Abstract
Viable cancer cells can commonly be recovered from surgical sites and venous blood during tumor resection. The adhesion of these cells to surrounding tissues may impact patient outcomes. Iatrogenic exposure to increased extracellular pressure modulates integrin binding affinity and stimulates colon cancer cell adhesion in vitro through an α-actinin-1-dependent signaling pathway. We hypothesized that preoperative small interfering RNA-mediated silencing of α-actinin-1 in tumor tissue could disrupt pressure-stimulated cancer cell adhesion to murine surgical wounds and thereby enhance subsequent tumor-free survival. Reducing α-actinin-1 in CT26 murine adenocarcinoma cells blocked cell adhesion to collagen in vitro and similarly inhibited pressure-induced CT26 implantation in murine surgical wounds in vivo. Surgical wound contamination with pressure-activated CT26 cells significantly reduced tumor-free survival compared to contamination with tumor cells maintained under ambient pressure. However, mice treated with pressure-activated CT26 cells preoperatively transfected with α-actinin-1-specific small interfering RNA displayed reduced surgical site implantation and increased tumor-free survival compared to mice exposed to pressure-activated cells expressing normal levels of α-actinin-1 protein. These results suggest that pressure activation of malignant cells promotes tumor development and impairs tumor-free survival. α-Actinin-1 may be an effective therapeutic target to inhibit perioperative pressure-stimulated tumor cell implantation.
Introduction
Iatrogenic tumor cell implantation within surgical wounds can compromise curative cancer surgery. Surgical dissemination of viable tumor cells within the peritoneal cavity, wound site, portal, and systemic venous circulation occurs in as many as 50% of patients during colon cancer resection [1–3]. Whereas the presence of free malignant cells represents a poor prognostic factor [4,5], the frequency at which these shed tumor cells result in perioperative metastases is difficult to quantify [6,7]. However, wound recurrence and distant metastasis due to perioperative tumor dissemination does occur [8,9]. Reducing the implantation of shed tumor cells may therefore be beneficial.
We have previously reported that exposure to physical forces including increased extracellular pressure, laminar, and nonlaminar shear stimulates colon cancer cell adhesion to matrix proteins, endothelial cell monolayers, and surgical wounds in vivo by modulating integrin binding affinity through a cytoskeleton- and focal adhesion complex-dependent signaling mechanism [10–14]. Shed tumor cells may be subjected to these mechanical stimuli during vascular and lymphatic transits, in the tumor microenvironment, or iatrogenically through surgical manipulation, laparoscopic insufflation, and postoperative bowel edema [15–19]. Cells from colon, breast, and glossal cancer lines, murine colonic adenocarcinomas, and primary human colon cancers all display similar pressure-mediated phenomena [10–12,20,21].
α-Actinin-1 is a crucial upstream component of the mechanical response pathway mediating pressure-stimulated cell adhesion [22]. α-Actinin is a ubiquitously expressed actin-cross-linking protein localized primarily along cytoskeletal filaments and at focal adhesion plaques in nonmuscle cells [23,24]. Increased pressure induces redistribution of α-actinin-1 to the membrane where it functions as an adapter protein and facilitates Src recruitment to β1-integrin-associated focal adhesions [22]. In this same study, a modest reduction in α-actinin-1 expression was able to completely disrupt pressure-mediated effects on tumor cell adhesion to type I collagen in vitro without significantly altering basal adhesion under ambient pressure conditions. These results suggested that α-actinin-1 expression is rate limiting under elevated pressures. The sensitivity of pressure-induced phenomena to α-actinin-1 manipulation suggests that α-actinin-1 may have potential as a therapeutic target for the inhibition of pressure-stimulated tumor cell adhesion.
We therefore postulated that preoperative reduction of α-actinin-1 expression in tumor tissue would block pressure-induced alterations in tumor cell adhesiveness and minimize wound implantation and tumor recurrence in an animal model. To test this hypothesis, we used small interfering RNA(siRNA) to selectively reduce α-actinin-1 protein in murine CT26 colon carcinoma cells. We assessed the effect of α-actinin-1 reduction on pressure-stimulated CT26 cell adhesion to collagen I in vitro and implantation in murine surgical wounds in vivo. Furthermore, using a previously characterized murine survival model [11], we assessed the impact of pressure activation of CT26 cells and α-actinin-1 reduction on tumor-free survival.
Materials and Methods
Cell Culture
CT26 is an N-nitroso-N-methylurethane-induced BALB/c undifferentiated colon carcinoma (American Type Culture Collection, Manassas, VA). CT26 cells were maintained as previously described [25].
Pressure Regulation
Pressure was controlled using an airtight Lucite box with an inlet valve for gas application and an outlet valve connected to a manometer [10]. The box was prewarmed to 37°C to prevent internal temperature and pressure fluctuations. Temperature was maintained within ±2°C and pressure within ±1.5 mm Hg of desired levels.
Cell Adhesion Assay
Suspended cells were allowed to adhere to collagen I-coated six-well plates (105 cells/well) for 30 minutes at 37°C under ambient and increased pressure (+15 mm Hg) conditions. After 30 minutes, non-adherent cells were gently washed away with warm phosphate-buffered saline (PBS), and adherent cells were formalin-fixed, hematoxylin-stained, and each well was counted in 20 or more random high-power fields using an inverted microscope [10].
Wound Implantation Studies
Bilateral 1-cm groin incisions were made in BALB/c mice anesthetized with 60 mg/kg intraperitoneal pentobarbital. Equal 100-µl suspensions of 105 51Cr-labeled CT26 cells were applied to each wound, with each mouse serving as its own control. Suspensions were aspirated after 30 minutes, and the wounds were vigorously washed with PBS. The mice were then euthanized, and the wounds were excised postmortem for the quantification of tumor adhesion. Radioactivity in the excised tissue was measured using an automated scintillation counter as previously detailed [26]. In parallel survival studies, equal suspensions of 104 unlabeled cells were placed on single-flank incisions. Suspensions were aspirated after 30 minutes, and the wounds were vigorously washed and closed. The mice were followed for 90 days to assess tumor development and were sacrificed once tumors achieved 100-mg size. Tumors were measured five times per week and mass were estimated from two-dimensional measurements. The tumor weight was calculated in mg = (a x b2)/2, where a and b are the tumor length and width in mm, respectively [27].
Transfection
Cells were transfected with 50 nM double-stranded siRNA directed toward the mRNA target 5′-CACAGAUCGAGAACAUCGAAG-3′ for α-actinin-1 (Dharmacon, Lafayette, CO) as previously described [22]. A Dharmacon siCONTROL Non-Targeting siRNA #1 sequence was used as a control. Cell transfectants were used for adhesion experiments after 48 hours.
Western Blot Analysis
Cell lysates were prepared as previously described [28]. Equal amounts of protein were resolved by SDS-PAGE and transferred to Hybond ECL nitrocellulose membrane (Amersham Pharmacia Biotech, Piscataway, NJ). Mouse monoclonal antibodies to α-actinin-1 (US Biological, Swampscott, MA) and glyceraldehyde-3-phosphate dehydrogenase (Biodesign International, Saco, MN) coupled with anti-mouse horseradish peroxidase-conjugated secondary antibodies (Cell Signaling, Beverly, MA) were used for the immunodetection of blotted proteins. Bands were detected with enhanced chemiluminescence (Amersham Pharmacia Biotech) and analyzed with a Kodak Image Station 440CF (Perkin Elmer, Boston, MA).
Cell Proliferation Assay
Cell proliferation was assessed by colorimetric analysis of 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT; ATCC) reduction every 24 hours. Absorbance values were obtained at a wavelength of 570 nm using a spectrophotometer.
Results and Discussion
Previous studies demonstrated that a 30-minute exposure to 15 mm Hg-increased extracellular pressure stimulates human colon cancer cell adhesion to collagen I by approximately 20% to 50% compared to cells under ambient conditions [10,12,26]. We initially sought to determine whether murine CT26 colon carcinoma cells respond similarly to increased pressure and whether siRNA-mediated reduction of α-actinin-1 blocks this effect. We used a previously characterized α-actinin-1-specific siRNA sequence (siACTN1) selected because of the perfect human-murine homology of the targeted region of mRNA [22]. Similar to previous observations, siRNA transfection of CT26 cells reduced total α-actinin-1 protein by 74 ± 6% (Figure 1A; n = 11; P < .01). Suspended CT26 cells were exposed to either elevated or ambient pressure conditions and evaluated for in vitro adhesion to collagen I (Figure 1B). Control nontargeting siRNA (siNT)-transfected CT26 cells displayed a 54 ± 9% (n = 6; P < .04) increase in adhesion after a 30-minute exposure to 15 mm Hg-elevated pressure. α-Actinin-1 silencing moderately reduced basal CT26 cell adhesion by 26 ± 5% (n = 6; P < .04) and completely blocked the increase in adhesion that accompanies exposure to elevated pressure.
Figure 1.
Effect of siRNA-mediated reduction of α-actinin-1 on pressure-stimulated murine colon cancer cell adhesion to collagen I. (A) Typical reduction of α-actinin-1 protein in CT26 cells transfected with siRNA targeted to α-actinin-1 (siACTN1) compared with cells transfected with a nontargeting sequence (siNT) as measured by Western blot analysis. Glyceraldehyde-3-phosphate dehydrogenase was used as a loading control, and α-actinin-1 protein expression is normalized against that of siNT-transfected cells (*P < .01; n = 11). (B) The 15 mm Hg-increased extracellular pressure (closed bars) stimulates siNT-transfected CT26 cell adhesion to collagen I compared with ambient pressure controls (open bars). CT26 cells transfected with siACTN1 fail to display any increase in adhesion due to pressure (*P < .04; n = 6; #P < .04 comparison of siNT versus siACTN1 adhesion under ambient pressure). Data from individual experiments were normalized to the siNT ambient pressure controls and graphically expressed as mean ± SEM. Statistical analysis was by Wilcoxon matched-pairs signed-ranks test.
Preliminary in vivo studies demonstrated that preexposure to increased pressure enhances murine tumor cell implantation to surgical wounds [26]. Therefore, we next confirmed that α-actinin-1 reduction also inhibits pressure-induced CT26 cell adhesion to murine tissues in vivo. Suspended CT26 cell transfectants were labeled with radioactive 51Cr and exposed to either ambient or increased pressure conditions. Equal aliquots of control and pressure-treated cell suspensions were placed in bilateral groin incisions, using each mouse as its own control, for 30minutes before the wounds were vigorously washed with PBS to remove nonadherent cells, and the surgical wounds were excised postmortem. Relative radioactivity of the excised wounds was used to quantify CT26 cell implantation (Figure 2). Preexposure to 15 mm Hg-elevated pressure increased siNT-transfected CT26 cell adhesion to murine wounds by 66 ± 14% (n = 5; P < .01) compared to those under ambient conditions. In contrast, siACTN1 transfection moderately reduced basal counts by 31 ± 9% (n = 5; differences not significant) and prevented the stimulation of adhesion by pressure.
Figure 2.
Effect of α-actinin-1 reduction on pressure-mediated CT26 tumor cell wound implantation. The 15 mm Hg-increased pressure (closed bars) stimulates the adhesion of siNT-transfected, 51Cr-labeled CT26 cells to murine surgical wounds compared with cells kept under ambient pressure conditions (open bars). Pressure failed to increase wound implantation of CT26 cells transfected with siACTN1 (*P < .01; n = 5). Adhesion was determined by the acquired radioactive counts per minute (cpm) of surgical wounds after a 30-minute exposure to 51Cr-labeled CT26 cells. Data were analyzed by paired Student's t test and graphically expressed as mean ± SEM.
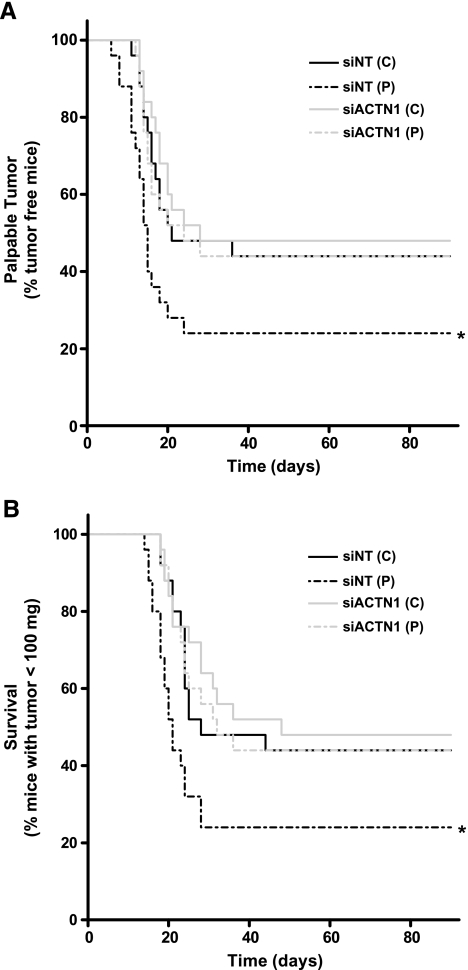
We next sought to determine whether the observed pressure-mediated increases in the number of cells adhering to surgical wounds have consequences for murine tumor development and tumor-free survival. As in the wound implantation studies, CT26 cells were transfected with either NTor ACTN1 siRNA in vitro, and after 48 hours, cell suspensions were exposed to ambient or increased pressure conditions. Equal aliquots of the cell suspensions were placed on single surgical incisions in the anesthetized mice. After 30 minutes, the wounds were washed and closed. Mice were examined daily for the development of a palpable tumor and growth was charted until a total tumor burden of 100 mg was reached to provide objective evidence of differences in rates of tumor development to correlate with the subjective impression of a palpable tumor (Figure 3). The 100-mg tumor burden was predetermined as a final endpoint in consultation with our veterinarians based on animal welfare considerations. Of the mice treated with siNT control cells, 56% developed tumors. On average, palpable tumors were observed by 21 days in the control group and a 100-mg tumor burden was reached by day 28. Of the mice exposed to pressure-activated CT26 cells, 76% developed tumors. The average tumor-free survival in the siNT pressure group was reduced to 15 days, and maximal tumor burden was reached by 21 days. The log-rank statistic for the plot of time to palpable tumor (Figure 3A) suggests that the difference in curves between siNT control and pressure groups is statistically significant (n = 25; P = .04). The curves depicting average time until a 100-mg tumor burden (Figure 3B) correlated with the time to palpable tumor curves and were similarly deemed to be significantly different between control and pressure groups (n = 25; P < .03).
Figure 3.
Effect of preoperative siRNA-mediated α-actinin-1 silencing on murine tumor development and survival after exposure to increased pressure. Exposure of CT26 cells to elevated pressure increases the rate of surgical wound implantation, whereas preoperative silencing of α-actinin-1 inhibits the pressure-mediated effect. (A) The Kaplan-Meier graph depicts the incidence of palpable tumor development over time. The solid black line plots the time to palpable tumor in mice exposed to siNT-transfected CT26 cells kept under ambient pressure. The interrupted black line represents exposure to pressure-activated siNT-transfected cells. The respective gray curves depict mice treated with parallel populations of CT26 cells transfected with siACTN1 (*P < .05; n = 25). (B) The Kaplan-Meier graph depicts the incidence of tumors reaching 100 mg over time. Pressure activation increases the rate at which the mice reach a critical tumor burden. siRNA-Mediated reduction of α-actinin-1 blocks this effect (*P < .05; n = 25). Survival data were analyzed by log-rank statistic.
The effect of siRNA-mediated α-actinin-1 reduction on murine tumor-free survival was examined in parallel (Figure 3). Of the mice exposed to siACTN1-treated control cells, 52% developed tumors. Palpable tumors were observed, on average, at 28 days and a 100-mg tumor burden was reached by day 42. No significant difference was found between siNT- and siACTN1-treated survival and time to palpable tumor curves under ambient pressure. Consistent with our previous data, only 56% of the mice exposed to pressure-activated, siACTN1-transfected CT26 cells developed tumors. The average tumor-free survival was 24 days and max tumor burden was reached by 37 days. Differences between siACTN1-transfected cells exposed to either ambient or increased pressure conditions were not significant. However, log-rank analysis of the difference between the curves for siNT- and siACTN1-treated, pressure-activated cells for both time to palpable tumor and 100-mg tumor burden are each statistically significant (n = 25; P < .05 for each). These results suggest that preoperative reduction of α-actinin-1 expression ablates the deleterious effects of pressure on wound implantation and enhances tumor-free survival.
Finally, we sought to address whether the altered rates of tumor development observed between experimental groups reflect only differences in cell adhesiveness during the surgical procedure or are additionally influenced by differences in CT26 cell proliferation from exposure to increased pressure or loss of α-actinin-1. Forty-eight hours after initial transfection, suspended siNT and siACTN1 transfectants were exposed to either ambient or increased pressure conditions for 30 minutes, mimicking the treatment of cells before their use in survival studies. CT26 cells were then lightly seeded and assessed for rates of proliferation determined by the colorimetric reduction of MTT in 24-hour increments (Figure 4). No significant differences were observed between any of the experimental groups (n = 4). This is consistent with our previous observation that increased pressure stimulates SW620 colon cancer cell proliferation only when sustained for at least 412 hours [29].
Figure 4.
Effect of increased pressure and α-actinin-1 reduction on CT26 cell proliferation. CT26 cells were transfected with either siACTN1 or siNT and, after 48 hours, were exposed to either 15 mm Hg-increased pressure or ambient pressure conditions. After exposure to pressure (time 0), cell proliferation of the respective populations was assessed by colorimetric analysis of MTT reduction every 24 hours. No significant difference in the rate of proliferation was observed between experimental groups (n = 4). All data are represented as mean ± SEM.
These results demonstrate that pressure activation of malignant cells has a biologic consequence for subsequent tumor development and tumor-free survival in an animal model. In addition, this study raises the possibility that perioperative therapeutic interventions can prevent this effect and reduce tumor recurrence. α-Actinin-1's lack of kinase activity makes it impervious to conventional pharmacologic perturbation and, therefore, an ideal target for RNA interference-based inhibitory strategies. While the therapeutic use of siRNA to silence disease-causing genes in humans is still in its infancy, preliminary reports from clinical trials are encouraging [30,31]. Although siRNA-mediated reduction of α-actinin-1 in this study was conducted in vitro and clinical translation would require consideration of preoperative access to the primary tumor, the reduction in basal adhesion and complete blockade of pressure-stimulated tumor cell adhesion accomplished through its silencing is proof-of-principle that α-actinin-like molecules may be suitable perioperative targets to reduce the metastasis of surgically disseminated cells.
Detection of free tumor cells in venous blood and peritoneal lavage from patients undergoing surgery for colorectal cancer is a significant predictor of tumor recurrence [5]. Factors influencing the subsequent implantation of shed tumor cells are of obvious clinical interest. Taken together with previous in vitro observations, our current results further suggest that perioperative exposure of malignant colonocytes to increased pressure and shear engendered by surgical manipulation, laparoscopic peritoneal insufflation, and intraabdominal third spacing may activate cancer cell adhesion and potentiate tumor metastasis.
Despite the relative rarity of wound recurrence, many other measures have been described in human surgery to avoid wound recurrence of resected tumors, including specialized techniques, mechanical irrigation, port site and intraabdominal chemotherapy, and even the secondary excision of the surgical wounds themselves [3,32–34]. Unfortunately, little data support the efficacy of such interventions. Although localized wound irrigation with calcium chloride can also reduce tumor cell implantation in mice [11], this strategy falls short in its ability to block pressure-induced integrin affinity modulation and has no effect on the adhesion of tumor cells shed into the vascular and lymphatic circulation. A means of systemically targeting the pressure-induced adhesion pathway preoperatively would likely be the most efficacious. Whether preoperative intratumoral or intravenous administration of α-actinin-1 siRNA could be a suitable therapeutic option requires further study.
There are obviously manifest differences between the surgical resection of CT26 colon carcinomas in mice and human colon cancers. The actual baseline incidence of clinically significant tumor recurrence in surgical wounds is much lower than the rates observed in this study. Here, we specifically titered the number of tumor cells seeded into each surgical wound to dramatically increase the number of tumor recurrences over that which is observed after human colectomy for cancer to increase the power of our study with a realistic sample size. Therefore, this model clearly demonstrates the long-term negative impact of increased pressure on colon cancer cell adhesion and supports the notion that effectively targeting this pathway can limit the implantation of shed tumor cells and enhance tumor-free survival.
Footnotes
This work was supported in part by the National Institutes of Health grant RO1DK06771 (M.D.B.) and by a VA Merit Review (M.D.B.).
References
- 1.Umpleby HC, Fermor B, Symes MO, Williamson RC. Viability of exfoliated colorectal carcinoma cells. Br J Surg. 1984;71:659–663. doi: 10.1002/bjs.1800710902. [DOI] [PubMed] [Google Scholar]
- 2.Allardyce R, Morreau P, Bagshaw P. Tumor cell distribution following laparoscopic colectomy in a porcine model. Dis Colon Rectum. 1996;39:S47–S52. doi: 10.1007/BF02053805. [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol. 1999;(43 Suppl):S15–S25. doi: 10.1007/s002800051093. [DOI] [PubMed] [Google Scholar]
- 4.Fujita S, Kudo N, Akasu T, Moriya Y. Detection of cytokeratin 19 and 20 mRNA in peripheral and mesenteric blood from colorectal cancer patients and their prognosis. Int J Colorectal Dis. 2001;16:141–146. doi: 10.1007/s003840100286. [DOI] [PubMed] [Google Scholar]
- 5.Guller U, Zajac P, Schnider A, Bosch B, Vorburger S, Zuber M, Spagnoli GC, Oertli D, Maurer R, Metzger U, et al. Disseminated single tumor cells as detected by real-time quantitative polymerase chain reaction represent a prognostic factor in patients undergoing surgery for colorectal cancer. Ann Surg. 2002;236:768–775. doi: 10.1097/00000658-200212000-00009. [Discussion 775–766] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes ES, McDermott FT, Polglase AL, Johnson WR. Tumor recurrence in the abdominal wall scar tissue after large-bowel cancer surgery. Dis Colon Rectum. 1983;26:571–572. doi: 10.1007/BF02552962. [DOI] [PubMed] [Google Scholar]
- 7.Reilly WT, Nelson H, Schroeder G, Wieand HS, Bolton J, O'Connell MJ. Wound recurrence following conventional treatment of colorectal cancer. A rare but perhaps underestimated problem. Dis Colon Rectum. 1996;39:200–207. doi: 10.1007/BF02068076. [DOI] [PubMed] [Google Scholar]
- 8.Group COoSTS, author. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 9.Turnbull RB, Jr, Kyle K, Watson FR, Spratt J. Cancer of the colon: the influence of the no-touch isolation technic on survival rates. Ann Surg. 1967;166:420–427. doi: 10.1097/00000658-196709000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basson MD, Yu CF, Herden-Kirchoff O, Ellermeier M, Sanders MA, Merrell RC, Sumpio BE. Effects of increased ambient pressure on colon cancer cell adhesion. J Cell Biochem. 2000;78:47–61. doi: 10.1002/(sici)1097-4644(20000701)78:1<47::aid-jcb5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.van der Voort van Zyp J, Conway WC, Thamilselvan V, Polin L, Basson MD. Divalent cations influence colon cancer cell adhesion in a murine transplantable tumor model. Am J Surg. 2005;190:701–707. doi: 10.1016/j.amjsurg.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Thamilselvan V, Basson MD. Pressure activates colon cancer cell adhesion by inside-out focal adhesion complex and actin cytoskeletal signaling. Gastroenterology. 2004;126:8–18. doi: 10.1053/j.gastro.2003.10.078. [DOI] [PubMed] [Google Scholar]
- 13.Thamilselvan V, Craig DH, Basson MD. FAK association with multiple signal proteins mediates pressure-induced colon cancer cell adhesion via a Src-dependent PI3K/Akt pathway. FASEB J. 2007;21:1730–1741. doi: 10.1096/fj.06-6545com. [DOI] [PubMed] [Google Scholar]
- 14.Thamilselvan V, Patel A, van der Voort van Zyp J, Basson MD. Colon cancer cell adhesion in response to Src kinase activation and actin-cytoskeleton by non-laminar shear stress. J Cell Biochem. 2004;92:361–371. doi: 10.1002/jcb.20072. [DOI] [PubMed] [Google Scholar]
- 15.Less JR, Posner MC, Boucher Y, Borochovitz D, Wolmark N, Jain RK. Interstitial hypertension in human breast and colorectal tumors. Cancer Res. 1992;52:6371–6374. [PubMed] [Google Scholar]
- 16.Nathan SS, DiResta GR, Casas-Ganem JE, Hoang BH, Sowers R, Yang R, Huvos AG, Gorlick R, Healey JH. Elevated physiologic tumor pressure promotes proliferation and chemosensitivity in human osteosarcoma. Clin Cancer Res. 2005;11:2389–2397. doi: 10.1158/1078-0432.CCR-04-2048. [DOI] [PubMed] [Google Scholar]
- 17.Dregelid E, Svendsen E. Endothelial cell injury in human saphenous veins after manipulation and tweezer grasping. J Cardiovasc Surg. 1988;29:464–469. [PubMed] [Google Scholar]
- 18.Wu JS, Brasfield EB, Guo LW, Ruiz M, Connett JM, Philpott GW, Jones DB, Fleshman JW. Implantation of colon cancer at trocar sites is increased by low pressure pneumoperitoneum. Surgery. 1997;122:1–7. doi: 10.1016/s0039-6060(97)90256-7. [DOI] [PubMed] [Google Scholar]
- 19.Moore-Olufemi SD, Xue H, Allen SJ, Moore FA, Stewart RH, Laine GA, Cox CS., Jr Effects of primary and secondary intra-abdominal hypertension on mesenteric lymph flow: implications for the abdominal compartment syndrome. Shock. 2005;23:571–575. [PubMed] [Google Scholar]
- 20.Conway WC, Van der Voort van Zyp J, Thamilselvan V, Walsh MF, Crowe DL, Basson MD. Paxillin modulates squamous cancer cell adhesion and is important in pressure-augmented adhesion. J Cell Biochem. 2006;98:1507–1516. doi: 10.1002/jcb.20819. [DOI] [PubMed] [Google Scholar]
- 21.Downey CAK, Thamilselvan V, Zhang L, Jiang Y, Rishi AK, Basson MD. Pressure stimulates breast cancer cell adhesion independently of cell cycle and apoptosis regulatory protein (CARP)-1 regulation of focal adhesion kinase. Am J Surg. 2006;192:631–635. doi: 10.1016/j.amjsurg.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Craig DH, Haimovich B, Basson MD. α-Actinin-1 phosphorylation modulates pressure-induced colon cancer cell adhesion through regulation of focal adhesion kinase-Src interaction. Am J Cell Physiol. 2007;293:1862–1874. doi: 10.1152/ajpcell.00118.2007. [DOI] [PubMed] [Google Scholar]
- 23.Lazarides E, Burridge K. α-Actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell. 1975;6:289–298. doi: 10.1016/0092-8674(75)90180-4. [DOI] [PubMed] [Google Scholar]
- 24.Langanger G, Moeremans M, Daneels G, Sobieszek A, De Brabander M, De Mey J. The molecular organization of myosin in stress fibers of cultured cells. J Cell Biol. 1986;102:200–209. doi: 10.1083/jcb.102.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Bronte V, Chen PW, Gritz L, Panicali D, Rosenberg SA, Restifo NP. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J Immunol. 1995;154:4685–4692. [PMC free article] [PubMed] [Google Scholar]
- 26.van der Voort van Zyp J, Thamilselvan V, Walsh M, Polin L, Basson MD. Extracellular pressure stimulates colon cancer cell adhesion in vitro and to surgical wounds by Src (sarcoma protein) activation. Am J Surg. 2004;188:467–473. doi: 10.1016/j.amjsurg.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Hazeldine ST, Polin L, Kushner J, Paluch J, White K, Edelstein M, Palomino E, Corbett TH, Horwitz JP. Design, synthesis, and biological evaluation of analogues of the antitumor agent, 2-(4-[(7-chloro-2-quinoxalinyl)oxy] phenoxy)propionic acid (XK469) J Med Chem. 2001;44:1758–1776. doi: 10.1021/jm0005149. [DOI] [PubMed] [Google Scholar]
- 28.Thamilselvan V, Basson MD. The role of the cytoskeleton in differentially regulating pressure-mediated effects on malignant colonocyte focal adhesion signaling and cell adhesion. Carcinogenesis. 2005;26:1687–1697. doi: 10.1093/carcin/bgi135. [DOI] [PubMed] [Google Scholar]
- 29.Walsh MF, Woo RK, Gomez R, Basson MD. Extracellular pressure stimulates colon cancer cell proliferation via a mechanism requiring PKC and tyrosine kinase signals. Cell Prolif. 2004;37:427–441. doi: 10.1111/j.1365-2184.2004.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novina CD, Sharp PA. The RNAi revolution. Nature. 2004;430:161–164. doi: 10.1038/430161a. [DOI] [PubMed] [Google Scholar]
- 31.Stevenson M. Therapeutic potential of RNA interference. N Engl J Med. 2004;351:1772–1777. doi: 10.1056/NEJMra045004. [DOI] [PubMed] [Google Scholar]
- 32.Sayfan J, Averbuch F, Koltun L, Benyamin N. Effect of rectal stump washout on the presence of free malignant cells in the rectum during anterior resection for rectal cancer. Dis Colon Rectum. 2000;43:1710–1712. doi: 10.1007/BF02236855. [DOI] [PubMed] [Google Scholar]
- 33.da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg. 2006;203:878–886. doi: 10.1016/j.jamcollsurg.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Katz MH, Barone RM. The rationale of perioperative intraperitoneal chemotherapy in the treatment of peritoneal surface malignancies. Surg Oncol Clin N Am. 2003;12:673–688. doi: 10.1016/s1055-3207(03)00034-6. [DOI] [PubMed] [Google Scholar]