Abstract
The intracellular signals governing cellular proliferation and developmental progression during lymphocyte development are incompletely understood. The tyrosine kinase Blk is expressed preferentially in the B lineage, but its function in B cell development has been largely unexplored. We have generated transgenic mice expressing constitutively active Blk [Blk(Y495F)] in the B and T lymphoid compartments. Expression of Blk(Y495F) in the B lineage at levels similar to that of endogenous Blk induced B lymphoid tumors of limited clonality, whose phenotypes are characteristic of B cell progenitors at the proB/preB-I to preB-II transition. Expression of constitutively active Blk in the T lineage resulted in the appearance of clonal, thymic lymphomas composed of intermediate single positive cells. Taken together, these results indicate that specific B and T cell progenitor subsets are preferentially susceptible to transformation by Blk(Y495F) and suggest a role for Blk in the control of proliferation during B cell development.
The pathways of B and T cell development reflect a common mechanism for generation of antigen receptor diversity (reviewed in ref. 1). In each lineage, functional rearrangement of a gene encoding the antigen receptor heavy chain—Ig μ or T cell receptor (TCR)-β—triggers expansion of lymphocyte progenitors by a factor of 10 or more (1–5). In B cell progenitors, this expansion is accompanied by a phenotypic transition from B220+CD43+ (proB/preB-I) to B220+CD43− (preB-II) (3, 4, 6). The analogous stage in T cell development is marked by a transition from the CD44loCD25+ to the CD44loCD25− phenotype (7, 8). In both lineages an immature form of the antigen receptor, containing the heavy chain and one or more surrogate light chains (9, 10), is essential for the proliferative expansion and its accompanying developmental transition (2, 3, 5, 11, 12). It is not clear whether the immature antigen receptor complexes play a direct role in triggering proliferation or deliver developmental cues that obligatorily precede cellular expansion.
Several members of the Src tyrosine kinase subfamily are associated with antigen receptor complexes and are activated upon antigen receptor engagement: Lck and Fyn with the TCR, and Blk, Lyn, and Fyn with the B cell receptor (13, 14). The potential functional redundancy of these kinases has made it difficult to define their individual roles in lymphocyte development and activation (15). Interference with Lck activity impedes T cell maturation at the CD4−CD8− (double negative or DN) to CD4+CD8+ (double positive or DP) transition (16, 17), whereas overexpression of wild-type or constitutively active Lck alters the normal T lymphoid maturation sequence, with suppression of TCR-β gene rearrangement and premature recombination at the TCR-α locus (18). Fyn and Lyn are not essential for lymphocyte development, but Fyn-deficient B cells respond poorly to interleukin 5 (IL-5) (19) whereas Lyn deficiency is associated with autoimmunity and reduced responsiveness of B cells to anti-Ig or lipopolysaccharide (20, 21). Blk is expressed preferentially in the murine B lineage (22, 23), but its function in B cell development has been largely unexplored. Here we describe transgenic mice expressing a constitutively active Blk mutant, Blk(Y495F), in the B and T lymphoid compartments. Our results indicate that analogous B and T cell progenitor subsets, corresponding in the B lineage to the proB/preB-I to preB-II transition and in T cell ontogeny to the intermediate single positive population, are preferentially susceptible to transformation by active Blk in vivo.
MATERIALS AND METHODS
Construction of Transgenic Mice.
Complementary DNA encoding Blk(Y495F) was generated by PCR as described (24) and inserted into the SalI restriction site of the vector pHSE3′ (25). The resulting construct was cleaved with XhoI to release a DNA fragment containing the mutated Blk coding sequence, flanked by the H-2K promoter and Ig μ intronic enhancer. The transgene was injected into fertilized eggs from B6SLJF1/J females and maintained by mating hemizygous founder animals with wild-type B6SJL/J mice.
Analysis of Transgene Expression.
Lymphoid organs were dispersed in RPMI 1640 medium supplemented with 10% fetal calf serum (RPMI-10). B cells, isolated by using anti-B220-conjugated magnetic beads (Miltenyi Biotec, Auburn, CA), were lysed at 107 cells/ml in 100 mM NaCl/30 mM Tris⋅Cl, pH 7.4/2 mM EDTA/1 mM Na3VO4/1 mM PMSF/1% Nonidet P-40 and 10 μg each of leupeptin, aprotinin, and pepstatin A. Lysates were cleared by centrifugation at 12,000 rpm for 10 min, and protein (106 cell equivalents) was fractionated by 8% SDS/PAGE. Blk was detected by immunoblotting in 1 μg/ml affinity-purified, polyclonal rabbit anti-Blk antibody Ab1306, which was raised against a glutathione S-transferase fusion protein containing amino acid residues 1–214 of Blk.
Fluorescence Cytometry.
Lymphoid organs were dispersed in RPMI-10. Cells (106) were collected by centrifugation and resuspended in 100 μl PBS containing 0.02% NaN3 and 1% BSA. After pretreatment with 1% normal mouse serum for 15 min on ice, cells were stained with fluorescein isothiocyanate (FITC) or phycoerythrin-conjugated antibodies against specific murine cell surface markers at 1 μg/ml for 30 min on ice. All antibodies were obtained from PharMingen except FITC-conjugated anti-IgM, which was purchased from Southern Biotechnology. Data were collected by using a FACScan analyzer (Becton Dickinson).
Analysis of DNA Rearrangements at Ig and TCR Loci.
TCR-β rearrangement was assayed by Southern hybridization to BglII-digested genomic DNA, using as a probe the 1.7-kb EcoRI fragment from plasmid pGEM5′β2I (26) (a gift of Jianzhu Chen, Massachusetts Institute of Technology). Ig κ rearrangement was analyzed by hybridization to genomic DNA digested with EcoRI and BamHI; the κ-specific probe was the 3.0-kb BamHI-HindIII fragment from plasmid pHBCκ (27) (a gift of Mark Schlissel, Johns Hopkins University School of Medicine). D-to JH and VH-to-DJH rearrangements at the Ig μ locus were analyzed by PCR as described (28): D-to-JH rearrangements were detected by using the primers DHL and J3; VH-to-DJH rearrangements were detected with a combination of primers VH559, VH7183, VHQ52, and J3. To assess length heterogeneity at coding joints, PCR products were isolated by agarose gel electrophoresis and reamplified in the presence of radiolabeled primer J3 and the corresponding unlabeled forward primers. Reamplified products were analyzed by electrophoresis through a 5% polyacrylamide-urea gel and detected by PhosphorImaging.
Transfer of Tumors to nu−/− Mice.
Cells (3 × 107) from lymphoid tumors or from normal lymphoid organs were injected intraperitoneally into nu−/− mice (Charles River Laboratories). Mice that showed signs of tumor growth were sacrificed 4–6 weeks later and autopsied. Mice without external signs of tumor growth were observed for up to 18 weeks.
Analysis of Phosphotyrosine-Containing Proteins.
Frozen cell suspensions were thawed on ice and lysed in a buffer containing 100 mM NaCl/30 mM Tris⋅Cl, pH 7.4/30 mM NaF/2 mM EDTA/1 mM Na3VO4/1 mM Na2MoO2/1% Nonidet P-40/1 mM PMSF and 10 μg each of leupeptin, aprotinin, and pepstatin A (Buffer L). Phosphotyrosine-containing proteins were detected by immunoblotting with antibody 4G10 as described (29). Antibodies against PLC-γ1 and 2 (Transduction Laboratories), the p85 subunit of PI 3-K (Upstate Biotechnology), or ZAP-70 (Transduction Laboratories) were affixed to protein A/G agarose (Santa Cruz Biotechnology); 10 μg antibody was incubated with cell lysates (5 × 107 cell equivalents each) for 4 hr at 4°C. Beads were collected by centrifugation and washed in Buffer L. Phosphotyrosine was detected by immunoblotting.
RESULTS
Construction of Transgenic Mice and Transgene Expression.
Blk(Y495F) was expressed under control of the murine H-2K promoter and the Ig μ intronic enhancer (Fig. 1A), which support transcription in the B lineage, T lineage, or both in a founder-specific fashion. Although Blk is expressed preferentially in the B lineage, construction of mice expressing Blk in the T lineage provided an opportunity to compare the effects of Blk overexpression with those previously observed for Lck. Founder animals, bearing 10–25 transgene copies, were mated to B6SJL/J mice.
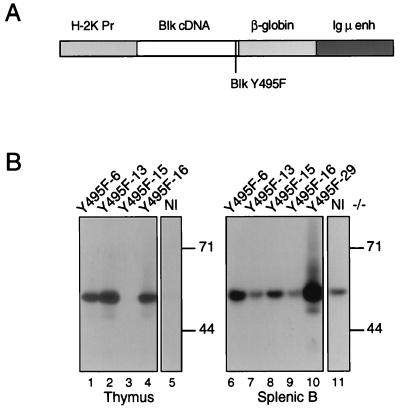
Figure 1.
Expression of mutant Blk proteins in transgenic mice. (A) Organization of Blk transgenes. The H2K promoter, Blk cDNA, β-globin splice donor and acceptor sequences, and the Ig heavy chain intronic enhancer are indicated by rectangles. The Y495F mutation is indicated by a vertical line. (B) Expression of constitutively active mutant Blk protein in transgenic mice. Thymocytes (lanes 1–5) or splenic B cells (lanes 6–11) from transgenic mice (lanes 1–4; lanes 6–10) and nontransgenic control animals (lanes 5 and 11) were assayed for Blk by immunoblotting. Individual transgenic mice from which samples were derived are identified across the top. Spleens from Blk(Y495F)-6, -13, -15, and -16 were macroscopically normal; tumor was evident in the spleen from founder animal Blk(Y495F)-29. The apparent sizes (in kDa) and positions of molecular mass standards are indicated at right.
Expression of Blk(Y495F) protein varied among transgenic lines. In purified splenic B cells from lines Blk(Y495F)-6, Blk(Y495F)-15, and Blk(Y495F)-29, total Blk accumulation was 4, 2, and 8 times greater, respectively, than that observed in normal littermates (Fig. 1B, compare lanes 6, 8, and 10 to lane 11). Splenic B cells derived from lines Y495F-13 and 16 showed no increase in Blk accumulation over that in normal mice (Fig. 1B, compare lanes 7 and 9 with lane 11). Transgene-derived Blk was detected in thymocytes from lines Blk(Y495F)-6, Blk(Y495F)-13, and Blk(Y495F)-16 (Fig. 1B, lanes 1, 2, and 4); thymic expression was undetectable in the Blk(Y495F)-15 transgenic line (Fig. 1B, lane 3).
Immature Lymphoid Tumors in Transgenic Mice Expressing Blk(Y495F).
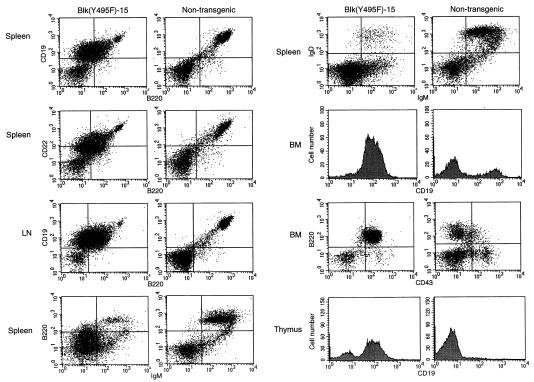
Eleven independent founder animals bearing the Blk(Y495F) transgene were generated. The founder mouse Blk(Y495F)-15F and its transgenic progeny, which expressed the constitutively active Blk mutant at a level similar to that of endogenous Blk (Fig. 1C, lane 8), harbored disseminated lymphomas of immature B lymphoid origin. Penetrance of the malignant phenotype was high, with 45% of animals exhibiting obvious tumors by 6 months. The Blk(Y495F)-15 founder, five transgenic progeny, and four normal mice were analyzed in detail; representative data are shown in Fig. 2. Profound enlargement of axillary and cervical lymph nodes (4- to 25-fold increases in cellularity) and moderate to severe splenomegaly (4.7- to 12-fold increases in cellularity) were the most striking macropathologic findings. The majority of splenic mononuclear cells from these animals expressed a CD19+CD22+B220lowsIg− phenotype; in the bone marrow CD19+ cells were greatly overrepresented, with B220+CD43+ cells comprising the predominant population (Fig. 2). Thus, the tumors arising in these animals expressed a surface phenotype characteristic of early B cell progenitors. Invasion of the thymus by B lymphoid tumor cells and a marked reduction in the proportion of intrathymic T cell progenitors were observed (Fig. 2).
Figure 2.
Expansion and dissemination of B220+CD43+ B cell progenitors in the Blk(Y495F)-15 transgenic line. Single-cell suspensions were prepared from spleen, lymph nodes, thymus, and bone marrow from lymphomatous Blk(Y495)-15 transgenic mice or from age-matched, nontransgenic littermates. Cells were stained, singly or in combination, with PE- (y axis) or FITC- (x axis) conjugated antibodies to CD19, B220, CD22, IgM, IgD, and CD43 as indicated. The founder, five transgenic progeny, and four normal mice were examined; representative results are presented.
The founder animal Blk(Y495F)-29F, which was sacrificed before a line could be established, also exhibited profound splenic and lymph node enlargement. More than 90% of lymph node-derived cells expressed the B220+CD19+sIg− phenotype, and the proportion of B220+ cells was increased 2.6-fold in the bone marrow (data not shown). As was observed in the Blk(Y495F)-15 transgenic line, displacement of intrathymic T cell progenitors by B lineage tumor cells was seen in the Blk(Y495F)-29F mouse (data not shown).
Six additional founder animals died by 8–12 weeks of age, before establishment of transgenic lines. On autopsy all six mice exhibited moderate to massive thymic enlargement. The three remaining founder mice, from which the transgenic lines Blk(Y495F)-6, Blk(Y495F)-13. and Blk(Y495F)-16 were derived, were sacrificed and analyzed. All three mice had enlarged thymuses with cellularity ranging from 2.3 to 8.5 times that of normal. These contained abundant cells that expressed the intermediate single positive phenotype CD3−TCRαβ−CD44loCD4−CD8+ and were heterogeneous for expression of CD25. The Blk(Y495F)-6 founder, four transgenic progeny, and three normal mice were examined; representative data are shown in Fig. 3. Thus the preponderance of cells in the thymic tumors resembled the intermediate single positive cells that normally appear at the DN-to-DP transition. Penetrance of the malignant phenotype was high: 100% of Blk(Y495F)-13, 60 percent of Blk(Y495F)-6, and 20 percent of Blk(Y495F)-16 transgenic progeny exhibited massive lymphoproliferative disease by 4 months of age.
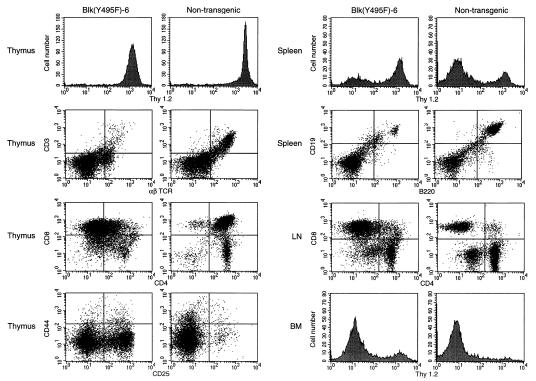
Figure 3.
Expansion and dissemination of CD3−CD4−CD8+ T cell progenitors in the Blk(Y495F)-6 transgenic line. Single-cell suspensions were made from thymus, spleen, lymph nodes, and bone marrow from thymomatous Blk(Y495)-6 transgenic mice or from age-matched, nontransgenic littermates. Cells were stained, singly or in combination, with PE- (y axis) and FITC- (x axis) conjugated antibodies to Thy1.2, CD3, αβ TCR, CD8, CD4, CD44, CD25, CD19, and B220 as indicated. The founder, four transgenic progeny, and three normal mice were examined; representative results are presented.
Some mice with thymic lymphomas also exhibited increases of up to 6-fold in lymph node cellularity and a corresponding increase in the fraction of CD4−CD8+ cells, consistent with the phenotype of the thymic tumors. In the spleens of these animals the fraction of Thy-1+ cells was increased, with a coordinate decrease in the fraction of B220+CD19+ cells (Fig. 3). An increase in the number of Thy-1+ cells was observed in the bone marrow of Blk(Y495F) transgenic mice bearing thymic lymphomas (Fig. 3).
Clonality of B and T Lymphomas in Blk(Y495F) Transgenic Mice.
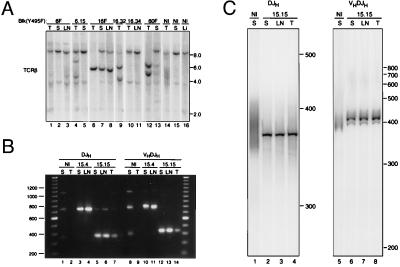
Tumor clonality was assessed by examining the configuration of Ig and TCR loci. Genomic DNA from thymus and lymph nodes of animals with T lymphoid tumors was analyzed for TCR-β rearrangement by Southern blotting. DNA from normal liver and spleen showed the expected germ-line band of about 10 kb (two faint background bands of about 2 and 4 kb were detected in all samples). In normal thymus the germ-line band was detected at reduced intensity, and polyclonal TCR-β rearrangement was evident as a series of bands, most prominent in the 4- to 6-kb range. In most thymic tumors one or more discrete, rearranged fragments and reduction of the germ-line signal were observed (Fig. 4A, lanes 6, 9, and 12), consistent with the presence of a dominant clone. Rearrangements identical to those found in thymus could be detected in peripheral lymphoid tissues (Fig. 4A, lanes 7, 8, and 13), indicating a common clonal origin for the tumor cells detected in these organs. In one mouse, Blk(Y495F)-16.34, thymic, and lymph node tumors were evident but discrete TCR-β rearrangements were not observed (Fig. 4A, lanes 10 and 11).
Figure 4.
Clonality of lymphoid tumors in Blk(Y495F) transgenic mice. (A) Analysis of TCR-β rearrangements. Genomic DNA was extracted from thymus (T), spleen (S), lymph node (LN), or liver (Li) of transgenic mice (lanes 1–13) or nontransgenic littermates (lanes 14–16). The following individual transgenic mice were assayed: Blk(Y495F)-6 founder (lanes 1–3); Blk(Y495F)-6.15 (lanes 4 and 5); the Blk(Y495F)-16 founder (lanes 6–8); Blk(Y495F)-16.32 (lane 9); Blk(Y495F)-16.34 (lanes 10 and 11); Blk(Y495F)-60 founder (lanes 12 and 13). DNA was digested with BglII and assayed for rearrangement by Southern hybridization using a probe derived from the Jβ-Cβ1 intron. (B) Analysis of Ig heavy chain rearrangements. Genomic DNA was extracted from lymphoid organs of Blk(Y495F)-15.4 (lanes 3, 4, 10, and 11), Blk(Y495F)-15.15 (lanes 5–7 and 12–14), or nontransgenic littermates (lanes 1, 2, 8, and 9). DJH and VHDJH rearrangements were amplified by PCR as described in ref. 28; products were fractionated by agarose gel electrophoresis and detected with ethidium bromide. Lanes 1–7, assays for DJH rearrangements; lanes 8–14, assays for VHDJH rearrangements. The sizes of DNA standards, in bp, are indicated at left. (C) Analysis of junctional heterogeneity in Ig heavy chain rearrangements. DJH3- and VHDJH3-containing PCR fragments from Blk(Y495F)-15.15 (lanes 2–4 and 6–8) or normal mice (lanes 1 and 5) were purified from an agarose gel and reamplified by using a radiolabeled J3 primer and D or VH primers as described (28). Products were fractionated by electrophoresis through a 5% polyacrylamide-urea gel and detected using a PhosphorImager. Sizes of DNA standards, in bp, are indicated at right.
In animal Blk(Y495F)-6.15 the thymus was not enlarged at the time of sacrifice but contained an increased fraction of cells with the intermediate single positive phenotype (data not shown) and overrepresentation of two novel hybridizing fragments (Fig. 4A, lane 4), consistent with outgrowth of a clonal population in situ. In agreement with this interpretation, only the germ-line TCR-β fragment was detected in lymph node DNA from this animal (Fig. 4A, lane 5).
To assess the rearrangement status of B lineage tumors, we used PCR-based assays (28) for DJH and VHDJH rearrangements (Fig. 4B). DJH rearrangements were assayed by using primers that detect all D rearrangements to JH1, JH2, or JH3 except for those involving DQ52 (28). As expected (28), amplification of genomic DNA from normal spleen produced fragments of approximately 330, 720, and 1,030 bp, corresponding to rearrangements involving JH1, JH2, and JH3 (Fig. 4B, lane 1); these products were not obtained with DNA from normal thymus (Fig. 4B, lane 2). In contrast, tumors from Blk(Y495F)-15.15 and Blk(Y495F)-15.4 each yielded a predominant fragment, corresponding to rearrangements involving JH3 and JH2, respectively. (Additional faint bands are consistent with the presence of nontumor cells in the tissue samples.) Notably, the size of the predominant fragment was consistent among samples taken from distinct sites within the same animal (Fig. 4B, lanes 3–7).
The tumor samples were also tested for VHDJH rearrangements (Fig. 4B, lanes 8–14). Normal spleen DNA yielded the expected (28) PCR fragments of about 350, 740, and 1,060 bp (Fig. 4B, lane 8), which were absent from a control reaction containing thymus DNA (Fig. 4B, lane 9). Samples from Blk(Y495F)-15.15 and Blk(Y495F)-15.4 each yielded a predominant fragment (Fig. 4B, lanes 10–14). As seen for DJH joints, the sizes of these fragments corresponded to rearrangements involving JH3 and JH2, respectively. The predominant use of a single JH segment by DJH and VHDJH rearrangements in a given tumor suggested that the malignancies arose by clonal expansion after D-to-JH rearrangement, but before VH-to-DJH rearrangement. To confirm this, PCR-amplified rearrangements from Blk(Y495F)15.15 or normal spleen were purified, reamplified in the presence of a radiolabeled primer and fractionated on a denaturing polyacrylamide gel. Products from normal spleen were heterogeneous in length, as expected of DJH and VHDJH rearrangements from a polyclonal source (Fig. 4C, lanes 1 and 5). In contrast, the reamplified DJH products from the Blk(Y495F)15.15 tumor samples yielded a predominant homogeneous product (Fig. 4C, lanes 2–4), consistent with the interpretation that D-to-JH rearrangement preceded clonal expansion. As predicted, VHDJH fragments from Blk(Y495F)15.15 exhibited some heterogeneity (Fig. 4C, lanes 6–8), albeit greatly restricted relative to the control. Interestingly, a similar pattern of VHDJH fragments was observed in all tumor samples, suggesting selective outgrowth of cells that had undergone VH-to-DJH rearrangement early in tumor development. The Ig κ locus remained in germ-line configuration in all tumors (data not shown), consistent with their immature surface phenotypes and with the rearrangement status of their Ig heavy chain loci.
Developmental Stage-Specific Transformation by Blk(Y495F) Does Not Result from Restricted Transgene Expression.
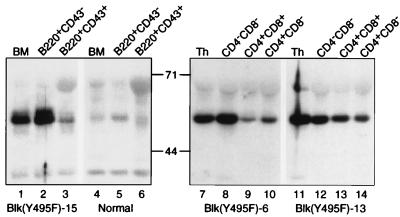
To confirm that developmental stage-restricted susceptibility to transformation by Blk(Y495F) was not the result of stage-restricted expression of the transgene, B220+CD43+ and B220+CD43− bone marrow cells were purified from normal mice and from tumor-free, 4-week-old Blk(Y495F)-15 transgenic mice. Identical numbers of cells were assayed for expression of Blk by immunoblotting. Blk was detected in total bone marrow cells, in the B220+CD43− population and less abundantly in the B220+CD43+ subset from normal mice (Fig. 5, lanes 4–6). In each of the samples from Blk(Y495F)-15 transgenic animals a greater quantity of Blk was detected than in normal mice, reflecting the sum of transgenic and endogenous protein (Fig. 5, lanes 1–3). The amount of Blk detected in the B220+CD43+ population was lower than in the B220+CD43− subset, despite preferential transformation of B220+CD43+ B cell progenitors in the Blk(Y495F)-15 transgenic line. We infer that the B220+CD43+ subset is preferentially sensitive to transformation by constitutively active Blk. Likewise, stage-restricted transgene expression does not account for the preferential appearance of intermediate single positive progenitors in Blk(Y495F)-induced T lymphoid tumors. Thymocytes from two independent lines of transgenic mice were harvested before the appearance of macroscopic tumors, sorted according to developmental stage, and assayed for expression of transgenic Blk protein. Although some variation was observed, the transgene was clearly expressed in the CD4−CD8−, CD4+CD8+, and CD4+CD8− populations (Fig. 5, lanes 8–10 and 12–14).
Figure 5.
Expression of Blk(Y495F) protein at sequential stages of lymphoid development. Bone marrow cells were obtained from macroscopically tumor-free Blk(Y495F)-15 transgenic mice (lanes 1–3) or nontransgenic littermates (lanes 4–6) at 4–6 weeks of age. B220+CD43+ (lanes 3 and 6) and B220+CD43− (lanes 2 and 5) populations were purified by FACS. Protein corresponding to 105 cells from unfractionated bone marrow (lanes 1 and 4) or sorted populations was assayed for Blk by immunoblotting. Thymocytes were prepared from macroscopically tumor-free Blk(Y495F)-6 (lanes 7–10) or Blk(Y495F)-13 (lanes 11–14) transgenic mice at 4–6 weeks of age. CD4−CD8− (lanes 8 and 12), CD4+CD8+ (lanes 9 and 13), and CD4+CD8− (lanes 10 and 14) populations were purified by FACS. Protein corresponding to 106 cells from unfractionated thymus (lanes 7 and 11) or sorted populations was assayed for Blk by immunoblotting. The apparent sizes (in kDa) and positions of molecular mass standards are indicated.
Transfer of Tumors to nu−/− Mice.
B lymphoid tumor cells from lymph nodes of three transgenic mice were injected intraperitoneally into a total of nine nu−/− mice; eight of nine recipients exhibited massive hepatosplenomegaly by 4 weeks after injection. Splenocytes from the same transgenic animals produced tumors in three of five recipients. Lymph node-derived T lymphoid tumor cells from a single transgenic animal produced tumors in two of two recipients. Thymus-derived T lymphoid tumors were also established in nu−/− recipients, but with somewhat lower efficiency (4/13). Control injections of normal thymocytes or splenic mononuclear cells yielded no tumors in recipient mice (0/8). Thus, malignantly transformed cells were present in all affected lymphoid organs tested.
Targets of Tyrosine Phosphorylation in Blk(Y495F)-Induced Tumors.
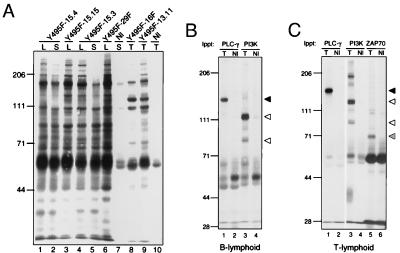
Catalytic activity is necessary for tumor induction by the Blk(Y495F) transgene, as an increase in the incidence of lymphoid tumors was not observed in more than 300 mice overexpressing catalytically inactive Blk, observed over a period of more than 18 months (S.N.M., D.I.D., J.R., H.D., and S.D., unpublished). Increased tyrosine phosphorylation of at least 20 distinct species was observed in Blk(Y495F)-associated B lymphoid tumors (Fig. 6A, lanes 1–6) relative to splenocytes or thymocytes from normal littermates (Fig. 6A, lanes 7 and 10). Similar sets of phosphotyrosine-containing proteins were detected in all B lymphoid tumor samples. A distinct but overlapping pattern of tyrosine phosphorylation was observed in T lymphoid tumors from two transgenic lines (Fig. 6A, lanes 8 and 9).
Figure 6.
Protein–tyrosine phosphorylation in Blk(Y495F)-induced lymphoid tumors. (A) Lymph nodes (lanes 1, 3, 4, and 6), spleens (lanes 2, 5, and 7), or thymuses (lanes 8–10) from Blk(Y495F) transgenic mice (lanes 1–6; lanes 8 and 9) or nontransgenic littermates (lanes 7 and 10) were dispersed and lysed. Phosphotyrosine-containing proteins in 106 cell equivalents were detected by immunoblotting. The following transgenic mice were examined: Blk(Y495F)-15.4 (lanes 1 and 2); Blk(Y495F)-15.15 (lane 3); Blk(Y495F)-15.3 (lanes 4 and 5); Blk(Y495F)-29F (lane 6); Blk(Y495F)-16F (lane 8); and Blk(Y495F)-13.11 (lane 9). (B and C) B lymphoid tumor-bearing lymph nodes (B, lanes 1 and 3) and T lymphoid tumor-bearing thymuses (C, lanes 1, 3, and 5) from Blk(Y495F) transgenic mice were dispersed; splenocytes (B, lanes 2 and 4) and thymocytes (C, lanes 2, 4, and 6) from nontransgenic littermates were prepared in parallel. PLC-γ, PI-3K, and ZAP-70 were immunoprecipitated from 5 × 107 cell equivalents of cell lysate, and phosphotyrosine was detected by immunoblotting as in A. The apparent sizes (in kDa) and positions of molecular mass standards are indicated at left. Arrowheads indicate individual proteins as described in the text.
Among the known targets of Src and related kinases are phospholipase C-γ (PLC-γ) and phosphatidylinositol 3-OH kinase (PI-3K) (see ref. 30 for review). The phosphotyrosine content of these proteins in tumor and control samples was assessed. Constitutive tyrosine phosphorylation of PLC-γ was detected in B and T lymphoid tumors (Fig. 6 B and C, lanes 1), but not in spleen or thymus from normal littermates (Fig. 6 B and C, lanes 2, solid arrowhead). Likewise, tyrosine phosphorylation of the p85 subunit of PI-3K was detected in tumors but not in control samples (Fig. 6 B and C, lanes 3 and 4, lower open arrowhead). A prominent phosphotyrosine-containing protein of higher molecular weight, possibly related to the p110 subunit of PI-3K, coprecipitated with p85 out of lysates from both tumor types (Fig. 6 B and C, lanes 3 and 4, upper open arrowhead). Targets for tyrosine phosphorylation by Blk(Y495F) could, in principle, include other tyrosine kinases. In support of this, tyrosine phosphorylation of the dual SH2-containing tyrosine kinase ZAP-70 was elevated markedly in Blk(Y495F)-induced T lymphoid tumor cells relative to normal thymus (Fig. 6C, lanes 5 and 6, hatched arrowhead).
DISCUSSION
In most tumors arising in Blk(Y495F) transgenic mice, the transformed populations resemble cells at specific, analogous periods of B or T lymphocyte development. The restricted clonality of DJH rearrangements, the patterns of VHDJH rearrangements, and retention of germ-line configuration at the Ig κ locus indicate that transformation of B lineage cells occurred after D-to-JH rearrangement but before VH-to-JH rearrangement or recombination of light chain genes. In this regard and with respect to cell surface phenotype, the B lymphoid transformants arising in Blk(Y495F) transgenic mice resemble B cell progenitors at the proB/preB-I to preB-II transition. By a similar argument we deduce that clonal expansion of Blk(Y495F)-induced T lymphoid tumors, which resemble cells at the DN-to-DP transition, occurred after initiation of rearrangement at the TCR-β locus. The clonality of Blk(Y495F)-associated tumors suggests that one or more heritable alterations, in addition to expression of the transgene, are required for transformation.
The cellular subsets transformed by Blk(Y495F) comprise a minor fraction of B or T progenitors in normal mice. The proB/preB-I population represents less than 10% of B lineage cells in the bone marrow (31); the intermediate CD8+ population represents fewer than 1% of all intrathymic T cell progenitors (32). Expression of the Blk(Y495F) transgene was not restricted to the developmental stages represented by lymphoid tumors; on the contrary, Blk(Y495F) protein was 5- to 10-fold less abundant in the B220+CD43+ target pool than in the larger B220+CD43− subset. Thus, preferential transformation of specific target pools appears to reflect their intrinsic susceptibility to the action of Blk(Y495F). The T lymphoid tumors of Blk(Y495F) transgenic mice are phenotypically similar to those observed in transgenic mice overexpressing wild-type or constitutively active Lck (33). Distinctions among Src-related kinases have been observed, however, because activated Fyn or Hck mutants fail to cause tumors when expressed in the same cellular compartment (33).
The expression of Blk throughout B cell development and its activation upon antigen receptor crosslinking have suggested roles in signaling through immature and mature forms of the B cell receptor, but direct evidence for this has been lacking. Overexpression of inactive or SH2-defective Blk in the B lineage does not perturb B cell development, antibody responses, or B cell receptor-dependent B cell proliferation; ectopic expression of inactive Blk in the T lineage has no effect on intrathymic T cell development (S.N.M., D.I.D., J.R., H.D., and S.D., unpublished). These observations are consistent with studies of homozygous Blk-deficient mice, which likewise exhibit normal B cell development and function (Texido, G., S.N.M., S.D., Rajewsky, K., and Tarakhovsky, S., unpublished). Thus, if Blk normally participates in the proliferative expansion of B progenitors at the proB/preB-I to preB-II transition, as is suggested by results presented here, then this function is likely to be redundant.
Mutations that activate Src-related kinases are expected to yield dominant phenotypes. Although expression of Blk is normally restricted to the B lineage in the mouse, transformation of T cell progenitors by Blk(Y495F) may have physiologic significance: ectopic expression of transforming proteins in the T lineage contributes to the generation of T lymphoid malignancies in humans (34, 35), and Blk is expressed in several T lymphoid tumor lines of human origin (36). These considerations, taken together with the high incidence and short latency of lymphoid tumors in Blk(Y495F) transgenic mice, may encourage investigation of Blk as a participant in initiation or progression of human B and T lymphoid neoplasms.
Acknowledgments
We thank Jean Richa (University of Pennsylvania) and Susan Brust (The Johns Hopkins University) for assistance in construction of transgenic mice. This work was supported by the Howard Hughes Medical Institute and by a grant from the National Cancer Institute.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: TCR, T cell receptor; PI-3K, phosphatidylinositol 3-OH kinase; PLC-γ, phospholipase C-γ.
References
- 1.Lin W-C, Desiderio S. Immunol Today. 1995;16:279–289. doi: 10.1016/0167-5699(95)80182-0. [DOI] [PubMed] [Google Scholar]
- 2.Shinkai Y, Koyasu S, Nakayama K, Murphy K M, Loh D Y, Reinherz E L, Alt F W. Science. 1993;259:822–825. doi: 10.1126/science.8430336. [DOI] [PubMed] [Google Scholar]
- 3.Karasuyama H, Rolink A, Shinkai Y, Young F, Alt F W, Melchers F. Cell. 1994;77:133–143. doi: 10.1016/0092-8674(94)90241-0. [DOI] [PubMed] [Google Scholar]
- 4.Rolink A, Grawunder U, Winkler T H, Karasuyama H, Melchers F. Int Immunol. 1994;6:1257–1264. doi: 10.1093/intimm/6.8.1257. [DOI] [PubMed] [Google Scholar]
- 5.Spanopoulou E, Roman C A J, Corcoran L M, Schlissel M S, Silver D P, Nemazee D, Nussenzweig M C, Shinton S A, Hardy R R, Baltimore D. Genes Dev. 1994;8:1030–1042. doi: 10.1101/gad.8.9.1030. [DOI] [PubMed] [Google Scholar]
- 6.Hardy R R, Carmack C E, Shinton S A, Kemp J D, Hayakawa K. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godfrey D I, Zlotnik A. Immunol Today. 1993;14:547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- 8.Godfrey D I, Kennedy J, Mombaerts P, Tonegawa S, Zlotnik A. J Immunol. 1994;152:4783–4792. [PubMed] [Google Scholar]
- 9.Groettrup M, von Boehmer H. Immunol Today. 1993;14:610–614. doi: 10.1016/0167-5699(93)90201-U. [DOI] [PubMed] [Google Scholar]
- 10.Melchers F, Karasuyama H, Haasner D, Bauer S, Kudo A, Sakaguchi N, Jameson B, Rolink A. Immunol Today. 1993;14:60–68. doi: 10.1016/0167-5699(93)90060-X. [DOI] [PubMed] [Google Scholar]
- 11.Fehling H J, Krotkova A, Saint-Ruf C, von Boehmer H. Nature (London) 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y, Davidson L, Alt F W, Baltimore D. Proc Natl Acad Sci USA. 1996;93:2169–2173. doi: 10.1073/pnas.93.5.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burkhardt A L, Stealey B, Rowley R B, Mahajan S, Prendergast M, Fargnoli J, Bolen J B. J Biol Chem. 1994;269:23642–23647. [PubMed] [Google Scholar]
- 14.Saouaf S, Mahajan S, Rowley R B, Kut S A, Fargnoli J, Burkhardt A L, Tsukada S, Witte O N, Bolen J B. Proc Natl Acad Sci USA. 1994;91:9524–9528. doi: 10.1073/pnas.91.20.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowell C A, Soriano P. Genes Dev. 1996;10:1845–1857. doi: 10.1101/gad.10.15.1845. [DOI] [PubMed] [Google Scholar]
- 16.Molina T J, Kishihara K, Siderovski D P, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige C J, Hartmann K-U, Veillette A, Davidson D, Mak T W. Nature (London) 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 17.Levin S D, Anderson S J, Forbush K A, Perlmutter R M. EMBO J. 1993;12:1671–1680. doi: 10.1002/j.1460-2075.1993.tb05812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson S J, Abraham K M, Nakayama T, Singer A, Perlmutter R M. EMBO J. 1992;11:4877–4886. doi: 10.1002/j.1460-2075.1992.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Appleby M W, Kerner J D, Chien S, Maliszewski C R, Bondadaá S, Perlmutter R M. J Exp Med. 1995;182:811–820. doi: 10.1084/jem.182.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hibbs M L, Tarlinton D M, Armes J, Grail D, Hodgson G, Maglitto R, Stacker S A, Dunn A R R. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 21.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 22.Dymecki S M, Zwollo P, Zeller K, Kuhajda F P, Desiderio S V. J Biol Chem. 1992;267:4815–4823. [PubMed] [Google Scholar]
- 23.Wasserman R, Li Y-S, Hardy R R. J Immunol. 1995;155:644–651. [PubMed] [Google Scholar]
- 24.Jones D H, Winistorfer S C. BioTechniques. 1992;12:528–534. [PubMed] [Google Scholar]
- 25.Pircher H, Mak T W, Lang R, Ballhausen W, Rüedi E, Hengartner H, Zinkernagel R M, Bürki K. EMBO J. 1989;8:719–727. doi: 10.1002/j.1460-2075.1989.tb03431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauxion F, Pray M G, Sen R. J Immunol. 1990;145:1577–1582. [PubMed] [Google Scholar]
- 27.Lewis S, Rosenberg N, Alt F, Baltimore D. Cell. 1982;30:807–816. doi: 10.1016/0092-8674(82)90285-9. [DOI] [PubMed] [Google Scholar]
- 28.Schlissel M S, Corcoran L M, Baltimore D. J Exp Med. 1991;173:711–720. doi: 10.1084/jem.173.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malek S N, Desiderio S. J Biol Chem. 1993;268:22557–22565. [PubMed] [Google Scholar]
- 30.Berridge M J. Nature (London) 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 31.Löffert D, Schaal S, Ehlich A, Hardy R R, Zou Y-R, Müller W, Rajewsky K. Immunol Rev. 1994;137:135–153. doi: 10.1111/j.1600-065x.1994.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald H R, Budd R C, Howe R C. Eur J Immunol. 1988;18:519–523. doi: 10.1002/eji.1830180405. [DOI] [PubMed] [Google Scholar]
- 33.Abraham K M, Levin S D, Marth J D, Forbush K A, Perlmutter R M. Proc Natl Acad Sci USA. 1991;88:3977–3981. doi: 10.1073/pnas.88.9.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisch P, Boehm T, Levenir I, Larson T, Arno J, Forster A, Rabbitts T H. Oncogene. 1992;7:2389–2397. [PubMed] [Google Scholar]
- 35.McGuire E A, Rintoul C E, Sclar G M, Korsmeyer S J. Mol Cell Biol. 1992;12:4186–4196. doi: 10.1128/mcb.12.9.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Islam K B, Rabbani H, Larsson C, Sanders R, Smith C I E. J Immunol. 1995;154:1265–1272. [PubMed] [Google Scholar]