Abstract
Emotions, stress, hunger, and circadian rhythms all promote wakefulness and behavioral arousal. Little is known about the pathways mediating these influences, but the orexin-producing neurons of the hypothalamus may play an essential role. These cells heavily innervate many wake-promoting brain regions, and mice lacking the orexin neurons have narcolepsy and fail to rouse in response to hunger (Yamanaka et al. [2003] Neuron 38:701–713). To identify the afferents to the orexin neurons, we first injected a retrograde tracer into the orexin neuron field of rats. Retrogradely labeled neurons were abundant in the allocortex, claustrum, lateral septum, bed nucleus of the stria terminalis, and in many hypothalamic regions including the preoptic area, dorsomedial nucleus, lateral hypothalamus, and posterior hypothalamus. Retrograde labeling in the brainstem was generally more modest, but labeling was strong in the periaqueductal gray matter, dorsal raphe nucleus, and lateral parabrachial nucleus. Injection of an anterograde tracer confirmed that most of these regions directly innervate the orexin neurons, with some of the heaviest input coming from the lateral septum, preoptic area, and posterior hypothalamus. In addition, hypothalamic regions preferentially innervate orexin neurons in the medial and perifornical parts of the field, but most projections from the brainstem target the lateral part of the field. Inputs from the suprachiasmatic nucleus are mainly relayed via the subparaventricular zone and dorsomedial nucleus. These observations suggest that the orexin neurons may integrate a variety of interoceptive and homeostatic signals to increase behavioral arousal in response to hunger, stress, circadian signals, and autonomic challenges.
Indexing terms: hypocretin, narcolepsy, sleep, lateral hypothalamus, appetite, arousal
Wakefulness and behavioral arousal are influenced by emotions, stress, hunger, circadian rhythms, and many other processes. In part, this arousal is mediated by classical ascending monoaminergic and cholinergic systems, but much recent research has highlighted the importance of orexin-A and -B (hypocretin-1 and -2) in controlling behavioral state. These neuropeptides are made by a small cluster of cells in the perifornical and lateral regions of the hypothalamus that project extensively to brain nuclei implicated in the control of behavioral state, appetite, and autonomic function (de Lecea et al., 1998; Peyron et al., 1998; Sakurai et al., 1998). In mice and humans, a loss of the orexin neurons produces all the symptoms of narcolepsy, including frequent naps, intrusions of cataplexy and other REM sleep-like phenomena into wakefulness, as well as mild obesity (Peyron et al., 2000; Thannickal et al., 2000; Hara et al., 2001; Crocker et al., 2005). In addition, mice lacking the orexin neurons fail to rouse in response to a lack of food (Yamanaka et al., 2003). Most likely, the orexin neurons help coordinate feeding, metabolism, and autonomic activity with the control of arousal at the appropriate time of day (Willie et al., 2001).
While it is clear that the orexin neurons are necessary for the control of behavioral state, surprisingly little is known about what regions govern their activity. A few studies have examined the effects of wake-promoting neurotransmitters such as monoamines and acetylcholine on the activity of the orexin neurons, while others have focused on appetite-regulating peptides (Yamanaka et al., 2002, 2003; Fu et al., 2004). However, the relative importance of these signals is difficult to estimate, as none of these studies have examined the full spectrum of inputs to the orexin neurons. To systematically determine the afferents to the orexin neurons, we used retrograde and anterograde tracing to identify direct inputs to the orexin neurons.
MATERIALS AND METHODS
Retrograde and anterograde tracing
These experiments used male Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) weighing 270–400 gm. Rats were anesthetized with chloral hydrate (350 mg/kg. i.p.) and placed in a stereotaxic apparatus. To identify potential afferents to the orexin neurons, we injected the retrograde tracer cholera toxin B (CTB) subunit (List Biological, Campbell, CA; 1% in saline) into the orexin field and nearby parts of the hypothalamus using a glass micropipette (tip diameter ~20 μm) and a compressed air delivery system (Picospritzer III, Intracel, Hertfordshire UK). Small volumes (6–9 nl) were delivered by directly measuring the displacement of the CTB meniscus with a calibrated reticule on an operating microscope. The orexin field injections were placed 2.5–3.5 mm caudal to bregma, 8.0–8.4 mm below the dura, and 1.2–1.8 mm lateral to the midline (Paxinos and Watson, 1997). We injected CTB into 65 rats and present results from six injections completely confined to the orexin neuron field and six injections adjacent to the field. To determine which retrogradely labeled regions specifically innervate the orexin neurons, we then injected the anterograde tracer biotinylated dextrans (BD; MW 10,000; Molecular Probes, Eugene OR; 25% in saline; 9–21 nl) into various nuclei of 186 rats. We present the results of 46 informative injections. All procedures were approved by the Harvard Medical School and Beth Israel Deaconess Medical Center Animal Care and Use Committees.
Tissue preparation
Seven days after tracer injections, rats were deeply anesthetized with chloral hydrate (700 mg/kg. i.p.) and transcardially perfused with 100–300 ml of 0.9% saline followed by 500 ml of neutral buffered 10% formalin (pH 7.0; Sigma, St. Louis, MO). Brains were removed, post-fixed for 4 hours in formalin, and cryoprotected overnight in 20% sucrose with 0.02% sodium azide (Sigma). Brains were then sectioned at 40 μm into a 1:4 series on a freezing microtome.
Immunohistochemistry
All tissue was treated with PBT (phosphate-buffered saline (PBS), pH 7.4, with 0.25% Triton X-100) containing 1% hydrogen peroxide for 30 minutes to inactivate endogenous peroxidases. After three 5-minute rinses in PBS, tissue for the retrograde tracing experiment was incubated overnight in goat anti-CTB primary antiserum (1: 100,000; List Biological). Sections were then incubated for 1 hour in biotinylated secondary antiserum (donkey antigoat IgG, 1:500; Jackson ImmunoResearch, West Grove, PA) followed by avidin-biotin complex (1:500; Vectastain ABC Elite Kit; Vector Laboratories, Burlingame, CA) for another 1 hour, with three 5-minute rinses in PBS between each step. CTB-immunoreactive (-IR) neurons were visualized by reaction with 3,3′-diaminobenzidine (DAB; SK-4100; Vector) for 6 minutes. To determine whether the CTB injection site was within the orexin field, sections were then incubated overnight with rabbit anti-orexin-A primary antiserum (1:3,000; Novus Biologicals, Littleton, CO) followed by a 1-hour incubation in biotinylated donkey antirabbit IgG secondary antiserum (1:500; Jackson ImmunoResearch). After 1 hour in ABC, sections were treated with Vector SG Blue kit (SK-4700; Vector) to produce a blue reaction product in orexin-IR neurons. Sections were then Nissl-stained with thionin to define nuclear boundaries. The orexin-A antiserum produced no staining in hypothalamic sections from prepro-orexin knockout mice (Chemelli et al., 1999). Incubation of 1 ml orexin-A antiserum with 50 μg of orexin-A peptide eliminated all immunoreactivity.
For the anterograde tracing experiment, after rinses and peroxidase treatment, sections were incubated in ABC for 1 hour and BD was visualized by reaction with DAB for 6 minutes. After PBS washes the tissue was incubated overnight in rabbit anti-orexin-A primary antiserum followed by a 1-hour incubation in biotinylated donkey antirabbit IgG secondary antiserum, 1 hour in ABC, and treatment with Vector SG Blue kit to produce a blue reaction product in orexin-IR neurons.
Sections were photographed with a digital camera (Nikon D100, Nikon, Tokyo, Japan) mounted directly on a Zeiss microscope. We adjusted only the sharpness, contrast, and brightness of images using PhotoShop (Adobe Systems, San Jose, CA). The distributions of cells were plotted from dark- and bright-field digital photomicrographs (Kodak DCS460) using Canvas (Deneba Systems, Miami, FL).
Analysis of retrograde labeling
To provide quantitative results, we selected six injections of CTB limited to the orexin field and counted every ipsilateral, retrogradely labeled cell in a 1:4 series from the olfactory bulb to the caudal medulla. We counted only those CTB-IR neurons containing a nucleus and then calculated the number of retrogradely labeled cells in each nucleus as a percentage of the total number of retrogradely labeled neurons. We did not count cells within the CTB injection site because CTB may have been taken up by local soma instead of terminals. Although each case received close to the same volume of CTB, the injections adjacent to the fornix intensely labeled neurons in the subiculum, most likely because CTB was taken up by fibers of the fornix. Therefore, labeling in the subiculum was excluded from the overall analysis. Because small regions may be underrepresented by this analysis, we also used a semiquantitative measure of labeling density: We counted the number of retrogradely labeled neurons in a representative 200 × 200 μm box and assigned a density measure from 0 (none) to 5 (densely packed cells). Specifically, we defined a density of 1 as 1–10 retrogradely labeled cells within the counting box, a density of 2 as 11–20 cells, a density of 3 as 21–30 cells, a density of 4 as 31–40 cells, and a density of 5 as > 40 cells.
Analysis of anterograde labeling
To measure the extent to which different regions innervate the orexin neurons, we counted the number of orexin neurons apposed by BD-labeled terminals. First, we examined appositions throughout the anteroposterior length of the orexin field and noted no rostral-caudal variations in the percent of orexin neurons receiving appositions; because these counts are very time-consuming, this anteroposterior analysis was performed on a subset of 10 cases with BD injections into the lateral septum, bed nucleus of the stria terminalis, medial preoptic area, anterior hypothalamic area, posterior hypothalamus, ventral tegmental area, and dorsal raphe. We then analyzed one section from the center of the orexin field (2.8–3.3 mm behind bregma) in every BD case. Orexin-IR neurons were examined for appositions at × 1000 and an apposition was defined as a BD-labeled bouton immediately abutting and in the same focal plane as the orexin neuron (Fig. 1A). Results are reported as the percent of orexin neurons in that section receiving at least one apposition. To measure whether inputs innervated different parts of the orexin field, we divided the field into three regions: a perifornical region defined by a 200 × 500 μm rectangle centered on the fornix with the bottom edge just below the fornix; a 400 × 500 μm medial region; and a 400 × 500 μm lateral region (Fig. 1B). These boxes were defined to apportion roughly equal numbers of orexin neurons into each of the three regions; across all anterograde tracing cases, the medial, perifornical, and lateral regions each contained an average of 65, 47, and 50 orexin-IR neurons/section, respectively.
Fig. 1.
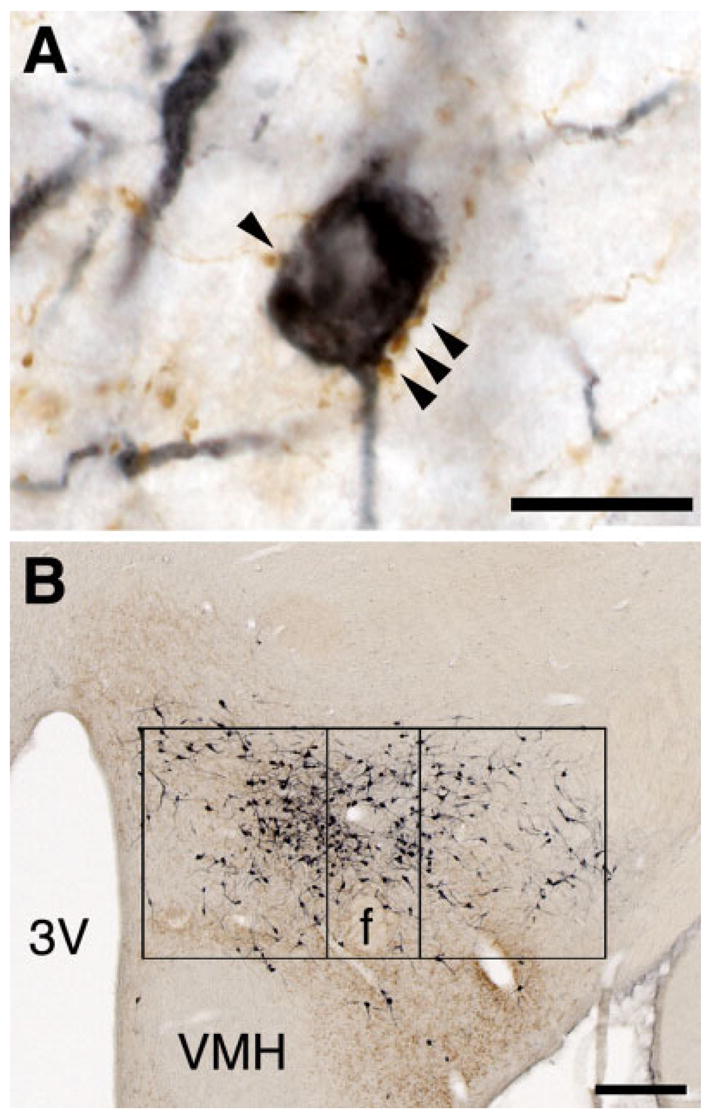
Innervation of the orexin neurons. A: With an injection of BD into the lateral septum, several brown, anterogradely labeled boutons closely apposed a dark blue, orexin-IR neuron. B: This injection labeled fibers in the medial and perifornical parts of the orexin field, with fewer fibers in the lateral part. Scale bars = 50 μm in A; 200 μm in B.
RESULTS
Experiment 1: retrograde tracing from the orexin field
One week after injection of CTB into the hypothalamus, we double-labeled brain sections for CTB and orexin and selected six injections limited to the orexin neuron field. The injections in cases 327 and 377 were centered in the perifornical part of the orexin neuron field, with slight extension of CTB towards the mammillothalamic tract in case 327 (Fig. 2). The CTB injections in cases 314 and 380 were located in the lateral hypothalamic portion of the orexin neuron field. The injections in cases 80 and 87 hit the medial part of the orexin field.
Fig. 2.
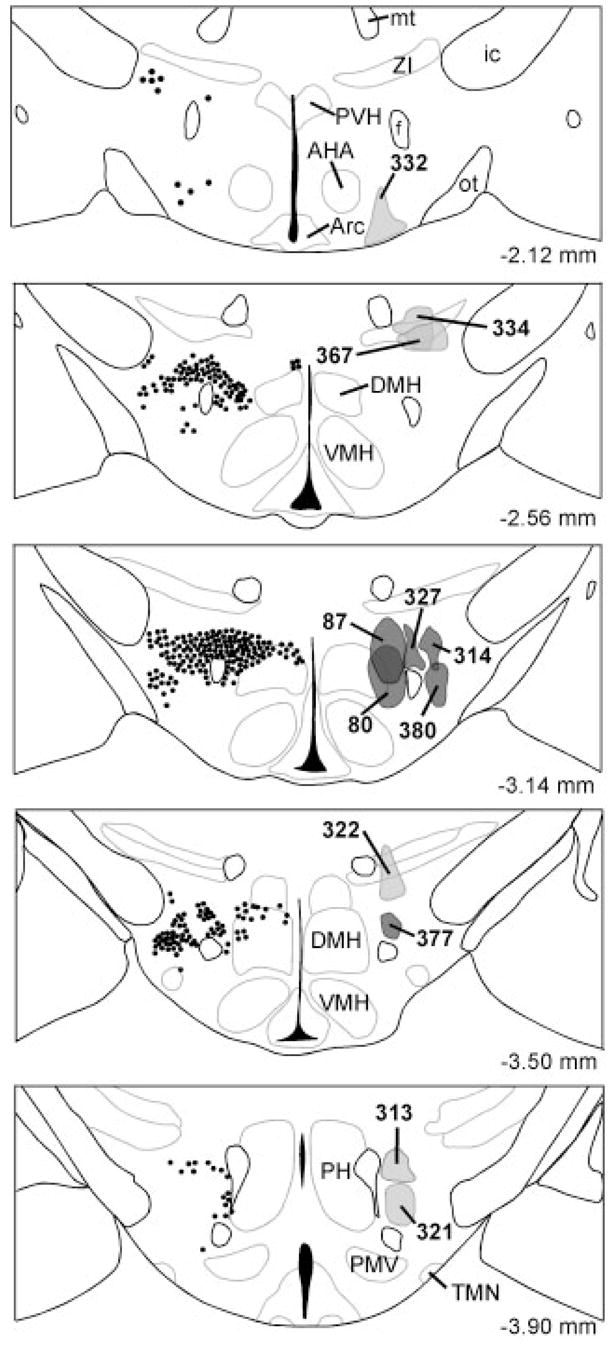
Distribution of CTB injection sites. The left side of each panel shows the typical distribution of orexin neurons. On the right side, injections into the orexin field are labeled in dark gray, and control injections adjacent to the field are labeled light gray. The distance behind bregma is noted at the bottom right.
General pattern of retrograde labeling
All six injections into the orexin neuron field retrogradely labeled a similar number of cells (17,100 ± 900 cells/series). Most labeled neurons were ipsilateral to the injection site, with about 20% of all cells in a similar distribution contralaterally. In general, each injection produced similar patterns of labeling, although injections into the medial part of the orexin field labeled more neurons in the amygdala, and lateral injections labeled more cells in the accumbens shell (AcbSh) and bed nucleus of the stria terminalis (BST). The perifornical cases had more retrograde labeling in the cortex.
Hypothalamic afferents
The hypothalamus contained the greatest number of CTB-labeled neurons, accounting for about one-third of all labeled cells throughout the brain (Table 1; Fig. 3). The medial preoptic area (MPA) contained a large number of labeled cells (5–7% of the number in the entire brain) that were uniformly distributed across the entire region. CTB-labeled neurons were also common in the lateral preoptic area (LPO; 3–5%), especially in the perifornical and lateral cases. The ventrolateral preoptic area (VLPO) and median preoptic nucleus contained a moderate density of labeled neurons. The subfornical organ and organum vasculosum of the lamina terminalis both contained <1% of all retrogradely labeled cells. In all six cases, the suprachiasmatic nucleus (SCN) contained only one or two retrogradely labeled cells. The subparaventricular zone contained few labeled neurons (<0.5%), but the adjacent anterior hypothalamic area contained about 1–2% of retrogradely labeled cells with a higher density in the medial and perifornical cases.
TABLE 1.
Main Sites of Retrograde Labeling after Injections of CTB into the Orexin Field1
| Medial region (cases 80, 87)
|
Perifornical region (cases 327, 377)
|
Lateral hypothalamic region (cases 314, 380)
|
||||
|---|---|---|---|---|---|---|
| % | Cell density | % | Cell density | % | Cell density | |
| Hypothalamus | 31.3 | 34.1 | 33.2 | |||
| Median preoptic nucleus | 1.1 | 3.0 | 0.8 | 3.0 | 1.4 | 3.0 |
| Medial preoptic area | 5.0 | 3.0 | 6.5 | 3.5 | 6.9 | 2.5 |
| Lateral preoptic area | 1.6 | 1.5 | 3.4 | 2.5 | 4.5 | 2.5 |
| Ventrolateral preoptic area | 0.2 | 1.0 | 0.2 | 4.0 | 0.1 | 3.0 |
| Anterior hypothalamic area | 2.4 | 3.0 | 1.4 | 3.0 | 0.7 | 1.0 |
| Paraventricular nucleus | 1.1 | 2.5 | 1.1 | 2.5 | 1.1 | 3.0 |
| Dorsomedial hypothalamic nucleus | 1.1 | 2.0 | 1.3 | 2.5 | 1.4 | 2.0 |
| Ventromedial hypothalamic nucleus | 4.4 | 4.0 | 2.2 | 4.5 | 2.2 | 4.0 |
| Arcuate nucleus | 2.8 | 2.5 | 1.3 | 3.0 | 1.5 | 3.0 |
| Lateral hypothalamic area | 3.4 | 1.5 | 6.3 | 5.0 | 5.0 | 4.0 |
| Posterior hypothalamic nucleus | 1.9 | 2.0 | 3.2 | 3.5 | 3.3 | 3.5 |
| Cortex | 18.9 | 21.0 | 14.1 | |||
| Medial orbital cortex | 1.1 | 1.5 | 1.4 | 3.0 | 1.1 | 3.5 |
| Prelimbic cortex | 1.6 | 1.5 | 2.3 | 3.5 | 0.9 | 2.0 |
| Dorsal peduncular cortex | 1.5 | 3.0 | 1.3 | 4.0 | 0.9 | 3.0 |
| Infralimbic cortex | 0.9 | 2.0 | 2.0 | 5.0 | 1.3 | 3.0 |
| Claustrum | 2.3 | 3.0 | 4.6 | 3.0 | 4.6 | 3.5 |
| Agranular and granular insular cortex | 2.1 | 1.5 | 1.9 | 2.5 | 2.7 | 3.0 |
| Basal ganglia | 5.5 | 5.3 | 9.9 | |||
| Accumbens nucleus, shell | 1.3 | 2.5 | 0.8 | 2.0 | 4.9 | 4.0 |
| Between accumbens shell and claustrum | 2.8 | 4.0 | 1.8 | 4.0 | 2.5 | 4.0 |
| Ventral pallidum | 1.2 | 1.0 | 1.6 | 3.0 | 2.3 | 2.5 |
| Septum | 13.2 | 12.8 | 11.9 | |||
| Lateral septal nucleus, dorsal part | 1.0 | 2.0 | 1.2 | 2.5 | 2.4 | 4.0 |
| Lateral septal nucleus, intermediate part | 9.9 | 3.5 | 10.7 | 5.0 | 8.1 | 4.5 |
| Lateral septal nucleus, ventral part | 1.6 | 1.5 | 0.6 | 1.0 | 0.7 | 1.0 |
| Bed nucleus of the stria terminalis | 6.7 | 5.5 | 9.8 | |||
| BST, medial division, anterior | 1.8 | 2.5 | 1.4 | 4.5 | 2.6 | 4.0 |
| BST, medial division, ventral | 0.5 | 2.5 | 0.3 | 2.5 | 1.1 | 4.5 |
| BST, medial division, posterolateral | 0.4 | 1.0 | 0.9 | 3.0 | 1.1 | 3.0 |
| BST, lateral division, ventral | 0.6 | 3.0 | 1.0 | 4.5 | 1.4 | 5.0 |
| BST, lateral division | 0.7 | 2.0 | 0.3 | 1.5 | 0.2 | 3.0 |
| BST, lateral division, posterior | 0.3 | 2.0 | 0.3 | 2.5 | 0.9 | 3.5 |
| Parastrial nucleus | 0.2 | 1.5 | 0.5 | 3.5 | 0.4 | 2.0 |
| Basal forebrain | 2.5 | 2.5 | 2.7 | |||
| Medial septum | 0.0 | 0.5 | 0.0 | 0.5 | 0.0 | 0.5 |
| Horizontal limb of the diagonal band | 0.1 | 1.0 | 0.4 | 2.0 | 0.4 | 1.5 |
| Substantia inominata | 2.0 | 1.0 | 1.4 | 3.0 | 1.7 | 2.5 |
| Amygdala | 7.1 | 4.9 | 4.9 | |||
| Medial amygdala | 3.9 | 4.0 | 1.3 | 2.5 | 0.6 | 1.5 |
| Central amygdaloid nucleus, medial div. | 0.2 | 1.0 | 0.5 | 3.5 | 1.3 | 3.5 |
| Central amygdaloid nucleus, lateral div. | 0.9 | 4.5 | 0.9 | 5.0 | 1.2 | 5.0 |
| Basolateral amygdaloid nucleus | 0.9 | 1.5 | 1.2 | 2.0 | 1.2 | 3.0 |
| Hippocampus | — | — | — | |||
| Subiculum | — | 4.5 | — | 5.0 | — | 3.0 |
| Brainstem | 12.9 | 13.2 | 16.2 | |||
| Substantia nigra | 0.3 | 1.0 | 0.9 | 2.0 | 0.9 | 2.0 |
| Ventral tegmental area | 0.6 | 1.5 | 0.2 | 1.5 | 0.3 | 2.5 |
| Lateral periaqueductal gray | 1.2 | 2.0 | 3.1 | 4.0 | 2.3 | 3.0 |
| Ventrolateral periaqueductal gray | 0.4 | 2.0 | 0.4 | 3.0 | 0.3 | 3.0 |
| Dorsal raphe | 0.4 | 1.5 | 0.5 | 3.5 | 0.8 | 3.5 |
| Median raphe | 0.3 | 1.5 | 0.2 | 1.5 | 0.3 | 1.5 |
| Pedunculopontine tegmental nucleus | 0.4 | 2.0 | 0.0 | 1.0 | 0.3 | 2.0 |
| Laterodorsal tegmental nucleus | 0.3 | 1.5 | 0.6 | 4.0 | 0.2 | 3.0 |
| Lateral parabrachial nucleus | 1.8 | 3.0 | 2.3 | 4.0 | 3.5 | 4.0 |
| Pre-coeruleus | 0.4 | 3.0 | 0.1 | 3.0 | 0.5 | 5.0 |
| Locus coeruleus | 0.1 | 1.0 | 0.2 | 2.0 | 0.0 | 2.5 |
After injections of CTB into the perifornical or lateral parts of the orexin field we counted every retrogradely labeled neuron from the olfactory bulb to the caudal medulla. The number of CTB-labeled neurons within each brain region is expressed as a percentage of the total number of retrogradely labeled cells along with the relative density of retrograde labeling. The hypothalamus contained about one-third of all CTB-IR cells, and the LS, BST, and limbic parts of the cortex were also moderately to heavily labeled. Relative cell counts were averaged for injections into the medial, perifornical, or lateral parts of the orexin field. Regions with relatively few retrogradely labeled neurons are not listed.
Fig. 3.
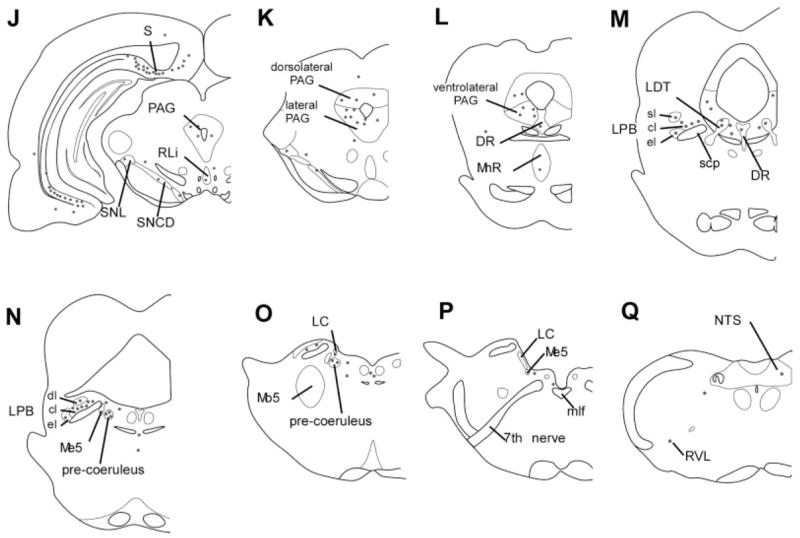
Distribution of retrogradely labeled neurons after an injection of CTB into the perifornical part of the orexin field (case 327). Each dot represents five neurons. The heaviest retrograde labeling was in the infralimbic and dorsal peduncular cortices, lateral septum, bed nucleus of the stria terminalis, amygdala, lateral and medial preoptic areas, hypothalamic regions (anterior hypothalamic area, VMH, DMH, PH), and brainstem regions including the PAG, DR, and pre-coeruleus. This injection extended slightly into the zona incerta and also labeled the cingulate cortex, an area not labeled with most other orexin field injections. sl, cl, el, dl, superior lateral, central lateral, external lateral, and dorsal lateral subnuclei of the LPB.
In the middle third of the hypothalamus, the parvocellular regions of the paraventricular nucleus (PVH) contained many CTB-IR neurons, but labeling of magnocellular neurons was rare. The entire dorsomedial nucleus (DMH) contained many cells (1–2%), even in the compact portion, and this labeling was clearly distinct from the injection sites. The ventromedial hypothalamic nucleus (VMH) also had heavy retrograde labeling (2–4%) throughout the nucleus. The arcuate nucleus (Arc) contained moderate to heavy labeling (1–3%), and cells were evenly distributed along its length. Labeling in the VMH and Arc was heavier with the medial injections of CTB. The dorsal hypothalamic area and ventrolateral hypothalamic area contained few (<0.3%) retrogradely labeled cells.
In the posterior third of the hypothalamus, the lateral hypothalamic area (LHA) and perifornical area contained many labeled neurons. We could not reliably count cells in these regions because CTB may have been taken up by dendrites or soma of nearby neurons, but adjacent to the injection sites the LHA and perifornical area were densely packed with hundreds of cells. The rostral portions of the posterior hypothalamic area (PH) also contained many densely packed CTB-IR neurons (2–3%), especially with CTB injections just dorsal to the fornix (cases 314, 327, and 377). The dorsomedial and ventrolateral subnuclei of the tuberomammillary nucleus contained a moderate density of retrogradely labeled cells. In all cases the dorsal and ventral premammillary nuclei were densely packed with labeled neurons. Labeling in the medial mammillary regions was light and variable, and the supramammillary nucleus was heavily labeled only in case 380.
Telencephalic afferents
The septum, BST, basal forebrain, amygdala, and limbic parts of the cortex accounted for most of the telencephalic inputs to the orexin neuron region.
Overall, the cortex contained 10–20% of all retrogradely labeled cells, and most of these were large neurons in the deeper layers of allocortex. All injections, but especially the perifornical cases, showed moderate labeling (1–2%) in the medial orbital, infralimbic (IL), and dorsal peduncular cortices. The perifornical injections produced moderate to heavy labeling in prelimbic (PrL) cortex, with light labeling in cingulate cortex. The cingulate cortex was heavily labeled only in cases 87 and 327, possibly because these dorsal injections extended slightly into the zona incerta. Layer 3 of the granular and agranular parts of insular cortex contained moderate to heavy labeling (2–3%), particularly in the lateral hypothalamic cases. With all injections the claustrum was very heavily labeled (2–5%), especially along the rostral edge of the AcbSh. The amygdalo-piriform transition area, amygdaloid hippocampal area, lateral entorhinal, dorsal tenia tecta, and dorsal endopiriform cortex contained minimal labeling (<1% each). Very few neurons were retrogradely labeled in most other cortical regions, including piriform, perirhinal, motor, somatosensory, and most association areas.
Labeling in the basal ganglia was limited to ventral structures that actually may be part of the extended amygdala (Alheid et al., 1995). The AcbSh contained moderate to heavy labeling (5%) with the lateral injections and light labeling (1%) in the perifornical and medial cases. Retrogradely labeled cells were rare in the accumbens core. The ventral pallidum contained moderate labeling (1–2%), especially with the lateral injections. No labeling was apparent in the caudate, putamen, or globus pallidus.
The lateral septum contained some of the densest retrograde labeling in the entire brain, accounting for 11–13% of all retrogradely labeled cells. The intermediate part of the lateral septum was densely labeled and contained most of these cells (8–11%). The dorsal part of the LS had fewer cells, but the labeling was still substantial and often dense, especially with injections into the lateral part of the orexin field. The medial injections produced some light labeling in the ventral part of the LS and septofimbrial nucleus, and other parts of the septum contained few cells.
The BST was another major source of afferents to the orexin neuron region, containing 10% of all retrogradely labeled cells with injections of CTB into the lateral part of the orexin field and 5–6% with medial and perifornical injections. This retrograde labeling was most prominent in the anterior and ventral parts of the medial division and in the ventral part of the lateral division. The median and posterior divisions of the BST, other lateral divisions, and the parastrial nucleus contained a moderate number of cells (0.5–1%) at a medium to high density.
The basal forebrain contained a moderate number of retrogradely labeled cells, and these were mainly scattered in the substantia inominata (1–2%). CTB-IR cells were uncommon in the horizontal and vertical limbs of the diagonal band and rare in the medial septum and magnocellular preoptic nucleus.
In the amygdala, the lateral subdivision of the central nucleus (CeA) was densely packed with retrogradely labeled cells in all cases. The medial subdivision of the CeA contained a moderate number of cells and the central subdivision was lightly labeled. All cases contained a moderate number of scattered cells in the basolateral nuclei of the amygdala and anterior amygdaloid area. Injections into the medial parts of the orexin field labeled many cells in the medial amygdaloid nuclei (4% vs. ~1% in the other cases).
Hippocampal afferents
Injections of CTB into the lateral or medial parts of the orexin field labeled 200–500 cells in the intermediate and ventral subiculum, with fewer cells in the dorsal part. In contrast, injections abutting the fornix labeled 2,000–11,000 cells in the subiculum. As shown below, anterograde labeling from the subiculum labels appositions on very few orexin neurons, and these high cell counts are most likely retrograde labeling from the dense field of subiculum terminals around the fornix (Kishi et al., 2000). Because of this methodologic artifact, we excluded cell counts in the subiculum from our total cell counts. Two injections (cases 80 and 380) also retrogradely labeled some cells in CA1 (~1%).
Thalamic and epithalamic afferents
The thalamus contained relatively few retrogradely labeled neurons, except for some moderate labeling in the paraventricular thalamic nucleus and the medial and lateral habenula. Scattered, rare cells were present in the reunions nucleus.
Brainstem afferents
The brainstem contained 13–16% of all retrogradely labeled cells in a distinct pattern. The periaqueductal gray matter (PAG) was moderately labeled beginning just behind the thalamus, and moving caudally, this labeling became concentrated in the lateral PAG (2–3%), with fewer cells in the ventrolateral PAG (<0.5%). With perifornical and lateral injections, the substantia nigra contained ~1% of all retrogradely labeled cells, mainly in its lateral and compact portions. The ventral tegmental area (VTA) contained a few cells. All injections moderately labeled the laterodorsal tegmental nucleus, but labeling in the pedunculopontine tegmental nucleus was light. The interpeduncular nucleus was lightly labeled only with injections into the lateral part of the orexin field.
The dorsal raphe nucleus (DR) contained moderately dense labeling and the perifornical injections mainly labeled cells in the central portion of the DR. The median raphe and raphe magnus nuclei contained a modest number of cells in all cases, but labeling was light to absent in the raphe pallidus, paramedian raphe, and pontine raphe nucleus. Lateral injections retrogradely labeled rostral and caudal linear nuclei of the raphe more than the perifornical and medial cases.
The lateral subdivision of the parabrachial nucleus (LPB) was densely labeled, especially with CTB injections into the lateral part of the orexin field (2–3%). Most cells were in the central lateral subnucleus, but the superior lateral, dorsal lateral, and rostral parts of external lateral subnuclei also contained occasional cells.
The locus coeruleus (LC) contained only 10–15 retrogradely labeled cells that were limited to its rostral pole. All injections, but especially lateral injections, densely labeled a small cluster of neurons just ventral to the rostral pole of the LC. These “pre-coeruleus” neurons are dorsolateral to Barrington’s nucleus and are probably lateral to the cluster of cells that produce neuropeptide S (Lu et al., 2004; Xu et al., 2004). The rostral portions of the ventrolateral medulla contained light labeling. Labeling in the nucleus of the solitary tract (NTS) was very light.
Injections adjacent to the orexin field
To control for spread of tracer outside the orexin field, we also examined the pattern of retrograde labeling produced by injections adjacent to the orexin field (Fig. 2). Compared to injections into the orexin neuron field, the distribution of CTB-labeled cells in these control cases had some similarities but many important differences.
An injection near the anteroventral edge of the orexin field (case 332) robustly labeled magnocellular neurons in the PVH and supraoptic nuclei. This injection also produced much heavier labeling in the SCN and subparaventricular zone. The VMH and Arc contained more labeled cells, but the LPO and LHA contained fewer. The medial orbital and PrL cortices contained fewer CTB-IR cells, as did the AcbSh and BST. Labeling in the amygdala was in the basal and medial regions rather than the CeA. In the brainstem, labeling was reduced in the substantia nigra, VTA, and lateral PAG, but there were more cells in the rostral parts of the NTS.
We examined three injections along the dorsal edge of the orexin field. Two injections (cases 367 and 334) extended into the zona incerta and produced more retrograde labeling in all cortical regions, especially orbital and cingulate cortex. In the hypothalamus, labeling was reduced in the MPO, LPO, VLPO, PVH, and DMH. The AcbSh and ventral pallidum contained few cells. With injections that touched the zona incerta the thalamus was heavily labeled in many regions, especially the parafascicular nucleus, habenular nuclei, pretectal nuclei, and midline nuclei. Within the brainstem, all three injections produced less labeling in the VTA and NTS.
One injection along the posterior edge of the orexin field (cases 313) labeled very few neurons in the preoptic area, BST, and CeA, but a slightly more ventral injection (case 321) produced a pattern similar to that seen with injections of CTB into the orexin field. Both injections produced much less labeling in medial orbital, PrL, infralimbic, and insular cortex. The PAG, DR, and LC all contained fewer cells.
These control injections demonstrate that regions adjacent to the orexin field receive distinct patterns of inputs, but because few of the inputs to the orexin field are unique, we used anterograde labeling to determine which nuclei specifically innervate the orexin neurons.
Experiment 2: anterograde tracing with BD
We injected the anterograde tracer BD into nearly 30 brain regions identified in Experiment 1 as projecting to the orexin field (Fig. 4). Most of these were nuclei with heavy retrograde labeling, but some were lightly labeled areas of physiologic interest. Appositions onto orexin neurons were most numerous with injections into the LS, BST, MPA, and PH (Fig. 5). Some regions such as the cingulate cortex, lateral PAG, and subiculum sent projections into the orexin field but mainly apposed non-orexin neurons.
Fig. 4.
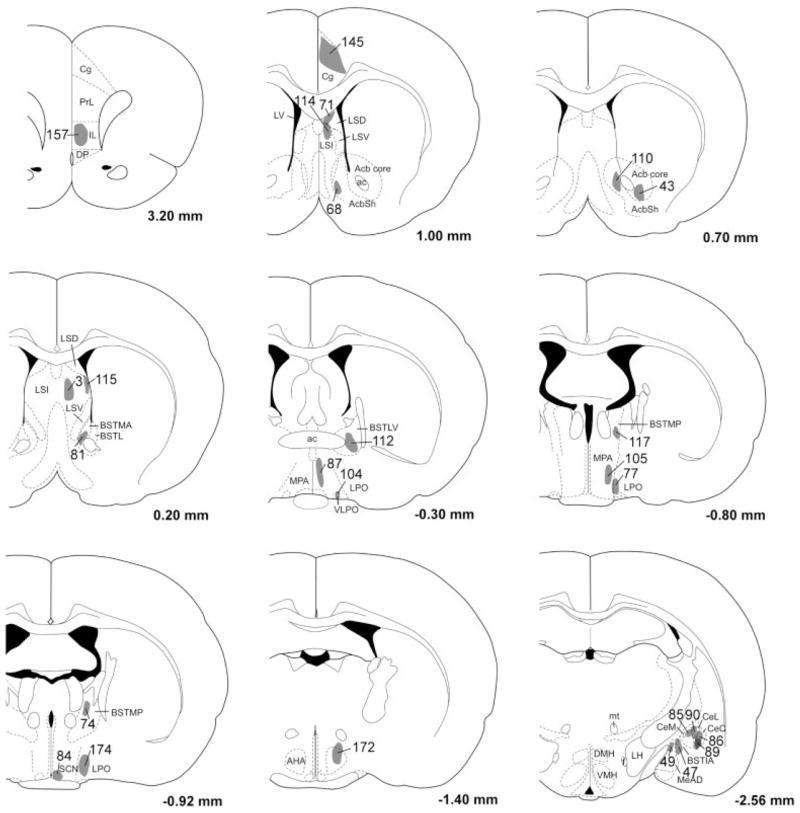
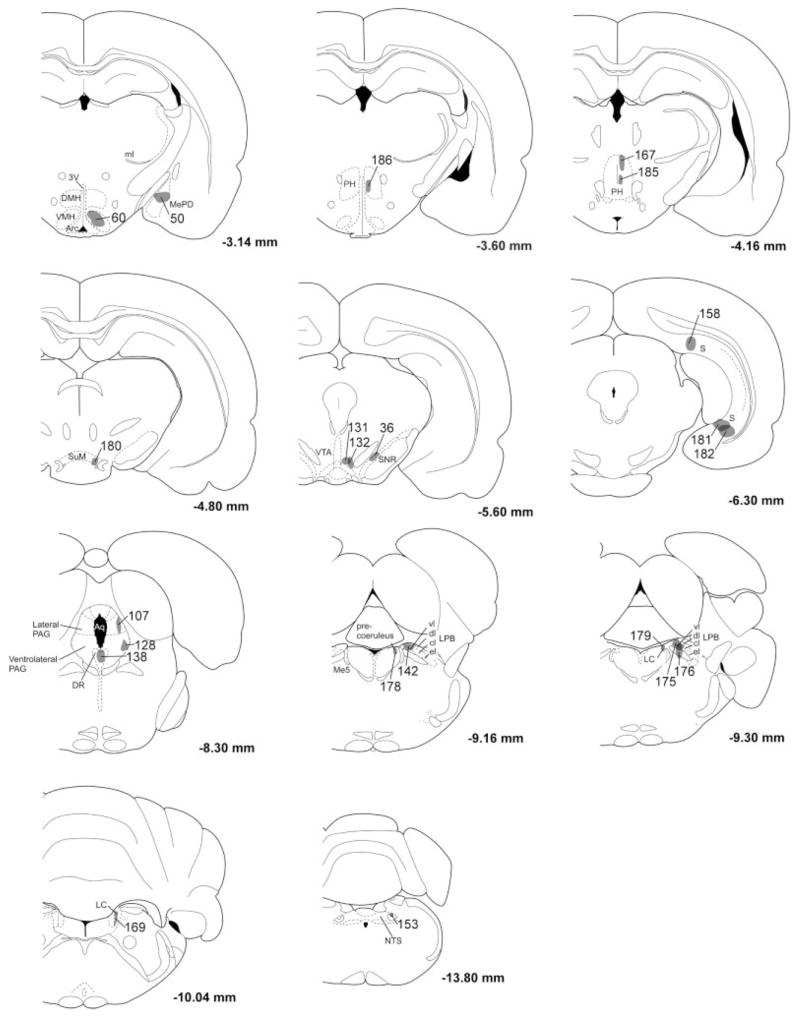
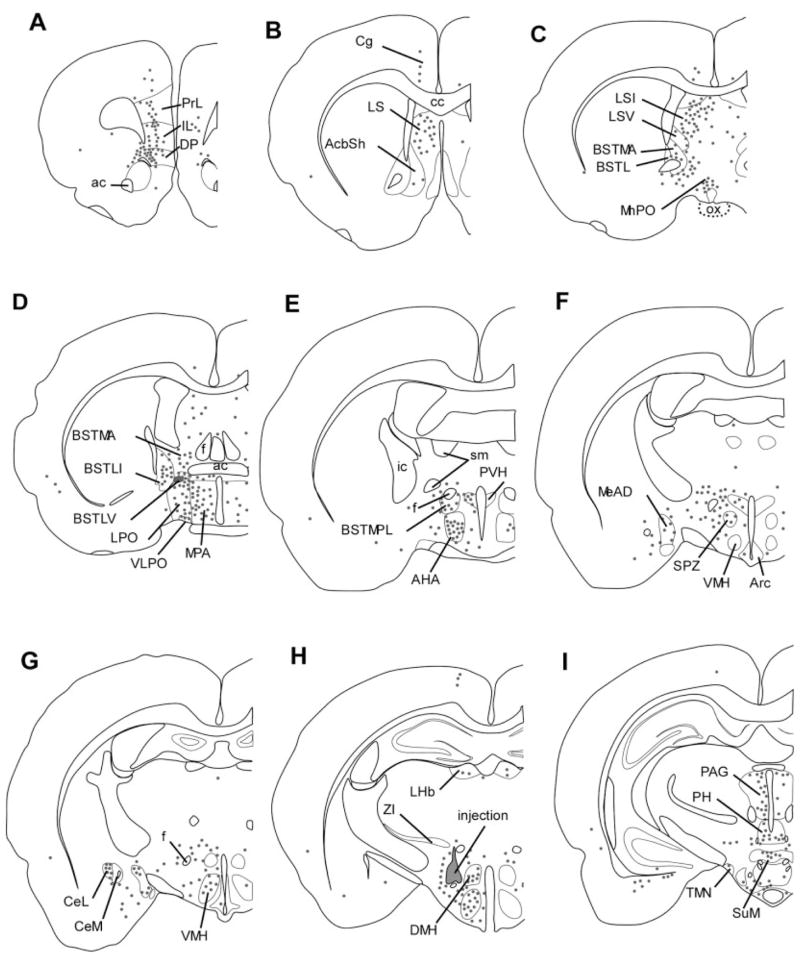
Distribution of BD injection sites. The anterograde tracer BD was injected into many brain regions thought to innervate the orexin neurons. Each BD injection site has been mapped onto template drawings (Paxinos and Watson, 1997), and to minimize clutter, some injections have been mapped to an adjacent section.
Fig. 5.
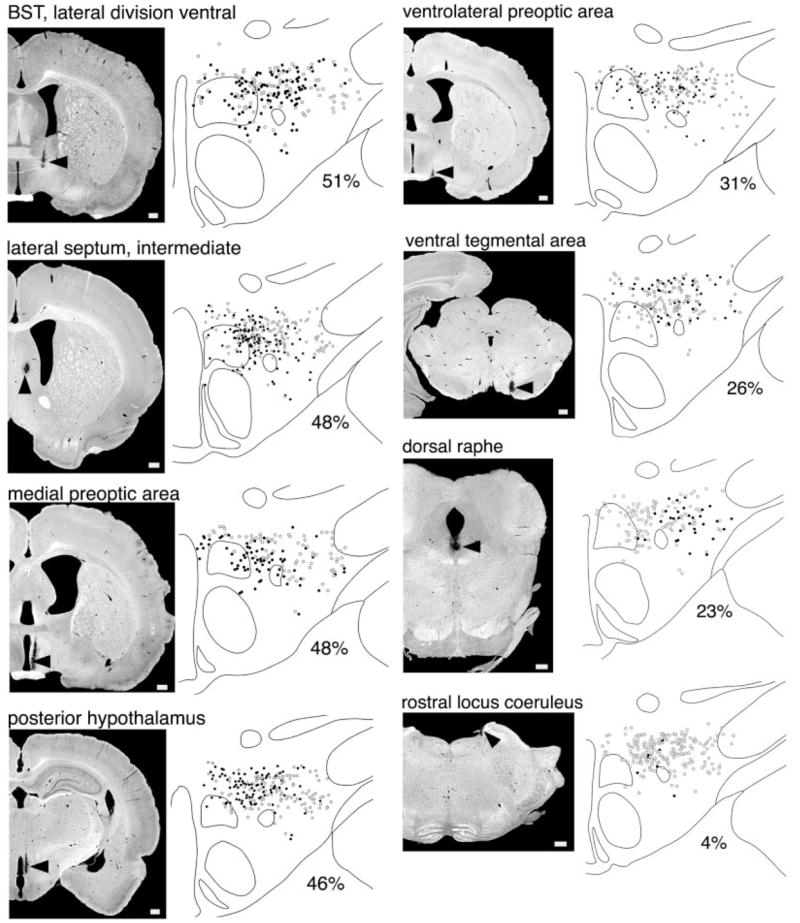
Anterograde labeling of afferents to the orexin neurons from several brain regions. The BD injection site is indicated by an arrow on the darkfield photomicrograph in each left-hand panel. The right-hand panel shows the distribution of orexin-IR neurons: black circles represent orexin neurons receiving appositions from the corresponding injection and white circles indicate orexin neurons with no apparent appositions. Below each drawing is noted the percent of all orexin neurons on that section that receive appositions. Hypothalamic inputs target orexin neurons in the medial and perifornical parts of the orexin field, whereas lighter inputs from brainstem regions tend to innervate orexin neurons in the lateral half of the field. Scale bars in the left panels = 500 μm.
Fibers from rostral regions generally followed two paths to the ipsilateral orexin field: a projection through the medial forebrain bundle (mfb), which extended dorsomedially up into the orexin field, and a medial path along the dorsal edge of the third ventricle that projected ventrolaterally through the dorsal hypothalamic area and DMH into the orexin field.
Hypothalamic inputs to the orexin neurons
Overall, the hypothalamus provided the heaviest innervation of the orexin neurons, with the densest inputs originating in the POA and PH. All hypothalamic regions that we injected preferentially innervated the medial part of the orexin field, with slightly fewer appositions in the perifornical region (Table 2). This pattern is consistent with prior studies of the medial zone of the hypothalamus, but even lateral regions (LPO and VLPO) sent few projections to the lateral orexin neurons.
TABLE 2.
Percent of Orexin Neurons Receiving Appositions in the Three Parts of the Orexin Field after Injections of BD1
| Part of the orexin field
|
||||
|---|---|---|---|---|
| Brain region | Number of orexin-IR neurons receiving appositions | Medial | Perifornical | Lateral |
| Ventrolateral preoptic area (2) | 56 | 30% | 24% | 16% |
| Medial preoptic area (2) | 71 | 55% | 43% | 39% |
| Lateral preoptic area (2) | 26 | 27% | 21% | 11% |
| Suprachiasmatic nucleus | 17 | 20% | 11% | 0% |
| Anterior hypothalamus | 21 | 21% | 17% | 5% |
| Ventromedial hypothalamus | 30 | 45% | 21% | 4% |
| Posterior hypothalamus (3) | 93 | 65% | 43% | 25% |
| Lateral septum, intermediate (4) | 112 | 63% | 43% | 26% |
| Nucleus accumbens, shell | 25 | 6% | 14% | 38% |
| Central amygdala, lateral subdivision | 39 | 14% | 30% | 30% |
| Medial amygdala, anterodorsal (2) | 16 | 9% | 11% | 20% |
| Substantia nigra | 42 | 16% | 24% | 60% |
| Ventral tegmental area (2) | 43 | 27% | 41% | 53% |
| Dorsal raphe | 31 | 3% | 24% | 42% |
Anterograde labeling with BD demonstrates that many nuclei preferentially innervate the medial or lateral parts of the orexin field. Hypothalamic nuclei mainly innervate orexin neurons in the medial and perifornical parts of the field, whereas inputs from the extended amygdala and brainstem tend to innervate neurons in the lateral part of the field. This pattern is exhibited by the 14 listed regions; 11 other regions appear to uniformly innervate the entire orexin field and are not listed. The heaviest innervation from each region is in bold. For some nuclei the numbers of appositions were averaged across several injections (number in parentheses).
Medial preoptic area
The MPA heavily innervated the orexin neurons. One injection (case 87; Fig. 5) into the lateral part of the medial preoptic nucleus labeled appositions on 48% of the orexin neurons and another into the caudal MPA (case 105) labeled boutons on 39% of the orexin-IR neurons. Both sites preferentially and heavily (five to six boutons/cell) innervated neurons in the medial and perifornical parts of the orexin field, especially just dorsomedial to the fornix. BD-labeled fibers mainly followed the mediodorsal path into the orexin field.
Lateral preoptic area
After an injection into the center of the VLPO (case 104), 31% of the orexin neurons received appositions. Neurons with appositions were most abundant dorsal and medial to the fornix, and many orexin neurons in the perifornical region received five or six boutons. Injections into the LPO just lateral and dorsal to the VLPO (cases 77 and 174) labeled appositions on 17% and 16%, respectively, of the orexin neurons in a similar distribution, although most neurons received only one or two appositions. In all cases, fibers coursed caudally through the mfb, but the VLPO injection also labeled fibers in the mediodorsal path.
Suprachiasmatic nucleus
An injection into the SCN that extended slightly into the ventral subparaventricular zone (case 84) lightly labeled boutons on 11% of the orexin neurons and all were medial to the fornix. These BD-labeled fibers densely innervated the periventricular nucleus and DMH but lightly innervated the orexin neurons.
Anterior hypothalamic area
Appositions contacted 13% of the orexin neurons after an injection of BD into the anterior hypothalamic area that extended partially into the subparaventricular zone (case 172). In the perifornical region, many orexin neurons received five or six contacts, but the density of appositions was less elsewhere. BD-labeled fibers ran through the medial aspect of the mfb to innervate the medial half of the orexin field.
Ventromedial hypothalamus
After an injection into the VMH (case 60), 23% of the orexin neurons received appositions. Most of these cells were medial to the fornix and had only one or two apparent synapses. BD-labeled fibers extended dorsally up into the medial part of the orexin field, DMH, and then into the dorsal hypothalamic area, with only rare fibers in the lateral part of the orexin field.
Posterior hypothalamus
Injection of BD into the PH of three animals (cases 167, 185, 186) labeled appositions on 32–58% of the orexin neurons, mostly dorsal and medial to the fornix. Two of these injections heavily (>5/cell) innervated orexin neurons in the perifornical and medial regions, although elsewhere in the field most orexin neurons received only one or two appositions. These fibers entered the dorsomedial part of the orexin field and then coursed in a ventrolateral direction, avoiding the DMH and VMH but innervating the orexin field.
Supramammillary nucleus
An injection into the supramammillary nucleus (case 180) targeted 17% of the orexin neurons with one or two appositions. BD-labeled fibers innervated orexin neurons in the perifornical and medial parts of the orexin field and also extended into the DMH, VMH, and Arc.
Telencephalic and diencephalic afferents to the orexin neurons
In contrast to inputs from the hypothalamus, most afferents from other forebrain regions innervated orexin neurons across the entire field, with slightly more appositions in the perifornical region.
Infralimbic cortex
After an injection into the IL (case 157), 22% of orexin-IR neurons received boutons, and these lightly innervated cells were located across the orexin neuron field even though fiber density was highest in the lateral hypothalamus.
Cingulate cortex
An injection into cingulate cortex area 1 (case 145) densely labeled fibers in the zona incerta and subincertal nucleus, but boutons apposed only 1% of the orexin neurons.
Nucleus accumbens
Projections from the AcbSh apposed orexin neurons in the lateral part of the field. One injection in the rostral part of the AcbSh (case 68) innervated a cluster of orexin neurons between the fornix and internal capsule, with many cells receiving five or six boutons, but overall, only 12% of the orexin neurons received appositions. Another injection, at the caudal edge of the AcbSh (case 110) lightly innervated 10% of the orexin neurons. Fibers from both injections ran through the lateral aspect of the mfb and extended mainly into the lateral part of the orexin field. An injection into the caudal core of the nucleus accumbens (case 43) labeled fibers along the medial edge of the internal capsule, but only one orexin neuron received an apposition.
Lateral septum
The intermediate and dorsal parts of the lateral septum heavily innervated the orexin neurons. Injections into the anterior or posterior portions of the intermediate part of the LS (cases 3 and 114) labeled boutons on 46–48% of the orexin neurons, especially medial and dorsal to the fornix with many cells receiving five or six appositions. In contrast, an injection into the posterolateral septum (case 115) lightly innervated only 10% of the orexin neurons. An injection into the anterior dorsal part of the LS (case 71) labeled appositions on 32% of the orexin neurons, with especially heavy input in the lateral and dorsal perifornical parts of the orexin field. In all cases, BD-labeled fibers coursed through the mfb and then radiated throughout the orexin field.
Bed nucleus of stria terminalis
The BST was a major source of inputs to the orexin neurons, but the intensity of anterograde labeling varied considerably between BST subnuclei. An injection into the ventral part of the lateral subdivision (case 112) innervated 51% of the orexin-IR cells, scattered across the field. An injection into the rostral, medial BST (case 81) innervated 20% of the orexin neurons. An injection into the posterior part of the medial division of the BST (case 117) innervated 37% of the orexin neurons, but an injection into the caudal edge of the medial division of the BST (case 74) innervated only 9% of the cells. With all BST injections, numerous BD-labeled fibers passed through the medial part of the mfb to appose orexin neurons across the field, especially in the perifornical region, where many neurons received five or six appositions.
Central amygdala
Injections of BD into the lateral subdivision of the CeA labeled appositions on 25% of the orexin neurons in case 90 and 13% of the orexin neurons with a slightly more anterior injection (case 85). With both injections, most innervated orexin neurons were in the lateral half of the orexin neuron field, and most of these cells received only one or two appositions. Fibers from the lateral subdivision coursed around the internal capsule to heavily innervate the perifornical region, but fiber density in other parts of the field was low. In contrast, with injections into the capsular part of the central nucleus only 2% of neurons were innervated in one case (case 86) and none in another (case 89).
Medial amygdala
Injections into the anterodorsal and posterodorsal parts of the medial amygdala (cases 49 and 50) labeled appositions on 15% and 10% of the orexin neurons, respectively. These cells were innervated in the lateral part of the orexin neuron field. Fibers from the medial amygdala coursed over the optic tract and around the internal capsule and spread across the entire orexin field. A slightly more lateral injection into the intraamygdaloid portion of the BST (case 47) lightly innervated only 4% of the orexin neurons.
Subiculum
With an injection into the dorsal subiculum (case 158), fibers were scarce in the orexin field and boutons apposed only 4% of the orexin neurons, mainly dorsal to the fornix. Two injections into the ventral subiculum (cases 181 and 182) labeled appositions on one or two orexin neurons, despite dense labeling of fibers in the fornix.
Brainstem afferents to the orexin neurons
Inputs from the brainstem ascended mainly in the dorsal tegmental bundle ventrolateral to the PAG or in a ventral pathway that ran past the subthalamic nucleus into the lateral aspect of the mfb. Projections from the substantia nigra, DR, and VTA preferentially innervated orexin neurons in the lateral part of the orexin field. Many of these brainstem inputs projected primarily to the ipsilateral orexin field, but fibers from the DR, ventrolateral PAG, and LC projected bilaterally.
Substantia nigra
An injection into the compact portion of the substantia nigra (case 36) labeled appositions on 31% of the orexin-IR neurons. Numerous BD-labeled fibers coursed through the LHA to heavily innervate orexin neurons in the lateral part of the field, but fibers and appositions were sparse in the medial and perifornical parts of the orexin field.
Ventral tegmental area
Injections of BD into the rostral (case 132) and caudal (case 131) VTA labeled one or two appositions on 26% and 33% of the orexin neurons across the field, but appositions were much more common in the lateral part of the field. Fibers from the VTA were sparse in the orexin field, DMH, and LHA but numerous just ventral to the fornix.
Periaqueductal gray matter
An injection into the ventrolateral PAG (case 128) labeled one or two boutons on 9% of the orexin neurons. These cells were scattered throughout the field but uncommon in the perifornical region. After an injection into the caudal lateral PAG (case 107), we noted a few boutons on 2% of the orexin neurons, all in the medial orexin field. With both injections, BD-labeled fibers ascended from the mfb into the dorsal hypothalamus, but fibers in the orexin field were sparse in case 107.
Dorsal raphe
An injection into the central portion of the anterior DR (case 138) very lightly innervated about 23% of the orexin neurons, mainly in the lateral orexin field. Fibers ascended bilaterally through the mfb and coursed through the orexin field with fewer fibers outside the field.
Lateral parabrachial nucleus
An injection into the rostral LPB (central lateral with some extension into the superior lateral subnucleus; case 142) bilaterally innervated 10% of the orexin neurons with one or two appositions. Slightly more caudal injections into the central lateral subnucleus (cases 175 and 176) lightly innervated 7% and 12% of the orexin neurons. In both cases, cells receiving appositions were scattered throughout the orexin field.
Locus coeruleus
After an injection into the rostral LC (case 179), the orexin field contained a few fibers, and 4% of the orexin neurons received one or two appositions. An injection into the caudal LC (case 169) labeled no appositions on the orexin neurons nor any fibers in the orexin field.
Pre-coeruleus
An injection into the pre-coeruleus that extended slightly into the rostral LC (case 178) labeled one or two appositions on 24% of the orexin neurons. Fibers lightly innervated the orexin field, forming appositions mainly in the perifornical and medial parts of the orexin field.
Nucleus of the solitary tract
An injection into the ventrolateral part of the caudal NTS (case 153) clearly labeled terminals in the PVH, but the orexin field contained no fibers.
DISCUSSION
Most prior research on the orexin neurons has focused on their interactions with systems controlling sleep/wake behavior or appetite, but we find that the orexin neurons receive afferents from a wide variety of brain regions (Fig. 6). The largest inputs are from other parts of the hypothalamus and from areas that regulate emotion and autonomic functions such as the infralimbic cortex, lateral septum, and BST. These observations suggest that the orexin neurons may be influenced by a variety of signals that govern homeostatic drives, behavioral state, and autonomic tone.
Fig. 6.
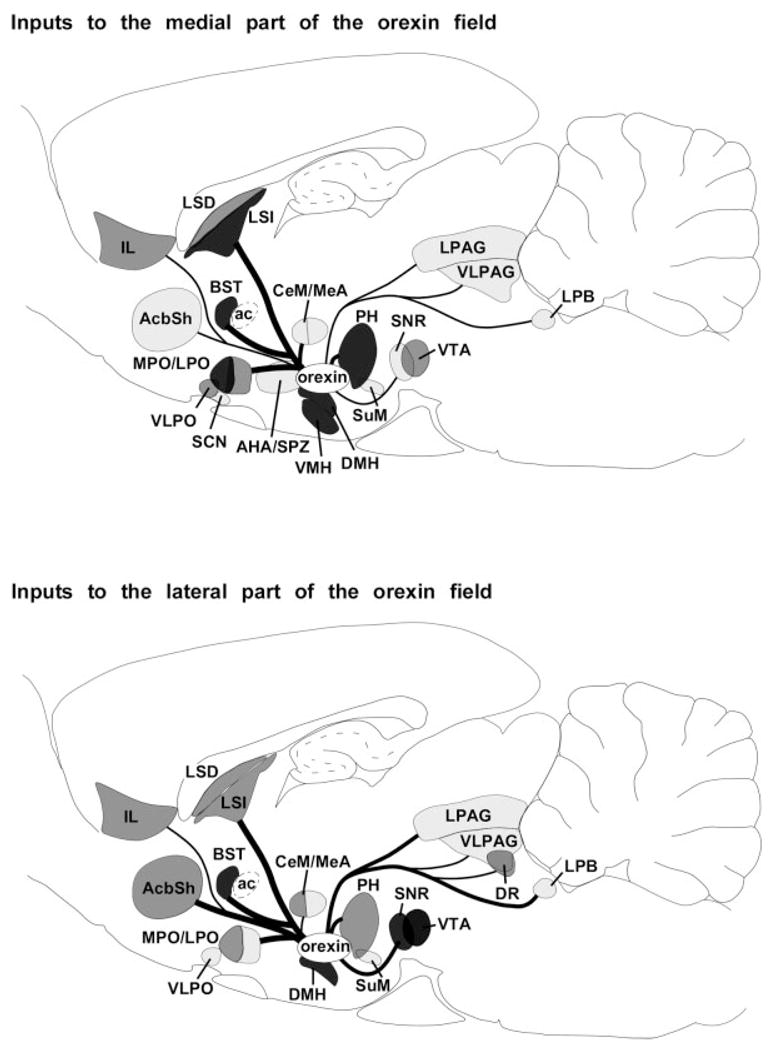
Summary of afferents to the orexin neurons. The upper panel shows inputs to orexin neurons in the medial part of the orexin field and the lower panel shows inputs to orexin neurons in the lateral part of the field. The heaviest inputs are from the pre-optic area, dorsomedial nucleus, posterior hypothalamus, lateral septum, and BST. This pattern suggests that the orexin neurons are influenced by signals regulating emotions, autonomic tone, appetite, circadian rhythms, and sleep/wake behavior. Regions labeled in dark, medium, and light gray innervate >45%, 25–44%, or 5–24% of the orexin neurons. Inputs that innervate <5% of the orexin neurons are not included. Line thickness indicates the relative number of retrogradely labeled neurons.
Methodological limitations
Our experiments provide a broad overview of the afferents to the orexin neurons, but some technical limitations warrant comment. First, our tracer injections cannot identify inputs from cells intermixed with the orexin neurons, and we may have overlooked local glutamatergic interneurons (Li et al., 2002). Second, the volume and spread of tracers varied slightly between injections, so our semiquantitative cell counts should be considered estimates. Nevertheless, the pattern of inputs appears reliable, as the intensity of anterograde labeling generally matched the retrograde labeling. Our quantitative analysis of appositions on the orexin neurons allowed us to identify a few exceptions to this pattern: the Cg, PAG, and subiculum project to the orexin field but provide little obvious input to the orexin neurons. Confocal microscopy might have demonstrated appositions more reliably, but electron microscopy will be needed to determine which of these appositions are true synapses and to examine inputs to distal dendrites. Still, as a broad survey of a wide range of afferents, our data provide a starting point for ultrastructural studies.
Comparison with prior studies
We find that the densest inputs to the orexin neurons originate from nearby hypothalamic regions in a pattern consistent with prior studies (Canteras et al., 1994; Vertes et al., 1995; Thompson and Swanson, 1998; Cvetkovic et al., 2003; Deurveilher and Semba, 2005). Some of the heaviest input comes from the LHA, PH, and DMH, and some of these LHA fibers may contain melanin-concentrating hormone (Guan et al., 2002), while some DMH fibers may contain glutamate and thyrotropin-releasing hormone (Chou et al., 2003). Innervation from the VMH and Arc was lighter, but these projections are interesting as they likely contain appetite-regulating peptides such as neuropeptide Y, agouti-related peptide, and α-melanocyte-stimulating hormone (Elias et al., 1998). Consistent with prior observations (Simerly and Swanson, 1988; Cvetkovic et al., 2003; Deurveilher and Semba, 2005), we find that preoptic regions such as the MPA and LPO heavily innervate the orexin neurons. Some preoptic projections may originate from sleep-promoting neurons (Sherin et al., 1998; McGinty et al., 2004), but others may arise from neurons that regulate thirst, body temperature, or sexual behavior.
Cortical inputs to the orexin field originate exclusively in regions governing emotion and autonomic functions, including the claustrum, insular, IL, dorsal peduncular, and medial orbital cortices. The IL moderately innervates orexin neurons in the perifornical part of the field in a pattern suggested by prior studies (Hurley et al., 1991; Vertes, 2004). The insular and dorsal peduncular cortices may preferentially innervate the lateral part of the orexin field (Floyd et al., 2001; Cvetkovic et al., 2003; Vertes, 2004), but these projections must be confirmed with anterograde tracing.
The lateral septum is one of the largest inputs to the orexin neurons, topographically innervating different parts of the orexin field. Consistent with earlier observations (Swanson and Cowan, 1979; Risold and Swanson, 1997; Cvetkovic et al., 2003), we find that the intermediate part of the LS mainly targets the medial part of the field and the dorsal part of the LS projects to the lateral part. In contrast, the central division of the extended amygdala (AcbSh, lateral BST, CeA) tends to innervate orexin neurons in the lateral half of the field, as predicted by previous experiments (Alheid et al., 1995; Petrovich et al., 2001).
Sakurai et al. (2005) recently examined the afferents to the orexin neurons using mice in which the human prepro-orexin promoter drives the expression of a tetanus toxin fragment fused to green fluorescent protein (TTC::GFP). This new technique is intended to induce abundant expression of tetanus toxin in the orexin neurons that should selectively label inputs to those cells. Just as in our study, this technique labeled neurons in the POA, VMH, DMH, and amygdala. However, TTC::GFP sometimes labeled regions with no known projections to the orexin field, including the medial septum and diagonal band of Broca, possibly from transport to second-order neurons or ectopic expression of the transgene. Injections of conventional tracers in mice could establish whether these discrepancies are due to species differences. The TTC::GFP technique also appears less sensitive than conventional tracers, as it failed to label many neurons in the IL, LS, VTA, or LPB—regions that probably innervate the orexin neurons as indicated by anterograde transport of BD. Conventional tracers such as CTB and BD have their limitations, and our results and those of Sakurai et al. still require validation with electron microscopy. Still, it is reassuring to see that the two techniques identify many of the same afferents, and future studies should help explain the differences.
Functional implications
The orexin neurons may promote arousal in response to signals regulating emotions and autonomic tone. Areas that control autonomic function including the IL, PrL, LS, BST, POA, DMH, and PH heavily innervate the orexin neurons, and orexin neuron activity is positively correlated with body temperature, heart rate, and blood pressure (Estabrooke et al., 2001; Marcus et al., 2003). In fact, high sympathetic tone delays the onset of sleep (Krauchi et al., 1999), and sympathetic activation may promote arousal by exciting the orexin neurons. Other inputs to the orexin neurons such as the IL, AcbSh, LS, BST, and amygdala also influence stress, aggression, and anxiety. Inhibition of the AcbSh with muscimol induces fos in orexin neurons lateral to the fornix (Zheng et al., 2003; Baldo et al., 2004), a pattern consistent with the projections of the AcbSh. Administration of corticotropin-releasing factor or stress from immobilization activates the orexin neurons and increases the production of prepro-orexin mRNA (Ida et al., 2000; Winsky-Sommerer et al., 2004). People with insomnia frequently experience anxiety, stress, and elevations in pulse, blood pressure, and metabolic rate (Bonnet and Arand, 1998, 2003), and excessive activation of the orexin neurons at night may contribute to their difficulty sleeping.
Sleep and wakefulness heavily influence the activity of the orexin neurons. The orexin neurons are active during wakefulness (Estabrooke et al., 2001; Yoshida et al., 2001; Zeitzer et al., 2003; Lee et al., 2005; Mileykovskiy et al., 2005), and we and others find moderately heavy inputs from wake-promoting brain regions including the DR and LDT (Sakurai et al., 2005). Appositions from the LC are rare, and while norepinephrine can inhibit the orexin neurons (Yamanaka et al., 2003; Li and van den Pol, 2005), the source, function, and importance of this norad-renergic input remains to be established. Among the regions that regulate behavioral state, the heaviest input originates in the VLPO and median preoptic nucleus, possibly in the sleep-promoting neurons that produce GABA (Sherin et al., 1998; McGinty et al., 2004). Most likely, these preoptic neurons inhibit the orexin neurons because perfusion of muscimol into the preoptic region increases fos in the orexin neurons (Satoh et al., 2003; Alam et al., 2004). The VLPO mainly projects to the medial and perifornical parts of the orexin field, and orexin neurons in these regions show the greatest reduction in activity during sleep (Estabrooke et al., 2001). Inputs from these wake- and sleep-active neurons may help ensure that the orexin neurons remain active during wakefulness and silent during sleep.
Regions that control metabolism and feeding such as the Arc, VMH, DMH, PeF, and LHA are also likely to influence the orexin neurons. Some of the projections from the Arc and VMH contain agouti-related peptide and neuropeptide Y (Elias et al., 1998), and both signaling molecules induce the expression of fos in the orexin neurons (Zheng et al., 2002; Campbell et al., 2003). The importance of these inputs as well as humoral signals (Cai et al., 1999; Lawrence et al., 2002; Yamanaka et al., 2002) is readily apparent in the behavioral response to food deprivation: wildtype mice have marked increases in locomotor activity and wakefulness with food deprivation, but mice lacking the orexin neurons show no increase in either behavior (Yamanaka et al., 2003). Most likely, the orexin neurons are an essential integrator of metabolic signals to promote arousal and food-seeking behavior.
Our anterograde tracing also suggests that different parts of the orexin field may respond to different stimuli. Hypothalamic regions preferentially innervate orexin neurons in the medial and perifornical parts of the field, but most projections from the brainstem target the lateral part of the field. Perhaps homeostatic drives (sleepiness, hunger, etc.) influence arousal via orexin neurons in the medial part of the field. Orexin neurons in the lateral part of the field are activated when an animal anticipates a reward such as drugs or food (Harris et al., 2005), and these neurons may be more influenced by regions that regulate reward, emotion, and autonomic function such as the ventral tegmental area, amygdala, or ascending visceral afferents. It remains to be established whether the orexin neurons in the medial and lateral parts of the field differ in their projections, but collectively, the orexin neurons may help provide an impetus through which motivating signals promote behavioral arousal.
CONCLUSIONS
By integrating a variety of interoceptive and homeostatic signals, the orexin neurons are well positioned to promote behavioral arousal in response to diverse challenges. In fact, the arousing influences of hunger, anxiety, and stress may require the orexin neurons. Conversely, homeostatic sleep drive and metabolic satiety may inhibit the orexin neurons, thus reducing arousal and promoting sleep.
Acknowledgments
We thank Clif Saper, Ida Llewellyn-Smith, Arthur Seelig, Jennifer Vlasaty, and Tom Chou for thoughtful comments on this article.
Grant sponsor: National Institutes of Health; Grant number: MH62589; Grant number: HL60292.
Abbreviations
- ac
Anterior commissure
- AcbSh
Nucleus accumbens, shell
- AHA
Anterior hypothalamic nucleus
- Arc
Arcuate nucleus
- BST
Bed nucleus of the stria terminalis
- BSTLP
BST, lateral division, posterior part
- BSTLV
BST, lateral division, ventral part
- BSTMA
BST, medial division, anterior part
- BSTMP
BST, medial division, posterior part
- BSTMV
BST, medial division, ventral part
- cc
Corpus callosum
- CeA
Central nucleus of the amygdala
- CeL
Central nucleus of the amygdala, lateral subdivision
- CeM
Central nucleus of the amygdala, medial subdivision
- Cg
Cingulate cortex
- DA
Dorsal hypothalamic area
- DMH
Dorsomedial nucleus of the hypothalamus
- DP
Dorsal peduncular cortex
- DR
Dorsal raphe
- f
Fornix
- ic
Internal capsule
- IL
Infralimbic cortex
- LC
Locus coeruleus
- LDT
Laterodorsal tegmental nucleus
- LHA
Lateral hypothalamic area
- LHb
Lateral habenula
- LPB
Lateral parabrachial nucleus
- LPO
Lateral preoptic area
- LS
Lateral septum
- LSD
Lateral septum, dorsal part
- LSI
Lateral septum, intermediate part
- LSV
Lateral septum. ventral part
- Me5
Mesencephalic trigeminal nucleus
- MeAD
Medial amygdaloid nucleus, anterodorsal
- mfb
Medial forebrain bundle
- mlf
Medial longitudinal fasciculus
- MnPO
Median preoptic nucleus
- MnR
Median raphe nucleus
- Mo5
Trigeminal motor nucleus
- MPA
Medial preoptic area
- mt
Mammillothalamic tract
- NTS
Nucleus of the solitary tract
- ox
Optic chiasm
- PAG
Periaqueductal gray
- PeF
Perifornical area
- PH
Posterior hypothalamus
- PMV
Ventral premammillary nucleus
- POA
Preoptic area
- PrL
Prelimbic cortex
- PVH
Paraventricular nucleus of the hypothalamus
- RLi
Rostral linear raphe nucleus
- RVL
Rostral ventrolateral reticular nucleus
- S
Subiculum
- SCN
Suprachiasmatic nucleus
- scp
Superior cerebellar peduncle
- sm
Stria medullaris
- SNCD
Substantia nigra, compact part
- SNL
Substantia nigra, lateral part
- SNR
Substantia nigra, reticular part
- SPZ
Subparaventricular zone
- SuM
Supramammillary nucleus
- TMN
Tuberomammillary nucleus
- VLPO
Ventrolateral preoptic area
- VMH
Ventromedial nucleus of the hypothalamus
- VTA
Ventral tegmental area
- ZI
Zona incerta
Footnotes
The first two authors contributed equally to this work.
LITERATURE CITED
- Alam N, Kumar S, Bashir T, Suntsova N, Szymusiak R, McGinty D. Muscimol microinjection into median preoptic nucleus (MnPN) activates c-fos in hypocretin and other perifornical-lateral hypothalamic area (PF-LHA) neurons. Annual Meeting of the Society for Neuroscience; San Diego. 2004. [Google Scholar]
- Alheid GF, de Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The rat nervous system. San Diego: Academic Press; 1995. pp. 495–578. [Google Scholar]
- Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci. 2004;19:376–386. doi: 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60:610–615. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Insomnia, metabolic rate and sleep restoration. J Intern Med. 2003;254:23–31. doi: 10.1046/j.1365-2796.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- Cai XJ, Widdowson PS, Harrold J, Wilson S, Buckingham RE, Arch JR, Tadayyon M, Clapham JC, Wilding J, Williams G. Hypothalamic orexin expression: modulation by blood glucose and feeding. Diabetes. 1999;48:2132–2137. doi: 10.2337/diabetes.48.11.2132. [DOI] [PubMed] [Google Scholar]
- Campbell RE, Smith MS, Allen SE, Grayson BE, Ffrench-Mullen JM, Grove KL. Orexin neurons express a functional pancreatic polypeptide Y4 receptor. J Neurosci. 2003;23:1487–1497. doi: 10.1523/JNEUROSCI.23-04-01487.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A, Espana RA, Papadopoulou M, Saper CB, Faraco J, Sakurai T, Honda M, Mignot E, Scammell TE. Concomitant loss of dynor-phin, NARP, and orexin in narcolepsy. Neurology. 2005;65:1184–1188. doi: 10.1212/01.wnl.0000168173.71940.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetkovic V, Poncet F, Fellmann D, Griffond B, Risold PY. Diencephalic neurons producing melanin-concentrating hormone are influenced by local and multiple extra-hypothalamic tachykininergic projections through the neurokinin 3 receptor. Neuroscience. 2003;119:1113–1145. doi: 10.1016/s0306-4522(03)00146-5. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FSn, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: Implications for the circadian control of behavioural state. Neuroscience. 2005;130:165–183. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK. Chemically defined projections linking the medio-basal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J Comp Neurol. 2001;432:307–328. doi: 10.1002/cne.1105. [DOI] [PubMed] [Google Scholar]
- Fu LY, Acuna-Goycolea C, van den Pol AN. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system. J Neurosci. 2004;24:8741–8751. doi: 10.1523/JNEUROSCI.2268-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JL, Uehara K, Lu S, Wang QP, Funahashi H, Sakurai T, Yanagizawa M, Shioda S. Reciprocal synaptic relationships between orexin-and melanin-concentrating hormone-containing neurons in the rat lateral hypothalamus: a novel circuit implicated in feeding regulation. Int J Obes Relat Metab Disord. 2002;26:1523–1532. doi: 10.1038/sj.ijo.0802155. [DOI] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A novel role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. Possible involvement of orexin in the stress reaction in rats. Biochem Biophys Res Commun. 2000;270:318–323. doi: 10.1006/bbrc.2000.2412. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Ono K, Yokota S, Ishino H, Yasui Y. Topographical organization of projections from the subiculum to the hypothalamus in the rat. J Comp Neurol. 2000;419:205–222. doi: 10.1002/(sici)1096-9861(20000403)419:2<205::aid-cne5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Krauchi K, Cajochen C, Werth E, Wirz-Justice A. Warm feet promote the rapid onset of sleep. Nature. 1999;401:36–37. doi: 10.1038/43366. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143:155–162. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, van den Pol AN. Direct and indirect inhibition by cat-echolamines of hypocretin/orexin neurons. J Neurosci. 2005;25:173–183. doi: 10.1523/JNEUROSCI.4015-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- Lu J, Devor M, Saper C. A pontine tegmental flip-flop switch for regulation of REM sleep. Annual Meeting of the Society for Neuroscience; 2004. pp. 895–896. [Google Scholar]
- Marcus J, Lu J, Williams T, Beuckmann D, Yanagisawa M, Saper C, Elmquist J. Orexin neurons are activated during increased sympathetic outflow. Annual Meeting of the Society for Neuroscience; 2003. p. 81.15. [Google Scholar]
- McGinty D, Gong H, Suntsova N, Alam MN, Methippara M, Guzman-Marin R, Szymusiak R. Sleep-promoting functions of the hypothalamic median preoptic nucleus: inhibition of arousal systems. Arch Ital Biol. 2004;142:501–509. [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers G, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Brain Res Rev. 1997;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Muraki Y, Tsujino N, Kageyama H, Koyama Y, Shioda S, Kunita S, Takahashi S, Yanagisawa M. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Satoh S, Matsumura H, Nakajima T, Nakahama K, Kanbayashi T, Nishino S, Yoneda H, Shigeyoshi Y. Inhibition of rostral basal forebrain neurons promotes wakefulness and induces FOS in orexin neurons. Eur J Neurosci. 2003;17:1635–1645. doi: 10.1046/j.1460-9568.2003.02577.x. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. The connections of the septal region in the rat. J Comp Neurol. 1979;186:621–655. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]
- Thannickal T, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with fluorogold and PHAL in the rat. Brain Res Brain Res Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Crane AM, Colom LV, Bland BH. Ascending projections of the posterior nucleus of the hypothalamus: PHA-L analysis in the rat. J Comp Neurol. 1995;359:90–116. doi: 10.1002/cne.903590107. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, de Lecea L, Civelli O. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Hara J, Tsujino N, Beuckmann C, Yanagisawa M, Sakurai T. Regulation of orexin neurons by peripheral nutritional signals: roles of leptin, ghrelin, and glucose. Sleep. 2002;25(Suppl):A356–357. [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Fujiki N, Nakajima T, Ripley B, Matsumura H, Yoneda H, Mignot E, Nishino S. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14:1075–1081. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23:3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Corkern MM, Crousillac SM, Patterson LM, Phifer CB, Berthoud HR. Neurochemical phenotype of hypothalamic neurons showing Fos expression 23 h after intracranial AgRP. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1773–1781. doi: 10.1152/ajpregu.00019.2002. [DOI] [PubMed] [Google Scholar]
- Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1436–1444. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]