Abstract
The endovascular perforation rodent model for experimental subarachnoid hemorrhage (SAH) studies is criticized for lack of control over bleeding. Presently, there is no practical grading system to categorize the severity of SAH depending on the amount of blood. We outline a simple and objective novel SAH grading system by examining the subarachnoid blood clots in the basal-cisterns, and evaluate for correlation with neurological status and cerebral vasospasm. Effects of simvastatin, known to reduce vasospasm, were examined using this grading system. Seventy-seven adult male Sprague-Dawley rats were divided randomly into 3 groups: sham-operated (n=24), SAH (n=32), and SAH+simvastatin (n=25). High resolution brain pictures were used to grade the severity of SAH and categorize animals into mild, moderate and severe groups. The SAH grades were compared with neurological scores and internal-carotid artery parameters such as diameter, perimeter and wall-thickness at 24hrs. Two investigators verified the grading system independently. The SAH grade showed linear correlation functionally with neurological status (r=0.42, p<0.01) and morphometrically with the degree of vasospasm (lrl>0.7, p<0.01), and also between two independent investigators (r=0.937, p<0.001). Simvastatin improved neurological score in moderate and severe (p<0.05) but not mild SAH groups (p=0.28). This grading system has the potential to be adopted for SAH experimental rodent models.
Keywords: subarachnoid hemorrhage, experimental rodent model, endovascular perforation, SAH grading, neurological score, cerebral vasospasm, simvastatin
Introduction
The subarachnoid hemorrhage (SAH) rodent model has been routinely employed for studying cerebral vasospasm, early brain injury and cerebral blood flow following SAH. The two major approaches for SAH modeling in rodents are the endovascular perforation model and autologous blood injection model. (Aladag et al., 2006; Bederson et al., 1995; Nikaido et al., 2004; Ostrowski et al., 2006; Prunell et al., 2003) The blood injection model can be used for quantitative experiments because the amount of SAH can be easily controlled. (Prunell et al., 2003) However, it is difficult to make drastic changes in intracranial pressure and cerebral blood flow using the injection model thus making it difficult to simulate the clinical scenario. To overcome this drawback, the endovascular perforation model is commonly used in experimental studies including vasospasm (Cahill et al., 2006; Megyesi et al., 2000; Nikaido et al., 2004; Yan et al., 2006). But the variability and lack of control over bleeding scale using the endovascular perforation approach (Prunell et al., 2003) makes it difficult to compare outcomes between different groups. Presently, there is no simple, practical, and useful SAH grading system to categorize the severity of SAH on the basis of subarachnoid blood.
In the present study we have outlined a simple and objective SAH grading system assessing the subarachnoid bleeding scale in the endovascular perforation model in rats. The practical utility of this grading system was evaluated functionally by correlating with neurological scores and morphometrically by correlating with internal carotid artery diameter. Furthermore, the reproducibility and consistency of this SAH grading system was verified by two different investigators. The sensitivity of this SAH grading was tested by adding a treatment group with simvastatin which has been previously shown to be neuroprotective (Cimino et al., 2005; Johnson-Anuna et al., 2007) and in ameliorating cerebral vasospasm (Lynch et al., 2005; McGirt et al., 2002; McGirt et al., 2006) in this model. We evaluated cerebral vasospasm and neurological deficits and their correlation with the amount of bleeding using this novel SAH grading system in the endovascular perforation rodent model.
Materials and Methods
Experimental Animals and Groups
All experiments were approved by the Institutional Animal Care and Use Committee of Loma Linda University. Eighty one adult male Sprague-Dawley rats (Harlan, Indianapolis, Ind) weighing between 250 and 300g were divided randomly into 3 weight-matched groups: sham-operated (sham n=24), subarachnoid hemorrhage (SAH) treated with 10% ethanol in normal saline as vehicle (SAH n=32), and SAH treated with simvastatin (Calbiochem, CA, USA) (SAH + simvastatin n=25). The neurological status of all animals was evaluated at 24 hrs post surgery using the scoring system elaborated in table 1 before euthanization and brain tissue harvesting. High resolution photographs of all brains were used for SAH grading. At least 6 brains from each group were used for histological analysis.
Induction of SAH
General anesthesia was induced with ketamine (100 mg/kg i.p.) and xylazine hydrochloride (10 mg/kg i.p.) followed by atropine (0.1 mg/kg s.c.). After intubation the animals were ventilated with an animal ventilator (Harvard Apparatus). A heating pad and a heating lamp were used to maintain the rectal temperature at 36.0±0.5°C.
SAH was induced by endovascular perforation of the internal carotid artery (ICA) bifurcation with a sharpened 4-0 nylon suture as previously described with slight modifications. (Bederson et al., 1995; Kusaka et al., 2004) Briefly, after exposing the left common carotid artery (CCA), external carotid artery (ECA), and ICA, the ECA was ligated and fashioned into a stump. The suture was advanced into ICA from the ECA stump through the common carotid bifurcation. The suture was further advanced into the intracranial ICA until resistance was felt (~15 to 18mm from common carotid bifurcation) and then pushed 3 mm further to perforate the ICA wall. Then the suture was withdrawn into the ECA and ICA was reperfused. Operative procedures were same for the sham group, except that the suture was removed once resistance was felt without puncture. The rats were allowed to recover after incision closure and housed individually until euthanization. All animals had free access to food and water.
Drug Administration
Thirty minutes after the procedure, SAH + simvastatin group received an intraperitoneal injection of simvastatin administered at a dose of 20 mg/kg. Sham and SAH groups received vehicle (1.5ml of 10% ethanol in normal saline).
Grading System for SAH
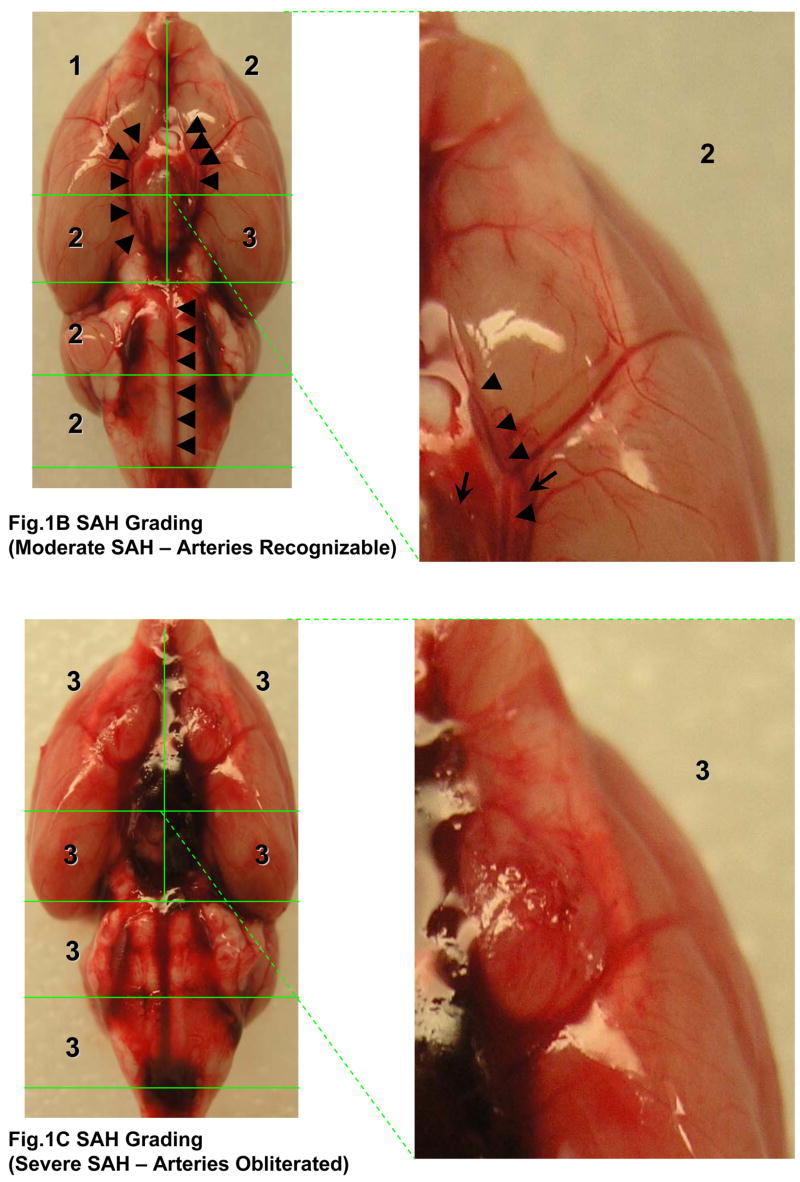
The rats were sacrificed under deep anesthesia at 24 hrs after surgery and the brains were removed rapidly. High resolution pictures of the base of the brain depicting the circle of Willis and basilar arteries were taken. In the photographs, the basal cistern was divided into 6 segments as shown in the Fig 1A. Each segment was allotted a grade from 0–3 depending on the amount of subarachnoid blood clot in the segment as follows: Grade 0: no subarachnoid blood, 1: minimal subarachnoid blood, 2: moderate blood clot with recognizable arteries, 3: blood clot obliterating all arteries within the segment as shown in the Fig 1B, and 1C. We identified and used the arteries within the basal cistern for this grading system: basilar artery (BA) and the circle of Willis composed of the anterior cerebral artery (ACA), the ICA, the proximal posterior cerebral artery (PCA) from ICA to posterior communicating artery (PcomA), and the PcomA. Middle cerebral artery (MCA), distal PCA and the superior cerebellar artery (SCA) were not included in the grading system. Only the arteries of the basal cistern were included for grading system to maintain reproducibility and consistency.
Fig 1.
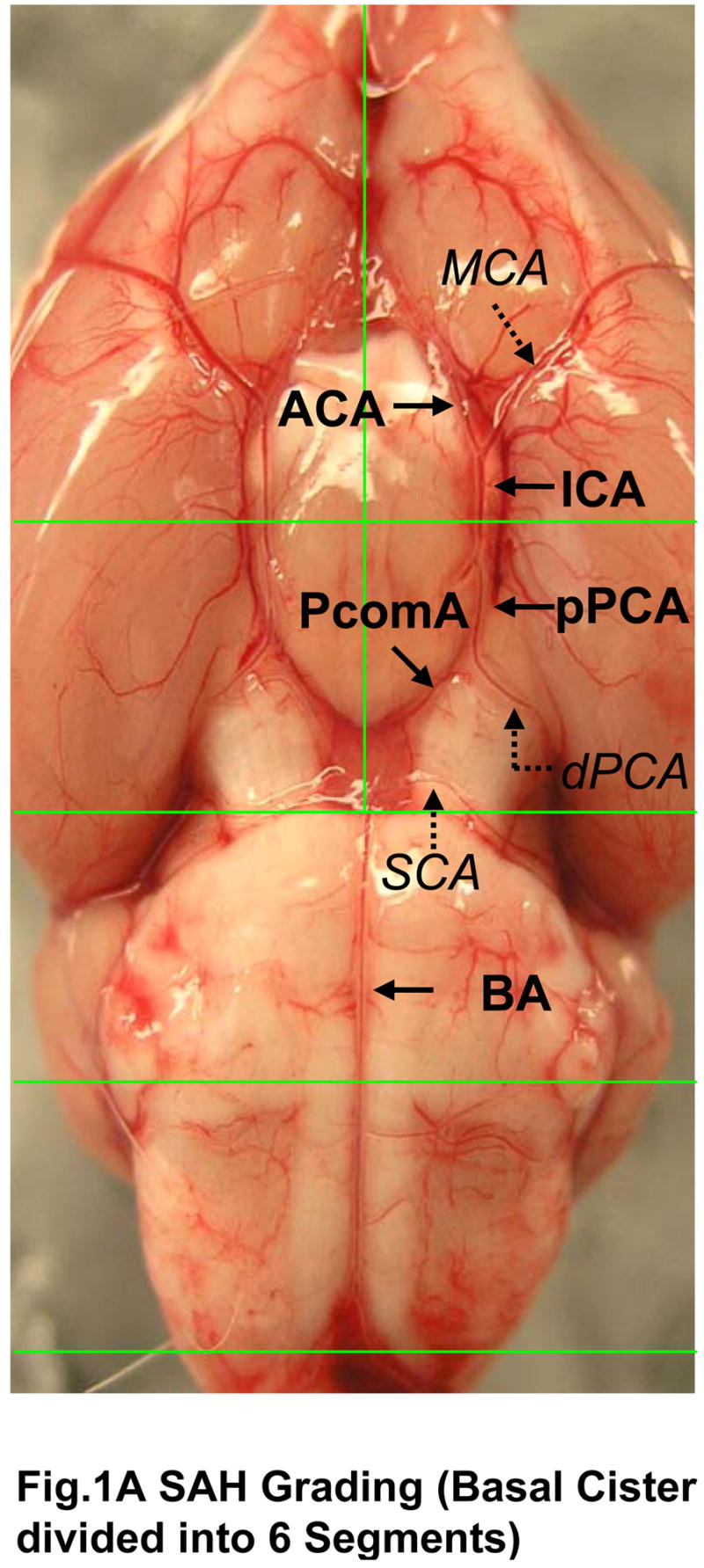
Photographs depicting SAH Grading. Fig 1A shows the division of the basal cistern into 6 segments. The subarachnoid blood clots are assessed in each of these segments to allot a score from 0–3 further explained in table 1. The arteries we identified in the basal cistern are as follows: the BA and circle of Willis composed of ACA, ICA, proximal PCA (from ICA to PcomA), and PcomA (indicated by black arrows). The MCA, distal PCA, and SCA (italicized and indicated by dotted arrows) were not used for the grading system. Fig 1B shows a representative moderate SAH. The arteries can be recognized in the magnified segment (marked by arrowheads). Arrows indicate the subarachnoid blood clots. The arteries are completely obliterated by the blood clot in Fig 1C.
ACA; anterior cerebral artery, BA; basilar artery, ICA; internal carotid artery, MCA; middle cerebral artery, PCA; posterior cerebral artery, PcomA; posterior communicating artery, SCA; superior cerebellar artery.
The animals received a total score ranging from 0 – 18 after adding the scores from all 6 segments. Depending on the final score the animals were divided into three groups as follows 0–7: mild SAH, 8–12: moderate SAH, and 13–18: severe SAH (Fig 2). This grading was done by a blinded observer. Sham operated animals did not have any hemorrhage (blood clots) and thus received a total score of 0.
Fig. 2.
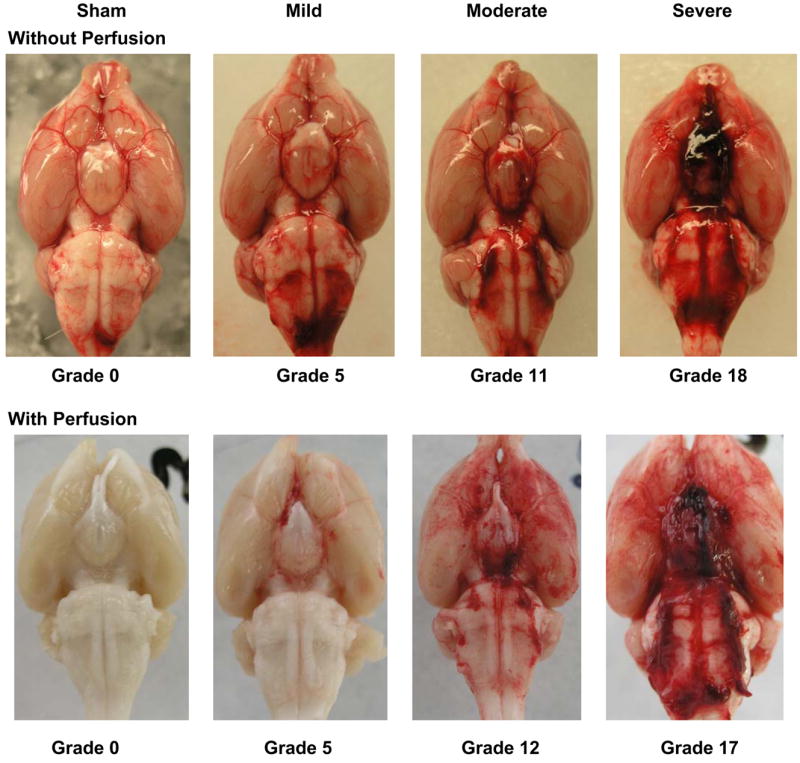
Degree of SAH Severity. Figure shows representative pictures of sham, mild, moderate and severe SAH groups with and without perfusion.
Neurological Scoring
Neurological scores were evaluated at 24 hours with a modification of scoring system reported by Garcia et al (Garcia et al., 1995) in a blinded fashion. An 18-point scoring system was used to evaluate the sensorimotor deficits as elaborated in the table I.
Table I. Neurological Evaluation after SAH.
Neurological Evaluation. The table shows the neurological scoring adopted to evaluate the neurological status of animals at 24 hours post-SAH. The maximal obtainable score is 18.
| Test | Score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Spontaneous Activity (in cage for 5 min) | No movement | Barely moves position | Moves but does not approach at least three sides of cage | Moves and approaches at least three sides of cage |
| Spontaneous movements of all limbs | No movement | Slight movement of limbs | Moves all limbs but slowly | Move all limbs same as pre-SAH |
| Movements of forelimbs (outstretching while held by tail) | No outreaching | Slight outreaching | Outreach is limited and less than pre-SAH | Outreach same as pre-SAH |
| Climbing wall of wire cage | Fails to climb | Climbs weakly | Normal climbing | |
| Reaction to touch on both side of trunk | No response | Weak response | Normal response | |
| Response to vibrissae touch | No response | Weak response | Normal response | |
Assessment of Cerebral Vasospasm
Cerebral vasospasm occurs 24 hours and lasts upto 72 hours after SAH in rat perforation model as previously shown (Cahill et al., 2006). Thus we examined cerebral vasospasm at 24 hours post SAH. Histological parameters of the ipsilateral ICA such as diameter, perimeter and wall thickness were used to assess cerebral vasospasm. Ipsilateral IC is in close vicinity to the puncture point as well as most likely to be surrounded by blood clots. At least 6 animals from each group were used for assessment. At 24 hours after surgery, under deep anesthesia the rats were transcardially perfused with 0.1 mol/L PBS (pH 7.4) followed by fixation with 10% formalin as described previously with the pressure of 60–80mmHg for 15 minutes. (Parra et al., 2002) The whole brains were quickly exenterated and postfixed in 10% formalin followed by 30% sucrose (w/v) for 3 days. Ten-micron-thick coronal sections cut by a cryostat (Leica Microsystems LM3050S) were mounted on poly-L-lysine-coated slides (Richard Allen, Kalamazoo, MI). These sections were stained with hematoxylin and eosin (H&E). We measured the major and minor axis, perimeter and wall thickness of ipsilateral intracranial ICA using Image J freeware from the National Institutes of Health, MD. The diameter of intracranial ICA is defined by taking average of major and minor axes.
Correlation between the SAH Grade and the Neurological Score
The SAH grade and the neurological score were compared by simple regression analysis and Spearman’s correlation coefficient by rank test in order to determine if this SAH grading system can be used for assessing the degree of SAH severity. We also compared the neurological score between SAH and SAH+simvastatin group using the grading system explained before in all three groups; mild, moderate, and severe SAH.
Variability between Investigators of this SAH Grading System
The SAH grades were determined by two different investigators to avoid bias as well as examine reproducibility of this grading system. All correlations were performed by simple regression analysis and Spearman’s correlation coefficient by rank test.
Correlation between the SAH Grade and Vasospasm
The SAH grade and these ICA diameters, perimeters and wall thicknesses were analyzed by simple regression analysis and Spearman’s correlation coefficient by rank test in order to determine if this SAH grade can be used to predict the degree of vasospasm.
Statistical Analysis
Simple regression analysis and Spearman’s correlation coefficient by rank test were performed to assess the correlation between the SAH grade and vasospasm, the SAH grade and neurological score, and between the SAH grades of each investigator. The data are expressed as mean±SEM. Statistical differences between the various groups were assessed with a 1-way ANOVA with Holm-Sidak posthoc analysis. Unpaired t test was used for comparisons between 2 groups. A value of P<0.05 was considered statistically significant. A value of IrI>0.4 was considered significant correlation and lrl>0.7 was considered strong correlation.
Results
Mortality
The mortality in this study is 0% in sham group, 15.6% in SAH group and 4.0% in SAH+simvastatin group at 24 hrs. Further analysis showed that the mortality is 0% in mild SAH group, 11% in moderate SAH group, and 23.5% in severe SAH group. Thus, if we consider severe SAH group, the mortality is comparable with previous reports (Cahill et al., 2006). Rats which did not survive up to 24 hrs postoperatively were not included for the grading system, neurological and morphological assessment.
Neurological Deficits
Significant neurological deficits were seen in moderate and severe SAH groups as compared to sham group at 24 hrs after SAH. The neurological scores in these groups were significantly improved with simvastatin treatment (Table II). Mild SAH group, however, did not show any neurological deficits as compared to sham group.
Table II. Neurological Score and Vasospasm.
Neurological Score and Vasospasm Histological Parameters. The table shows mean±SEM of each individual group. Moderate and severe SAH groups showed significant neurological deficits which were significantly improved by simvastatin treatment. However mild SAH group did not show any significant neurological deficits. Simvastatin treatment improves all histological parameters.
| Neurological Score | Lt IC Diameter (μm) | Lt IC Perimeter (μm) | Lt IC wall thickness (μm) | ||||
|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Overall | ||||
| Sham | 18.0±0.0 | 18.0±0.0 | 18.0±0.0 | 18.0±0.0 | 259.7±10.6 | 865.4±39.5 | 9.5±0.5 |
| SAH + Vehicle | 17.1±0.4 | 15.3±0.7 # | 13.3±0.9 # | 14.8±0.5 | 214.8±10.8 ## | 688.9±31.8 ## | 26.1±2.8 ## |
| SAH + Simvastatin | 16.5±1.4 | 17.2±0.3* | 16.0±0.5 * | 16.5±0.2* | 267.4±8.0** | 882.4±30.0** | 11.8±1.5** |
indicate p<0.05 vs Sham and SAH+vehicle respectively;
represent p<0.01 vs Sham and SAH+vehicle respectively.
Quantification of Vasospasm
All three histological parameters namely diameter, perimeter and thickness of ipsilateral IC in SAH+vehicle group were significantly different from the sham group. The diameter and perimeter were attenuated whereas the thickness was increased in the SAH+vehicle group as compared with the sham group. Simvastatin treatment significantly reversed all these changes (Table II).
Correlation between the SAH Grade and the Neurological Score
There is a correlation between the SAH grade and neurological score (r=0.42, p<0.01) (Fig 3A). There is a significant difference between SAH and SAH+simvastatin group in overall SAH grade (14.8±3.0 vs 16.5±1.3, p<0.05). On dividing the groups depending on the severity of SAH, there is significant difference between the simvastatin-untreated and treated groups in moderate (15.3±1.9 vs 17.2±1.0, p<0.05) and severe (13.3±3.429 vs 16.0±1.5, p<0.05) SAH, however not in mild SAH (17.1±0.40 vs 16.5±1.0, p=0.282) (Fig 3B).
Fig. 3.
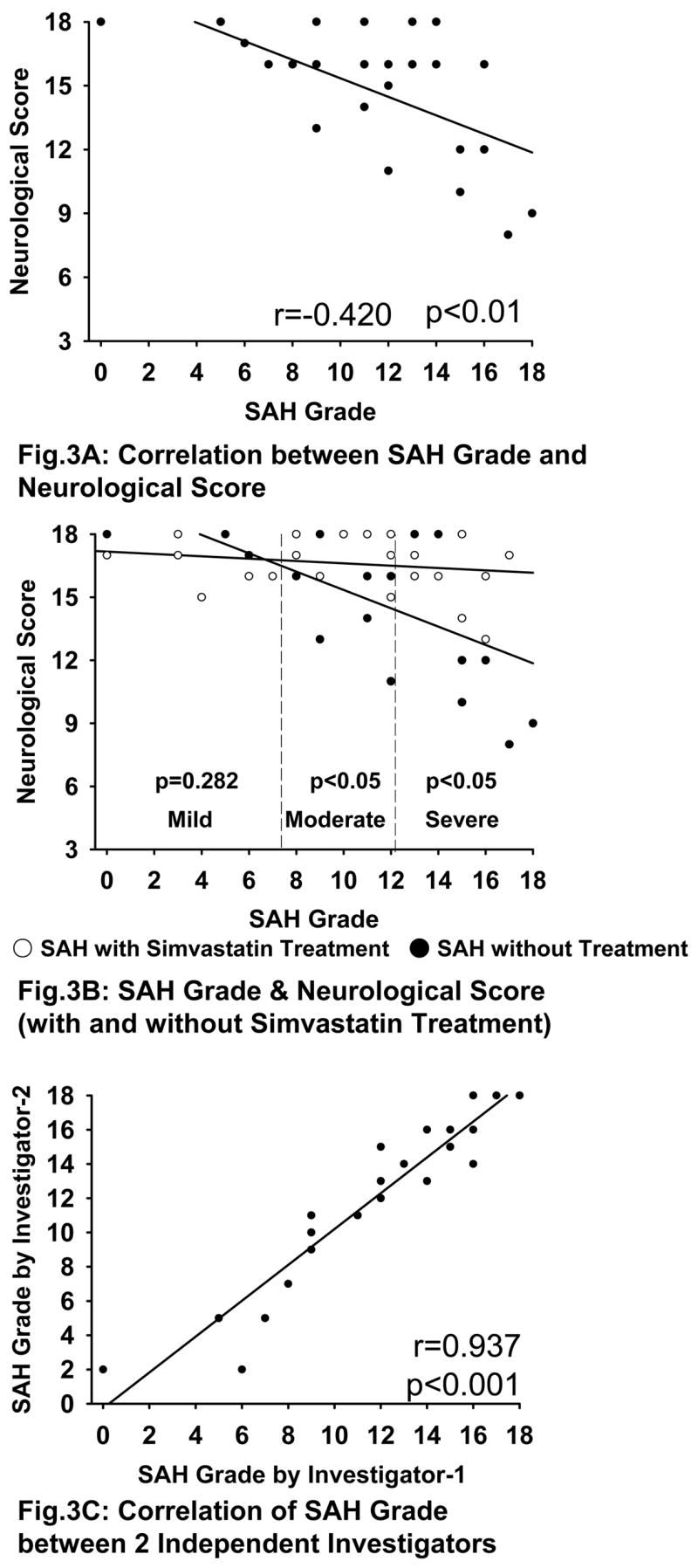
Correlation of SAH Grade with Neurological Score and between independent investigators. Fig 3A shows that there is a significant correlation between SAH grade and neurological score (r=0.42, p<0.01). Fig 3B shows a significant difference between SAH+vehicle and SAH+simvastatin groups in over all SAH grade (p<0.05). There is also significant difference between these groups in moderate (p<0.05) and severe (p<0.05) SAH but not mild SAH (p=0.282). Fig 3C shows a significantly strong linear correlation for SAH grading between two independent investigators (r=0.937, p<0.001).
Variability between Investigators for this SAH Grading System
This SAH grading system is consistent and correlates well between two different investigators (r=0.937, p<0.001) (Fig 3C).
Correlation between the SAH Grade and Vasospasm
There is a strong correlation between the SAH grade and intracranial IC diameter (r<−0.789, p<0.01), perimeter (r<−0.86, p<0.01) and wall thickness (r>0.901, p<0.01) (Fig 4A–C). Significant difference is noted between SAH and SAH+simvastatin group in all of diameter (203.2±25.4 vs 267.4±19.6μm, p<0.01), perimeter (652.7±71.0 vs 882.4±73.5μm, p<0.01) and wall thickness (29.5±5.95 vs 11.87±3.83μm, p<0.01) (Fig 5A–C).
Fig. 4.
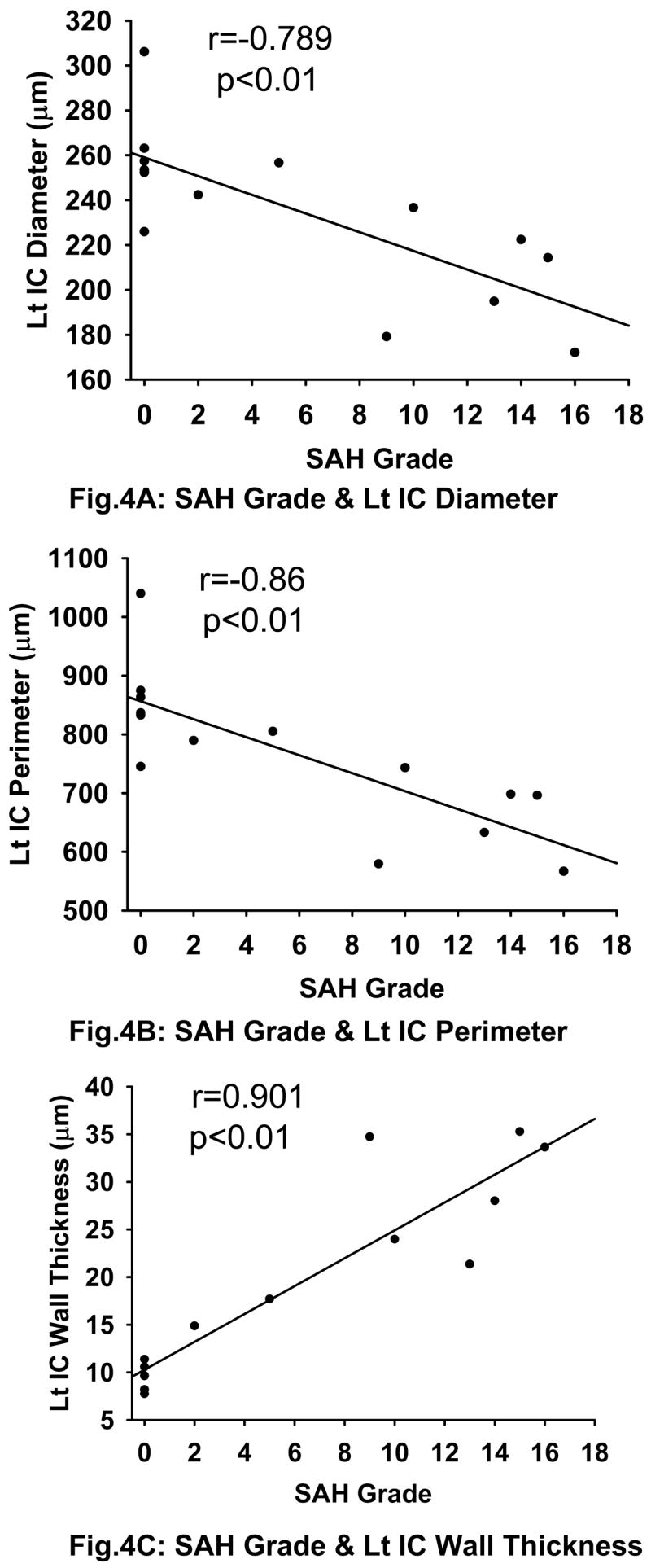
Correlation between SAH Grade and Cerebral Vasospasm. There is a strong linear correlation between the SAH grade and intracranial IC diameter (Fig 4A) (r=−0.789, p<0.01), perimeter (Fig 4B) (r=−0.860, p<0.01) and wall thickness (Fig 4C) (r=0.901, p<0.01). All the three figures also include data from the sham group which is clustered at grade 0 on the x-axis as expected. Lt IC indicates the left / ipsilateral internal carotid artery.
Fig. 5.
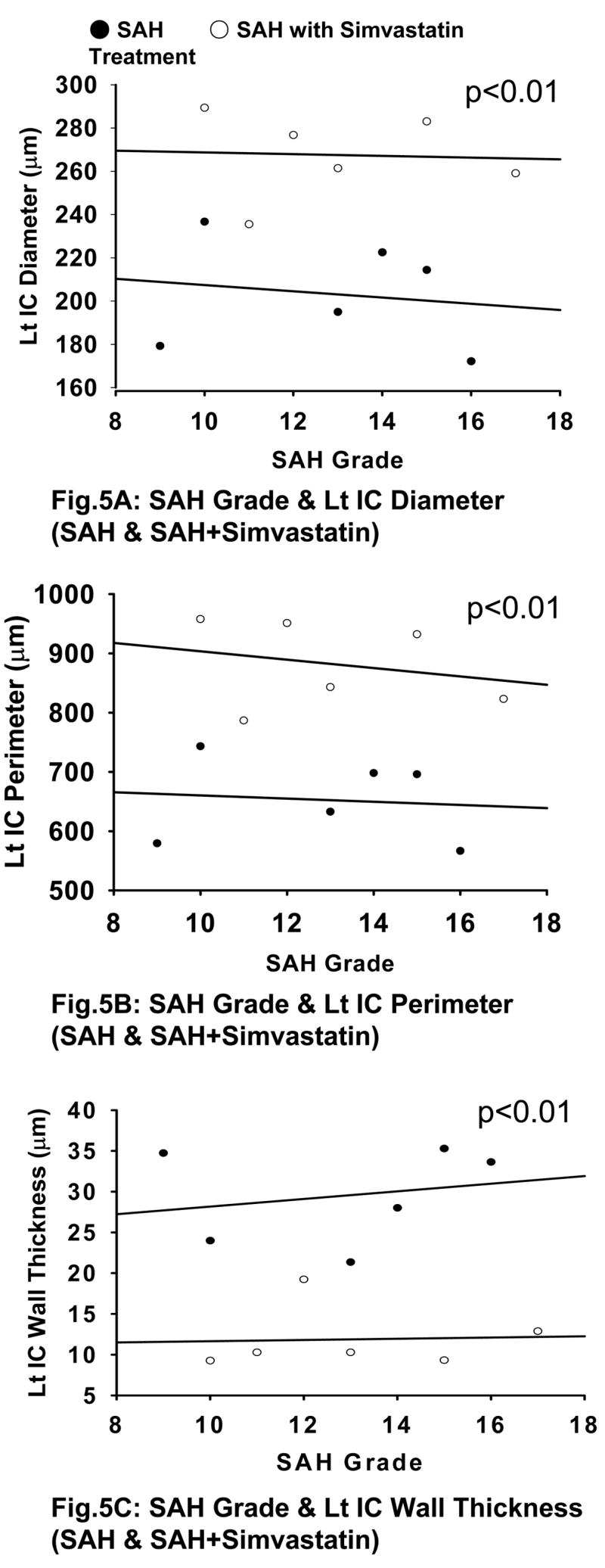
SAH Grade and Cerebral Vasospasm (with and without simvastatin treatment). There is a significant difference between SAH and SAH+simvastatin groups in IC diameter (Fig 5A), IC perimeter (Fig 5B), and IC wall thickness (Fig 5C) (p<0.01). Lt IC indicates the left / ipsilateral internal carotid artery.
Discussion
The present study introduces a novel grading system for experimental SAH using the endovascular perforation model in rodents. The new SAH grading system is consistent between different investigators and shows strong functional correlation with neurological score and morphological correlation with cerebral vasospasm. This SAH grading system is practical and easy to use in experimental models.
The SAH grading system allows animals to be categorized by severity of bleeding. The extent of subarachnoid blood clots correlates strongly with neurological outcomes. Thus, in experimental SAH studies various treatments can be evaluated between comparable groups. This will enhance the confidence that the results are an accurate reflection of the treatments and limit the variability.
The present SAH grading system is straightforward, reproducible, and consistent. Pictures preclude the need for time consuming or tedious procedures such as hematocrit and blood clot volume analysis or use of microscopes, magnifiers, and other measuring tools. Brain samples can be used for morphology or molecular biology because the measurements will be done on the photos without damage the samples. This SAH grading system is convenient because it has the potential to be adopted as a grading tool for experimental rodent models.
The results obtained from the simvastatin effects on neurological deficit show that treatment is effective in overall SAH grade. However, further analysis showed that simvastatin treatment is effective only in moderate and severe SAH groups but not in the mild SAH group. Furthermore, there is no significant difference between sham and both treated and untreated mild SAH groups. These results suggest that mild SAH may not be able to produce sufficient brain and vascular damage in experimental in-vivo models. Future studies may require more caution when comparing mild SAH groups to assess real drug efficacy.
Presently, the endovascular perforation model is commonly used only in rats and mice. However, intracranial arterial perforation models have been used in the past in rabbits, cats and also primates (Megyesi et al., 2000). This grading system can be adopted for all perforation models wherein there is little control over the bleeding after arterial perforation. Our goal is to extend this grading system to other perforation models of SAH as well.
Summary
We report a novel, simple and consistent grading system for evaluating SAH bleeding scale in endovascular perforation rodent model. The grading system showed strong correlation with neurological status and degree of cerebral vasospasm. This grading system has the potential to be adopted as a grading system for SAH experimental rodent models.
Acknowledgments
This study was partially supported by grants from NIH NS45694, NS43338, and NS53407 to JHZ.
Abbreviations
- ACA
anterior cerebral artery
- BA
basilar artery
- CCA
common carotid artery
- ECA
external carotid artery
- ICA
internal carotid artery
- Lt IC
left internal carotid
- MCA
middle cerebral artery
- PBS
phosphate buffered saline
- PCA
posterior cerebral artery
- PcomA
posterior communicating artery
- SAH
subarachnoid hemorrhage
- SCA
superior cerebellar artery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Takashi Sugawara: None
Robert Ayer: None
Vikram Jadhav: None
John H. Zhang: Research Grants from NIH NS45694, NS43338, NS53407
Reference List
- 1.Aladag MA, Turkoz Y, Ozcan C, Sahna E, Parlakpinar H, Akpolat N, et al. Caffeic acid phenethyl ester (CAPE) attenuates cerebral vasospasm after experimental subarachnoidal haemorrhage by increasing brain nitric oxide levels. Int J Dev Neurosci. 2006;24:9–14. doi: 10.1016/j.ijdevneu.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26:1086–1091. doi: 10.1161/01.str.26.6.1086. [DOI] [PubMed] [Google Scholar]
- 3.Cahill J, Calvert JW, Solaroglu I, Zhang JH. Vasospasm and p53-induced apoptosis in an experimental model of subarachnoid hemorrhage. Stroke. 2006;37:1868–1874. doi: 10.1161/01.STR.0000226995.27230.96. [DOI] [PubMed] [Google Scholar]
- 4.Cimino M, Balduini W, Carloni S, Gelosa P, Guerrini U, Tremoli E, et al. Neuroprotective effect of simvastatin in stroke: a comparison between adult and neonatal rat models of cerebral ischemia. Neurotoxicology. 2005;26:929–933. doi: 10.1016/j.neuro.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 6.Johnson-Anuna LN, Eckert GP, Franke C, Igbavboa U, Muller WE, Wood WG. Simvastatin protects neurons from cytotoxicity by up-regulating Bcl-2 mRNA and protein. J Neurochem. 2007;101:77–86. doi: 10.1111/j.1471-4159.2006.04375.x. [DOI] [PubMed] [Google Scholar]
- 7.Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH. Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:916–925. doi: 10.1097/01.WCB.0000125886.48838.7E. [DOI] [PubMed] [Google Scholar]
- 8.Lynch JR, Wang H, McGirt MJ, Floyd J, Friedman AH, Coon AL, et al. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: results of a pilot randomized clinical trial. Stroke. 2005;36:2024–2026. doi: 10.1161/01.STR.0000177879.11607.10. [DOI] [PubMed] [Google Scholar]
- 9.McGirt MJ, Lynch JR, Parra A, Sheng H, Pearlstein RD, Laskowitz DT, et al. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke. 2002;33:2950–2956. doi: 10.1161/01.str.0000038986.68044.39. [DOI] [PubMed] [Google Scholar]
- 10.McGirt MJ, Pradilla G, Legnani FG, Thai QA, Recinos PF, Tamargo RJ, et al. Systemic administration of simvastatin after the onset of experimental subarachnoid hemorrhage attenuates cerebral vasospasm. Neurosurgery. 2006;58:945–951. doi: 10.1227/01.NEU.0000210262.67628.7E. [DOI] [PubMed] [Google Scholar]
- 11.Megyesi JF, Vollrath B, Cook DA, Findlay JM. In vivo animal models of cerebral vasospasm: a review. Neurosurgery. 2000;46:448–460. [PubMed] [Google Scholar]
- 12.Nikaido H, Tsunoda H, Nishimura Y, Kirino T, Tanaka T. Potential role for heat shock protein 72 in antagonizing cerebral vasospasm after rat subarachnoid hemorrhage. Circulation. 2004;110:1839–1846. doi: 10.1161/01.CIR.0000142615.88444.31. [DOI] [PubMed] [Google Scholar]
- 13.Ostrowski RP, Tang J, Zhang JH. Hyperbaric oxygen suppresses NADPH oxidase in a rat subarachnoid hemorrhage model. Stroke. 2006;37:1314–1318. doi: 10.1161/01.STR.0000217310.88450.c3. [DOI] [PubMed] [Google Scholar]
- 14.Parra A, McGirt MJ, Sheng H, Laskowitz DT, Pearlstein RD, Warner DS. Mouse model of subarachnoid hemorrhage associated cerebral vasospasm: methodological analysis. Neurol Res. 2002;24:510–516. doi: 10.1179/016164102101200276. [DOI] [PubMed] [Google Scholar]
- 15.Prunell GF, Mathiesen T, Diemer NH, Svendgaard NA. Experimental subarachnoid hemorrhage: subarachnoid blood volume, mortality rate, neuronal death, cerebral blood flow, and perfusion pressure in three different rat models. Neurosurgery. 2003;52:165–175. doi: 10.1097/00006123-200301000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Yan J, Chen C, Lei J, Yang L, Wang K, Liu J, et al. 2-methoxyestradiol reduces cerebral vasospasm after 48 hours of experimental subarachnoid hemorrhage in rats. Exp Neurol. 2006;202:348–356. doi: 10.1016/j.expneurol.2006.06.009. [DOI] [PubMed] [Google Scholar]