Abstract
Nuclear factor kappa B (NF-κB) is a key mediator of inflammation. Unchecked NF-κB signalling can engender autoimmune pathologies and cancers. Here, we show that Tax1-binding protein 1 (TAX1BP1) is a negative regulator of TNF-α- and IL-1β-induced NF-κB activation and that binding to mono- and polyubiquitin by a ubiquitin-binding Zn finger domain in TAX1BP1 is needed for TRAF6 association and NF-κB inhibition. Mice genetically knocked out for TAX1BP1 are born normal, but develop age-dependent inflammatory cardiac valvulitis, die prematurely, and are hypersensitive to low doses of TNF-α and IL-1β. TAX1BP1−/− cells are more highly activated for NF-κB than control cells when stimulated with TNF-α or IL-1β. Mechanistically, TAX1BP1 acts in NF-κB signalling as an essential adaptor between A20 and its targets.
Keywords: A20, NF-κB, Tax, TAX1BP1, TRAF6
Introduction
Chronic inflammation is frequently mediated via the nuclear factor kappa B (NF-κB) pathway (Beg et al, 1995; Schulze-Osthoff et al, 1997; Karin and Ben-Neriah, 2000; Greten, 2004; Karin, 2006). The NF-κB family encompasses five members, NF-κB1 (p105/p50), NF-κB2 (p100/p52), RelA (p65), RelB, and cRel (Pomerantz and Baltimore, 2002), which are sequestered in the cytoplasm as inactive complexes with inhibitors of kappa B (IκB) proteins (Pomerantz and Baltimore, 2002). Cytoplasmic NF-κB complexes can be activated by a variety of stimuli, including viral and bacterial pathogens, cytokines, and stress-inducing agents (Karin and Lin, 2002; Pomerantz and Baltimore, 2002). Recent studies have characterized ubiquitination as a mechanism for NF-κB activation (Chen, 2005; Haglund and Dikic, 2005). Hence, TNF-α and IL-1β stimulation of cells is followed by K63-linked polyubiquitination of TRAF2 (Shi and Kehrl, 2003), RIP1 (Legler et al, 2003; Lee et al, 2004), and TRAF6 (Wang, 2001; Dunne and O'Neill, 2003). K63 ubiquitination does not trigger proteasome-dependent degradation, but rather creates a docking site on TRAF/RIP1 for downstream NF-κB signalling proteins (Wullaert et al, 2006) (Supplementary Figure 1). Conversely, de-ubiquitination of signalling proteins serves a negative regulatory mechanism. Thus, de-ubiquitinases A20 and CYLD have been reported to de-ubiquitinate specific substrates, including TRAF6 and RIP1 (Boone, 2004; Evans et al, 2004; Jono et al, 2004; Wertz et al, 2004b), to downmodulate signalling events.
Tax1-binding protein 1 (TAX1BP1; also known as TXBP151 or T6BP) was originally identified as a molecule that binds the human T-cell leukaemia virus type 1 Tax oncoprotein (Chin et al, 2007). Tax is a potent NF-κB activator, and it efficiently immortalizes mammalian peripheral blood lymphocytes ex vivo (Yoshida, 2001; Matsuoka, 2005; Matsuoka and Jeang, 2007). Previous studies have shown that TAX1BP1 binds the NF-κB inhibitory protein A20 (De Valck et al, 1999), and in an IL-1β-specific manner the NF-κB signalling protein TRAF6 (Ling and Goeddel, 2000). These findings suggest that TAX1BP1 plays a regulatory role in NF-κB activation.
To understand better the physiological function of TAX1BP1, we created TAX1BP1 knockout (KO) mice. TAX1BP1-deficient mice are born developmentally normal; however, TAX1BP1−/− animals develop age-dependent inflammatory cardiac valvulitis and skin dermatitis, die prematurely, and are hypersensitive to low doses of TNF-α and IL-1β. Cultured TAX1BP1−/− cells exposed to TNF-α or IL-1β activate NF-κB more robustly than TAX1BP1+/+ cells. Furthermore, TAX1BP1 inhibits RIP1 and TRAF6 polyubiquitination and recruits the ubiquitin-editing protein A20 to these molecules. Finally, we characterized a novel ubiquitin-binding domain in TAX1BP1, which is needed for the interaction of TAX1BP1 with TRAF6. Our findings support TAX1BP1 as an essential adaptor for linking A20 to TRAF6 and other factors in order to influence NF-κB activation.
Results
Characterization of TAX1BP1-deficient mice
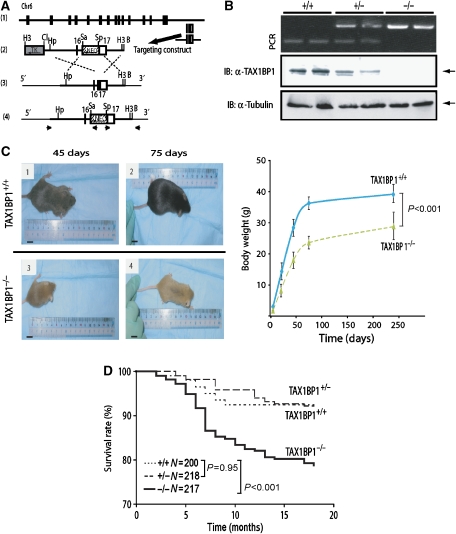
TAX1BP1-deficient (TAX1BP1−/−) mice were created by gene targeting (Figure 1A). Exon 17 of the Tax1bp1 gene in embryonic stem (ES) cells was removed, and this was confirmed by PCR (Figure 1B). Chimaeric mice from blastocyst injection and germline transmission of the mutated Tax1bp1 (KO) allele were established. The Tax1bp1 KO allele segregated in a Mendelian fashion (data not shown). Using TAX1BP1-specific antiserum in western blotting, we confirmed the expression of the 86-kDa TAX1BP1 protein in TAX1BP1+/+ and TAX1BP1+/−, but not in TAX1BP1−/−, mice (Figure 1B, middle panel).
Figure 1.
Generation of TAX1BP1−/− mice. (A) Targeted disruption of the mouse TAX1BP1 gene. The targeting vector pMm151(NEO)KO, the thymidine kinase cassette (TK) located at the 5′ end of the targeting vector (indicated by a shaded box), and the Neo cassette (indicated by a hatched box) are indicated. Arrows show the sense direction for each ORF. Homologous recombination at the TAX1BP1 locus was confirmed by PCR by using specific primers indicated by small arrows. H3: HindIII; Cl: ClaI; Hp: HpaI; Sa: SalI; Sp: SpeI; B: BamHI. (B) TAX1BP1 KO was confirmed by PCR (upper panel) or by blotting using a polyclonal antibody against TAX1BP1 (middle panel). Proteins were normalized using anti-tubulin (lower panel). (C) Left: gross appearance of representative 45- and 75-day TAX1BP1+/+ and TAX1BP1−/− mice. Right: growth curves of TAX1BP1+/+ and TAX1BP1−/− mice. TAX1BP1−/− and WT controls were weighed at 2-week intervals for 75 days and again at 240 days. Ten animals were used per group. Data represent mean weight±s.e. (P<0.0001). (D) Death of mice over an 18 months follow-up. The Kaplan–Meier curves show that TAX1BP1−/− mice have lower survival than WT or TAX1BP1+/− mice (log-rank P<0.001).
TAX1BP1+/+, TAX1BP1+/−, and TAX1BP1−/− mice showed no overt anomalies at birth. However, TAX1BP1−/− mice failed to thrive and grew noticeably smaller than their wild-type (WT) siblings (Figure 1C). By 18 months, a statistically significant difference in survival emerged between TAX1BP1−/− (KO) and WT littermates (Figure 1D). At necropsy, all expired TAX1BP1−/− mice had hypertrophic cardiac valvulitis with dramatically thickened valve leaflets (Figure 2A). These tissues were infiltrated with macrophages, neutrophils, and T cells (Figure 2B). Because valvulitis was not found in deliberately killed new born and young (21 days) TAX1BP1−/− mice (Figure 2A, left panels), this phenotype appears not to be a developmental pathology, but a consequence of age-dependent inflammatory changes. Consistent with an inflammation model, adult TAX1BP1−/− mice, compared to WT siblings, also had increased prevalence of treatment-refractory skin inflammation (Figure 2C). The inflamed skin tissues were infiltrated similarly with T cells (positive anti-CD3 staining), neutrophils (positive anti-myeloperoxidase staining), and macrophages (positive anti-F4/80 staining) (Figure 2C).
Figure 2.
TAX1BP1−/− mice show severe cardiac valvulitis and skin dermatitis with inflammatory cellular infiltrates. (A) Valvulitis in haematoxylin and eosin (H&E)-stained valves (Va) from 21-day, 6-month-, and 12-month-old TAX1BP1−/− mice. In panels 5 and 6, valve leaflets from TAX1BP1−/− mice are grossly hypertrophic with cellular infiltration and collagen invasion. (B) Immunostaining of valve leaflets from TAX1BP1−/− mice using anti-F4/80 (F4/80 is a macrophage-restricted cell surface glycoprotein) and anti-CD3, revealing the presence of macrophages (panel 1) and T cells (panel 2) (arrowheads). H&E (panel 3) and anti-myeloperoxidase (panel 4) stainings show mononuclear cells, neutrophils (arrowheads), and cellular debris near small capillary (Ca); magnification × 400. Arteritis and periarteritis in TAX1BP1−/− mice are shown. The lower magnification (panel 5) shows heart blood vessel (Ve) with surrounding infiltration of mononuclear cells and neutrophils; magnification × 200. The enlarged panel 6 shows severe inflammation characterized by the infiltration of mononuclear cells and neutrophils near a small capillary; magnification × 400. (C) Skin inflammation in TAX1BP1 KO mice. Inflammation observed in H&E-stained skin from TAX1BP1−/− mice (panels 1 and 2). Sections of TAX1BP1 KO skin were stained with H&E (panel 3) and for T cells (CD3) (panel 4), macrophages (F4/80) (panel 5), and neutrophils (myeloperoxidase) (panel 6); magnification × 100. Enlargement of panels 4–6 shows the presence of T cells (anti-CD3 staining), neutrophils (anti-myeloperoxidase staining), and macrophages (anti-F4/80 staining); magnification × 400.
Hypersensitivity of TAX1BP1−/− mice to TNF-α and IL-1β
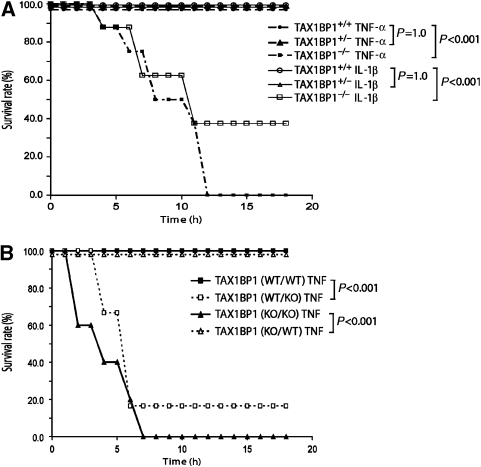
To better understand in vivo physiology, we next challenged TAX1BP1−/− mice with low doses of IL-1β or TNF-α. Provocatively, 8 out of 14 TAX1BP1−/− mice injected intraperitoneally with 0.5 mg IL-1β per kg body weight and 14 out of 14 TAX1BP1−/− mice injected with 0.5 mg TNF-α per kg body weight expired within 12 h (Figure 3A). By contrast, none of the 28 TAX1BP1+/+ or the 28 TAX1BP1+/− mice responded untowardly to IL-1β or TNF-α injections (Figure 3A).
Figure 3.
Increased hypersensitivity of TAX1BP1−/− mice to inflammatory challenges. (A) Kaplan–Meier curves of TAX1BP1 mice after sublethal TNF-α or IL-1β challenges. TAX1BP1−/− mice succumbed to low doses of TNF-α and IL-1β. Twelve-week-old TAX1BP1+/+, TAX1BP1+/−, and TAX1BP1−/− littermates were challenged with proinflammatory cytokine TNF-α (0.5 mg/kg) (TAX1BP1+/+, closed circle; TAX1BP1+/−, closed triangle; TAX1BP1−/−, closed square) or IL-1β (0.5 mg/kg) (TAX1BP1+/+, open circle; TAX1BP1+/−, open triangle; TAX1BP1−/−, open square). Survival was monitored every 2 h, and the experiment was terminated after 24 h. The number of mice in each experimental group was n=14. No control mice (+PBS n=14) died (data not shown); the experiments were performed twice. (B) Kaplan–Meier curves of TAX1BP1 bone marrow chimaera mice after TNF-α challenge. Five- to 6-week-old female TAX1BP1−/− or TAX1BP1+/+ recipient mice were first sublethally irradiated and then transplanted with the indicated donor bone marrow. Eight weeks after transplantation, bone marrow chimaera mice were challenged with TNF-α (0.5 mg/kg body weight, intraperitoneal injection). Survival was monitored every 2 h and the experiment was terminated after 24 h. The number of mice in each experimental group was n=12; the experiments were performed twice. Statistical analysis was performed using a log-rank test (P<0.001).
To assess if the observed ‘hypersensitivity' was haematologically mediated, we created chimaeric mice whereby TAX1BP1-deficient animals were first subjected to total body irradiation with 1100 rad and then transplanted with either WT (KO/WT) or TAX1BP1−/− (KO/KO) bone marrow. As controls, we irradiated WT mice and reconstituted them with either WT (WT/WT) or TAX1BP1−/− (WT/KO) marrow. We then examined the TNF-α responses of these chimaeric mice. All 24 irradiated TAX1BP1−/− mice became ‘protected' after transplantation with WT marrow. Hence, irradiated TAX1BP1−/− mice with WT marrow (KO/WT) (Figure 3B, open triangle) had TNF-α morbidity indistinguishable from that of WT/WT control mice (Figure 3B, closed square). However, irradiated WT mice reconstituted with TAX1BP1−/− marrow (WT/KO) acquired TNF-α mortality rates (Figure 3B, open square) similar to those of irradiated TAX1BP1−/− mice with TAX1BP1−/− marrow (KO/KO; Figure 3B, filled triangle). Thus, TNF-α induced hyperinflammation tracks with donor haematopoietic cells irrespective of the genotype of the recipient.
TAX1BP1 regulates NF-κB but not JNK
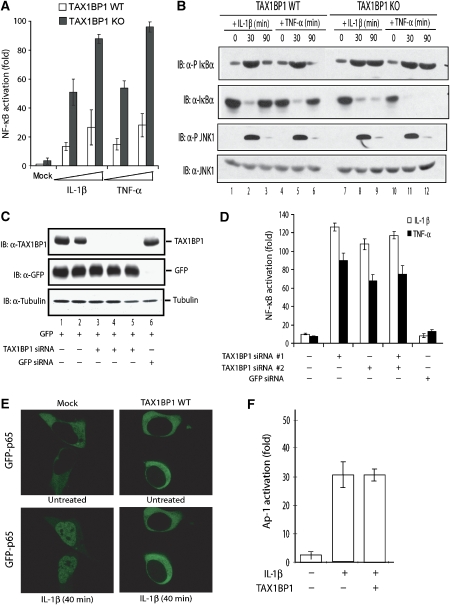
In previous studies, TAX1BP1 bound A20 (De Valck et al, 1999) and TRAF6 (Ling and Goeddel, 2000), indicating a role in TRAF6–NF-κB signalling. The above hypersensitivity findings in TAX1BP1−/− mice could be explained by TAX1BP1 negatively regulating TNF-α or IL-1β activation of NF-κB. This explanation predicts that TNF-α and IL-1β would elicit higher NF-κB activity in TAX1BP1−/− than WT MEFs. Indeed, when tested, an NF-κB reporter was expressed ∼4-fold higher in IL-1β- or TNF-α-treated TAX1BP1−/− than WT MEFs (Figure 4A). To confirm this finding, we also assessed NF-κB activity in peritoneal macrophages isolated from WT and KO mice. Here, induction of IκBα phosphorylation and its subsequent degradation were used as markers of NF-κB activation. Consistently, KO (versus WT) macrophages treated with IL-1β or TNF-α showed significantly enhanced IκBα phosphorylation and degradation (Figure 4B, top two rows).
Figure 4.
TAX1BP1 inhibits TNF-α- and IL-1β-triggered NF-κB activation. (A) TAX1BP1 KO MEFs show higher NF-κB responses to TNF-α and IL-1β than WT MEFs. MEFs were transfected with an NF-κB-luc reporter and an RSV-β-gal plasmid as an internal control. At 16 h after transfection, increasing concentrations of IL-1β (10–50 ng/ml) and TNF-α (10–50 ng/ml) were added for 8 h and the cells were harvested for luciferase assay. (B) Macrophages from TAX1BP1 KO mice show a higher NF-κB response to TNF-α and IL-1β than WT macrophages. Peritoneal macrophages from WT and KO mice were stimulated for 30 and 90 min at 37°C with IL-1β (100 ng/ml) and TNF-α (100 ng/ml) and cell lysates were examined by immunoblotting (IB) using anti-phospho (P) Ser 32/36 IκBα, anti-IκBα, anti-phospho (P) T183 JNK1, or anti-JNK1, as indicated. (C) 293T cells were co-transfected with 2 μg TAX1BP1-specific siRNA (lanes 3–5) or control GFP siRNA (lane 6) in a DNA transfection mix of 100 ng of GFP plasmid, 50 ng of NF-κB-luc, and 50 ng of RSV-β-gal and adjusted to equal concentrations with pCDNA3. TAX1BP1, GFP, and tubulin expressions were verified by IB. (D) To analyse NF-κB activation, cells transfected with or without the indicated siRNA were treated with either IL-1β (50 ng/ml) or TNF-α (50 ng/ml) and NF-κB-luciferase reporter expression was assayed 8 h later. (E) TAX1BP1 blocks IL-1-triggered nuclear translocation of GFP-p65. 293-IL1R cells were transfected with 250 ng of GFP-p65 and then with either 500 ng of control pcDNA3 (mock) or TAX1BP1 (TAX1BP1 WT). At 24 h after transfection, cells were treated with IL-1β (100 ng/ml) for 40 min. Green fluorescence (GFP-p65) was monitored for 40 min with a Leica laser-scanning microscope. The top row shows untreated cells at the beginning; the bottom row shows cells after 40 min of IL-1β treatment. (F) TAX1BP1 has no effect on IL-1β-induced AP-1 activation. 293T cells were transfected with 1.6 μg of total plasmid DNA containing a mixture of AP-1-luciferase reporter and RSV-galactosidase plasmid, without (−) or with (+) TAX1BP1 plasmid. At 40 h after transfection, cells were treated with IL-1β (10 ng/ml) for 8 h. Cells were then harvested for luciferase assay.
Next, to extend mouse findings to human cells, we performed siRNA knockdown of cell endogenous TAX1BP1 (Figure 4C) and overexpression of transfected TAX1BP1 in 293-IL1R cells (Cao et al, 1996) (Figure 4E). Cells transfected with TAX1BP1-specific siRNA or GFP-specific siRNA (as control; Figure 4C) and an NF-κB-dependent luciferase reporter were stimulated with either IL-1β (50 ng/ml) or TNF-α (50 ng/ml). When compared to control (GFP) siRNA transfection, the three TAX1BP1-siRNA knockdowns each resulted in >20-fold increases in IL-1β- or TNF-α-induced NF-κB-dependent luciferase activity (Figure 4D). Conversely, when transfected TAX1BP1 was overexpressed, IL-1β-induced translocation of NF-κB p65/RelA into the nucleus was inhibited (Figure 4E). Altogether, the results support a pivotal TAX1BP1 function in moderating IL-1β or TNF-α activation of NF-κB.
To ask if TAX1BP1 is specific for NF-κB, we also checked for JNK activation. KO and WT peritoneal macrophages treated with IL-1β or TNF-α were assessed for JNK status using a phospho-JNK-specific antibody (Figure 4B, bottom two rows). Interestingly, IL-1β- or TNF-α-treated TAX1BP1−/− and WT macrophages showed neither enhanced nor persistent JNK phosphorylation (Figure 4B, bottom two rows). Moreover, because JNK activation increases AP-1 activity (Derijard et al, 1994), we employed an AP-1-dependent luciferase assay to check the role of TAX1BP1 role in JNK signalling. Overexpression of TAX1BP1 failed to influence IL-1β activation of an AP-1-dependent reporter (Figure 4F). Collectively, these results exclude a role for TAX1BP1 in JNK regulation, supporting its specificity for NF-κB signalling.
Enhanced in vivo NF-κB activation in TAX1BP1−/− mice
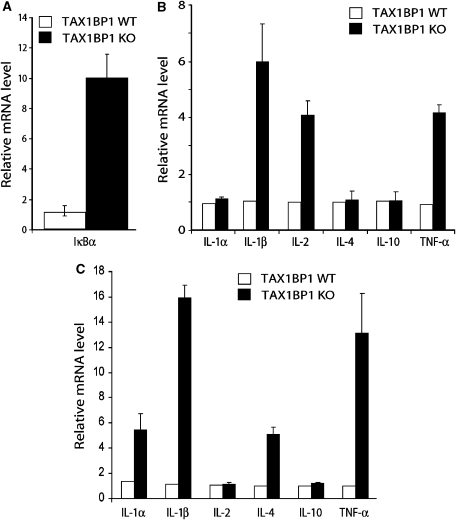
To correlate the results from cultured cells (Figure 4) with the valvulitis and dermatitis in animals (Figure 2), we re-analysed heart valve tissues from WT and KO mice probing for evidence of in vivo NF-κB activation. Using IκBα mRNA induction as a measure of NF-κB activation, this mRNA was found to be increased in KO compared to WT heart valves (Figure 5A). KO (versus WT) heart tissue was markedly elevated in proinflammatory cytokines IL-1β, IL-2, and TNF-α, but not IL-1α, IL-4, or IL-10 (Figure 5B). Inflamed KO skin tissue was similarly increased for several of these cytokines (Figure 5C). These results are consistent with in vivo NF-κB activation in TAX1BP1−/− cells enhancing proinflammatory cytokine expression.
Figure 5.
Increased expression of proinflammatory cytokines TNF-α and IL-1β in TAX1BP1−/− tissue. (A) Real-time PCR analysis of IκBα mRNA expression in heart tissue isolated from four control and five TAX1BP1 KO mice. (B) Real-time PCR analysis of proinflammatory cytokines in heart tissue isolated from four control and five TAX1BP1 KO mice. (C) Real-time PCR analysis of proinflammatory cytokines in skin isolated from four control and five KO mice. All the results are the mean values relative to GADPH; error bars indicate SE of triplicate samples.
The ubiquitin-binding domain of TAX1BP1 serves a critical regulatory role
TAX1BP1 has three coiled-coil regions (amino acids 132–604) and two C-terminal zinc (Zn) fingers (Figure 6A). To investigate its NF-κB modulatory mechanism, we constructed a series of TAX1BP1 deletion mutants (Supplementary Figure 2A) seeking to identify the protein region important for regulating activation of NF-κB. In this analysis, full-length TAX1BP1 and TAX1BP1 mutants that preserved the C-terminal half of the protein retained function, while mutants in which the C-terminal Zn fingers of TAX1BP1 were removed did not (Supplementary Figure 2B). Co-immunoprecipitations indicated that carboxyl amino acids 388–747 of TAX1BP1, which include the two Zn fingers, were needed for TAX1BP1 to associate with TRAF6 (Supplementary Figure 2C).
Figure 6.
The second Zn finger of TAX1BP1 contains a novel ubiquitin-binding domain. (A) Schematic representations of TAX1BP1 WT, which contains three coiled-coil (CC) and two ubiquitin-binding domains (open rectangles), and four TAX1BP1 ubiquitin-binding domain mutants (UBZ1 mutant: F737A; UBZ2 mutant: F764A; UBZ1+2 mutant: F737A+F764A; UBZ* mutant: C757A+C760A), which are mutated (X) in the indicated first (UBZ1) or second (UBZ2) Zn finger(s). (B) Binding of TAX1BP1 to ubiquitin. HA-TAX1BP1 was expressed in 293T cells and cell lysates were prepared 40 h later. Total cell lysates were incubated for 8 h at 4°C with either GST-ubiquitin (Ub) (lane 3) or GST-ubiquitin Ile44 mutant (UBI44A) (lane 4) immobilized on glutathione Sepharose. The binding of TAX1BP1 was visualized by immunoblotting (IB) with anti-HA. (C) Mutation of the UBZ2 domain disrupts the ubiquitin-binding potential of TAX1BP1. FLAG-TAX1BP1, FLAG-TAX1BP1 UBZ1, FLAG-TAX1BP1 UBZ2, or FLAG-TAX1BP1 UBZ* was transfected into 293T cells. Binding to immobilized GST (cont) or GST-Ub (Ub) was analysed by IB with anti-FLAG. (D) The UBZ2 domain is sufficient to bind K48- and K63-linked ubiquitin. Purified polyubiquitin chains (K48- or K63-linked) were incubated with immobilized intact individual UBZ domains fused to GST (GST-UBZ2, GST-UBZ1) or both UBZ domains fused to GST (GST-UBZ1+2) for 2 h at 4°C. Binding of polyubiquitin chains to GST fusion proteins was visualized by IB using anti-Ub. Ponseau S stained the GST fusion proteins used in the binding assays.
The above results attribute functional importance to the C-terminal half of TAX1BP1, which sequence analyses indicate contains ubiquitin-interacting motifs. Indeed, in yeast two-hybrid experiments, we repeatedly identified the C terminus of TAX1BP1 to bind WT ubiquitin, but not ubiquitin in which Ile44 in the hydrophobic patch was mutated to alanine (data not shown). In pulldowns, we confirmed that TAX1BP1 bound GST-ubiquitin, but not GST-ubiquitin I44A (Figure 6B). To characterize the two Zn fingers in TAX1BP1 for ubiquitin binding, we individually mutated two conserved Phe residues to Ala respectively in each Zn finger (UBZ1: F737A; UBZ2: F764A) separately (UBZ1 and UBZ2; Figure 6A) or together (UBZ1+2; Figure 6A). The binding of TAX1BP1 to GST-ubiquitin was more severely abolished by mutation in the second than in the first Zn finger (Figure 6C, compare lanes 2, 5, and 8). Bioinformatics analyses show that UBZ2 belongs to the ubiquitin-binding Zn finger (UBZ) family, originally identified in the translesion synthesis polymerase η (Bienko et al, 2005). Accordingly, fusion of the UBZ2 domain or UBZ1+UBZ2 domains to GST (Figure 6D, lanes 3, 5, 8, and 10), but not UBZ1 domain to GST (Figure 6D, lanes 4 and 9) bound avidly K63-linked and K48-linked polyubiquitin chains. To further confirm the importance of UBZ2 in ubiquitin binding, we constructed another UBZ2 mutant in which both cysteine 757 and 760 were changed to alanines (UBZ*; Figure 6A). Similar to TAX1BP1 UBZ2, TAX1BP1 UBZ* did not bind GST-ubiquitin (Figure 6C, lane 11).
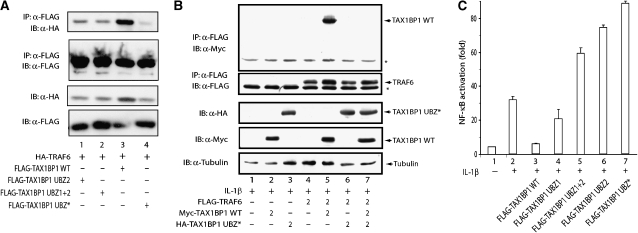
Because TAX1BP1 was shown to bind TRAF6, which is ubiquitinated in response to overexpression or IL-1β stimulation, we checked WT TAX1BP1 and TAX1BP1 UBZ* to co-immunoprecipitate with TRAF6. WT TAX1BP1 co-immunoprecipitated, but TAX1BP1 UBZ* did not, with TRAF6 (Figure 7A). Moreover, TAX1BP1 UBZ* overexpression prevented the physical association between WT TAX1BP1 and TRAF6 (Figure 7B, compare lanes 5 and 7), demonstrating an important role for the UBZ2 domain in TRAF6–TAX1BP1 binding. Next, we compared the ability of overexpressed TAX1BP1 and its several UBZ mutants to inhibit IL-1β activation of NF-κB. While TAX1BP1 overexpression inhibited IL-1β-induced NF-κB activation (Figure 7C, lane 3), TAX1BP1 proteins mutated in the UBZ2 domain actually enhanced IL-1β signal (Figure 7C, lanes 5–7). This latter finding is consistent with a dominant-negative effect exerted by TAX1BP1 UBZ2 domain mutants on TAX1BP1–TRAF6 binding (Figure 7B; data not shown). Collectively, these data point to TRAF6 as an important point of interference for TAX1BP1 in the IL-1β signalling pathway.
Figure 7.
TAX1BP1's ubiquitin-binding domain is required for inhibition of IL-1β triggered NF-κB activation. (A) UBZ2 mutation disrupts the binding of TAX1BP1 to TRAF6. 293T cells were co-transfected with HA-tagged TRAF6 and the indicated FLAG-tagged TAX1BP1 UBZ mutants. 24 h after transfection, cell lysates were subjected to immunoprecipitation (IP) with 1 μg of mouse monoclonal anti-FLAG antibody (α-FLAG), followed by immunoblotting (IB) with mouse monoclonal anti-FLAG antibody coupled to HRP (α-FLAG-HRP), or mouse monoclonal anti-HA (α-HA). Transfection efficiency was verified by IB with anti-FLAG-HRP and anti-HA. (B) TAX1BP1 UBZ* exerts a dominant-negative effect on TAX1BP1–TRAF6 binding. Cells were transfected with FLAG-TRAF6 and/or Myc-TAX1BP1 or HA-TAX1BP1 UBZ*. After 48 h, cell lysates were immunoprecipitated with anti-FLAG, followed by IB with anti-Myc or anti-FLAG (upper panels). Protein amounts were verified by IB with anti-tubulin (lower panel). * indicates a background band. (C) The UBZ2 domain is essential for TAX1BP1's NF-κB inhibitory potential. 293T cells were transfected with 2 μg of total plasmid DNA containing a mixture of NF-κB luciferase reporter with pActβ-galactosidase plasmid and the indicated WT or TAX1BP1 UBZ mutant plasmids. After 24 h, cells were stimulated for 6 h with 20 ng/ml IL-1β and assayed for luciferase. Values shown are normalized against β-galactosidase activity.
Interaction of TAX1BP1 with A20 reduces TRAF6 ubiquitination
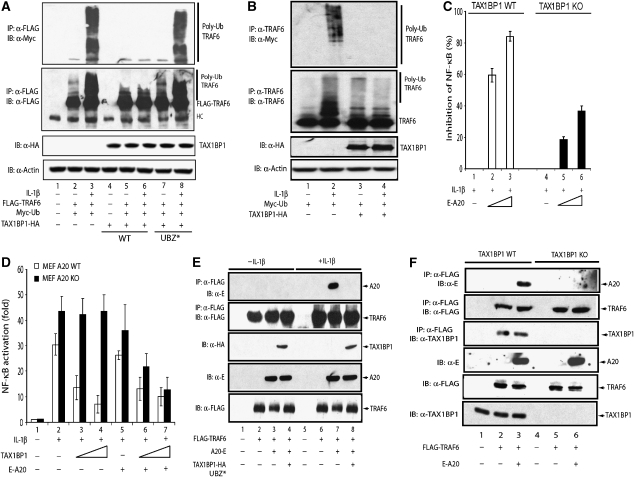
IL-1β activates NF-κB through K63 polyubiquitination of TRAF6 (Chen, 2005). Our above observations point to TRAF6 as a target for TAX1BP1. We next asked whether the inhibitory effect of TAX1BP1 on IL-1β activation of NF-κB resulted from an effect on TRAF6 polyubiquitination. 293 cells were transfected with FLAG-TRAF6, Myc-Ub, and TAX1BP1-HA, and the ubiquitination status of TRAF6 was queried by first immunoprecipitating TRAF6 followed by immunoblotting with anti-Myc (Figure 8A). TRAF6 was richly polyubiquitinated in IL-1β-treated cells; however, coexpression of TAX1BP1-HA strongly reduced TRAF6 polyubiquitination (Figure 8A, compare lanes 3 and 6; Figure 8B, compare lanes 2 and 4). In contrast, the ubiquitination status of TRAF6 was not affected by coexpression of the TAX1BP1 UBZ* mutant (Figure 8A, compare lanes 6–8), which was not capable of binding TRAF6. Thus, TAX1BP1 expression decreases TRAF6 polyubiquitination, which could be due to enhanced de-ubiquitination or by an interference with the growth of the ubiquitin chains on TRAF6.
Figure 8.
Expression of TAX1BP1 reduces ubiquitination of TRAF6. (A) TAX1BP1 overexpression inhibits ubiquitination of ectopically expressed TRAF6. 293T cells were transfected with FLAG-TRAF6, HA-TAX1BP1 (WT; lanes 4–6), or HA-TAX1BP1 UBZ* (UBZ*; lanes 7 and 8) with Myc-Ub. At 40 h after transfection, cells were stimulated with IL-1β for 8 h. A 300 μg portion of cell lysate was subjected to immunoprecipitation (IP) with 40 μl of anti-FLAG-conjugated agarose beads, followed by immunoblotting (IB) with either rabbit polyclonal anti-Myc (top panel) or rabbit polyclonal anti-FLAG (second panel). HA-TAX1BP1 in the cell lysates was assessed by IB using anti-HA, and protein loadings were normalized by IB with anti-actin (lower panels). (B) TAX1BP1 overexpression inhibits IL-1β-induced ubiquitination of cell endogenous TRAF6. 293T cells were transfected with Myc-Ub (lanes 1 and 2) or co-transfected with HA-TAX1BP1 (WT) (lanes 3 and 4) and Myc-Ub. At 40 h after transfection, cells were stimulated as indicated with IL-1β (50 ng/ml) for 8 h (lanes 2 and 4). A 300 μg portion of cell lysate was subjected to IP with 40 μl of anti-TRAF6-conjugated agarose beads, followed by IB with either rabbit polyclonal anti-TRAF6 (middle panel) or rabbit polyclonal anti-Myc (upper panel). HA-TAX1BP1 in the cell lysates was assessed by IB using anti-HA, and protein loadings were normalized by IB with anti-actin (lower panel). (C) Reduced ability of A20 to moderate IL-1β's induction of NF-κB in TAX1BP1−/− MEF cells. WT and TAX1BP1−/− MEFs were transfected with NF-κB-luc and RSV-β-gal, with or without increasing amounts of A20 plasmid. At 40 h after transfection, cells were treated with IL-1β (50 ng/ml) for 8 h and then harvested for luciferase analysis. (D) TAX1BP1 fails to moderate IL-1β's induction of NF-κB in A20−/− MEFs. WT and A20−/− MEFs were transfected with NF-κB-luc and RSV-β-gal, with or without increasing amounts of TAX1BP1 or A20 plasmids. At 40 h after transfection, cells were treated with IL-1β (50 ng/ml) for 8 h and then harvested for luciferase analysis. (E) IL-1β induces the binding of A20 to TRAF6, which is abolished by overexpression of TAX1BP1 UBZ*. 293T cells were transfected with FLAG-TRAF6, E-tagged A20, and HA-tagged TAX1BP1 UBZ*, as indicated. Cells were stimulated with IL-1β (50 ng/ml) for 8 h. Binding of TRAF6 to A20 was analysed by co-immunoprecipitation. Total expression of FLAG-TRAF6, HA-TAX1BP1 UBZ*, and E-A20 in the cell lysate was assessed by IB using anti-FLAG, anti-HA, and anti-E. (F) TRAF6 and A20 do not bind each other in TAX1BP1 KO cells. TAX1BP1+/+ and TAX1BP1−/− MEFs were transfected with E-A20 and FLAG-TRAF6. Binding of A20 to TRAF6 was analysed as described in (E).
We and others have reported that TAX1BP1 binds A20 (Heyninck et al, 1999). A20 can moderate NF-κB activity by de-ubiquitinating TRAF6 (Boone, 2004) and other signalling molecules (Wertz, 2004; Heyninck and Beyaert, 2005). The above results suggest a role for TAX1BP1 as an adaptor to bridge A20 and TRAF6, resulting in reduced ubiquitination of TRAF6 (Supplementary Figure 1). This interpretation predicts that the ability of A20 to inhibit IL-1β-induced NF-κB activity would be attenuated in TAX1BP1-deficient cells. Indeed, A20 inhibited NF-κB activity three-fold less well in TAX1BP1−/− than TAX1BP1+/+ MEFs (Figure 8C). Moreover, overexpression of TAX1BP1 failed to inhibit IL-1β-induced NF-κB activity in A20−/− MEFs (A20 KO; Figure 8D).
An A20–TAX1BP1–TRAF6 bridging model offers a second prediction: expression of the dominant-negative TAX1BP1 UBZ* mutant should reduce A20–TRAF6 association in IL-1β-treated cells. We verified the binding between A20 and TRAF6 in IL-1β-treated cells and found that TAX1BP1 UBZ* overexpression did abolish this IL-1β-induced A20+TRAF6 complex (Figure 8E, compare lanes 7 and 8). In contrast, overexpression of WT TAX1BP1 promoted the interaction between TRAF6 and A20 (Supplementary Figure 3A). Consistent with the above observations and the adaptor role of TAX1BP1 in A20–TRAF6 binding, co-immunoprecipitation of A20 and TRAF6 was compromised in TAX1BP1−/− compared to WT MEFs (Figure 8F, compare lanes 3 and 6). We have already shown that the UBZ2 domain is important for TRAF6 binding. Comparison of the ability of WT TAX1BP1 and different UBZ2 mutants to co-immunoprecipitate with A20 revealed that UBZ2 also mediates the interaction of TAX1BP1 with A20 (Supplementary Figure 3B).
TAX1BP1 promotes A20-dependent reduction of RIP1 ubiquitination
Our TAX1BP1−/− mouse data also implicated a role for TAX1BP1 in TNF-α signalling. TAX1BP1 does not interact with TRAF2 (Ling and Goeddel, 2000), but possibly, TAX1BP1 could affect TNF-α-induced NF-κB signalling by influencing RIP1 ubiquitination. To test this hypothesis, we first established an association between TAX1BP1 and RIP1 by co-immunoprecipitation (Figure 9A). We next queried for the effect of overexpressed TAX1BP1 on RIP1 ubiquitination. RIP1 was polyubiquitinated in response to TNF-α treatment (Figure 9B, lane 4); however, polyubiquitination of RIP1 was significantly decreased when TAX1BP1-HA was overexpressed (Figure 9B, lane 6).
Figure 9.
TAX1BP1 binds RIP1 and inhibits TNF-α-induced ubiquitination of RIP1. (A) TNF-α induces the binding of TAX1BP1 to RIP1. 293T cells were transfected with FLAG-RIP1, HA-TAX1BP1, and E-TRAF2. At 40 h after transfection, cells were stimulated with TNF-α for 8 h. Total expression of transfected proteins was assessed by immunoblotting (IB) using anti-FLAG, anti-HA, and anti-E respectively. A 300 μg portion of cell lysate was subjected to immunoprecipitation (IP) with 40 μl of anti-HA-conjugated agarose beads, followed by IB with rabbit polyclonal anti-FLAG, anti-HA, or anti-E as indicated. * indicates a background band. (B) Overexpression of TAX1BP1 inhibits TNF-α-induced RIP1 ubiquitination. 293T cells were co-transfected with HA-TAX1BP1 and FLAG-RIP1. At 48 h after transfection, cells were stimulated with TNF-α (50 ng/ml) for 30 min (lanes 2, 4, and 6). RIP1 was immunoprecipitated with anti-FLAG. Binding of RIP1 and TAX1BP1 was revealed by IB with anti-HA (middle panel) and RIP1 ubiquitination was revealed by IB with anti-FLAG (top panel). HA-TAX1BP1 in the cell lysates was assessed by IB using anti-HA. (C) TAX1BP1 is needed for A20–RIP1 complex formation. 293T cells were transfected with control or TAX1BP1-siRNA. Cells were stimulated for 30 min at 37°C with TNF-α (50 ng/ml). A 300 μg portion of cell lysates was immunoprecipitated with 40 μl of anti-FLAG-conjugated agarose beads, followed by IB with either rabbit polyclonal anti-FLAG or anti-E. TAX1BP1 in the cell lysates was assessed by IB using anti-TAX1BP1, and protein loadings were normalized by IB with anti-tubulin (lower panel). (D) TAX1BP1 fails to moderate TNF-α-induced NF-κB activation in A20−/− MEFs. WT and A20−/− MEFs were transfected with an NF-κB-luc plasmid and an RSV-β-gal plasmid, with FLAG-RIP1, HA-TAX1BP1, or E-A20 plasmids. At 40 h after transfection, cells were treated with TNF-α (10 ng/ml) for 8 h and then harvested for luciferase assay.
A20 can moderate NF-κB activity through reducing RIP1 ubiquitination (Wertz et al, 2004a). Our finding that TAX1BP1–RIP1 binding correlated with an inhibitory effect of TAX1BP1 on TNF-α-induced RIP1 polyubiquitination further suggests a bridging function for TAX1BP1 and RIP1–A20 association in TNF-α signalling. Indeed, we could show that siRNA knockdown of TAX1BP1 prevented the TNF-α-induced formation of an RIP1–A20 complex (Figure 9C). Moreover, TAX1BP1 overexpression was unable to inhibit RIP1-induced NF-κB activation in A20-deficient MEF cells (Figure 9D). While additional experiments are needed to refine mechanistic details, our current results reveal important and complex roles for TAX1BP1 in regulating the inhibitory effect of A20 on TRAF6- and RIP1-mediated NF-κB signalling.
Discussion
Worldwide, chronic rheumatic heart disease is estimated to affect 5–30 million children and young adults, and 90 000 patients die from this disease each year (WHO, 2004). Valve pathology is the most common finding in inflammatory heart disease (Rullan and Sigal, 2001). To date, approximately four million valve operations have been performed globally on patients of all ages, with about 300 000 procedures performed annually in the United States. As yet, a full understanding of the molecular basis for valvulitis and an animal model for this pathology are wanting.
Here, we report a model of cardiac valvulitis characterized by age-dependent morbid valve disease and intractable skin inflammation in TAX1BP1−/− mice. TAX1BP1−/− mice show inflammation-induced valve changes that resemble the valve disease in human rheumatic fever (Roberts et al, 2001; Rullan and Sigal, 2001) with infiltrations of T cells and macrophages in the cardiac valves and myocardium of TAX1BP1-deficient mice. TAX1BP1−/− mice also display skin inflammation, and skin tissues are infiltrated with T cells, neutrophils, and macrophages.
TAX1BP1 is an 86 kDa protein that was previously shown to bind the NF-κB inhibitory protein A20 (De Valck et al, 1999) and the NF-κB signalling molecule TRAF6 (Ling and Goeddel, 2000). Our current study shows that TAX1BP1 is a novel ubiquitin-binding protein that negatively modulates IL-1β- and TNF-α-induced NF-κB signalling. While signalling through IL-1β and TNF-α is critical for host defences against pathogens (Dinarello, 2004; Kollias, 2005), unchecked signalling can have catastrophic consequences (Liew et al, 2005). Accordingly, a major in vivo consequence of loss of TAX1BP1 function in moderating inflammatory signalling is illustrated by fatal hypersensitivities experienced by TAX1BP1−/− mice when dosed with amounts of TNF-α and IL-1β that are otherwise innocuous to WT mice.
Our current results implicate TAX1BP1 as an adapter that recruits A20 to TRAF6 and/or RIP1. A result of this recruitment is a reduction in polyubiquitinated TRAF6 and RIP1. Polyubiquitination of TRAF6 and RIP1 has been reported to be involved in their interaction with the adaptor proteins NEMO and TAB2 (Kanayama, 2004; Wu et al, 2006), which are essential for downstream NF-κB signalling. Ubiquitin binding to NEMO and TAB2 is facilitated by specific ubiquitin-binding motifs. Similarly, we delineated a novel ubiquitin-binding motif in TAX1BP1 that is essential for its binding to A20 and TRAF6, and its ability to downmodulate TNF-α- and IL-1β-induced NF-κB activation. A20 has previously been shown to negatively regulate NF-κB activation by de-ubiquitinating TRAF6 and RIP1. Our data indicate a role for polyubiquitination of TRAF6 and RIP in the binding of TAX1BP1, which recruits the de-ubiquitinating protein A20. The latter might then de-ubiquitinate TRAF6 and RIP1, thus terminating NF-κB signalling. However, we cannot exclude an alternative mechanism in which TAX1BP1+A20 interfere with the growth of the ubiquitin chain on TRAF6. As both TRAF6 and A20 seem to bind the same UBZ2 domain of TAX1BP1, one can question how two molecules can bind simultaneously to the same region in TAX1BP1. We have found that TAX1BP1 can form homodimers (data not shown), and we propose that TAX1BP1 functions as a dimer with one molecule binding A20 and the other binding TRAF6. This would also explain the dominant-negative effect of overexpression of a TAX1BP1 UBZ2 mutant that we observed. The identification of TAX1BP1 as a ubiquitin-dependent negative regulator of NF-κB signalling expands the versatility of ubiquitin binding as a mechanism for regulating both positive and negative signalling (Haglund and Dikic, 2005). Interestingly, TAX1BP1 provides a means for pathway-specific regulation since we demonstrated that TAX1BP1 is a negative regulator of NF-κB but not JNK signalling. The underlying mechanistic basis for this specificity remains to be clarified. It can be speculated that TAX1BP1 also recruits A20 to other polyubiquitinated proteins. In this context, it is worth mentioning that TAX1BP1 has previously been shown to mediate the anti-apoptotic activities of A20 in TNF signalling (De Valck et al, 1999). It will therefore be interesting to identify specific ubiquitinated targets for TAX1BP1 and A20 in the TNF cell death signalling pathway.
The phenotype of TAX1BP1 KO mice is largely different from the phenotype of A20 KO mice, which succumb shortly after birth due to strong inflammation in different organs. Moreover, the strong cardiac pathology of TAX1BP1-deficient mice is peculiar in view of the critical function of NF-κB in the normal physiology of different organs. One reason for the more tissue-restricted phenotype of TAX1BP1-deficient mice might be the redundancy with other A20 adaptor proteins. In this context, A20 has been shown to bind three different ABIN family members, which have been suggested to be involved in the NF-κB inhibitory function of A20 (Heyninck and Beyaert, 1999; Van Huffel et al, 2001; Wullaert et al, 2007). Moreover, ABIN-1 was recently shown to facilitate the binding of A20 to NEMO and to be essential for NEMO de-ubiquitination by A20 (Mauro et al, 2006). Interestingly, we recently demonstrated the presence of a novel ubiquitin-binding motif in ABINs that is essential for their NF-κB inhibitory function (Wagner et al, submitted for publication). Speculatively, ABINs may fulfil a similar bridging function between A20 and its specific ubiquitinated targets as shown here for TAX1BP1.
After our study was completed, we noted the publication of a paper by Shembade et al (2007), who reported on an alternate approach to generate homozygous TAX1BP1-deficient mice. There are significant technical and scientific differences between our work and Shembade et al (2007). First, Shembade et al employed a random viral insertion strategy to ‘knock out' the TAX1BP1 gene. In this strategy, a viral vector is inserted into ES cells to trap upstream exons, resulting in a fused mRNA that directs the expression of a fusion protein comprised of the sequences encoded in the trapped exons, an antibiotic (neomycin) gene, and a colorimetric marker (β-galactosidase). This approach does not produce a ‘cleanly' targeted KO but rather creates a mutant genotype (i.e. termed m/m by Shembade et al) that expresses an artefactual protein–antibiotic marker–galactosidase fusion moiety that could produce phenotype(s) not simply due to loss of gene function. Second, the above technical nuance could be biologically confounding because Shembade et al's TAX1BP1m/m mouse is embryonic lethal while our TAX1BP1−/− mouse is born alive. Third, unlike TAX1BP1−/− MEFs, Shembade et al's m/m cells are not viable for propagation unless first transformed with SV40 large T antigen. A T antigen transformation event can produce cryptic secondary changes in m/m cells, which complicate biochemical analyses. This may explain why Shembade et al found that TAX1BP1 is critical to both NF-κB and JNK signalling, while our results restrict TAX1BP1 to only NF-κB and exclude its involvement in JNK signalling. Other differences between the studies could arise from different genetic backgrounds in the mice; nevertheless, on balance, our results agree with Shembade et al that TAX1BP1 is a novel adaptor protein that recruits A20 to cognate substrate(s).
In conclusion, in vivo loss of TAX1BP1 engenders A20-dependent hyperinflammation through NF-κB dysregulation. While NF-κB is likely important to all tissues and cells, the observation that TAX1BP1−/− mice that expire prematurely exhibit serious cardiac valvulitis suggests a hitherto unexplored importance of NF-κB signalling to the normal physiology of valve leaflets. This suggestion will need to be investigated further. However, TAX1BP1−/− mice could provide a candidate inflammatory cardiac valvulitis animal model that could be useful for checking whether drugs that specifically interrupt NF-κB activity (May et al, 2000) can be used to treat rheumatic heart disease.
Materials and methods
Cells and media
293, 293-IL1R, and MEFs were maintained in DMEM/high glucose with 10% fetal bovine serum.
Plasmids and siRNA
FLAG-TRAF6, E-A20 (De Valck et al, 1999), Myc-TAX1BP1, HA-TAX1BP1, E-TRAF2 (LMBP 5245), and FLAG-RIP1 (LMBP 4850) expression vectors are available from the BCCM/LMBP plasmid collection, Department of Molecular Biology, Ghent University, Belgium (http://bccm.belspo.be/about/lmbp.htm). The cysteine to alanine substitution at amino acids 757 and 760 of human TAX1BP1 (UBZ*) and the phenylalanine to alanine substitution at amino acids 737 (UBZ1) and 764 (UBZ2) were generated by PCR. All constructs were verified by sequencing. Primer sequences, PCR and ligation conditions, and cloning details for all constructions are available on request. siRNA sequences used are as follows: TAX1BP1-siRNA-1, CTGGAATGTCATTACACCTTA; TAX1BP1-siRNA-2, AAGAAGAACTGTTAAAGTTAA. Control GFP siRNA was CGGCAAGCTGACCCTGAAGTTCAT.
Co-immunoprecipitation and immunoblotting
At 24 h after transfection, cells were lysed with buffer (0.5% Nonidet P-40, 50 mM HEPES (pH 7.3), 150 mM NaCl, 2 mM EDTA, 1 mM phenylmethylsulphonyl fluoride, 0.1 mM sodium orthovanadate, 1 mM sodium fluoride, and protease inhibitor mix (Roche)). Lysates were incubated with monoclonal anti-FLAG (M2, Sigma) for at least 3 h at 4°C, or with the indicated antibodies for 3 h followed by protein G Sepharose for another 2 h. Proteins were detected by anti-FLAG (Sigma), anti-HA (Sigma), anti-E (Amersham Biosciences), or anti-Myc (Sigma).
Antibodies and reagents
Anti-GFP (Sigma), anti-CD3 (A0452, Dako), anti-myeloperoxidase (A0398, Dako), anti-CD45R/B220 (553086, Pharmingen), anti-F4/80 (RM2900, CALTAG), anti-phospho-Ser 32/36 IκBα (9246, Cell Signaling), anti-IκBα (4814, Cell Signaling), anti-phospho-T183 JNK1 (ab47337, Abcam), and anti-JNK1 (ab10664, Abcam) were purchased.
Generation of TAX1BP1−/− mice
A 12 kb EcoRI–BamHI fragment that contained exons 16 and 17 of Tax1bp1 was used for the generation of a targeting plasmid. The translated region of exon 17 was replaced by a neomycin gene cassette (NEO). The linearized targeting vector was electroporated into E14.1 ES cells and selected in G418. Four independent ES clones were injected into fertilized blastocysts from C57BL/6 female mice. Chimaeric mice were backcrossed for six generations with C57BL/6 female mice for germinal transmission. Heterozygous mice were intercrossed to obtain homozygotes. Homologous recombination was confirmed by PCR by using the following primers: Neo FW CGCCTTCTATCGCCTTCTTGACGAGTTCTT; 5′FW ATTAATTAATTCAACTTGTACACTGACAGG; 3′Rev GGGAACATTAATTCACAGAGGGGACATTTC.
In vitro GST pulldown assay
GST pulldown assay was performed as described previously (Bienko et al, 2005). All of the GST-UBZ constructs were generated using pGEX-4T1 (Amersham Bioscience).
Animal studies, histology, and immunohistochemistry
Experiments were carried out in accordance with the NIH guidelines for animal treatment, housing, and euthanasia. Animals were euthanized by CO2 and tissues were collected in 10% neutral buffered formalin and stained with haematoxylin and eosin. A phenotyping bioassay was performed by a board-certified veterinary pathologist. Slides were deparaffinized, rehydrated, and endogenous peroxidase was blocked with H2O2/methanol. Heat-induced epitope retrieval (HIER) with citrate buffer pH 6.0 was used for CD3, CD45R, and F4/80, whereas HIER with dH2O was used for myeloperoxidase. Peroxidase-based IgG Vectastain Elite kits from Vector Labs were used as per the instructions, except for F4/80 in which a rabbit anti-rat, -mouse adsorbed secondary was substituted.
Bone marrow transplantation
Five- to six-week-old female recipient TAX1BP1−/− or TAX1BP1+/+ mice received 1100 rad of total body irradiation. Bone marrow cells were harvested aseptically from the femora and tibiae of either TAX1BP1−/− or TAX1BP1+/+ donor mice and red blood cells were lysed. Recipient mice received 4 × 106 nucleated cells intravenously within 3 h of irradiation. From 2 days before injection, host mice were housed under sterile conditions with water containing 25 mg/l neomycin sulphate and 13 mg/l polymyxin B sulphate to allow bone marrow reconstitution. Eight weeks after transplantation, blood (approximately 100 μl) was collected from the tails of the bone marrow recipients and from control mice to check the quality of the reconstitution.
Quantitative RT–PCR
Real-time quantitative reverse transcription–PCR (ABI Prism 7300 Sequence Detection System; Applied Biosystems, Foster City, CA) was used to determine the mRNA levels of 6-carboxyfluorescent-labelled IL-1α, IL-1β, IL-2, IL-4, IL-10, TNF-α, and IκBα (ABI Prism, Pre-Developed TaqMan Assay Reagents).
Isolation of peritoneal macrophages
Macrophages were collected by peritoneal lavage (10 ml) from unstimulated mice. The peritoneal cells were suspended in RPMI-1640 medium containing 2 mM L-glutamine, 25 mM HEPES, 100 μg/ml streptomycin, 100 U penicillin G-sodium per millilitre, and 10% FCS, and plated on 18 mm glass coverslips. Two sets of experiments were performed (n=2). For each set, six animals per group were used and for each animal, nearly 70–80% of the peritoneal cells were identified as adherent macrophages by microscopic observation, and 96% were identified as viable by Trypan blue exclusion.
Statistical analysis
Statistical analyses were performed using SAS version 8.02 (SAS Institute Inc., Cary, NC, USA).
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the US Government.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Acknowledgments
We thank Drs J Inoue, K Matsumoto, and S Akira for providing plasmids; C Viboud for statistical analysis; K Hoffman for bioinformatics analysis; Y Belkaid for helpful discussion and technical advice; AB White, R Plishka, and C Erb for technical support; A Ma for providing MEF A20−/− cells; and A Dayton, W Leonard, and members of the Jeang laboratory for critical reading of the manuscript. LV and KH are respectively predoctoral and postdoctoral FWO research associates. FI is a postdoc of the Alexander von Humboldt and the Uehara Memorial Foundation. This research was supported by the DFG, FWO, IAP, GOA, NCI contract N01-CO-12400, and funding from the NIAID/NIH intramural programme. HI, J-MP, LV, GZ, FI, CDS, MFS, VY, and KH did the experiments and ID, RB, and K-TJ provided ideas and wrote the paper. Work in K-TJ's laboratory is supported by NIH, NIAID intramural funding. This project has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under contract no. N01-C0-12400.
References
- Beg AA, Sha WC, Bronson RT, Baltimore D (1995) Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev 9: 2736–2746 [DOI] [PubMed] [Google Scholar]
- Bienko M, Green CM, Crosetto N, Rudolf F, Zapart G, Coull B, Kannouche P, Wider G, Peter M, Lehmann AR, Hofmann K, Dikic I (2005) Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science 310: 1821–1824 [DOI] [PubMed] [Google Scholar]
- Boone DL (2004) The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol 5: 1052–1060 [DOI] [PubMed] [Google Scholar]
- Cao Z, Henzel WJ, Gao X (1996) IRAK: a kinase associated with the interleukin-1 receptor. Science 271: 1128–1131 [DOI] [PubMed] [Google Scholar]
- Chen ZJ (2005) Ubiquitin signalling in the NF-[kappa]B pathway. Nat Cell Biol 7: 758–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K-T, Chun ACS, Ching Y-P, Jeang K-T, Jin D-Y (2007) Human T-cell leukemia virus oncoprotein tax represses nuclear receptor-dependent transcription by targeting coactivator TAX1BP1. Cancer Res 67: 1072–1081 [DOI] [PubMed] [Google Scholar]
- De Valck D, Jin DY, Heyninck K, Van de Craen M, Contreras R, Fiers W, Jeang KT, Beyaert R (1999) The zinc finger protein A20 interacts with a novel anti-apoptotic protein which is cleaved by specific caspases. Oncogene 18: 4182–4190 [DOI] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ (1994) JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76: 1025–1037 [DOI] [PubMed] [Google Scholar]
- Dinarello CA (2004) Therapeutic strategies to reduce IL-1 activity in treating local and systemic inflammation. Curr Opin Pharmacol 4: 378–385 [DOI] [PubMed] [Google Scholar]
- Dunne A, O'Neill LA (2003) The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE 2003: re3. [DOI] [PubMed] [Google Scholar]
- Evans PC, Ovaa H, Hamon M, Kilshaw PJ, Hamm S, Bauer S, Ploegh HL, Smith TS (2004) Zinc-finger protein A20, a regulator of inflammation and cell survival, has de-ubiquitinating activity. Biochem J 378: 727–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten FR (2004) IKK[beta] links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118: 285–296 [DOI] [PubMed] [Google Scholar]
- Haglund K, Dikic I (2005) Ubiquitylation and cell signaling. EMBO J 24: 3353–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyninck K, Beyaert R (1999) The cytokine-inducible zinc finger protein A20 inhibits IL-1-induced NF-kappaB activation at the level of TRAF6. FEBS Lett 442: 147–150 [DOI] [PubMed] [Google Scholar]
- Heyninck K, Beyaert R (2005) A20 inhibits NF-[kappa]B activation by dual ubiquitin-editing functions. Trends Biochem Sci 30: 1–4 [DOI] [PubMed] [Google Scholar]
- Heyninck K, De Valck D, Vanden Berghe W, Van Criekinge W, Contreras R, Fiers W, Haegeman G, Beyaert R (1999) The zinc finger protein A20 inhibits TNF-induced NF-kappaB-dependent gene expression by interfering with an RIP- or TRAF2-mediated transactivation signal and directly binds to a novel NF-kappaB-inhibiting protein ABIN. J Cell Biol 145: 1471–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jono H, Lim JH, Chen LF, Xu H, Trompouki E, Pan ZK, Mosialos G, Li JD (2004) NF-kappaB is essential for induction of CYLD, the negative regulator of NF-kappaB: evidence for a novel inducible autoregulatory feedback pathway. J Biol Chem 279: 36171–36174 [DOI] [PubMed] [Google Scholar]
- Kanayama A (2004) TAB2 and TAB3 activate the NF-[kappa]B pathway through binding to polyubiquitin chains. Mol Cell 15: 535–548 [DOI] [PubMed] [Google Scholar]
- Karin M (2006) Nuclear factor-kappaB in cancer development and progression. Nature 441: 431–436 [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y (2000) Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol 18: 621–663 [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A (2002) NF-kappaB at the crossroads of life and death. Nat Immunol 3: 221–227 [DOI] [PubMed] [Google Scholar]
- Kollias G (2005) TNF pathophysiology in murine models of chronic inflammation and autoimmunity. Semin Arthritis Rheum 34: 3–6 [DOI] [PubMed] [Google Scholar]
- Lee TH, Shank J, Cusson N, Kelliher MA (2004) The kinase activity of Rip1 is not required for tumor necrosis factor-alpha-induced I[kappa]B kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J Biol Chem 279: 33185–33191 [DOI] [PubMed] [Google Scholar]
- Legler DF, Micheau O, Doucey MA, Tschopp J, Bron C (2003) Recruitment of TNF receptor 1 to lipid rafts is essential for TNF[alpha]-mediated NF-[kappa]B activation. Immunity 18: 655–664 [DOI] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O'Neill LA (2005) Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 5: 446–458 [DOI] [PubMed] [Google Scholar]
- Ling L, Goeddel DV (2000) T6BP, a TRAF6-interacting protein involved in IL-1 signaling. Proc Natl Acad Sci USA 97: 9567–9572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M (2005) Human T-cell leukemia virus type I (HTLV-I) infection and the onset of adult T-cell leukemia (ATL). Retrovirology 2: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M, Jeang KT (2007) Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer 7: 270–280 [DOI] [PubMed] [Google Scholar]
- Mauro C, Pacifico F, Lavorgna A, Mellone S, Iannetti A, Acquaviva R, Formisano S, Vito P, Leonardi A (2006) ABIN-1 binds to NEMO/IKKgamma and co-operates with A20 in inhibiting NF-kappaB. J Biol Chem 281: 18482–18488 [DOI] [PubMed] [Google Scholar]
- May MJ, D'Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S (2000) Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science 289: 1550–1554 [DOI] [PubMed] [Google Scholar]
- Pomerantz JL, Baltimore D (2002) Two pathways to NF-[kappa]B. Mol Cell 10: 693–695 [DOI] [PubMed] [Google Scholar]
- Roberts S, Kosanke S, Terrence Dunn S, Jankelow D, Duran CM, Cunningham MW (2001) Pathogenic mechanisms in rheumatic carditis: focus on valvular endothelium. J Infect Dis 183: 507–511 [DOI] [PubMed] [Google Scholar]
- Rullan E, Sigal LH (2001) Rheumatic fever. Curr Rheumatol Rep 3: 445–452 [DOI] [PubMed] [Google Scholar]
- Schulze-Osthoff K, Ferrari D, Riehemann K, Wesselborg S (1997) Regulation of NF-kappa B activation by MAP kinase cascades. Immunobiology 198: 35–49 [DOI] [PubMed] [Google Scholar]
- Shembade N, Harhaj NS, Liebl DJ, Harhaj EW (2007) Essential role for TAX1BP1 in the termination of TNF-alpha-, IL-1- and LPS-mediated NF-kappaB and JNK signaling. EMBO J 26: 3910–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CS, Kehrl JH (2003) Tumor necrosis factor (TNF)-induced germinal center kinase-related (GCKR) and stress-activated protein kinase (SAPK) activation depends upon the E2/E3 complex Ubc13-Uev1A/TNF receptor-associated factor 2 (TRAF2). J Biol Chem 278: 15429–15434 [DOI] [PubMed] [Google Scholar]
- Van Huffel S, Delaei F, Heyninck K, De Valck D, Beyaert R (2001) Identification of a novel A20-binding inhibitor of nuclear factor-kappa B activation termed ABIN-2. J Biol Chem 276: 30216–30223 [DOI] [PubMed] [Google Scholar]
- Wang C (2001) TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412: 346–351 [DOI] [PubMed] [Google Scholar]
- Wertz IE (2004) De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-[kappa]B signalling. Nature 430: 694–699 [DOI] [PubMed] [Google Scholar]
- Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM (2004a) De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-[kappa]B signalling. Nature 430: 694–699 [DOI] [PubMed] [Google Scholar]
- Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM (2004b) De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 430: 694–699 [DOI] [PubMed] [Google Scholar]
- WHO (2004) Diagnosis of rheumatic fever. Rheumatic fever and rhematic heart disease. Report of a WHO Expert Consultation. WHO Technical Report Series, Vol. 923, pp 20–40. Geneva, Switzerland: World Health Organization
- Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD (2006) Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected]. Nat Cell Biol 8: 398–406 [DOI] [PubMed] [Google Scholar]
- Wullaert A, Heyninck K, Janssens S, Beyaert R (2006) Ubiquitin: tool and target for intracellular NF-kappaB inhibitors. Trends Immunol 27: 533–540 [DOI] [PubMed] [Google Scholar]
- Wullaert A, Verstrepen L, Van Huffel S, Adib-Conquy M, Cornelis S, Kreike M, Haegman M, El Bakkouri K, Sanders M, Verhelst K, Carpentier I, Cavaillon JM, Heyninck K, Beyaert R (2007) LIND/ABIN-3 is a novel lipopolysaccharide-inducible inhibitor of NF-kappaB activation. J Biol Chem 282: 81–90 [DOI] [PubMed] [Google Scholar]
- Yoshida M (2001) Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu Rev Immunol 19: 475–496 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3