Abstract
Important targets for cAMP signalling in the heart are hyperpolarization-activated and cyclic nucleotide-gated (HCN) channels that underlie the depolarizing ‘pacemaker' current, If. We studied the role of If in mice, in which binding of cAMP to HCN4 channels was abolished by a single amino-acid exchange (R669Q). Homozygous HCN4R669Q/R669Q mice die during embryonic development. Prior to E12, homozygous and heterozygous embryos display reduced heart rates and show no or attenuated responses to catecholaminergic stimulation. Adult heterozygous mice display normal heart rates at rest and during exercise. However, following β-adrenergic stimulation, hearts exhibit pauses and sino-atrial node block. Our results demonstrate that in the embryo, HCN4 is a true cardiac pacemaker and elevation of HCN4 channel activity by cAMP is essential for viability. In adult mice, an important function of HCN4 channels is to prevent sinus pauses during and after stress while their role as a pacemaker of the murine heart is put into question. Most importantly, our results indicate that HCN4 channels can fulfil their physiological function only when cAMP is bound.
Keywords: cardiology, cyclic nucleotides, heart, ion channel, signalling
Introduction
Spontaneous activity of the mammalian heart is generated in the sino-atrial node (SAN). How pacemaker activity is generated in SAN cells and how pacemaking is regulated by the autonomous nervous system is still a matter of debate. A number of different ion channels and signalling events have been implicated in pacemaker activity (for review see, Couette et al, 2006). Key players controlling the diastolic depolarization are the hyperpolarization-activated current If and the L-type calcium current ICa,L (DiFrancesco, 1993; Verheijck et al, 1999; Biel et al, 2002; Zhang et al, 2002; Mangoni et al, 2003; Barbuti et al, 2007).
The If current is mediated by hyperpolarization-activated and cyclic nucleotide-gated (HCN) channels. These channels have a number of particular features that enable them to open at voltages near the maximal diastolic potential (MDP). First, channels are activated on hyperpolarization of the membrane potential. Second, channel activity is dependent on the intracellular cAMP concentration ([cAMP]i) (DiFrancesco and Tortora, 1991). When cAMP binds to the cyclic nucleotide-binding domain (CNBD) of HCN channels, the activation curve shifts towards more positive voltages, thereby enhancing channel activity. In addition, channels with cAMP bound activate faster and deactivate slower. Thus, an increase of [cAMP]i, for example, during β-adrenergic stimulation, enhances If, whereas a decrease of [cAMP]i, for example, during muscarinic stimulation, attenuates If (DiFrancesco et al, 1986; DiFrancesco and Tromba, 1988). It has been proposed that cAMP modulation of If is the primary pathway by which the autonomous nervous system controls the heart rate (Bucchi et al, 2003). Consistent with this model, mutations in the HCN4 channel gene, the major isoform in the SAN, are associated with sinus bradycardia and arrhythmia (Schulze-Bahr et al, 2003; Milanesi et al, 2006).
The other key player in cardiac pacemaking is the voltage-dependent Ca2+ channel Cav1.3 that carries ICa,L. The ICa,L is activated during the early phase of the diastolic depolarization, and inhibition of ICa,L induces bradycardia in vivo (Lande et al, 2001). Moreover, Cav1.3 knockout mice are bradycardic and show sino-atrial arrhythmia (Platzer et al, 2000; Zhang et al, 2002; Mangoni et al, 2003). These observations have led to the proposal that in SAN cells Cav1.3 channels generate a significant electrical signal contributing to the diastolic depolarization (Couette et al, 2006). Within the framework of this model, the increase of ICa,L during cAMP stimulation enhances the pacing component.
In addition to If and ICa,L, at least three other ionic pathways contribute to the regulation of the heart rate. One pathway is ICa,T, which is almost exclusively mediated by Cav3.1 channels (Mangoni et al, 2006). Cav3.1 knockout mice exhibit pronounced bradycardia and a significant slowing of atrioventricular (AV) conduction (Mangoni et al, 2006). Another current implied in pacemaker generation is a sustained inward current, Ist, which was identified in spontaneously beating SAN cells (Guo et al, 1995). Its proposed role in cardiac pacemaking is largely based on numerical simulations of pacemaker activity (Shinagawa et al, 2000; Zhang et al, 2002). The impact of Ist on pacing the heart is difficult to estimate due to its unidentified molecular nature and the lack of specific pharmacological tools to target this current. Finally, rhythmic Ca2+ oscillations have been proposed to be involved in cardiac pacemaking. This proposal challenges the major role of If in primary pacemaker activity of the SAN and its role in the autonomous regulation of the heartbeat (Bogdanov et al, 2001; Kodama et al, 2002; Lipsius and Bers, 2003). Instead of If, electrogenic Na+/Ca2+ exchange has been proposed to provide the major depolarizing pacemaking current. According to this model, SAN cells generate rhythmic, submembrane Ca2+ oscillations via spontaneous opening of ryanodine receptors in the sarcoplasmatic reticulum (SR) (Bogdanov et al, 2001). Thereby, the intracellular Ca2+ concentration is locally increased and the Na+/Ca2+ exchanger in the plasma membrane is activated. An inward current is generated that depolarizes the cell. The frequency of spontaneous Ca2+ release events is tightly controlled by the efficiency of Ca2+ uptake into the SR by the Ca2+ ATPase SERCA. The pumping rate of SERCA is highly sensitive to changes in [cAMP]i. An increase of [cAMP]i stimulates SERCA activity (for review see, Colyer, 1998; Kamp and Hell, 2000), leading to a higher SR Ca2+ uptake and spontaneous Ca2+ events occur more frequently. The heart beats faster.
Here, we investigate the role of HCN4 in cardiac pacemaking of mice using a knock-in mouse model in which cAMP binding to the HCN4 channel has been abolished. Our results are both intriguing and unexpected. They demonstrate that (1) in the embryo, HCN4 is a powerful pacemaker but only when cAMP is bound and (2) in adult mice, HCN4 does not seem to contribute to cardiac pacemaking, but rather ensures stable heart rhythm during and after stress.
Results
Generation of HCN4R669Q knock-in mice
To define the physiological function of If and its modulation by cAMP more precisely, we generated knock-in mice that harbour a single amino-acid exchange (R669Q) in the CNBD of the HCN4 channel. This arginine residue is crucial for the binding of cAMP because it interacts with the negatively charged phosphate group (McKay et al, 1982; Bubis et al, 1988; Zagotta et al, 2003). To ascertain that the mutation abolishes regulation by cAMP, we analysed the wild-type HCN4 and the mutant HCN4R669Q channel in Flp-In-293 cells. In the absence of cAMP, the voltage-dependent activation of HCN4R669Q mutant and wild-type channels was similar (Figure 1A). Voltages of half-maximal activation V1/2 were −94.3±3.7 mV (n=8) for wild-type and −99.5±3.4 mV (n=7) for HCN4R669Q channels. Cyclic AMP shifted the activation curve of the wild-type HCN4 channel by +24 mV (V1/2=−70.6±2.7 mV (n=3)), whereas no shift was observed for the HCN4R669Q mutant (V1/2=−96.3±7.1 mV (n=5)). We introduced the mutation into the HCN4 gene locus of mice via homologous recombination in embryonic stem cells (Figure 1B). Integration of the targeting vector, germ-line transmission, and deletion of the neomycin resistance gene were confirmed by Southern blot and PCR analysis (Figure 1C and D, and data not shown). Heterozygous HCN4+/R669Q mice were viable, bred normally, and were indistinguishable from wild-type littermates. However, no homozygous HCN4R669Q/R669Q pups were born from heterozygous matings (HCN4+/+: 57/155; HCN4+/R669Q: 98/155). Analysis of timed matings revealed that HCN4R669Q/R669Q embryos developed normally until E11, but were dead from E12 on (Figure 1E), indicating that homozygous embryos die between E11 and E12.
Figure 1.
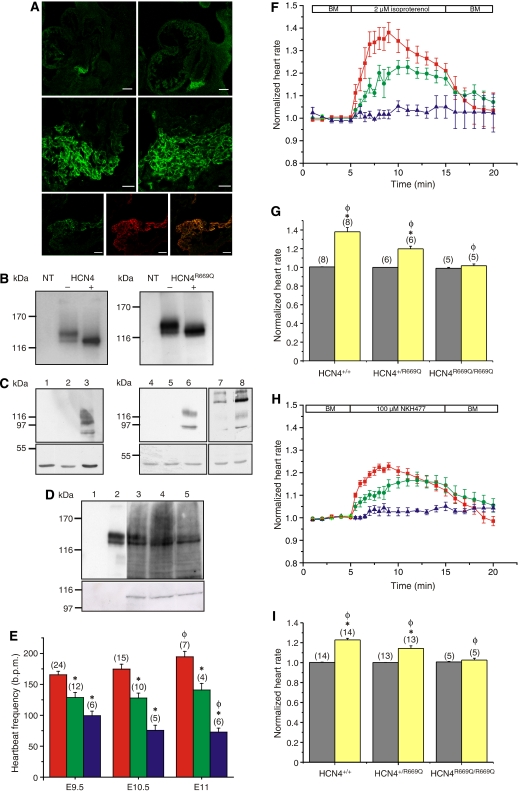
Embryonic death of HCN4R669Q/R669Q mice. (A) Voltage-dependent activation of heterologously expressed HCN4 (circles) and HCN4R669Q channels (squares). Voltages of half-maximal activation (±s.d.) in the absence (open symbols) and presence (filled symbols) of cAMP (100 μM) were −94.3±3.7 (n=8) and −70.6±2.7 mV (n=3) for HCN4, and −99.5±3.4 (n=7) and −96.3±7.1 mV (n=5) for HCN4R669Q, respectively. (B) Targeting strategy. Only exons IV–VIII of the HCN4 locus are shown (white boxes). The targeting vector carried the mutation in exon VII (red arrow) and the neomycin resistance gene (neo), flanked by two loxP elements (red arrowheads). neo was deleted by Cre-mediated recombination. Homologous recombination and deletion of neo were detected through Southern blot using the XbaI restriction sites (X) and the 3′ probe (black box), and through PCR (primers 3 and 4, grey arrows). XbaI fragments that are recognized by the 3′ probe are depicted in blue. (C) Southern blot and PCR analysis of genomic DNA from HCN4+/+ (lane 1), HCN4+/neoR669Q (lane 2), and HCN4+/R669Q (lane 3) mice. Upper panel: Southern hybridization of XbaI-digested DNA with the 32P-radiolabelled 3′ probe. Middle panel: amplification of the wild-type allele (primers 1 and 2, 586 bp). Lower panel: amplification of the recombinant allele (primers 3 and 4, 1804 or 610 bp). (D) Genotyping of embryos from one litter. Upper panel: PCR amplifying the wild-type allele (primers 1 and 2). Middle panel: PCR amplifying the recombinant allele (primers 3 and 4). Lower panel: genotypes of analysed litter. (E) Number of embryos found alive (E9.5–E12.5; red: HCN4+/+; green: HCN4+/R669Q; blue: HCN4R669Q/R669Q).
The amino-acid exchange R669Q does not alter protein expression of HCN4
We were concerned about the similar lethal phenotype of HCN4−/− (Stieber et al, 2003) and HCN4R669Q/R669Q mice. To investigate whether the pattern or level of expression of the channel mutant is altered, we analysed the cellular HCN4 distribution during embryonic development prior to E11.5. Different HCN4-specific antibodies labelled the same region in the heart of HCN4+/+ and HCN4R669Q/R669Q embryos (Figure 2A). No obvious difference existed in the cellular distribution of the HCN4 channel in wild type compared to the HCN4R669Q channel in homozygous embryos (Figure 2A, middle panel). A monoclonal, HCN4-specific antibody (specificity of HCN4 antibodies shown in Figure 2C) labelled two bands in western blots of membrane proteins from Flp-In-293 cells that expressed the HCN4 or the HCN4R669Q channel (Figure 2B). Treatment with PNGase F revealed that the upper band represents a glycosylated form of the HCN4 channel. In western blots of proteins isolated from wild-type, heterozygous, and homozygous embryonic hearts, the antibody recognized the same protein bands with equal intensities, demonstrating that the mutation does not affect the level of HCN4 expression (Figure 2D).
Figure 2.
The HCN4R669Q channel alters the embryonic heartbeat. (A) Upper panels: cryo-sections from HCN4+/+ (left) and HCN4R669Q/R669Q embryos (right) stained with an HCN4-specific antibody (PPc73K; bar: 100 μm). Middle panels: higher magnification of the labelled region (bar: 20 μm). Lower panels: Two independent HCN4-specific antibodies label identical structures in the heart of HCN4R669Q/R669Q embryos (left: PPc73K; middle: SHG-1E5; right: overlay; bar: 20 μm). (B) Western blot analysis of membrane proteins from Flp-In-293 cells, expressing HCN4 (left) or HCN4R669Q (right), labelled with the HCN4-β antibody. NT: non-transfected cells, +: treatment with PNGase F. (C) Left, upper panel: lysates from HEK293-mHCN1 cells. The following antibodies have been used to show the isoform specificity: 1, HCN4-β; 2, HCN4-specific PG2-7H9; 3, HCN1-specific RTQ-7C3. Middle, upper panel: lysates from HEK293-mHCN2 cells. Antibodies used: 4, HCN4-β; 5, HCN4-specific PG2-7H9; 6, HCN2-α. Right, upper panel: lysates from HEK293-mHCN4 cells. Antibodies used: 7, HCN4-β; 8, HCN4-specific PG2-7H9. All lower panels: loading control with anti-actin antibody. (D) Upper panel, lanes 1 and 2: membrane proteins from Flp-In-293 cells (1, non-transfected; 2, transfected with mHCN4) labelled with the HCN4-specific antibody PG2-7H9 (exposure time: 1 min). Lanes 3–5: total proteins from embryonic hearts (3, HCN4+/+; 4, HCN4+/R669Q; 5, HCN4R669Q/R669Q; 10 hearts per genotype) labelled with PG2-7H9 (exposure time: 10 min). Lower panel: loading control with anti-actinin antibody. (E) Basal embryonic heart rate at E9.5, E10.5, and E11 (HCN4+/+, red; HCN4+/R669Q, green; HCN4R669Q/R669Q, blue). The number of embryos analysed is indicated. Student's t-test: *P<0.05 compared to HCN4+/+ at the same developmental stage; φP<0.05 compared to the same genotype at E9.5. (F) Effect of isoproterenol (2 μM) on the embryonic heart rate (HCN4+/+, red; HCN4+/R669Q, green; HCN4R669Q/R669Q, blue). Data have been normalized to the rates during superfusion with BM. (G) Increase in heart rate (at 9 min) during perfusion with isoproterenol. Data have been normalized to the corresponding heart rate during superfusion with BM (grey, basal heart rate; yellow, heart rate at 9 min). Statistical analysis: student's t-test; *P<0.05 compared to basal heart rate; φP<0.05 compared to heart rate of the other genotypes at 9 min. (H) See F for NKH477 (100 μM). (I) See G for NKH477. Data represent mean±s.e.m.
HCN4R669Q/R669Q embryonic mice display reduced basal heart rates
We analysed the spontaneous beat frequency of hearts isolated from HCN4+/+, HCN4+/R669Q, and HCN4R669Q/R669Q embryos prior to E11.5. Under basal conditions, hearts from heterozygous and homozygous embryos beat regularly without obvious arrhythmias; however, the heart rate was significantly slower compared to hearts from wild-type embryos (Figure 2E and Table I). Furthermore, in wild-type embryos, the heart rate increased from E9 to E11 (Figure 2E and Table I), thereby enhancing supply of nutrients and oxygen necessary for proper development. This increase was not observed in HCN4+/R669Q embryos, and in HCN4R669Q/R669Q embryos, the heart rate was even further reduced from day 9.5 to 11.5 (Figure 2E). Notably, heart rates from HCN4R669Q/R669Q and HCN4−/− embryos at E9.5 (Stieber et al, 2003) are virtually identical, indicating that the Arg669Gln exchange completely eliminates the pacing function of the HCN4 channel.
Table 1.
Basal embryonic heart rate
| Basal heart beat (b.p.m.) | |||
|---|---|---|---|
| E9.5 | E10.5 | E.11 | |
| HCN4+/+ | 164.5±5.9 | 174.6±7.8 | 194.5±8.7 |
| HCN4+/R669Q | 129.7±9.0 | 127.9±7.9 | 141±10.5 |
| HCN4R669Q/R669Q | 99.3±7.3 | 75.6±8.2 | 72.2±6.5 |
Adrenergic stimulation does not accelerate the heart rate of HCN4R669Q/R669Q embryonic mice
Isoproterenol superfusion increased the rate of isolated hearts from HCN4+/+ and HCN4+/R669Q embryos (37.4±4.5% (n=8) and 19.8±2.8% (n=6), respectively), whereas no increase was observed in hearts from HCN4R669Q/R669Q embryos (2.8±2.5% (n=5); Figure 2F and G). Similarly, superfusion with NKH477, a drug that specifically activates adenylyl cyclases, increased the heart rate from HCN4+/+ and HCN4+/R669Q embryos (22.6±1.7% (n=14) and 14.2±2.6% (n=13), respectively), but not from HCN4R669Q/R669Q embryos (1.9±1.9% (n=5); Figure 2H and I). These results demonstrate that HCN4 is the principal target for cAMP during the embryonic stages analysed in this study. An acceleration of heartbeat frequency during β-adrenergic stimulation is only possible when cAMP binds to HCN4.
The If of HCN4R669Q/R669Q cardiomyocytes activates slower and deactivates faster
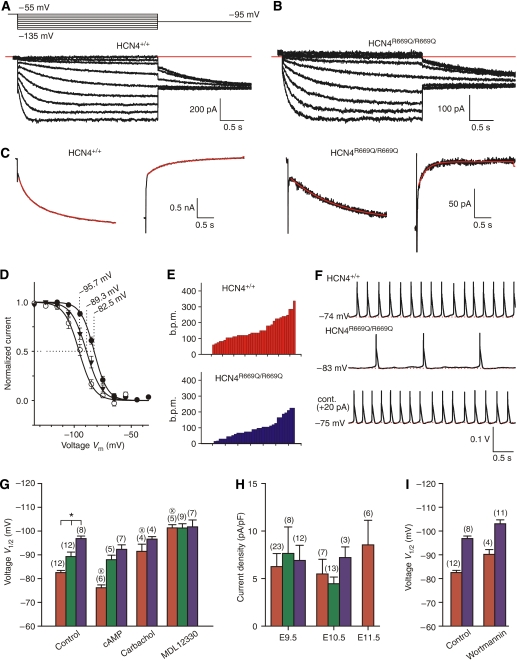
To characterize genotypic differences of cardiomyocytes, we recorded If currents and action potentials from cultured cells of HCN4+/+, HCN4+/R669Q, and HCN4R669Q/R669Q embryonic hearts. Isolated beating cells were studied with the patch-clamp technique in the whole-cell configuration. The If current was activated by hyperpolarizing voltage steps from a holding voltage of −55 mV to test voltages up to −135 mV. Subsequently, a voltage step to −95 mV was applied to probe the activation state of HCN channels. We observed robust If currents in all three genotypes. Figure 3A and B shows currents from cardiomyocytes of wild-type and homozygous embryos, respectively. The If current of wild-type cardiomyocytes activated faster and more completely than that of HCN4R669Q/R669Q cells (Figure 3A and B). Therefore, we analysed the kinetics of If current activation and deactivation in more detail. Figure 3C shows current responses of wild-type and homozygous cells to steps from the holding voltage (−55 mV) to the test voltage (−95 mV) and back to the holding voltage. We fitted simple exponential models to the respective current traces ignoring the initial delay at the onset of activation (see Figure 3C). While for the homozygous genotype a single exponential with a time constant of 1757±132 ms (n=16) was sufficient to describe activation, two time constants of 264±35 ms (relative amplitude 0.35) and 1771±474 ms (relative amplitude 0.65) (n=11) were necessary to describe wild-type currents. Similarly, deactivation of homozygous currents was well described by a single time constant of 172±23 ms (n=9), while for wild-type currents two time constants of 200±38 and 1690±314 ms (n=8) were necessary for a satisfactory fit. The additional time constant for wild-type currents was fast in the case of activation and slow in the case of deactivation, demonstrating that in wild type, currents activated faster and deactivated slower compared to currents of homozygous cells. Table II summarizes the results of the kinetic analysis. We have included in Table II also the kinetic analysis of the activation of heterologously expressed HCN4 and HCN4R669Q channels. The activation properties of wild-type cardiomyocytes are strikingly similar to those of heterologously expressed HCN4 currents in the presence of cAMP (see Table II).
Figure 3.
If currents and action potentials recorded from isolated embryonic cardiomyocytes. (A) Upper panel: voltage protocol for the recording of If. Lower panel: If current in an HCN4+/+ cell. Tail currents at −95 mV were used to analyse the voltage dependence of activation. The red line in the current progression indicates the zero current level. (B) If current in an HCN4R669Q/R669Q cell. The red line in the current progression indicates the zero current level. (C) Left part: activation and deactivation of HCN4+/+ channels. Right part: activation and deactivation of HCN4R669Q/R669Q channels. The time course of activation and deactivation of If was fitted with two (HCN4+/+) or a single (HCN4R669Q/R669Q) exponential term (red traces). (D) Voltage-dependent activation of If from HCN4+/+ (filled circles), HCN4+/R669Q (filled triangles), and HCN4R669Q/R669Q (open circles) cells. The solid line was calculated with the Boltzmann equation with the following parameters (mean±s.d.): HCN4+/+, V1/2=−82.5±3.2 mV and s=5.8±2.4 mV (n=12); HCN4+/R669Q, V1/2=−89.3±6.6 mV and s=6.6±1.1 mV (n=12); HCN4R669Q/R669Q, V1/2=−95.7±4.5 mV and s=6.5±2.6 mV (n=9). (E) Summary of beat frequencies (in b.p.m.) of isolated cells recorded in current-clamp mode. Upper panel: frequencies recorded from HCN4+/+ cells. Lower panel: frequencies recorded from HCN4R669Q/R669Q cells. (F) Action potentials of an HCN4+/+ (upper panel) or an HCN4R669Q/R669Q (middle and lower panels) cell. In the experiment shown in the lower panel, a depolarizing current of 20 pA was injected. (G) Voltages V1/2 of half-maximal activation of HCN4+/+ (red), HCN4+/R669Q (green), and HCN4R669Q/R669Q (blue) under control conditions, with 500 μM cAMP (HCN4+/+ and HCN4R669Q/R669Q) or 100 μM cAMP (HCN4+/R669Q) in the pipette solution, or with 10 μM carbachol in the bath, or with 10 μM MDL12330 in the pipette solution. (H) Current densities at −135 mV (determined as If, −135 mV per cell capacitance) of cells from HCN4+/+ (red), HCN4+/R669Q (green), and HCN4R669Q/R669Q (blue) embryonic hearts at defined developmental stages. Recordings were made between 24 and 36 h after preparation. (I) Voltages V1/2 of half-maximal activation of If from HCN4+/+ (red) and HCN4R669Q/R669Q (blue) cells under control conditions (data from G) and from cells pre-incubated with wortmannin (10 μM) for at least 30 min. Data represent mean±s.e.m. except when explicitly stated; *P<0.05 as indicated; ⊗P<0.05 compared to the same genotype under control conditions.
Table 2.
Kinetics of If activation and deactivation
| τfast (ms) | Relative amplitude A1/(A1+A2) | τslow (ms) | Relative amplitude A2/(A1+A2) | n | |
|---|---|---|---|---|---|
| Activation | |||||
| HCN4a | — | — | 1873±132 | 1.0 | 19 |
| HCN4 (100 μM cAMP)a | 290±32 | 0.55±0.09 | 1522±426 | 0.45±0.09 | 7 |
| HCN4R669Q a | — | — | 1749±185 | 1.0 | 9 |
| HCN4+/+ b | 264±35 | 0.35±0.03 | 1771±474 | 0.65±0.03 | 11 |
| HCNR669Q/R669Q b | — | — | 1757±132 | 1.0 | 16 |
| Deactivation | |||||
| HCN4+/+ b | 200±38 | 0.43±0.04 | 1690±314 | 0.57±0.04 | 8 |
| HCNR669Q/R669Q b | 172±23 | 1.0 | — | — | 9 |
| aRecorded in HEK293 cells. | |||||
| bGenotype of embryonic cardiomyocytes. | |||||
| The equation I(t)=A0+A1exp(−t/τfast)+A2exp(−t/τslow) was used to describe the activation and deactivation of hyperpolarization activated currents (A1 and A2, amplitudes of the fast and slow components, respectively; τfast and τslow, fast and slow time constants, respectively). | |||||
The voltage-dependent activation of If in cardiomyocytes differs among genotypes
Next, we analysed tail currents as in Figure 3A and B to determine the voltage dependence of activation of If for the different genotypes. The voltage of half-maximal activation (V1/2) in the three genotypes differed significantly: V1/2 was −82.5±3.2 mV (n=12) for wild-type, −89.3±6.6 mV (n=12) for heterozygous, and −95.7±4.5 mV (n=9) for homozygous cardiomyocytes (Figure 3D). This result is reminiscent of the differences observed with heterologously expressed HCN4 and HCN4R669Q channels in the presence of cAMP (compare Figure 2A). However, for the experiments presented here, no exogenous cAMP was added. We interpret this result to indicate that even in the unstimulated state, the If in wild-type and heterozygous cardiomyocytes is significantly upregulated by cAMP.
Isolated HCN4R669Q/R669Q cardiomyocytes beat with lower frequencies
We recorded action potentials from isolated cells of wild-type and homozygous hearts to investigate whether the differences in If are reflected in the spontaneous rates of cardiomyocytes. Action potentials were highly variable from cell to cell, indicating that cardiomyocytes at this developmental stage have already undergone substantial differentiation. Spontaneous rates of HCN4+/+ cells ranged from 60 to 337 b.p.m. (Figure 3E, upper panel), the action potential duration (APD) varied from 30 to 360 ms, and the MDP varied from −62 to −87 mV. Rates from HCN4R669Q/R669Q cells varied from 15 to 225 b.p.m. (Figure 3E, lower panel), ADPs from 5 to 800 ms, and MDPs from −61.5 to −87 mV. On average, rates were significantly lower in HCN4R669Q/R669Q cells (99±11 b.p.m. (n=32)) than in HCN4+/+ cells (156±11 b.p.m. (n=37), P<0.05). We reasoned that cells with the highest spontaneous rates eventually pace the embryonic heart. In wild-type embryos, we identified pacemaker-like cardiomyocytes (9 of 32 cells) with particularly high average rates of 211±20 b.p.m. (n=9), MDPs of −75.7±0.7 mV, APDs<100 ms, and robust If currents when voltage clamped. In the presence of cAMP (100 μM in the pipette solution), this cell population displayed beat frequencies of 338±41 b.p.m., indicating a pronounced stimulatory effect of cAMP. Action potentials of a typical wild-type cell from this population are shown in Figure 3F (upper panel). The cell is spontaneously active at about 240 b.p.m.
We then searched for HCN4R669Q/R669Q cells that produced the same action potential shape (APD<100 ms) characteristic of this particular class of cells. In total, 8 of 32 cells were found with action potential shapes similar to the ones we were looking for. These cells were characterized by a relatively slow rate of 92±9 b.p.m. (n=8) and MDPs of −82.6±0.7 mV. Figure 3F (middle panel) shows a typical example. The cell was active with 75 b.p.m. and had an MDP of −83 mV. When we injected a depolarizing current of 20 pA into this cell (Figure 3F, lower panel) to compensate for the poor activation of the HCN4R669Q/R669Q channel, action potential rates increased drastically to values otherwise not observed in HCN4R669Q/R669Q cells. Under these conditions, rate, action potential shape, and MDP were very similar to the wild-type pacemaker cell types at this developmental stage.
The If of HCN4R669Q/R669Q cardiomyocytes is unresponsive to changes in cAMP
We next studied If currents in the presence of exogenous cAMP (500 μM). Under this condition, the V1/2 value of If currents from wild-type cardiomyocytes was further shifted by 6 to −76.2±2.9 mV (n=6), while that from homozygous cardiomyocytes was largely unchanged (V1/2=92.3±4.9 mV, n=6) (Figure 3G). The small shift of V1/2 by 6 mV in wild-type cells compared to a shift by 24 mV for heterologously expressed HCN4 channels supports the notion that the level of cAMP in cardiomyocytes is unusually high in the resting state. Therefore, the dynamic range of changes in cAMP is restrained and the extent of regulation of If is accordingly smaller.
We reasoned that inhibition of basal adenylyl cyclase activity should abolish the genotypic differences in V1/2. Muscarinic modulation is known to inhibit adenylyl cyclase. We stimulated cells with carbachol (10 μM in the bath solution) and studied the effect on If currents in wild-type and homozygous cardiomyocytes. While If of homozygous cardiomyocytes was unaffected by carbachol, some wild-type cells displayed If with a more negative activation curve. However, the action of carbachol was variable (range of V1/2 of wild-type If from −84 to −98 mV), indicating that the muscarinic system may not be homogeneously expressed in all cardiomyocytes. Consequently, we used the adenylyl cyclase inhibitor MDL12330 (10 μM in the pipette solution) to directly inhibit cAMP synthesis in cardiomyocytes. In the presence of MDL12330, all genotypes displayed the same V1/2 of −101 mV (Figure 3G). This result strongly supports the notion that a high resting cAMP level is responsible for the differences in If and, accordingly, for the differences in heart rate between the three genotypes.
If current densities do not differ among genotypes
Some mutations in the CNBD of HERG channels have been proposed to induce trafficking defects, that is, fewer channels are incorporated into the plasma membrane (for review, see Thomas et al, 2003). To investigate whether Arg669 is also important for trafficking, we determined If current densities of embryonic cardiomyocytes at different developmental stages. We used the time-resolved component of If at −135 mV (full activation) for this analysis. Figure 3H summarizes the results. We observed no significant genotypic differences of If current density at days E9.5 or E10.5. Furthermore, no significant increase of If current density was observed in wild-type cells from E9.5 to E11.5, indicating that the increase of heart rate observed during this developmental window is probably not caused by an enhanced expression of If currents. HCN currents have been shown to exhibit an instantaneous current component (Gauss et al, 1998; Macri and Accili, 2004; Mistrik et al, 2006; Proenza and Yellen, 2006; Lin et al, 2007). We determined the current density of the instantaneous current (including leak) at −95 mV for the three genotypes. We observed no genotypic differences (mean (median)±s.d. (number of experiments); wild-type, 2.5 (1.8)±2.5 pA/pF (n=38); HCN4+/R669Q, 1.8 (1.6)±1.3 pA/pF (n=24); HCN4R669Q/R669Q, 2.0 (2.0)±1.3 pA/pF (n=15)).
PI3 kinase is not responsible for the genotypic differences of If
Finally, we studied whether If currents of wild-type and homozygous cardiomyocytes are differently regulated by phosphoinositide 3 (PI3) kinase. Recently, it has been shown that PIP2 can act as a ligand that allosterically opens HCN channels by shifting the activation curve to more positive potentials (Pian et al, 2006; Zolles et al, 2006). We studied the activation of If after incubation of cardiomyocytes with wortmannin (10 μM in the bath solution) for at least 30 min. While the V1/2 values of both, wild type and HCN4R669Q/R669Q, were shifted to more negative values by wortmannin treatment (7.7 and 6.2 mV, respectively), the difference of V1/2 among genotypes was unchanged (Figure 3I). We conclude that there is no differential regulation of wild type and mutant If by PI3 kinase. Taken together, our results indicate that wild-type If is enhanced by cAMP at rest and that this acceleration is indispensable for pacemaker activity of If.
Heart rates in adult mice do not differ between genotypes
The differences in basal heart rate and in the activation of If between wild-type and heterozygous embryos prompted us to study the heart rate in freely moving adult HCN4+/+ and HCN4+/R669Q mice by telemetric recording of electrocardiogram (ECG). Neither the heart rate at rest nor during exercise or mental stress was different between genotypes (Figure 4A and C). The heart rate of sedated animals and the intrinsic rate of the isolated heart were also not different between genotypes (Figure 4B and C), nor was the electrophysiology of the AV node and the ventricle (Figure 4C). After exercise, HCN4+/R669Q mice developed pauses and sino-atrial block more often than their wild-type littermates (HCN4+/R669Q: 9±7 pauses/55 min, median 6.5, range 2–25; HCN4+/+: 6.5±5 pauses/55 min, median 1, range 0–14, P<0.05). Sino-atrial block and sinus pauses were also found in spontaneously beating, Langendorff-perfused hearts, mostly during washout of the β-adrenoceptor agonist orciprenaline (baseline: pauses in 4/10 HCN4+/R669Q hearts versus 1/9 HCN4+/+ hearts; during orciprenaline infusion: pauses in 5/10 HCN4+/R669Q versus 1/9 HCN4+/+ hearts; during washout of orciprenaline: pauses in 8/10 HCN4+/R669Q versus 0/9 HCN4+/+ hearts, P<0.05 for HCN4+/R669Q versus HCN4+/+).
Figure 4.
HCN+/R669Q mice display a sino-atrial block. (A) Representative examples of telemetric ECG recordings in freely moving mice carrying an implanted ECG transmitter. All recordings were obtained during the 55-min recovery period after stress tests (air jets or swimming). Animal numbers are indicated. (B) Representative recording of spontaneous rhythm in isolated, Langendorff-perfused hearts. Ventricular monophasic action potential (MAP), right atrial electrogram (RA), and lead II of the tissue bath ECG (ECG) are shown. The WT heart shows a constant sinus rhythm, the HCN4+/R669Q heart shows three pauses with doubling of cycle length, consistent with sino-atrial block. (C) Electrophysiological measurements in sedated animals, freely moving animals and in the isolated, beating heart. All values are given as mean±s.e.m. There were no significant differences among groups. Rates in the isolated hearts include hearts with sino-atrial block. This explains the relatively large s.d. in this group during orciprenaline treatment.
Discussion
The role of If in embryonic and adult cardiomyocytes is a matter of a long-lasting debate (Kodama et al, 2002; Lipsius and Bers, 2003). While some groups favour If as the principle pacemaker current of SAN cells, others attribute only a minor modulatory function to this current. We studied here knock-in mice that carry a single amino-acid exchange in the CNBD of the HCN4 channel, which represents the major component of the If current in SAN cells (Stieber et al, 2003). Our results provide the following insights.
First, hearts from HCN4R669Q/R669Q embryos show virtually no response to catecholaminergic stimulation, indicating that the HCN4 channel is the major if not only target for cAMP-mediated acceleration of the heart rate by catecholamines. Furthermore, this result shows that protein phosphorylation by another important target of cAMP signalling, cAMP-dependent protein kinase, is not involved in cardiac pacemaking at this developmental stage. The β-adrenergic acceleration of the heart rate is crucial for survival of the embryo (Portbury et al, 2003; Chandra et al, 2006). Adrenergic stimulation maintains the embryonic heart rate during transient hypoxia that occurs during gestation. Hypoxia induces a life-threatening bradycardia unless catecholamine release compensatorily accelerates the heart rate (Portbury et al, 2003).
The fatal outcome of the HCN4R669Q/R669Q knock-in model demonstrates the outstanding role of HCN4 as the key transducer of catecholaminergic signalling in the embryonic heart ensuring survival of the embryo.
Another key observation of our study is that even in the absence of adrenergic stimulation, the rates of heterozygous and homozygous hearts were significantly slower than those of wild-type hearts. This genotypic difference persisted in isolated cells where the If current displayed different activation properties: the activation curve of wild-type If was significantly shifted towards more positive voltages compared to that of homozygous cells. In heterozygous cells, this shift was smaller. The shift can be accounted for by high basal adenylyl cyclase activity resulting in high cAMP levels. Indeed, blocking adenylyl cyclase activity with MDL12330 completely eliminates differences in the activation curves of If. Moreover, Vinogradova et al (2006) have recently shown that the basal cAMP level in rabbit SAN cells is markedly higher than in other cardiac cell types. Finally, even in non-cardiac cells, namely GH(3) immortalized pituitary cells, basal cAMP levels significantly contribute to HCN channel activity (Kretschmannova et al, 2006).
One of the most striking observations was that the resting beat frequency of embryonic HCN4R669Q and HCN4−/− (Stieber et al, 2003) hearts did not differ. The HCN4R669Q channel, while present at similar density as the HCN4 channel in wild-type cardiomyocytes and proven functional, seems not to contribute to the pacing of the embryonic heart. Obviously, additional pacemaker mechanisms besides If must exist in the embryonic heart that are responsible for the residual heart rate found in homozygous embryos. We investigated the beating properties of wild-type and homozygous cells in detail. In HCN4+/+ cells, we identified a cell type with especially high beating rates that we consider the pacemaker cells of the embryonic heart. A cell population with very similar action potentials was also found in the HCN4R669Q/R669Q preparation. In this respect, our knock-in mouse model differs from the HCN4 knockout model, in which no ‘mature' pacemaker cells were found (Stieber et al, 2003). The rates of these ‘pacemaker-like' HCN4R669Q/R669Q cells were much lower than those in wild-type cells. Indeed, other cell types of the HCN4R669Q/R669Q heart exhibited higher rates than this particular cell population. Most of these other cells displayed If currents, however, some were devoid of If. We speculate that in the HCN4R669Q/R669Q heart probably these other cell populations take over to pace the heart, albeit with reduced rates.
If we assume that cells with If are involved in pacemaking, why are the rates of HCN4R669Q/R669Q and HCN4−/− identical? First, the MDP of most of the cells recorded here adopts values of about −75 mV. At this voltage the open probability of the HCN4R669Q channel is only ∼4%, as determined from the voltage dependence of activation. Most likely, the depolarizing action of the HCN4R669Q/R669Q channel is too small to be of any significance. The channel, in the absence of cAMP, becomes physiologically silent. Second, in HCN4−/− as well as HCN4R669Q/R669Q embryos other mechanisms may compensate the effect of the deletion or mutation of the HCN4 channel. This is expected to result in the same heart rate in both mouse lines. However, other HCN channel isoforms are not upregulated in HCN4 knockout mice (Stieber et al, 2003; Herrmann et al, 2007) and other potential mechanisms have not been studied so far.
In light of these findings and models of heart-rate regulation in the SAN (Robinson et al, 2006), the normal heart rate of adult heterozygous HCN4R669Q mice at rest and during exercise is unexpected. While hearts of heterozygous embryos display significantly reduced rates and attenuated responses to catecholamines, no such genotypic differences exist in adult hearts. Different scenarios can be envisioned to interpret this result. First, during development, other pacemakers may compensate the bradycardic effect of the channel mutation. In humans, the situation might be different. Mutations in or near the CNBD of HCN4 have been found to lead to a profound bradycardic phenotype in humans (Schulze-Bahr et al, 2003; Milanesi et al, 2006). The molecular phenotype of one of these mutations was somewhat milder than the one investigated here. The mutated channels in this study are activated at more negative voltages than wild-type channels (Milanesi et al, 2006). Interestingly, the negative shift of V1/2 was only 8.4 mV, less than the shift we observed for the If in homozygous cardiomyocytes (13 mV). Thus, a putative compensatory mechanism is lacking in humans. While a compensatory mechanism cannot be discarded, we favour a different interpretation of the identical heart rates in wild-type and heterozygous mice. During embryonic development of the mouse, the role of If may switch from that of a major pacemaker to one that backs up other pacing elements in the adult. In fact, while this manuscript was in preparation, Herrmann et al (2007) published their work on a mouse model in which the HCN4 channel has been deleted cardiac specifically in adult mice. Mutant mice showed no defect in heart-rate regulation during sympathetic stimulation, but exhibit cardiac arrhythmia characterized by sinus pauses. The phenotype is similar to that of adult heterozygous HCN4+/R669Q mice. It will be interesting to investigate the cardiac phenotype of adult homozygous HCN4R669Q/R669Q mice to study whether these animals display the phenotype observed in knockout mice. This experiment would test the idea whether cAMP binding is indispensable for the physiological function of HCN4 channels rather than only modulatory. From our results and the similar observation of Herrmann et al (2007), we conclude that in adult mice, HCN4 channels serve a back-up mechanism that maintains a stable heart beat in situations during and after stress. HCN4 channels are probably no longer involved in sympathetic stimulation of the heart rate.
In humans, the role of HCN4 obviously is different. How can we explain these species-dependent differences? The most obvious difference in cardiac function between mice and humans is the frequency of the beating heart (60–200 b.p.m. in humans versus 500–1000 b.p.m. in mice). The beating frequency in humans is similar to the one in embryonic mice, where we have shown that HCN4 is a true pacemaker. We suggest that HCN4 can only serve as pacemaker at low beating frequencies; at higher frequencies, its slow activation characteristics are not suitable to support higher heart rates.
Materials and methods
Cloning of the targeting vector for the generation of HCN4 knock-in mice
A genomic clone containing exons IV–VIII of the HCN4 gene was isolated from a mouse genomic 129/SvJ library (Stratagene, Amsterdam, The Netherlands). The library was probed with a cDNA fragment encoding the S5 to S6 region of mHCN4. A clone of roughly 11.7 kb was subcloned into pBluescript SK(−) (Stratagene), sequenced, and used for the construction of the targeting vector. The vector was constructed in neo-flox-8 by using a 1.8-kb genomic fragment as short arm of homology and a 4.4-kb genomic fragment as long arm of homology. The short arm was generated by ligating an AatII/HindIII fragment of the genomic clone together with a HindIII/XbaI-digested PCR product (forward: 5′-TCAGAAGCTTGGAGGGACCC-3′; reverse: 5′-CCACTtctAGAACCCTCAATGGAA-3′) into the AatII/XbaI-digested neo-flox-8. The long arm was generated by ligating an SphI/NsiI fragment of the genomic clone with a NotI/Asp718-digested PCR product (forward: 5′-TCTCAGATGGCgGccgcAGCACTTGTGTT-3′; reverse: 5′-ACTGGGTACCAGCAGCACCC3′) as well as an Asp718/SphI-digested PCR product that carries the mutation CG to AG in exon VII into the NotI/NsiI-digested neo-flox-8 containing the short arm. The mutated PCR fragment was generated by subcloning an Asp718/NarI fragment into pBluescript SK(−) and using this subclone as a template for a recombinant PCR performed with the ‘QuikChange site-directed mutagenesis kit' (Stratagene) (forward: 5′-GACCCGGGGTCGGCagACAGCCAGCGTCAG-3′; reverse: 5′-CTGACGCTGGCTGTctGCCGACCCCGGGTC-3′). For negative selection, a diphtheria toxin A (DTA) cassette was inserted at the 5′ end of the short arm. The DTA cassette has been amplified on pGEMTeasyDTA2 (forward: 5′-AATGACGTCCGGTACCTCGACG-3′; reverse: 5′-ACTGACGTCCGGTACCTTAATTAA-3′) and subcloned into pBluescript. An AatII fragment was excised and cloned into the AatII site of the targeting vector.
Generation of HCN4 knock-in mice
The vector was linearized with NsiI and electroporated into V6.5 embryonic stem cells (Rideout et al, 2000). Positive clones were identified by Southern blot analysis with 5′ and 3′ probes. Blastocyst injection was performed for three independent clones. Male chimeras were crossed with C57BL/6J females (Jackson Laboratories, Bar Harbor, ME, USA). The neomycin resistance gene was removed by crossing heterozygous mice with deleter-cre mice (Schwenk et al, 1995). Heterozygous mice were backcrossed for 10 generations to a C57BL/6J background.
Embryos were genotyped by PCR of yolk-sac DNA, and mice by PCR of tail DNA using the following primer set: primer no. 1: 5′-CTTAGTGGTAGACTGTTGTGGTT-3′ and primer no. 2: 5′-GCTGGCTGTGCGCCGACCC-3′ amplifying a 586-bp wild-type fragment, primer no. 3: 5′-TCTGACGCTGGCTGTCTG-3′ and primer no. 4: 5′-CCATTGAGGGTTCTAGACTC-3′ amplifying a 1804-bp fragment in HCN4+/neoR669Q mice and a 610-bp fragment after excision of the neomycin resistance gene.
Isolation and culture of embryonic hearts and cardiomyocytes
Embryos from timed matings were removed from pregnant females and transferred to pre-warmed PBS (in mM): NaCl 137; KCl 2.7; Na2HPO4 10; and KH2PO4 1.8. For analysis of the beat frequency, hearts were dissected, placed into DMEM (Gibco, Karlsruhe, Germany) containing 10% FBS (Gibco), and cultured over night at 37°C, 10% CO2. During measurements, hearts were superfused with DMEM containing 10 mM HEPES/NaOH pH 7.4 (basal medium, BM). Drugs were added to BM (isoproterenol: 2 μM; NKH477: 100 μM). For isolation of cardiomyocytes, hearts were dissected and placed in a solution containing (in mM): NaCl 120, KCl 5.4, MgSO4 5, sodium pyruvate 5, taurine 20, HEPES 10, glucose 20, CaCl2 0.03, and 1 mg/ml collagenase B (Roche Diagnostics, Mannheim, Germany). The incubation was stopped with DMEM containing 20% FBS, 1% non-essential amino acids (Gibco), 1% penicillin/streptomycin (Gibco), and 100 μM β-mercaptoethanol (Sigma, Seelze, Germany). Cardiomyocytes were placed in a shaker at 37°C for 30 min, plated onto 3 cm dishes, and incubated at 37°C, 5% CO2 for 24–72 h.
Western blot analysis and immunohistochemistry
Ten embryonic hearts from each genotype were pooled and homogenized in lysis buffer (in mM): NaCl 10, EDTA 2, HEPES 25, DTT 3, supplemented with 0.5 mg/ml Pefabloc SC. HEK293 cells were lysed in lysis buffer (in mM): Tris–HCl (pH 7.6) 10, NaCl 140 and 1% Triton X-100, supplemented with 0.5 mg/ml Pefabloc SC. Membrane proteins from Flp-In-293mHCN4 and Flp-In-293mHCN4R669Q cells were isolated as described (Wachten et al, 2006). Proteins were separated on a 7.5% SDS–PAGE, blotted, and probed with one of the following antibodies: monoclonal HCN4-specific antibody (PG2–7H9, rat, 1:2) (Harzheim, 2006; Mataruga, 2006), polyclonal, affinity-purified HCN4-specific antibody (HCN4-β, rabbit, 1:2000) (Mataruga, 2006), monoclonal HCN1-specific antibody (RTQ-7C3, rat, 1:100) (Scholten, 2001), polyclonal, affinity-purified HCN2-specific antibody (HCN2-α, rabbit, 1:1000) (Mataruga, 2006) by using a chemiluminescence detection system. For reprobing with either a monoclonal α-actinin antibody (sarcomeric, mouse; Sigma, 1:1000) or a polyclonal anti-actin antibody (rabbit; Sigma, 1:200) the membranes were incubated in stripping buffer (in mM): Tris–HCl (pH 6.7) 62.5, β-mercaptoethanol 100, and 2% SDS for 30 min at 65°C, washed with PBS and probed again. For immunohistochemical analysis, embryos were fixed in 5% paraformaldehyde, cryo-protected in 10 and 30% sucrose, and embedded in Tissue Tek (Sakura Finetek, Zoeterwoude, The Netherlands) at −20°C. Frozen sections (16 μm, sagittal) were pre-incubated in blocking buffer (PBS, 0.5% Triton X-100, 5% Chemiblocker). Sections were incubated with the HCN4-specific antibodies (SHG-1E5: monoclonal, rat, 1:5: PPc73K: polyclonal, rabbit, 1:400 (Scholten, 2001)) overnight and with the secondary antibodies (Alexa Fluor 488 goat anti-rat IgG; Molecular Probes, Karlsruhe, Germany; CY3 donkey anti-rabbit IgG, 1:500; Dianova, Hamburg, Germany) for 1 h in phosphate buffer containing 5% Chemiblocker. The labelled cryo-sections were analysed with a confocal laser-scanning microscope (Leica TCS, Solms, Germany).
Electrophysiological measurements
We recorded from cells with the patch-clamp technique in the whole-cell configuration. The pipette solution contained (in mM): K+ aspartate 130, NaCl 10, MgCl2 2, EGTA 1, HEPES 10, pH 7.2 (KOH). cAMP or MDL12330 was added to the pipette solution as indicated. Cardiomyocytes were bathed in BM. Wortmannin or carbachol were added to BM as indicated. Flp-In-293 cells expressing HCN4 and HCN4R669Q channels were bathed in a solution containing (in mM): NaCl 140, KCl 5.4, CaCl2 1.8, MgCl2 1, HEPES 5, glucose 10, pH 7.4 (NaOH). All recordings were performed at 37°C. All voltages given were corrected for liquid junction potentials.
ECG and telemetry
Six-lead surface ECGs were recorded in littermate pairs of mice (12 weeks old) during isoflurane inhalation (1–1.5%). Recordings of 10 s were used for signal averaging. To record an ECG in freely roaming animals, a telemetric ECG transmitter (DSI, St Paul, MN, USA) was implanted. The telemetric ECG was analysed during normal activity and periods of stress, that is, swimming and warm air jets, according to published protocols (Kirchhof et al, 2003, 2006; Knollmann et al, 2003; Fabritz et al, 2004). All recordings were digitized (EMKA, Falls Church, VA, USA) at 2 kHz and analysed off-line.
Electrophysiological study of the isolated heart
To assess intrinsic heart rate and AV nodal conduction, the heart was rapidly excised and retrogradely perfused in a Langendorff apparatus. Ventricular monophasic action potentials, atrial and ventricular electrograms, and tissue bath ECGs were simultaneously recorded during spontaneous rhythm and during programmed atrial stimulation (incremental atrial pacing, constant pacing at 100 and 140 ms cycle length) at baseline and during perfusion with orciprenaline (1.7 μM) following published techniques (Kirchhof et al, 2003, 2006; Kuhlmann et al, 2006).
All original recordings were scrutinized for arrhythmias. We analysed heart rate, AV nodal conduction, APD, and QT interval semi-automatically using custom-designed software (LabVIEW and EMKA). Sino-atrial block (2:1) was defined following accepted prespecified clinical criteria, that is, a sudden two-fold increase of PP interval. All analyses were blinded to genotype.
Statistical analysis
Data are expressed as mean±s.d. or mean±s.e.m. Statistical comparisons were carried out with Student's t-test.
Acknowledgments
We thank J Deussing for providing the neo-flox-8 vector, A Mataruga and A Scholten for the generation and characterization of the HCN4-specific antibodies, and C Aretzweiler for superb technical assistance and help with the animals. DH was a fellow of the Boehringer Ingelheim Fonds, TB was supported by the Land Nordrhein-Westfalen, and PK is supported by the IZKF Münster.
References
- Barbuti A, Baruscotti M, DiFrancesco D (2007) The pacemaker current: from basics to the clinics. J Cardiovasc Electrophysiol 18: 342–347 [DOI] [PubMed] [Google Scholar]
- Biel M, Schneider A, Wahl C (2002) Cardiac HCN channels: structure, function, and modulation. Trends Cardiovasc Med 12: 206–212 [DOI] [PubMed] [Google Scholar]
- Bogdanov KY, Vinogradova TM, Lakatta EG (2001) Sinoatrial nodal cell ryanodine receptor and Na+–Ca2+ exchanger: molecular partners in pacemaker regulation. Circ Res 88: 1254–1258 [DOI] [PubMed] [Google Scholar]
- Bubis J, Neitzel JJ, Saraswat LD, Taylor SS (1988) A point mutation abolishes binding of cAMP to site A in the regulatory subunit of cAMP-dependent protein kinase. J Biol Chem 263: 9668–9673 [PubMed] [Google Scholar]
- Bucchi A, Baruscotti M, Robinson RB, DiFrancesco D (2003) If-dependent modulation of pacemaker rate mediated by cAMP in the presence of ryanodine in rabbit sino-atrial node cells. J Mol Cell Cardiol 35: 905–913 [DOI] [PubMed] [Google Scholar]
- Chandra R, Portbury AL, Ray A, Ream M, Groelle M, Chikaraishi DM (2006) Beta1-adrenergic receptors maintain fetal heart rate and survival. Biol Neonate 89: 147–158 [DOI] [PubMed] [Google Scholar]
- Colyer J (1998) Phosphorylation states of phospholamban. Ann NY Acad Sci 853: 79–91 [DOI] [PubMed] [Google Scholar]
- Couette B, Marger L, Nargeot J, Mangoni ME (2006) Physiological and pharmacological insights into the role of ionic channels in cardiac pacemaker activity. Cardiovasc Hematol Disord Drug Targets 6: 169–190 [DOI] [PubMed] [Google Scholar]
- DiFrancesco D (1993) Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol 55: 455–472 [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Ferroni A, Mazzanti M, Tromba C (1986) Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol 377: 61–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D, Tortora P (1991) Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature 351: 145–147 [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Tromba C (1988) Inhibition of the hyperpolarization-activated current (if) induced by acetylcholine in rabbit sino-atrial node myocytes. J Physiol 405: 477–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabritz L, Kirchhof P, Fortmüller L, Auchampach J, Baba H, Schmitz W, Breithardt G, Neumann J, Boknik P (2004) Gene dose-dependent atrial arrhythmias, heart block and atrial brady-cardiomyopathy in mice overexpressing the A3-adenosine receptor. Cardiovasc Res 62: 500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss R, Seifert R, Kaupp UB (1998) Molecular identification of a hyperpolarization-activated channel in sea urchin sperm. Nature 393: 583–587 [DOI] [PubMed] [Google Scholar]
- Guo J, Ono K, Noma A (1995) A sustained inward current activated at the diastolic potential range in rabbit sino-atrial node cells. J Physiol 483: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzheim D (2006) Der Einfluss von cAMP auf die physiologische Funktion von HCN4-Schrittmacherkanälen in der Maus. Thesis/dissertation, Universität Köln, Fatultät Math.Wat., Köln
- Herrmann S, Stieber J, Stöckl G, Hofmann F, Ludwig A (2007) HCN4 provides a ‘depolarization reserve' and is not required for heart rate acceleration in mice. EMBO J 26: 4423–4432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp TJ, Hell JW (2000) Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res 87: 1095–1102 [DOI] [PubMed] [Google Scholar]
- Kirchhof P, Fabritz L, Fortmüller L, Lankford AR, Matherne G, Baba HA, Schmitz W, Breithardt G, Neumann J, Boknik P (2003) Decreased chronotropic response to exercise and atrio-ventricular nodal conduction delay in mice overexpressing the A1-adenosine receptor. Am J Physiol 285: H145–H153 [DOI] [PubMed] [Google Scholar]
- Kirchhof P, Fabritz L, Zwiener M, Witt H, Schafers M, Zellerhoff S, Paul M, Athai T, Hiller KH, Baba HA, Breithardt G, Ruiz P, Wichter T, Levkau B (2006) Age- and training-dependent development of arrhythmogenic right ventricular cardiomyopathy in heterozygous plakoglobin-deficient mice. Circulation 114: 1799–1806 [DOI] [PubMed] [Google Scholar]
- Knollmann BC, Kirchhof P, Sirenko SG, Degen H, Greene AE, Schober T, Mackow JC, Fabritz CL, Potter JD, Morad M (2003) Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2+-dependent action potential remodeling. Circ Res 92: 428–436 [DOI] [PubMed] [Google Scholar]
- Kodama I, Honjo H, Boyett MR (2002) Are we lost in the labyrinth of the sinoatrial node pacemaker mechanism? J Cardiovasc Electrophysiol 13: 1303–1305 [DOI] [PubMed] [Google Scholar]
- Kretschmannova K, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS (2006) Dependence of hyperpolarisation-activated cyclic nucleotide-gated channel activity on basal cyclic adenosine monophosphate production in spontaneously firing GH3 cells. J Neuroendocrinol 18: 484–493 [DOI] [PubMed] [Google Scholar]
- Kuhlmann MT, Kirchhof P, Klocke R, Hasib L, Stypmann J, Fabritz L, Stelljes M, Tian W, Zwiener M, Mueller M, Kienast J, Breithardt G, Nikol S (2006) G-CSF/SCF reduces inducible arrhythmias in the infarcted heart potentially via increased connexin43 expression and arteriogenesis. J Exp Med 203: 87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande G, Demolombe S, Bammert A, Moorman A, Charpentier F, Escande D (2001) Transgenic mice overexpressing human KvLQT1 dominant-negative isoform. Part II: Pharmacological profile. Cardiovasc Res 50: 328–334 [DOI] [PubMed] [Google Scholar]
- Lin W, Laitko U, Juranka PF, Morris CE (2007) Dual stretch responses of mHCN2 pacemaker channels: accelerated activation, accelerated deactivation. Biophys J 92: 1559–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsius SL, Bers DM (2003) Cardiac pacemaking: If versus Ca2+, is it really that simple? J Mol Cell Cardiol 35: 891–893 [DOI] [PubMed] [Google Scholar]
- Macri V, Accili EA (2004) Structural elements of instantaneous and slow gating in hyperpolarization-activated cyclic nucleotide-gated channels. J Biol Chem 279: 16832–16846 [DOI] [PubMed] [Google Scholar]
- Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, Nargeot J (2003) Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci USA 100: 5543–5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangoni ME, Traboulsie A, Leoni AL, Couette B, Marger L, Le Quang K, Kupfer E, Cohen-Solal A, Vilar J, Shin HS, Escande D, Charpentier F, Nargeot J, Lory P (2006) Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/alpha1G T-type calcium channels. Circ Res 98: 1422–1430 [DOI] [PubMed] [Google Scholar]
- Mataruga A (2006) Licht- und elektronenmikroskopische Analyse von Bipolarzelltypen in der Mausretina. Thesis/dissertation, Universität Köln, Fatultät Math.Wat., Köln
- McKay DB, Weber IT, Steitz TA (1982) Structure of catabolite gene activator protein at 2.9-Å resolution. Incorporation of amino sequence and interactions with cyclic AMP. J Biol Chem 257: 9518–9524 [PubMed] [Google Scholar]
- Milanesi R, Baruscotti M, Gnecchi-Ruscone T, DiFrancesco D (2006) Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N Engl J Med 354: 151–157 [DOI] [PubMed] [Google Scholar]
- Mistrik P, Pfeifer A, Biel M (2006) The enhancement of HCN channel instantaneous current facilitated by slow deactivation is regulated by intracellular chloride concentration. Pflügers Arch 452: 718–727 [DOI] [PubMed] [Google Scholar]
- Pian P, Bucchi A, Robinson RB, Siegelbaum SA (2006) Regulation of gating and rundown of HCN hyperpolarization-activated channels by exogenous and endogenous PIP2. J Gen Physiol 128: 593–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J (2000) Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102: 89–97 [DOI] [PubMed] [Google Scholar]
- Portbury AL, Chandra R, Groelle M, McMillian MK, Elias A, Herlong JR, Rios M, Roffler-Tarlov S, Chikaraishi DM (2003) Catecholamines act via a beta-adrenergic receptor to maintain fetal heart rate and survival. Am J Physiol Heart Circ Physiol 284: H2069–H2077 [DOI] [PubMed] [Google Scholar]
- Proenza C, Yellen G (2006) Distinct populations of HCN pacemaker channels produce voltage-dependent and voltage-independent currents. J Gen Physiol 127: 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout WM III, Wakayama T, Wutz A, Eggan K, Jackson-Grusby L, Dausman J, Yanagimachi R, Jaenisch R (2000) Generation of mice from wild-type and targeted ES cells by nuclear cloning. Nat Genet 24: 109–110 [DOI] [PubMed] [Google Scholar]
- Robinson RB, Brink PR, Cohen IS, Rosen MR (2006) If and the biological pacemaker. Pharmacol Res 53: 407–415 [DOI] [PubMed] [Google Scholar]
- Scholten A (2001) Charakterisierung von hyperpolarisations-aktivierten und zyklisch Nukleotid-gesteuerten Ionenkanälen (HCN-Kanäle) in der Retina und im Gehirn der Ratte. Thesis/dissertation, Universität Köln, Fatultät Math.Wat., Köln
- Schulze-Bahr E, Neu A, Friedrich P, Kaupp UB, Breithardt G, Pongs O, Isbrandt D (2003) Pacemaker channel dysfunction in a patient with sinus node disease. J Clin Invest 111: 1537–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K (1995) A Cre-transgenic mouse strain for the ubiquitous deletion of IoxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res 23: 5080–5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa Y, Satoh H, Noma A (2000) The sustained inward current and inward rectifier K+ current in pacemaker cells dissociated from rat sinoatrial node. J Physiol 523: 593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieber J, Herrmann S, Feil S, Löster J, Feil R, Biel M, Hofmann F, Ludwig A (2003) The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci USA 100: 15235–15240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Kiehn J, Katus HA, Karle CA (2003) Defective protein trafficking in hERG-associated hereditary long QT syndrome (LQT2): molecular mechanisms and restoration of intracellular protein processing. Cardiovasc Res 60: 235–241 [DOI] [PubMed] [Google Scholar]
- Verheijck EE, van Ginneken AC, Wilders R, Bouman LN (1999) Contribution of L-type Ca2+ current to electrical activity in sinoatrial nodal myocytes of rabbits. Am J Physiol 276: H1064–H1077 [DOI] [PubMed] [Google Scholar]
- Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou YY, Xiao RP, Cheng H, Stern MD, Maltsev VA, Lakatta EG (2006) High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res 98: 505–514 [DOI] [PubMed] [Google Scholar]
- Wachten S, Schlenstedt J, Gauss R, Baumann A (2006) Molecular identification and functional characterization of an adenylyl cyclase from the honeybee. J Neurochem 96: 1580–1590 [DOI] [PubMed] [Google Scholar]
- Zagotta WN, Olivier NB, Black KD, Young EC, Olson R, Gouaux E (2003) Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature 425: 200–205 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu Y, Song H, Rodriguez J, Tuteja D, Namkung Y, Shin HS, Chiamvimonvat N (2002) Functional roles of Ca(v)1.3 (alpha(1D)) calcium channel in sinoatrial nodes: insight gained using gene-targeted null mutant mice. Circ Res 90: 981–987 [DOI] [PubMed] [Google Scholar]
- Zolles G, Klöcker N, Wenzel D, Weisser-Thomas J, Fleischmann BK, Roeper J, Fakler B (2006) Pacemaking by HCN channels requires interaction with phosphoinositides. Neuron 52: 1027–1036 [DOI] [PubMed] [Google Scholar]