Abstract
The crystal structure at 2.0-Å resolution of the complex of the Escherichia coli chemotaxis response regulator CheY and the phosphoacceptor-binding domain (P2) of the kinase CheA is presented. The binding interface involves the fourth and fifth helices and fifth β-strand of CheY and both helices of P2. Surprisingly, the two heterodimers in the asymmetric unit have two different binding modes involving the same interface, suggesting some flexibility in the binding regions. Significant conformational changes have occurred in CheY compared with previously determined unbound structures. The active site of CheY is exposed by the binding of the kinase domain, possibly to enhance phosphotransfer from CheA to CheY. The conformational changes upon complex formation as well as the observation that there are two different binding modes suggest that the plasticity of CheY is an essential feature of response regulator function.
The Escherichia coli chemotaxis signal transduction pathway is a member of the widespread family of two-component regulatory systems (reviewed in ref. 1). Two-component signal transduction pathways are defined by the conservation of an autophosphorylating histidine kinase and a response regulator with a conserved aspartate phosphorylation site. Propagation of the signal occurs through both covalent protein modification and noncovalent protein–protein interactions in a simple pathway (reviewed in refs. 2 and 3).
In the chemotaxis signaling pathway, the histidine autokinase CheA serves as the phosphodonor for two response regulator proteins, CheY and CheB (4). CheB is a methylesterase involved in signal attenuation. CheY functions as a phosphorylation-dependent switch in the excitation response. A single domain of the kinase CheA (residues 159–227, hereafter referred to as P2) is likely to be responsible for localizing the phosphoacceptors in the vicinity of the kinase for subsequent phosphorylation, because protein fragments containing the P2 domain of CheA can bind to CheY with an affinity similar to that of the full-length protein (5, 6). CheB binds to a fragment of CheA (residues 1–233) that contains both the P2 domain and the N-terminal phosphotransfer domain with an affinity similar to that of full-length CheA (6).
We are interested in the mechanism of signal transduction in the chemotaxis pathway and in two-component systems in general. Of particular interest are the conformations achieved during the signaling process and the ways in which discrimination can be achieved among pathways containing homologous members. Previous works have determined the structure of CheY and of the P2 domain in the free state (7, 8). Recently, an independently determined crystal structure of CheY and the P2 domain of CheA was reported (9). The heterodimer structure reported by Welch et al. (9) at 3.0-Å resolution is of a slightly different construct of CheA than that described here, and crystallizes in a different space group. In this article we describe the crystal structure at 2.0-Å resolution of the complex of CheY and P2 that reveals that this interaction interface has two binding modes. These two binding modes and the conformational changes between the free and bound forms yield insight into the nature of protein–protein interactions that may be important for signaling.
MATERIALS AND METHODS
Protein Expression and Purification.
CheY was expressed in E. coli and purified to homogeneity by the standard methods (10). CheY was dialyzed into 10 mM Tris⋅HCl, pH 7.0/0.02% sodium azide and concentrated to 18 mg/ml.
A gene encoding residues 156–229 of full-length CheA, plus an N-terminal methionine residue, was generated by using standard PCR methods using the plasmid pTM22 (11) as a template. The selection of residues encompassing the P2 domain was based on sequence comparison that identified conserved regions (11) and the NMR analysis of residues 124–257 that identified those residues that are structured in solution (12). Protein was expressed and purified in a manner similar to that reported by McEvoy et al. (12), with the addition of an ammonium sulfate precipitation step before application to a G50 sizing column as the last purification step. P2 was dialyzed against 10 mM Tris⋅HCl, pH 7.0/0.02% sodium azide and concentrated to 30 mg/ml.
Crystallization, Data Collection, and Refinement.
Crystals were grown in hanging drops containing 1 μl of CheY (15 mg/ml), 1 μl of P2 (9 mg/ml), and 2 μl of well solution (0.5 ml of 0.63 M NaH2PO4/1.17 M K2HPO4/10 mM NH4Cl, pH 7.0) in vapor equilibrium with the well solution at room temperature. Maximum crystal dimensions were 1.2 × 0.5 × 0.03 mm. Data were collected at room temperature. Native data and the selenomethionine-CheY and K2PtI6 derivative data were collected at the Stanford Synchrotron Radiation Laboratory on beam lines 7-1 and 9-1. The imaging plate data were processed with the programs denzo and scalepack (13). Data for the crystals soaked with HgCl2 were collected in house on a Xuong–Hamlin multiwire area detector and processed by using the supplied software (14).
Initial phase information was obtained from three derivatized crystals. Heavy atom derivatives were obtained by soaking the crystals in reservoir buffer containing 1 mM HgCl2 or 1 mM K2PtI6 for 15 min or 6 months before data collection, respectively. Selenomethionine containing CheY protein was expressed and purified essentially as described above, with modifications for selenomethionine incorporation (15). Initial multiple isomorphous replacement phases were calculated at 3.0-Å resolution (figure of merit = 0.51) by using the protein program (16). Solvent flattening of the electron density maps was carried out using the density modification function of the ccp4 software package (17). A real space search (S.J.R., unpublished program) of the electron density map using the α-carbon backbone of E. coli apo-CheY (3chy; ref. 7) as the search model showed two prominent peaks 13 and 10 SDs above the mean. The R-factor after placement of the two CheY molecules was 0.58 for the resolution range 8.0–5.0 Å. The positions of the two CheY molecules were refined as a rigid body by using the software package tnt (18). A real space search using the α-carbon backbone of the NMR-derived P2 domain (1fwp; ref. 8) as a model showed a peak 7 SDs above the mean. The second P2 molecule was placed by rotation and translation to the comparable position on the other CheY molecule. Rigid body refinement of the positions of all four molecules in the asymmetric unit was performed, then positional refinement. Model building was done by using the program o (19). Phase combination was performed and reduced-bias 2Fo − Fc maps were calculated by using sigmaa (20). Native and derivative data and refinement statistics are given in Table 1.
Table 1.
Native and derivative data collection and structure refinement statistics
| Space group | P212121 | |||
| Unit cell parameters, Å | a = 54.5, b = 64.2, c = 158.0 | |||
| Vm, Å3/Da | 3.1 | |||
| Native | Selenomethionine CheY | HgCl2 | K2PtI6 | |
| No. of crystals | 2 | 1 | 1 | 1 |
| Data source | SSRL (7-1) | SSRL (7-1) | CuKα (U. Oregon) | SSRL (9-1) |
| Resolution, Å | 2.0 | 1.95 | 3.0 | 2.8 |
| No. of reflections | 146,840 | 93,775 | 23,101 | 34,326 |
| No. of unique reflections | 30,701 | 36,778 | 10,092 | 12,878 |
| Rmerge, % | 5.5 | 5.1 | 4.5 | 8.9 |
| Overall completeness, % | 78 | 77 | 73 | 81 |
| No. of heavy atom sites | NA | 6 | 2 | 2 |
| RC† | NA | 0.70 | 0.58 | 0.82 |
| Phasing power | NA | 1.36 | 1.74 | 0.82 |
| Final R-factor,‡ % | 21.7 | |||
| No. of non-H protein atoms | 2934 | |||
| No. of water molecules | 124 | |||
| Deviations from restraints | ||||
| Ideal bond lengths, Å | 0.022 | |||
| Ideal bond angles, ° | 2.89 | |||
| Correlated B values, Å2 | 4.55 | |||
SSRL, Stanford Synchrotron Radiation Laboratory; NA, not applicable.
Rmerge = 100(∑|I − 〈I〉|/∑|I|), where 〈I〉 is the average intensity of equivalent reflections.
RC = ∑∥Fder| − |Fnat + fH∥/∑∥Fder| − | Fnat∥, where Fder and Fnat are the derivative and native structure factor amplitudes, respectively, and fH is the heavy atom scattering factor.
R-factor = 100(∑∥Fo| − |Fc∥/|Fo|), where Fo and Fc are the observed and calculated structure factor amplitudes within the set of reflections used for refinement. Refinement was carried out using the data between 20.0 Å and 2.0 Å.
The final model consists of residues 2–129 of CheY-A, residues 2–129 of CheY-B, residues 159–225 of P2-A, and residues 158–226 of P2-B, and 124 water molecules. The side chains of the following residues do not show clear electron density and are omitted: 19, 22, 26, 34, 89, 91, and 118 of CheY-A; 19, 26, and 118 of CheY-B, 168 and 224 of P2-A and 168 and 224 of P2-B.
Solvent-accessible surface area and secondary structures were calculated by using the program dssp (21). Overlay coordinates were generated with the program edpdb (22). Hydrogen bonds were evaluated by using the program hbplus (23), which defines hydrogen bonds according to standard geometric criteria. The alignment of the CheY and CheB protein sequences was generated by using the GCG package (version 8.0; Genetics Computer Group, Madison, WI).
RESULTS
Overall Structure of the Heterodimers.
The structure of the CheY/P2 complex has been determined at 2.0-Å resolution by using a combination of multiple isomorphous replacement and molecular replacement. The final statistics for native and derivative data collection and structural refinement are given in Table 1.
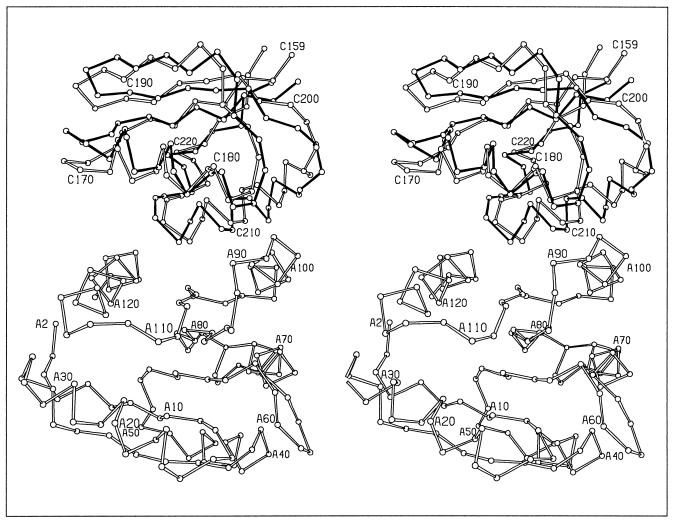
There are two CheY/P2 heterodimers in the asymmetric unit, which surprisingly show two different modes of association (Fig. 1). While the heterodimer interfaces both involve association of the fourth and fifth helices and the fifth β-strand of CheY with the two helices of the P2 domain, a superposition of the two CheY molecules does not superimpose the two P2 molecules. A further 10.7° rotation of one P2 molecule relative to the other is required to optimally align the two molecules. The differences between the two heterodimers, termed the CheY/P2-A and CheY/P2-B heterodimers, are discussed in detail in the following section.
Figure 1.
Two modes of CheY/P2 interactions. Stereoview of the α-carbon backbone of the A heterodimer (open bonds) superimposed with P2 of the B heterodimer (solid bonds) based on alignment of the CheY molecules. CheY residues are numbered from A2 to A120. P2 residues are numbered from C159 to C220.
Though the mode of association of CheY and P2 is different in the two heterodimers, the overall structures of the two CheY and P2 molecules in the asymmetric unit are similar, having rms deviations of 1.0 Å and 0.63 Å for CheY and P2, respectively. The greatest difference in the structures of the two CheY molecules is in the position of the fourth helix relative to the first helix. The fourth helix is one of the helices forming the binding interface and therefore the two binding modes observed influence the local structure in this region, causing differences in CheY structure.
Details of the Binding Modes.
While the two heterodimers have similar interface regions, the details of the interactions differ. The hydrogen bonds in each heterodimer are listed in Table 2. In total, there are nine hydrogen-bonding interactions in the A-heterodimer and six in the B-heterodimer. Of these, five involve the same partners, though the distances differ. In the B-heterodimer, there is a hydrogen bond between K122 of CheY and E171 of P2, though in the A-heterodimer K122 is forming a water-mediated hydrogen bond to the backbone carbonyl of A169. K119 of CheY is hydrogen bonded to E178 of P2 in the A-heterodimer, while no similar interaction is present in the B-heterodimer. There is a hydrogen bond from K92 of CheY to the backbone carbonyl of D202 of P2 in the A-heterodimer, but in the B-heterodimer K92 is interacting with the side chain of E93 and a water molecule is hydrogen bonding to the backbone carbonyl of D202. Each heterodimer buries similar amounts of surface area upon complex formation: 1250 Å2 and 1218 Å2 for the A and B heterocomplexes, respectively.
Table 2.
Hydrogen bonds in the two CheY/P2 heterodimers
| Heterodimer A
|
Heterodimer B
|
||||
|---|---|---|---|---|---|
| CheY | P2 | Length, Å | CheY | P2 | Length, Å |
| Direct | |||||
| K92 Nζ | D202 O | 3.3 | |||
| Q100 Nɛ2 | D207 Oδ1 | 2.9 | Q100 Nɛ2 | D207 Oδ1 | 2.3 |
| Y106 OH | E178 Oɛ2 | 2.7 | |||
| Y106 OH | H181 Nδ1 | 2.7 | Y106 OH | H181 Nδ1 | 3.3 |
| K119 Nζ | E178 Oɛ2 | 3.4 | |||
| K122 Nζ | E171 Oɛ2 | 3.2 | |||
| K126 Nζ | C213 O | 2.4 | K126 Nζ | C213 O | 2.3 |
| K126 Nζ | I216 O | 2.6 | K126 Nζ | I216 O | 2.7 |
| Water-mediated | |||||
| S104 O | HOH 306 | 3.4 | S104 O | HOH 306 | 3.2 |
| HOH 306 | F214 O | 3.3 | HOH 306 | F214 O | 2.8 |
| K122 Nζ | HOH 362 | 2.9 | |||
| HOH 362 | A169 O | 2.7 | |||
Overall CheY Structural Changes.
Moderate changes are seen in the overall structure of CheY by a comparison of the α-carbon backbones of CheY in the P2-bound heterodimer and the unbound E. coli apo-CheY structure (7). While the overall secondary structure elements are retained, concerted changes have occurred between secondary structure elements (Fig. 2). The overall backbone rms deviation is 1.8 Å between the α-carbon atoms of E. coli apo-CheY (3chy; ref. 7) and CheY of either heterocomplex. The rms deviation of the α-carbon backbones of CheY in the heterodimer and other E. coli CheY crystal structures of various metal-bound or mutant states varies from 1.8 Å to 2.2 Å (7, 24–27). The overall relative changes in CheY structure are greater in the P2-bound structures of CheY than those observed between any of the other structures of CheY that are available. Large localized structural alterations are observed in E. coli CheY in the Mg2+ bound form (24), but the magnitude of the overall distortion of the backbone is not as large as is observed in CheY when in complex with the P2-domain.
Figure 2.
(A and B) Stereoviews of superpositions of the α-carbon backbone of E. coli apo-CheY (7) (open bonds) and CheY-A and CheY-B of the heterodimers (solid bonds), respectively. Superpositions were based on the most similar regions, which were residues 2–40, 85–95, and 100–129 for apo-CheY and CheY-A and residues 2–35, 50–70, and 100–129 of apo-CheY and CheY-B.
The largest changes between the free and P2-bound CheY structures are in the positions of the helices. In general, the helices have undergone an expansion compared with their positions in E. coli apo-CheY as determined by analysis of a δ-distance plot (data not shown). The first helix has moved with respect to both the second and third helices, and the third helix is farther from the fifth helix in the heterodimer structures. The helices of both the CheY-A and CheY-B molecules have undergone these conformational changes relative to E. coli apo-CheY.
CheY Active Site.
Previous structural and functional studies have implicated a number of areas in CheY that may be crucial for activation, both within and distant from the active site. The conformational changes observed between the structure of CheY bound to P2 and the E. coli apo-CheY structure (3chy; ref. 7) are reported in the following paragraphs. Because P2 preferentially interacts with the unphosphorylated form of CheY (28, 29), these changes may influence the rate of phosphotransfer.
In general, the active sites of E. coli apo-CheY and P2-bound CheY are similar. The active site of CheY is distinct from the P2 binding face, and therefore is not sterically obstructed by P2 binding. The aspartates in the active site of the free and P2-bound structures of CheY have equivalent conformations. The electron density map shows a well-ordered feature in each CheY active site, at a position comparable to that of a bound water molecule in the active site of the E. coli apo-CheY structure.
In the E. coli apo-CheY structure, a salt bridge is formed between D57 and K109 (7), which will necessarily be disrupted as a consequence of phosphorylation of D57. The disruption of the D57/K109 interaction has been suggested to be involved in switching from the inactive to active signaling state (29). In the E. coli apo-CheY structure, the distance from the carboxyl group of D57 to the amino group of K109 is 2.7 Å (7), and the distances are 3.3 Å and 3.4 Å in the A- and B-heterodimers, respectively.
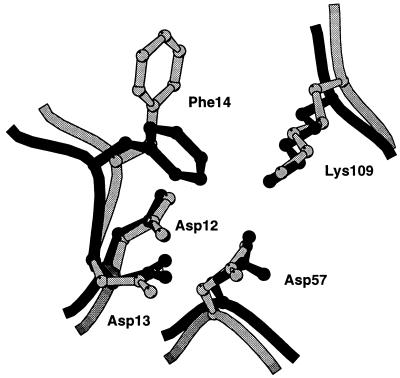
In addition to localizing CheY near the phosphorylation domain of CheA, binding of the P2 domain might facilitate phosphotransfer by opening up the active site of CheY for phosphorylation by CheA. Upon binding of the P2 domain, the solvent accessible surface area of D57 increases 2-fold, from 8 Å2 in the E. coli apo-CheY structure (7), to about 16 Å2 when bound to P2. Phenylalanine 14, which partially closes the active site in the apo and Mg2+ bound structures of E. coli CheY (7, 24), adopts a more open conformation in the heterocomplex, thereby providing more access to the active site than in the unbound CheY structure (Fig. 3).
Figure 3.
Active site region of CheY. The overlay of CheY free (dark) and P2-bound (light) was based on a superposition of the entire α-carbon backbone.
The accessibility of the active site in the P2-bound conformation of CheY is evidenced by a crystal contact between two symmetry-related CheY-A molecules. The carboxyl group of a glutamate side chain (E5) of a CheY-A molecule projects into the active site region of an adjacent CheY-A molecule. The electron density for this glutamate side chain is weak, but clearly oriented toward the active site.
Mutational analyses (29, 30) suggest that there may be residues, such as Y106, that participate both in P2 interaction and interaction with switch components in the flagellar basal body. Tyrosine-106 is located in the fifth β-strand on the putative switch binding surface of CheY (7, 29). The conformation of this tyrosine side chain may be important for CheY-induced switching of the flagellar motor, because mutations of CheY have been isolated that force the tyrosine to preferentially occupy the solvent-exposed position, and these show defects in motor switching (25, 26, 31). In E. coli apo-CheY, Y106 is observed to occupy two states, being found in an exposed solvent accessible position and buried in a more hydrophobic pocket (7). In both of the heterodimers, Y106 occupies the solvent accessible rotamer, and makes a hydrogen bond to a P2 residue.
Structure of the P2 Domain of CheA.
The structure of the P2 domain when bound to CheY is similar to the unbound structure determined in solution by NMR methods (8). The rms deviations of the α-carbon backbone between the average solution structure and the P2-A and P2-B domains in the crystal structure are 2.4 Å and 2.2 Å, respectively, over residues 159–225. In P2 of the heterocomplexes, the helices are shifted from their positions relative to the unbound structure (8), causing moderate overall changes in the structure of the protein. These conformational differences could possibly be due to conformational changes induced by CheY binding, a reflection of the different environments (solution or crystalline), or the accuracy of the solution structure determined by NMR methods. Determination of the relative contribution of these factors is not possible now.
Interface Residue Conservation.
CheA interacts with the methylesterase CheB with a binding affinity similar to that of CheY (6). Based on the similar binding affinity and the presence of a response regulator domain of CheB that is homologous to CheY (26% residue identity), it is not unreasonable to hypothesize that the interface-forming residues of CheY may be conserved in CheB. The fifth helix is generally highly variable, possibly because in many other response regulators this regions serves as a transition point to a C-terminal domain (32). Surprisingly, few of the interface forming residues of CheY are identical or similar to corresponding residues in CheB (Fig. 4).
Figure 4.
Sequence alignment of CheY and the response regulator domain of CheB. The interface forming residues in CheY of either heterodimer have a bar over the residue. Those residues that form side-chain hydrogen bonds in either heterodimer are indicated by ★ above the residue.
DISCUSSION
Heterodimer Interactions.
The crystal structure of the CheYP2 complex reveals that there are two binding modes that involve the same interface. The observation of two different binding modes involving different hydrogen bonding schemes is remarkable and suggests that flexibility of the interface may be functionally important. These differing interactions can be likened to domain motions with respect to each other as have been observed in crystal structures of multidomain proteins.
An NMR analysis of CheY bound to P2 in solution identified a number of residues in the C-terminal region of the protein that may form a CheA binding interface based on chemical shift changes of CheY resonances in the presence of P2 (5). Many of these residues (I96, A103, G105, K122, I123, and K126) are also observed to be interface residues in the crystal structure of the heterocomplexes. The additional residues in the fourth β-strand of CheY identified by the NMR analysis that have little exposed surface area (M85, V86, and T87) are likely to have perturbed chemical shift values in the presence of P2 due to indirect effects of structural rearrangement. The general agreement between the residues identified by NMR and x-ray diffraction suggests that the contacts seen between CheY and P2 in the crystal are present in the complex in solution.
A mutational analysis of CheY identified a cluster of residues on CheY that lead to altered CheA binding (D13, T87, A90, E93, Y106, V108, F111, T112, and E117) (30). These interface residues are not the same interface residues observed in the crystal structure of the heterocomplexes, with the exception of residues A90 and Y106. Based on the observation of the overall plasticity of CheY in the heterocomplex, it now seems likely that these mutationally identified residues cause structural modifications that are propagated through CheY structure to alter the binding affinity.
Overall CheY Structure.
It is likely that the conformational changes of CheY seen in the heterodimer with P2 reflect those conformations that are the most easily phosphorylated. The moderate structural changes in the P2-bound CheY structure may enhance the rate of phosphotransfer. Because other structures of CheY determined under different crystallization conditions and in different space groups do not show structural changes on the order of those found in the heterodimers we conclude that the conformational changes we observe in CheY are induced by binding to the P2 domain, rather than an artifact of crystallization.
After phosphorylation has occurred, at least some aspects of the phospho-CheY structure are no longer optimal for kinase binding because affinity is reduced (6, 28). The site of phosphorylation is not in contact with P2 so the reduction in affinity must reflect changes in the P2-binding interface of CheY. One way to reduce the interaction with P2 would be to move Y106 to an inaccessible site upon phosphorylation, resulting in the loss of the Y106 hydrogen bond to H181 in P2.
CheY Active Site.
The binding of P2 may have induced conformational changes in the active site of CheY that enhance the phosphotransfer reaction. One of these conformational changes is the lengthening of the D57/K109 salt bridge. Phosphotransfer from CheA to CheY has a requirement for divalent cation (33). In two Mg2+-bound CheY structures, the D57K109 salt bridge is disrupted by the coordination of Mg2+ in the active site (24, 34). The lengthening of the salt bridge in the P2-bound CheY structure may represent a conformation that is poised for Mg2+ binding and subsequent phosphotransfer. Another active site formational change is in the position of F14, which increases the accessible surface area of the phosphorylation residue, D57. NMR studies of E. coli CheY suggest that there is flexibility in this region (35), and therefore the binding of P2 may induce the open conformation preferentially.
The penetration of a glutamate side chain from a symmetry-related CheY molecule into the active site of one of the CheY molecules demonstrates the accessibility of the active site to a negatively charged group from another molecule when CheY is bound to P2. This may reflect the interactions taking place during the phosphotransfer reaction. The phosphohistidine from the phosphotransfer domain (CheA1–134) of CheA requires access to the active site, and the phosphohistidine from the surface of the P1 domain may penetrate the active site of CheY much as the glutamate side chain of the symmetry-related CheY molecule does.
Interface Residue Conservation.
The P2-interface forming residues of CheY are not conserved in the homologous domain of the methylesterase CheB. If the P2 binding site on CheB is in a similar region to that of CheY, this seems to exclude the possibility that there is a molecular “code” of amino acids that are used by the kinase to recognize the cognate response regulators. The plasticity of CheY and P2 demonstrated by the different binding modes of the two heterodimers may be essential for recognition of CheB using different specific contacts.
Concluding Remarks.
The interactions of CheY and the P2 domain of CheA exhibit flexibility in their mode of interaction, which may be a functionally relevant feature. In addition to the two binding modes that are presented here, the recently reported heterodimer structure of Welch et al. (9) seems to present yet another mode of interaction. The heterodimer structure of Welch et al. is similar to those described here in overall features, and equivalent conformations of F14 and Y106 are also observed. The details of the interface hydrogen bonding differ, and the amount of buried surface area is slightly less than what is observed in either of the two heterodimers presented here.
The crystal structure of the CheY/P2 complex reveals unexpected plasticity in the overall CheY structure as well as in the details of interactions with P2. This result, combined with the lack of sequence conservation of key residues in the proposed CheB/P2 binding interface, suggests that structural malleability is a vital feature of protein-protein interactions in response regulators. This observation is consistent with the NMR evidence for long-range conformational changes upon phosphorylation (10, 36), suggesting that the observed plasticity of CheY itself is essential to its role in signal transduction.
Acknowledgments
We thank Eric Bertelsen for comments on the manuscript. This work was supported by National Research Service Award predoctoral training grants from the National Institutes of Health to M.M.M. and A.C.H. and by National Institutes of Health Grant AI17808 to F.W.D. All diffraction data except for the Hg-derivative data were collected at the Stanford Synchrotron Radiation Laboratory, which is funded by the Department of Energy, Office of Basic Energy Sciences. The Biotechnology Program at the Stanford Synchrotron Radiation Laboratory is supported by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program and by the Department of Energy, Office of Biological and Environmental Research.
Footnotes
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, Biology Department, Brookhaven National Laboratory, Upton, NY 11973 (reference 1eay).
References
- 1.Parkinson J S, Kofoid E C. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 2.Blair D F. Annu Rev Microbiol. 1995;49:489–522. doi: 10.1146/annurev.mi.49.100195.002421. [DOI] [PubMed] [Google Scholar]
- 3.Falke J J, Bass R B, Butler S L, Chervitz S A, Danielson M A. Annu Rev Cell Dev Biol. 1997;13:457–512. doi: 10.1146/annurev.cellbio.13.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess J F, Oosawa K, Kaplan N, Simon M I. Cell. 1989;53:79–87. doi: 10.1016/0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- 5.Swanson R V, Lowry D F, Matsumura P, McEvoy M M, Simon M I, Dahlquist F W. Nat Struct Biol. 1995;2:906–910. doi: 10.1038/nsb1095-906. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Swanson R V, Simon M I, Weis R M. Biochemistry. 1995;34:14626–14636. doi: 10.1021/bi00045a003. [DOI] [PubMed] [Google Scholar]
- 7.Volz K, Matsumura P. J Biol Chem. 1991;266:15511–15519. doi: 10.2210/pdb3chy/pdb. [DOI] [PubMed] [Google Scholar]
- 8.McEvoy M M, Muhandiram D R, Kay L E, Dahlquist F W. Biochemistry. 1996;35:5633–5640. doi: 10.1021/bi952707h. [DOI] [PubMed] [Google Scholar]
- 9.Welch M, Chinardet N, Mourey L, Birck C, Samama J-P. Nat Struct Biol. 1998;5:25–29. doi: 10.1038/nsb0198-25. [DOI] [PubMed] [Google Scholar]
- 10.Lowry D F, Roth A F, Rupert P B, Dahlquist F W, Moy F J, Domaille P J, Matsumura P. J Biol Chem. 1994;269:26358–26362. [PubMed] [Google Scholar]
- 11.Morrison T B, Parkinson J S. Proc Natl Acad Sci USA. 1994;91:5485–5489. doi: 10.1073/pnas.91.12.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEvoy M M, Zhou H, Roth A R, Lowry D F, Morrison T B, Kay L E, Dahlquist F W. Biochemistry. 1995;34:13871–13880. doi: 10.1021/bi00042a019. [DOI] [PubMed] [Google Scholar]
- 13.Otwinowski Z. In: Proceedings of the CCP4 Study Weekend. Sawyer L, Isaac N, Bailey S, editors. Warrington, U.K.: Science and Engineering Council Daresbury Laboratory; 1993. pp. 56–62. [Google Scholar]
- 14.Howard A J, Nielsen C, Xuong N H. Methods Enzymol. 1985;114:452–471. doi: 10.1016/0076-6879(85)14030-9. [DOI] [PubMed] [Google Scholar]
- 15.Van Duyne G D, Standaert R F, Karplus P A, Schreiber S L, Clardy J. J Mol Biol. 1993;229:105–124. doi: 10.1006/jmbi.1993.1012. [DOI] [PubMed] [Google Scholar]
- 16.Steigemann W. Doctoral thesis. Munich: Technical University; 1974. [Google Scholar]
- 17.Collaborative Computational Project, Number 4. Acta Crystallogr D. 1994;50:760–763. [Google Scholar]
- 18.Tronrud D E, Ten Eyck L F, Matthews B W. Acta Crystallogr A. 1987;43:489–501. [Google Scholar]
- 19.Jones T A, Zou J Y, Cowan S W, Kjeldgaard M. Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 20.Read R J. Acta Crystallogr A. 1986;42:140–149. [Google Scholar]
- 21.Kabsch W, Sander C. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X-J, Matthews B W. J Appl Crystallogr. 1995;28:624–630. [Google Scholar]
- 23.Thornton J M, Edwards M S, Taylor W R, Barlow D J. EMBO J. 1986;5:409–413. doi: 10.1002/j.1460-2075.1986.tb04226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellsolell L, Prieto J, Serrano L, Coll M. J Mol Biol. 1994;238:489–495. doi: 10.1006/jmbi.1994.1308. [DOI] [PubMed] [Google Scholar]
- 25.Ganguli S, Wang H, Matsumura P, Volz K. J Biol Chem. 1995;270:17386–17393. [PubMed] [Google Scholar]
- 26.Zhu X, Rebello J, Matsumura P, Volz K. J Biol Chem. 1997;272:5000–5006. doi: 10.1074/jbc.272.8.5000. [DOI] [PubMed] [Google Scholar]
- 27.Jiang M, Bourret R B, Simon M I, Volz K. J Biol Chem. 1997;272:11850–11855. doi: 10.1074/jbc.272.18.11850. [DOI] [PubMed] [Google Scholar]
- 28.Schuster S C, Swanson R V, Alex L A, Bourret R B, Simon M I. Nature (London) 1993;365:343–347. doi: 10.1038/365343a0. [DOI] [PubMed] [Google Scholar]
- 29.Roman S J, Meyers M, Volz K, Matsumura P. J Bacteriol. 1992;174:6247–6255. doi: 10.1128/jb.174.19.6247-6255.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shukla D, Matsumura P. J Biol Chem. 1995;270:24414–24419. doi: 10.1074/jbc.270.41.24414. [DOI] [PubMed] [Google Scholar]
- 31.Zhu X, Amsler C D, Volz K, Matsumura P. J Bacteriol. 1996;178:4208–4215. doi: 10.1128/jb.178.14.4208-4215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volz K. Biochemistry. 1993;32:11741–11753. doi: 10.1021/bi00095a001. [DOI] [PubMed] [Google Scholar]
- 33.Lukat G S, Stock A M, Stock J B. Biochemistry. 1990;29:5436–5442. doi: 10.1021/bi00475a004. [DOI] [PubMed] [Google Scholar]
- 34.Stock A M, Martinez-Hackert E, Rasmussen B F, West A H, Stock J B, Ringe D, Petsko G A. Biochemistry. 1993;32:13375–13380. doi: 10.1021/bi00212a001. [DOI] [PubMed] [Google Scholar]
- 35.Moy F J, Lowry D F, Matsumura P, Dahlquist F W, Krywko J E, Domaille P J. Biochemistry. 1994;33:10731–10742. doi: 10.1021/bi00201a022. [DOI] [PubMed] [Google Scholar]
- 36.Drake S K, Bourret R B, Luck L A, Simon M I, Falke J J. J Biol Chem. 1993;268:13081–13088. [PMC free article] [PubMed] [Google Scholar]