Abstract
The essential contribution of the antidepressant-sensitive serotonin (5-HT) transporter SERT (which is encoded by the SLC6A4 gene) to platelet 5-HT stores suggests an important role of this transporter in platelet function. Here, using SERT-deficient mice, we have established a role for constitutive SERT expression in efficient ADP- and thrombin-triggered platelet aggregation. Additionally, using pharmacological blockers of SERT and the vesicular monoamine transporter (VMAT), we have identified a role for ongoing 5-HT release and SERT activity in efficient human platelet aggregation. We have also demonstrated that fibrinogen, an activator of integrin αIIbβ3, enhances SERT activity in human platelets and that integrin αIIbβ3 interacts directly with the C terminus of SERT. Consistent with these findings, knockout mice lacking integrin β3 displayed diminished platelet SERT activity. Conversely, HEK293 cells engineered to express human SERT and an activated form of integrin β3 exhibited enhanced SERT function that coincided with elevated SERT surface expression. Our results support an unsuspected role of αIIbβ3/SERT associations as well as αIIbβ3 activation in control of SERT activity in vivo that may have broad implications for hyperserotonemia, cardiovascular disorders, and autism.
Introduction
Before gaining attention as a neurotransmitter, serotonin (5-hydroxytryptamine [5-HT]) was first detected in the blood and initially studied for its ability to trigger vasoconstriction (1). Although platelets store large quantities of 5-HT, the amine is actually synthesized in the gut, where it is released after the activation of presynaptic neurons or the stimulation of enterochromaffin cells (2). Once 5-HT enters the circulation, it is sequestered inside platelets by antidepressant-sensitive 5-HT transporters (SERTs, SLC6A4) and then into secretory granules by the vesicular monoamine transporter (VMAT). Chronic use of 5-HT selective reuptake inhibitors (SSRIs) decreases platelet 5-HT content and can exacerbate bleeding (3, 4). Secreted 5-HT, acting on platelet 5-HT2a receptors, has been proposed to augment the effects of other prothrombotic agents that act through integral membrane receptor proteins, including the αIIbβ3 complex (also known as glycoprotein GPIIb/IIIa) (5). These actions of 5-HT may also represent a pathway for platelet hyperactivity and spontaneous aggregation that is observed in patients with atherosclerotic disease (6). Whether continuous SERT activity is needed to sustain platelet aggregation is unknown, though the recognition that serotonylation of intracellular Rac and Rho proteins supports platelet granule secretion suggests a role for SERT in sustaining platelet aggregation independent of 5-HT2a receptors (7). Together these studies provide indirect support for SERT as a key player in platelet 5-HT homeostasis and sustaining thrombosis, conclusions reinforced by association of SERT polymorphisms with cardiovascular disease (8).
SERTs in the brain, platelets, and transfected cells are increasingly recognized to be regulated by complex signaling mechanisms that involve the actions of multiple Ser/Thr kinases and phosphatases (9, 10) as well as scaffolding/adaptor proteins (11). These interactions can rapidly redistribute the transporter among distinct membrane subdomains, alter transporter cell surface expression, and/or control SERT catalytic activity (11–13). Additionally, SERT proteins are phosphorylated at several different sites, where the balance between phosphorylation by PKC and PKG and dephosphorylation by protein phosphatase 2A (PP2A) influence different aspects of SERT catalytic activity and trafficking (12, 14, 15). Tonic activation p38 MAPK signaling pathways, without direct phosphorylation of the transporter, controls basal surface SERT expression levels, whereas acute p38 MAPK activation induces trafficking-independent stimulation of SERT transport activity (12, 16).
SERT is now recognized as a downstream effector of multiple cell-surface receptors (17, 18). In platelets, the activation of thrombin and ADP receptors initiates “inside-out” signaling, leading to integrin αIIbβ3 serine and tyrosine phosphorylation and integrin receptor activation and clustering (19). Consequently, binding of αIIbβ3 ligand (e.g., fibrinogen) triggers “outside-in” signaling that promotes further cytoskeletal (CS) reorganization and granule secretion (20). The propagation of outside-in signaling leads to the formation of focal adhesion structures, continued granule secretion, and thrombus formation (21). The current studies, which establish physical associations between SERT and αIIbβ3, reveal the regulation of SERT trafficking and 5-HT as additional facets of integrin-mediated platelet activation.
Genetic variation leading to altered αIIbβ3 activity is associated with platelet dysfunction, exemplified by the association of constitutively active forms of αIIbβ3 with elevated blood 5-HT and arterial thrombosis and myocardial infarction (22, 23). Here we report that a constitutively active allele of integrin β3, encoded by the human gene ITGB3, modulates SERT surface and subcellular localization via a PP2A- and p38 MAPK–dependent signaling pathway. Our findings provide a mechanistic basis for established genetic interactions of ITGB3 and SLC6A4 polymorphisms in setting 5-HT blood levels (24). Additionally, they reveal an unsuspected coordination between αIIbβ3 signaling and SERT activity that can sustain platelet aggregation and may contribute to the recognized comorbidities between cardiovascular and brain disorders.
Results
Platelet SERT expression and function are involved in platelet aggregation.
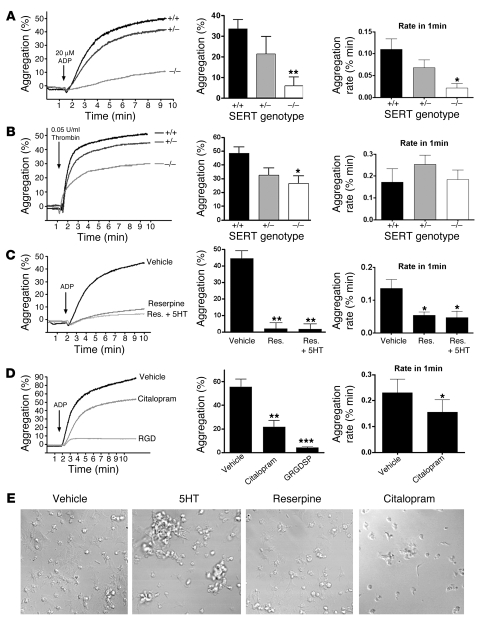
To examine the role for SERT in thrombus formation, we isolated platelets from wild-type mice or mice lacking SERT, which have significantly reduced blood 5-HT levels (25, 26). Platelet aggregation assays conducted with wild-type mouse platelets using 20 μM ADP or 0.05 U/ml thrombin demonstrated approximately 50% aggregation within approximately 10 minutes. In contrast, SERT–/– mice showed an 80% reduction in ADP-induced aggregation over the same time period and a significantly slower rate of platelet aggregation (t = 10 minutes, P = 0.0179, n = 4; rate: P = 0.0108; Figure 1A). In response to thrombin, platelets lacking SERT had an approximately 50% reduction in aggregation (t = 10 minutes, P = 0.0171, n = 4; Figure 1B). In contrast to ADP, thrombin did not induce a decrease in aggregation rates in SERT–/– platelets, suggesting a more prominent role of the transporter in maintaining aggregation. These data reveal agonist-dependent roles for SERT in the rate and extent of platelet aggregation.
Figure 1. Altered platelet aggregation in platelets with reduced SERT uptake and/or reduced granule content.
In vitro aggregation studies in SERT+/+, SERT +/–, and SERT–/– mouse platelets. Platelets were washed and exposed to either 20 μM ADP (A) or 0.05 U/ml thrombin (B). SERT-null mouse platelets have diminished aggregation response to ADP, as established by the analysis of initial aggregation rates. For A and B, bar plots represents mean ± SEM of 4 mice/genotype. (C) Depletion of granular pools of 5-HT by incubation with 10 nM reserpine (30 minutes at 37°C) dramatically reduces ADP-mediated (20 μM) aggregation in human platelets. (D) Acute SERT blockade by 4.5 μM citalopram reduces ADP-mediated platelet aggregation. Washed human platelets were incubated at 37°C for 10 minutes with citalopram or 1 mM GRGDSP peptide. n = 6. For A–D, final aggregation data represent mean ± SEM at t = 10 minutes. Rates are represented as mean ± SEM. One-way ANOVA with Dunnett’s post-test, *P < 0.05, **P < 0.01, and ***P < 0.005. (E) Confocal microscopy of human platelets plated on glass coverslips coated with fibrinogen. Platelets were preincubated with 10 μM 5-HT, 4.5 μM citalopram for 10 minutes, or 10 nM reserpine for 30 minutes at 37°C; seeded onto coverslips; and activated with 20 μM ADP. Images shown are representative of 4 independent experiments. Original magnification, ×65.
To examine whether loss of sensitivity to ADP in SERT–/– platelets arises from loss of SERT protein per se versus loss of intracellular 5-HT, we treated normal platelets with the VMAT inhibitor reserpine (27). Acute (30-minute) reserpine treatments led to a loss of granule stores but overall retention of 5-HT in the cytoplasm, and reserpine-treated platelets remained competent for attachment and spreading on fibrinogen-coated plates (Figure 1E). However, reserpine-treated platelets exhibited a marked reduction in aggregation rate and extent that could not be reversed by acute incubation in 10 μM external 5-HT (t = 10 minutes, P < 0.0001, n = 4; rate analysis: P = 0.0169, n = 4; Figure 1C). Platelets with normal intracellular stores of 5-HT that were treated acutely with the SSRIs citalopram or fluoxetine at concentrations required to fully block platelet SERT attenuated ADP-induced platelet aggregation, reducing the initial rate of aggregation (Figure 1, D and E; t =10 minutes, P = 0.006, n = 6; rate analysis: P = 0354, n = 8) and also diminishing spreading of platelets onto fibrinogen-coated plates (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI33374DS1). These findings indicate that 5-HT recycling via SERT is required to sustain stable rates of platelet aggregation. Although we did not observe a significant enhancement of platelet aggregation in samples preincubated with 10 μM 5-HT in vitro (data not shown), the treatment did promote the formation of platelet aggregates (Figure 1E). Taken together, our findings indicate that SERT function plays a role not only in the acquisition of 5-HT to load granule stores but also in 5-HT recycling that supports platelet aggregation.
Fibrinogen binding activates SERT uptake in human platelets.
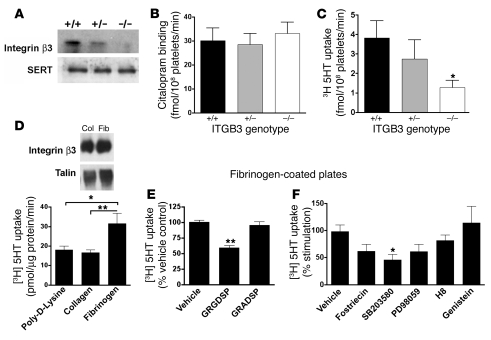
As ITGB3 has been identified as a quantitative trait locus for whole-blood 5-HT levels (24), we studied the effect of genetic ablation of murine Itgb3 on platelet SERT activity. The absence of integrin β3 in Itgb3-null (Itgb3–/–) mice did not impact the levels of SERT protein as assessed by immunoblots of total platelet protein (Figure 2A) or analysis of 3[H]citalopram binding to platelet membranes (Figure 2B). Remarkably, homozygous disruption of Itgb3 led to an approximately 70% decrease in 5-HT uptake (2-tailed, unpaired Student’s t test, P < 0.05, n = 5; Figure 2C). The impact of Itgb3 gene dosage on SERT activity was not an indirect result of changes in levels of platelet 5-HT, as HPLC analysis of these levels showed no difference between Itgb3+/+ and Itgb3–/– animals (data not shown). These findings reveal that αIIbβ3 expression or signaling is required for normal platelet SERT function.
Figure 2. Integrin regulation of SERT function.
(A and B) SERT expression levels are conserved regardless of the Itgb3 genotype. (A) Western blot of native SERT from platelet lysates from Itgb3+/+, Itgb3+/–, and Itgb3–/– mice. (B) Platelets were isolated from Itgb3+/+, Itgb3+/–, and Itgb3–/– mice and incubated with [3H]citalopram (20 nM) for 20 minutes at 4°C. (C) Integrin αIIbβ3 loss of function dramatically reduces SERT uptake activity. Platelets were isolated from Itgb3+/+, Itgb3+/–, and Itgb3–/– mice and [3H]5-HT (50 nM) transport assayed. For B and C, data are shown as mean ± SEM. One-way ANOVA with Dunnett’s post-test, *P < 0.05, n = 5 per group. (D) Immobilized fibrinogen, but not collagen, enhances human platelet SERT uptake activity. Human platelets were seeded on plates coated with either poly-d-lysine, collagen, or fibrinogen and [3H]5-HT uptake measured. One-way ANOVA with Bonferroni’s post-test, *P < 0.05. (E) The competing peptide GRGDSP (1 mM) reverses the fibrinogen-mediated enhancement of SERT activity in seeded human platelets. n = 4. (F) The fibrinogen-mediated enhancement of SERT uptake activity can be partially reversed by the p38 MAPK inhibitor SB203580. Platelets attached to fibrinogen-coated plates were incubated for 10 minutes with fostriecin (1 nM), SB203580 (20 μM), PD98059 (10 μM), H8 (0.1 μM), or genistein (20 μM) before measurement of [3H]5-HT uptake. n = 8. The data for D–F are presented as mean ± SEM with 1-way ANOVA and Dunnett’s post-test, *P < 0.05 and **P < 0.005.
The activation of inside-out pathways by platelet aggregation agonists triggered widespread signaling cascades within platelets, leading to different effects on SERT function (thrombin, ADP, Ca2+, and PKC activation; shown in Supplemental Figure 2, A and B). To examine whether αIIbβ3 signaling imparts changes in SERT activity, we took advantage of the ability of immobilized fibrinogen to induce αIIbβ3 clustering and localized outside-in signaling in nonactivated platelets (28). Whereas the α2β1 ligand collagen failed to stimulate SERT activity to a greater extent than immobilized poly-d-lysine, platelets seeded on αIIbβ3 ligand fibrinogen showed an enhanced level of 5-HT uptake as measured 30 minutes after plating (P = 0.0403, n = 4; Figure 2D). Soluble fibrinogen also enhanced SERT activity, though higher concentrations were required (Supplemental Figure 2C). We found no evidence of SERT regulation by soluble collagen (Supplemental Figure 2D). The specificity of immobilized fibrinogen effects was further confirmed by blocking available binding sites with the αIIbβ3-binding peptide GRGDSP, which reduced platelet 5-HT uptake to poly-d-lysine levels (P < 0.0001, n = 4; Figure 2E). The control peptide GRADSP had no effect on fibrinogen-induced stimulation of SERT activity. To explore αIIbβ3-signaling pathways that support fibrinogen-induced SERT activation, we treated platelets for 10 minutes with inhibitors of tyrosine kinase (genistein, 20 μM), ERK (PD98059, 10 μM), PKG (H8, 0.1 μM), p38 MAPK (SB203580, 20 μM), or PP2A (fostriecin, 1nM) prior to fibrinogen incubation. Only the p38 MAPK inhibitor SB203580 effected a significant attenuation of fibrinogen stimulation (P = 0.0244, n = 8; Figure 2F). These data suggest that signaling arising from fibrinogen-bound αIIbβ3 can enhance SERT surface expression and/or catalytic function through pathways linked to p38 MAPK.
SERT participates in the αIIbβ3 complex through direct protein-protein interactions.
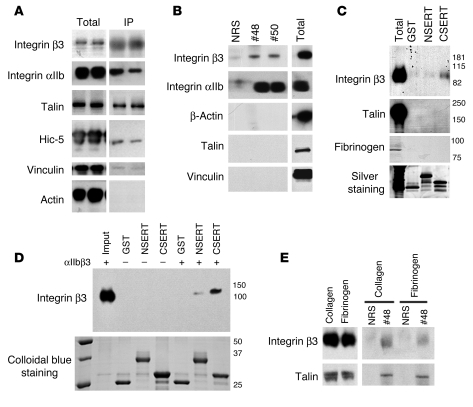
Our evidence of a functional relationship between αIIbβ3 and SERT led us to ask whether a physical complex exists that could support coordinated receptor-transporter signaling. Indeed, proteomic analysis of SERT fusion protein complexes following incubation with human platelet extracts revealed multiple peptides for components of the αIIbβ3 complex (Supplemental Table 1), including both αIIb and β3 subunits. To validate these findings, we immunoprecipitated full-length SERT from detergent extracts of platelets and also recovered both αIIb and β3 integrins, as well as the integrin β3–associated proteins talin and adaptor protein Hic-5 (Figure 3A), suggesting recovery of a larger protein complex. To assess the specificity of this complex, we lysed human platelets with a high-stringency buffer (RIPA, containing 1% SDS) and isolated SERT using 2 different SERT antibodies (nos. 48 and 50). Using both SERT antisera, we recovered significant quantities of αIIbβ3 protein, whereas no αIIbβ3 signal was detected when using normal rabbit serum (Figure 3B). Notably, under these more stringent conditions, talin was not retained in coimmunoprecipitations, suggesting that it associates indirectly or is more detergent-sensitive. To localize a domain in SERT that might support these associations, we performed pull-down assays from platelet detergent extracts using glutathione-S-transferase (GST) or GST fused to the N or C terminus of rat SERT. In these assays, we found that the SERT C terminus selectively supported integrin β3 associations (Figure 3C). To assess whether αIIbβ3 directly binds to the C terminus of SERT, we performed in vitro binding experiments using purified SERT GST fusion proteins with purified αIIbβ3 (10 μg). We were able to recover both integrin αIIb and β3 in samples containing SERT C terminus, as confirmed by Western blots against the β3 subunit (Figure 3D), suggesting a direct interaction between the C terminus of SERT and αIIbβ3. To investigate whether the stimulation of SERT mediated by fibrinogen binding to αIIbβ3 reflects a change in levels of SERT/αIIbβ3 complexes, we immunoprecipitated SERT from platelets seeded onto fibrinogen or collagen-coated plates. No differences in the recovery of the SERT/αIIbβ3 complexes were observed when comparing fibrinogen and collagen (Figure 3E), suggesting that inside-out signaling to activate SERT takes advantage of a preassembled integrin/SERT complex.
Figure 3. Physical interactions between SERT and integrin αIIbβ3.
Western blots showing the association of SERT with members of the focal adhesion complex. Human platelet lysates were prepared under low- (A, 1% Triton X-100, n = 2 shown) and high-stringency (B, RIPA buffer) conditions, and SERT immunocomplexes were isolated (normal rabbit serum [lane 1, NRS] or 2 different anti-SERT polyclonal sera [nos. 48 and 50; see ref. 49 for details] were used). The focal adhesion proteins integrin αIIbβ3, vinculin, talin, and actin were identified by immunoblotting with specific antibodies. Only integrin αIIbβ3 interacts with SERT under high-stringency conditions. (C) GST pulldowns performed in human platelets under high-stringency conditions (RIPA buffer) demonstrate that the C terminus of SERT (CSERT) mediates the SERT/αIIbβ3 interaction. (D) GST pulldowns performed using purified αIIbβ3 (Enzyme Research) demonstrate that the β3 subunit directly interacts with the C terminus of SERT. (E) The fibrinogen-mediated enhancement of SERT uptake activity is not mediated by increased interactions with the focal adhesion complex. No significant differences were found between collagen and fibrinogen samples. For A–E, representative blots from at least 3 independent experiments are shown.
The ITGB3 PlA2 (Pro33) variant enhances SERT uptake activity via enhanced transport surface expression.
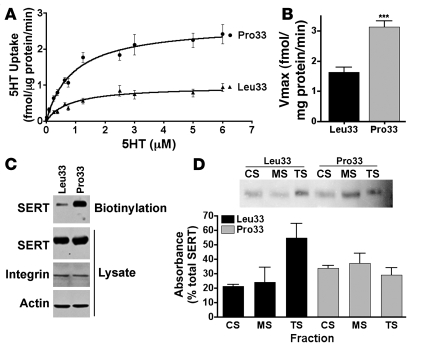
The ITGB3 PlA1 and PlA2 polymorphisms correspond to Leu33 and Pro33 coding variants, respectively. Platelets carrying the Pro33 isoform show increased αIIbβ3 activation, fibrinogen binding, and platelet reactivity upon ADP stimulation (29). Given our evidence of a physical and functional interaction between SERT and αIIbβ3, we asked whether these 2 ITGB3 alleles differentially impact SERT function. HEK293 cells stably expressing either the αIIbLeu33 or αIIbPro33 were transfected with human SERT and evaluated for 5-HT transport activity. Saturation analyses revealed that the Pro33 allele conferred a significant (260%) increase in 5-HT uptake activity, compared with the Leu33 cells (2-way ANOVA, P < 0.0001; Figure 4A). This increase was supported by an approximately 2-fold increase in Vmax (unpaired Student’s t test, P = 0.0002, n = 10; Figure 4B), with no significant change in Km (data not shown). This increase in Vmax was correlated with elevated SERT plasma membrane expression as assessed by surface biotinylation experiments (Figure 4C).
Figure 4. Genetic variation in ITGB3 alters SERT transport activity, surface trafficking, and subcellular localization.
(A) Saturation analyses of HEK293 cells expressing SERT, αIIb, and either the Leu33 or the Pro33 isoforms of integrin β3. Data are presented as mean ± SEM. Two-way ANOVA, P < 0.0001, n = 10. (B) Pro33β3 expression increases SERT Vmax. Unpaired Student’s t test, ***P < 0.0001, n = 10. (C) The increase in SERT Vmax by Pro33β3 is reflected by enhancement of SERT plasma membrane expression. Intact HEK293 cells were exposed to biotin, and surface proteins were captured by streptavidin beads. Surface SERT was detected by Western blot analysis. No significant differences were found in total SERT or αIIbβ3 expression. Representative blot from 3 independent experiments. (D) SERT subcellular distribution in platelets from Leu33 or Pro33 homozygous subjects. Human platelets were fractionated, and CS and MS fractions were collected. SERT is evenly distributed among the CS, MS, and TS compartments in Pro33 subjects. Data are presented as mean ± SEM. Two-way ANOVA, P < 0.05, n = 3 per group.
Differential SERT subcellular localization associated with integrin β3 alleles.
Previously, we found that modification of intracellular pathways alters the distribution of SERT protein between platelet subcellular compartments (11), with a quantitatively similar distribution evident between mouse and human platelets. Most (88%) of the protein is localized in intracellular, Triton X-100–soluble (TS) vesicles, whereas only 7%–10% is localized at the cell surface, where it is associated with the membrane skeleton (MS). We also identified a transient compartment where SERT-containing vesicles associate with the CS. To analyze whether Leu33 and Pro33 alleles differentially impact SERT subcellular distribution, we prepared CS, MS, and TS fractions from platelets from subjects homozygous for either allele. The distribution of SERT protein in Leu33 platelets reveals the majority of the transporter to be localized in the TS fraction, predominantly composed of intracellular vesicles. In contrast, Pro33 platelets exhibit a strikingly different pattern, redistributing SERT from TS to both CS and MS fractions (2-way ANOVA, P = 0.0237; Figure 4D). These findings are consistent with an increase in SERT activity accompanying coexpression with Pro33 ITGB3 and are consistent with whole-blood 5-HT levels found in Pro33 subjects (24, 30).
The ITGB3 PlA2 (Pro33)–enhanced transport surface trafficking is mediated by PP1/PP2A and p38 MAPK signaling.
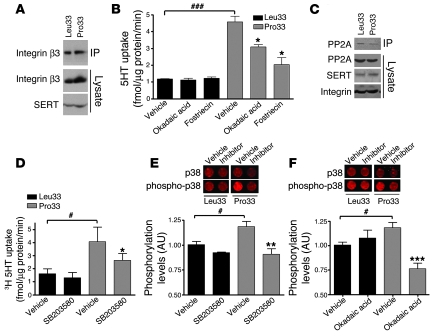
Coimmunoprecipitation experiments showed that the elevated SERT activity did not arise from an allele-dependent effect on the formation/stability of SERT/integrin αIIbβ3 complexes (Figure 5A). Since the prothrombotic phenotype in Pro33 platelets is thought to be a consequence of altered serine/threonine phosphatase signaling, also observed in HEK293 cells stably expressing this particular isoform (31), we examined whether SERT stimulation is sensitive to serine/threonine phosphatase antagonists. Whereas incubation for 10 minutes with the PP1/PP2A inhibitor okadaic acid (250 nM) or the specific PP2A inhibitor fostriecin (1 nM) did not alter basal SERT activity in Leu33 cells, both treatments resulted in a significant reduction in SERT activity in Pro33 cells (P = 0.0273, okadaic acid, n = 6; P = 0.0305, fostriecin, n = 4; Figure 5B and Supplemental Figure 4B). These data are interesting in light of our prior findings that PP2A associates with SERT and acute inhibition of PP2A increases SERT phosphorylation and internalization. Since coimmunoprecipitation studies did not reveal a difference between SERT/Pro33 and SERT/Leu33 cells in the relative levels of SERT/PP2A complexes, it is possible that Pro33 signaling targets preformed SERT/PP2A complexes (or possibly other pools of PP2A) to support SERT trafficking (Figure 5C).
Figure 5. ITGB3 Pro33 regulates SERT transport activity via serine/threonine protein phosphatases and p38 MAPK signaling.
(A) Coimmunoprecipitation experiment showing no changes in the SERT/αIIbβ3 complexes between SERT/Leu33 and SERT/Pro33 cells. (B) Reduction of SERT uptake activity in Pro33, but not Leu33, cells by inhibition of serine/threonine phosphatases. HEK293 cells expressing SERT and either Leu33 or Pro33 were incubated for 10 minutes with vehicle, okadaic acid (250 nM), or fostriecin (1 nM) before measurement of [3H]5-HT uptake. Data are presented as mean ± SEM. Paired Student’s t test: *P < 0.05, 1-way ANOVA with Dunnett’s post-test: ###P < 0.0005, n = 6. (C) Coimmunoprecipitation experiment showing no changes in the SERT/PP2A complexes between Leu33 and Pro33 cells. (D) Reduction of SERT uptake activity in Pro33 by inhibition of p38 MAPK. HEK293 cells expressing SERT and either Leu33 or Pro33 were incubated for 10 minutes with SB203580 (20 μM) before measurement of [3H]5-HT uptake. Data are presented as mean ± SEM. Paired Student’s t test: *P < 0.05; 1-way ANOVA with Dunnett’s post-test: #P < 0.05; n = 6. (E and F) Enhanced p38 MAPK phosphorylation levels in Pro33 cells are sensitive to SB203580 and okadaic acid (250 nM). Data were normalized to total p38 MAPK levels and are presented as mean ± SEM. One-way ANOVA with Dunnett’s post-test: #P < 0.05; paired Student’s t test: **P < 0.01, ***P < 0.005; n = 3.
Enhanced cell adhesion and migration attributed to integrin β3 Pro33 can be abolished by MAPK inhibition (31), suggesting that activated MAPKs could play a role in the Pro33 regulation of SERT. Therefore, we investigated the role of MAPK signaling in the enhancement of SERT activity by incubating SERT/Leu33 and SERT/Pro33 cells for 10 minutes with either a specific ERK2 inhibitor (PD98059, 10 μM) or the p38 MAPK inhibitor SB203580 (20 μM). The ERK inhibitor PD98059 failed to reduce Pro33-stimulated 5-HT transport (Supplemental Figure 4C). In contrast, SERT activity was reduced upon treatment with the p38 MAPK inhibitor SB203580 specifically in SERT/Pro33 cells (Figure 5D, P = 0.0365, n = 6; Supplemental Figure 4F). These studies support the idea that p38 MAPK is upregulated or activated in Pro33 cells. Indeed, whereas basal and phosphorylated ERK levels did not differ between Pro33 and Leu33 cells (Supplemental Figure 4D), Pro33 cells demonstrated a consistent elevation of p38 MAPK phosphorylation, relative to Leu33 cells (Figure 5E; P = 0.0238, n = 3). Treatments with either SB203580 (Figure 5E; P = 0.0045, paired Student’s t test, n = 3) or okadaic acid (Figure 5F; P = 0.0068, paired Student’s t test, n = 3) eliminated this increase. In contrast to the Pro33-supported stimulation of 5-HT uptake, p38 MAPK phosphorylation was insensitive to fostriecin (Supplemental Figure 4G), suggesting a role for PP1 in sustaining p38 MAPK phosphorylation (32). Taken together, our findings indicate that the Pro33-triggered elevation of 5-HT uptake arises from enhanced p38 MAPK signaling supported by both PP1 and PP2A.
Discussion
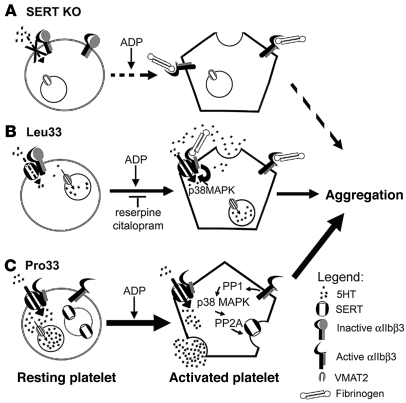
In the present study, we demonstrate that SERT expression supports efficient platelet aggregation; we identify the integrin receptor αIIbβ3 as a novel component of SERT regulatory protein complexes and demonstrate that SERT trafficking and transport activity are influenced by functional ITGB3 coding variants. Genetic ablation of platelet 5-HT uptake in SERT–/– mice, SSRI blockade of human SERT, and depletion of platelet 5-HT granules with reserpine significantly reduced the rate and extent of platelet aggregation, findings best explained by an ongoing need for platelets to recycle releasable stores of 5-HT (Figure 6, A and B). The effects of genetic SERT elimination on aggregation were more pronounced than those observed with ex vivo SSRI treatments, consistent with the preservation of intracellular 5-HT stores during acute SSRI treatments and the more profound aggregation impact observed with reserpine-induced granule depletion. The prothrombotic human integrin β3 polymorphism Pro33 (PlA2) induced elevated SERT surface expression and enhanced 5-HT uptake activity (Figure 6C), effects that plausibly contribute to elevated 5-HT blood levels. The recognition of an important role for SERT in platelet aggregation and identification of αIIbβ3/SERT interactions that can impact transporter trafficking and 5-HT uptake capacity provides a mechanistic basis for established interactions between SLC6A4 and ITGB3 genetic variation in hyperserotonemia and cardiovascular disease.
Figure 6. Model for αIIbβ3 regulation of SERT function and 5-HT signaling in platelets.
(A) SERT-KO platelets lack intracellular 5-HT stores that can be released upon integrin activation. This absence of granule 5-HT leads to diminished aggregation induced by ADP. (B) Leu33 platelets exhibit low basal 5-HT uptake activity, sufficient to maintain granule stores of 5-HT. Binding of immobilized fibrinogen to αIIbβ3 leads to changes in SERT uptake activity that can enhance granule secretion through serotonylation of small Rho GTPases. The depletion of granule stores by reserpine or inhibition of SERT uptake activity by citalopram diminishes platelet aggregation. (C) Integrin β3 Pro33 isoform enhances SERT uptake activity and membrane expression, leading to elevated platelet 5-HT levels. The molecular mechanism for the Pro33 cells involve enhanced PP1 and basal p38 MAPK phosphorylation levels and consequent altered PP2A pathways.
In addition to associated proteins, SERT is responsive to multiple kinase-mediated signaling pathways including those linked to PKC, PKG, and p38 MAPK activation (10, 12, 14), as well as dephosphorylation events mediated by PP2A (9). Although extensive work has been done regarding the identification and characterization of signaling pathways that regulate SERT, only PKC and PKG have been shown to directly phosphorylate the transporter (14, 15), suggesting that multiple pathways may interact to affect transporter function and trafficking. Our implication of p38 MAPK pathways in both rapid, fibrinogen-modulated SERT activity and the constitutive effects of ITGB3 Pro33 elevation of SERT surface expression is consistent with recent findings that p38 MAPK controls both basal SERT catalytic function (18) and trafficking (16). Despite the role for p38 MAPK in fibrinogen stimulation of SERT, as implicated by attenuation of stimulation by SB203580, fibrinogen stimulation does not elevate total p38 MAPK phosphorylation levels (data not shown). This finding is not surprising if only a limited pool of p38 MAPK, perhaps associated with the transporter, is responsible for SERT catalytic modulation. Since a stable complex is evident between αIIbβ3 and SERT, it seems altogether possible that fibrinogen binding can impact SERT conformations directly, stabilizing a state that is more susceptible to existing modulators (such as p38 MAPK).
Although we could not detect acute activation of p38 MAPK by fibrinogen, basal p38 MAPK phosphorylation in Pro33 cells was clearly elevated above levels found in Leu33 cells. That this activation was related to increased SERT trafficking and activity became evident with the ability of SB203580 to significantly reduce 5-HT uptake in the SERT/Pro33 cells but not the SERT/Leu33 cells. A constitutive elevation in p38 MAPK phosphorylation that drives elevated SERT surface expression and increased 5-HT uptake has been previously demonstrated by Samuvel and coworkers (16) using overexpression of the upstream p38 MAPK activator MKK3b. p38 MAPK is known to activate PP2A (32, 33), and the specific PP2A inhibitor fostriecin reduced the stimulation of SERT evident in SERT/Pro33 cells. Interestingly, fostriecin had no effect on the elevation of p38 MAPK phosphorylation found in Pro33 cells, though the PP1/PP2A inhibitor okadaic acid was effective. Thus, we suspect that PP1 activation downstream of Pro33 may be responsible for p38 MAPK activation, whereas transmission of kinase activation to SERT upregulation involves PP2A (Figure 6C) (34).
The decrease in platelet aggregation in SERT-null mice is reminiscent of the diminished 5-HT content found in subjects chronically exposed to SSRIs (35) and the reduced platelet activation observed in depressed patients after chronic SSRI treatment (36). Additionally, short-term, open-label treatment of depressed patients with the SSRI paroxetine leads to a significant reduction in the number of functional αIIbβ3 and plasma concentrations of proaggregatory factors (37). Whereas acute blockade of SERT did not compromise αIIbβ3 activation per se, as assessed by activated αIIbβ3 antibody binding (data not shown), acute SSRI treatments reduced platelet aggregation in vitro. It is possible that SERT activity is necessary not only for maintaining the granular 5-HT pools, but also to resupply cytoplasmic pools utilized for the amplification of signaling pathways that facilitate platelet aggregation. Indeed, high cytoplasmic 5-HT concentrations are necessary for rapid exocytosis of α-granules and controlled serotonylation of small G proteins during irreversible platelet aggregation (7, 38, 39). This idea is consistent with a differential effect of reserpine and citalopram on platelet spreading on fibrinogen-coated plates. A more detailed study of how various platelet agonists trigger platelet aggregation will provide a deeper understanding of how SERT activity collaborates with the multiple pathways that drive platelet aggregation. These ideas may also contribute to the negative correlation between cardiovascular disease and SSRI-induced remission of depression (40, 41).
Our studies have focused on platelet SERT and integrin β3 interactions due to the genetic interactions evident between ITGB3 and SLC6A4 for hyperserotonemia and also due to the tractability of platelets as a model system. Although the association of ITGB3 PlA1/A2 polymorphisms with acute thrombosis and stroke remains controversial, with contradictory findings in the general population, it may enhance the risk in populations already susceptible to coronary disease, such as in the elderly (42–44). It has not escaped our attention that similar mechanisms may also play a role in the regulation of SERT in other tissues, such as the gut or the brain. Integrin β3 is expressed in the developing and mature CNS and acts as a modulator of neurite formation and synaptic plasticity (45). In this regard, the enhanced SERT activity supported by αIIbβ3 polymorphisms may generate behavioral phenotypes similar to those of hSERT polymorphisms associated with altered SERT gene and protein expression (46, 47). In this regard, recent studies indicate a genetic interaction between ITGB3 and SLC6A4 in mediating autism susceptibility (30, 48). The finding that SERT and integrin β3 interact functionally and physically reveals an entirely unsuspected function for adhesion proteins in 5-HT homeostasis and represents a mechanism worthy of further investigation as a component of comorbidities between cardiovascular and mental illness. Studies of mice expressing human coding variants of ITGB3 and SLC6A4 should be useful in further analysis of this possibility.
Methods
Reagents and antibodies.
Most reagents, including thrombin from bovine plasma, adenosine 5ι-diphosphate (ADP), collagen type I from rat tail, β-actin, vinculin, and talin mouse monoclonal antibodies were purchased from Sigma-Aldrich. Fibrinogen from human plasma, αIIbβ3-binding peptide GRGDSP, and the control peptide GRADSP were purchased from Calbiochem (EMD Biosciences). Mouse monoclonal antibodies against integrin αIIb, integrin β3, Hic-5, and focal adhesion kinase (FAK) were obtained from BD Transduction Laboratories (BD Biosciences). Mouse monoclonal antibodies against p44/42 MAPK (ERK1/2), phospho-p44/42 (phospho-ERK1/2), p38 MAPK, and phospho–p38 MAPK were purchased from Cell Signaling Technology Inc. SERT antisera (nos. 48 and 50) have been previously characterized elsewhere (49).
Preparation of platelets.
Outdated human platelet-rich plasma (PRP) concentrates were obtained from the American Red Cross. Platelets were used within 72 hours and tested initially for aggregation by thrombin (0.01–0.5 U/ml), to ensure that platelets were viable and competent for aggregation (50). Studies with mouse platelets were performed in accordance with humane guidelines established by the Vanderbilt IACUC under an approved protocol (M/04/376). For these studies, we used littermates as controls, although both Itgb3 and Slc6a4 mice have already been backcrossed 10 generations onto a C57BL/6 background. Mouse platelets were collected in a tube containing acid citrate dextrose (39 mM citric acid, 75 mM sodium citrate, and 135 mM glucose, pH 7.4) from the superior vena cava after euthanasia. Whole blood was centrifuged immediately at 190 g for 15 minutes at room temperature to obtain PRP. The PRP was centrifuged at 2,500 g for 15 minutes at 22°C, and the resulting platelet pellet was gently resuspended in PBS buffer, pH 7.4, and used immediately.
Platelet aggregation.
Platelet aggregations were measured using washed platelets. The pelleted platelets were resuspended in Tyrode buffer and adjusted to a concentration of 3 × 108 platelets/ml using a Coulter counter (Beckman Coulter). Platelets were tested for aggregation in a thrombin concentration curve and compared with fresh samples activated with 0.5 U/ml thrombin, where no significant differences were found. Some platelets were treated with 1 mM GRGDSP, 10 μM 5-HT, 1 μM citalopram for 10 minutes or 10 nM reserpine for 30 minutes at 37°C. After stimulation with either 0.05 U/ml thrombin or 20 μM ADP, the change in light transmission was monitored with an aggregometer (Chronolog Inc.). Each result is representative of at least 4 independent experiments. Representative aggregation profiles are shown in Figure 1. Rate analysis was performed as a linear regression 60 seconds after the initial changes in light transmission by addition of ADP/thrombin (minutes 2–3 after agonist). We did not use the data for the initial minute after ADP addition due to solution turbidity that artifactually impacted aggregation measurements.
Mouse platelet transport and binding assays.
Approximately 2 × 108 platelets were used in each binding assay. For 5-HT transport, we followed the protocol described by Zhu et al. (12).To assess total SERT levels, we quantitated the binding of the SSRI [3H]citalopram (20 nM) to intact platelets on ice for 20 minutes in binding buffer (100 mM Tris-HCl, pH 7.4; 100 mM NaCl). Both uptake and binding were terminated by filtering the sample through GF/B Whatman paper using a BRANDEL cell harvester (Biomedical Research and Development Laboratories Inc.). The counts obtained from the filtered samples were corrected for the nonspecific binding/uptake obtained using parallel samples incubated with fluoxetine (1 μM). Experiments were performed in duplicate and replicated at least 4 times.
Immobilized platelet 5-HT transport assays.
Transport activity was assayed in 24-well plates (40,000 cells/well) prepared 24 hours prior to the 5-HT uptake assay. Plates were coated overnight at 4°C with 20 μg/ml poly-d-lysine, 100 μg/ml collagen, or 100 μg/ml fibrinogen in PBS. Human platelets were seeded for 30 minutes at 37°C for adhesion of platelets to the bottom of the plates. Serum was removed by aspiration, and platelets were washed once with modified Krebs-Ringer HEPES (KRH) buffer with or without modifiers (as described in Results). Transport assays were performed as described above (details in Supplemental Methods). Data were normalized to protein levels assayed for each plating condition to assure that the data obtained were due to changes in SERT activity and not to differences in total number of attached platelets. Western blot analyses of talin and integrin β3 indicated that the total number of platelets was similar in all conditions assayed. Data are shown as mean ± SEM of 3 or more independent experiments.
Immunoprecipitations and GST pulldowns.
Immunoprecipitation and immunoblots were performed from human platelet detergent extracts as described by Carneiro and Blakely (11). Details pertinent to this study are outlined in Supplemental Methods. For GST pulldowns, 100 μg of platelet RIPA lysates were incubated for 1 hour at 4°C with 20 μg GST fusion proteins containing either the COOH- (aa 1–86) or the N-terminal (aa 594–680) region of rat SERT coupled to glutathione beads. The beads were washed 3 times with PBS, and proteins were eluted in Laemmli buffer. To assess direct binding of αIIbβ3, the same amount of GST fusion proteins were incubated with 20 μg of purified αIIbβ3 (Enzyme Research). The samples were washed and separated by SDS-PAGE and identified by colloidal blue staining. To assure that the bands identified in the colloidal blue staining represented αIIbβ3 and not contaminating bacterial proteins, we performed Western blot analysis using the integrin β3 antibody. All the biochemical data are shown as representative sample of 3 independent experiments.
MAPK phosphorylation assays.
To monitor phosphorylation levels of MAPK in intact cells, HEK293 cells expressing either αIIbLeu33β3 or αIIbPro33β3 were seeded in 96-well plates (105 cells/well) and cultured in DMEM containing 10% FBS, 300 μg/ml Geneticin G418 (Gibco), and 100 μg/ml Zeocin (Invitrogen) in a 37°C incubator with 5% CO2 for 16–24 hours. Medium was removed by aspiration, and cells were washed once with PBS and then incubated at 37°C in PBS with or without SB203580 (20 μM), PD98095 (10 μM), okadaic acid (250 nM), or fostriecin (1 nM) for 10 minutes. We assessed phosphorylation levels by in cell Western blot analysis using the Odyssey system as described by the manufacturer (Li-Cor Biosciences). Details for the in-cell Western blot conditions are described in Supplemental Methods. After the final wash, the plate was scanned, and the captured image of the signal was processed and quantified with the Odyssey Infrared Imaging System (Li-Cor Biosciences). Phosphorylated protein levels were assessed by normalizing for total levels of each specific MAPK. Each experiment was conducted in duplicate, and data are presented as mean ± SEM of 3 or more experiments.
Isolation and analysis of platelet fractions.
Human blood of ITGB3 genotyped subjects was collected under an approved institutional review board protocol (University of Illinois at Chicago). All subjects provided informed consent for research studies. Platelet CS and MS isolation methods have been described in detail elsewhere (11). Graphs show SERT levels in each fraction, where CS + MS + TS = 100%. Statistical analysis was performed using 2-way ANOVA with Bonferroni’s multiple comparison tests in GraphPad Prizm 4 software (GraphPad Software Inc.).
Statistics.
Most of the data analysis was performed using 1-way ANOVA with Dunnett’s multiple comparison post-tests. For Figure 1, we analyzed the different aggregation profiles of the averaged data ± SEM using 2-way ANOVA and chose the profile that best resembled the averaged data as a representative plot. The t = 10 minutes graph represents the final aggregation levels observed in all experiments after 10 minutes of addition of ADP/thrombin. The initial aggregation rate was calculated as the slope of the linear regression from second to the third minute after addition of ADP, since we observed an increased in light transmission during the first minute (due to changes in platelet morphology). For Figure 1D, we only compared the aggregation rates between vehicle- and citalopram-treated platelets, as the slope for RGD-treated samples was 0. In this case, we used paired Student’s t test. For Figure 5, we performed 1-way ANOVA for all groups tested, where n = 6 represents the number of independent experiments performed. We also performed paired Student’s t tests to analyze the effect of kinase and phosphatase inhibitors on p38 MAPK phosphorylation levels on Pro33 cells and Leu33 cells, independently. We also used paired Student’s t tests to analyze the okadaic acid and the fostriecin effects on SERT activity, because these experiments were done independently. All Student’s t test analyses were performed using 2-tailed parameters. All the analyses were performed using GraphPad Prizm for Mac OS X (GraphPad Software Inc.). P values of less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Vinod Vijayan for provision of HEK293 cells stably transfected with the αIIbLeu33β3 or αIIbPro33β3 integrins. We also acknowledge Bryan Voss for help with the platelet aggregation assays, Jane Wright for animal husbandry and genotyping, and Tammy Jessen, Angela Steele, and Qiao Han for general laboratory support. We thank Mary Zutter and Sam Santoro for reagent provision and advice on experiments and early stages of our analyses and Jeremy Veenstra-Vander Weele for critical reading of the final manuscript. The authors have been supported as follows: NARSAD Young Investigator Award and NIDA DA007390 to A.M.D. Carneiro; the NIMH Intramural Program for D.L. Murphy; NICHD/CPEA U19 HD35482 to E.H. Cook; and NIMH P50 MH078028 to R.D. Blakely.
Footnotes
Nonstandard abbreviations used: CS, cytoskeleton/cytoskeletal; GST, glutathione-S-transferase; 5-HT, serotonin; ITGB3, integrin β3; MS, membrane skeleton/skeletal; PP2A, protein phosphatase 2A; SERT, 5-HT transporter; SSRI, 5-HT selective reuptake inhibitor; TS, Triton X-100–soluble; VMAT, vesicular monoamine transporter.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:1544–1552 (2008). doi:10.1172/JCI33374
References
- 1.Hilton B.P., Cumings J.N. An assessment of platelet aggregation induced by 5-hydroxytryptamine. J. Clin. Pathol. 1971;24:250–258. doi: 10.1136/jcp.24.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gershon M.D. Review article: serotonin receptors and transporters — roles in normal and abnormal gastrointestinal motility. Aliment. Pharmacol. Ther. 2004;20(Suppl. 7):3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 3.Sauer W.H., Berlin J.A., Kimmel S.E. Effect of antidepressants and their relative affinity for the serotonin transporter on the risk of myocardial infarction. Circulation. 2003;108:32–36. doi: 10.1161/01.CIR.0000079172.43229.CD. [DOI] [PubMed] [Google Scholar]
- 4.Roose S.P., Miyazaki M. Pharmacologic treatment of depression in patients with heart disease. Psychosom. Med. 2005;67(Suppl. 1):S54–S57. doi: 10.1097/01.psy.0000163455.43226.bf. [DOI] [PubMed] [Google Scholar]
- 5.Nishihira K., et al. Inhibition of 5-hydroxytryptamine receptor prevents occlusive thrombus formation on neointima of the rabbit femoral artery. J. Thromb. Haemost. 2006;4:247–255. doi: 10.1111/j.1538-7836.2005.01702.x. [DOI] [PubMed] [Google Scholar]
- 6.Trip M.D., Cats V.M., van Capelle F.J., Vreeken J. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N. Engl. J. Med. 1990;322:1549–1554. doi: 10.1056/NEJM199005313222201. [DOI] [PubMed] [Google Scholar]
- 7.Walther D.J., et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell. 2003;115:851–862. doi: 10.1016/S0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- 8.Nakatani D., et al. Influence of serotonin transporter gene polymorphism on depressive symptoms and new cardiac events after acute myocardial infarction. Am. Heart J. 2005;150:652–658. doi: 10.1016/j.ahj.2005.03.062. [DOI] [PubMed] [Google Scholar]
- 9.Bauman A.L., et al. Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J. Neurosci. 2000;20:7571–7578. doi: 10.1523/JNEUROSCI.20-20-07571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blakely R.D., et al. Regulated phosphorylation and trafficking of antidepressant-sensitive serotonin transporter proteins. Biol. Psychiatry. 1998;44:169–178. doi: 10.1016/S0006-3223(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 11.Carneiro A.M., Blakely R.D. Serotonin-, protein kinase C-, and Hic-5-associated redistribution of the platelet serotonin transporter. J. Biol. Chem. 2006;281:24769–24780. doi: 10.1074/jbc.M603877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu C.B., Carneiro A.M., Dostmann W.R., Hewlett W.A., Blakely R.D. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J. Biol. Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- 13.Jayanthi L.D., Samuvel D.J., Blakely R.D., Ramamoorthy S. Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol. Pharmacol. 2005;67:2077–2087. doi: 10.1124/mol.104.009555. [DOI] [PubMed] [Google Scholar]
- 14.Ramamoorthy S., Samuvel D.J., Buck E.R., Rudnick G., Jayanthi L.D. Phosphorylation of threonine residue 276 is required for acute regulation of serotonin transporter by cyclic GMP. . J. Biol. Chem. 2007;282:11639–11647. doi: 10.1074/jbc.M611353200. [DOI] [PubMed] [Google Scholar]
- 15.Ramamoorthy S., Blakely R.D. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999;285:763–766. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- 16.Samuvel D.J., Jayanthi L.D., Bhat N.R., Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J. Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansah T.A., Ramamoorthy S., Montanez S., Daws L.C., Blakely R.D. Calcium-dependent inhibition of synaptosomal serotonin transport by the alpha 2-adrenoceptor agonist 5-bromo-N-[4,5-dihydro-1H-imidazol-2-yl]-6-quinoxalinamine (UK14304). J. Pharmacol. Exp. Ther. 2003;305:956–965. doi: 10.1124/jpet.102.047134. [DOI] [PubMed] [Google Scholar]
- 18.Zhu C.B., Hewlett W.A., Feoktistov I., Biaggioni I., Blakely R.D. Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol. Pharmacol. 2004;65:1462–1474. doi: 10.1124/mol.65.6.1462. [DOI] [PubMed] [Google Scholar]
- 19.Phillips D.R., Nannizzi-Alaimo L., Prasad K.S. Beta3 tyrosine phosphorylation in alphaIIbbeta3 (platelet membrane GP IIb-IIIa) outside-in integrin signaling. Thromb. Haemost. 2001;86:246–258. [PubMed] [Google Scholar]
- 20.DeMali K.A., Wennerberg K., Burridge K. Integrin signaling to the actin cytoskeleton. Curr. Opin. Cell Biol. 2003;15:572–582. doi: 10.1016/S0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 21.de Virgilio M., Kiosses W.B., Shattil S.J. Proximal, selective, and dynamic interactions between integrin alphaIIbbeta3 and protein tyrosine kinases in living cells. J. Cell Biol. 2004;165:305–311. doi: 10.1083/jcb.200402064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams M.S., Bray P.F. Genetics of arterial prothrombotic risk states. Exp. Biol. Med. (Maywood). 2001;226:409–419. doi: 10.1177/153537020122600504. [DOI] [PubMed] [Google Scholar]
- 23.Vijayan K.V., Bray P.F. Molecular mechanisms of prothrombotic risk due to genetic variations in platelet genes: enhanced outside-in signaling through the Pro33 variant of integrin beta3. Exp. Biol. Med. (Maywood). 2006;231:505–513. doi: 10.1177/153537020623100504. [DOI] [PubMed] [Google Scholar]
- 24.Weiss L.A., et al. Genome-wide association study identifies ITGB3 as a QTL for whole blood serotonin. Eur. J. Hum. Genet. 2004;12:949–954. doi: 10.1038/sj.ejhg.5201239. [DOI] [PubMed] [Google Scholar]
- 25.Bengel D., et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol. Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- 26.Eddahibi S., et al. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J. Clin. Invest. 2000;105:1555–1562. doi: 10.1172/JCI8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fishkes H., Rudnick G. Bioenergetics of serotonin transport by membrane vesicles derived from platelet dense granules. J. Biol. Chem. 1982;257:5671–5677. [PubMed] [Google Scholar]
- 28.Gawaz M.P., et al. Ligand bridging mediates integrin alpha IIb beta 3 (platelet GPIIB-IIIA) dependent homotypic and heterotypic cell-cell interactions. J. Clin. Invest. 1991;88:1128–1134. doi: 10.1172/JCI115412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vijayan K.V., Goldschmidt-Clermont P.J., Roos C., Bray P.F. The Pl(A2) polymorphism of integrin beta(3) enhances outside-in signaling and adhesive functions. J. Clin. Invest. 2000;105:793–802. doi: 10.1172/JCI6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss L.A., et al. Variation in ITGB3 is associated with whole-blood serotonin level and autism susceptibility. Eur. J. Hum. Genet. 2006;14:923–931. doi: 10.1038/sj.ejhg.5201644. [DOI] [PubMed] [Google Scholar]
- 31.Vijayan K.V., Liu Y., Sun W., Ito M., Bray P.F. The Pro33 isoform of integrin beta3 enhances outside-in signaling in human platelets by regulating the activation of serine/threonine phosphatases. J. Biol. Chem. 2005;280:21756–21762. doi: 10.1074/jbc.M500872200. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q., Hofmann P.A. Protein phosphatase 2A-mediated cross-talk between p38 MAPK and ERK in apoptosis of cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H2204–H2212. doi: 10.1152/ajpheart.01050.2003. [DOI] [PubMed] [Google Scholar]
- 33.Avdi N.J., Malcolm K.C., Nick J.A., Worthen G.S. A role for protein phosphatase-2A in p38 mitogen-activated protein kinase-mediated regulation of the c-Jun NH(2)-terminal kinase pathway in human neutrophils. J. Biol. Chem. 2002;277:40687–40696. doi: 10.1074/jbc.M204455200. [DOI] [PubMed] [Google Scholar]
- 34.Vijayan K.V., Liu Y., Li T.T., Bray P.F. Protein phosphatase 1 associates with the integrin alphaiib subunit and regulates signaling. J. Biol. Chem. 2007;279:33039–33042. doi: 10.1074/jbc.C400239200. [DOI] [PubMed] [Google Scholar]
- 35.Maurer-Spurej E., Pittendreigh C., Solomons K. The influence of selective serotonin reuptake inhibitors on human platelet serotonin. Thromb. Haemost. 2004;91:119–128. doi: 10.1160/TH03-05-0330. [DOI] [PubMed] [Google Scholar]
- 36.Serebruany V.L., et al. Selective serotonin reuptake inhibitors yield additional antiplatelet protection in patients with congestive heart failure treated with antecedent aspirin. Eur. J. Heart Fail. 2003;5:517–521. doi: 10.1016/S1388-9842(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 37.Musselman D.L., et al. Platelet reactivity in depressed patients treated with paroxetine: preliminary findings. Arch. Gen. Psychiatry. 2000;57:875–882. doi: 10.1001/archpsyc.57.9.875. [DOI] [PubMed] [Google Scholar]
- 38.Matsusaka S., Wakabayashi I. 5-Hydroxytryptamine as a potent migration enhancer of human aortic endothelial cells. FEBS Lett. 2005;579:6721–6725. doi: 10.1016/j.febslet.2005.10.068. [DOI] [PubMed] [Google Scholar]
- 39.Guilluy C., et al. Transglutaminase-dependent RhoA activation and depletion by serotonin in vascular smooth muscle cells. J. Biol. Chem. 2007;282:2918–2928. doi: 10.1074/jbc.M604195200. [DOI] [PubMed] [Google Scholar]
- 40.Nair G.V., et al. 1999Depression, coronary events, platelet inhibition, and serotonin reuptake inhibitors. Am. J. Cardiol. 84321–323, A328. . 10.1016/S0002-9149(99)00284-2 [DOI] [PubMed] [Google Scholar]
- 41.Glassman A.H. Depression and cardiovascular comorbidity. Dialogues Clin. Neurosci. 2007;9:9–17. doi: 10.31887/DCNS.2007.9.1/ahglassman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mikkelsson J., Perola M., Laippala P., Penttila A., Karhunen P.J. Glycoprotein IIIa Pl(A1/A2) polymorphism and sudden cardiac death. . J. Am. Coll. Cardiol. 2000;36:1317–1323. doi: 10.1016/S0735-1097(00)00871-8. [DOI] [PubMed] [Google Scholar]
- 43.Mikkelsson J., Perola M., Penttila A., Goldschmidt-Clermont P.J., Karhunen P.J. The GPIIIa (beta3 integrin) PlA polymorphism in the early development of coronary atherosclerosis. Atherosclerosis. 2001;154:721–727. doi: 10.1016/S0021-9150(00)00683-3. [DOI] [PubMed] [Google Scholar]
- 44.Kastrati A., et al. PlA polymorphism of glycoprotein IIIa and risk of adverse events after coronary stent placement. J. Am. Coll. Cardiol. 2000;36:84–89. doi: 10.1016/S0735-1097(00)00709-9. [DOI] [PubMed] [Google Scholar]
- 45.Chavis P., Westbrook G. Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature. 2001;411:317–321. doi: 10.1038/35077101. [DOI] [PubMed] [Google Scholar]
- 46.Hanna G.L., et al. Serotonin transporter and seasonal variation in blood serotonin in families with obsessive-compulsive disorder. Neuropsychopharmacology. 1998;18:102–111. doi: 10.1016/S0893-133X(97)00097-3. [DOI] [PubMed] [Google Scholar]
- 47.Ozaki N., et al. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol. Psychiatry. 2003;8:933–936. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- 48.Coutinho A.M., et al. Evidence for epistasis between SLC6A4 and ITGB3 in autism etiology and in the determination of platelet serotonin levels. Hum. Genet. 2007;121:243–256. doi: 10.1007/s00439-006-0301-3. [DOI] [PubMed] [Google Scholar]
- 49.Qian Y., Melikian H.E., Rye D.B., Levey A.I., Blakely R.D. Identification and characterization of antidepressant-sensitive serotonin transporter proteins using site-specific antibodies. . J. Neurosci. 1995;15:1261–1274. doi: 10.1523/JNEUROSCI.15-02-01261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DiMinno G., Silver M.J., Murphy S. Stored human platelets retain full aggregation potential in response to pairs of aggregating agents. Blood. 1982;59:563–568. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.