Abstract
Evidence from human and animal studies support the hypothesis that psychological stress can be a co-factor for the initiation and progression of cancer. Recent work from our laboratory and others have shown that the catecholamine hormone, norepinephrine (NE), may influence tumor progression of some solid epithelial tumors including nasopharyngeal carcinoma (NPC) and ovarian cancer by modulating the expression of proangiogenic and pro-metastatic factors, such as vascular endothelial growth factor (VEGF). In this study, we determined whether NE can likewise modulate the expression of VEGF in a lymphoid tumor, multiple myeloma (MM), a cancer of plasma cells. Three MM-derived cell lines, NIH-H929, MM-M1, and FLAM-76, were studied. The presence of β1- and β2-adrenergic receptors (ARs) was assessed using Western blotting. Cells were treated with 0, 1, and 10 μM NE for 1, 3, 6, and 24 hours and the levels of VEGF in culture supernatants were measured by ELISA. Immunoblots of cell lysates revealed the presence of β1- and β2-ARs in all three MM-derived cell lines. However, these MM-derived cell lines exhibited varying degrees of NE-dependent regulation of VEGF expression with FLAM-76 (the only IL-6-dependent cell line among the three) exhibiting the most significant stimulation, followed by MM-M1 cells and then NIH-H929. The data suggest that the ability of NE to regulate the expression of VEGF is not limited to solid epithelial tumors and suggests a possible regulatory role of catecholamine stress hormones in MM progression.
Keywords: vascular endothelial growth factor, multiple myeloma, angiogenesis, norepinephrine, psychological stress
1. Introduction
There is evidence that psychological factors can affect the incidence and progression of some cancers (Kiecolt-Glaser and Glaser, 1999; Lewis et al., 2002; Reiche et al., 2004). The hypothesis that stress could be a co-factor is supported by data obtained from animal models (Riley, 1975; Saul et al., 2005). Studies in the field of psychoneuroimmunology (PNI) have shown that psychological stress can induce endocrine-mediated dysregulation of many aspects of cellular immune function (including the inhibition of T-cell responses to tumor-associated antigens on tumor cells of immunogenic tumors); these interactions have been shown to be bidirectional (Ader, 2007; Padgett and Glaser, 2003; Rabin, 1999).
There is now accumulating evidence to suggest that stress can also have effects that may contribute to tumor progression independent of its effects on the immune system (Lutgendorf et al., 2003; Lutgendorf et al., 2002; Sood et al., 2006; Yang et al., 2006). Our work and that of others have shown that matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF) are factors that can be modulated by catecholamine hormones. For example, work by Sood, Lutgendorf and others have shown that norepinephrine (NE) and epinephrine (E) may influence the progression of ovarian cancer by modulating the expression of MMPs and the pro-angiogenic cytokine, VEGF, in ovarian cancer cells (Lutgendorf et al., 2003; Lutgendorf et al., 2002; Sood et al., 2006). The ability of NE to promote progression of other cancers is supported by our recent work showing that NE enhanced the invasive and pro-angiogenic properties of nasopharyngeal carcinoma (NPC) cells by stimulating the secretion of VEGF, MMP-2, and MMP-9 (Yang et al., 2006). These observations support the hypothesis that stress-associated activation of the sympathetic-adrenal medullary (SAM) axis can, in part, promote tumorigenesis by modulating the expression of pro-angiogenic and pro-metastatic factors. As these studies were performed with solid epithelial tumors, we wanted to determine if other tumor cell types distinctly different from carcinomas expressed β-ARs and how they would respond to NE; we chose the blood cell tumor multiple myeloma (MM).
Multiple myeloma is a systemic disease of plasma cells, comprising 10% of all hematological cancers and is characterized by the abundance of abnormal plasma cells in the bone marrow. Symptoms exhibited by patients with MM include hypercalcemia, anemia, and impaired production of normal immunoglobulin as well as osteoporosis in the pelvis, spine, ribs, and skull. It is an incurable disease with a median survival of about 3–4 years with conventional chemotherapy. The American Cancer Society estimates that about 19,900 new cases of MM (10,960 in men and 8,940 in women) will be diagnosed during 2007 with about 10,790 Americans (5,550 men and 5,240 women) expected to die of this disease in 2007 (American Cancer Society, 2007).
As in other cancers, angiogenesis has been implicated in the pathogenesis and progression of MM (Jakob et al., 2006). For example, evidence suggest that increased angiogenesis in bone marrow of MM patients is a prognostic marker for disease progression (reviewed in (Hanamura et al., 2001)). VEGF has been shown to be an important factor for the angiogenic process during MM progression (Podar and Anderson, 2005). Therefore, the goal of this study was to examine the regulatory role of the stress hormone NE on the expression of VEGF in human MM-derived cell lines.
2. Materials and Methods
2.1 Cell Lines and Culture Conditions
The established human multiple myeloma cell lines, NIH-H929, MM-M1 and FLAM-76 were used in the study. NIH-H929 is a differentiated, highly secretory human plasma cell line established from a malignant effusion in a patient with IgAk myeloma (Gazdar et al., 1986). MM-M1 was established from a patient in the terminal stage of multiple myeloma (Suzuki et al., 1991). FLAM-76 was established from a patient with aggressive nonsecretory plasma cell leukemia; of the three cell lines this cell line does not produce IL-6, and is dependent for growth on exogenous IL-6 (Kubonishi et al., 1992). The NIH-H929, MM-M1and FLAM-76 cells were kindly provided by Dr. Michael Kuehl, Center for Cancer Research, NCI. Cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, L-glutamine, and 1X antibiotic-antimycotic (Invitrogen Life Technologies, Carlsbad, CA) at 37°C in a humidified atmosphere with 5% CO2. Culture medium for FLAM-76 cells was further supplemented with 2 ng/ml recombinant human IL-6 (PeproTech Inc., Rocky Hill, NJ). Cells were seeded in 10 ml round-bottom polypropylene tubes, with 1 × 106 cells/tube, cultured for 1 day in serum-free media, and treated with NE (Sigma-Aldrich Co., St. Louis, MO) at 0, 1, and 10 μM. To assess the effects NE on levels of VEGF, culture supernatants were removed after 1, 3, 6, and 24 hours, centrifuged, and frozen at −80°C until assayed.
2.2 Determination of VEGF Protein Concentration in Culture Supernatants
The concentration of VEGF was measured using Human VEGF Quantikine ELISA Kits (R&D Systems, Minneapolis, MN) following the manufacturer’s protocol. These assays employ the quantitative sandwich enzyme immunoassay technique. The resultant color was read at 450 nm using a Labsystems Multiskan MCC/340 plate reader. The concentrations of VEGF in a sample were determined by interpolation from a standard curve. The minimum detectable level for VEGF was < 5.0 pg/ml. The experiments were performed in duplicate and performed at least twice.
2.3 Analysis of β-adrenergic receptor expression by Western blotting
The expression of β-ARs in MM-derived cells was assessed using Western blotting. The Western blot analysis of β-AR expression was performed as previously described (Yang et al., 2006). Briefly, cell lysates were prepared from 5 × 106 cells in 200 μl buffer containing 100 mM Tris-HCl, pH 7.6; 0.5% Triton X-100, 2 mM PMSF, and 1 μl of Protease Inhibitor Cocktail. PMSF and Protease Inhibitor Cocktail were purchased from Sigma-Aldrich Corp. (St. Louis, MO; Cat. nos. 78830 and P8340, respectively). Proteins were electrophoretically separated in 10% polyacrylamide gels, transferred to Hybond-ECL membranes (Amersham Biosciences Corp., Piscataway, NJ), and probed with 1.0 μg/ml of the V-19 rabbit anti-β1-AR polyclonal antibody (sc-568) overnight at 4°C. Blots were subsequently stripped with 100 mM 2-mercaptoethanol, 2% sodium dodecyl sulfate, 62.8 mM Tris, pH 6.8 and probed with 1.0 μg/ml of the H-73 rabbit anti-β2-AR polyclonal antibody (sc-9042). Blots were then incubated in 1.0 μg/ml of anti-rabbit IgG Peroxidase Conjugate (Calbiochem, San Diego, CA) for 1 hr at room temperature. As negative controls, blots were incubated in 1.0 μg/ml of normal rabbit IgG (sc-2027). Antibodies sc-568, sc-9042, and sc-2027 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). As a loading control, the stripped blots were reprobed with 0.2 ng/ml of the anti-actin (Ab-1) mouse mAb (JLA20) (Calbiochem) followed by a goat anti-mouse IgG antibody (Calbiochem). Immunoreactive bands were visualized using the Phototope-HRP Western Detection System (Cell Signaling Technology, Inc., Danvers, MA).
2.4 Statistical Analysis
Continuous variables were compared with analysis of variance (ANOVA) with an LSD post-hoc test using SPSS™ v15.0 (SPSS science, Chicago, IL). P ≤ 0.05 was considered significant.
3. Results
As a first step to examine whether the MM-derived cell lines NIH-H929, MM-M1 and FLAM-76 have the potential to respond to NE, we examined the expression of β1- and β2-ARs. The three cell lines expressed similar patterns for β-ARs with all three cell lines expressing the β1-AR at greater abundance than β2-AR (Fig. 1). The NIH-H929, MM-M1 and FLAM-76 cell lines exhibited equivalent amounts of a 75 kDa immunoreactive band recognized by the anti-β1-AR antibody. However, NIH-H929 and MM-M1 cells exhibited additional immunoreactive bands that manifested as streaks migrating at relative molecular weights greater than 75 kDa. When blots of cell lysates were probed for β2-AR, slight differences were also observed in the immunoreactive bands expressed by the three cell lines. FLAM-76 cells exhibited three distinct bands with molecular weights of 44, 65, and 100 kDa recognized by the anti-β2-AR antibody, corresponding to the unglycosylated monomer, the glycosylated monomer, and the dimeric forms, respectively. The NIH-H929 and MM-M1 cells exhibited significantly lower levels of the three configurations of β2-AR. In addition, the latter cells exhibited, albeit in very low levels, additional bands with apparent molecular weights greater than 100 kDa. The presence of β1- and β2-ARs in these MM-derived cells suggest that NIH-H929, MM-M1 and FLAM-76 have the potential to respond to NE.
Figure 1.
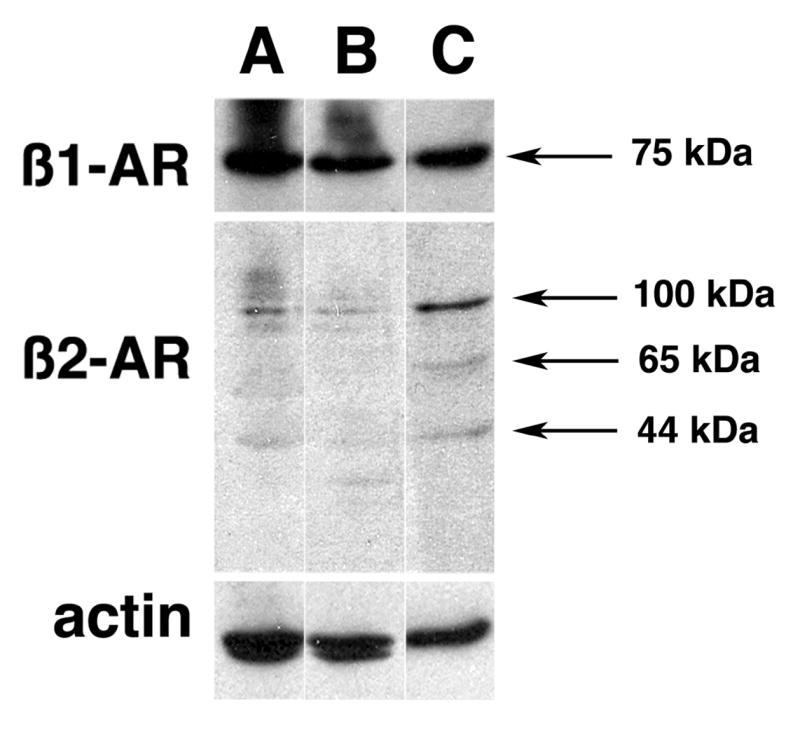
Expression of β-ARs in multiple myeloma (MM)-derived cell lines. Western blot analysis of β1-AR and β2-AR expression in A) MM NCI-H929, B) MM-M1, C) FLAM-76 cell lines. Cell lysates from all MM cell lines probed for β1-AR revealed a band with an apparent molecular weight of 75 kDa. Three major bands corresponding to the unglycosylated monomer (44 kDa), the glycosylated monomer (65 kDa), and dimer (100 kDa) were observed when cell lysates were probed for β2-AR. Actin expression in each cell line was utilized to monitor variability in loading. The bands were not observed in blots incubated with normal rabbit serum (not shown).
Having shown that all three cell lines express the β1- and β2-ARs, we tested whether treatment of NIH-H929, MM-M1 and FLAM-76 with NE results in the upregulation of VEGF levels in culture supernatants as observed in the ovarian cancer and NPC cell lines. No significant upregulation of VEGF levels in culture supernatants was observed after NE treatment of NIH-H929 cells (Fig. 2A). In contrast to the other two MM-derived cell lines (see below), an apparent downregulation of VEGF levels was observed in NIH-H929 after NE treatment. This was most apparent after 24 hours of treatment (F(2, 15) = 7.368, p < 0.05); the greatest downregulation of 41.31 ± 20.62% of control levels was observed after the 24-hour treatment with 10 μM NE (p < 0.05).
Figure 2.

VEGF concentrations in culture supernatants from MM-derived cell lines after treatment with NE. Levels of VEGF protein in culture supernatants were measured after treatment with 0, 1, and 10 μM NE of A) MM NCI-H929, B) MM-M1, C) FLAM-76 cells for 1, 3, 6, and 24 hours. Values are presented as percent of untreated control levels; Bars, SE.
There was an effect of NE treatment on VEGF levels in cultures of MM-MM1 cells at the 24 hour time point (F(2, 21) = 5.717, p < 0.05) (Fig. 2B). For example, MM-M1 cells treated with 1 μM NE for 24 hours resulted in the greatest upregulation of up to 150.06 ± 17.04% (p < 0.05) of control levels. Treatment with higher concentrations of NE result in gradually lower levels of VEGF with treatment for 24 hours with 10 μM NE resulting in 76.05 ± 19.53% of control levels.
Among the three MM-derived cell lines, FLAM-76 cells exhibited the greatest upregulation of VEGF in response to NE treatment. While one-hour treatment of FLAM-76 cells with all concentrations of NE resulted in significantly lower levels of VEGF in culture supernatants F(2, 3) = 10.25, p < 0.05 (with 1 μM NE resulting in 64.12 ± 39% of control VEGF levels), longer treatment times resulted in the significant upregulation of VEGF levels (3h: F(2, 15) = 4.844, p < 0.05, 6h: F(2, 27) = 6.400, p < 0.05, 24h: F(2, 27) = 4.896, p < 0.05) (Fig. 2C). For example, the greatest upregulation of VEGF levels was observed after the treatment with 10 μM NE for 3 hours (153.44 ± 14.87% of control VEGF, p < 0.05).
4. Discussion
There is accumulating evidence suggesting that stress-induced increases in levels of the catecholamine stress hormones, NE and E, can have direct impact on the growth and expansion of tumor cells that are independent of the effects of the hormones on the immune system. Sood and others have shown that NE and E can modulate the expression of MMPs and VEGF in ovarian cancer cells thereby influencing ovarian tumor progression (Lutgendorf et al., 2003; Lutgendorf et al., 2002; Sood et al., 2006). We have recently described the NE-dependent upregulation of VEGF, MMP-2, and MMP-9 expression in the NPC cell line HONE-1 (Yang et al., 2006) and have further shown that NE can likewise stimulate the expression of VEGF, interleukin (IL)-6 and IL-8 in the human melanoma cell line C8161 (unpublished data). These published studies describe the effects of catecholamine hormones on carcinomas. Our laboratory is exploring how widespread these interactions are among other types of tumor cells.
In this study, we show that β1- and β2-ARs are expressed in NIH-H929, MM-M1, and FLAM-76 cells. We further showed that there are differences in the response to exposure to NE among the three MM-derived cell lines. We observed that among the MM-derived cell lines tested, FLAM-76 exhibited the greatest NE-dependent response by upregulating the level of VEGF in culture supernatants after treatment for 3, 6, and 24 h with 1 μM NE. This was followed by MM-M1 and NIH-H929.
Derived from mutated post-germinal center B-lymphocytes, MM tumor cells are attracted to the bone marrow microenvironment through a complex interplay of multiple cytokines, including VEGF. MM cells proliferate in the bone marrow, causing cytopenias, pain, bone loss and fractures. Late in the disease course, as tumor cells acquire secondary mutations, they may enter the peripheral blood circulation and manifest as extramedullary disease in advanced settings. VEGF is a crucial cytokine that directs and promotes tumorogenesis and potentiation in the marrow. VEGF levels correlate with overall prognosis and associated bone destruction, which contributes substantially to clinical morbidity (Alexandrakis et al., 2007). Highlighting this idea, in fact, VEGF is the target of at least 4 novel therapies in development (Podar et al., 2007). This clinical spectrum of such progressive disease is well represented in the three cell lines studied in the present work.
First, of the MM-derived cell lines tested, FLAM-76 molecularly represents MM in the earliest (and comparatively longest) clinical phase – i.e., where disease is confined to the bone marrow. This notion is supported by the idea that the cells are IL-6 dependent for growth, suggesting a requirement for stromal cell support in vivo. Additionally, FLAM-76 harbors cytogenetic mutations commonly associated with early stage clinical disease. Specifically, FLAM-76 contains the t(11;14) translocation, often present at diagnosis, as well as a karyotypic deletion of chromosome 13, and which has been strongly associated with marrow neovascularization, increased angiogenesis, and richer overall bone marrow microvascular density (Schreiber et al., 2000). That NE stimulation via β1 and β2-AR on MM cells leads to increased VEGF levels suggests a novel, additional mechanism by which MM cells contribute to a conducive ambient microenvironment, although NE-dependent upregulation of VEGF in FLAM-76 cells is not as extensive as those previously observed in HONE-1 cells (Yang et al., 2006). For the first time, our results suggest NE may play a facilitative, potentiating role in the paracrine feedback loops that drive the microenvironment to stimulate MM cell proliferation and progression. It is of interest that among the three MM-derived cell lines, the FLAM-76 cell line is the only cell line that did not exhibit β1-AR bands larger than 100 kDa and also exhibited more of the dimeric form of β2-AR. Whether or not these differences in β-AR expression exert an influence on the greater NE-dependent upregulation of VEGF observed in FLAM-76 cells is not known and needs further examination. As FLAM-76 cells seem to have retained characteristics of the original primary tumor despite having gone through several generations in culture, including the ability to upregulate VEGF expression in response to NE, whether primary MM tumors and other IL-6 dependent MM-derived cell lines will exhibit the same ability to upregulate the expression of VEGF in response to NE needs to be explored.
Second, MM-M1 exhibits a complex karyotype with multiple deletions, indicative of a progressive state of clinical disease and it lacks the classic t(11;14) translocation described above in regards to FLAM-76. Interestingly, our results suggest MM-M1 is less responsive to NE stimulation than FLAM-76; moreover, the line seems to express relatively less β2-AR. Whether this relationship is significant is a matter of ongoing research, however, suggests that β2-AR expression may be particularly important in mediating VEGF production in response to NE.
The MM-derived cell line NIH-H929 represents MM in its clinically most advanced state. The line was initiated from extramedullary myeloma, from a malignant pleural effusion just weeks before the patient expired. Cytogenetically, the line harbors a rearranged c-myc proto-oncogene, a molecular finding often associated with aggressive, accelerating clinical disease and poor prognosis (Bergsagel and Kuehl, 2001). Germane to our findings, functional c-myc is necessary for MM cell VEGF production and related angiogenesis (Podar et al., 2006). This may partially account for the lack of VEGF production observed in response to NE stimulation in the NIH-H929 line. In fact, it is interesting to note that our observations suggest that longer treatment times and higher concentrations of NE result in a downregulation of VEGF levels in culture supernatants of NIH-H929 cells.
Data presented here show that the FLAM-76 cell line can be stimulated to release VEGF after treatment with at least 1 μM NE, a concentration that is 10 times the level detectable in human serum during periods of stress (Bierhaus et al., 2003). It is well established that the bone marrow, along with other lymphoid organs are innervated by the sympathetic nervous system (reviewed in (Bellinger et al., 2006)). Furthermore, Tang et al. showed that sympathetic activation in mice by cold exposure or Pseudomonas aeruginosa infection increased NE turnover rates in the bone marrow by 36% and 131%, respectively, thus demonstrating that the adrenergic innervation of the bone marrow is functional and is responsive to environmental stressors and infectious agents resulting in the local release of NE (Tang et al., 1999). Whether the stress-associated activation of the sympathetic nervous system results in the upregulation of NE levels in the bone marrow is unknown. However, observations described here suggest the potential for a stress-associated stimulation of pro-angiogenic properties of MM cells through the upregulation of NE levels.
Finally, the mechanism involved in the NE-dependent increase in VEGF release by MM cells is not known. There are at least nine isoforms of VEGF generated through the alternative splicing of the VEGF mRNA. Most VEGF producing cells appear to preferentially express the VEGF121, VEGF165, VEGF189 isoforms with VEGF165 being the predominant form. These isoforms differ in their ability to bind heparin and the extracellular matrix but possess the same biological activity (Takahashi and Shibuya, 2005; Yamazaki and Morita, 2006). Furthermore, it has been shown that certain factors, including NE, can regulate the various isoforms of VEGF in a differential manner (Asano et al., 2001; Asano et al., 1997; Laitinen et al., 1997). The VEGF ELISA kit used in this study has been shown to recognize both the VEGF121 and VEGF165 isoforms. Since all the isoforms share the same N-terminus generated from exons 1–5 of the VEGF gene, it is likely that our assays were able to detect all the VEGF isoforms released into the culture supernatant. Whether NE can differentially regulate the expression of the various isoforms of VEGF in MM cells (and in other cancer cells) at the transcriptional, post-transcriptional, translational or post-translational level is not known and is a question of great interest to our laboratory. Alternatively, the possibility of NE-dependent regulation of β-AR expression as a way of further controlling the expression of VEGF in MM cells is being explored.
Taken together, we present novel evidence of β1-AR and β2-AR expression on MM-derived cell lines representing progressive stages of clinical disease with decreasing dependence on angiogenesis for growth and progression. These receptors are functionally responsive to NE, in that VEGF expression is seen in response to NE, in particular in the FLAM-76 line which is best representative phenotypically and genotypically of MM in its most common, clinical disease state. The preliminary observations of the differences in the NE-dependent regulation of VEGF expression among three MM-derived cell lines described here suggest that elucidation of the mechanism(s) underlying these differences may lead to intervention modalities that can slow the growth (and perhaps metastasis) of tumor cells that express β-ARs and increase the efficacies of treatment regimens for MM at its earlier, angiogenesis-dependent stages by alleviating the effects of stress-associated increase in catecholamine levels. These data support further inquiry into the clinical effects of β-AR blocking agents in patients with MM.
Acknowledgments
This study was supported in part by NCI CA100243 and The Gilbert and Kathryn Mitchell Endowment to RG, NCI CA100243-01A2S1 to EVY and The Ohio State University Comprehensive Cancer Center Core Grant CA16058 (NCI). We would like to thank Dr. W. Michael Kuehl (Center for Cancer Research, NCI) for providing the NIH-H929, MM-M1 and FLAM-76 cell lines.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ader R, editor. Psychoneuroimmunology. Elsevier/Academic Press; Amsterdam; Boston: 2007. [Google Scholar]
- Alexandrakis MG, Sfiridaki A, Miyakis S, Pappa C, Kandidaki E, Alegakis A, Margioris AN. Relationship between serum levels of vascular endothelial growth factor, hepatocyte growth factor and matrix metalloproteinase-9 with biochemical markers of bone disease in multiple myeloma. Clinica Chimica Acta; International Journal of Clinical Chemistry. 2007;379:31–35. doi: 10.1016/j.cca.2006.11.024. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer Facts & Figures 2007. American Cancer Society; Atlanta: 2007. [Google Scholar]
- Asano A, Irie Y, Saito M. Isoform-specific regulation of vascular endothelial growth factor (VEGF) family mRNA expression in cultured mouse brown adipocytes. Molecular and Cellular Endocrinology. 2001;174:71–76. doi: 10.1016/s0303-7207(00)00450-0. [DOI] [PubMed] [Google Scholar]
- Asano A, Morimatsu M, Nikami H, Yoshida T, Saito M. Adrenergic activation of vascular endothelial growth factor mRNA expression in rat brown adipose tissue: implication in cold-induced angiogenesis. The Biochemical Journal. 1997;328(Pt 1):179–183. doi: 10.1042/bj3280179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, Molinaro C, Lubahn C, Lorton D. Innervation of lymphoid organs: Clinical implications. Clinical Neuroscience Research. 2006;6:3–33. [Google Scholar]
- Bergsagel PL, Kuehl WM. Chromosome translocations in multiple myeloma. Oncogene. 2001;20:5611–5622. doi: 10.1038/sj.onc.1204641. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar AF, Oie HK, Kirsch IR, Hollis GF. Establishment and characterization of a human plasma cell myeloma culture having a rearranged cellular myc proto-oncogene. Blood. 1986;67:1542–1549. [PubMed] [Google Scholar]
- Hanamura I, Iida S, Akano Y, Hayami Y, Kato M, Miura K, Harada S, Banno S, Wakita A, Kiyoi H, Naoe T, Shimizu S, Sonta SI, Nitta M, Taniwaki M, Ueda R. Ectopic expression of MAFB gene in human myeloma cells carrying (14;20)(q32;q11) chromosomal translocations. Japanese Journal of Cancer Research: Gann. 2001;92:638–644. doi: 10.1111/j.1349-7006.2001.tb01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob C, Sterz J, Zavrski I, Heider U, Kleeberg L, Fleissner C, Kaiser M, Sezer O. Angiogenesis in multiple myeloma. European Journal of Cancer (Oxford, England: 1990) 2006;42:1581–1590. doi: 10.1016/j.ejca.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J, Glaser R. Psychoneuroimmunology and cancer: fact or fiction? European Journal of Cancer. 1999;35:1603–1607. doi: 10.1016/s0959-8049(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Kubonishi I, Seto M, Shimamura T, Enzan H, Miyoshi I. The establishment of an interleukin-6-dependent myeloma cell line (FLAM-76) carrying t(11;14)(q13;q32) chromosome abnormality from an aggressive nonsecretory plasma cell leukemia. Cancer. 1992;70:1528–1535. doi: 10.1002/1097-0142(19920915)70:6<1528::aid-cncr2820700614>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Laitinen M, RistimAki A, Honkasalo M, Narko K, Paavonen K, Ritvos O. Differential Hormonal Regulation of Vascular Endothelial Growth Factors VEGF, VEGF-B, and VEGF-C Messenger Ribonucleic Acid Levels in Cultured Human Granulosa-Luteal Cells. Endocrinology. 1997;138:4748–4756. doi: 10.1210/endo.138.11.5500. [DOI] [PubMed] [Google Scholar]
- Lewis CE, O’Brien RM, Barraclough J, editors. Psychoimmunology of Cancer. Oxford University Press; Oxford: 2002. [Google Scholar]
- Lutgendorf SK, Cole S, Costanzo E, Bradley S, Coffin J, Jabbari S, Rainwater K, Ritchie JM, Yang M, Sood AK. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clinical Cancer Research. 2003;9:4514–4521. [PubMed] [Google Scholar]
- Lutgendorf SK, Johnsen EL, Cooper B, Anderson B, Sorosky JI, Buller RE, Sood AK. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95:808–815. doi: 10.1002/cncr.10739. [DOI] [PubMed] [Google Scholar]
- Padgett DA, Glaser R. How stress influences the immune response. Trends in immunology. 2003;24:444–448. doi: 10.1016/s1471-4906(03)00173-x. [DOI] [PubMed] [Google Scholar]
- Podar K, Anderson KC. The pathophysiologic role of VEGF in hematologic malignancies: therapeutic implications. Blood. 2005;105:1383–1395. doi: 10.1182/blood-2004-07-2909. [DOI] [PubMed] [Google Scholar]
- Podar K, Richardson PG, Chauhan D, Anderson KC. Targeting the vascular endothelial growth factor pathway in the treatment of multiple myeloma. Expert Review of Anticancer Therapy. 2007;7:551–566. doi: 10.1586/14737140.7.4.551. [DOI] [PubMed] [Google Scholar]
- Podar K, Tonon G, Sattler M, Tai YT, Legouill S, Yasui H, Ishitsuka K, Kumar S, Kumar R, Pandite LN, Hideshima T, Chauhan D, Anderson KC. The small-molecule VEGF receptor inhibitor pazopanib (GW786034B) targets both tumor and endothelial cells in multiple myeloma. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19478–19483. doi: 10.1073/pnas.0609329103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin BS. Stress, immune function, and health: the connection. Wiley-Liss; New York: 1999. [Google Scholar]
- Reiche EMV, Nunes SOV, Morimoto HK. Stress, depression, the immune system, and cancer. The Lancet Oncology. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- Riley V. Mouse mammary tumors: alteration of incidence as apparent function of stress. Science. 1975;189:465–467. doi: 10.1126/science.168638. [DOI] [PubMed] [Google Scholar]
- Saul AN, Oberyszyn TM, Daugherty C, Kusewitt D, Jones S, Jewell S, Malarkey WB, Lehman A, Lemeshow S, Dhabhar FS. Chronic Stress and Susceptibility to Skin Cancer. J Natl Cancer Inst. 2005;97:1760–1767. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S, Ackermann J, Obermair A, Kaufmann H, Urbauer E, Aletaha K, Gisslinger H, Chott A, Huber H, Drach J. Multiple myeloma with deletion of chromosome 13q is characterized by increased bone marrow neovascularization. British Journal of Haematology. 2000;110:605–609. doi: 10.1046/j.1365-2141.2000.02248.x. [DOI] [PubMed] [Google Scholar]
- Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S, Cole SW. Stress Hormone-Mediated Invasion of Ovarian Cancer Cells. Clin Cancer Res. 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Takahashi T, Okuno Y, Fukumoto M, Fukui H, Koishihara Y, Ohsugi Y, Ohno Y, Imura H. Estimation of interleukin 6 production by reverse transcriptase-polymerase chain reaction in four human myeloma cell lines. Leukemia Research. 1991;15:1043–1050. doi: 10.1016/0145-2126(91)90110-f. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clinical Science (London, England: 1979) 2005;109:227–241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- Tang Y, Shankar R, Gamelli R, Jones S. Dynamic norepinephrine alterations in bone marrow: evidence of functional innervation. Journal of Neuroimmunology. 1999;96:182–189. doi: 10.1016/s0165-5728(99)00032-6. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Morita T. Molecular and functional diversity of vascular endothelial growth factors. Molecular Diversity. 2006;10:515–527. doi: 10.1007/s11030-006-9027-3. [DOI] [PubMed] [Google Scholar]
- Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh PE, Lemeshow S, Glaser R. Norepinephrine Up-regulates the Expression of Vascular Endothelial Growth Factor, Matrix Metalloproteinase (MMP)-2, and MMP-9 in Nasopharyngeal Carcinoma Tumor Cells. Cancer Research. 2006;66:10357–10364. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]


