Abstract
“Radiation recall”—also called “radiation recall dermatitis”—has been defined as the “recalling” by skin of previous radiation exposure in response to the administration of certain response-inducing drugs. Although the phenomenon is relatively well known in the medical world, an exact cause has not been documented. Here, we report a rare occurrence of the radiation recall phenomenon in a breast cancer patient after palliative radiotherapy for bone, brain, and orbital metastases.
Keywords: Radiation recall dermatitis, breast cancer, orbital metastases
1. HISTORY
A 55-year-old woman was diagnosed with breast adenocarcinoma in August 2006. In late September, she complained of back pain and slight numbness. A bone scan revealed a mild increase in activity in the thoracic spine and the proximal fourth and anterior sixth ribs. Magnetic resonance imaging (mri) of the spine confirmed metastatic involvement in the tenth thoracic vertebra. The patient received radiotherapy with 20 Gy in 5 fractions in October 2006. She tolerated the treatment very well with complete pain relief.
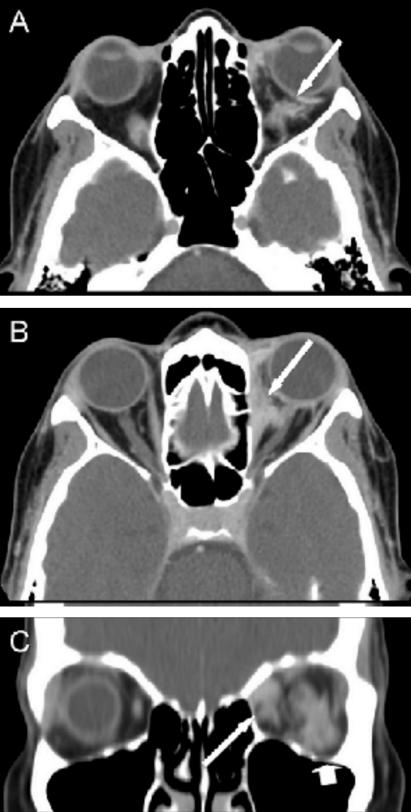
The woman returned to her medical oncologist for systemic therapy. A combination of paclitaxel (175 mg/m2) and gemcitabine (1000 mg/m2) was commenced in November 2006. After receiving a single dose, the patient complained of double vision. A computed tomography examination confirmed left orbital metastases (Figure 1) and multiple intraparenchymal brain metastases in the left frontal lobe and left cerebellum. The woman was treated with whole-brain radiotherapy (wbrt), including the left orbit, which received a dose of 20 Gy in 5 fractions. The initial chemotherapy treatment took place 13 days before the commencement of the wbrt. No adverse reactions were observed immediately after the radiation treatment.
FIGURE 1.
Retrobulbar metastasis, axial, and coronal computed tomography images with contrast. (A) Axial view of the inferior orbits demonstrates enhancing abnormal soft tissue posterior and lateral to the left globe (arrow). (B) Axial view of the upper orbits demonstrates enhancing abnormal soft tissue in the medial left orbit inseparable from the medial rectus muscle (arrow). (C) Coronal view demonstrates abnormal soft tissue in the medial left orbit inseparable from the medial rectus (arrow) and in the lateral inferior left orbit inseparable from the inferior rectus and in contact with the optic nerve (arrowhead).
Ten days after completion of the wbrt, the patient received her second dose of paclitaxel and gemcitabine. Within 2 days, the patient detected discoloured and inflamed skin limited to the region that had previously been irradiated. She also experienced swelling in the left ear, muffled hearing, and discomfort in the eyes as a result of the reaction. Surprisingly, increased pigmentation also occurred in the area of the thoracic bone metastases treated with palliative radiotherapy approximately 7 weeks earlier. Silver sulphadiazine cream and hydrocortisone eardrops were prescribed to treat external symptoms. All chemotherapy was put on hold.
Approximately 4 weeks after development of the skin reaction, the patient developed new cervical nodes compatible with clinical progression of her breast cancer. Once the external skin reaction had improved significantly, with only mild discolouration remaining, chemotherapy was resumed. At this time, nearly 6 weeks had passed since the appearance of the radiation recall dermatitis (rrd). A chemotherapy regimen of cyclophosphamide (600 mg/m2), epirubicin (100 mg/m2), and 5-fluorouracil (600 mg/m2) replaced the paclitaxel and gemcitabine. Dexamethasone (Decadron: Merck, Whitehouse Station, NJ, U.S.A) was administered at 20 mg before the first chemotherapy treatment and at 10 mg before each subsequent treatment. No adverse reactions have occurred since. At follow-up, the patient’s double vision had improved, and a computed tomography scan revealed a stable appearance in the orbital metastases. New mri examination of the brain, orbits, and spine revealed no demyelination corresponding to the areas affected by the rrd reaction.
2. DISCUSSION
“Radiation recall”—also called rrd—is defined as the “recalling” by skin of previous radiation exposure in response to the administration of certain response-inducing drugs 1. In the medical world, the rrd phenomenon has been termed anything from “moderately rare” to “moderately common.” No exact cause or incidence has been documented2.
D’Angio and colleagues originally documented rrd in 1959 3; the trigger for the abnormal reaction was dactinomycin 1. Cytotoxics are common instigators 1. Some medications have been documented to be more commonly involved with rrd: docetaxel, doxorubicin, gemcitabine, and paclitaxel (Tables I and II). Although the association is only a loose one, Camidge and Price proposed that more-severe skin reactions are more common when the period between radiation and the recall-triggering drug is smaller 1. According to Putnik et al. 60, the median time between the conclusion of radiation treatment and the materialization of rrd is 39 days. In the present case, materialization of the rrd occurred within 2 days.
TABLE I.
I Radiation recall dermatitis (rrd): case summaries
| Reference | Condition treated | Radiation dose | Drug leading to rrda | Time to rrd | Treatment a |
|---|---|---|---|---|---|
| Tan et al., 1959 4 | Ewing sarcoma of the left hip | 10 Gy to the left knee and 17.5 Gy to the spine | Dactinomycin (75 μg/kg) 7 days after completion of rt | Unspecified | Unspecified |
| D’Angio, 1962 3 | Wilms tumour | rt to the left lung and right paracardiac | Dactinomycin | During administra- tion of response- inducing drug | Unspecified |
| Von Essen et al., 1963 5 | Breast carcinoma | 30 Gy | 5-Fluorouracil (15 mg/kg daily for 4 days) 7 weeks after rt | 2 Weeks | Unspecified |
| Sears, 1964 6 | Wilms tumour | Postsurgical tumour-bed irradiation | Hydroxyurea (60 mg/kg daily) 1 month after rt | 5 Days | Unspecified |
| Wilms tumour | Radiation for pulmonary metastasis | Hydroxyurea (60 mg/kg daily) 1 month after rt | 9 Days | Unspecified | |
| Rhabdomyosarcoma of the cervical area | 30 Gy | Hydroxyurea (60 mg/kg daily) 47 days after rt | 8 Days | Unspecified | |
| Rhabdomyosarcoma of the cervical area | 16 Gy to site of pulmonary metastasis | Hydroxyurea (60 mg/kg daily) 7 days after rt | 16 Days | Unspecified | |
| Lampkin, 1969 7 | Rhabdomyosarcoma of the right middle ear | 56.44 Gy to the right face 28.32 Gy to the left side | Vinblastine (0.2 mg/kg) 2 months after rt | 1 Day | Unspecified; same reaction occurred 2 weeks later |
| Jaffe et al., 1973 8 | Osteogenic sarcoma | 26.25 Gy | Methotrexate (400 mg/kg) 24 hours after rt | Unspecified | Unspecified; rechallenged 2–3 weeks later with no recurrence |
| Donaldson et al., 1974 9 | Fibrosarcoma of the right mandible | 59.5 Gy | Doxorubicin (Adriamycin: 60 mg/m2) 5 weeks after rt | 7 Days | Unspecified; rechallenged at weeks 7 and 15 at the same and reduced doses with identical reaction |
| Osteosarcoma of the fibula | 59.5 Gy | Doxorubicin (Adriamycin: 60 mg/m2) 1 week after rt | 7 Days | Unspecified; rechallenged after 12 weeks with identical reaction | |
| Etcubanas and Wilbur, 1974 10 | Mandibular fibrosarcoma | Unspecified | Doxorubicin (Adriamycin: 60 mg/m2) 4 weeks after rt | Unspecified | Unspecified; after second cycle, a milder skin reaction occurred |
| Unspecified | Unspecified | Doxorubicin (Adriamycin: 60 mg/m2) 1 week after rt | Unspecified | Unspecified; rechallenged twice, with identical reaction each time | |
| Cassady et al., 1975 11 | Lymphoma to the right axilla and supraclavicular fossa | 12 Gy to mantle field | Doxorubicin (Adriamycin: 75 mg/m2) 26 days after rt | Hours | Unspecified |
| Osteosarcoma to the left proximal humerus | 24 Gy | Doxorubicin (Adriamycin: 30 mg/m2 daily for 3 days) 18 days after rt | 7 Days | Unspecified | |
| Rosen et al., 1975 12 | Osteogenic sarcoma | 16 Gy | Methotrexate (200 mg/kg) 8 weeks after rt | 5 Days | Unspecified; rechallenged twice with similar reactions |
| Mayer et al., 1976 13 | Metastatic breast cancer | 45 Gy to the spine | Doxorubicin (Adriamycin: 80 mg/injection, 1 injection per month for 9 months) given 7 years after rt | 7 Months (at the time of the 7th injection) | Unspecified |
| Fontana, 1979 14 | Small-cell lung cancer | 38 Gy | Etoposide (100 mg/m2 on days 1–3) 7 days after rt | 18 Hours | Unspecified; rechallenged 3 weeks later, resulting in the same reaction |
| Solberg et al., 1980 15 | Acute myelomonocytic leukemia and leukemia cutis | 21 Gy whole-body irradiation | Doxorubicin (Adriamycin: 35 mg/m2 daily for 3 days) given 2 days after rt | 4 Days | Death related to toxicity |
| Weiss et al., 1986 16 | Advanced cancers | Unspecified | Intravenous trimetrexate (80 mg/m2 over 24 hours) every 28 days | Unspecified | Unspecified |
| Unspecified | Intravenous trimetrexate (200 mg/m2 over 24 hours) every 28 days | Unspecified | Unspecified | ||
| Unspecified | Intravenous trimetrexate (200 mg/m2 over 24 hours) every 28 days | Unspecified | Unspecified | ||
| Unspecified | Intravenous trimetrexate (200 mg/m2 over 24 hours) every 28 days | Unspecified | Unspecified | ||
| Jolivet et al., 1987 17 | Lung cancer | 40 Gy | Trimetrexate (2 mg/m2 bolus) for 9 consecutive days every 28 days for 2 cycles; begun 10 months after completion of rt | Unspecified | Unspecified |
| Kellie et al., 1987 18 | Embryonal rhabdomyosarcoma of the legs | 54 Gy | Melphalan (200 mg/m2) 6 weeks after completion of rt | 24 Hours | Unspecified |
| Nemechek and Corder, 199219 | Man (age 34) with hiv and a large Kaposi sarcoma lesion on the left foot | 27 Gy in 15 fractions | Intravenous vinblastine (10 mg/m2), begun 10 months after rt | 48 Hours | Healed by the 5th day after chemotherapy |
| Parry, 1992 20 | Woman (age 70) with breast cancer | Wide local excision and adjuvant rt 2 years earlier | Tamoxifen (20 mg daily) started 2 years after rt | 5 Days | Discontinued tamoxifen; resolved in 2 weeks; rechallenged at 10 mg daily, with mild recurrence |
| Raghavan et al., 1993 21 | Recurrent breast carcinoma | 61.2 Gy to the chest wall; 65.3 Gy to the supraclavicular region | Paclitaxel (130 mg/m2 over 24 h), begun 2 days after rt | 5 Days | Antibiotics, chest wall debridement |
| Stelzer et al., 199322 | aids-related Kaposi sarcoma | Each lesion randomized to 1 of 3 possible radiation fractionation schemes:1) 40 Gy in 20 fractions2) 40 Gy in 20 fractions3) 8 Gy in 1 fraction4) 20 Gy in 5 fractions | Intravenous bleomycin (10 mg/m2) on a weekly basis | 1) 3 Days after second injection2) 3 Days after second injection3) No rrd4) No rrd | In 1) and 2), exacerbated by oral etoposide therapy started 4 days after appearance of the skin reaction |
| Shenkier and Gelman, 1994, as cited by | Advanced gastric cancer | 44 Gy | Paclitaxel (90 mg/m2) 7 months after rt | 3 Hours | Mild recurrence when paclitaxel given in 7 further cycles |
| Camidge and Price, 2001 1 | Advanced gastric cancer | 44 Gy | Paclitaxel (90 mg/m2) 8 months after rt | 6 Hours | No recurrence when paclitaxel given in 7 further cycles |
| Abadir and Liebmalen 1995 23 | Woman (age 60) with adenocarcinoma of the gallbladder | Tumour dose was 61.2 Gy in 34 fractions | Simvastatin for hypercholesterolemia (20 mg daily), 11 months after rt | 2–3 Days | Prednisone and cephalotin |
| Extermann et al., 1995 24 | Man (age 55) with ductal carcinoma of the right breast | Tamoxifen (20 mg daily), plus 48.25 Gy with a 15-Gy boost to the tumour bed | Isoniazid 400 mg, plus rifampicin 600 mg, plus pyrazinamide 2 mg to treat nasopharyngeal tuberculosis, 4 months after rt | During 4th week of treatment | All medications were continued, and the reaction gradually regressed in the weeks following |
| Perez et al., 1995 25 | Woman (age 32) with metastatic breast cancer | 30 Gy to the lumbar spine | Edatrexate (100 mg/m2 biweekly), begun 6 weeks after rt | After 3 doses (11 days) | Topical therapy, nsaids; rechallenged with prednisone with mild recurrence |
| Phillips, 1995, as cited by Camidge and Price, 2001 1 | Unknown | 25 Gy | Paclitaxel (90 mg/m2) given 27 days after rt | 3 Days | No recurrence when paclitaxel given in 3 further cycles |
| Schweitzer et al., 1995 26 | Woman (age 61) with metastatic lung adenocarcinoma | 43.2 Gy to the mediastinum; 46 Gy to the ribs | Paclitaxel (175 mg/m2 over 3 hours), begun 12 days after rt completion | Hours | Dexamethasone (Decadron: 20 mg), diphenhydramine (50 mg); paclitaxel given with Decadron after 2 weeks with no recurrence |
| Bokenmeyer et al., 1996 27 | Woman (age 55) with breast cancer | 50 Gy to the breast; 54 Gy to the lymph nodes | Paclitaxel (175 mg/m2 over 3 h), 13 months after rt | 5 Days | Discontinuation of paclitaxel |
| McCarty et al., 1996 28 | Woman (age 51) with invasive lobular breast carcinoma | 50.4 Gy in 28 fractions; mild skin erythema developed | Paclitaxel (200 mg/m2), 7 days after rt | 4 Days | Healing within 10 days; treatment unspecified |
| Yeo et al., 1997 29 | Woman (age 51) with breast cancer | 30 Gy in 10 fractions to T10–L4 spine and pelvis | Docetaxel (100 mg/m2) on 3-weekly basis and prior oral dexamethasone (Decadron) for 5 days | 4 Days after second injection | Dose reduction; no recurrence of rrd |
| Bostrom et al., 1999 30 | Woman (age 48) with highly differentiated tuboloductal breast cancer | 54 Gy | Tamoxifen (20 mg/m2 daily) 28 months after rt | 2 Months | Local steroid cream, mometasone furoate, once daily for 10 days; skin appeared normal 7 weeks after discontinuing tamoxifen; after 8 weeks, restarted on toremifene without recurrence |
| Wilson et al., 1999 31 | Woman (age 46) with breast cancer | Unspecified | Epirubicin | 2 Weeks | Surgical debridement and microvascular free-flap reconstruction |
| Camidge & Kunkler, 2000 32 | Woman (age 50) with breast cancer | 50 Gy in 20 fractions | Cycle 2 of docetaxel (100 mg/m2) with dexamethasone (Decadron: 8 mg once daily for 3 days), 16 days after end of rt | Within 7 days | Docetaxel reduced to 75% and given 21 days later; steroids for 7 days without recurrence |
| Castellano et al., 2000 33 | Man (age 61) with stage iv nsclc | 24 Gy in 8 fractions | Gemcitabine (1250 mg/m2 on days 1, 8, 15 per 28-day cycle) 4 weeks after completion of rt | 8 Days (second dose) | Oral dexamethasone (Decadron) and diphenhydramine; resolved 10 days later; treatment continued with other chemotherapies |
| Giesel et al., 2000 34 | Woman with breast cancer | Whole-brain irradiation: 2 Gy for 5 days weekly, up to 50 Gy | Docetaxel restarted (30 mg/m2 weekly) | Unspecified | Unspecified |
| Woman with breast cancer | Whole-brain irradiation: 2 Gy for 5 days weekly, for up to 50 Gy | Docetaxel re-started (100 mg/m2 weekly) | Unspecified | Unspecified | |
| Kharfan et al., 2000 35 | Woman (age 25) with stage iv nodular sclerosis Hodgkin disease | 30 Gy to lumbar spine and right proximal femur | Methotrexate (10 mg/m2 on day +1 after bone marrow transplant; 15 mg/m2 on days +3, +6, and +11) | 7 Days after transplant | Hydrating emulsions (treated symptomatically) |
| Chan et al., 2001 36 | Man (age 50) with a sigmoid carcinoma | 41.4 Gy in 23 days | Oxaliplatin-based chemotherapy (oxaliplatin, plus 5-fluorouracil, plus folinic acid), resumed 8 days after completion on rt | 3 Days | Aqueous cream and sodium fusidate ointment (Fucidin); chemotherapy discontinued and reaction settled after 2 weeks; 5-fluorouracil plus folinic acid alone resumed without recurrence |
| Kennedy and McAleer, 2001 37 | Malignant melanoma to the right temple | 30 Gy | Dacarbazine (800 mg/m2 once every 3 weeks), begun 2 months after rt | 10 Days | Unspecified |
| Bar-Sela et al., 2001 38 | Man (age 65) with lung adenocarcinoma | RT to mediastinum and upper lobe | Gemcitabine | Unspecified | Unspecified |
| Jeter et al., 2002 39 | Woman (age 41) with breast cancer | 30 Gy in 10 fractions to lumbar spine | Gemcitabine (1000 mg/m2 every 2 weeks), plus trastuzumab (Herceptin) weekly for 4 weeks, 5.5 months after rt | 2 Weeks | Discontinuation of gemcitabine slowly resolved the skin reaction |
| Man (age 79) with nsclc | 30 Gy in 10 fractions | Gemcitabine (1000 mg/m2)
11 days after rt |
10 Days | Supportive care, alginate gel pads, bowel rest | |
| Woman (age 63) with metastatic adenocarcinoma of unknown primary | 30 Gy in 10 fractions, plus 25 Gy in 2 fractions (boost) | Gemcitabine (1000 mg/m2), 3.4 months after rt | 3 Days | Intravenous steroids for 2 days with minimal response | |
| Morkas et al., 2002 40 | Woman (age 39) with infiltrating ductal carcinoma | 50.4 Gy in 28 fractions, plus 10 Gy to tumour bed and 2 cm surrounding | Docetaxel (100 mg/m2), plus prophylactic dexamethasone (Decadron) | 10 Days | Methylprednisone (80 mg twice daily); docetaxel at 75% induced a less severe reaction |
| Ortmann and Hohenberg, 2002 41 | Woman (age 56) with breast cancer | 30 Gy in 10 fractions to right hip | Capecitabine (2000 mg twice daily for 14 days) | 3 Days after completion of first course | Unspecified |
| Piroth et al., 2002 42 | Woman (age 40) with breast cancer | 30.9 Gy upper-body irradiation, plus whole-brain and pelvis | Docetaxel (30 mg/m2) started 1 week after rt | 14 Days | Discontinuation and anti-inflammatory agents |
| Thomas and Stea, 2002 43 | Woman (age 29) with malignant melanoma of the scalp | Excision, plus biweekly treatments of 6 Gy, totalling 30 Gy | Intravenous interferon alfa-2b (20×109 IU) administered 5 days after completion of rt | 6 Days | Occlusive dressings with wound gel; resolved in 7 days |
| Ee and Yosipovitch, 2003 44 | Woman (age 55) with metastatic breast cancer | Photo-recall | Chemotherapy with taxanes | Unspecified | Unspecified |
| Jimeno et al., 2003 45 | Woman (age 53) with stage IV infiltrating ductal carcinoma | 30 Gy to left femur | Pegylated liposomal doxorubicin (40 mg/m2 on day 1 every 28 days), 4 weeks after completion of rt | 12 Days | Topical steroids (betamethasone dipropionate); completely resolved 14 days later |
| Keung et al., 2003 46 | Woman (age 49) with breast cancer | 50 Gy in 25 fractions following modified mastectomy | Arsenic trioxide (0.15 mg/kg daily), for 5 days each week for 5 weeks | Day 2 of week 3 | Arsenic trioxide discontinued, topical triamcinolone/silver sulfadiazine cream started |
| Schwartz et al., 2003 47 | Woman (age 37) with recurrent ovarian adenocarcinoma | Palliative whole-pelvis rt: 45 Gy in 25 fractions | 3 Months later, started on gemcitabine (800 mg/m2), every other week; reduced to 600 mg/m2 because of severe neutropenia | Unspecified | Ciprofloxacin (250 mg twice daily) with slight improvement; 2nd cycle after 2 weeks produced the same reaction within 24 hours |
| Muggia, 200448 | Woman with breast cancer | rt to supraclavicular, internal mammary, and axillary areas | Doxorubicin with weekly trastuzumab | 2–4 Weeks | None; continued liposomal doxorubicin |
| Singer et al., 2004 49 | Woman (age 88) with infiltrating ductal carcinoma | 50.4 Gy, plus 10 Gy to tumour bed | Tamoxifen (20 mg daily) | 3 Months | None; continued on tamoxifen; completely resolved 3 months later |
| Borgia et al., 2005 50 | Woman (age 63) with infiltrating ductal carcinoma | 50 Gy over 5 weeks | Docetaxel (100 mg/m2), every 3 weeks started 1 week after rt | 4 Days after second course | Oral methylprednisone resulted in 10-day complete remission |
| Kandemir et al., 2005 51 | Woman (age 55) with breast cancer | 50 Gy over 5 weeks | Docetaxel (100 mg/m2), plus oral dexamethasone (Decadron) for 3 days | 11 Days | None; complete resolution after 6 days; continued docetaxel with no recurrence |
| Marisavljevic et al., 2005 52 | Woman (age 32) with stage iib Hodgkin lymphoma | Total dose of 60 Gy | Gemcitabine (1250 mg/m2 on days 1, 8, 15), plus oral dexamethasone (Decadron: 8 mg on days 1, 2, 8, 9, 15, 16) more than 2 years after rt | 2 Days | Skin reaction faded over 10 days without specific treatment; mild recurrence after each gemcitabine administration |
| Ash and Videtic, 2006 53 | Woman (age 56) with infiltrating ductal carcinoma | 50 Gy in 25 fractions, plus additional 10 Gy in 5 fractions to lumpectomy site | Phentermine 1 year after rt | Unspecified | Prednisone 30 mg daily for 2 weeks; minimal discolouration after 4 weeks |
| Ayoola and Lee, 2006 54 | Woman (age 54) with lung squamous cell carcinoma | 64.8 Gy to thorax and mediastinum | Cefotetan upon admission to hospital for cholecystitis | 3 Days | Cefotetan withdrawn; free of pain in 4 days |
| Barlesi et al., 2006 55 | Woman (age 75) with primary lung adenocarcinoma; treated for breast cancer 27 years earlier | Lumpectomy and adjuvant radiation to the breast 27 years earlier | Pemetrexed (500 mg/m2); oral prednisone (40 mg) twice daily the day before, the day of, and the day after chemotherapy | 3 Days | Steroids (prednisone 1 mg/kg daily); improvement in 48 hours; resolution at 2 weeks |
| Fakih, 2006 56 | White man (age 52) with pancreatic adenocarcinoma | 1.8 Gy daily for 50.4 Gy total | Gemcitabine (1000 mg/m2), for 3 weeks every 4-week-cycle | Cycle 5 | Withdrawal of gemcitabine resulted in spontaneous resolution |
| Kaya et al., 2006 57 | Woman (age 41) with non-Hodgkin lymphoma | UV radiation | Methotrexate (high dose), plus cytarabine (high dose) | Unspecified | Cold compress; lesions resolved within a week (with hyperpigmentation) |
| Kundranda and Daw, 2006 58 | Woman (age 48) with well-differentiated infiltrating ductal carcinoma | 50 Gy with a boost of 14 Gy to the tumour bed | Tamoxifen (20 mg daily) | Within 1 week | Oral cephalexin did not provide relief; tamoxifen discontinued, diphenhydramine given; after 12 weeks, restarted on tamoxifen with mild itchiness, but no recurrence |
| Mizumoto et al., 2006 59 | Woman (age 76) with diffuse large B cell lymphoma of the left neck | 36 Gy in 18 fractions to the left neck | Docetaxel (60 mg/m2) every 3 weeks 1 year after rt | 6 Days | Gargle with a local anesthetic and topical corticosteroids; 80% of docetaxel dose was given 2 weeks later; milder recall phenomenon recurred after 1 week |
| Woman (age 60) with breast cancer | 50 Gy in 20 fractions | Docetaxel (30 mg/m2 weekly) restarted 14 days after rt | Day 6 after second course of chemotherapy | Topical corticosteroids; continued docetaxel therapy for 9 cycles | |
| Putnik et al., 2006 60 | Man (age 65) with squamous cell carcinoma of the epiglottis | 64.8 Gy | Hypericin | 4 Weeks after rt, then again 1 year after rt | Symptoms controlled by steroid cream, but disappeared only when hypericin was discontinued |
| Hird et al., 2007 (present article) | Woman (age 55) with metastatic breast adenocarcinoma | 1) 20 Gy in 5 fractions to the thoracic spine (October 2006) 2) Whole-brain radiation: 20 Gy in 5 fractions (November 2006) | Paclitaxel (175 mg/m2) and gemcitabine (1000 mg/m2) administered 1.5 weeks after completion of whole-brain radiation | 2 Days | Silver sulphadiazine cream and hydrocortisone eardrops; discolouration still apparent after 8 weeks; started on cef with concurrent dexamethasone (Decadron) without recurrence |
Holders of named pharmaceutical trademarks: Adriamycin: Pharmacia, Kalamazoo, MI, U.S.A.; Decadron: Merck and Co., Whitehouse Station, NJ, U.S.A.; Fucidin: Leo Pharma, Ballerup, Denmark; Herceptin: Genentech, San Francisco, CA, U.S.A.
rt = radiotherapy; nsaids = nonsteroidal anti-inflammatory drugs; nsclc = non-small-cell lung cancer; uv = ultraviolet; cef = cyclophospha-mide, epirubicin, 5-fluorouracil.
TABLE II.
| Drug | Frequency | |
|---|---|---|
| (n) | (%) | |
| Docetaxel | 10 | 13 |
| Doxorubicin | 10 | 13 |
| Gemcitabine | 8 | 11 |
| Paclitaxel | 8 | 11 |
| Trimetrexate | 5 | 7 |
| Methotrexate | 4 | 5 |
| Hydroxyurea | 4 | 5 |
| Tamoxifen | 4 | 5 |
| Dactinomycin | 2 | 3 |
| Vinblastine | 2 | 3 |
| Others | 18 | 24 |
Although the precise mechanism of action for rrd is not known, several mechanisms that may, or may not, lead to the development of radiation recall have been proposed. These mechanisms include changes in vascularization, dna repair, radiation-impaired epithelial function of stem cells, and increased sensitivity to drugs 1. Corticosteroids have been suggested to have some protective effects 61. We found that steroids are commonly used in the treatment of external symptoms and with the intention of preventing recurrent reactions during subsequent chemotherapy administration 23,25,26,30,32,33,39,40,43,45,46,50,53,55,59,60.
Most recall reactions have occurred when radiotherapy and chemotherapy are separated by less than 2 months (Table I). The present case demonstrates a maximum time frame of 7 weeks separating radiation and resumption of chemotherapy treatment. Of the total reported cases of rrd outlined here, only 27% (20/75) demonstrated a duration greater than 7 weeks in terms of time passed between completion of radiotherapy and commencement of chemotherapy 1,5–7,12,13,17,19,20,23,24,27,30,37,39,52,53,59.
Although rrd is a rare phenomenon, it poses a significant barrier to treatment for patients. The condition creates a paradox: patients and clinicians alike wish to proceed with the most desirable treatment in the given circumstances, but are unable to do so because of the rare skin reaction. The present report serves as a reminder to palliative health care professionals of the possible danger of a recall reaction if an insufficient period has passed between radiotherapy and commencement of a potential recall-inducing drug.
Acknowledgments
3. ACKNOWLEDGMENT
This study was supported by the Michael and Karen Goldstein Cancer Research Fund.
4. REFERENCES
- 1.Camidge R, Price A. Characterizing the phenomenon of radiation recall dermatitis. Radiother Oncol. 2001;59:237–45. doi: 10.1016/s0167-8140(01)00328-0. [DOI] [PubMed] [Google Scholar]
- 2.Ortmann E, Hohenberg G. Treatment side effects. Case 1. Radiation recall phenomenon after administration of capecitabine. J Clin Oncol. 2002;20:3029–34. doi: 10.1200/JCO.2002.20.13.3029. [DOI] [PubMed] [Google Scholar]
- 3.D’Angio GJ. Clinical and biologic studies of actinomycin D roentgen irradiation. Am J Roentgenol. 1962;87:106–9. [PubMed] [Google Scholar]
- 4.Tan CT, Dargeon HW, Burchenal JH. The effect of actinomycin D on cancer in childhood. Pediatrics. 1959;24:544–61. [PubMed] [Google Scholar]
- 5.Von Essen CF, Kligerman MM, Calabresi P. Radiation and 5-fluorouracil: a controlled clinical study. Radiology. 1963;81:1018–26. doi: 10.1148/81.6.1018. [DOI] [PubMed] [Google Scholar]
- 6.Sears ME. Erythema in areas of previous irradiation in patients treated with hydroxyurea (nsc-32065) Cancer Chemother Rep. 1964;40:31–2. [PubMed] [Google Scholar]
- 7.Lampkin BC. Skin reaction to vinblastine. Lancet. 1969;1:891. doi: 10.1016/s0140-6736(69)91941-2. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe N, Paed D, Farber S, et al. Favorable response of metastatic osteogenic sarcoma to pulse high-dose methotrexate with citrovorum rescue and radiation therapy. Cancer. 1973;31:1367–73. doi: 10.1002/1097-0142(197306)31:6<1367::aid-cncr2820310611>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson SS, Glick JM, Wilbur JR. Letter: Adriamycin activating a recall phenomenon after radiation therapy. Ann Intern Med. 1974;81:407–8. doi: 10.7326/0003-4819-81-3-407. [DOI] [PubMed] [Google Scholar]
- 10.Etcubanas E, Wilbur JR. Letter: uncommon side effects of Adriamycin (nsc-123127) Cancer Chemother Rep. 1974;58:757–8. [PubMed] [Google Scholar]
- 11.Cassady JR, Richter MP, Piro AJ, Jaffe N. Radiation– Adriamycin interactions: preliminary clinical observations. Cancer. 1975;36:946–9. doi: 10.1002/1097-0142(197509)36:3<946::aid-cncr2820360316>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Rosen G, Tefft M, Martinez A, Cham W, Murphy ML. Combination chemotherapy and radiation therapy in the treatment of metastatic osteogenic sarcoma. Cancer. 1975;35:622–30. doi: 10.1002/1097-0142(197503)35:3<622::aid-cncr2820350313>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 13.Mayer EG, Poulter CA, Aristizabal SA. Complications of irradiation related to apparent drug potentiation by Adriamycin. Int J Radiat Oncol Biol Phys. 1976;1:1179–88. doi: 10.1016/0360-3016(76)90091-2. [DOI] [PubMed] [Google Scholar]
- 14.Fontana JA. Radiation recall associated with vp-16–213 therapy. Cancer Treat Rep. 1979;63:224–5. [PubMed] [Google Scholar]
- 15.Solberg LA, Jr, Wick MR, Bruckman JE. Doxorubicin-enhanced skin reaction after whole-body electron-beam irradiation for leukemia cutis. Mayo Clin Proc. 1980;55:711–15. [PubMed] [Google Scholar]
- 16.Weiss RB, James WD, Major WB, Porter MB, Allegra CJ, Curt GA. Skin reactions induced by trimetrexate, an analog of methotrexate. Invest New Drugs. 1986;4:159–63. doi: 10.1007/BF00194596. [DOI] [PubMed] [Google Scholar]
- 17.Jolivet J, Landry L, Pinard MF, McCormack JJ, Tong WP, Eisenhauer E. A phase i study of trimetrexate, an analog of methotrexate, administered monthly in the form of nine consecutive daily bolus injections. Cancer Chemother Pharmacol. 1987;20:169–72. doi: 10.1007/BF00253973. [DOI] [PubMed] [Google Scholar]
- 18.Kellie SJ, Plowman PN, Malpas JS. Radiation recall and radiosensitization with alkylating agents. Lancet. 1987;1:1149–50. doi: 10.1016/s0140-6736(87)91711-9. [DOI] [PubMed] [Google Scholar]
- 19.Nemechek PM, Corder MC. Radiation recall associated with vinblastine in a patient treated for Kaposi sarcoma related to acquired immune deficiency syndrome. Cancer. 1992;70:1605–6. doi: 10.1002/1097-0142(19920915)70:6<1605::aid-cncr2820700627>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Parry BR. Radiation recall induced by tamoxifen. Lancet. 1992;340:49. doi: 10.1016/0140-6736(92)92460-w. [DOI] [PubMed] [Google Scholar]
- 21.Raghavan VT, Bloomer WD, Merkel DE. Taxol and radiation recall dermatitis. Lancet. 1993;341:1354. doi: 10.1016/0140-6736(93)90871-d. [DOI] [PubMed] [Google Scholar]
- 22.Stelzer KJ, Griffin TW, Koh WJ. Radiation recall skin toxicity with bleomycin in a patient with Kaposi sarcoma related to acquired immune deficiency syndrome [abstract] Cancer. 1993;71:1322–5. doi: 10.1002/1097-0142(19930215)71:4<1322::aid-cncr2820710425>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 23.Abadir R, Leibmann J. Radiation reaction recall following simvastatin therapy: a new observation. Clin Oncol (R Coll Radiol) 1995;7:325–6. doi: 10.1016/s0936-6555(05)80545-x. [DOI] [PubMed] [Google Scholar]
- 24.Extermann M, Vogt N, Forni M, Dayer P. Radiation recall in a patient with breast cancer treated for tuberculosis. Eur J Pharmacol. 1995;48:77–8. doi: 10.1007/BF00202177. [DOI] [PubMed] [Google Scholar]
- 25.Perez EA, Campbell DL, Ryu JK. Radiation recall dermatitis induced by edatrexate in a patient with breast cancer. Cancer Invest. 1995;13:604–7. doi: 10.3109/07357909509024929. [DOI] [PubMed] [Google Scholar]
- 26.Schweitzer VG, Julliard GJ, Bajada CL, Parker RG. Radiation recall dermatitis and pneumonitis in a patient treated with paclitaxel. Cancer. 1995;76:1069–72. doi: 10.1002/1097-0142(19950915)76:6<1069::aid-cncr2820760623>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Bokemeyer C, Lampe C, Heneka M, Schabet M, Bamberg M, Kanz L. Paclitaxel-induced radiation recall dermatitis. Ann Oncol. 1996;7:755–77. doi: 10.1093/oxfordjournals.annonc.a010730. [DOI] [PubMed] [Google Scholar]
- 28.McCarty MJ, Peake MF, Lillis P, Vukelja SJ. Paclitaxel-induced radiation recall dermatitis. Med Pediatr Oncol. 1996;27:185–6. doi: 10.1002/(SICI)1096-911X(199609)27:3<185::AID-MPO9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 29.Yeo W, Leung SF, Johnson PJ. Radiation-recall dermatitis with docetaxel: establishment of a requisite radiation threshold. Eur J Cancer. 1997;33:698–9. doi: 10.1016/s0959-8049(96)00461-3. [DOI] [PubMed] [Google Scholar]
- 30.Bostrom A, Sjolin–Forsberg G, Wilking N, Bergh J. Radiation recall: another call with tamoxifen. Acta Oncologica. 1999;38:955–9. doi: 10.1080/028418699432653. [DOI] [PubMed] [Google Scholar]
- 31.Wilson J, Carder P, Gooi J, Nishikawa H. Recall phenomenon following epirubicin [abstract] Clin Oncol (R Coll Radiol) 1999;11:424–5. doi: 10.1053/clon.1999.9099. [DOI] [PubMed] [Google Scholar]
- 32.Camidge DR, Kunkler IH. Docetaxel-induced radiation recall dermatitis and successful rechallenge without recurrence. Clin Oncol (R Coll Radiol) 2000;12:272–3. [PubMed] [Google Scholar]
- 33.Castellano D, Hitt R, Cortes–Funes H, Romero A, Rodriguez– Peralto JL. Side effects of chemotherapy: case 2. Radiation recall induced by gemcitabine. J Clin Oncol. 2000;18:693–8. doi: 10.1200/JCO.2000.18.3.695. [DOI] [PubMed] [Google Scholar]
- 34.Giesel BU, Kutz GG, Thiel HJ. Recall dermatitis caused by re-exposure to docetaxel following irradiation of the brain. Case report and review of the literature [abstract, German] Strahlenther Onkol. 2001;117:487–93. doi: 10.1007/pl00002431. [DOI] [PubMed] [Google Scholar]
- 35.Kharfan Dabaja MA, Morgensztern D, Markoe AM, Bartlett– Pandite L. Radiation recall induced by methotrexate with in a patient with Hodgkin’s disease. Am J Clin Oncol. 2000;23:531–3. doi: 10.1097/00000421-200010000-00020. [DOI] [PubMed] [Google Scholar]
- 36.Chan RT, Au GK, Ho JW, Chu KW. Radiation recall with oxaliplatin: report of a case and review of the literature. Clin Oncol (R Coll Radiol) 2001;13:55–7. doi: 10.1053/clon.2001.9216. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy RD, McAleer JJ. Radiation recall dermatitis in a patient treated with dacarbazine. Clin Oncol (R Coll Radiol) 2001;13:470–2. doi: 10.1053/clon.2001.9316. [DOI] [PubMed] [Google Scholar]
- 38.Bar-Sela G, Beny A, Bergman R, Kuten A. Gemcitabine-induced radiation recall dermatitis: case report [abstract] Tumori. 2001;87:428–30. doi: 10.1177/030089160108700614. [DOI] [PubMed] [Google Scholar]
- 39.Jeter MD, Janne PA, Brooks S, et al. Gemcitabine-induced radiation recall. Int J Radiat Oncol Biol Phys. 2002;53:394–400. doi: 10.1016/s0360-3016(02)02773-6. [DOI] [PubMed] [Google Scholar]
- 40.Morkas M, Fleming D, Hahl M. Challenges in oncology. Case 2. Radiation recall associated with docetaxel. J Clin Oncol. 2002;20:867–9. doi: 10.1200/JCO.2002.20.3.867. [DOI] [PubMed] [Google Scholar]
- 41.Ortmann E, Hohenberg G. Treatment side effects: case 1. Radiation recall phenomenon after administration of capecitabine. J Clin Oncol. 2002;20:3029–30. doi: 10.1200/JCO.2002.20.13.3029. [DOI] [PubMed] [Google Scholar]
- 42.Piroth MD, Krempien R, Wannenmacher M, Zierhut D. Radiation recall dermatitis from docetaxel [abstract] Onkologie. 2002;25:438–40. doi: 10.1159/000067438. [DOI] [PubMed] [Google Scholar]
- 43.Thomas R, Stea B. Radiation recall dermatitis from high-dose interferon alfa-2b. J Clin Oncol. 2002;20:355–7. doi: 10.1200/JCO.2002.20.1.355. [DOI] [PubMed] [Google Scholar]
- 44.Ee HL, Yosipovitch G. Photo recall phenomenon: an adverse reaction to taxanes. Dermatology. 2003;207:196–8. doi: 10.1159/000071795. [DOI] [PubMed] [Google Scholar]
- 45.Jimeno A, Cirelos EM, Castellano D, Caballero B, Rodriguez– Peralto JL, Cortes–Funes H. Radiation recall dermatitis induced by pegylated liposomal doxorubicin. Anticancer Drugs. 2003;14:575–6. doi: 10.1097/00001813-200308000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Keung YK, Lyerly ES, Powell BL. Radiation recall phenomenon associated with arsenic trioxide. Leukemia. 2003;17:1417–36. doi: 10.1038/sj.leu.2402992. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz BM, Khuntia D, Kennedy AW, Markman M. Gemcitabine-induced radiation recall dermatitis following whole pelvic radiation therapy. Gynecol Oncol. 2003;91:421–2. doi: 10.1016/s0090-8258(03)00404-9. [DOI] [PubMed] [Google Scholar]
- 48.Muggia FM. Re: “Radiation recall dermatitis induced by pegylated liposomal doxorubicin. Anticancer Drugs. 2004;15:35. doi: 10.1097/00001813-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Singer EA, Warren RD, Pennanen MF, Collins BT, Hayes DF. Tamoxifen-induced radiation recall dermatitis. Breast J. 2004;10:170–1. doi: 10.1111/j.1075-122x.2004.21222.x. [DOI] [PubMed] [Google Scholar]
- 50.Borgia F, Guarneri C, Guarneri F, Vaccaro M. Radiation recall dermatitis after docetaxel administration: absolute indication to replace the drug? Br J Dermatol. 2005;153:674–5. doi: 10.1111/j.1365-2133.2005.06801.x. [DOI] [PubMed] [Google Scholar]
- 51.Kandemir EG, Karabudak O, Maydagli A. Docetaxel-induced radiation recall dermatitis. Swiss Med Wkly. 2005;135:34–5. doi: 10.4414/smw.2005.10896. [DOI] [PubMed] [Google Scholar]
- 52.Marisavljevic D, Ristic B, Hajder J. Gemcitabine-induced radiation recall dermatitis in a patient with resistant Hodgkin lymphoma. Am J Hematol. 2005;80:87–93. doi: 10.1002/ajh.20379. [DOI] [PubMed] [Google Scholar]
- 53.Ash RB, Videtic GM. Radiation recall dermatitis after the use of anorexiant phentermine in a patient with breast cancer. Breast J. 2006;12:186–7. doi: 10.1111/j.1075-122X.2006.00235.x. [DOI] [PubMed] [Google Scholar]
- 54.Ayoola A, Lee YJ. Radiation recall dermatitis with cefotetan: a case study. Oncologist. 2006;11:1118–20. doi: 10.1634/theoncologist.11-10-1118. [DOI] [PubMed] [Google Scholar]
- 55.Barlesi F, Tummino C, Taset AM, Astoul P. Unsuccessful rechallenge with pemetrexed after previous radiation recall dermatitis. Lung Cancer. 2006;54:423–5. doi: 10.1016/j.lungcan.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 56.Fakih MG. Gemcitabine-induced rectus abdominus radiation recall. JOP. 2006;7:306–10. [PubMed] [Google Scholar]
- 57.Kaya TI, Tiftik N, Tursen U, Ikizoglu G, Yalcin A. Ultraviolet recall phenomenon associated with methotrexate and cytarabine. J Eur Acad Dermatol Venereol. 2006;20:353–4. doi: 10.1111/j.1468-3083.2006.01432.x. [DOI] [PubMed] [Google Scholar]
- 58.Kundranda MN, Daw HA. Tamoxifen-induced radiation recall dermatitis. Am J Clin Oncol. 2006;29:637–8. doi: 10.1097/01.coc.0000189693.23157.8d. [DOI] [PubMed] [Google Scholar]
- 59.Mizumoto M, Harada H, Asakura H, et al. Frequency and characteristics of docetaxel-induced radiation recall phenomenon. Int J Radiat Oncol Biol Phys. 2006;66:1187–91. doi: 10.1016/j.ijrobp.2006.05.073. [DOI] [PubMed] [Google Scholar]
- 60.Putnik K, Stadler P, Schafer C, Koelbl O. Enhanced radiation sensitivity and radiation recall dermatitis (rrd) after hypericin therapy—case report and review of the literature. Radiat Oncol. 2006;1:32. doi: 10.1186/1748-717X-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Azria D, Magne N, Zouhair A, et al. Radiation recall: a well recognized but neglected phenomenon. Cancer Treat Rev. 2005;31:555–70. doi: 10.1016/j.ctrv.2005.07.008. [DOI] [PubMed] [Google Scholar]