Abstract
Upregulation of group IIA phospholipase A2 (sPLA2-IIA) correlates with prostate tumor progression suggesting prooncogenic properties of this protein. Here, we report data on expression of three different sPLA2 isozymes (groups IIA, V, and X) in normal (PrEC) and malignant (DU-145, PC-3, and LNCaP) human prostate cell lines. All studied cell lines constitutively expressed sPLA2-X, whereas sPLA2-V transcripts were identified only in malignant cells. In contrast, no expression of sPLA2-IIA was found in PrEC and DU-145 cells, but it was constitutively expressed in LNCaP and PC-3 cells. Expression of sPLA2-IIA is upregulated in PC-3 and in PrEC cells by IFN-γ in a signal transducer and activator of transcription-1-dependent manner, but not in LNCaP cells. Additional signaling pathways regulating sPLA2-IIA expression include cAMP/protein kinase A, p38 mitogen-activated protein kinase, protein kinase C, Rho-kinase, and mitogen-activated/extracellular response protein kinase / extracellular signal-regulated kinase. No deletions were revealed in the sPLA2-IIA gene from DU-145 cells lacking the expression of sPLA2-IIA. Reexpression of sPLA2-IIA was induced by 5-aza-2′-deoxycytidine demonstrating that DNA methylation is implicated in the regulation of sPLA2-II. Together, these data suggest that sPLA2-IIA and sPLA2-V, but not sPLA2-X, are differentially expressed in normal and malignant prostate cells under the control of proinflammatory cytokines; epigenetic mechanisms appear involved in the regulation of sPLA2-IIA expression, at least in DU-145 cells.
Introduction
Secretory phospholipases A2 (sPLA2, phosphatide sn-2-acylhydrolases; EC 3.1.1.4) belong to a growing and structurally heterogenous superfamily of phospholipases that play a critical role in a number of relevant physiological processes including defense mechanisms, production of bioactive lipids, and cell signaling [1–3]. At present, 10 different human sPLA2 isozymes are known. Among these, the group IIA isozyme, sPLA2-IIA, is the best established to contribute to the pathogenesis of inflammatory diseases such as sepsis and septic shock, rheumatoid arthritis, and atherosclerosis [1–3]. Other studies suggest that sPLA2-IIA is also important for the genesis of different cancers [4–11]. For example, increased expression of sPLA2-IIA correlates with tumor grade in human colon cancer and Barrett's adenocarcinoma [12,13]. Furthermore, increased levels of this enzyme have been found in the extracellular microenvironment of prostate cancer tissues and cell lines [4–6] correlating with poor survival [7] and high aggressiveness [8].
The role of sPLA2-IIA in tumorigenesis, however, remains unresolved. A protective function of sPLA2-IIA has been suggested from data in Min mice, in which mutations of the adenomatous polyposis coli gene lead to the development of multiple adenomas throughout their small and large intestines [14]. However, this function could not be proven in humans where sPLA2-IIA gene mutations do not appear to play a major role in the development of colorectal cancers [15,16]. Otherwise, in human pancreatic cancer cells and gastric adenocarcinoma, the expression of sPLA2-IIA was associated with prolonged survival and less frequent metastasis [14,17,18]. In contrast, prooncogenic properties of sPLA2-IIA upregulation have been suggested from studies on different tumor cell lines and xenograft tumor models [6,19,20].
The expression of sPLA2-IIA is regulated through a number of growth peptides, cytokines, and transcription factors in a cell-type-specific manner [1–3,21]. However, the role of proinflammatory cytokines and related signaling pathways in the regulation of sPLA2-IIA in prostate cancer cell lines remains to be determined. This question has special interest because of the well-known relationship between progression of cancers and levels of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and interleukin-6 (IL-6) [22–26]. It was suggested that transcription factors such as nuclear factor-kappa B (NF-κB) and members of the signal transducer and activator of transcription (STAT) family play pivotal roles in transmitting proinflammatory cytokine signals during tumor development [27]. These transcription factors are necessary at the same time for sPLA2-IIA promoter activity [3].
The potential contribution of proinflammatory cytokines to the expression of sPLA2 in prostate cancer cells provided the premise for the present study. To this end, we analyzed the expression of three sPLA2 isozymes in malignant prostate cell lines and compared the expression pattern with that found in normal prostate epithelial cells.
Materials and Methods
Materials
Recombinant human interleukin-1β (IL-1β), IL-6, TNF-α, and IFN-γ were purchased from Roche Diagnostics Applied Science (Mannheim, Germany). 2′-Amino-3′-methoxyflavone (PD98059), Janus kinase (JAK) inhibitor-I, 4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole (SB-202190), phorbol-12-myristate 13-acetate (PMA), and (s)-(+)-2-methyl-1-[(4-methyl-5-isoquinolinyl)sulfonyl]-homopiperazine, 2HCl (H-1152) were obtained from Calbiochem (San Diego, CA). Forskolin, N-acetyl-l-cysteine, 5-aza-2′-deoxycytidine (5-aza-dC), mithramycin A, and caffeic acid phenethyl ester (CAPE) were purchased from Sigma-Aldrich (Deisenhofen, Germany). Phorbol-12-myristate 13-acetate, PD98059, forskolin, SB-202190, mithramycin A, JAK inhibitor-I, and CAPE were dissolved in dimethyl sulfoxide (DMSO). The final concentrations of solvents were 0.3% or less. Controls using DMSO alone were run in all cases.
Cell Culture and Incubation
Normal human prostate epithelial cells (PrEC; Cambrex Bio Science, Walkersville, MD) were maintained up to a maximum of six passages in prostate epithelial growth medium supplemented with bovine pituitary extract, epidermal growth factor, insulin, transferrin, hydrocortisone, retinoic acid, epinephrine, triiodothyronine, and gentamicin-amphotericin solution on dishes coated with collagen type I (BioCoat; BD Falcon, Heidelberg, Germany). Every 2 to 3 days, the medium was changed, and before reaching confluence, the cells were passaged using trypsin/ethylenediaminetetraacetic acid.
Human prostatic malignant cell lines (PC-3, DU-145, and LNCaP cells) were purchased from the German Collection of Microorganisms and Cell Cultures (Berlin, Germany). They were cultured in a standard cell culture medium RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2.
For 5-aza-dC treatment, DU-145 cells were cultured in RPMI 1640 cell medium containing 10% FCS and 5-aza-dC added to the final concentration of 1 to 10 µM from a freshly prepared 10-mM stock solution. After 2 to 4 days, the cells were harvested and analyzed for sPLA2-IIA protein and mRNA levels [28].
RNA Extraction and Reverse Transcription-Polymerase Chain Reaction Analysis
RNA was isolated after lysing cells in TRI Reagent (Sigma-Aldrich) according to the manufacturer's instructions. Isolated RNA was converted to cDNA using the GeneAmp RNA-PCR Kit (PerkinElmer LAS GmbH, Jügesheim, Germany). A portion of the reverse transcription (RT) reaction products was then amplified for the identification of sPLA2-IIA, -V, -X, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a housekeeping gene using polymerase chain reaction (PCR). The applied primer pairs were 5′-GTG ATC ATG ATC TTT GGC CTA CTG CA-3′ and 5′-TCT CCC TCG TGG GGA GCA ACG ACT-3′ for sPLA2-IIA, giving PCR products with a length of 411 bp, 5′-GGG CTG CAA CAT TCG CAC AC-3′ and 5′-CCT CTC TCA GGA ACC AGG CAG-3′ for sPLA2-V, giving PCR products with a length of 278 bp, 5′-CCA TCG CCT ATA TGA AAT ATG G-3′ and 5′-TAG GAA CTG GGG GTA GAA GAG-3′ for sPLA2-X, giving PCR products with a length of 295 bp, and 5′-CGG AGT CAA CGG ATT TGG TCG TAT TG-3′ and 5′-GCA GGA GGC ATT GCT GAT GAT CTT G-3′ for GAPDH amplifying products with a length of 439 bp. The oligonucleotides for the analysis of mRNA were synthesized according to the published nucleotide sequences of human sPLA2-IIA, sPLA2-V, sPLA2-X, and GAPDH genes [21,29,30]. In the PCR, primer pairs were applied to a final concentration of 0.8 µM. The conditions for amplification were as follows: 40 cycles at 94°C for 30 seconds, 68°C for 30 seconds, and 72°C for 1 minute. The buffers and reagents used were from GeneAmp Kit (PerkinElmer LAS GmbH). After amplification, products were analyzed by electrophoresis on agarose gels. GAPDH mRNA was determined in every sample as a reference.
Enzyme-Linked Immunosorbent Assays Specific for sPLA2-IIA and Transcription Factors
The sPLA2-IIA protein released into the medium was determined using an enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (Cayman Chemical, MI). Culture medium was removed, the cell surface-bound sPLA2-IIA was extracted with ice-cold PBS, pH 7.4, containing 1 M NaCl, and the extract was collected with cell culture medium, centrifuged for 10 minutes at 400g to remove cell debris, and was used for sPLA2-IIA protein determination in the ELISA. Total cell protein was determined using a Bicinchoninic Acid assay kit (Sigma-Aldrich).
For the determination of NF-κB, CAAT-enhancer-binding protein-β (C/EBP-β), specificity protein 1 (SP1), and STAT1 DNA binding activities, cells were incubated in six-well plates. After incubation, nuclear extracts were prepared using a nuclear extraction kit (Active Motif, Carlsbad, CA). The DNA binding capacities of NF-κB p65 and C/EBP-β in nuclear extracts were estimated using ELISA-based assays (Active Motif ) according to the manufacturer's instructions. STAT1 and SP1 DNA binding activities were studied using STAT1 and SP1 ELISA kits from Panomics, Inc. (Fremont, CA). The phosphorylation of STAT1 was measured using a cell-based ELISA kit (RayBiotech, Inc., Norcross, GA) according to the manufacturer's instructions.
DNA Sequencing
DNA sequences were analyzed with a genetic analyzer (ABI PRISM 310; Perkin-Elmer Applied Biosystems, Foster City, CA).
Data Analysis
Data were analyzed by two-tailed and unpaired Student's t test to calculate the indicated P values. Differences were considered significant at P < .05.
Results
Basal and Cytokine-Induced Expression of Group IIA, V, and X Phospholipases A2 in Normal and Malignant Prostate Cells
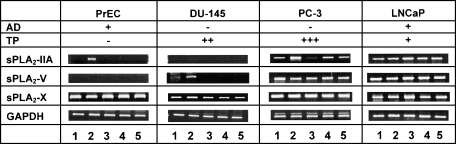
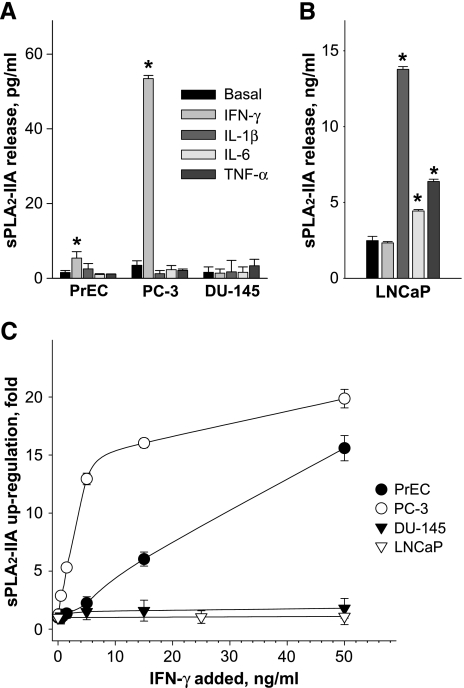
In prostate cancer cells, PC-3 and LNCaP, sPLA2-IIA mRNA was constitutively expressed, whereas in normal PrEC and malignant DU-145 cells, no sPLA2-IIA transcripts were detectable (Figure 1). However, exposure to IFN-γ led to the induction of sPLA2-IIA expression in PrEC and to further upregulation in PC-3 cells. No cytokinemediated induction of sPLA2-IIA transcripts occurred in DU-145 cells. Similar results were also obtained at the protein level where negligible amounts of sPLA2-IIA were released from DU-145 cells after incubation with cytokines (Figure 2). In LNCaP cells, sPLA2-IIA protein was produced under normal conditions at about 700-fold higher levels compared to PC-3 cells (Figure 2, A and B). This high basal expression in LNCaP cells was further increased by IL-1β, TNF-α, and IL-6 treatments by 5.0-, 2.0-, and 1.5-fold, respectively, but not by IFN-γ.
Figure 1.
Agarose gel electrophoresis showing amplifiers of different secretory phospholipase A2 isozymes (sPLA2-IIA, sPLA2-V, and sPLA2-X) and GAPDH mRNA after RT-PCR analysis expressed in normal and malignant prostate cell lines under basal conditions and after exposure to proinflammatory cytokines. Cells were incubated for 24 hours in medium containing 10% FCS alone and additions of cytokines at a final concentration of 25 ng/ml. Lanes 1 to 5 are controls (without additions of cytokines), IFN-γ, IL-1β, IL-6, and TNF-α, respectively. Data are representative of at least three independent experiments giving similar results. AD and TP indicate the androgen dependence and tumorigenicity potential according to literature [32,34].
Figure 2.
Effects of proinflammatory cytokines on sPLA2-IIA expression in normal (PrEC) and malignant (DU-145, PC-3, and LNCaP) prostate cells. Cytokines were added in a final concentration of 25 ng/ml. The sPLA2-IIA amounts released into the medium by PrEC, PC-3, and DU-145 cells (A) were measured after 48 hours of incubation. LNCaP cells (B) were incubated for 24 hours. *P < .05 versus cells incubated without cytokines (basal expression). (C) Concentration dependence of IFN-γ on sPLA2-IIA upregulation in prostate cells. The results are expressed as changes relative to the basal level of sPLA2-IIA secretion without IFN-γ treatment. The basal levels of sPLA2-IIA (pg/mg cell protein) in PrEC, PC-3, DU-145, and LNCaP cells were 10.05 ± 1.34; 13.11 ± 0.95; 2.50 ± 2.91, and 9898.15 ± 171.43, respectively. The prostate cells were incubated with indicated concentrations of IFN-γ for 48 hours. Results are the means ± SD of analysis in quadruplicate and are representative of three independent experiments.
Transcripts of sPLA2-V were consistently detected in PC-3 and LNCaP cells, but levels were relatively insensitive to the addition of cytokines (Figure 1). In PrEC cells, no sPLA2-V transcripts were detected, and these could not have been induced by any of the investigated cytokines. Under basal conditions, moderate levels of sPLA2-V mRNA were detected in DU-145 cells; these levels were strongly induced by IFN-γ. No inductive effects on sPLA2-V expression were observed in this cell line with IL-1β, IL-6, and TNF-α (Figure 1). The sPLA2-X isozyme was strongly expressed in all studied cell lines, and no marked response to the added cytokines was observed. In all samples, levels of GAPDH mRNA were comparable (Figure 1).
Analysis of the active concentration range for IFN-γ indicated that IFN-γ-mediated upregulation of sPLA2-IIA in normal PrEC and malignant PC-3 cells was dose-dependent (Figure 2C). A half-maximal effect was observed at ∼4 ng/ml IFN-γ in PC-3 cells and at ∼12 ng/ml IFN-γ in PrEC cells.
Regulation of sPLA2-IIA Expression by the Activity of Signaling Pathways
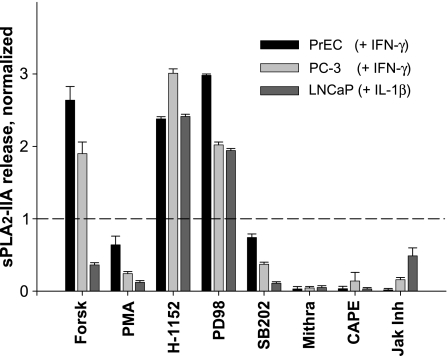
Because, in DU-145 cells, no basal or cytokine-induced sPLA2-IIA expressions were identifiable, we analyzed signaling components only in PrEC, PC-3, and LNCaP cells. A marked increase in release of sPLA2-IIA protein was observed in PrEC and PC-3 cells when these cells were simultaneously stimulated with both IFN-γ and forskolin, in comparison to cells exposed to IFN-γ alone (Figure 3). In contrast, forskolin attenuated the sPLA2-IIA expression induced by IL-1β in LNCaP cells. Phorbol-12-myristate 13-acetate, an activator of protein kinase C (PKC), inhibited the cytokine-stimulated expression of sPLA2-IIA in all three analyzed cell types. H-1152, an inhibitor of Rho-kinase, and PD-98059, an inhibitor of mitogenactivated/extracellular response protein kinase, increased further the cytokine-induced sPLA2-IIA production in PrEC, PC-3, and LNCaP cells. Conversely, a significant suppression of sPLA2-IIA occurred in prostate cell lines after exposure to SB-202190, mithramycin A, JAK inhibitor-1, and CAPE, which inhibit p38 mitogen-activated protein kinase (MAPK), SP1 binding activity, the JAK/STAT pathway, and NF-κB, respectively. Other NF-κB inhibitors, such as pyrrolidine dithiocarbamate, N-acetyl-l-cysteine, and 20S proteasome inhibitor, also decreased the amounts of sPLA2-IIA released into the medium (data not shown).
Figure 3.
Effects of activators and inhibitors of cell signaling pathways on the sPLA2-IIA expression in normal PrEC and malignant PC-3 cells stimulated with IFN-γ and in malignant LNCaP cells stimulated with IL-1β. Cells were incubated for 48 hours. The sPLA2-IIA levels (pg/mg cell protein) induced by corresponding cytokines were 19.57 ± 2.96; 268.49 ± 4.61; 23640.0 ± 130.0 in PrEC, PC-3, and LNCaP cells, respectively. Forskolin (Forsk; 10 µM), PMA (30 ng/ml), H-1152 (10 µM), PD98059 (PD98; 50 µM), SB-202190 (SB202; 50 µM), mithramycin A (Mithra; 250 nM), CAPE (25 µM), or JAK inhibitor-I (JAK Inh; 1 µM) was added simultaneously with cytokines. Results are expressed as changes relative to the control (treated just with cytokine) that was assigned a value of 1.0. The data shown are the means ± SD of analysis in triplicate and are representative of four independent experiments.
Activities of Transcription Factors in IFN-γ-Treated Prostate Cells
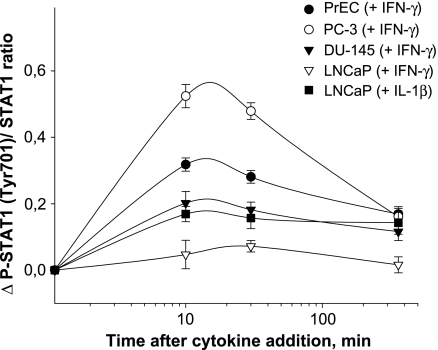
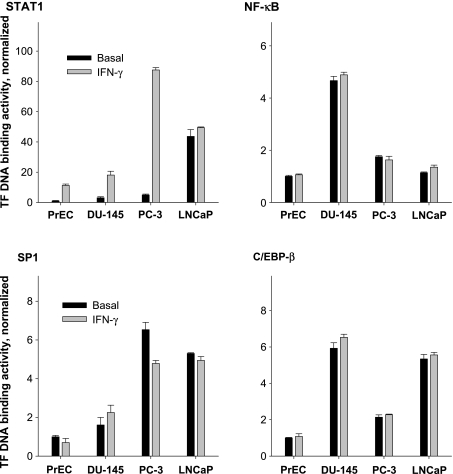
Using a cell-based ELISA, we showed a marked difference in the time course of cellular STAT1 phosphorylation (P-STAT1) induced by IFN-γ (Figure 4). After 15 minutes of incubation, the highest reactivity in P-STAT1 values was found in PC-3 cells followed by PrEC and DU-145 cells. No increased P-STAT1 signals were detected during the whole incubation time-course in LNCaP cells (Figure 4). In addition to STAT1 phosphorylation, we studied the DNA binding activities of STAT1 using nuclear extracts of cells treated with IFN-γ (Figure 5). There was a ∼20-fold activation of STAT1 in PC-3 cells after exposing to IFN-γ for 2 hours. In PrEC cells, this activation averaged ∼10-fold, and in DU-145 cells, it averaged ∼ 6-fold. In LNCaP cells, again no significant activation of STAT1 occurred.
Figure 4.
Signal transducer and activator of transcription-1 (STAT1) phosphorylation induced by IFN-γ in PrEC, PC-3, DU-145, and LNCaP cells. The STAT1 phosphorylation was determined by cell-based ELISA technique. Each point represents the mean of cellular STAT1 activation expressed as the ratio P-STAT1/STAT1 and measured after 0, 10, 30, and 360 minutes of incubation with 25 ng/ml IFN-γ or 25 ng/ml IL-1β. The results are the means ± SD of analysis in triplicate and are representative of three independent experiments.
Figure 5.
Effects of IFN-γ on cellular STAT1, NF-κB, SP1, and C/EBP-β activities in nuclear extract of normal (PrEC) and malignant prostate cells (PC-3, DU-145, and LNCaP). In nuclear cell extracts, the STAT1, NF-κB, SP1, and C/EBP-β DNA binding activities were measured after a 2-hour exposure of cells to 25 ng/ml IFN-γ. Obtained data for each transcription factor were normalized toward the signal level obtained in PrEC cells and are shown as means ± SD of the analyses in triplicate and are representative of three independent experiments.
Large differences were found in the basal binding activities of STAT1, NF-κB, C/EBP-β, and SP1 between the different prostate cell lines (Figure 5). In contrast to STAT1 activation, however, no significant changes in DNA binding activities of NF-κB, C/EBP-β, and SP1 were found, with the exception of SP1, the binding activity of which decreased in PC-3 cells after incubation with IFN-γ (Figure 5). In LNCaP cells, treatment with IL-1β did not result in STAT1 activation (data not shown).
Reexpression of sPLA2-IIA in DU-145 Cells After Treatment with a DNA Methyltransferase Inhibitor
DU-145 cells do not express sPLA2-IIA either constitutively or after exposure to cytokines. Therefore, we addressed the question of whether failure of sPLA2-IIA expression in this cell line may be due to PLA2G2A gene mutations. For this reason, we analyzed the sequence of the proximal part of the promoter region [-319, +111] and exons 2 to 5 of the PLA2G2A gene (access codes M22431 and AY462114, transcription start site according to the study of Andreani et al. [31]). Our data did not show substantial differences between DU-145 DNA probes and the wild-type sequence (data not shown).
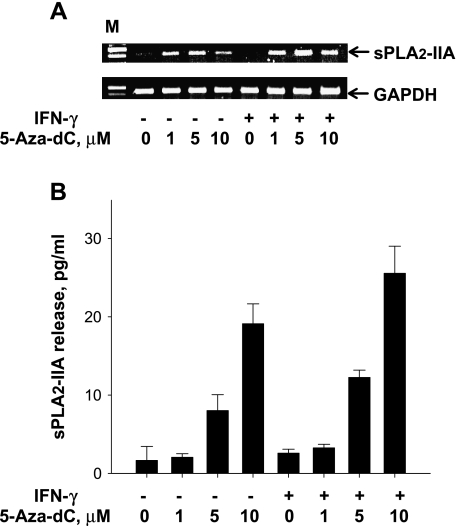
To define the contribution of epigenetic mechanisms, malignant DU-145 cells were incubated with the DNA methyltransferase inhibitor, 5-aza-dC, for 48 to 96 hours. No transcripts specific for sPLA2-IIA were present in DU-145 cells treated with DMSO as a control, but transcripts became detectable after incubating with 5-aza-dC (Figure 6A). The recovery of sPLA2-IIA expression after 48 to 96 hours of treatment with the DNA-demethylating agent was confirmed by measuring the sPLA2-IIA protein released into the cell culture medium (Figure 6B).
Figure 6.
Reexpression of sPLA2-IIA in DU-145 cells following the treatment with 5-aza-dC as inhibitor of DNA methyltransferase. (A) Agarose gel electrophoresis showing amplified sPLA2-IIA mRNA of DU-145 cells treated with increased concentrations of 5-azadC. Cells were incubated for 72 hours without or with IFN-γ and indicated amounts of 5-aza-dC. M, 100-bp molecular weight ladder. (B) sPLA2-IIA protein levels released by DU-145 cells into the medium at increased concentrations of 5-aza-dC. Cells were incubated for 72 hours without or with IFN-γ and indicated amounts of 5-aza-dC. Results are the means ± SD of analysis in triplicate and are representative of four independent experiments.
Discussion
In the current study, we present data on the expression of three sPLA2 isoforms (IIA, V, and X) in normal human prostate cells and in three cancer cell lines, PC-3, DU-145, and LNCaP, with differing tumorigenicity [32–34]. The results show that the sPLA2 isozymes are differentially expressed and induced in different manners in prostate cells. In normal PrEC prostate cells no basal expression of sPLA2-IIA and sPLA2-V was detectable, whereas both isozymes were constitutively expressed in malignant LNCaP and PC-3 cell lines. Expression of sPLA2-IIA, but not the expression of sPLA2-V, was inducible by IFN-γ in PC-3 and PrEC cells. Here, the IFN-γ-mediated increase of sPLA2-IIA expression correlated strongly with STAT1 activation.
In contrast to PC-3 and PrEC cells, in LNCaP cells, the expression of sPLA2-IIA was completely insensitive to IFN-γ. This unresponsiveness agrees with the observation that LNCaP cells are unable to initiate IFN-γ signaling due to the lack of JAK1 expression caused by an epigenetic mechanism [35]. For this reason, it was impossible to find a significant STAT1 activation by IFN-γ in LNCaP cells. Nevertheless, IL-1β, TNF-α, and IL-6 increased sPLA2-IIA expression in this cell line. In PC-3 and PrEC cells, synthesis of sPLA2-IIA was completely unresponsive to IL-1β, TNF-α, and IL-6, implying that corresponding signaling events and transcription factors are not functionally active in these cell lines. The finding that the JAK inhibitor-I, which suppresses both JAK1 and JAK2, significantly decreased sPLA2-IIA expression in LNCaP cells, although these cells do not possess JAK1 activity, suggests that in addition to JAK1, a JAK2-dependent signaling pathway is involved in the regulation of sPLA2-IIA.
Besides STAT1, other transcription factors, such as NF-κB, SP1, and C/EBP-β, also exhibit specific binding sites in the sPLA2-IIA promoter region [31,36]. Although the IFN-γ-mediated upregulation of sPLA2-IIA in PrEC and PC-3 cells did not correlate with the activation of NF-κB, SP1, and C/EBP-β, for an optimal expression of sPLA2-IIA, at least NF-κB and SP1 are absolutely necessary. This conclusion is based on a nearly complete inhibition of sPLA2-IIA by mithramycin A, an inhibitor of SP1, and CAPE, an inhibitor of NF-κB, in PrEC, PC-3, and LNCaP cells. In the case of C/EBP-β, no specific inhibitors were available. Nevertheless, with the exception of DU-145 cells, the basal DNA binding activities of C/EBP-β correlated strongly with the sPLA2-IIA expression in prostate cells. This underlines that C/EBP-β is also important for an optimal sPLA2-IIA expression.
In DU-145 cells, genomic analysis showed that both the failure of basal sPLA2-IIA expression (see also Sved et al. [6]) and the distinct lack of all tested cytokines to induce sPLA2-IIA expression at mRNA and protein levels could not be explained by deletions in the sPLA2-IIA gene. Our experiment on exposure of DU-145 cells to an inhibitor of methyltransferases (5-aza-dC) indicated, for the first time, that the involvement of epigenetic mechanisms in the regulation of in sPLA2-IIA expression. In cancer cells, epigenetic silencing of different genes is caused by the aberrant methylation of cytosines belonging to CpG islands, stretches of DNA rich in CpG dinucleotides often associated with gene promoters [19,37–41]. This methylation may perturb the expression of genes critical to the regulation of cell proliferation. In this context, one possible explanation for the activation of the sPLA2-IIA in 5-aza-dC-treated DU-145 cells was that this gene promoter contains CpG islands and that methylation of these may be responsible for sPLA2-IIA gene silencing. However, analysis of the PLA2G2A gene promoter region (-1000 to +200 bp from the transcription start site according to the study of Andreani et al. [31]) performed using the EMBOSS-CpGPlot program (EMBL-European Bioinformatics Institute; http://www.ebi.ac.uk) reveals no CpG islands and a relatively low number of CpG sites. Nevertheless, it has been shown that the presence of a single methylated CpG dinucleotide within the binding sites of crucial transcription factors can be sufficient for gene inactivation as described, for example, in the case of the SP1 binding site in the thymidine kinase gene [42]. Therefore, this mechanism may also be responsible for sPLA2-IIA gene silencing even when no CpG islands are present in the promoter region. In agreement with this suggestion, our results show that blockage of SP1 binding sites by mithramycin A treatment almost completely abolished the expression of sPLA2-IIA in PrEC, PC-3, and LNCaP cells (Figure 3).
Additional investigations have to clarify whether an aberrant methylation in DU-145 cells is taking place directly at selected CpG sites within PLA2G2A promoter itself or indirectly through CpG methylation in the promoter of corresponding transcription factors that regulate sPLA2-IIA transcription. The failed effect of DNA methyl-transferase inhibition on sPLA2-IIA expression in rat mesangial cells stimulated with cytokine IL-1β [43] suggests that perhaps such a mechanism is absent in cells that normally express sPLA2-IIA.
A number of upstream pathways in both normal and malignant prostate cells are involved in the activation of STAT1, NF-κB, C/EBP-β, and SP1 transcription factors, with subsequent capacity to modulate sPLA2-IIA expression. According to our data, p38 MAPK kinase activates the sPLA2-IIA expression, whereas PKC, RhoA/Rho-kinase, and mitogen-activated/extracellular response protein kinase / extracellular signal-regulated kinase 1/2 pathways possess negative regulatory effects. It is known that PKC interferes with other signaling pathways leading to an inhibition of sPLA2-IIA expression. This may relate to the sPLA2-IIA downregulation found in mesangial cells [44]. Both p38 MAPK and survival kinase Akt pathways play critical roles as downstream effectors of PKC isozymes in prostate cancer cells [45]. Activation of PKC promotes the dephosphorylation and inhibition of the Akt pathway, which is constitutively activated in tumor cells.
In our study, we found a strong modulating effect of cAMP/protein kinase A pathway on sPLA2-IIA expression in prostate cells. Forskolin, an activator of this signaling cascade, either facilitated the induction of sPLA2-IIA expression by IFN-γ in PrEC and PC-3 cells or repressed the induction of sPLA2-IIA expression by IL-1β in LNCaP cells. The reason of this forskolin-mediated opposite action remains unknown.
What relevance does different expression patterns of sPLA2-IIA and sPLA2-V in prostate cell lines have for tumorigenic potential? PC-3 and DU-145 cells have been identified as highly aggressive, whereas the LNCaP cell line has a low aggressive phenotype [32,33]. The metastatic potentials of these prostate cancer cell lines correlates with the expression of proangiogenic genes [34]. Based on these properties, the expression profiles of sPLA2 isozymes suggest that the sPLA2-IIA plays, if any, only a secondary role in tumorigenicity. DU-145 cells expressed, instead of sPLA2-IIA, the sPLA2-V isozyme, particularly after the exposure of cells to IFN-γ. Therefore, it is conceivable that sPLA2-V compensates for the lack of expression of sPLA2-IIA DU-145 cells and contributes to oncogenesis in this cell line. In this context, it is noteworthy that sPLA2-V isozyme acts similarly to sPLA2-IIA at both arachidonic acid release [46] and arachidonic acid-mediated signaling transduction in sPLA2-IIA-negative cell types [47,48]. Further studies are necessary to elucidate the contribution of various sPLA2 isozymes to proliferation, migration, invasion, cell-to-cell interaction, and other activities in prostate cancer cells.
In summary, our study has elucidated several novel aspects about expression of sPLA2 isozymes in normal (PrEC) and malignant (DU-145, PC-3, and LNCaP) human prostate cell lines: (1) sPLA2-IIA, sPLA2-V, and sPLA2-X are expressed in both normal prostate cells and malignant prostate cell lines, but in a differential manner; (2) expression of sPLA2-IIA and sPLA2-V, but not of sPLA2-X, is controlled by proinflammatory cytokines in prostate cells, but different cell signaling pathways are involved in the regulation of sPLA2-IIA; 3) no significant expression of sPLA2-IIA was found in the DU-145 cell line, and epigenetic mechanisms seem to be responsible for the silencing of sPLA2-IIA in this cell line. Additional investigations may clarify whether an aberrant methylation in DU-145 cells is taking place directly at the promoter of the sPLA2-IIA gene itself or indirectly through CpG methylation in the promoter of corresponding transcription factors that regulate the synthesis of sPLA2-IIA in these cells.
Acknowledgments
The authors are grateful to Margot Vogel for technical assistance. The authors also thank Graeme Eisenhofer for helpful discussions.
Abbreviations
- 5-aza-dC
5-aza-2′-deoxycytidine
- C/EBP-β
CAAT-enhancer-binding protein-β
- CAPE
caffeic acid phenethyl ester
- DMSO
dimethyl sulfoxide
- ELISA
enzyme-linked immunosorbent assay
- FCS
fetal calf serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IFN-γ
interferon-γ
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- JAK
Janus kinase
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor-kappa B
- PKC
protein kinase C
- PMA
phorbol-12-myristate 13-acetate
- RT-PCR
reverse transcription-polymerase chain reaction
- SP1
specificity protein 1
- sPLA2-IIA
secretory phospholipase A2 of group IIA
- STAT1
signal transducer and activator of transcription-1
- TNF-α
tumor necrosis factor-alpha
References
- 1.Dennis EA, Deems RA, Yu L. Extracellular phospholipase A2. Adv Exp Med Biol. 1992;318:35–39. doi: 10.1007/978-1-4615-3426-6_4. [DOI] [PubMed] [Google Scholar]
- 2.Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 2002;68–69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 3.Menschikowski M, Hagelgans A, Siegert G. Secretory phospholipase A2 of group IIA: is it an offensive or a defensive player during atherosclerosis and other inflammatory diseases? Prostaglandins Other Lipid Mediat. 2006;79:1–33. doi: 10.1016/j.prostaglandins.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Kallajoki M, Alanen KA, Nevalainen M, Nevalainen TJ. Group II phospholipase A2 in human male reproductive organs and genital tumors. Prostate. 1998;35:263–272. doi: 10.1002/(sici)1097-0045(19980601)35:4<263::aid-pros5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Jiang JZ, Neubauer BL, Graff JR, Chedid M, Thomas JE, Roehm NW, Zhang S, Eckert GJ, Koch MO, Eble JN, et al. Expression of group IIA secretory phospholipase A2 is elevated in prostatic intraepithelial neoplasia and adenocarcinoma. Am J Pathol. 2002;160:667–671. doi: 10.1016/S0002-9440(10)64886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sved P, Scott KF, McLeod D, King NJ, Singh J, Tsatralis T, Nikolov B, Boulas J, Nallan L, Gelb MH, et al. Oncogenic action of secreted phospholipase A2 in prostate cancer. Cancer Res. 2004;64:6934–6940. doi: 10.1158/0008-5472.CAN-03-3018. [DOI] [PubMed] [Google Scholar]
- 7.Graff JR, Konicek BW, Deddens JA, Chedid M, Hurst BM, Colligan B, Neubauer BL, Carter HW, Carter JH. Expression of group IIa secretory phospholipase A2 increases with prostate tumor grade. Clin Cancer Res. 2001;7:3857–3861. [PubMed] [Google Scholar]
- 8.Morgenbesser SD, McLaren RP, Richards B, Zhang M, Akmaev VR, Winter SF, Mineva ND, Kaplan-Lefko PJ, Foster BA, Cook BP, et al. Identification of genes potentially involved in the acquisition of androgen-independent and metastatic tumor growth in an autochthonous genetically engineered mouse prostate cancer model. Prostate. 2007;67:83–106. doi: 10.1002/pros.20505. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita S, Ogawa M, Sakamoto K, Abe T, Arakawa H, Yamashita J. Elevation of serum group II phospholipase A2 levels in patients with advanced cancer. Clin Chim Acta. 1994;228:91–99. doi: 10.1016/0009-8981(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 10.Gorovetz M, Baekelandt M, Berner A, Trope' CG, Davidson B, Reich R. The clinical role of phospholipase A2 isoforms in advanced-stage ovarian carcinoma. Gynecol Oncol. 2006;103:831–840. doi: 10.1016/j.ygyno.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 11.Miki Y, Mukae S, Murakami M, Ishikawa Y, Ishii T, Ohki H, Matsumoto M, Komiyama K. Butyrate inhibits oral cancer cell proliferation and regulates expression of secretory phospholipase A2-X and COX-2. Anticancer Res. 2007;27:1493–1502. [PubMed] [Google Scholar]
- 12.Kennedy BP, Soravia C, Moffat J, Xia L, Hiruki T, Collins S, Gallinger S, Bapat B. Overexpression of the nonpancreatic secretory group II PLA2 messenger RNA and protein in colorectal adenomas from familial adenomatous polyposis patients. Cancer Res. 1998;58:500–503. [PubMed] [Google Scholar]
- 13.Lagorce-Pagès C, Paraf F, Wendum D, Martin A, Fléjou JF. Expression of inflammatory secretory phospholipase A2 and cytosolic phospholipase A2 in premalignant and malignant Barrett's oesophagus. Virchows Arch. 2004;444:426–435. doi: 10.1007/s00428-004-1003-7. [DOI] [PubMed] [Google Scholar]
- 14.MacPhee M, Chepenik KP, Liddell RA, Nelson KK, Siracusa LD, Buchberg AM. The secretory phospholipase A2 gene is a candidate for the Mom1 locus, a major modifier of ApcMin-induced intestinal neoplasia. Cell. 1995;81:957–966. doi: 10.1016/0092-8674(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 15.Riggins GJ, Markowitz S, Wilson JK, Vogelstein B, Kinzler KW. Absence of secretory phospholipase A2 gene alterations in human colorectal cancer. Cancer Res. 1995;55:5184–5186. [PubMed] [Google Scholar]
- 16.Dobbie Z, Muller H, Scott RJ. Secretory phospholipase A2 does not appear to be associated with phenotypic variation in familial adenomatous polyposis. Hum Genet. 1996;98:386–390. doi: 10.1007/s004390050226. [DOI] [PubMed] [Google Scholar]
- 17.Kashiwagi M, Friess H, Uhl W, Berberat P, Abou-Shady M, Martignoni M, Anghelacopoulos SE, Zimmermann A, Buchler MW. Group II and IV phospholipase A2 are produced in human pancreatic cancer cells and influence prognosis. Gut. 1999;45:605–612. doi: 10.1136/gut.45.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung SY, Chen X, Chu KM, Yuen ST, Mathy J, Ji J, Chan AS, Li R, Law S, Troyanskaya OG, et al. Phospholipase A2 group IIA expression in gastric adenocarcinoma is associated with prolonged survival and less frequent metastasis. Proc Natl Acad Sci USA. 2002;99:16203–16208. doi: 10.1073/pnas.212646299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong Q, Patel M, Scott KF, Graham GG, Russell PJ, Sved P. Oncogenic action of phospholipase A2 in prostate cancer. Cancer Lett. 2006;240:9–16. doi: 10.1016/j.canlet.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Belinsky GS, Rajan TV, Saria EA, Giardina C, Rosenberg DW. Expression of secretory phospholipase A2 in colon tumor cells potentiates tumor growth. Mol Carcinog. 2007;46:106–116. doi: 10.1002/mc.20271. [DOI] [PubMed] [Google Scholar]
- 21.Lindbom J, Ljungman AG, Lindahl M, Tagesson C. Increased gene expression of novel cytosolic and secretory phospholipase A2 types in human airway epithelial cells induced by tumor necrosis factor-α and IFN-γ. J Interferon Cytokine Res. 2002;22:947–955. doi: 10.1089/10799900260286650. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 23.Singh RP, Agarwal R. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocr Relat Cancer. 2006;13:751–778. doi: 10.1677/erc.1.01126. [DOI] [PubMed] [Google Scholar]
- 24.Corcoran NM, Costello AJ, Hovens CM. Interfering with cellsurvival signalling as a treatment strategy for prostate cancer. BJU International. 2006;97:1149–1153. doi: 10.1111/j.1464-410X.2006.06198.x. [DOI] [PubMed] [Google Scholar]
- 25.Balasoiu M, Turculeanu A, Avramescu C, Comanescu V, Simionescu C, Mogoanta L. Cytokines levels in prostate adenocarcinomas. Rom J Morphol Embryol. 2005;46:179–182. [PubMed] [Google Scholar]
- 26.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshimura A. Signal transduction of inflammatory cytokines and tumor development. Cancer Sci. 2006;97:439–447. doi: 10.1111/j.1349-7006.2006.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menschikowski M, Hagelgans A, Heyne B, Hempel U, Neumeister V, Goez P, Jaross W, Siegert G. Statins potentiate the IFN-γ induced upregulation of group IIA phospholipase A2 in human aortic smooth muscle cells and HepG2 hepatoma cells. Biochim Biophys Acta. 2005;1733:157–171. doi: 10.1016/j.bbalip.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Crowl R, Stoner C, Stoller T, Pan YC, Conroy R. Isolation and characterisation of cDNA clones from human placenta coding for phospholipase A2. Adv Exp Med Biol. 1990;279:173–184. doi: 10.1007/978-1-4613-0651-1_11. [DOI] [PubMed] [Google Scholar]
- 30.Ercolani L, Florence B, Denaro M, Alexander M. Isolation and complete sequence of a functional human glyceraldehyde-3-phosphate dehydrogenase gene. J Biol Chem. 1988;263:15335–15341. [PubMed] [Google Scholar]
- 31.Andreani M, Olivier JL, Berenbaum F, Raymondjean M, Bereziat G. Transcriptional regulation of inflammatory secreted phospholipases A2. Biochim Biophys Acta. 2000;1488:149–158. doi: 10.1016/s1388-1981(00)00117-7. [DOI] [PubMed] [Google Scholar]
- 32.Webber MM, Bello D, Quader S. Immortalized and tumorigenic adult human prostatic epithelial cell lines: characteristics and applications: Part 3. Oncogenes, suppressor genes, and applications. Prostate. 1997;30:136–142. doi: 10.1002/(sici)1097-0045(19970201)30:2<136::aid-pros9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 33.Engl T, Relja B, Blumenberg C, Müller I, Ringel EM, Beecken WD, Jonas D, Blaheta RA. Prostate tumor CXC-chemokine profile correlates with cell adhesion to endothelium and extracellular matrix. Life Sci. 2006;78:1784–1793. doi: 10.1016/j.lfs.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Aalinkeel R, Nair MP, Sufrin G, Mahajan SD, Chadha KC, Chawda RP, Schwartz SA. Gene expression of angiogenic factors correlates with metastatic potential of prostate cancer cells. Cancer Res. 2004;64:5311–5321. doi: 10.1158/0008-5472.CAN-2506-2. [DOI] [PubMed] [Google Scholar]
- 35.Dunn GP, Sheehan KC, Old LJ, Schreiber RD. IFN unresponsiveness in LNCaP cells due to the lack of JAK1 gene expression. Cancer Res. 2005;65:3447–3453. doi: 10.1158/0008-5472.CAN-04-4316. [DOI] [PubMed] [Google Scholar]
- 36.Yang TT, Ung PM, Rincón M, Chow CW. Role of the CCAAT/enhancer-binding protein NFATc2 transcription factor cascade in the induction of secretory phospholipase A2. J Biol Chem. 2006;281:11541–11552. doi: 10.1074/jbc.M511214200. [DOI] [PubMed] [Google Scholar]
- 37.Cookson MS, Reuter VE, Linkov I, Fair WR. Glutathione S-transferase PI (GST-pi) class expression by immunohistochemistry in benign and malignant prostate tissue. J Urol. 1997;157:673–676. [PubMed] [Google Scholar]
- 38.Millar DS, Ow KK, Paul CL, Russell PJ, Molloy PL, Clark SJ. Detailed methylation analysis of the glutathione S-transferase pi (GSTP1) gene in prostate cancer. Oncogene. 1999;18:1313–1324. doi: 10.1038/sj.onc.1202415. [DOI] [PubMed] [Google Scholar]
- 39.Lodygin D, Epanchintsev A, Menssen A, Diebold J, Hermeking H. Functional epigenomics identifies genes fre Ruently silenced in prostate cancer. Cancer Res. 2005;65:4218–4227. doi: 10.1158/0008-5472.CAN-04-4407. [DOI] [PubMed] [Google Scholar]
- 40.Chetcuti A, Margan SH, Russell P, Mann S, Millar DS, Clark SJ, Rogers J, Handelsman DJ, Dong Q. Loss of annexin II heavy and light chains in prostate cancer and its precursors. Cancer Res. 2001;61:6331–6334. [PubMed] [Google Scholar]
- 41.Nelson WG, Yegnasubramanian S, Agoston AT, Bastian PJ, Lee BH, Nakayama M, De Marzo AM. Abnormal DNA methylation, epigenetics, and prostate cancer. Front Biosci. 2007;12:4254–4266. doi: 10.2741/2385. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Hattar J, Jiricny J. Methylation of single CpG dinucleotides within a promoter element of the Herpes simplex virus tk gene reduces its transcription in vivo. Gene. 1988;65:219–227. doi: 10.1016/0378-1119(88)90458-1. [DOI] [PubMed] [Google Scholar]
- 43.Xin C, Ren S, Eberhardt W, Pfeilschifter J, Huwiler A. FTY720 suppresses interleukin-1β-induced secretory phospholipase A2 expression in renal mesangial cells by a transcriptional mechanism. Br J Pharmacol. 2007;150:943–950. doi: 10.1038/sj.bjp.0707171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scholz K, Vlachojannis GJ, Spitzer S, Schini-Kerth V, Van Den Bosch H, Kaszkin M, Pfeilschifter J. Modulation of cytokine-induced expression of secretory phospholipase A2-type IIA by protein kinase C in rat renal mesangial cells. Biochem Pharmacol. 1999;58:1751–1758. doi: 10.1016/s0006-2952(99)00279-8. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka Y, Gavrielides MV, Mitsuuchi Y, Fujii T, Kazanietz MG. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. J Biol Chem. 2003;278:33753–33762. doi: 10.1074/jbc.M303313200. [DOI] [PubMed] [Google Scholar]
- 46.Murakami M, Shimbara S, Kambe T, Kuwata H, Winstead MV, Tischfield JA, Kudo I. The functions of five distinct mammalian phospholipase A2s in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2s are functionally redundant and act in concert with cytosolic phospholipase A2. J Biol Chem. 1998;273:14411–14423. doi: 10.1074/jbc.273.23.14411. [DOI] [PubMed] [Google Scholar]
- 47.Bernatchez PN, Winstead MV, Dennis EA, Sirois MG. VEGF stimulation of endothelial cell PAF synthesis is mediated by group V 14 kDa secretory phospholipase A2. Br J Pharmacol. 2001;134:197–205. doi: 10.1038/sj.bjp.0704215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tessier C, Hichami A, Khan NA. Implication of three isoforms of PLA2 in human T-cell proliferation. FEBS Lett. 2002;520:111–116. doi: 10.1016/s0014-5793(02)02779-5. [DOI] [PubMed] [Google Scholar]