiments for each protocol) and C: near-membrane indicator FFP18 (N=44 trials in 3 independent experiments). Sperm were perfused with media HS containing 0 or 2 mM CaCl2 and 0 or 15 mM as indicated. The high potassium, high pH medium K8.6 depolarizes sperm to open pHi- and voltage-gated calcium channels and allow entry of Ca2+.
Fig. 2A also shows that when Ca2+ was restored to the perfusing medium, internal [Ca2+] rose to reestablish cellular homeostasis. Finally, cells received a brief depolarizing stimulus, opening pHi- and voltage-gated Ca2+ entry channels and elevating cytosolic [Ca2+]. Together these results show that brief removal of external Ca2+ did not affect sperm Ca2+ homeostasis or Ca2+ channel activity adversely.
does not evoke Ca2+ entry
Cellular responses that require the presence of extracellular Ca2+ often involve an evoked Ca2+ entry that elevates intracellular [Ca2+]. Therefore, we monitored intracellular [Ca2+] while small groups of sperm were perfused with medium HS alone or with HS containing 15 mM NaHCO3. The fura-2 indicator did not detect an increase in [Ca2+] evoked by (Fig. 2B). However, sperm have little cytoplasm so it is possible that sequestration of fura-2 into intracellular organelles might obscure small changes in cytosolic [Ca2+] produced by entry of Ca2+ across the plasma membrane. Therefore we also monitored [Ca2+] using the near-membrane Ca2+ indicator FFP18 (Fig. 2C). As for fura-2, the FFP18 reported no -evoked elevation of intracellular Ca2+.
Uncaging intracellular Ca2+ does not rescue action in Ca2+-deficient medium
We reasoned that if an undetected localized elevation of intracellular [Ca2+] is required for to accelerate the flagellar beat, then raising bulk intracellular [Ca2+] by photolytic uncaging should obviate the requirement for extracellular Ca2+. For sperm loaded with NP-EGTA-AM, the NP-EGTA produced by esterolysis presumably binds cytosolic Ca2+. A normal resting cyosolic [Ca2+] of ~150 nM was observed in the sperm co-loaded with fura-2 and NP-EGTA (Fig. 3A), presumably because leakage of external Ca2+ had replenished any intracellular free Ca2+ that was bound by the chelator during loading. Intense irradiation of NP-EGTA produces a photocleavage product with a much lower affinity for Ca2+ and thus a rapid release (‘uncaging’) of bound Ca2+ (Ellis-Davies and Kaplan, 1994). A 3-s irradiation with 365 nm light raised cytosolic [Ca2+] from ~150 to ~400 nM in sperm that had been loaded with NP-EGTA and perfused with a Ca2+-deficient medium containing 15 mM NaHCO3. Fig. 3B shows that [Ca2+] fell little in the 6 s following photolysis, consistent with the slow recovery (t1/2~ 60 s) of cytosolic [Ca2+] from Ca2+ loads imposed by depolarizing stimuli (Wennemuth et al., 2003). Sperm examined 20 s after irradiation, when [Ca2+] presumably was still elevated, were still beating at the same ~2.5 Hz frequency as observed 20 s before irradiation. They increased their beat rate only after external Ca2+ was restored. Thus, we find that sperm loaded with NP-EGTA and fura-2 increased their intracellular Ca2+, but not their beat frequency, in response to UV photolysis. Elevation of cytosolic [Ca2+] did not rescue the activation of sperm by from blockade by removal of external Ca2+.
Fig. 3. Elevation of intracellular Ca2+ does not activate sperm.

A: Averaged beat frequency (open cirlcles, left axis) and intracellular [Ca2+] (closed circles right axis) for individual sperm sequentially examined before and after uncaging of Ca2+ by photolysis. Dashed lines indicate predicted Ca2+ concentrations during beat frequency measurements. (N = 9 in 2 independent experiments). Boxed area and jagged arrow indicate the 3 s of continuous exposure to 365 nm light. B: Averaged [Ca2+] before and after uncaging on an expanded time scale.
Ca2+ is required for cAMP production, not for cAMP action
Our past work indicates that the cAMP messenger mediates the activation of the sperm flagellar beat by the anion. We asked now whether removal of external Ca2+ blocks the action of by preventing elevation of sperm cAMP content. Fig. 4A compares the cAMP content of sperm extracts prepared 30 s after treatment with media differing only in the presence or absence of added CaCl2 and NaHCO3. In HS with 2 mM CaCl2, the cAMP content was 1.6 ± 0.2 pmol/107 cells. With 15 mM NaHCO3 also present, the content increased >2-fold to 3.4 ± 0.7 pmol/107 cells. In the absence of added Ca2+, the cAMP content was 0.9 ± 0.1 pmol/107 cells whether examined in the presence or absence 15 mM NaHCO3. Hence extracellular Ca2+ is required for to increase cAMP production.
Fig. 4. Ca2+ is required for cAMP production not action.
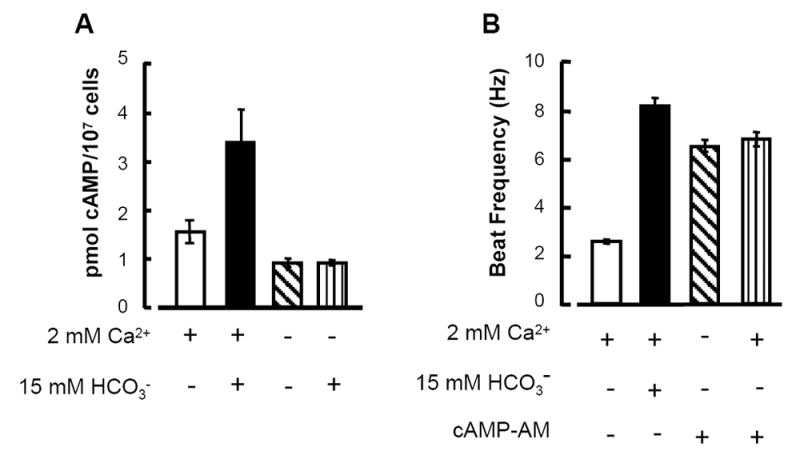
A: The cAMP content of sperm populations extracted after a 30 s treatment with 0 or 2 mM Ca2+ and 0 or 15 mM NaHCO3 as indicated (mean ± sem, N=11-15 samples, 3 repetitions). B: Beat frequencies of sperm bathed in medium Na7.4 alone or fortified with 15 mM NaHCO3 were compared to those of sperm transferred to HS after 30 min incubation with 60 μM cAMP-AM in medium HS that contained or lacked 2 mM Ca2+ (mean ± sem, N=17-28 cells, 3 animals).
To exclude the possibility that extracellular Ca2+ has additional required actions downstream of cAMP production, we examined sperm whose cAMP content was increased by loading with the cAMP-AM ester. Fig. 4B shows that after loaded sperm were transferred to medium lacking Ca2+, their accelerated beat (6.6 ± 1.3 Hz) was indistinguishable from that of sperm transferred to medium containing 2 mM Ca2+ (6.8 ± 1.3 Hz). Lack of a difference between these two treatments indicates that extracellular Ca2+ is not needed downstream of cAMP production.
2’-Hydroxyestradiol inhibits stimulation of SACY by
Figs. 4A and 1A indicate that extracellular Ca2+ is required for to elevate the cAMP content and to accelerate the flagellar beat of mouse sperm. These actions of are absent from sperm that lack SACY, the atypical adenylyl cyclase of sperm (Xie et al., 2006). As a pharmacologic test for the involvement of SACY, we asked if 2’ hydroxyestradiol (2’OH-E), an inhibitor of SACY (Braun, 1990), would block the -evoked rise in beat frequency. Fig. 5A shows that sperm treated with 3 mM NaHCO3 alone beat at 8.2 ± 0.5 Hz, whereas after a 30 min pre-incubation with 100 μM 2’OH-E, sperm treated with 3 mM NaHCO3 together with 100 μM 2’OH-E had a beat of only 3.7 ± 0.3 Hz. The 2’OH-E was less effective when applied at 70 or 30 μM. Treatment with 2’OH-E attenuated actions on sperm in a concentration-dependent manner.
Fig. 5. evoked beat acceleration is mediated by SACY and cAMP.
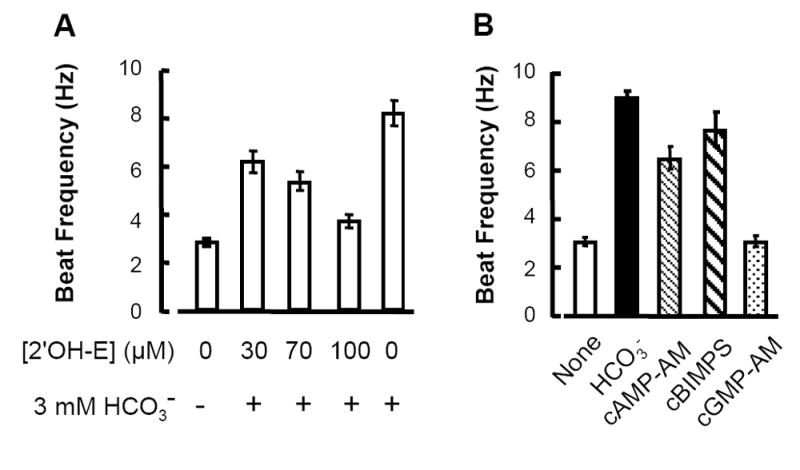
A: The SACY-inhibitor 2‘hydroxyestradiol (2’OH-E) attenuates the accelerating action of . Sperm were bathed with medium HS and the indicated concentrations of inhibitor for 30 min, 3 mM NaHCO3 in HS then was added and images of randomly-selected sperm were sampled in the following 1-5 min (mean ± sem, N=6-19 cells in 3 independent experiments). B: Sperm were incubated 30-60 min in medium HS alone (none, ), or for 30 min with 60 μM cAMP-AM or 60 μM cGMP-AM, or for 5 min with 50 μM cBIMPS. Sperm were then transferred to a bath containing HS. As a positive control, 15 mM NaHCO3 was applied to otherwise untreated sperm (mean ± sem, N=5-16 cells in 2-3 independent experiments).
acceleration of the flagellar beat is cAMP-specific
To verify that the cyclic nucleotide cAMP specifically mediates activation of mouse sperm, we compared the response of sperm to various cyclic nucleotide analogs (Fig. 5B). Sperm in medium HS alone had a beat frequency of 3.1 ± 0.2 Hz that increased to 9.0 ± 0.3 Hz after addition of 15 mM NaHCO3. Treatment with 60 μM cAMP-AM or 50 μM cBiMPS also speeded the beat (to 6.4 ± 0.4 and 7.7 ± 0.7 Hz respectively; Fig. 5B). By contrast, the beat frequency of sperm loaded with 60 μM cGMP-AM (3.1 ± 0.2 Hz) was indistinguishable from that of untreated controls.
Activation is specifically signaled by
Available evidence indicates that flagellar responses to NaHCO3 result primarily from engagement of a cAMP-mediated signaling pathway. Two additional observations argue against involvement of changes in osmolarity or pH. First, sperm bathed in HS or in HS supplemented with 15 mM additional NaCl had almost identical beat rates (3.1 ± 0.2 vs 3.0 ± 0.3 Hz). Hence, the ~10% increase in osmolarity produced by supplementation with 15 mM NaHCO3 does not contribute to its stimulatory action. Second, the intracellular pH of single sperm did not change during exposure to 15 mM NaHCO3 (Fig. 6B). Sperm in HS had an average intracellular pH of 6.80 ± 0.03 before, and 6.82 ± 0.04 after 30 s exposure to 15 mM NaHCO3. As controls, a 30-60 s exposure to HS with 15 mM sodium propionate or 15 mM NH4Cl, decreased or increased sperm pH by ≥0.3 pH units (Figs 6C&D).
Fig. 6. Bicarbonate does not alter intracellular pH.
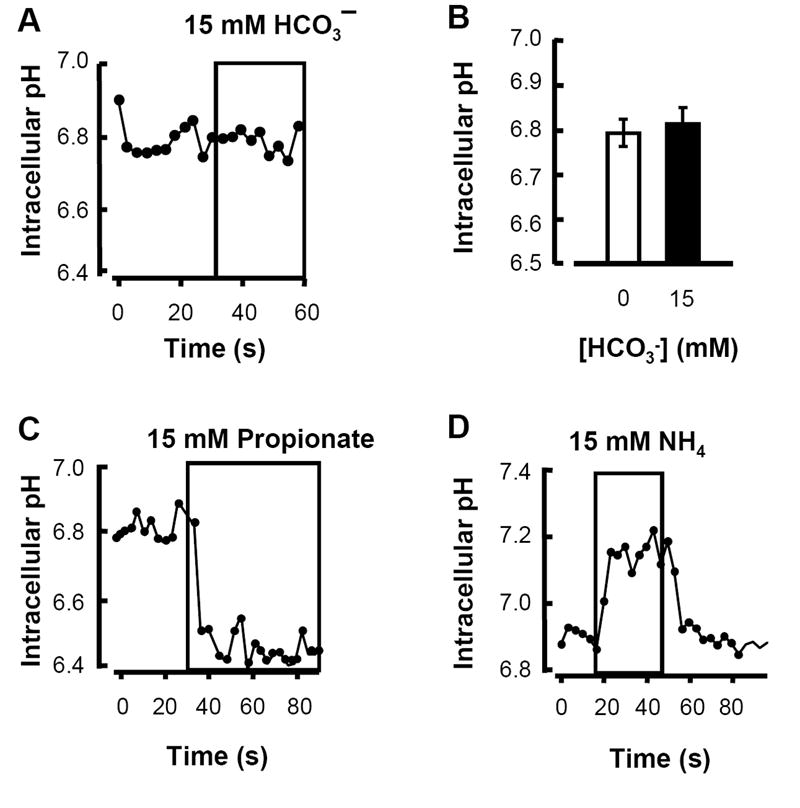
Intracellular pH of single sperm as indicated by BCECF. A: Intracellular pH of an example sperm before and during local perfusion with 15 mM NaHCO3. B: Averaged intracellular pH of single sperm before and during exposure to 15 mM NaHCO3, as in A (mean ± sem, N=24 cells in 3 independent experiments). C, D: Example traces from control experiments that validated probe responses to local perfusion with HS fortified with either 15 mM sodium propionate or 15 mM ammonium chloride as indicated.
Diffusion of CO2 into sperm provides intracellular
For to activate SACY, it must enter the sperm. As shown schematically in Fig. 7A, we considered two possible mechanisms for entry: 1) by anion transport across the cell membrane via a carrier or; 2) by direct diffusion of CO2 through the membrane with subsequent hydration to . In Fig. 7B we applied 1 mM of the broad-spectrum anion exchange inhibitor 4-acetamido-4’-isothiocyanatostilbene-2,2’-disulfonic acid (SITS) for 60 s before and during treatment with NaHCO3. We chose the concentration of 3 mM, rather than 15 mM, to provided a longer time course, thereby enhancing our ability to detect changes in the onset of activation. The SITS neither slowed the onset of response to NaHCO3 nor reduced the extent of the beat acceleration. In the presence or absence of SITS the beat frequency reached a near maximum of ~9 Hz by 35 s.
Fig. 7. Slowing of action by an inhibitor of carbonic anhydrase.
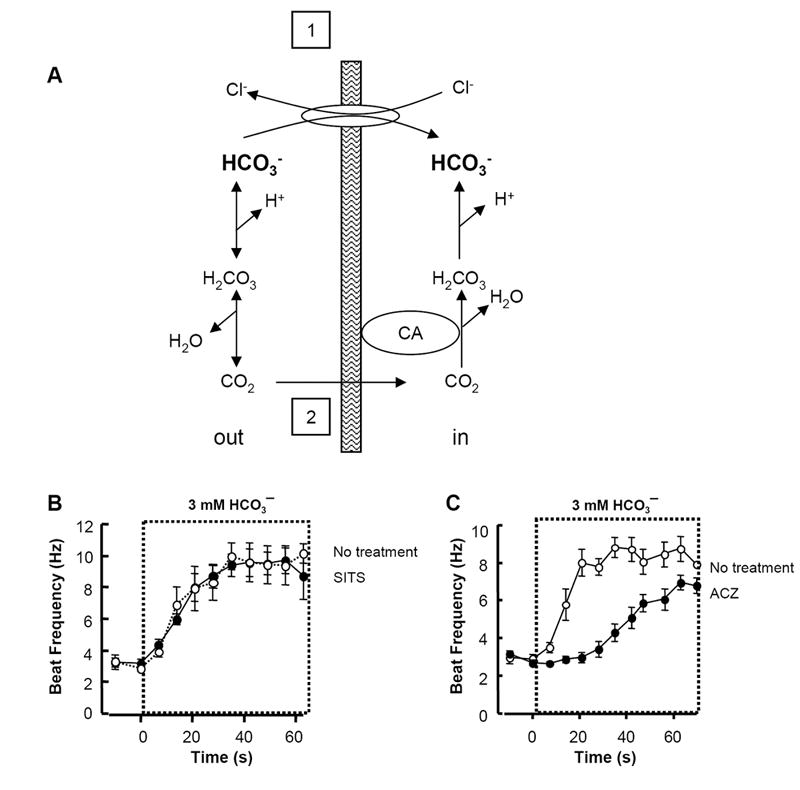
A: Possible routes (1 and 2) for entry of mediated by anion transporters or for entry of equivalents by diffusional entry of CO2. Carbonic anhydrase promotes rapid hydration of CO2 to generate the anion and a proton and the reconversion of and a proton to CO2 and H2O. B: Sperm incubated with 0 (open circles) or 1 mM SITS (closed circles) for 60 s before and during application of 3 mM NaHCO3 (mean ± sem, N=6-7 cells in 3 independent experiments). C: Sperm continuously incubated with 0 (open circles) or 1 mM of the carbonic anhydrase inhibitor acetazolamide (ACZ) (closed circles) for 30 s before and during exposure to 3 mM NaHCO3 (mean ± sem, N=6-11 cells in 3 independent experiments). Boxed areas indicate periods of local perfusion with media that contained NaHCO3.
By contrast, the accelerating action of was slowed dramatically for sperm treated with 1 mM of the carbonic anhydrase inhibitor acetazolamide (ACZ). Whereas untreated sperm reached their maximal frequency by ~35 s, sperm treated with ACZ required ~60 s of exposure to NaHCO3 to reach a near-maximal beat rate (Fig. 7C). At pH 7, acetazolamide inhibits carbonic anhydrase with an IC50 of ~160 nM (Lindskog and Thorslund, 1968). Therefore, most intracellular carbonic anhydrase activity should be inhibited by 1 mM ACZ, even if the drug is not highly membrane-permeant.
Spontaneous hydration of intracellular CO2 presumably explains the ability of to slowly increase the beat frequency even in the presence of a strong blockade of carbonic anhydrase activity. At 25°C, the spontaneous reaction has a rate constant of 0.037 s-1 (Roughton, 1941), meaning that it should take 27 s to achieve 1/e of the maximal response. Indeed we find that 27 s after the maximal response seen when treating with alone, the beat frequency of sperm in 1 mM ACZ and is ~6 Hz. We also treated sperm with the cell-impermeant carbonic anhydrase inhibitor benzolamide to explore the possibility that an extracellular carbonic anhydrase is required for this signaling pathway. We find that at 30 μM, benzolamide did not slow the onset of the -evoked acceleration of beat (data not shown).
Another possible route for entry of is via the CFTR anion channel recently found in sperm (Xu et al, 2007, Hernandez-Gonzalez et al. 2007). However, Table 1 shows that CFTR-i172, a selective blocker of this channel did not prevent the acceleration of the flagellar beat by , consistent with its reported inability to prevent bicarbonate-dependent increases in protein phosphotyrosine content (Hernandez-Gonzalez et al., 2007).
Table 1.
CFTR-inhibitor 172 does not prevent acceleration of the beat by NaHCO3
| Medium | Beat Frequency (Hz)
(Mean ± SEM) |
|---|---|
| HS | 2.33 ± 0.23a,c |
| HS + 15 mM NaHCO3 | 6.70 ± 0.32b,c |
| HS + 10 μM CFTR-i172 | 2.84 ± 0.15a,d |
| HS + 10 μM CFTR-i172 + 15 mM NaHCO3 | 6.25 ± 0.30b.d |
Not significantly different (P>0.15, paired t-test)
Not significantly different (P>0.35, paired t-test)
Significantly different (P<0.001, paired t-test)
Sperm were bathed for 5-10 min with medium HS in the presence or absence of 10 μM CFTR-i172. Representative sperm were selected for waveform analysis then perfused for 1 min with the same medium supplemented with 15 mm NaHCO3 and resampled.
N= 20 cells for each group in 2 independent experiments
Our data suggest that stilbene-sensitive anion exchangers are not involved in the entry of for sperm activation. Instead, they suggest that the predominant route of entry into sperm is via diffusion of CO2, followed by its intracellular re-hydration facilitated by carbonic anhydrase.
Discussion
is the signal that activates sperm
is a physiologically-relevant stimulus for activation of sperm motility. The luminal fluids of the epididymis and vas deferens have a low content which is thought to help maintain sperm in a quiescent state during transit and storage (Jones and Murdoch, 1996). Consistent with this proposed suppressive role, active reabsorption of occurs in the caput epididymis (Breton, 2001). At mating, sperm move from an environment with low [ ] and encounter elevated [ ] in the male and female reproductive fluids (Boatman and Robbins, 1991; David et al., 1969; David et al., 1973; Okamura et al., 1985). The importance of a high uterine [ ] is supported by the possible contribution of a low uterine content to female infertility in cystic fibrosis patients (Wang et al., 2003b). Here we have examined activation of mouse sperm motility by applied at the 15 mM concentration found to produce near-maximal acceleration of the flagellar beat in vitro. In vivo, at the prevailing elevated temperature and pH, sperm may encounter higher concentrations that may produce additional effects.
enters sperm via diffusion of CO2
The role of anion exchangers in sperm capacitation is not clear. Several reports indicate that the broad-spectrum anion exchange inhibitor SITS blocked transport (Okamura et al., 1988) and inhibited various actions of on sperm including the enhanced cAMP accumulation evoked by phorbol esters (Visconti and Kopf, 1998; Visconti et al., 1990). However, another stilbene derivative 4,4’-diisothiocyanostilbene-2,2’-disulfonic acid (DIDS) reportedly enhanced cAMP accumulation, respiration, and motility of porcine sperm (Tajima and Okamura, 1990). This action of DIDS was proposed to result from inhibition of anion exchangers that removed endogenous CO2 derived from oxidative metabolism. In other work, hyperpolarization of the sperm membrane potential by was attributed to its electrogenic entry via a DIDS-sensitive cotransporter (Demarco et al., 2002) or via the CFTR anion channel (Xu et al., 2007a).
Still other work has proposed involvement of carbonic anhydrase activity in the action of on sperm motility (Brook et al., 1996; Tajima et al., 1987). Indeed, the ubiquitous, cytosolic carbonic anhydrase type II (CAH2) is abundant in sperm (Mezquita et al., 1999; Parkkila et al., 1991). A careful and comprehensive immunohistological study of the mouse epididymis indicates that principal cells of the corpus epididymis also transfer CAH4 protein to the external surface of the mouse sperm flagellum (Ekstedt et al., 2004). The CAH4 is retained on membranes of mouse sperm from the cauda epididymis (Stein et al., 2006) and of human ejaculated sperm (Ficarro et al., 2003) as revealed with proteomic analysis using mass spectroscopy. We have now shown that a cell-permeant carbonic anhydrase inhibitor slows the onset of beat acceleration. increases the beat frequency of sperm treated with the carbonic anhydrase inhibitor ACZ, albeit with a slower time course, indicating that carbonic anhydrase is not required for actions on sperm. Not suprisingly, CAH2-deficient male mice have no reported deficits in fertility. However, we would predict that, CAH2-deficient sperm would respond to with a slower time course than that of wild-type sperm. We propose that intracellular CAH2 may promote rapid activation of sperm motility at mating.
A role for extracellular CAH4 remains uncertain. CAH4 knockout male mice remain fertile (Shah et al., 2005). Although CAH4 therefore is not essential for fertilization, its ability to promote responses to might similarly confer a competitive advantage for early progress of sperm through the female reproductive tract. Cell-impermeant CA inhibitors might allow test of this proposal. However, in our limited trials (data not shown) 30 μM benzolamide did not alter onset of the speeding action of .
Our work indicates that the that activates motility rapidly enters sperm via diffusion of CO2. The intracellular rehydration of CO2 to releases a proton in the net reaction: , this reaction should decrease intracellular pH. Conversely, if the anion enters sperm via a cotransporter or anion channel, then intracellular pH should increase. As assessed by imaging of single immobilized cells (Xu et al., 2007a), by fluorimetric responses from cell suspensions (Demarco et al., 2002), or by photometry from loosely-tethered sperm (our present work), evoked large increases, small increases, or no change in sperm intracellular pH, respectively. The lack of an observable pH change upon challenge with may indicate that internal pH of sperm is buffered strongly enough to preclude such a -associated decrease in intracellular pH. Indeed early work indicates that sperm have a strong pH buffering capacity (Babcock, 1983). Additional work will be required to resolve the important question of whether pH within the sperm flagellum rises or falls on the ~10-s time scale during which evokes speeding of the flagellar beat.
The sperm-specific Na+/H+ exchanger sNHE (Wang et al., 2003a) presumably functions in cytosolic pH homeostasis. Curiously, cAMP-AM but not activates sNHE-null sperm. Perhaps misregulation of pH in the sNHE null sperm precludes from stimulating the SACY without affecting cAMP actions downstream of SACY. Alternatively, sNHE may associate and functionally interact with the carbonic anhydrases of sperm as the NHE isoforms apparently do in some other tissues (Becker et al., 2007). The sNHE also may associate with and stabilize the full-length form of SACY (Wang et al., 2007).
Ca2+ is a cofactor in signaling
Consistent with our prior work (Carlson et al., 2003), we confirm that removal of external Ca2+ reversibly prevents from speeding the flagellar beat. Now we also show that for sperm already stimulated with , removal of external Ca2+ causes gradual loss of the rapid beat, demonstrating that and Ca2+ must be present simultaneously and continuously in the bathing medium. We do not find any evidence here or in past work to support the simple explanation that external Ca2+ is needed because evokes an entry of Ca2+ that is required for the cAMP-mediated acceleration of the flagellar beat. In fact, in a direct test of the hypothesis that rapid actions of require an elevation of cytosolic Ca2+, we find that photolytic uncaging of Ca2+ fails to restore the accelerating action of to sperm examined in Ca2+-deficient medium. Although the possibility remains that required increases in [Ca2+] take place in a hypothetical microdomain that is inaccessible to both indicator dyes and to Ca2+ released by uncaging, the simpler interpretation is that entry of Ca2+ is not required for to directly stimulate SACY to produce the activation of motility early in capactiation. Nonetheless, the potentiation of SACY activity that is produced by Ca2+ with in vitro assays (Jaiswal and Conti, 2003) may have relevance in later stages of sperm capacitation, serving to link a slow rise in cytosolic [Ca2+] to a secondary rise in cAMP content.
Our data suggest that Ca2+ may be acting on the extracellular surface of the sperm. It is possible that external Ca2+ is required to engage a transmembrane Ca2+ -sensing receptor. Such Ca2+-sensing receptors are expressed in many tissues (Chattopadhyay and Brown, 2000), and regulate diverse functions such as parathyroid hormone secretion by the parathyroid gland, calcitonin secretion by C-cells in the thyroid, Ca2+ and H2O transport in the mammary gland, and fluid fluxes in the gastrointestinal tract ((Pi et al., 2005) and refs. within). We find that 0.5 mM spermine, an agonist of the classical Ca2+ sensing receptor, does not replace Ca2+ in signaled activation (data not shown). Although the mouse epididymis reportedly expresses a novel Ca2+-sensing receptor (Pi et al., 2005) we can only speculate that this or a similar receptor may be added to the sperm surface during spermiogenesis or epididymal transit. An attractive feature of this hypothesis is that it could explain the shared requirement for extracellular Ca2+ in the accelerating actions of and of catecholamines and nucleosides (Schuh et al., 2006). This hypothetical extracellular Ca2+-sensing receptor presumably would couple to the sperm adenylyl cyclase SACY.
Signaling specificity in the activation evoked by
We also find that cAMP-analogs increase beat frequency and cGMP does not. Numerous past reports proposed roles for cAMP as a regulatory messenger in sperm (Garbers et al., 1982). The recent demonstration that the sperm adenylyl cyclase SACY is stimulated by explained how could increase internal cAMP content. However, the details of the signaling path are not completely understood. Here we compared cAMP-analog cBiMPS with the cAMP-AM and cGMP-AM esters. We chose cBiMPS because it is relatively cell-permeant, highly resistant to hydrolysis by phosphodiesterases, and has a much higher affinity for PKA than for protein kinase G (Holt and Harrison, 2002; Sandberg et al., 1991). The ability of cBIMPS and cAMP-AM to speed the beat in the absence of indicates that actions in sperm activation are strictly the result of elevated cAMP.
Here we explored alternative explanations of action, specifically examining the possibilities that evokes mouse sperm activation by changing extracellular osmolarity or intracellular pH. We found that increasing extracellular medium osmolarity alone does not increase the flagellar beat frequency. Further, treatment does not detectably alter intracellular pH. However, increased intracellular pH has been associated with mammalian sperm capacitation (Cross and Razy-Faulkner, 1997; Xu et al., 2007; Zeng et al., 1996). In fact, sperm fail to capacitate when internal alkalinization is prevented (Parrish et al., 1989). These reported pH changes are associated with long term (~90 min) capacitating incubations at 37 °C, whereas we were looking at actions only within the first minute of exposure at room temperature. It is probable that we would have observed increased intracellular pH following a longer capacitating incubation with -containing medium.
In summary, the work presented here contributes to the emerging picture that control of flagellar functions in sperm uses numerous unique or atypical components that interact in complex and sometimes unusual ways to alter the Ca2+, pH and cAMP mediators that control and coordinate the flagellar waveform and swimming behavior of sperm. In particular they implicate sperm carbonic anhydrase and an unidentified cell-surface Ca2+-binding protein as probable components of the machinery that determines content of the cAMP mediator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babcock DF. Examination of the intracellular ionic environment and of ionophore action by null point measurements employing the fluorescein chromophore. J Biol Chem. 1983;258:6380–6389. [PubMed] [Google Scholar]
- Becker HM, et al. Carbonic Anhydrase II increases the activity of the human electrogenic cotransporter. J Biol Chem. 2007;282:13508–13521. doi: 10.1074/jbc.M700066200. [DOI] [PubMed] [Google Scholar]
- Bedford JM, Yanagimachi R. Initiation of sperm motility after mating in the rat and hamster. J Androl. 1992;13:444–449. [PubMed] [Google Scholar]
- Boatman DE, Robbins RS. Bicarbonate: Carbon-dioxide regulation of sperm capacitation, hyperactivated motility, and acrosome reactions. Biol Reprod. 1991;44:806–813. doi: 10.1095/biolreprod44.5.806. [DOI] [PubMed] [Google Scholar]
- Braun T. Inhibition of the soluble form of testis adenylate cyclase by catechol estrogens and other catechols. Proc Soc Exp Biol Med. 1990;194:58–63. doi: 10.3181/00379727-194-43055. [DOI] [PubMed] [Google Scholar]
- Braun T, et al. Mn2+-sensitive, soluble adenylate cyclase in rat testis. Differentiation from other testicular nucleotide cyclases. Biochim Biophys Acta. 1977;481:227–235. doi: 10.1016/0005-2744(77)90155-3. [DOI] [PubMed] [Google Scholar]
- Breton S. The cellular physiology of carbonic anhydrases. JOP. 2001;2:159–164. [PubMed] [Google Scholar]
- Brook PF, et al. Measurement of intracellular pH in human spermatozoa by flow cytometry with the benzo[c]xanthene dye SNAFL-1: a novel, single excitation, dual emission, molecular probe. Mol Hum Reprod. 1996;2:18–25. doi: 10.1093/molehr/2.1.18. [DOI] [PubMed] [Google Scholar]
- Buck J, et al. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci USA. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson AE, et al. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci USA. 2003;100:14864–14868. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay N, Brown EM. Cellular “sensing” of extracellular calcium : emerging roles in regulating diverse physiological functions. Cell Signal. 2000;12:361–366. doi: 10.1016/s0898-6568(00)00082-6. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- Cross NL, Razy-Faulkner P. Control of human sperm intracellular pH by cholesterol and its relationship to the response of the acrosome to progesterone. Biol Reprod. 1997;56:1169–1174. doi: 10.1095/biolreprod56.5.1169. [DOI] [PubMed] [Google Scholar]
- David A, et al. Composition of rabbit oviduct fluid in ligated segments of the Fallopian tube. J Reprod Fertil. 1969;19:285–289. doi: 10.1530/jrf.0.0190285. [DOI] [PubMed] [Google Scholar]
- David A, et al. Chemical composition of human oviduct fluid. Fertil Steril. 1973;24:435–439. [PubMed] [Google Scholar]
- Demarco IA, et al. Involvement of a cotransporter in mouse sperm capacitation. J Biol Chem. 2003;278:7001–7009. doi: 10.1074/jbc.M206284200. [DOI] [PubMed] [Google Scholar]
- Ekstedt E, et al. Carbonic anhydrase in mouse testis and epididymis; transfer of isozyme IV to spermatozoa during passage. J Mol Histol. 2004;35:167–173. doi: 10.1023/b:hijo.0000023387.02793.af. [DOI] [PubMed] [Google Scholar]
- Ellis-Davies G, Kaplan J. Nitrophenyl-EGTA, a photolabile chelator that selectively binds Ca2+ with high affinity and releases it rapidly upon photolysis. Proc Natl Acad Sci USA. 1994;91:187–191. doi: 10.1073/pnas.91.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, et al. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci USA. 2004;101:2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficarro S, et al. Phosphoproteome analysis of capacitated human sperm. Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J Biol Chem. 2003;278:11579–11589. doi: 10.1074/jbc.M202325200. [DOI] [PubMed] [Google Scholar]
- Garbers DL, et al. A requirement of bicarbonate for the Ca2+-induced elevation of cAMP in guinea pig spermatozoa. J Biol Chem. 1982;257:8980–8994. [PubMed] [Google Scholar]
- Grynkiewicz G, et al. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hamner CE, Williams WL. Composition of Rabbit Oviduct Secretions. Fertil Steril. 1965;16:170–176. doi: 10.1016/s0015-0282(16)35523-6. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez EO, et al. Involvement of cystic fibrosis transmembrane conductance regulator in mouse sperm capacitation. J Biol Chem. 2007;282:24397–24406. doi: 10.1074/jbc.M701603200. [DOI] [PubMed] [Google Scholar]
- Hess KC, et al. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005;9:249–259. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt WV, Harrison RA. Bicarbonate stimulation of boar sperm motility via a protein kinase A-dependent pathway: between-cell and between-ejaculate differences are not due to deficiencies in protein kinase A activation. J Androl. 2002;23:557–565. [PubMed] [Google Scholar]
- Jaiswal BS, Conti M. Identification and functional analysis of splice variants of the germ cell soluble adenylyl cyclase. J Biol Chem. 2001;276:31698–31708. doi: 10.1074/jbc.M011698200. [DOI] [PubMed] [Google Scholar]
- Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci USA. 2003;100:10676–10681. doi: 10.1073/pnas.1831008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RC, Murdoch RN. Regulation of the motility and metabolism of spermatozoa for storage in the epididymis of eutherian and marsupial mammals. Reprod Fertil Dev. 1996;8:553–568. doi: 10.1071/rd9960553. [DOI] [PubMed] [Google Scholar]
- Lindskog S, Thorslund A. On the interaction of bovine cobalt carbonic anhydrase with sulfonamides. Eur J Biochem. 1968;3:453–460. doi: 10.1111/j.1432-1033.1967.tb19552.x. [DOI] [PubMed] [Google Scholar]
- Mezquita P, et al. Novel transcripts of carbonic anhydrase II in mouse and human testis. Mol Hum Reprod. 1999;5:199–205. doi: 10.1093/molehr/5.3.199. [DOI] [PubMed] [Google Scholar]
- Nolan MA, et al. Sperm specific protein kinase A catalytic subunit Cα2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci USA. 2004;101:13483–13488. doi: 10.1073/pnas.0405580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N, et al. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J Biol Chem. 1985;260:9699–9705. [PubMed] [Google Scholar]
- Okamura N, et al. Decrease in bicarbonate transport activities during epididymal maturation of porcine sperm. Biochem Biophys Res Commun. 1988;157:1280–1287. doi: 10.1016/s0006-291x(88)81013-1. [DOI] [PubMed] [Google Scholar]
- Patton C, et al. Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium. 2004;35:427–431. doi: 10.1016/j.ceca.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Parkkila S, et al. A high activity carbonic anhydrase isoenzyme CA II. is present in mammalian spermatozoa. Histochemistry. 1991;95:477–482. doi: 10.1007/BF00315743. [DOI] [PubMed] [Google Scholar]
- Parrish JJ, et al. Capacitation of bovine sperm by heparin: inhibitory effect of glucose and role of intracellular pH. Biol Reprod. 1989;41:683–699. doi: 10.1095/biolreprod41.4.683. [DOI] [PubMed] [Google Scholar]
- Pi M, et al. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem. 2005;280:40201–40209. doi: 10.1074/jbc.M505186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughton FJW. The kinetics and rapid thermochemistry of carbonic acid. J Am Chem Soc. 1941;63:2930–2934. [Google Scholar]
- Sandberg M, et al. Characterization of Sp-5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole- 3’,5’-monophosphorothioate (Sp-5,6-DCl-cBiMPS) as a potent and specific activator of cyclic-AMP-dependent protein kinase in cell extracts and intact cells. Biochem J. 1991;279(Pt 2):521–527. doi: 10.1042/bj2790521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh SM, et al. Signaling pathways for modulation of mouse sperm motility by adenosine and catecholamine agonists. Biol Reprod. 2006;74:492–500. doi: 10.1095/biolreprod.105.047837. [DOI] [PubMed] [Google Scholar]
- Shah GN, et al. Carbonic anhydrase IV and XIV knockout mice: roles of the respective carbonic anhydrases in buffering the extracellular space in brain. Proc Natl Acad Sci USA. 2005;102:16771–16776. doi: 10.1073/pnas.0508449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair ML, et al. Specific expression of soluble adenylyl cyclase in male germ cells. Mol Reprod Dev. 2000;56:6–11. doi: 10.1002/(SICI)1098-2795(200005)56:1<6::AID-MRD2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Stein KK, et al. Proteomic analysis of sperm regions that mediate sperm-egg interactions. Proteomics. 2006;6:3533–3543. doi: 10.1002/pmic.200500845. [DOI] [PubMed] [Google Scholar]
- Tajima Y, Okamura N. The enhancing effects of anion channel blockers on sperm activation by bicarbonate. Biochim Biophys Acta. 1990;1034:326–332. doi: 10.1016/0304-4165(90)90059-6. [DOI] [PubMed] [Google Scholar]
- Tajima Y, et al. The activating effects of bicarbonate on sperm motility and respiration at ejaculation. Biochim Biophys Acta. 1987;924:519–529. doi: 10.1016/0304-4165(87)90168-1. [DOI] [PubMed] [Google Scholar]
- Thomas JA, et al. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Kopf GS. Regulation of Protein Phosphorylation during Sperm Capacitation. Biol Reprod. 1998;59:1–6. doi: 10.1095/biolreprod59.1.1. [DOI] [PubMed] [Google Scholar]
- Visconti PE, et al. Bicarbonate dependence of cAMP accumulation induced by phorbol esters in hamster spermatozoa. Biochim Biophys Acta. 1990;1054:231–236. doi: 10.1016/0167-4889(90)90246-a. [DOI] [PubMed] [Google Scholar]
- Wang D, et al. A sperm-specific Na+/H+ exchanger sNHE. is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase sAC. Proc Natl Acad Sci USA. 2007;104:9325–9330. doi: 10.1073/pnas.0611296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, et al. A new sperm-specific Na+/H+ Exchanger required for sperm motility and fertility. Nat Cell Biol. 2003;5:1117–1122. doi: 10.1038/ncb1072. [DOI] [PubMed] [Google Scholar]
- Wennemuth G, et al. Bicarbonate actions on flagellar and Ca2+ -channel responses: Initial events in sperm activation. Development. 2003;130:1317–1326. doi: 10.1242/dev.00353. [DOI] [PubMed] [Google Scholar]
- Xie F, et al. Soluble adenylyl cyclase sAC. is indispensable for sperm function and fertilization. Dev Biol. 2006;296:353–362. doi: 10.1016/j.ydbio.2006.05.038. [DOI] [PubMed] [Google Scholar]
- Xu WM, et al. Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc Natl Acad Sci USA. 2007;104:9816–9821. doi: 10.1073/pnas.0609253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi R. Mammalian Fertilization. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press; New York: 1994. pp. 189–317. [Google Scholar]
- Zeng Y, et al. pH regulation in mouse sperm: Identification of Na+-, Cl--, and -dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev Biol. 1996;173:510–520. doi: 10.1006/dbio.1996.0044. [DOI] [PubMed] [Google Scholar]


