Abstract
Multiple lines of evidence have indicated that the inability of adult mammalian central nervous system (CNS) axons to regenerate after injury is partly due to the growth inhibitory property of central myelin. Three prototypical myelin-associated inhibitors of neurite outgrowth have been identified, including Nogo, myelin-associated glycoprotein (MAG), oligodendrocyte-myelin glycoprotein (OMgp). These inhibitory ligands, their receptors and signaling pathways are being intensively investigated for their roles in CNS axon regeneration failure. In addition, several members of the axon guidance molecules have been implicated in restricting CNS axon regeneration, some of which are expressed by mature oligodendrocytes. Here we review in vitro and in vivo studies of these molecules in neurite growth and in axon regeneration failure and discuss the implications of these studies. While the increasing number of potential axon regeneration inhibitors highlights the complexity of the restrictive CNS environment, it provides new windows of opportunity as well as new challenges for therapeutic development for spinal cord injury and related neurological conditions.
Keywords: axon regeneration, spinal cord injury, myelin inhibitor, neurite outgrowth, central nervous system repair, corticospinal tract
The non-permissive nature of the CNS
“Once development was ended, the founts of growth and regeneration of the axons and dendrites dried up irrevocably. In adult centers, the nerve paths are something fixed, ended, immutable. Everything may die. Nothing may be regenerated.” – Santiago Ramón y Cajal
Cajal was among the first in the modern era to articulate the observation that the adult mammalian central nervous system (CNS) has poor capability to regenerate axons after injury (Ramón y Cajal, 1928). He also described the retraction bulbs, the swollen structure at the end of severed axons that was indicative of a failed attempt at regeneration. In contrast, functionally significant regeneration is widely observed in other systems. These include CNS axons in fish and tailed amphibians, embryonic CNS axons in mammals, and peripheral nervous system (PNS) axons in adult mammals (Schwab and Bartholdi, 1996). These observations, together with Cajal’s depiction of the failed regenerative attempt in the adult mammalian CNS, prompted the idea that CNS axons are intrinsically capable of regeneration, although their attempt to regenerate fails for reasons that are specific to the adult CNS in higher vertebrates. Studies in the early 1980’s by Aguayo and colleagues (David and Aguayo, 1981; Richardson et al., 1982) clearly showed that after CNS injury in adult rats, axons can regenerate into a permissive peripheral nerve graft, demonstrating the opposing regenerative environment in the CNS and the PNS. Schwab and colleagues found that dissociated neurons were able to grow into sciatic but not optic nerve explants in a culture system, reinforcing the idea that CNS and PNS possess differential conducibility to axon regeneration and paving the way for a biochemical analysis of such inhibitory cues (Schwab and Thoenen, 1985). As it is now well accepted, a major source of inhibitory factors is CNS myelin made by oligodendrocytes; another is the glial scar that forms at the injury site. This review will focus on the role of myelin-derived (or white matter) inhibitors in regeneration failure, while that of the glial scar inhibitors, especially the chondroitin sulfate proteoglycans (CSPGs), will be the topic of the article by Silver and colleagues in the current issue. It should be mentioned that several terms have been used to describe the effect of myelin on axon growth: non-permissive, restrictive and inhibitory. These terms are used here interchangeably since they are referring to the same property of the CNS myelin in discussions of axon regeneration.
The protein components of CNS myelin and white matter are inhibitory
There have been many studies indicating that myelin contributes to the non-permissive nature of the CNS. Berry was the first to postulate that breakdown products of the myelin after CNS injury might be to blame for axon regeneration failure (Berry, 1982). Two in vivo studies supported the hypothesis that myelin and oligodendrocytes are inhibitory to regeneration. First, regeneration of descending tract axons after lesion was enhanced in postnatal rats after depleting oligodendrocytes and CNS myelin with γ-irradiation (Savio and Schwab, 1990). Second, after adult mice were immunized with myelin so that their own immune system was stimulated to produce polyclonal antibodies including those that presumably block myelin-associated inhibitors, regeneration of corticospinal (CST) axons was observed following injury (Huang et al., 1999).
In a series of seminal studies that would eventually lead to the identification of Nogo, Schwab and colleagues showed that 1) CNS myelin, differentiated oligodendrocytes and white matter are non-permissive substrates for neurite growth in vitro (Caroni and Schwab, 1988a; Savio and Schwab, 1989); 2) the non-permissiveness of mature oligodendrocytes can also be demonstrated in a growth cone collapse assay (Bandtlow et al., 1990); 3) importantly, this non-permissive property appeared to be associated with the protein but not lipid components of myelin since it is sensitive to protease treatment (Caroni and Schwab, 1988b). Size fractionation with SDS-PAGE led to the identification of two protein bands (35 kd and 250 kd) with strong growth inhibitory activities. A monoclonal antibody, IN-1, was raised against these proteins (Caroni and Schwab, 1988a). The IN-1 antibody was shown to neutralize the non-permissiveness of CNS myelin, oligodendrocytes and white matter in vitro. In vivo administration of the IN-1 antibody in rats was shown to improve axonal regeneration of descending tract axons and behavioral recovery after a partial spinal cord injury (Schnell and Schwab, 1990; Bregman et al., 1995). The possibility that one or two myelin-derived inhibitors account for most of the CNS axon regeneration failure was appealing as it would allow for a relatively straightforward approach to promote regeneration by simply neutralizing the one or few “bad guys” in CNS.
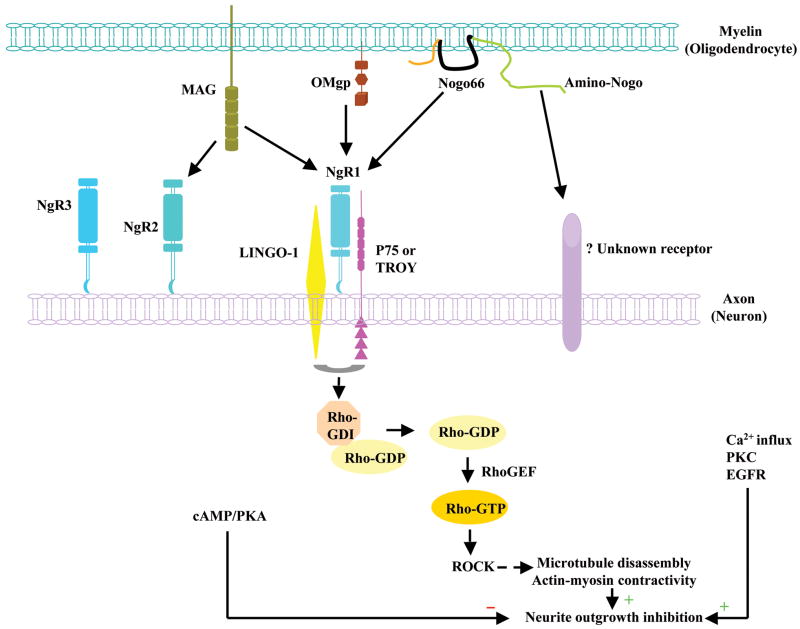
We now know that there are at least half a dozen myelin-derived inhibitors of neurite growth. Three of these, Nogo, MAG and OMgp, were initially identified based on their inhibitory activity on neurite growth. Because the identification of these molecules was instrumental in developing the concept of myelin inhibition of axon regeneration, we refer to these three inhibitors here as the prototypical myelin-derived inhibitors. Extensive in vitro studies have depicted a biochemical pathway for the prototypical myelin inhibitors to exert their effect on neurite growth (Fig. 1). Briefly, the three inhibitors, while sharing no homology, can bind to a single neuronal receptor, NgR1, which together with co-receptors p75NTR or TROY, and LINGO-1, mediates the inhibitory effect (Yiu and He, 2006). MAG can also bind to NgR2 (Venkatesh et al., 2005). There are possibly other, yet unidentified receptors. Downstream effectors include RhoA, Rho kinase (ROCK), Protein Kinase C (PKC) and Epidermal Growth Factor Receptor (EGFR) (Yiu and He, 2006). Here we first discuss the role of the prototypical myelin inhibitors and their receptors in CNS axon regeneration. We then discuss the more recent discovery that repulsive axon guidance molecules may also serve as myelin-derived axon growth inhibitors.
Figure 1.
The prototypical myelin-derived neurite growth inhibitors, their receptors and intracellular signaling pathway. Oligodendrocyte-expressed Nogo, MAG, and OMgp all bind to GPI-anchored protein NgR1, which recruits co-receptor p75 or TROY and LINGO-1 to form a receptor complex to transduce the inhibitory signals in neurons. MAG also binds to NgR2, where the identity of co-receptors has not been confirmed. In neurons, the NgR1 receptor complex stimulates the dissociation of Rho-GDP from GDI and the subsequent formation of active Rho-GTP, which with additional downstream effectors leads to rearrangement of cytoskeletons to inhibit neurite outgrowth. Other downstream effectors for axon growth inhibition include PKC and EGFR, which are related to calcium influx. However, cAMP/PKA plays an opposite role by relieving the growth inhibition.
Nogo
Partial amino acid sequence of Nogo was identified based on a purified myelin-derived inhibitory activity on neurite outgrowth that can be neutralized with the IN-1 antibody (Spillmann et al., 1998). This allowed three labs to simultaneously identify the Nogo gene as encoding a fourth member of the Reticulon family of proteins, so named as these proteins are predominantly localized in the endoplasmic reticulum (ER) due to their ER-retention motif (Chen et al., 2000; GrandPre et al., 2000; Prinjha et al., 2000). Three major protein isoforms, Nogo-A, -B, -C, are generated via alternate splicing and differential promoter usage of the Nogo gene. The inhibitory action of Nogo on neurite growth is mediated by at least two domains: one is an N-terminal region specific to Nogo-A; the other is an extracellular 66 amino acid loop (also known as Nogo-66) between the two hydrophobic segments in a C-terminal region that is shared by all three isoforms (GrandPre et al., 2000; Oertle et al., 2003). Between the two inhibitory domains, Nogo-66 appears to be more potent in a growth cone collapse assay and its effect is more neuron-specific (Fournier et al., 2001). Nogo is highly expressed by CNS oligodendrocytes but not PNS Schwann cells, consistent with its proposed role as a CNS myelin-specific inhibitor of axon regeneration.
Prior to the cloning of the Nogo gene, most work concerning its role in CNS axon regeneration was conducted with the IN-1 antibody. Following the original studies where administration of the IN-1 antibody was shown to enhance CST regeneration and functional recovery after a partial spinal cord injury in rats (Schnell and Schwab, 1990; Bregman et al., 1995), numerous studies have been published, primarily by Schwab and colleagues, where administration of the IN-1 antibody was shown to enhance axonal plasticity (i.e., regeneration and/or sprouting). For example, the infusion of a recombinant, humanized IN-1 antibody Fab fragment (rIN-1 Fab) into a spinal cord injury site was able to promote long-distance regeneration of injured axons in the spinal cord of adult rats (Brosamle et al., 2000). Application of IN-1 in adult cerebellum resulted in the sprouting of uninjured Purkinje cell axon, suggesting that a normal function for such an inhibitor is to maintain the proper targeting by axonal terminals (Buffo et al., 2000). Behavior outcome such as locomotor recovery also demonstrated improvement after IN-1 application (Merkler et al., 2001). When the CST was damaged, IN-1 antibody treatment led to a doubling of the number of collaterals innervating cervical spinal cord by an undamaged fiber tract, the rubrospinal tract, which was associated with an almost complete recovery of precision movements of the forelimb and fingers (Raineteau et al., 2001). Thus, both axonal regeneration by an injured fiber system and axonal sprouting by an uninjured fiber system appear to contribute to the beneficial effect of IN-1 antibody treatment.
After Nogo was cloned, several additional reagents were developed to investigate the role of Nogo in spinal axon regeneration. Since IN-1 has limited specificity for Nogo, the development of these new reagents provided the opportunity to examine more specifically the role of Nogo. New antibodies specifically targeted for Nogo were developed, and for the most part, appeared to work much like IN-1 both in vitro and in vivo (Chen et al., 2000; Liebscher et al., 2005). A peptide inhibitor of Nogo, NEP1-40, was developed to interfere with the interaction between Nogo and its receptor NgR1. Intrathecal administration of NEP1-40 was shown to lead to enhanced CST regeneration and functional recovery in a spinal cord dorsal hemisection model in rats (GrandPre et al., 2002). In this study, numerous ectopic CST fibers were found in the white matter in addition to the grey matter caudal to the injury site. In a second study subcutaneous injection of NEP1-40 was shown to enhance CST regeneration in mice, even when the peptide treatment was applied one week after the injury (Li and Strittmatter, 2003). Interestingly, regenerating CST axons in NEP1-40 subcutaneously injected mice appeared to differ in their organization from those in rats that received intrathecal infusion of NEP1-40 in that the latter group exhibited a strong pattern of ectopic CST fibers in the white matter both above and below injury (GrandPre et al., 2002) while axonal sprouting in NEP1-40 treated mice is mainly restricted to grey matter (Li and Strittmatter, 2003). It is not known whether such a difference in the pattern of regenerating CST fibers reflects a difference between species or in the experimental paradigms applied.
While neutralizing agents have been invaluable in dissecting the function of myelin-derived inhibitors, targeted mutagenesis in mice has been widely accepted as the “gold standard” for determining gene function in mammals (Capecchi, 2005). With mouse knockouts, one can avoid potential complications derived from the variable delivery efficiency or non-specific effects of neutralizing agents. The rationale for such genetic analysis is straightforward: if Nogo were indeed a major inhibitor of axon regeneration, Nogo-deficient mice would be expected to exhibit greatly enhanced axon regeneration. Three groups independently generated Nogo deficient mice. In vitro studies of all three groups concluded that Nogo-deficient myelin has reduced inhibitory effect on neurite outgrowth, indicating that the inhibitory activity of myelin is attributable at least partially to Nogo (Kim et al., 2003; Simonen et al., 2003; Zheng et al., 2003). However, the findings of the in vivo spinal cord injury studies on these different mutants have been less consistent.
One group did not observe enhanced CST sprouting or regeneration in two different Nogo mutants: a Nogo-A,B targeted mutant and a Nogo-A,B,C targeted mutant (Zheng et al., 2003). The Nogo-A,B mutant is a null for Nogo-A and –B, while the Nogo-A,B,C mutant represents a null for Nogo-C and a null or severe hypomorph for Nogo-A,B. The second group focused their analysis on a Nogo-A targeted mutant which inadvertently resulted in the upregulation of Nogo-B (Simonen et al., 2003). In a mixed genetic background, about one quarter of the Nogo-A mutants showed a trend for more regenerating corticospinal axons caudal to the lesion. However, the mutant group as a whole did not exhibit a statistically significant difference from their wild type controls. Axonal sprouting rostral to the lesion had mixed results in the Nogo-A mutant: one measure was elevated in the mutants and another decreased in the mutants with most other measures indistinguishable between mutants and controls (Simonen et al., 2003). A more recent study by the same group indicated that axon regeneration caudal to the injury site in the same mutant, but one that had been bred into a pure genetic background (either 129X1/SvJ or C57BL/6), is statistically significantly enhanced when compared with strain-matched controls (Dimou et al., 2006). This study indicates that 1) the strain background is an important consideration when analyzing mouse mutants of myelin inhibitors; 2) the effect of disrupting Nogo appears to be less robust than one would expect if Nogo serves as the predominant impediment to regeneration. It should be noted, however, that the interpretation of these results has been complicated by the upregulation of Nogo-B in this mutant.
The third group analyzed a gene trap mutant that resulted from a retroviral insertion into a Nogo-A specific exon, and both Nogo-A and Nogo-B transcripts were disrupted in this mutant (Kim et al., 2003). Strikingly, ectopic fibers, sometimes numerous (up to over a thousand axons), were found both rostral and caudal to the injury site in the lateral and ventral white matter in about half of the mutants examined (Kim et al., 2003). The ectopic location of these labeled fibers was initially taken as compelling evidence that they reflect regeneration rather than sparing. However, a recent study has called into question whether these ectopic fibers indeed reflect regeneration. Instead, it appears that such a pattern of ectopic fibers resulted from accidental injection of the neuronal tracer into the cerebral ventricle of the mice (Steward et al., 2007). This ectopic pattern of labeling has no genotype-phenotype correlation such that both wild type, heterozygous and homozygous mutants display this pattern of labeling when injection was performed too deep into the cortex. Thus, further studies are required to resolve the degree of any enhanced axon regeneration in this mutant with a method that will avoid artifactual labeling. Regardless, any axon regeneration in this Nogo-A,B mutant appears to be much more limited than previously thought (Cafferty et al., 2007; Steward et al., 2007). As a whole, axon regeneration in Nogo-deficient mice is much less pronounced and consistent than rodents treated with Nogo neutralizing agents. It might be possible that neutralizing IN-1 or peptide affects more than one inhibitor at a time, or it has other effects in addition to the release of inhibition. Future studies with genetic knockout mice will benefit from the use of inducible knockout mice so that the effect of acute gene deletion can be assessed in order to circumvent any possible compensatory mechanisms in germline knockout mice (Zheng et al., 2006).
MAG
MAG is a transmembrane myelin protein with a large extracellular fragment that contains five immunoglobulin-like domains. MAG is expressed both by CNS oligodendrocytes and PNS Schwann cells, but its level in the CNS is much higher than that in the PNS (Trapp, 1990). The inhibition of MAG on neurite outgrowth in vitro depends on the age of the neuronal culture. While MAG promotes the growth of neurons derived at early stages (embryonic and neonate), it inhibits neurite outgrowth from more mature neurons (McKerracher et al., 1994; Mukhopadhyay et al., 1994; Domeniconi et al., 2002; Hasegawa et al., 2004). For example, MAG is inhibitory on adult DRG neurons while promoting neurite growth from neonatal DRG neurons (Mukhopadhyay et al., 1994). MAG binding to NgR1-expressing cells is GPI-dependent and not sialic acid-dependent. In contrast, MAG binding to an NgR1 homolog, NgR2, is sialic acid-dependent, and NgR2 can also mediate the inhibitory effect of MAG in vitro (Venkatesh et al., 2005). MAG has been extensively used to mimic the effect of CNS myelin on neurite growth (Filbin, 2003). However, there has been a lack of evidence demonstrating MAG’s role as an inhibitor of CNS axon regeneration based on in vivo studies either using MAG mutant mice (Bartsch et al., 1995) or function blocking agents.
OMgp
OMgp is a glycosylphosphotidylinositol (GPI)- anchored protein and contains a series of tandem leucine-rich repeats (LRR). Initially thought to be present in compact myelin, it was later shown that OMgp is expressed in the membrane surrounding the Nodes of Ranvier made by oligodendrocyte-like cells (Huang et al., 2005). Furthermore, most of OMgp expression appears to be in the neurons (Habib et al., 1998). Interestingly, OMgp deficient mice exhibit elevated collateral sprouting from the CNS nodes of Ranvier, suggesting a more general role for OMgp in restricting axonal sprouting in development and physiology (Huang et al., 2005). However, a role for OMgp in adult CNS axon regeneration after injury has not been demonstrated.
NgR1
NgR1 (also known as NgR) was initially identified in a screen for Nogo-66 interacting proteins by Strittmatter and colleagues (Fournier et al., 2001). It was later found that not only Nogo, but the other two prototypical myelin inhibitors of neurite growth, MAG and OMgp, can also bind to NgR1 even though the three inhibitors share no homology (Domeniconi et al., 2002; Liu et al., 2002; Wang et al., 2002b). Expressed at the surface of various neurons, NgR1 is a GPI-linked LRR-containing protein just as OMgp. Since the inhibitory signals from the three myelin inhibitors may converge at NgR1, disrupting NgR1 was expected to be more effective in releasing myelin inhibition and consequently on promoting axon regeneration.
Intrathecal delivery of an NgR1 ectodomain that acts as an NgR1 antagonist by competing with endogenous NgR1 in binding the inhibitory ligands (Nogo, MAG and OMgp) leads to enhanced CST and serotonergic fiber regeneration and sprouting, and improved functional recovery in rats (Li et al., 2004; Li et al., 2005). Similar results were obtained by transgenic expression of the same NgR1 ectodomain in mice (Li et al., 2005). The pattern of axonal sprouting in these two studies appears to differ from that of the study with intrathecal administration of NEP1-40 in rats where ectopic CST fibers in the white matter above and below injury are the hallmark. Nevertheless, these two studies suggest that administrating such a dominant negative NgR1 fragment alone is sufficient to elicit significant spinal axon regrowth. In a study with the optic nerve crush model, Benowitz and colleagues found that viral delivery of a similar dominant negative NgR1 expression construct had no effect on retinal axon regeneration on its own (Fischer et al., 2004). Rather, only when combined with a regeneration-inducing lens injury did dominant negative NgR1 expression lead to several fold increase in axon regeneration (Fischer et al., 2004). This study indicates that NgR1 contributes to the restrictive environment in the CNS but in order to achieve detectable axon regeneration in the CNS other manipulations might be required.
Compared to the dominant negative form of NgR1, loss of function experiments with NgR1 generated much less axon regeneration overall. Two groups independently generated and characterized NgR1 null mice. Both groups failed to observe enhanced axon regeneration in the CST of NgR1 mutants, an unexpected finding given that NgR1 can mediate the inhibitory effect of all three prototypical myelin inhibitors (Kim et al., 2004; Zheng et al., 2005). Interestingly, one group found enhanced regeneration of the raphespinal and rubrospinal fiber tracts and improved motor function after complete transection (Kim et al., 2004). The selective enhancement of regeneration in certain axonal tracts in this NgR1 mutant may reflect differences in the intrinsic regenerative capacity of various axonal populations or a differential response of axonal tracts to NgR1 deletion. The latter possibility, if true, would imply the presence of some cell-type specificity in receiving and interpreting myelin inhibitory signals, which would be in line with the fact that NgR1 is not expressed by all neurons in the adult CNS. In the corticospinal neurons, however, NgR1 is expressed and yet CST axons in NgR1 deficient mice do not exhibit enhanced regeneration. Functional redundancy with other neuronal receptors may therefore be responsible for the continued inhibition of axon regeneration in these mice.
In a growth cone collapse assay, NgR1-deficient postnatal dorsal root ganglion neurons are less sensitive than wild type neurons to solubilized myelin inhibitors (Kim et al., 2004). However, in a neurite outgrowth assay, NgR1 deficient postnatal cerebellar postnatal granule neurons or dorsal root ganglion neurons are as inhibited as wild type neurons by immobilized myelin or Nogo-66 substrate (Zheng et al., 2005). Investigating the differential requirement for NgR1 in mediating the effect of myelin inhibitors in a growth cone collapse vs. neurite outgrowth assay might lead to important insight into the signaling mechanism of myelin inhibition. Nevertheless, the ligand binding domain (LBD) of NgR1 promotes neurite outgrowth independent of the genotype of the neurons, that is, it promotes neurite growth from both wild type and NgR1-deficient neurons (Zheng et al., 2005). This result indicates that exogenous LBD of NgR1 can mask inhibitory epitopes that do not usually signal through endogenous NgR1 and/or other receptors are redundant with NgR1.
Other ligands and ligand binding receptors
There are two sequence homologues of NgR1 in the mammalian genome, NgR2 and NgR3, which are expressed in both embryonic and adult PNS and CNS neurons. Like NgR1, they are GPI-linked proteins that contain LRR domains. One piece of evidence supporting the possibility of other receptors mediating the inhibitory effect of myelin is that neurite outgrowth from NgR1 deficient neurons is not disinhibited when compared with that from wild type neurons in neurite outgrowth assays (Zheng et al., 2005). Indeed, it has been shown that not only NgR1, but at least NgR2, can mediate the inhibitory signal from MAG (Venkatesh et al., 2005). While no biochemical interaction has been identified between NgR3 and the three prototypical myelin inhibitors, the involvement of NgR3 cannot be excluded. It will be interesting to know if mice deficient in more than one NgR family member (e.g. NgR1 and NgR2) will exhibit enhanced CST regeneration. Interestingly, it was recently found that the interacting networks in the Nogo/NgR pathways are potentially more complex than previously thought (Lauren et al., 2007). For instance, OMgp can bind to MAG; Rtn2 and Rtn3, two paralogues of Nogo, can bind to NgR1 with high affinity. The functional significance of such interactions has not been tested with neurite inhibition assays. However, if confirmed, these interactions will significantly increase the complexity of the molecular interacting networks in myelin inhibition of axonal growth.
p75NTR, TROY, LINGO-1
That NgR1 is a GPI-linked protein suggests the existence of a transmembrane protein to transduce the inhibitory signaling upon ligand binding. p75NTR has been shown to interact with NgR1, forming a receptor complex to mediate the inhibitory response of three myelin inhibitors (Wang et al., 2002a; Wong et al., 2002). The inhibition of neurite outgrowth by myelin was blocked in neurons from p75NTR knockout mice and the interference of NgR1-p75NTR interaction reduced neuron’s response to myelin inhibitors (Wang et al., 2002a). Neurons from p75NTR deficient mice also fail to respond to MAG-induced neurite outgrowth inhibition (Yamashita et al., 2002). However, p75NTR-deficient mice do not exhibit enhanced regeneration in the CST or ascending sensory fibers (Song et al., 2004; Zheng et al., 2005).
Since p75NTR is expressed only in a subpopulation of mature CNS neurons, a search for an additional co-receptor led to the identification of TROY (also known as TAJ), an orphan receptor in the TNF family, as a alternative co-receptor that associates with NgR1 and LINGO to mediate the inhibitory cellular response to myelin components in neurons not expressing p75NTR (Park et al., 2005; Shao et al., 2005). The role of TROY in axon regeneration in vivo has not been demonstrated.
Unlike in neurons, in a reconstituted non-neuronal system such as COS-7 cells, NgR1/p75NTR fails to act as a functional receptor complex to transduce the inhibitory response to myelin inhibitors. This suggests that an additional component(s) might participate in the formation of the receptor complex to confer myelin inhibition. A nervous system-specific transmembrane protein LINGO-1 has been identified and found to be this additional functional component. Co-expression of NgR1, p75NTR, and LINGO-1 in COS-7 cells confers inhibitory response to OMgp (Mi et al., 2004). In primary neuronal cultures, dominant negative form of LINGO-1 alleviates the inhibition (Mi et al., 2004). Intrathecal infusion of a soluble form of LINGO that acts as an antagonist was shown to promote CST axon sprouting after dorsal hemisection spinal cord injury in rats, in particular around the area of the ventral CST (Ji et al., 2006). This study suggests that LINGO-1 is involved in axonal plasticity after spinal cord injury. It will be interesting to generate LINGO-1 mutants and to examine their regenerative potential after spinal cord injury, provided that they are viable,.
Taken together, these studies illustrate a scenario where a single inhibitor or receptor is unlikely to explain the general failure of axon regeneration in the CNS. Functional redundancy at the ligand level might contribute to the failure of regeneration in mutant mice where a single inhibitor has been knocked out. The same might be true for the receptors. Whether disrupting multiple inhibitory molecules – either at the ligand or receptor level – will enhance regeneration is a critical question. Specifically, triple knockouts of Nogo, MAG, OMgp, mice deficient in all NgR homologues, as well as mice deficient in both p75NTR and TROY, may provide the clue as to how much myelin inhibition alone accounts for the failure of axon regeneration in the CNS. In addition to the complex molecular interactions within the Nogo/NgR inhibitory pathway (Lauren et al., 2007), a study by Giger and colleagues reinforced the idea of molecular specificity in inhibiting neurite growth (Venkatesh et al., 2007). Therefore, different axonal tracts or neuronal types may differ in their complement of receptors to receive myelin inhibitory signals.
Intracellular signaling
As all three prototypical myelin inhibitors can bind to a common receptor NgR1, they might also activate the same downstream signaling pathways. One of the known intracellular intermediates of the NgR1/p75NTR/LINGO-1 signaling is RhoA, a Rho family of small GTPase that switches between a GDP-bound inactive form and a GTP-bound active form. RhoA activates its downstream effector Rho kinase (Rock) to modify actin cytoskeleton rearrangement. In vitro studies indicate that Y27632, a synthetic ATP competitive antagonist of ROCK, promotes axonal growth from dorsal root ganglia by 5 to 10 fold (Borisoff et al., 2003). In vivo administration of C3 transferase, a RhoA inhibitor, or Y27632 in a protein adhesive at the injury site resulted in an improved locomotion, enhanced long-distance CST fiber regeneration and local sprouting (Dergham et al., 2002).
It has been found that elevated cAMP can overcome inhibition by MAG and myelin (Song et al., 1998; Cai et al., 1999). Moreover, injection of cAMP into the cell body of DRG neurons resulted in the regeneration of injured spinal axons from the cAMP-injected cell bodies (Neumann et al., 2002; Qiu et al., 2002). Subsequent studies by Filbin and colleagues identified target genes and transcription factors in response to increased cAMP, such as Arg1 and CREB, which have been shown to counteract the inhibitory effect of myelin (Cai et al., 2002; Gao et al., 2004).
Other molecules that are involved in myelin inhibitory signaling include PKC and EGFR. Applying pharmacological inhibitors of PKC or expression of its dominant negative form overcomes myelin inhibition in primary neurons. Intrathecal infusion of Gö6976, a PKC inhibitor, stimulated robust regeneration of injured dorsal column axons (Sivasankaran et al., 2004). Both in vitro and in vivo results indicate that PKC is one of the signaling mediators of myelin inhibition. Another study also by He and colleagues concluded that EGFR mediates myelin inhibition (Koprivica et al., 2005). They showed that myelin inhibitors trigger EGFR phosphorylation and in vivo administration of EGFR inhibitors stimulate optic nerve regeneration. Since both PKC and EGFR are calcium-related signaling molecules, it will be interesting to examine the role of Ca2+ influx and other molecules that are affected by Ca2+ change in mediating the inhibitory response to myelin.
It is important to note that many of the downstream effectors for myelin inhibitors are shared with those for CSPGs, a class of glial scar-derived regeneration inhibitors (Silver, this issue). For instance, Rho/ROCK, PKC and EGFR appear to be common mediators of neurite inhibition by both the prototypical myelin inhibitors and the CSPGs (Lehmann et al., 1999; Monnier et al., 2003; Sivasankaran et al., 2004; Koprivica et al., 2005). These observations indicate that such common downstream effectors may offer certain advantages as therapeutic targets.
Physiological function of the Nogo/NgR1 pathway
The study of the Nogo/NgR1 pathway has been mostly focused on the role of these molecules in the event of an injury. Our understanding of their role during development or under physiological conditions is much more limited. However, several studies have started to unravel some interesting aspects of the role of myelin inhibitory molecules in normal development and physiology. Interestingly, Nogo and NgR1 appear to play a role in closing the period of ocular dominance plasticity in mice (McGee et al., 2005). Furthermore, OMgp functions to restrict axonal sprouting from the Nodes of Ranvier as discussed above (Huang et al., 2005). Both of these two functions are in line with a general role in axonal plasticity for these neurite inhibitors in the absence of an injury. Finally, Rapoport and colleagues have proposed that Nogo and other Reticulons play a role in stabilizing highly curved ER membrane tubules (Voeltz et al., 2006). It is not clear how this basic cell biological function relates to the neurite inhibitory function of Nogo. Studies of the normal developmental or physiological roles of the Nogo/NgR1 pathway may provide important clues as to how these molecules might function in restricting neurite inhibition after injury.
Axon guidance molecules: potential new roles for old molecules
Axon guidance molecules play important roles in growth cone navigations during development (Tessier-Lavigne and Goodman, 1996). They serve as attractive or repulsive cues that are sensed and interpreted by receptor complexes on the surface of growth cones, which consequently turn towards appropriate directions. There are four families of classical axon guidance molecules including Netrins, Slits, Semaphorins and Ephrins along with their respective receptors. In addition, Repulsive Guidance Molecule (RGM) and morphogens such as WNTs, Sonic Hedgehog (SHH) and Bone Morphogenetic Proteins (BMPs) can serve as axon guidance cues (Yaron and Zheng, 2007). It has long been speculated that some axon guidance molecules may play a role in the adult nervous system under physiological or pathological conditions where axonal plasticity is involved (Yaron and Zheng, 2007). On the one hand, axon guidance cue/receptor pairs may be established ectopically to coerce axons to grow in preconceived directions or patterns, as discussed by Smith and colleagues in this special issue. On the other hand, since chemorepulsive axon guidance molecules can retract and repel growth cones, they may function as endogenous growth inhibitory factors in the injured CNS, much like the prototypical myelin-derived inhibitors. Recent evidence suggests that this may indeed be the case. A subset of the repulsive axon guidance molecules are expressed in intact and/or injured CNS. Some of these are expressed by the scar tissue such as class 3 Semaphorins (De Winter et al., 2002), while others are expressed by oligodendrocytes, including Ephrin-B3, Sema4D, Sema5A, Netrin-1 and RGM. Here we discuss the potential role of guidance molecules that are expressed by oligodendrocytes after CNS injury.
Ephrin-B3 is a class B Ephrin and can interact with the class B Eph receptors as well as EphA4, a class A Eph receptor. During corticospinal tract development, Ephrin-B3 is concentrated at the spinal cord midline and prevents EphA4-expressing axon collaterals of the corticospinal tract from re-crossing the midline since they have already crossed once at the spinomedullary junction (Davy and Soriano, 2005). CST axons in either Ephrin-B3 or EphA4 mutant mice exhibit bilateral innervation of spinal grey matter due to the loss of the midline barrier (Ephrin-B3) or the receptor to sense the barrier (EphA4) (Dottori et al., 1998; Kullander et al., 2001; Yokoyama et al., 2001). Benson et al. reported the expression of Ephrin-B3 in postnatal myelinating oligodendrocytes in the mouse spinal cord and provided evidence that this myelin component confers sensitivity to postnatal cortical neurons that express EphA4 (Benson et al., 2005). Moreover, in vitro neurite outgrowth studies demonstrated that a significant part of myelin inhibition is mediated by Ephrin-B3, which remarkably equals to the inhibitory activity of Nogo, MAG, and OMgp combined – at least for the postnatal cortical neurons tested (Benson et al., 2005). Interestingly, an independent group reported robust axonal regeneration and improved functional recovery in mice lacking EphA4, the receptor for Ephrin-B3 during CST development (Goldshmit et al., 2004). Furthermore, several potential mechanisms for improved axon regeneration and functional recovery were suggested, including a loss of EphA4-mediated axon growth inhibition, reduced astrogliosis and altered vascular remodeling (Goldshmit et al., 2004; Goldshmit et al., 2006). Taken together, these studies illustrated a new role for developmentally critical axon guidance molecules in axon growth inhibition and other important aspects of CNS injury and repair.
In addition to Ephrin-B3, several other guidance cues are expressed by oligodendrocytes. Sema4D, a transmembrane class 4 Semaphorin, is selectively expressed in the postnatal mouse brain by oligodendrocytes and this expression peaks with myelin formation (Moreau-Fauvarque et al., 2003). Its expression is strongly and transiently upregulated in oligodendrocytes that surround the lesion site after adult CNS injury. In vitro, Sema4D is a potent inhibitor for postnatal DRG and granule cell axons. These results suggest that Sema4D acts as a myelin-based molecule that might participate in the inhibition of CNS axonal regeneration.
Another member of the Semaphorin family, Sema5A, has also been shown to be expressed by oligodendrocytes (Goldberg et al., 2004). In addition, Sema5A induces growth cone collapse and inhibits neurite growth of postnatal retinal ganglion cells (RGCs). Antibodies against Sema5A partially relieves inhibition of RGC axon growth by optic nerve explants, indicating that Sema5A contributes to axon growth inhibition. What is unique about Sema5A is that it is not expressed or enriched in myelin. Therefore, these data suggest that oligodendrocyte lineage cells might contribute to axon inhibition even in the absence of myelin.
Netrin-1 can act either as a chemoattractive or a chemorepulsive guidance cue during development depending on the receptor composition. Its receptor DCC mediates Netrin attraction when presented alone while UNC5H receptors mediate repulsion either alone or in conjunction with DCC. Netrin-1 is constitutively expressed by neurons and myelinating oligodendrocytes in the adult rat spinal cord (Manitt et al., 2001). After spinal cord injury, the persistent expression of Netrin-1 by oligodendrocytes surrounding the lesion and the shift in expression levels in neurons towards the repulsive receptor UNC5H over the attractive receptor DCC suggest a role for Netrin-1 in myelin inhibition (Manitt 2006). As for Sema4D and Sema5A above, no in vivo regeneration study has been reported that investigates whether Netrin-1 indeed inhibits axon regeneration after spinal cord injury.
RGM, or Repulsive Guidance Molecule, is thought to play a role in retinotectal topographic map in the chick (Monnier et al., 2002; Matsunaga et al., 2006). The role of its three mammalian homologues, RMGa, RGMb and RGMc, in axon guidance is less well understood. However, a recent study indicates that RGMa is expressed by oligodendrocytes and CNS myelin in adult rats and its expression is upregulated around the injury site after spinal cord injury (Hata et al., 2006). RGMa inhibits neurite growth from postnatal cerebellar neurons and this inhibition can be relieved by an anti-RGMa antibody. Importantly, intrathecal administration of this neutralizing antibody leads to enhanced CST regeneration/sprouting and improved functional recovery (Hata et al., 2006). These data provided evidence that RGM acts as another myelin-derived inhibitor of axon regeneration.
Taken together, mounting evidence suggests that axon guidance molecules play a role in restricting axon plasticity after adult CNS injury. Guidance molecules that are expressed by oligodendrocytes and CNS myelin may contribute to axon regeneration failure. In addition, the role of guidance molecules is not limited to those that are expressed by oligodendrocytes. A recent study indicates that administration of a small molecule inhibitor of Sema3A, a guidance molecule that is expressed by the fibroblast component of the scar tissue (De Winter et al., 2002), leads to multiple beneficial effects including enhanced regenerative response from axons (Kaneko et al., 2006). Thus, the potential role of axon guidance molecules in restricting axon regeneration after CNS injury is not limited to white matter or myelin (see the review by Smith and colleagues in the current issue).
Concluding remarks
Studies by Schwab and colleagues starting in the 1980’s formulated the concept of myelin inhibitors of axon regeneration. The initial hope was that only one or two axon regeneration inhibitors would be present such that unleashing the regenerative potential of the CNS axons would require the manipulation of only one or two major inhibitors. There is now a growing list of axon growth inhibitors that are produced by oligodendrocytes and present in the myelin, which suggests a high level of functional redundancy in growth inhibition. These molecules inhibit neurite growth in vitro, and for some of these, there is in vivo data supporting a role in restricting axon regeneration. However, their relative contribution to regeneration in vivo remains unknown. Do all neurite inhibitors make equal or even comparable contributions? Do we need to neutralize all these inhibitors in order to achieve significant and consistent axon regeneration? Or, are there dominant players among these inhibitors? In other words, are there only one or two inhibitory molecules that contribute to the majority of the inhibitory influence? Is there any molecular specificity or distinction in regeneration inhibition such that certain inhibitors preferentially affect a subset of axonal pathways (e.g. those that express the receptors for the inhibitors of interest)? Indeed, a recent study suggests that there is a cell type specific mechanism for growth inhibition such that each cell type uses a distinct complement of receptors to mediate growth inhibition (Venkatesh et al., 2007). Finally, how do the myelin-derived neurite growth inhibitors interact with other glia-derived inhibitory factors such as CSPGs in restricting axon regeneration? Are these simply successive roadblocks both of which have to be overcome before successful axon regeneration can occur?
To address these questions, both pharmacological methods and genetic tools provide valuable approaches. While the former offers the possibility of a relatively speedy functional test in vivo and sometimes may even directly lead to useful therapeutic agents, genetic analysis often provides a more rigorous test of gene function. In genetic analysis, an inducible knockout system might be used to overcome any developmental compensation in the germline mutant of a particular inhibitor (Zheng et al., 2006).
A frequent challenge in regeneration research is the dichotomy between in vitro and in vivo phenotypes. A robust in vitro phenotype such as in a neurite outgrowth assay is often not readily translated into a corresponding in vivo phenotype. The relative simplicity of dissociated neuronal culture simply cannot match the complexity of spinal cord injury. In the end, the criterion for a proven role for an inhibitory ligand or receptor lies in the robustness and reproducibility of the in vivo data. The inherent variability in experimental spinal cord injury should be carefully considered. New technologies, such as in vivo imaging of spinal axons with advanced microscopy, will provide much-needed arsenal to examine axon regeneration (Kerschensteiner et al., 2005). Only with a thorough understanding of the role of myelin inhibitors in regeneration failure can we design rational, safe and effective therapeutic intervention to target these molecules to promote recovery and repair in spinal cord injury and related neurological conditions.
Acknowledgments
We thank Jae Lee and Julia Herrmann for helpful comments. Research in the authors’ laboratory is supported by grants from the Roman Reed Spinal Cord Injury Research Fund of California, the Dana Foundation, the International Spinal Research Trust, the California Institute of Regenerative Medicine and NIH/NINDS (NS054734) to B.Z. F.X. is supported by a postdoctoral fellowship from the Christopher and Dana Reeve Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandtlow C, Zachleder T, Schwab ME. Oligodendrocytes arrest neurite growth by contact inhibition. J Neurosci. 1990;10:3837–3848. doi: 10.1523/JNEUROSCI.10-12-03837.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch U, Bandtlow CE, Schnell L, Bartsch S, Spillmann AA, Rubin BP, Hillenbrand R, Montag D, Schwab ME, Schachner M. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron. 1995;15:1375–1381. doi: 10.1016/0896-6273(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci U S A. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. Post-injury myelin-breakdown products inhibit axonal growth: an hypothesis to explain the failure of axonal regeneration in the mammalian central nervous system. Bibl Anat. 1982:1–11. [PubMed] [Google Scholar]
- Borisoff JF, Chan CC, Hiebert GW, Oschipok L, Robertson GS, Zamboni R, Steeves JD, Tetzlaff W. Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci. 2003;22:405–416. doi: 10.1016/s1044-7431(02)00032-5. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- Brosamle C, Huber AB, Fiedler M, Skerra A, Schwab ME. Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J Neurosci. 2000;20:8061–8068. doi: 10.1523/JNEUROSCI.20-21-08061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A, Zagrebelsky M, Huber AB, Skerra A, Schwab ME, Strata P, Rossi F. Application of neutralizing antibodies against NI-35/250 myelin-associated neurite growth inhibitory proteins to the adult rat cerebellum induces sprouting of uninjured purkinje cell axons. J Neurosci. 2000;20:2275–2286. doi: 10.1523/JNEUROSCI.20-06-02275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Kim JE, Lee JK, Strittmatter SM. Response to correspondence: Kim et al., “axon regeneration in young adult mice lacking Nogo-A/B.” Neuron 38, 187–199. Neuron. 2007;54:195–199. doi: 10.1016/j.neuron.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Deng K, Mellado W, Lee J, Ratan RR, Filbin MT. Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth MAG and myelin in vitro. Neuron. 2002;35:711–719. doi: 10.1016/s0896-6273(02)00826-7. [DOI] [PubMed] [Google Scholar]
- Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- Capecchi MR. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat Rev Genet. 2005;6:507–512. doi: 10.1038/nrg1619. [DOI] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988a;1:85–96. doi: 10.1016/0896-6273(88)90212-7. [DOI] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988b;106:1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Davy A, Soriano P. Ephrin signaling in vivo: look both ways. Dev Dyn. 2005;232:1–10. doi: 10.1002/dvdy.20200. [DOI] [PubMed] [Google Scholar]
- De Winter F, Oudega M, Lankhorst AJ, Hamers FP, Blits B, Ruitenberg MJ, Pasterkamp RJ, Gispen WH, Verhaagen J. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp Neurol. 2002;175:61–75. doi: 10.1006/exnr.2002.7884. [DOI] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Schnell L, Montani L, Duncan C, Simonen M, Schneider R, Liebscher T, Gullo M, Schwab ME. Nogo-A-deficient mice reveal strain-dependent differences in axonal regeneration. J Neurosci. 2006;26:5591–5603. doi: 10.1523/JNEUROSCI.1103-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Dottori M, Hartley L, Galea M, Paxinos G, Polizzotto M, Kilpatrick T, Bartlett PF, Murphy M, Kontgen F, Boyd AW. EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proc Natl Acad Sci U S A. 1998;95:13248–13253. doi: 10.1073/pnas.95.22.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Fischer D, He Z, Benowitz LI. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J Neurosci. 2004;24:1646–1651. doi: 10.1523/JNEUROSCI.5119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, Spencer T, Mellado W, Kandel ER, Filbin MT. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44:609–621. doi: 10.1016/j.neuron.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Vargas ME, Wang JT, Mandemakers W, Oster SF, Sretavan DW, Barres BA. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J Neurosci. 2004;24:4989–4999. doi: 10.1523/JNEUROSCI.4390-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, Galea MP, Bartlett PF, Turnley AM. EphA4 regulates central nervous system vascular formation. J Comp Neurol. 2006;497:864–875. doi: 10.1002/cne.21029. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Galea MP, Wise G, Bartlett PF, Turnley AM. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J Neurosci. 2004;24:10064–10073. doi: 10.1523/JNEUROSCI.2981-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPre T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Habib AA, Marton LS, Allwardt B, Gulcher JR, Mikol DD, Hognason T, Chattopadhyay N, Stefansson K. Expression of the oligodendrocyte-myelin glycoprotein by neurons in the mouse central nervous system. J Neurochem. 1998;70:1704–1711. doi: 10.1046/j.1471-4159.1998.70041704.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Fujitani M, Hata K, Tohyama M, Yamagishi S, Yamashita T. Promotion of axon regeneration by myelin-associated glycoprotein and Nogo through divergent signals downstream of Gi/G. J Neurosci. 2004;24:6826–6832. doi: 10.1523/JNEUROSCI.1856-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata K, Fujitani M, Yasuda Y, Doya H, Saito T, Yamagishi S, Mueller BK, Yamashita T. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J Cell Biol. 2006;173:47–58. doi: 10.1083/jcb.200508143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, McKerracher L, Braun PE, David S. A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron. 1999;24:639–647. doi: 10.1016/s0896-6273(00)81118-6. [DOI] [PubMed] [Google Scholar]
- Huang JK, Phillips GR, Roth AD, Pedraza L, Shan W, Belkaid W, Mi S, Fex-Svenningsen A, Florens L, Yates JR, 3rd, Colman DR. Glial membranes at the node of Ranvier prevent neurite outgrowth. Science. 2005;310:1813–1817. doi: 10.1126/science.1118313. [DOI] [PubMed] [Google Scholar]
- Ji B, Li M, Wu WT, Yick LW, Lee X, Shao Z, Wang J, So KF, McCoy JM, Pepinsky RB, et al. LINGO-1 antagonist promotes functional recovery and axonal sprouting after spinal cord injury. Mol Cell Neurosci. 2006;33:311–320. doi: 10.1016/j.mcn.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, Okano HJ, Ikegami T, Moriya A, Konishi O, et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006;12:1380–1389. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- Kim JE, Li S, GrandPre T, Qiu D, Strittmatter SM. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Koprivica V, Cho KS, Park JB, Yiu G, Atwal J, Gore B, Kim JA, Lin E, Tessier-Lavigne M, Chen DF, He Z. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science. 2005;310:106–110. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- Kullander K, Croll SD, Zimmer M, Pan L, McClain J, Hughes V, Zabski S, DeChiara TM, Klein R, Yancopoulos GD, Gale NW. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev. 2001;15:877–888. doi: 10.1101/gad.868901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren J, Hu F, Chin J, Liao J, Airaksinen MS, Strittmatter SM. Characterization of myelin ligand complexes with neuronal Nogo-66 receptor family members. J Biol Chem. 2007;282:5715–5725. doi: 10.1074/jbc.M609797200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, Tigyi G, McKerracher L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kim JE, Budel S, Hampton TG, Strittmatter SM. Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury. Mol Cell Neurosci. 2005;29:26–39. doi: 10.1016/j.mcn.2004.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liu BP, Budel S, Li M, Ji B, Walus L, Li W, Jirik A, Rabacchi S, Choi E, et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebscher T, Schnell L, Schnell D, Scholl J, Schneider R, Gullo M, Fouad K, Mir A, Rausch M, Kindler D, et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- Manitt C, Colicos MA, Thompson KM, Rousselle E, Peterson AC, Kennedy TE. Widespread expression of netrin-1 by neurons and oligodendrocytes in the adult mammalian spinal cord. J Neurosci. 2001;21:3911–3922. doi: 10.1523/JNEUROSCI.21-11-03911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga E, Nakamura H, Chedotal A. Repulsive guidance molecule plays multiple roles in neuronal differentiation and axon guidance. J Neurosci. 2006;26:6082–6088. doi: 10.1523/JNEUROSCI.4556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Merkler D, Metz GA, Raineteau O, Dietz V, Schwab ME, Fouad K. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J Neurosci. 2001;21:3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Monnier PP, Sierra A, Macchi P, Deitinghoff L, Andersen JS, Mann M, Flad M, Hornberger MR, Stahl B, Bonhoeffer F, Mueller BK. RGM is a repulsive guidance molecule for retinal axons. Nature. 2002;419:392–395. doi: 10.1038/nature01041. [DOI] [PubMed] [Google Scholar]
- Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci. 2003;22:319–330. doi: 10.1016/s1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
- Moreau-Fauvarque C, Kumanogoh A, Camand E, Jaillard C, Barbin G, Boquet I, Love C, Jones EY, Kikutani H, Lubetzki C, et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci. 2003;23:9229–9239. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Oertle T, van der Haar ME, Bandtlow CE, Robeva A, Burfeind P, Buss A, Huber AB, Simonen M, Schnell L, Brosamle C, et al. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci. 2003;23:5393–5406. doi: 10.1523/JNEUROSCI.23-13-05393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Yiu G, Kaneko S, Wang J, Chang J, He XL, Garcia KC, He Z. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45:345–351. doi: 10.1016/j.neuron.2004.12.040. [DOI] [PubMed] [Google Scholar]
- Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Raineteau O, Fouad K, Noth P, Thallmair M, Schwab ME. Functional switch between motor tracts in the presence of the mAb IN-1 in the adult rat. Proc Natl Acad Sci U S A. 2001;98:6929–6934. doi: 10.1073/pnas.111165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. Degeneration and Regeneration of the Nervous System. New York: Hafner; 1928. [Google Scholar]
- Richardson PM, McGuinness UM, Aguayo AJ. Peripheral nerve autografts to the rat spinal cord: studies with axonal tracing methods. Brain Res. 1982;237:147–162. doi: 10.1016/0006-8993(82)90563-7. [DOI] [PubMed] [Google Scholar]
- Savio T, Schwab ME. Rat CNS white matter, but not gray matter, is nonpermissive for neuronal cell adhesion and fiber outgrowth. J Neurosci. 1989;9:1126–1133. doi: 10.1523/JNEUROSCI.09-04-01126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savio T, Schwab ME. Lesioned corticospinal tract axons regenerate in myelin-free rat spinal cord. Proc Natl Acad Sci U S A. 1990;87:4130–4133. doi: 10.1073/pnas.87.11.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J Neurosci. 1985;5:2415–2423. doi: 10.1523/JNEUROSCI.05-09-02415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, Allaire N, Thill G, Levesque M, Sah D, McCoy JM, et al. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Simonen M, Pedersen V, Weinmann O, Schnell L, Buss A, Ledermann B, Christ F, Sansig G, van der Putten H, Schwab ME. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38:201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB, Xu XM, He Z. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci. 2004;7:261–268. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Song XY, Zhong JH, Wang X, Zhou XF. Suppression of p75NTR does not promote regeneration of injured spinal cord in mice. J Neurosci. 2004;24:542–546. doi: 10.1523/JNEUROSCI.4281-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillmann AA, Bandtlow CE, Lottspeich F, Keller F, Schwab ME. Identification and characterization of a bovine neurite growth inhibitor (bNI-220) J Biol Chem. 1998;273:19283–19293. doi: 10.1074/jbc.273.30.19283. [DOI] [PubMed] [Google Scholar]
- Steward O, Zheng B, Banos K, Yee KM. Response to: Kim et al., “axon regeneration in young adult mice lacking Nogo-A/B.” Neuron 38, 187–199. Neuron. 2007;54:191–195. doi: 10.1016/j.neuron.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Trapp BD. Myelin-associated glycoprotein. Location and potential functions. Ann N Y Acad Sci. 1990;605:29–43. doi: 10.1111/j.1749-6632.1990.tb42378.x. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Chivatakarn O, Lee H, Joshi PS, Kantor DB, Newman BA, Mage R, Rader C, Giger RJ. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J Neurosci. 2005;25:808–822. doi: 10.1523/JNEUROSCI.4464-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh K, Chivatakarn O, Sheu SS, Giger RJ. Molecular dissection of the myelin-associated glycoprotein receptor complex reveals cell type-specific mechanisms for neurite outgrowth inhibition. J Cell Biol. 2007;177:393–399. doi: 10.1083/jcb.200702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002a;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002b;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Wong ST, Henley JR, Kanning KC, Huang KH, Bothwell M, Poo MM. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat Neurosci. 2002;5:1302–1308. doi: 10.1038/nn975. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Higuchi H, Tohyama M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J Cell Biol. 2002;157:565–570. doi: 10.1083/jcb.200202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaron A, Zheng B. Navigating their way to the clinic: Emerging roles for axon guidance molecules in neurological disorders and injury. Dev Neurobiol. 2007 doi: 10.1002/dneu.20512. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama N, Romero MI, Cowan CA, Galvan P, Helmbacher F, Charnay P, Parada LF, Henkemeyer M. Forward signaling mediated by ephrin-B3 prevents contralateral corticospinal axons from recrossing the spinal cord midline. Neuron. 2001;29:85–97. doi: 10.1016/s0896-6273(01)00182-9. [DOI] [PubMed] [Google Scholar]
- Zheng B, Atwal J, Ho C, Case L, He XL, Garcia KC, Steward O, Tessier-Lavigne M. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]
- Zheng B, Lee JK, Xie F. Genetic mouse models for studying inhibitors of spinal axon regeneration. Trends Neurosci. 2006;29:640–646. doi: 10.1016/j.tins.2006.09.005. [DOI] [PubMed] [Google Scholar]