Abstract
The presence of certain bacteria in the gastrointestinal tract influences behavior and brain function. For example, challenge with live Campylobacter jejuni (C. jejuni), a common food-born pathogen, reduces exploration of open arms of the plus maze, consistent with anxiety-like behavior, and activates brain regions associated with autonomic function, likely via a vagal pathway. As yet, however, little is known regarding the interface of immune sensory signals with brain substrates that mediate changes in behavioral states. To address this issue, we challenged mice with either C. jejuni or saline, and 7-8 hours later assessed anxiety-like behavior using the open hole board, and used immunohistochemical detection of the protein c-Fos as an activation marker in the brain. C. jejuni treatment was associated with increased avoidance of the center regions of the hole board, compared to saline-treated controls. Exposure to the hole board induced activation in multiple brain regions previously implicated in anxiety-like behavior, including the lateral septum (LS), paraventricular (PVN) and dorsomedial (DMH) hypothalamic nuclei, basolateral and central nuclei of the amygdala (BLA, CEA), bed nucleus of the stria terminalis (BST) and periaquiductal grey (PAG), compared to homecage controls. In C. jejuni-treated animals c-Fos induction also occurred in autonomic regions, as previously reported. The PVN, BLA, parts of the BST, medial prefrontal (mPFC) and anterior cingulate responded to both C. jejuni-treatment and the hole board, suggesting a role for these regions in the enhanced anxiety-like behavior observed. In saline-treated animals, anxiety-like behavior was predicted by activation in the CEA and BLA, whereas in C. jejuni-treated animals, c-Fos expression in the BST predicted the degree of anxiety-like behavior. These findings implicate the PVN, amygdala and BST as interfaces between gastrointestinal pathogenic challenge and brain regions that mediate behavioral responses to stress, and reinforce these nuclei as anatomical substrates by which viscerosensory stimuli can influence behavior.
1. Introduction
Oral administration of certain bacteria, including the common food-borne bacterial pathogen Campylobacter jejuni (C. jejuni) enhances anxiety-like behavior in mice (Lyte et al. 1998; Lyte et al. 2006). Although this local gastrointestinal infection does not provoke evidence of a systemic immune response in these mice, it induces c-Fos expression (an activation marker) in vagal sensory neurons that likely innervate the gut in the first few hours following administration (Goehler et al. 2005; Lyte et al. 2006). Thus, vagal sensory pathways likely provide a major conduit by which this gastrointestinal infection can influence behavior. Consistent with this idea, infection with C. jejuni increased c-Fos expression in several brain regions that include the nucleus of the solitary tract (NTS), the lateral parabrachial nucleus (LPB), central amygdala (CEA) and the hypothalamic paraventricular nucleus (PVN), the bed nucleus of the stria terminalis (BST), and the medial prefrontal cortex (mPFC; Gaykema et al. 2004; Goehler et al. 2005). These brain regions comprise prominent components of the network of brain regions that process visceral/autonomic information, including those that are typically activated following vagal stimulation (Naritoku et al. 1995).
Although it is becoming increasingly evident that viscerosensory information regarding states such as peripheral arousal, pain, satiety, or inflammation can modulate ongoing behavior and mood (e.g. Craig 2002; Goldstein & Silverman 2006; Miyashita & Williams 2006), as yet the neural substrates that allow this interaction are not established. “Bottom-up” viscerosensory (systemic/interoceptive) challenges with e.g. satiety hormones (Rinaman 2003; Viltart et al. 2006), glucoprivation (Ritter et al. 2001) or immune challenge (Gaykema et al. 2007), activate ascending neural projections from the caudal brainstem NTS and ventrolateral medulla (VLM) that target a constellation of brain regions associated with the autonomic network, including the brainstem nuclei locus coeruleus (LC), LPB, and periaquiductal grey (PAG) as well as forebrain regions including the PVN and CEA (e.g. Billig et al. 2001; Gaykema et al. 2004; Goehler et al. 2005; Elmquist et al. 1993, 1996; Wan et al. 1993). This pattern overlaps with brain regions previously implicated in mediation of fear and anxiety (e.g. hypothalamus, amygdala and BST; Walker et al. 2003). Because these areas seem to serve as integrators of autonomic responses to stimuli signaling exteroceptive threats (Herman et al. 2003), they constitute key candidates for an interface between viscerosensory signals and affective states.
The previous studies investigating neuronal responses (c-Fos induction) to bacterial infection assessed animals resting their home cages, and thus reported only the c-Fos response to infection. Because c-Fos induction was not assessed following exposure to a novel environment, or any other potentiall anxiogenic situation, it was not possible to determine any relationship between c-Fos expression in the brain and anxiety-like behavior. Therefore, the present study aimed at identifying brain regions that may serve to integrate exteroceptive and interoceptive challenges (e.g., novel environment and infection) and potentially mediate the effects of immune-related viscerosensory modulation of anxiety-like behavior. We inoculated mice with C. jejuni orally and evaluated exploratory behavior using the hole board (HB). This apparatus is designed to provide a novel, potentially dangerous environment, in which inhibition of exploration of the center, the most exposed part, is taken as an index of anxiety-like behavior. Induction of c-Fos protein in the brain was used as a neuronal activity marker. Behavior and associated brain c-Fos expression patterns of mice following oral administration of either saline or C. jejuni were compared to identify potential areas that may mediate the effects of intestinal infection on anxiety-like behavior. Based on the established overlap of viscerosensory and stress-responsive functions, we expected that a convergence of signals relevant to anxiety-like behavior and C. jejuni treatment would be revealed via c-Fos expression in the amygdala (CEA and BLA), BST and PVN. In addition, other brain regions implicated in viscerosensory processing and anxiety-like behavior were assessed as well.
2. Methods
2.1. Animals
Thirty-eight 5-week-old CF-1 male mice were purchased from Charles River Laboratories (Wilmington, DE). Upon arrival, the mice were housed one animal per cage (width: 16 cm, length 22cm, and height 13cm; made of opaque polypropylene) and placed in a 12-hour light/dark cycle (dark: 2100-0900 hours, light: 0900-2100 hours). Food and water were available ad libitum. The last cage and bedding changes were done at least 48 hours preceding experiments. Prior to testing, the animals were acclimated to experimental procedures once a day for three consecutive days, by transporting their cages to the adjacent testing room. This testing room was divided by a heavy curtain into two sections: one section containing the holeboard apparatus, and the other containing the rack holding animal cages. For habituation, the mice were briefly removed from the cage, handled, and returned to their cage, without exposure to the testing apparatus itself. This acclimation took 2-5 minutes for each mouse. The last habituation was completed 3 days prior to infectious challenge and subsequent behavioral testing. All treatment procedures were approved by the Minnesota Medical Research Foundation Institutional Animal Care and Use Committee.
2.2. Per oral challenge
Two days before challenge, a stock culture of Campylobacter jejuni (#29428, American Type Culture Collection, Manassas, VA) was grown overnight in Luria-Bertani (LB) broth. Both bacteria containing and control tubes were incubated overnight at 37 °C in a 5% CO2 incubator. On the day of the experiment, both the C. jejuni-containing and control tubes were centrifuged at 5000 x g for 10 minutes, the supernatant discarded, and the bacterial pellet (C. jejuni tube) or not (control tube) resuspended in pre-warmed phosphate-buffered saline. The tubes were centrifuged and washed an additional 2X. Based on optical density the concentration of C. jejuni was adjusted to a final density of approximately 108 CFU per ml. Mice were per orally challenged with 0.2 ml of either C. jejuni containing solution (approximately 2 × 107 CFU per mouse, n= 19) or control saline solution (n= 19) using a ball-tipped, stainless steel feeding needle.
2.3. Holeboard apparatus
Exploratory behavior was assessed with the use of a plexiglass holeboard apparatus manufactured by Hamilton Kinder LLC (Poway, CA). The arena consisted of a square enclosure (40×40 cm) with a smooth, opaque floor, containing nine holes (2.5 cm in diameter and 7.5 cm deep, and arranged in three rows of 3 equidistant holes) evenly spaced in the floor, approximately 10 cm apart. The middle hole was baited with a piece of fruit loop cereal. The center zone was defined as the 20×20 cm area in the middle of the enclosure, and the periphery zone as the part of the arena outside the center zone adjoining the perimeter of the enclosure. In addition, an additional mid-center zone of 5×5 cm was defined in the immediate vicinity of the middle hole. The arena was equipped with two arrays of photo beams for automated assessment, with the lower array to record of the animals’ movements across the field and the upper one to record rearing. In addition the holes were each equipped with photo beams to record pokes into each hole. The animal’s movements on the open field, including rearing and pokes into individual holes, were detected by breakage of individual photo beam paths. Automated data acquisition on a real-time basis from the hole-board was achieved through connection via an SBC-I/O converter (Hamilton-Kinder Model MM100CC) to a computer in the test room. The apparatus provided data on locomotion (distance traveled (cm) in peripheral and center zones, cumulative time spent in the different zones (seconds), number of pokes into each of the nine holes, and the number of entries in the various zones.
2.4. Behavioral testing and tissue collection
Twenty-two mice were tested in the holeboard apparatus between 6 and 7 hours after per oral inoculation divided over two successive days. To control for order effect, testing alternated between the 11 C. jejuni-treated and 11 saline-treated subjects. Another group of sixteen mice (8 C. jejuni-treated and 8 saline-treated subjects) served as home-cage controls and were left in the animal holding room until sacrifice. The adjacent behavior testing room was lit with a diffuse overhead light with the light intensity in the holeboard apparatus set at 100 lux. The testing apparatus was initially cleaned with 20% isopropyl alcohol solution and thoroughly dried, and cleaning was repeated between test subjects. Animals in their home cages were brought into the testing room 30 minutes prior to testing. When not in the apparatus, the mice remained in their opaque polypropylene cages, covered with a filter-top that blocked smells, on the side of the curtained room that did not contain the holeboard apparatus. At the onset of the testing, each animal was placed in the same corner of the apparatus facing the center of the open field, and was allowed to explore the hole-board for a total duration of 5 minutes. The animals were then returned to their cage and transported back to the adjacent holding room. One hour after the start of testing (7-8 hours following per oral inoculation), the mice were brought to the perfusion room, deeply anesthetized with the inhalant isoflurane, and transcardially perfused with buffered 0.9% buffered saline followed by 4% paraformaldehyde (in 0.1 M. phosphate buffer, pH 7.4). The home cage controls were anesthetized and perfused between 7 and 8 h following inoculation. After perfusion fixation, the brains were dissected, post-fixed overnight, and stored in 0.1 M phosphate buffer containing 0.1% sodium azide.
2.4. Tissue preparation and c-Fos protein immunohistochemistry
Each brain was sectioned using a vibratome sectioning thickness of 50 μm. Brain sections were stored in 0.1 M phosphate buffer (pH 7.4) with sodium azide (0.1%). Sections through the entire brain were collected sequentially in six sets so that each set contained a rostrocaudal series of sections evenly spaced apart (300 μm). All sections were rinsed between incubation steps in phosphate buffered saline (PBS, 0.9% sodium chloride and 0.01 M, phosphate buffer, pH 7,4). Antibody solutions consisted of PBS with Triton X-100 (0.5%), normal goat serum (Serotec, 5%), and sodium azide (0.1%) added. To reduce nonspecific staining, sections were treated in a mixture of hydrogen peroxide (0.3%) and sodium azide (0.1%), followed by blocking of non-specific labeling with normal goat serum (5%) and Fab’ fragment of goat anti-rat IgG (1:500) in PBS with 0.5% triton X-100. The sections from each first and fourth set were processed for c-Fos as previously described (Goehler et al., 2005). Briefly, sections were incubated in anti-Fos (Oncogene, Ab5, 1:50,000 for 48-72 h), in biotinylated goat anti-rabbit IgG (Jackson Immunoresearch, 1:750, overnight), followed by avidin-biotin-peroxidase complex (ABC kit Elite, Vector, 1:500, 4h or overnight). Immunoreactivity for c-Fos was visualized using nickel-enhanced 3,3′-diaminobenzidine (DAB, 0.025%, nickelous ammonium sulfate 0.15%) yielding a black reaction product following the addition of glucose oxidase (Sigma) and β-D-glucose. After staining, the sections were mounted, dehydrated, coverslipped, and examined with an Olympus BX51 microscope with bright-field illumination. Images were collected with a digital camera (Magnafire, Optronics) attached to the microscope. The brain activation patterns in the different groups were obtained by semi-quantitative analysis of c-Fos expression in 16 brain regions as detailed below.
Quantification of c-Fos-positive cell nuclei was done with the NIH Image software (version 1.61 on a Macintosh PowerPC computer, OS9.2) of digital images captured by the digital camera. The numbers of c-Fos immunoreactive nuclei were assessed in 16 brain regions that were associated with viscerosensory functions (i.e., detection of infection), exploratory behavior and/or or anxiety-like/defensive responses (e.g. Charney, 2003). The regions analyzed included (with reference to corresponding rostro-caudal coordinates from the Mouse Brain Atlas of Paxinos and Franklin, 2001): the nucleus of the solitary tract (NTS, one level at bregma -7.48 mm), lateral parabrachial nucleus (LPB, one level at bregma -5.34 mm), locus coeruleus (LC, one level at bregma -5.52 mm), periaquiductal grey area (PAG; because the PAG is distinct functionally and anatomically, we subdivided into dorsolateral, lateral, and ventrolateral parts, three levels at bregma -4.24, -4.48, and -4.72 mm), dorsal raphe nucleus (DRN, one level at -4.48 mm), paraventricular hypothalamus (PVN, one level at bregma -0.94 mm) anterior hypothalamic area (AHA, one level at bregma -0.70 mm), posterior hypothalamus (PH, one level at bregma -2.30 mm), dorsomedial hypothalamus (DMH, one level at bregma -1.94 mm), central (CEA) and basolateral nuclei (BLA) of the amygdala (three levels at bregma -1.22, -1.34, and -1.46 mm), bed nucleus of the stria terminalis (BST) divided because of functional and anatomical differences into dorsolateral, ventrolateral, and medial subdivisions (three levels at bregma -0.26, -0.14, and -0.02 mm), lateral septum (LS, one level at bregma +0.38 mm), anterior cingulate cortex (ACC, at bregma +1.18 mm), infralimbic and prelimbic cortex (ILC and PLC, two levels at bregma +1.78 and 1.94 mm).
2.5 Data analysis
The behavioral measures were compared between between C. jejuni- and saline-treated mice using one-way analysis of variance (ANOVA; SPSS). Behavioral variables included total distance traveled (general measure of activity), and indices of anxiety-like behavior such as distance in the center zone, total time spent in the center zone, number of entries into the center zone. The differences in the numbers of c-Fos-immunoreactive profiles in each brain region were analyzed using a two-way ANOVA with treatment (C. jejuni or saline) and test condition (holeboard exploration or home cage condition) as between-subject variables. Post-hoc t-tests (Bonferroni) were performed to reveal significant differences between separate groups (corrected for multiple comparisons). Statistical significance was defined as probability less than 0.05. To assess potential relationships between c-Fos expression in brain regions and behavioral scores, c-Fos counts of the above-mentioned brain regions were correlated with behavior measures using multiple regression (SPSS) separately for the saline- and C. jejuni-treated mice, with probability less than 0.05 used as criterion for statistical significance. Because several brain regions showed a pattern of correlation with multiple indices of anxiety-like behavior that approached, but did not reach statistical criterion, to provide a more comprehensive picture of the pattern of brain regions in which activation may contribute to anxiety-like behavior we define probability values less than 0.10 as a trend toward or of marginal significance.
3. Results
C. jejuni increases anxiety-like behavior in the holeboard
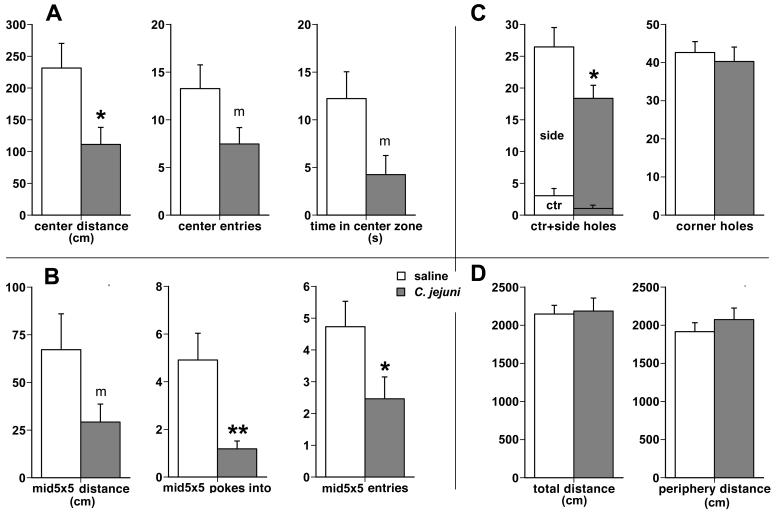
Although both the saline and C. jejuni-treated mice spent most of the time in the periphery zone of the holeboard, the avoidance of the 20×20 cm center zone was more pronounced in C. jejuni-treated animals (Fig. 1A). The infected mice traversed a reduced distance in the center zone (111 +/- 27 cm) as compared to the saline-treated mice (231 +/- 39 cm; main treatment effect F1,20 = 6.48, p = 0.02), and entered the center zone less frequently (7.5 +/- 1.7 vs. 13.3 +/- 2.5 entries, F1,20 = 3.68, p = 0.07). Infected mice also spent less total time in the center zone (8.8 +/- 2.2 vs. 20.2 +/- 5.9 s) and this difference was marginally significant F1,20 = 3.27, p = 0.08). Increased avoidance of the center was also reflected in decreased number of visits by the C. jejuni-treated mice of the very middle 5×5 cm zone immediately surrounding the center hole (Fig. 1B). The infected mice entered this zone less frequently than the uninfected controls (2.5 +/- 0.7 vs. 4.7 +/- 0.8; F1,20 = 4.62, p = 0.04), and made fewer partial entries or “pokes into” this zone (1.2 +/- 0.3 vs. 4.9 +/- 1.1; F1,20 = 10.15, p = 0.005). Infected mice traveled a shorter distance inside this middle zone (29.2 +/- 9.5 cm vs 67.2 +/- 18.8 cm), albeit this difference was marginally significant (F1,20 = 3.26, p = 0.08).
Fig. 1.
Infection with Campylobacter jejuni increases anxiety-like behavior during exploration in the holeboard. A. Reduced exploration by C. jejuni-treated mice (the darkly shaded bars) of the 20×20 cm center area (or zone) is reflected by decreased distance traveled within, entries into, and time spent inside the center zone, all in comparison to the vehicle-treated mice (open bars). B. Infected mice also engaged in diminished exploration of the very middle 5×5 cm zone as shown by reduced distance traveled, fewer partial entries (or pokes into) and full entries into the very middle zone. C. Infected mice made fewer visits and pokes into the mostly exposed center and moderately exposed side holes than the uninfected ones, but poked into the most protected corner holes as frequently as the saline-treated mice. D. There were no differences between the C. jejuni- and the saline-treated groups in general locomotor activity as reflected in the total distance traveled in the entire holeboard and in the distance traveled in the peripheral zone of the holeboard, indicating the absence of sickness behavior. * p < 0.05, ** p < 0.005, m: marginal significance, p < 0.086.
In line with the above-mentioned differences in overall exploration of the holeboard, C. jejuni-treated mice made fewer nose pokes into the less protected center and side holes (18.4 +/- 2.0 vs. 26.5 +/- 3.0; F1,20 = 4.84, p = 0.04; Fig. 1C). In contrast, the infected and the control groups explored the more protected corner holes with equal frequency (40.3 +/- 3.8 vs. 42.6 +/- 2.8).
Measures of overall activity, including total distance traveled, distance traveled in the peripheral zone (Fig. 1D), basic and fine movements, rearing, did not differ between the infected and control groups, indicating that the increased avoidance of the center region was not likely due to hypo-arousal and decreased activity due to psychomotor slowing, a common symptom of sickness.
Effects of C. jejuni challenge and hole board exploration on c-Fos expression
To determine candidate brain regions potentially contributing to enhancement of innate anxiety-like behavior triggered by C. jejuni challenge, numbers of c-Fos positive neurons were assessed in selected brain regions previously associated with anxiety-like or fear behavior, and autonomic regions that typically respond to immune activation. We compared brain c-Fos patterns in groups of mice that either were exposed to the holeboard (HB) or remained in their home cage and were either orally challenged with C. jejuni or saline (vehicle) solution. Based on these comparisons, the brain regions analyzed showed either an increased c-Fos expression associated with the behavioral testing alone, with C. jejuni infection alone, or responded with increased expression of c-Fos to both the treatment and behavioral testing.
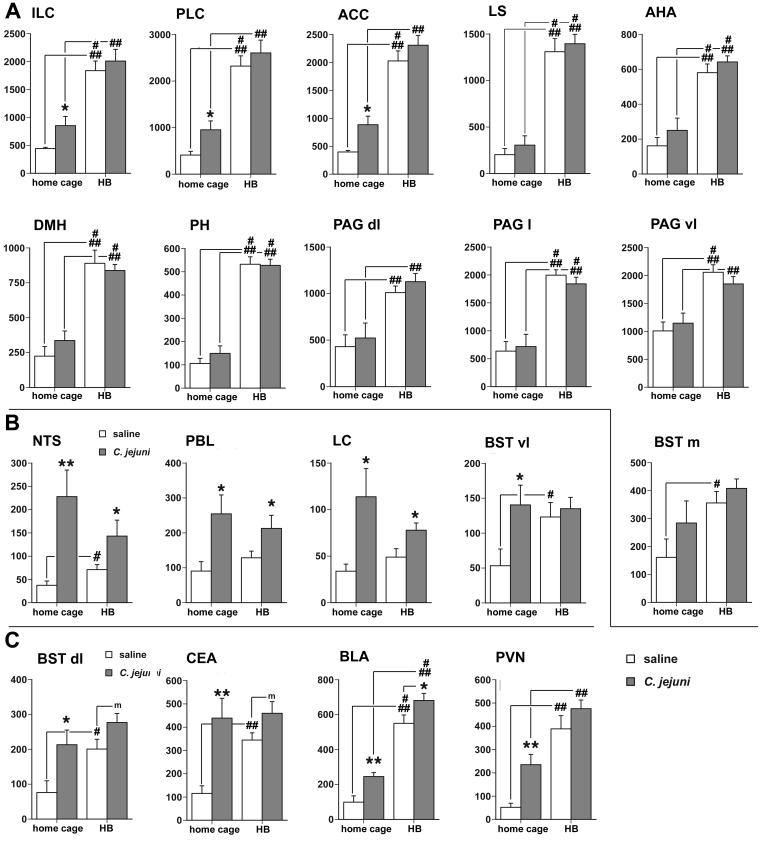
Brain regions that show statistically significant main effects associated with an increase in c-Fos protein only in response to behavioral testing on the HB include the medial prefrontal cortex (ILC: F1,34= 29.28, p < 0.0001; PLC: F1,34= 34.16, p < 0.0001; and ACC: F1,34= 48.97, p < 0.0001), the lateral septum (LS: F1,34= 92.18, p < 0.0001), and the medial portion of the BST (BSTm: F1,34 = 8.91, p <0.005), the anterior hypothalamic area (AHA: F1,34 = 64.27, p < 0.0001), the dorsomedial and posterior hypothalamic nuclei (DMH: F1,34= 47.33, p < 0.0001; PH: F1,34= 84.90, p < 0.0001), and the periaqueductal gray (dorsolateral PAG: F1,34 = 29,28, p < 0.0001; lateral PAG: F1,34 = 70.64, p < 0.0001; ventrolateral PAG: F1,34 = 33.87, p < 0.0001), as depicted in Fig. 2A. Although no main effects of C. jejuni challenge were apparent in this collection of brain regions, a small, marginally significant increase in c-Fos expression in response to C. jejuni infection in the ACC (p = 0.09). In addition, when comparing the home cage groups alone (Bonferroni), C. jejuni challenge produced a smaller, but significant increase in c-Fos expression in the ILC, PLC, and ACC. These data are illustrated with photomicrographs of representative sections in Figs. 3A-F (LS, DMH, and PH) and 4A-D (medial prefrontal cortex).
Fig. 2.
A. Dramatic increases in the number of cells displaying c-Fos expression in selected brain areas in response to exposure to the holeboard (significant main effect of behavioral test, but not main treatment, effect in two-way ANOVA). In comparison to the home cage condition, the holeboard test leads to strong increases in c-Fos-positive cells in the medial prefrontal cortex (ILC, PLC, and ACC), the lateral septum, the medial (m) portion of the BST (ventromedial and dorsomedial parts combined), the hypothalamus (AHA, DMH, and PH), and the PAG (in ventrolateral, lateral, and dorsomedial quadrants. In addition, a smaller, but significant, increase in c-Fos expression due to C. jejuni infection was noticeable among the home cage controls in the medial prefrontal cortex (ILC, PLC, and ACC). B. Increases in the number of c-Fos-positive cells in selected brain regions primarily in response to C. jejuni challenge (significant main treatment, but not main test condition, effect in two-way ANOVA). Brain regions include the NTS, PBL, LC, and the ventrolateral portion of the BST. In addition, the NTS and BSTvl showed a holeboard exposure-related increase in c-Fos expression relative to the home cage condition, which was only apparent in the saline-treated groups. C. Increase in the number of c-Fos-immunoreactive cells in response to both C. jejuni challenge and holeboard exposure (both significant main treatment and test condition effects in two-way ANOVA) was evident in select brain regions, including the dorsolateral BST, CEA, BLA, and the PVN. Only in the CEA, the two-way ANOVA revealed a significant interaction (p < 0.05), as there was no further increase in c-Fos expression as a result of holeboard exposure in the infected mice beyond what was induced by the bacterial challenge. C. jejuni challenge vs. saline treatment: * p < 0.05, ** p < 0.005. Holeboard vs. home cage condition: # p < 0.05, ## p < 0.005, ### p < 0.0005.
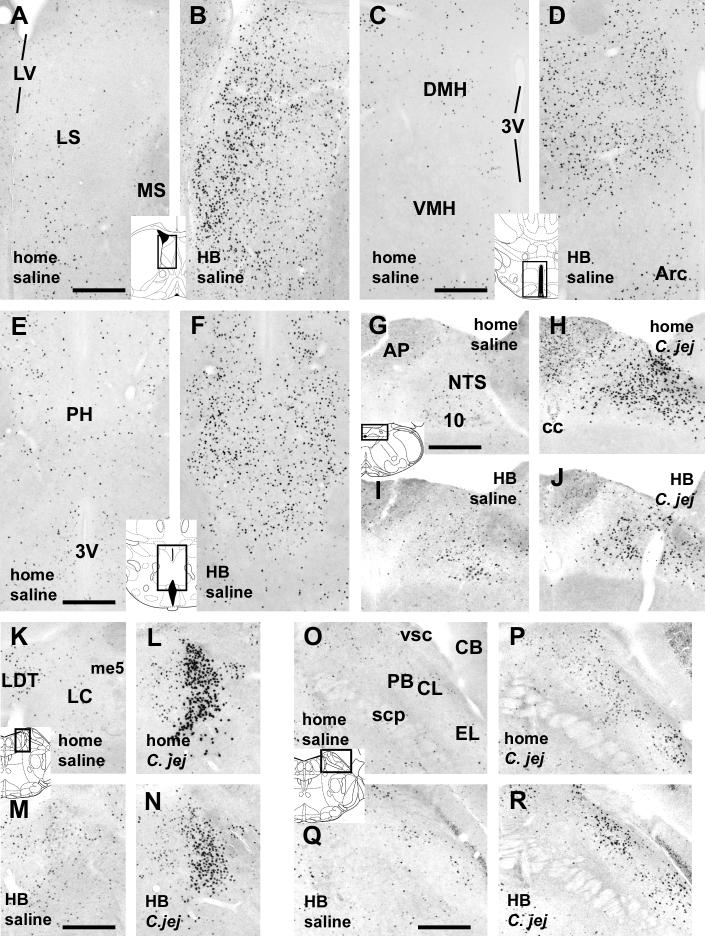
Fig. 3.
A-F. Photomicrographs showing increased c-Fos staining in the LS (A,B), DMH (C,D), and PH (E,F) in mice exposed to the holeboard (HB, in B,D,F) in comparison to the home cage controls (home, in A,C,E). No effects of C. jejuni challenge were observed in these regions in either home cage or holeboard groups (not shown). G-R. Infection with C. jejuni increased c-Fos staining in brainstem regions, including the NTS (G-J), LC (K-N), and PBL (O-R). This effect of infection (panels labeled C. jej in H,J [NTS], L,N [LC], and P,R [PBL]) was seen in both home cage (panels labeled “home”) and holeboard (HB) groups when compared with the saline-treated groups (panels labeled “saline” in G,I [NTS], K,M [LC], and O,Q [PBL]). Inserts depicting brain section diagrams show the locations of the photomicrographs (modified after Paxinos and Franklin, 2001). Abbreviations: 3V: third ventricle; 10: dorsal motor nucleus of the vagus; Arc: arcuate nucleus; AP: area postrema; CB: cerebellum; C.jej: C. jenuni-challenged. DMH: dorsomedial hypothalamus; LC: locus coeruleus; LDT: laterodorsal tegmental nucleus; LS: lateral septum; LV lateral ventricle; me5: mesencephalic trigeminal tract; MS: medial septum; NTS: nucleus of the solitary tract; PBL: lateral parabrachial nucleus; scp: superior cerebellar peduncle; VMH: ventromedial hypothalamus; vsc: ventral spinocerebellar tract. Scale bars: 250 μm.
Brain regions that responded with significant increased c-Fos expression to only the bacterial challenge included the nucleus of the solitary tract (NTS: main effect of treatment, F1,34= 16.49, p.< 0.0003), the lateral parabrachial nucleus (PBL: F1,34= 11.05, p < 0.002), the locus coeruleus (LC: F1,34= 13.3, p < 0.001). Although the ventrolateral portion of the bed nucleus of the stria terminalis (BSTvl) also showed significant main effects for C. jejuni treatment (F1,34= 5.03, p < 0.03), it should be noted that the interaction was marginally significant (p >0.09). These findings are depicted in Fig. 2B (graphs), and further illustrated in Fig. 3G-R.
Finally, the brain regions that showed significant increase in the expression of c-Fos in response to both C. jejuni challenge and behavioral testing included the dorsolateral BST (F1,34 (test)= 4.31, p.< 0.04; F1,34 (treatment) = 11.30, p.< 0.001), the central nucleus of the amygdala (CEA-F1,34 (test) = 5.83, p.< 0.02; F1,34 (treatment) = 17.91, p.< 0.0002), the basolateral nucleus of the amygdala (BLA-F1,34 (test) = 96.76, p.< 0.0001; F1,34 (treatment) = 12.57, p.< 0.001), and the paraventricular nucleus of the hypothalamus (PVH-F1,34 (test)= 46.4, p.< 0.0001; F1,34 (treatment) = 10.5, p.< 0.003), as illustrated by the graphs in Fig. 2C, and photomicrographs in Fig. 4E-L (PVN and amygdala) and Fig. 5 (BST). For the BST, PVN and BLA, the increase in number of c-Fos-positive cells as a result of both the bacterial challenge and behavioral testing was additive, and no significant interactions were apparent in these behavior- and infection-responsive regions. However, the CEA showed a significant interaction between the treatment and test conditions (F1,34= 5.15, p. < 0.02). In this nucleus, the treatment effect on c-Fos expression was prominent in the home cage controls, but much smaller in the mice tested in the holeboard. A summary diagram is provided in Fig. 6, and depicts the pattern of activated brain regions (as determined with c-Fos protein induction) as a consequence of the bacterial, behavioral, or both challenges.
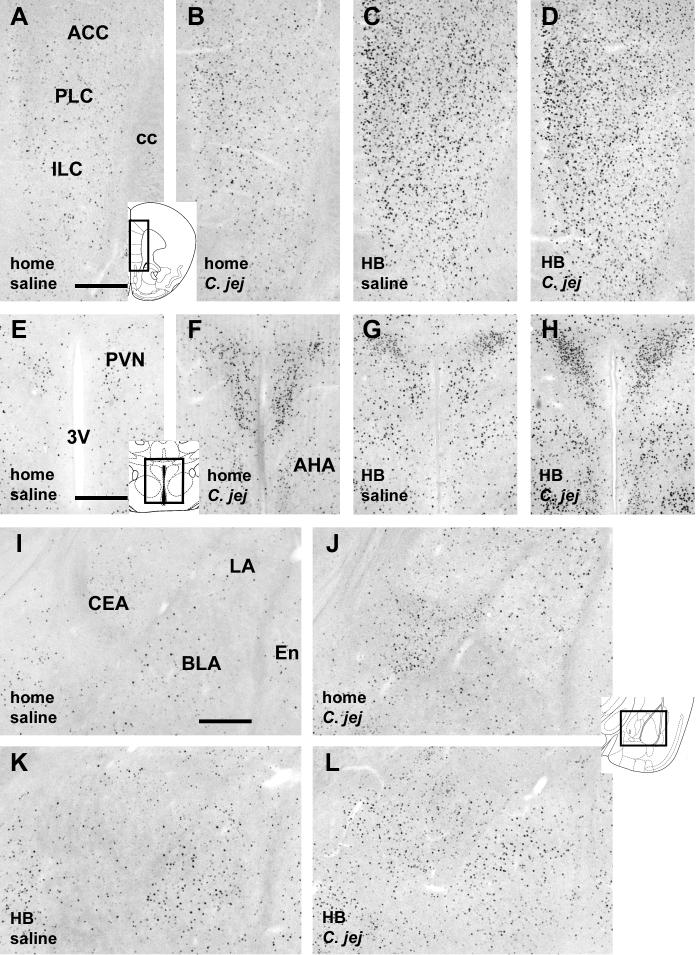
Fig. 4.
Photomicrographs of the medial prefrontal cortex (A-D), PVN (E-H), and the amygdala (I-L) showing increase in c-Fos-immunoreactive cells in response to C. jejuni challenge as well as holeboard exposure. For each brain region, the four panels depict each of the four experimental conditions indicated in the bottom left of the panels. Inserts depicting diagrams of brain sections show the locations of the photomicrographs (modified after Paxinos and Franklin, 2001). Abbreviations: 3V: third ventricle; ac: anterior commisure; ACC: anterior cingulate cortex; AHA: anterior hypothalamic area; BLA: basolateral nucleus of the amygdala; BST: bed nucleus of the stria terminalis; CEA: central nucleus of the amygdala; f: fornix; ILC: infralimbic cortex; LA: lateral nucleus of the amygdala; LV: lateral ventricle; PLC: prelimbic cortex; PVN: paraventricular nucleus of the hypothalamus. Scale bars in A, E, I: 250 μm, and apply to other corresponding panels.
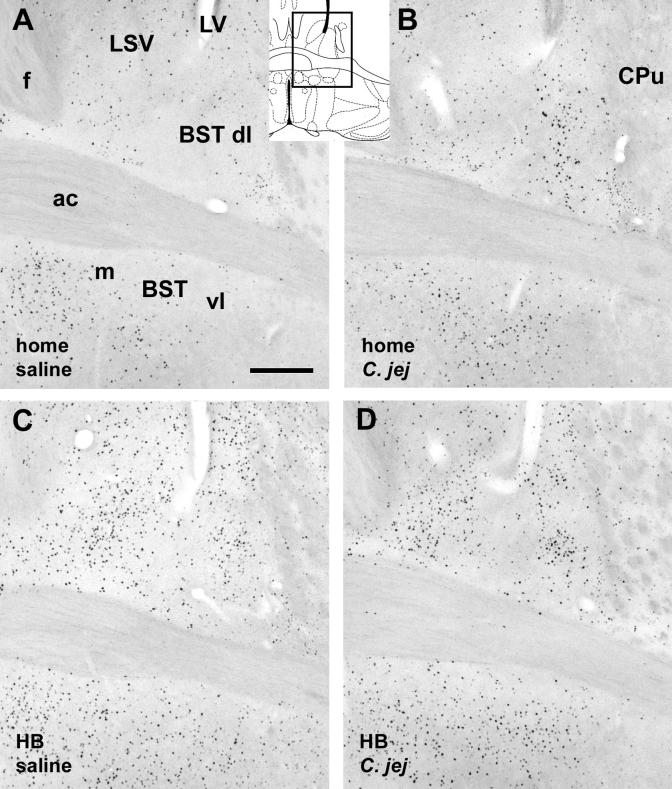
Fig. 5.
Photomicrographs of the bed nucleus of the stria terminalis (A-D), showing increase in c-Fos-immunoreactive cells in response to C. jejuni challenge (predominantly in the dorso- and ventrolateral parts) and to holeboard exposure. Each panel depicts one of the four experimental conditions indicated in the bottom left of the panels. Insert depicting a diagram of a brain section shows the location of the photomicrographs (modified after Paxinos and Franklin, 2001). Abbreviations: ac: anterior commissure; BST: bed nucleus of the stria terminalis, dl: dorsolateral part, vl: ventrolateral part; m: medial part; C. jej: treated with C. jejuni; CPu: caudate putamen; f: fornix; HB: holeboard exposure: LSV: lateral septal nucleus, ventral part. Scale bar in A: 250 μm, applies to all panels.
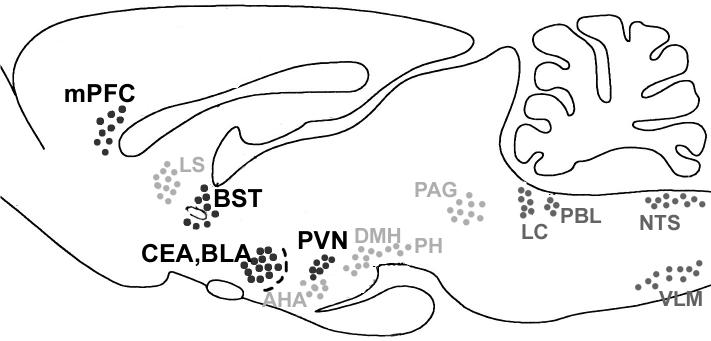
Fig. 6.
Summary diagram of brain regions that are activated primarily in response to C. jejuni challenge (brainstem nuclei in dark grey), in response to behavioral challenge (holeboard exposure, brain regions in light grey), or both (brain regions in black). The latter regions likely represent the interface of visceral and exteroceptive input through which behavioral responses to environmental challenge, such as exposure to the holeboard, may be modified by viscerosensory challenge.
Correlations between c-Fos expression and anxiety-like behavior
To determine the potential contribution of specific brain regions to the potentially anxiety-related aspects of exploratory behavior, we assessed correlations between c-Fos counts in specific brain regions and indices of center zone exploration that reflect the level of anxiety-like state in the individual mice. To eliminate interference by C. jejuni challenge on c-Fos induction, the correlations were assessed in each treatment group separately. Among the saline-treated animals, c-Fos counts in the BLA, CEA, PVN and ventrolateral PAG (PAGvl) were negatively correlated with exploratory behavior (Table 1). That is, the greater the activation of neurons in these brain regions, the less likely were the mice to explore the center regions of the holeboard. In particular c-Fos counts in the BLA showed strong negative correlation with multiple indices, such as distance traveled and time spent in the center zone, as well as entries into the very middle 5×5 cm zone, time spent and distance traveled in the very middle. The c-Fos counts in the CEA correlated negatively with entries into and distance traveled within the 20×20 cm center area, as well as entries into the very middle 5×5 cm zone. The c-Fos expression in the PVN and the PAGvl correlated with the distance traveled in the 20×20 cm center area (both PVN and PAGvl), time spent in the center area (PVN) and entries into the 5×5 cm middle zone (PAGvl). Lastly, c-Fos counts in the medial prefrontal cortex (PLC and ILC) showed marginal negative correlations with distance traveled in the 20×20 cm center area and entries into the 5×5 cm middle zone (ILC only).
Table 1.
Correlations between c-Fos counts and behavioral measures
| A. Saline group | BLA | CEA | PVN | PAGvl | ILC | PLC | |
|---|---|---|---|---|---|---|---|
| center area 20×20 cm | distance |
r = -.821 p = .001 |
r = -.745 p = .006 |
r = -.63 p = .036 |
r = -.60 p = .048 |
r = -.561 p = .07 |
r = -.549 p = .08 |
| time spent |
r = -.748 p = .006 |
r = -.655 p = .027 |
|||||
| entries into | r = -.536 p = .09 |
r = -.695 p = .015 |
|||||
| middle zone 5×5 cm | distance |
r = -.733 p = .008 |
r = -.548 p = .08 |
||||
| time spent |
r = -.71 p = .012 |
r = -.562 p = .07 |
|||||
| entries |
r = -.691 p = .016 |
r = -.742 p = .007 |
r = -.62 p = .04 |
r = -.585 p = .058 |
|||
| center hole | pokes |
r = -.617 p = .042 |
| B. C. jejuni group | BST | CEA | NTS | |
|---|---|---|---|---|
| center area 20×20 cm | distance | r= -.521 p = .10 |
r= +.594 p = .053 |
|
| time spent |
r= +.606 p = .047 |
|||
| entries into |
r= -.636 p = .033 |
|||
| middle zone 5×5 cm | distance | r= -.538 p = .088 |
||
| time spent | ||||
| center hole | pokes |
In contrast, this pattern of multiple negative correlations found in the saline-treated mice was absent in the C. jejuni-treated group. Rather, c-Fos expression in the BST (and only the BST) predicted avoidance of the center regions of the holeboard (entries into the 20×20 cm center area and trend towards distance traveled in the center area). Notably, c-Fos counts in the BLA, CEA, PVN and PAGvl did not correlate remotely close to any of the behavioral measures described above, which may be due to the altered induction of c-Fos as a direct consequence of C. jejuni infection (as this treatment resulted in increased c-Fos expression in these regions, see previous section). In addition, activation in the NTS was correlated positively with entries and pokes into the 20×20 cm center zone, and distance traveled in this center zone.
4. Discussion
The results from this study show that challenge with the bacterial pathogen C. jejuni increased anxiety-like responses in the open field/hole board exploration task compared to saline treated controls, (as expected based on previous studies) in that infected mice displayed a reduced willingness to explore the center of the holeboard. This difference in exploratory style may follow from induction of c-Fos protein in the CEA, the BLA, the BST, and the PVN, which responded to both the exploration task and the bacterial challenge. Notably, these regions form part of a network that has been established to integrate internal challenges (such as infection) with autonomic, neuroendocrine, and behavioral (e.g. anxiety or fear-like) responses to processive, or exteroceptive, challenges (Herman et al., 2003; Sawchenko et al., 2000). Further evidence supporting the involvement of these regions in anxiety-like behavior is provided by the observation that the number of c-Fos positive neurons in the BLA, CEA, PVN and PAGvl was associated with avoidance of the center regions of the OF/HB in saline treated animals, suggesting direct influences of, or on, these brain regions in the inhibition of exploratory behavior. In contrast, the BST was the only brain region in which activation was correlated with measures of anxiety-like responses (avoidance of the center area) to the novel environment the in C. jejuni challenged animals. Thus, the findings reported here support the PVN, CEA, BLA and especially the BST as interfaces by which viscerosensory signals influence brain regions mediating responses to “processive” stressors, which in the case of infection may lead to a shift in exploratory strategy toward preference for safety over e.g. foraging.
Methodological considerations: neural activation correlates of behavior
Previous findings assessing patterns of c-Fos induction tend to support roles for the mPFC, medial core of the hypothalamus (i.e., AHA, PVN, DMH), amygdala (CEA, BLA), and the PAG, in different paradigms thought to mediating and/or modulating anxiety-like behavior (Adamec et al., 2005; Charney, 2003; Salome et al., 2004; Singewald and Sharpe, 2000; Walker et al., 2003). However, conclusions need to be drawn carefully regarding the interaction of neural substrates contributing to anxiety (or any other behavior or brain function). For instance, different patterns of brain activity can emerge depending on the experimental model (e.g. conditioned fear vs. innate anxiety), as the specific demands of the behavioral assessment varies depending on the paradigm used. Similarly, the use of protein activation markers (e.g., c-Fos) can provide general information regarding patterns of activated brain regions that may underlie changes in behavior, but these are correlational, do not provide information about flow of activation through a network, and may overlook populations of neurons that are inhibited as a result of the behavioral challenge, or that are moderately activated but do not produce immunohistochemically detectable c-Fos protein. Nonetheless, response patterns as elucidated by e.g. c-Fos analysis can provide templates for further studies, and can support commonalities of specific brain regions with behaviors.
C. jejuni effects on behavior
The finding that C. jejuni inoculation enhances anxiety-like behavior in the HB supports our previous findings using another species of bacteria, Citrobacter rodentium, as well as previous reports of anxiety-like behavior following immune challenge (Lacosta et al. 1999; Kusnecov et al. 1999; Rossi-George et al. 2004). The holeboard test used in this experiment is believed to assess innate apprehension by rodents of open spaces, and avoidance of the center region of the apparatus is thought to represent a type of anxiety-like behavior. This task can be considered a type of “processive” challenge, involving a more cognitive or “top-down” flow of information through the neural networks involved in initiating or modulating behavior. The finding that the presence of bacteria in the intestine enhances avoidance of the center region of the holeboard indicates that “bottom up” sensory information from the body can modulate, in this case enhance, ongoing responses to “top down” neuronal processing in response to exteroceptive (processive) challenges such as a novel environment.
Patterns of neuronal activation following the open field/holeboard test
The constellation of brain regions activated following exposure to the holeboard is consistent with findings from previous studies investigating brain regions activated in situations provoking “innate anxiety” (Salome et al., 2004). Among these are nuclei of the “defensive core”: the septal area, amygdala, hypothalamus and periaquiductal grey, that are critical for endocrine, autonomic and behavioral responses to fear- or anxiety- provoking situations (Brandao et al. 2003). In addition, several subregions of the amydala, including the CEA and BLA, as well as the BST, have been consistently implicated in the experience of fear or anxiety (Campbell and Merchant 2003; Hale et al., 2006; Morilak et al., 2005; Walker and Davis 2003). More recently, the medial PFC, including the dorsal (cingulate and prelimbic) and ventral (infralimbic) parts have been recognized as critical contributors to stress responses and coping (Herman et al., 2003; Spencer et al., 2003; Amat et al., 2006). Together, components of this network co-ordinate perceptual and emotional sequelae of processive stressors with autonomic and behavioral responses.
In animals exposed both to the processive (holeboard exposure) and viscerosensory (C. jejuni) challenges, c-Fos expression in the PVN, BLA and BST (brain regions that respond to both challenges) was additive, as numbers of c-Fos positive neuronal nuclei were slightly elevated compared to their respective controls, but there was no statistically significant interaction. That is, viscerosensory challenge with C. jejuni enhanced c-Fos responses to exposure to the holeboard (or vice versa). These findings are consistent with the idea that these different types of challenges recruit stress-related neurocircuitry via different initial pathways, and that these brain regions serve as interfaces between categorically different stressors (e.g. Emmert and Herman 2001; Sawchenko et al., 2000). How this leads to enhanced avoidance of the center of the holeboard is not clear, but may derive from enhanced salience of the emotional cues associated with the novel environment (McNaughton and Corr, 2003). Salience could be enhanced as a result of neuroendocrine or autonomic responses mediated by the PVN that could increase peripheral arousal, which could feed then back to e.g. the extended amygdala. For example, Williams and colleagues have shown that systemic injection of epinephrine, a correlate of peripheral arousal, activates the vagus nerve (Miyashita and Williams, 2006) and, via projections from the NTS enhances performance in a passive avoidance task (Hassert et al., 2004; Williams and McGaugh, 1993; Williams et al., 2000). Notably, this pathway parallels the likely pathway by which gut bacteria influence brain functions. Additionally or alternatively, gut bacteria could enhance salience via increasing negative affect communicated through the amygdala and/or BST (see below).
Whereas c-Fos expression in the amygdala (CEA and BLA) predicted avoidance of the center (open) part of the open field/hole board in saline treated animals, in C. jejuni-treated animals, (which showed significantly increased avoidance of the center region), this behavior was instead correlated with c-Fos induction in the BST. How this might have translated into enhanced avoidance of the center region is unclear. Clues to a mechanism, however, derive from studies investigating the neural basis of increased negative affect associated with opiate withdrawal (Delfs et al., 2000; Harris and Aston-Jones, 2007). These studies have indicated a critical role of the BST, and in particular, its catecholaminergic inputs from the caudal brainstem. Blockade of adrenergic receptors in the BST prevented development of place aversions in rats undergoing morphine withdrawal (Harris and Aston-Jones, 2007). Like morphine withdrawal, immune challenge with lipopolysaccharide (a component of gram-negative bacterial cell walls) activates caudal brainstem projection neurons (A1/C1 cell group in the VLM and the A2/C2 cell group in the NTS) that target the BST directly (Gaykema et al., 2007). Because C. jejuni treatment also activates catecholaminergic neurons of the A2/C2 groups (Goehler et al., 2005), enhanced drive on their projections to the BST following C. jejuni treatment may have intensified negative affective responses to the novel, but potentially dangerous, environment.
Immune challenge as contributor to “bottom-up” drive on affective state
Taken together these findings imply an adaptive link between viscerosensory, including immune sensory, signaling and brain systems specialized for behavioral responses to threats or potentially threatening situations. If the presence of an infection increases the salience, or valence (McNaughton and Corr, 2004) associated with potential danger in the holeboard, infections may lead to a perception of greater threat, and thus more cautious behavior. Because sick animals can be compromised in coping with physical threats, more cautious behavior may lead to enhanced survival. Thus, viscerosensory input may provide bottom-up drive on affective states as a means to bias an animal’s response toward avoiding dangerous situations.
This early response to potential illness can be considered to be an adaptive defensive strategy. The holeboard provides a conflict situation in which mice choose safety (by remaining along the walls of the maze) or exploration, which could lead to finding novel sources of food (foraging). Infected animals seem more inclined to choose safety over foraging, which indicates a change in exploratory behavior pattern that could represent a change in motivational state, as has been suggested for classic sickness behavior (Aubert, 1999; Dantzer, 2005; Hart, 1988).
Although peripheral infection can potentiate anxiety-like behavior in the context of processive stressors, the converse could also be true. That is, autonomic responses to infection could also be enhanced by ongoing stressors. This possibility could contribute stress effects on gastrointestinal ulcers, which although are largely caused by infection with Helicobacter pylorii, can be exacerbated by processive stress (Levenstein, 2000). Indeed, additional drive on e.g. the hypothalamus-pituitary adrenal axis via enhancement of PVN activation might also influence autonomic and immune responses to infection.
Conclusion/perspectives
The findings presented here demonstrate that bacterial infection in the GI tract activates central viscerosensory pathways that interface with stress-related and “defensive” network nuclei in the hypothalamic PVN, the amygdala (BLA and CEA), and the BST. Whereas these interface regions have been previously established as nodal points for the integration of psychological or processive stress with behavioral responses to potential threats or threatening situations, the infection-induced enhancement of anxiety-like behavior underlines the idea that visceral “bottom up” input markedly modulates behavior, and provides anatomical substrates for mechanisms by which peripheral inflammation can influence responses to stress.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamec RE, Blundell J, Burton P. Neural circuit changes mediating lasting brain and behavioral changes to predator stress. Neursci. Biobehav. Rev. 2005;29:1225–1241. doi: 10.1016/j.neubiorev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of ventral medial prefrontal cortex. J. Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert A. Sickness and behavior in animals: a motivational perspective. Neursci. Biobehav. Rev. 1999;12:1029–1036. doi: 10.1016/s0149-7634(99)00034-2. [DOI] [PubMed] [Google Scholar]
- Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. J. Neurosci. 2006;26:11442–11453. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billig I, Yates BJ, Rinaman L. Plasma hormone levels and central c-Fos expression in ferrets after systemic administration of cholecytokinin. Am. J. Physiol. Regulatory integrative Comp. Physiol. 2001;281:R1243–R1255. doi: 10.1152/ajpregu.2001.281.4.R1243. [DOI] [PubMed] [Google Scholar]
- Branao ML, Troncoso AC, de Souza Silva MA, Huston JP. The relevance of neuronal substrates of defense in the midbrain tectum to anxiety and stress: empirical and conceptual considerations. Eur. J. Pharmacol. 2003;463:225–233. doi: 10.1016/s0014-2999(03)01284-6. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain Res. 2003;993:1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- Castex N, Fioramonti J, Fargeas MJ, Bueno L. c-fos expression in specific rat brain nuclei after intestinal anaphylaxis: involvement of 5-HT3 receptors and vagal afferent fibers. Brain Res. 1995;688:149–160. doi: 10.1016/0006-8993(95)00526-v. [DOI] [PubMed] [Google Scholar]
- Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr. Scand. 2003;108:38–50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur. J. Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Somatization: A psychoneuroimmune perspective. Psychoneuroendocrinol. 2005;30:947–952. doi: 10.1016/j.psyneuen.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Ackerman MK, Register KB, Rimler RB, Ross LR, Jacobson CD. Induction of Fos-like immunoreactivity in the rat brain following Pastuerella multicida endotoxin administration. Endocrinol. 1993;133:3054–3057. doi: 10.1210/endo.133.6.8243337. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Saper CB. Activation of neurons projecting to the paraventricular hypothalamic nucleus by intravenous lipopolysaccharide. J. Comp. Neurol. 1996;374:315–331. doi: 10.1002/(SICI)1096-9861(19961021)374:3<315::AID-CNE1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Emmert MH, Herman JP. Differential forebrain c-fos mRNA induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Res. 1999;845:60–67. doi: 10.1016/s0006-8993(99)01931-9. [DOI] [PubMed] [Google Scholar]
- Gaykema RPA, Goehler LE, Lyte M. Brain response to cecal infection with Campylobacter jejuni: analysis with Fos immunohistochemistry. Brain Behav. Imm. 2004;18:238–245. doi: 10.1016/j.bbi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Gaykema RPA, Chen C-C, Goehler LE. Organization of immune-responsive medullary projections to the bed nucleus of stria terminalis, central amygdala, and paraventricular nucleus of the hypothalamus: Evidence for parallel viscerosensory pathways in the rat brain. Brain Res. 2007;1130:130–145. doi: 10.1016/j.brainres.2006.10.084. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RPA, Anderson K, Hansen MK, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemoreceptive pathway. Auton. Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RPA, Opitz N, Reddaway R, Badr NA, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav. Immun. 2005;19:334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Goldstein MA, Silverman ME. Autonomics and cognition. Clin. Auton. Res. 2006;16:86–89. doi: 10.1007/s10286-006-0339-2. [DOI] [PubMed] [Google Scholar]
- Hale MW, Bouwknecht JA, Spiga F, Shekhar A, Lowry CA. Exposure to high- and low-light conditions in an open-field test of anxiety increases c-Fos expression in specific subdivisions of the rat basolateral amygdaloid complex. Brain Res. Bull. 2006;71:174–182. doi: 10.1016/j.brainresbull.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Activation in extended amygdala corresponds to altered hedonic processing during protracted morphine withdrawal. Behav. Brain Res. 2007;176:251–258. doi: 10.1016/j.bbr.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci .Biobehav. Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav. Neurosci. 2004;188:79–88. doi: 10.1037/0735-7044.118.1.79. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Meuller NK, Ulrich-Lai Y, Ostrnader MM, Choi D, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front. Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H. Behavioral and neurochemical consequences of lipopolysaccharide in mice: anxiogenic-like effects. Brain Res. 1999;818:291–303. doi: 10.1016/s0006-8993(98)01288-8. [DOI] [PubMed] [Google Scholar]
- Levenstein S. The very model of a modern etiology: A biopsychosocial view of peptic ulcer. Psychosomatic Med. 2000;62:176–185. doi: 10.1097/00006842-200003000-00003. [DOI] [PubMed] [Google Scholar]
- Lyte M, Varcoe JJ, Bailey MT. Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiol. Behav. 1998;65:63–68. doi: 10.1016/s0031-9384(98)00145-0. [DOI] [PubMed] [Google Scholar]
- Lyte M, Wang L, Opitz N, Gaykema RPA, Goehler LE. Anxiety-like behavior during initial stage of infection with agent of colonic hyperplasia Citrobacter rodentium. Physiol. Behav. 2006;89:350–357. doi: 10.1016/j.physbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J.. Clin. Psychiatry. 2001;62:28–36. [PubMed] [Google Scholar]
- Mayer EA, Tillisch K, Bradesi S. Review article: modulation of the brain-gut axis as a therapeutic approach in gastrointestinal disease. Aliment. Pharmacol. Ther. 2006;24:919–933. doi: 10.1111/j.1365-2036.2006.03078.x. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci. Biobehav. Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Williams CL. Epinephrine administration increases neural impulses propagated along the vagus nerve: Role of peripheral α-adrenergic receptors. Neurobiol. Learning Memory. 2006;85:116–124. doi: 10.1016/j.nlm.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Myers EA, Banihashemi L, Rinaman L. The anxiogenic drug yohimbine activates central viscerosensory circuits in rats. J. Comp. Neurol. 2005;492:426–441. doi: 10.1002/cne.20727. [DOI] [PubMed] [Google Scholar]
- Naritoku DK, Terry WJ, Helfert RH. Regional induction of fos immunoreactivity in the brain by anticonvulsant stimulation of the vagus nerve. Epilepsy Res. 1995;22:53–62. doi: 10.1016/0920-1211(95)00035-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Second ed. Academic Press; San Diego, CA, U.S.A.: 2001. [Google Scholar]
- Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central c-Fos expression in rats after gastric viscerosensory stimulation. J. Neurosci. 2003;23:10084–10092. doi: 10.1523/JNEUROSCI.23-31-10084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J. Comp. Neurol. 2001;432:197–216. doi: 10.1002/cne.1097. [DOI] [PubMed] [Google Scholar]
- Rossi-George A, Urbach D, Colas D, Goldfarb Y, Kusnecov AW. Neuronal, endocrine and anorexic responses to the T-cell superantigen staphylococcal enterotoxin A: Dependence on tumor-necrosis factor-α. J. Neurosci. 2005;25:5314–5322. doi: 10.1523/JNEUROSCI.0687-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salome N, Salchner P, Viltart O, Sequeira H, Wigger A, Landgraf R, Singewald N. Neurobiological correlates of high (HAB) versus low anxiety-related behavior (LAB): differential Fos expression in HAB and LAB rats. Biol. Psychiatry. 2004;55:715–723. doi: 10.1016/j.biopsych.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Li H-Y, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Progr. Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- Singewald N, Sharpe T. Neuroanatomical targets of anxiogenic drugs in the hindbrain as revealed by Fos immunocytochemistry. Neurosci. 2000;98:759–770. doi: 10.1016/s0306-4522(00)00177-9. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: possible role of the bed nucleus of the stria terminalis. J. Comp. Neurol. 2005;481:363–376. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- Viltart O, Sartor DM, Verberne AJM. Chemical stimulation of visceral afferents activates medullary neurones projecting to the central amygdala and periaqueductal grey. Brain Res. Bull. 2006;71:51–59. doi: 10.1016/j.brainresbull.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur. J. Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wan W, Janz L, Vriend CY, Sorensen CM, Greenberg AH, Nance DM. Differential induction of c-Fos immunoreactivity in hypothalamus and brain stem nuclei following central and peripheral administration of endotoxin. Brain Res. Bull. 1993;32:581–587. doi: 10.1016/0361-9230(93)90158-8. [DOI] [PubMed] [Google Scholar]
- Williams CL, McGaugh JL. Reversible lesions of the nucleus of the solitary tract attenuate the memory-modulating effects of post-training epinephrine. Behav. Neurosci. 1993;107:955–962. [PubMed] [Google Scholar]
- Williams CL, Men D, Clayton EC. The effects of noradrenergic activation of the nucleus tractus solitarius on memory and in potentiating norepinephrine release in the amygdala. Behav. Neurosci. 2000;114:1131–1144. doi: 10.1037//0735-7044.114.6.1131. [DOI] [PubMed] [Google Scholar]