Abstract
Signal transducer and activator of transcription 3 (STAT3) is a transcriptional factor that performs a broad spectrum of biological functions in response to various stimuli. However, no specific coactivator that regulates the transcriptional activity of STAT3 has been identified. Here we report that CR6-interacting factor 1 (Crif1) is a specific transcriptional coactivator of STAT3, but not of STAT1 or STAT5a. Crif1 interacts with STAT3 and positively regulates its transcriptional activity. Crif1−/− embryos were lethal around embryonic day 6.5, and manifested developmental arrest accompanied with defective proliferation and massive apoptosis. The expression of STAT3 target genes was markedly reduced in a Crif1−/− blastocyst culture and in Oncostatin M-stimulated Crif1-deficient MEFs. Importantly, the key activities of constitutively active STAT3-C, such as transcription, DNA binding, and cellular transformation, were abolished in the Crif1-null MEFs, suggesting the essential role of Crif1 in the transcriptional activity of STAT3. Our results reveal that Crif1 is a novel and essential transcriptional coactivator of STAT3 that modulates its DNA binding ability, and shed light on the regulation of oncogenic STAT3.
Keywords: Crif1, embryogenesis, STAT3, transcriptional coactivator, tumorigenesis
Introduction
Signal transducer and activator of transcription 3 (STAT3) is a latent cytoplasmic transcriptional factor that can be activated by cytokines and growth factors. Disruption of STAT3 by gene targeting generated early embryonic lethality (∼embryonic day 6.5) (Takeda et al, 1997), and tissue-specific removal of STAT3 revealed its broad functional spectrum, including cell proliferation, differentiation, and apoptosis (Levy and Darnell, 2002). STAT3 has also gained attention because it is persistently active in a high proportion of human cancers and is required for tumor cell survival (Bromberg, 2001). STAT3 activates the transcription of several genes involved in cell cycle progression, such as Myc, Pim1, and Fos, and also upregulates antiapoptotic genes such as Bcl-2 and Bcl-XL (Yu et al, 2007).
Upon cytokine stimulation, STAT3 is activated through phosphorylation by Janus kinase (Jak) family members, dimerizes, and then translocates into the nucleus, where it binds specific promoter sequences of target genes and induces their transcription (Levy and Darnell, 2002; Levy and Lee, 2002). Significant progress has been made in the elucidation of the positive and negative regulation of STAT3 signalling (Levy and Darnell, 2002; Heinrich et al, 2003). Negative regulators, such as SOCS3, PIAS3, and GRIM-19, play important roles in cellular function by limiting cytokine signals (Chung et al, 1997; Lufei et al, 2003; Alexander and Hilton, 2004). In contrast, EZI and Gfi-1 have been suggested as positive regulators of STAT3 signalling. EZI, a nuclear zinc-finger protein, augments STAT3 activity by keeping it in the nucleus (Nakayama et al, 2002), and another zinc-finger protein, Gfi-1, also enhances STAT3 signalling by interacting with the STAT3 inhibitor PIAS3 (Rodel et al, 2000). Although the STAT3-signalling pathway from the plasma membrane to the nucleus has been delineated in detail, the molecular bases that govern gene transcription by STAT3 require further elucidation.
Like many other transcription factors, STAT3 associates with the transcriptional coactivators cAMP response element binding protein-binding protein/p300 (CBP/p300) and steroid receptor coactivator 1 (NcoA/SRC1a). These interactions enhance the transcriptional activity of STAT3 (Nakashima et al, 1999; Giraud et al, 2002). In addition, other transcriptional activators, such as c-Jun and glucocorticoid receptor, function synergistically with STAT3 to activate gene expression (Shuai, 2000). However, these coactivators are not specific to STAT3, but are also implicated in the functions of other STAT family members as well as in oncoproteins (such as Myb, Jun, and Fos), transforming viral proteins (such as E1A, E6, and large T antigen) and tumor-suppressor proteins (such as p53, E2F, Rb, Smads, RUNX, and BRCA1) (Shuai, 2000; Litterst and Pfitzner, 2002; Iyer et al, 2004). Recently, MCM5 and CoaSt6 have been suggested as specific coactivators of STAT1 and STAT6, respectively, although their in vivo relevance needs to be determined (Snyder et al, 2005; Goenka and Boothby, 2006). These findings raise the possibility of the existence of an additional coactivator that binds specifically to STAT3 and modulates its transcriptional activity.
Using yeast two-hybrid screening, we identified CR6-interacting factor 1 (Crif1) as a novel binding partner of STAT3, and found that overexpression of Crif1 enhances the transcriptional activity of STAT3. Crif1 interacts with Gadd45α,β,γ, and Nur77, and has been suggested as a potential regulator of cell cycle progression and cell growth (Chung et al, 2003; Park et al, 2005). To determine the role of Crif1 in vivo, we generated mice with a disruption in the gene encoding Crif1. Interestingly, Crif1-deficient embryos showed early embryonic lethality before the gastrulation stage, and Crif1-deficient blastocysts exhibited reduced expression of STAT3 target genes. Mouse embryonic fibroblasts (MEFs) with the Crif1 gene disrupted using Cre recombinase exhibited impaired STAT3 transcriptional activities, although the upstream signalling events were intact. Chromatin immunoprecipitation (ChIP) experiments and an electrophoretic mobility shift analysis (EMSA) showed that Crif1 is essential for the DNA-binding activity of STAT3. Furthermore, cellular transformation by the constitutively active form of STAT3 was completely abolished in the Crif1-null MEFs. Our findings revealed that Crif1 is an essential and specific transcriptional coactivator of STAT3.
Results
STAT3 interacts with Crif1
A yeast two-hybrid screen identified Crif1 as a novel binding partner of STAT3 (Supplementary Figure 1A). To confirm this interaction, Myc-tagged Crif1 was transfected into HEK 293 cells, and lysates were immunoprecipitated with an anti-Myc antibody and then immunoblotted with an anti-STAT3 antibody to detect endogenous STAT3 (Figure 1A). As a result, Crif1 indeed interacted with STAT3. To test whether Crif1 also interacts with other STAT proteins, we used MEFs overexpressing HA-tagged Crif1 (HA-Crif1). In contrast to the strong binding with STAT3, Crif1 did not interact with STAT1 and STAT5a, suggesting a specific functional link between Crif1 and STAT3 (Figure 1B and C). To identify the domain of Crif1 responsible for STAT3 binding, we generated three HA-Crif1 deletion mutants (Supplementary Figure 1B). These HA-Crif1 mutants were each expressed in HEK 293 cells and immunoprecipitated, which revealed that the C-terminal coiled-coil domain (CCD) of Crif1 interacted with STAT3 (Figure 1D). We used a yeast two-hybrid system to define the domain of STAT3 that interacts with Crif1. The analysis revealed that the CCD of STAT3 was sufficient for the interaction with Crif1 (Figure 1E). Taken together, these results suggest that Crif1 and STAT3 interact via their CCDs.
Figure 1.
Interaction of Crif1 with STAT3. (A) Interaction between Crif1 and STAT3. HEK 293T cells were transfected with Myc-Crif1 and immunoprecipitated (IP) with an anti-Myc Ab, followed by western blotting (IB) with an anti-STAT3 Ab. (B, C) Specific binding of Crif1 with STAT3. MEFs overexpressing mock or HA-Crif1 were mock-stimulated (B) or stimulated with IFNγ (20 ng/ml) for 30 min (C), and lysates were immunoprecipitated with an anti-HA Ab, followed by western blotting with anti-STAT3 (B), STAT5a (B), and STAT1 (C) Abs. (D) HA-tagged Crif1 deletion mutants were transfected into HEK 293T cells and then immunoprecipitated with an anti-HA Ab. Total cell extracts and immunoprecipitates were blotted with anti-HA and anti-STAT3 Abs. (E) Schematic representation of the STAT3 constructs and their interactions with full-length Crif1 in a yeast two-hybrid system. NTD, N-terminal domain; CCD, coiled-coil domain; DBD, DNA-binding domain; LK, linker domain; SH2, SH2 domain; TAD, transactivation domain.
Oncostatin M stimulation enhances the interaction between Crif1 and STAT3
To examine the spatio-temporal interaction of Crif1-STAT3 upon stimulation with Oncostatin M (OSM), a member of the IL-6 cytokine subfamily, we generated NIH3T3 cell lines stably expressing HA-Crif1 (HA-Crif1 NIH3T3), and examined the localization of HA-Crif1 and STAT3 in the HA-Crif1 NIH3T3 cells before and after the OSM stimulation. Consistent with previous reports, Crif1 was localized predominantly in the nucleus (Gornemann et al, 2002; Chung et al, 2003). Without OSM stimulation, STAT3 was found in both the nucleus and cytoplasm, but OSM stimulation resulted in the accumulation of STAT3 in the nucleus, where Crif1 was located (Figure 2A). The merged pictures of Crif1 and STAT3 indicated the colocalization of both proteins in the nucleus after OSM stimulation. Furthermore, when the cell lysates were immunoblotted with an anti-phospho-STAT3 antibody or immunoprecipitated with an anti-HA antibody, followed by an anti-STAT3 antibody, STAT3 phosphorylation was detected at 5 min after treatment with OSM, and began to diminish at 60 min (Figure 2B). Interestingly, the amount of STAT3 bound to Crif1 apparently increased upon OSM stimulation, suggesting that the interaction between Crif1 and STAT3 is dependent on the OSM stimulation.
Figure 2.
Crif1 enhances the transcriptional activity of STAT3. (A) Colocalization of Crif1 and STAT3. HA-Crif1 expressing NIH3T3 cells were stimulated with OSM (10 ng/ml) and immunostained with anti-HA (in green) and anti-STAT3 (in red) Abs. (B) Enhanced interaction between Crif1 and STAT3 upon OSM stimulation. HA-Crif1-expressing NIH3T3 cells were stimulated with OSM (10 ng/ml), and lysates were immunoprecipitated with an anti-HA Ab, followed by western blotting with an anti-STAT3 Ab. (C–H) Enhanced transcriptional activity of STAT3 by Crif1. NIH 3T3 cells were cotransfected with the Crif1 expression vector and the STAT3 responsive m67-luciferase construct, and were cotransfected with the STAT3 expression vector (C) or were stimulated with LIF (D) and OSM (E) for 8 h. HCT116 (F), SNU387 (G) and MDA-MB 468 (H) human cancer cell lines were cotransfected with the Crif1 expression vector and the STAT3 responsive m67-luciferase construct. Luciferase activity was measured 36 h after transfection. The results are representative of three independent experiments (*P<0.05, **P<0.002, ***P<0.02). A full-colour version of this figure is available at The EMBO Journal Online.
Since Crif1 resides and might function in the nucleus, we tested whether the interaction between Crif1 and STAT3 upon OSM stimulation results from the nuclear accumulation of activated STAT3 using STAT3Y705F, which has a mutation at a phosphorylation site for dimerization (Kaptein et al, 1996; Bhattacharya and Schindler, 2003). V5-tagged STAT3 and V5-tagged STAT3Y705F plasmids were each co-electroporated with HA-tagged Crif1 into the MEFs, which were then stimulated with OSM for 20 min. Anti-HA immunoprecipitates from the whole-cell extracts were subjected to a western blot analysis with an anti-V5 antibody. OSM stimulation enhanced the Crif1–STAT3 interaction, but not the Crif1–STAT3Y705F interaction (Supplementary Figure 1F). However, the basal level of interaction between Crif1 and STAT3Y705F might represent the basal import pathway (Bhattacharya and Schindler, 2003). In addition, Crif1 interacted with TAD domain-deleted STAT3, which lacks phosphorylation sites for dimerization (Supplementary Figure 1C and D). These results suggest that the nuclear enrichment of STAT3 by OSM stimulation enhances the interaction between Crif1 and STAT3.
Crif1 enhances the transcriptional activity of STAT3
The stimulation-dependent interaction between Crif1 and STAT3 might affect the biological activity of STAT3, as a transcriptional factor. To evaluate whether Crif1 alters the transcriptional activity of STAT3, we performed reporter gene assays, using a luciferase reporter construct containing four STAT-binding sites (m67-luc) (Bromberg et al, 1999). Crif1 alone slightly enhanced the STAT3-mediated transcriptional activity in NIH3T3 cell lines. However, when Crif1 was coexpressed with full-length STAT3, Crif1 substantially increased the STAT3-mediated transcriptional activity (Figure 2C). Furthermore, Crif1 enhanced the STAT3-mediated transcriptional activity in the presence of LIF and OSM (Figure 2D and E). We also examined the effect of Crif1 using human cancer cell lines, HCT116 (human colon cancer cell line), SNU387 (human hepatic cancer cell line), and MDA-MB 468 (human breast cancer cell line), which have constitutively phosphorylated STAT3 (Yoshida et al, 1996; Garcia et al, 1997; Siddiquee et al, 2007). As expected, the transcriptional activity of STAT3 in these cell lines was dramatically increased, by over 20-fold, by the ectopic expression of Crif1 without any stimulation (Figure 1F–H). The relatively small induction in the NIH3T3 cell line by ectopic Crif1 expression might be due to different cellular contexts, such as the endogenous expression of Crif1. These results suggest that Crif1 positively regulates the STAT3-mediated transcriptional activity.
Generation of Crif1−/− mice
STAT3 is essential and sufficient to maintain the pluripotency of murine embryonic stem cells (Niwa et al, 1998), and its disruption causes embryonic lethality before gastrulation (Takeda et al, 1997). To elucidate the physiological relevance of the STAT3-Crif1 interaction, Crif1−/− mice were generated (Supplementary Figure 2). The Crif1+/− mice were healthy and fertile. However, when the heterozygous mice were intercrossed, no Crif1−/− mice were detected among 330 offspring, indicating that Crif1 deficiency results in postnatal or embryonic lethality. To determine when the Crif1−/− mice died, time-pregnant heterozygous females were killed at different gestational stages. Among 66 embryos analysed from E7.5 to E9.5, none were Crif1−/−. Among 30 embryos assayed from E6.5 embryos, only three were Crif1−/− (Supplementary Figure 2D). In contrast, Crif1−/− blastocysts were morphologically normal and appeared in the expected Mendelian ratio (Supplementary Figure 2D). These data indicate that Crif1−/− embryos die around E6.5.
Crif1−/− embryos show defective proliferation and massive apoptosis
At E6.5, all of the Crif1−/− embryos were smaller and developmentally retarded, as compared with their control littermates (Figure 3A). A histological analysis of 18 E6.5 decidua generated from heterozygous intercrosses revealed two distinct morphological classes. Fifteen embryos (83.3%) exhibited normal cellularity and cytoarchitecture in all embryonic and extra-embryonic structures (Figure 3B), and three (16.7%) were severely growth retarded and showed abnormal structures, such as the absence of a proamniotic cavity (Figure 3C). Therefore, we examined the rate of proliferation using BrdU incorporation and the extent of apoptosis by performing TUNEL assays in Crif1 mutant and control littermates at E6.5. BrdU labelling showed that the proliferation rate in the Crif1−/− embryos was significantly reduced relative to that of the control littermates (Figure 3D and G). Furthermore, few apoptotic cells were observed in the control embryos, but many TUNEL-positive cells were scattered throughout the Crif1−/− embryos at E6.5 (Figure 3E and H). These results suggest that the growth deficit of the Crif1−/− embryos resulted from both defective cellular proliferation and increased cell death.
Figure 3.
Defective proliferation and massive apoptosis in Crif1−/− embryos. (A) Gross appearance of PCR-verified E6.5 Crif1+/− (left) and Crif1−/− embryos (right). The genotypes of both embryos, determined by PCR analysis, are indicated at the bottom of the figure. (B–I) Sagittal sections of E6.5 Crif1+/− (B, D–F) and Crif1−/− (C, G–I) embryos were stained with haematoxylin and eosin (B, C), anti-BrdU Ab (D, G), TUNEL (E, H), and Hoechst (F, I).
Defective outgrowth of the inner cell mass from Crif1−/− blastocysts
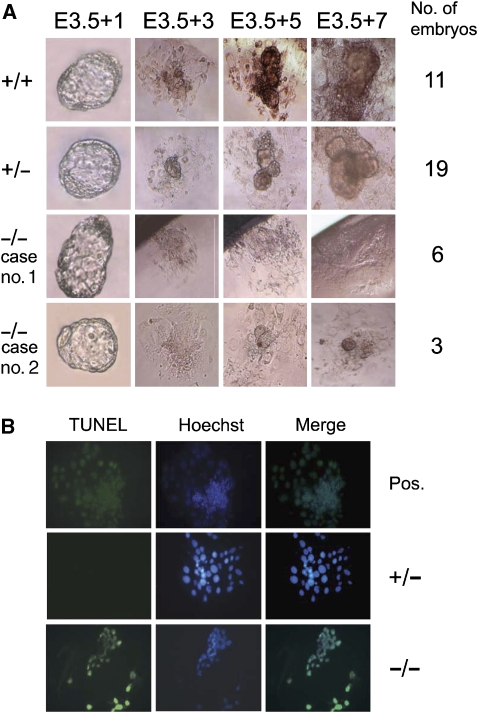
To directly assess the growth capability of Crif1−/− embryos, we collected E3.5. blastocysts from heterozygote intercrosses and cultured them individually for 7 days. Two days after culture, all of the blastocysts had successfully attached to the bottom of culture dish and hatched from the zona pellucida. After 3 days in culture, all of the blastocysts produced apparently normal trophoblast giant cells, a process necessary to induce the decidual reaction during implantation. However, the inner cell mass (ICM), which forms the future embryonic tissues, did not exhibit outgrowth in the Crif1−/− blastocysts. Longer periods of blastocyst culture confirmed the inability of ICM outgrowth from 6 of the 9 Crif1−/− blastocysts (Figure 4A, case no. 1). Although the other Crif1−/− blastocysts showed marginal ICM outgrowth, these ICM-like cells did not grow further (Figure 4A, case no. 2). At 3 days of culture, we also examined whether the Crif1−/− blastocysts were undergoing apoptosis. Whereas the Crif1+/− blastocysts showed no apoptotic cells, the Crif1−/− blastocysts displayed many TUNEL-positive apoptotic cells (Figure 4B). Taken together, these results suggest that Crif1 is required for the normal ICM outgrowth of blastocysts.
Figure 4.
Defective outgrowth of ICM (A) and massive apoptosis (B) in Crif1−/− blastocysts. (A) Crif1+/+ (+/+), Crif1+/− (+/−), and Crif1−/− (−/−) blastocysts from Crif1+/− intercrosses were individually cultured in LIF-containing ES medium for 7 days. The outgrowths of ICM were inspected daily and photographed on the indicated day. (B) Crif1+/− (+/−), and Crif1−/− (−/−) blastocysts cultured for 3 days were subjected to a TUNEL assay. A DNase I-treated Crif1+/− blastocyst was used as a positive control for DNA fragmentation (Pos).
Expression of Crif1 in the early developmental stages
Crif1 mRNA was previously reported to be expressed ubiquitously, and at notably high levels in the thyroid gland, heart, lymph nodes, trachea, and adrenal tissues (Chung et al, 2003). In the blastocysts, Crif1 was expressed in the ICM, similarly to Oct4 and Nanog (Supplementary Figure 3A and B; data not shown). During early development, Crif1 was expressed in embryonic and extra-embryonic tissues, and the highest level of Crif1 expression was seen in the chorion, allantois, and amnion (Supplementary Figure 3C). Since Crif1 is critical for ICM outgrowth, we examined whether Crif1−/− blastocysts have an intact ICM. Sixty-nine blastocysts from seven heterozygote intercrosses were randomly divided into three groups and subjected to in situ hybridization and genotyping (Supplementary Figure 3E and F). One group (n=16) was genotyped; as expected, four blastocysts were Crif1−/−, consistent with the expected Mendelian ratio. The second group (n=26) was assayed for the presence of Oct4 expression: all were positive, indicating that all of the blastocysts had an apparently normal ICM (Supplementary Figure 3E). The third group (n=27) was assayed for Crif1 expression. Unexpectedly, it was detected in all of them, whereas 25% were expected to be homozygous mutants. This result suggests the possibility that an initial supply of Crif1 mRNA might be delivered maternally (Supplementary Figure 3F).
Interestingly, the Crif1 mRNA in the Crif1−/− blastocysts disappeared after 3 days of culture (Supplementary Figure 4). Therefore, we examined the Crif1 mRNA expression patterns before the blastocyst stage, using an RT–PCR analysis. As expected, Crif1 mRNA was detected in unfertilized eggs and persisted until the blastocyst stage, although its expression level was decreased (Supplementary Figure 3D). These results suggest that the maternally derived Crif1 mRNA is not degraded until the blastocyst stage, and thus it might contribute to the initial outgrowth and survival of the ICM in Crif1−/− blastocysts.
Decreased expression of STAT3 target genes in cultured Crif1−/− blastocysts
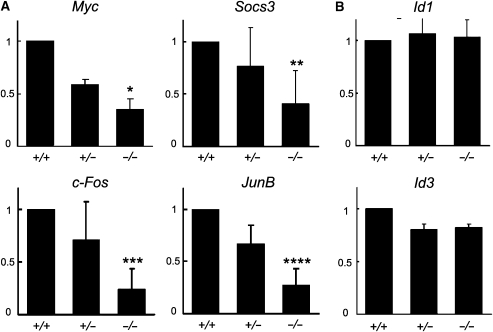
Since Crif1 interacted with STAT3, and regulated its transcriptional activity, we predicted that the lethality would, at least partially, result from impaired STAT3 signalling. To investigate this possibility, we examined the expression levels of STAT3 target genes in cultured blastocysts, because STAT3 is responsible for the ICM outgrowth in this period (Takeda et al, 1997). Three days after the blastocyst culture in the presence of LIF, one-third of an individual colony was used for genotyping by genomic PCR, and the rest of the colony with the same genotype was pooled. RNA was extracted from each pooled colony lysate and analysed by semi-quantitative RT–PCR and real-time RT–PCR. Although the maternally derived Crif1 mRNA still remained in the Crif1−/− blastocysts before the culture (Supplementary Figure 3F), it was undetectable in the Crif1−/− colonies 3 days after the blastocyst culture, whereas the Crif1+/− colonies showed about half of the expression, as compared with that of the Crif1+/+ colonies (Supplementary Figure 4). Intriguingly, the expression levels of four known STAT3 target genes, Myc, Socs3, c-Fos, and JunB, were significantly decreased in the Crif1−/− colonies, as compared with the Crif1+/+ and Crif1+/− colonies (Supplementary Figure 4). In contrast, the expression levels of unrelated genes (Oct4, Id1, Id3, and Sox2) were not affected. Real-time RT–PCR also showed decreased expression levels of STAT3 target genes (Myc, Socs3, c-Fos, and JunB), whereas those of unrelated genes, Id1 and Id3, were not changed (Figure 5). These results indicate that Crif1 is critical for the expression of STAT3 target genes, and that it might work as a positive regulator of STAT3.
Figure 5.
Decreased expression of STAT3 target genes in colonies from Crif1−/− blastocysts. (A, B) Real-time RT–PCR analyses of STAT3 target genes (Myc, Socs3, c-Fos, and JunB) and unrelated genes (Id1 and Id3). Crif1+/+ (+/+), Crif1+/− (+/−), and Crif1−/− (−/−) blastocysts were individually cultured in LIF-containing ES medium for 3 days. One-third of an individual colony was used for genotyping by genomic PCR, and the rest of the colony with the same genotype was pooled. RNA was extracted from each pooled colony lysate and analysed by real-time RT–PCR. The error bars indicate the standard deviation. Oct4 was used for normalization. The results are representative of three independent experiments. Significant differences are *P<0.0001, **P<0.01, ***P<0.0005, and ****P<0.0001.
Conditional inactivation of Crif1 in MEFs
To further explore the molecular mechanism of Crif1 in the STAT3 signalling, we generated a conditional Crif1-knockout allele, Crif1flox, in which exon 2 is flanked by two loxp sites, as described in Supplementary Figure 5. While STAT3 is dispensable for the normal growth of MEFs, its target genes are readily induced by various stimuli (Schlessinger and Levy, 2005). Thus, MEFs can be an ideal tool for studying the precise mechanism of STAT3 signalling. We generated Crif1flox/+ and Crif1flox/− MEFs from E13.5 embryos by parental crosses of Crif1+/− and Crif1flox/flox mice. Crif1+/Δ and Crif1−/Δ MEFs were derived from Crif1flox/+ and Crif1flox/− MEFs, respectively, by an MSCV-Cre retrovirus (Supplementary Figure 5C and D).
Specific role of Crif1 in STAT3 transcriptional activity
To clarify the molecular mechanism of Crif1 in STAT3 signalling, we first examined the phosphorylation status of STAT3 after OSM stimulation. As expected, STAT3 phosphorylation was readily observed in both the Crif1+/Δ and Crif1−/Δ MEFs after OSM stimulation (Supplementary Figure 6A). To examine the dimerization of STAT3 in the Crif1−/Δ MEFs, we performed a co-immunoprecipitation assay after co-electroporation of flag-tagged STAT3 and V5-tagged STAT3 plasmids into the Crif1+/Δ and Crif1−/Δ MEFs. Anti-flag immunoprecipitates from the whole-cell extracts were subjected to a western blot analysis with an anti-V5 antibody. After OSM stimulation, both the Crif1+/Δ and Crif1−/Δ MEFs showed increased interaction between flag-tagged STAT3 and V5-tagged STAT3 (Supplementary Figure 6B). When we also tested the nuclear translocation of STAT3 after OSM stimulation, most of the STAT3 in both the Crif1+/Δ and Crif1−/Δ MEFs was localized in the nucleus 20 min after OSM stimulation, and it still existed in the nucleus at 60 min (Supplementary Figure 6C). Taken together, these data indicate that the activation events, phosphorylation, dimerization, and nuclear translocation, of STAT3 in response to OSM were not affected by the disruption of the Crif1 gene.
The impaired expression of STAT3 target genes in the Crif1−/− blastocyst culture prompted us to examine STAT3-dependent transcriptional activation in the Crif1−/Δ MEFs by quantitative real-time PCR. The expression of STAT3 target genes (c-Fos, JunB, and Socs3) in response to OSM was specifically increased in Crif1+/Δ MEFs 1–2 h after OSM treatment. However, the Crif1−/Δ MEFs showed only slight induction of these genes, as compared to the induction in Crif1+/Δ MEFs (Figure 6A; Supplementary Figure 7A–C). The residual induction of STAT3 target genes in the Crif1−/Δ MEFs might be due to either the incomplete disruption of the Crif1 gene (Supplementary Figure 5D) or the STAT3-independent pathway responsive to OSM (Kuropatwinski et al, 1997).
Figure 6.
Impaired transcriptional activity of STAT3 in Crif1−/Δ MEFs. (A) Impaired expression of STAT3 target genes. Crif1+/Δ and Crif1−/Δ MEFs were treated with OSM (5 ng/ml) for the indicated times, and the Socs3 gene expression was analysed by real-time RT–PCR; *P<0.01. (B) Impaired transcriptional activity of STAT3. Crif1+/Δ and Crif1−/Δ MEFs cotransfected with the m67-luc/pRL-TK vectors were treated with various concentrations of OSM, and the luciferase activity was measured 36 h after transfection. The results are presented as fold induction of luciferase activity from triplicate experiments, and the error bars represent the standard deviations. The results are representative of four independent experiments; *P<0.01. (C, D) Abolishment of transcriptional activity by the constitutively active form of STAT3 (STAT3-C) in the absence of Crif1. Crif1+/Δ and Crif1−/Δ MEFs were transfected with the m67-luc/pRL-TK vectors and the STAT-C construct (C), and luciferase activity was measured 36 h after transfection. In panel D, the full length and a Crif1 mutant with a minimal STAT3 interaction domain (C1) were also each cotransfected into Crif1−/Δ MEFs. The results are presented as in panel C, and are representative of three independent experiments; **P<0.003. (E) Crif1+/Δ and Crif1−/Δ MEFs were treated with INFγ (20 ng/ml) for the indicated times, and the Irf1 gene expression was analysed by real-time RT–PCR.
We also performed reporter gene assays, using the m67-luc reporter and the pRL-TK control reporter. Whereas STAT3-mediated transcriptional activity by OSM stimulation was readily observed in the Crif1+/Δ MEFs in a dose-dependent manner, its activity was severely decreased in the Crif1−/Δ MEFs (Figure 6B). Since OSM activates other factors besides STAT3 (Kuropatwinski et al, 1997), and the m67-luc reporter is also responsive to STAT1 (Sironi and Ouchi, 2004), the residual response by OSM in Crif1−/Δ MEFs might be due to STAT1 activation. To exclude the influence of STAT1 after OSM stimulation, we used a constitutively active form of STAT3, STAT3-C (bridged by S–S linkages between cysteines, instead of phosphotyrosines), which can induce cellular transformation of fibroblasts (Bromberg et al, 1999). Intriguingly, the transcriptional activity of STAT3-C was abolished in the Crif1−/Δ MEFs (Figure 6C). This impaired transcriptional activity of STAT3-C was completely rescued by the introduction of the full-length Crif1 cDNA into the Crif1−/Δ MEFs, but not by the N-terminal deletion mutant C1, which has a minimal binding domain of STAT3 (Figure 6D). These data indicate that Crif1 is indispensable for the transcriptional activity of STAT3, and that the binding motif of Crif1 is not sufficient to activate STAT3.
To further clarify whether Crif1 is specifically involved in the induction of STAT3 target genes, we examined the induction of Irf-1 and Socs3 after IFNγ treatment, which is mainly mediated through STAT1 (Fujita et al, 1989; Starr et al, 1997). Whereas Irf-1 is induced by STAT1, Socs3 is activated by both STAT1 and STAT3 (Shen et al, 2004). As expected, Irf-1 and Socs3 were readily induced by IFNγ in the Crif1+/Δ and Crif1−/Δ MEFs (Figure 6E; Supplementary Figure 7E). Interestingly, without OSM and IFNγ stimulation, the expression levels of the STAT3 target genes (Myc, Socs3, JunB, and c-Fos) in the Crif1−/Δ MEFs were lower than those in the Crif1+/Δ MEFs, whereas the expression levels of the unrelated genes (Id1, Id3, Cd44, and Irf-1) were comparable (Figure 6A and E; Supplementary Figure 7). The lower expression levels of the STAT3 target genes in the Crif1−/Δ MEFs might be due to impaired STAT3-mediated transcription in response to LIF and/or other unidentified factors produced by MEFs. To exclude the possibility that a reduced level of STAT3 could affect the expression of STAT3 target genes, we examined the expression levels of endogenous STAT3 after OSM stimulation in the Crif1+/Δ and Crif1−/Δ MEFs. Both of them showed slightly increased levels of STAT3 six hours after OSM treatment (Supplementary Figure 6D). Thus, the decreased transcriptional activity of STAT3 in Crif1−/Δ MEFs did not result from a reduced level of STAT3. Since the Stat3 gene can be expressed in STAT3-independent manners (Narimatsu et al, 2001), the Crif1−/Δ MEFs could harbour an increased level of STAT3 after OSM stimulation. Collectively, these data suggest that Crif1 specifically regulates the transcriptional activity of STAT3.
Impaired DNA-binding activity of STAT3 in Crif1-null MEFs
Since Crif1 resides in the nucleus constitutively, and the STAT3 activation events, phosphorylation, dimerization and nuclear translocation, are not affected by the disruption of the Crif1 gene, Crif1 must work within the nucleus to regulate the transcriptional activity of STAT3. Thus, we examined whether Crif1 could influence the DNA binding of STAT3, which recognizes a conserved response element in the promoters of the genes encoding Socs3, c-Fos and Myc (Barre et al, 2003; Yang et al, 2003; Zhang et al, 2006). Crif1+/Δ and Crif1−/Δ MEFs were stimulated with OSM for 10∼30 min, and chromatin, prepared using a formaldehyde cross-linking protocol, was immunoprecipitated with an anti-STAT3 antibody. The occupancy of the promoters was quantified by specific pairs of primers spanning the STAT3-responsive regions on the Socs3 promoter. The OSM stimulation resulted in STAT3 occupancy of the promoters in as early as 10 min in the Crif1+/Δ MEFs (Figure 7A; Supplementary Figure 8A). However, Crif1−/Δ MEFs displayed about a 3.6-fold decrease in STAT3 recruitment to the Socs3 promoter (Figure 7A). The occupancy of STAT3 on the Myc and c-Fos promoters was also decreased in Crif1−/Δ MEFs (Supplementary Figure 8A).
Figure 7.
Defective DNA-binding activity of STAT3 in Crif1−/Δ MEFs. (A) ChIP. Soluble chromatin was prepared from Crif1+/Δ and Crif1−/Δ MEFs treated with OSM (5 ng/ml) for the indicated times and was immunoprecipitated with the anti-STAT3 Ab. The final DNA extracts were amplified, using pairs of primers that cover the STAT3-binding sites in the Socs3 promoter, by real-time PCR; *P<0.01. (B) Abolishment of DNA binding by STAT3-C. The Crif1+/Δ and Crif1−/Δ MEFs were electroporated with STAT-C and were subjected to ChIP with the anti-STAT3 Ab. DNA amplification was performed as in panel A; **P<0.01. (C) Crif1+/Δ and Crif1−/Δ MEFs were treated with OSM (10 ng/ml) for the indicated times. The nuclear extracts were subjected to EMSAs with radioactively-labelled STAT3 gel-shift oligonucleotides. Below the blot, the bar indicates the relative density of each lane with respect to the control, which has an arbitrary value of 1. (D) The recruitment of HA-Crif1 to the STAT3-responsive region. Wild-type MEFs electroporated with HA-Crif1 were stimulated with OSM (5 ng/ml) for the indicated times. Chromatin complexes were immunoprecipitated with anti-HA or anti-STAT3 Abs. DNA amplification was performed as in panel A. (E) Anchorage-independent growth. Immortalized Crif1+/Δ and Crif1−/Δ MEFs stably overexpressing STAT-C cells (1 × 104) were suspended in soft agarose and plated in 6-well dishes. After 20 days of incubation, colonies larger than 0.2 mm in diameter were counted. Columns, average number of colonies (experiments done in triplicate); bars, ±s.d.
We extended our study to the DNA binding of constitutively active STAT3-C. We immortalized Crif1+/Δ and Crif1−/Δ MEFs using SV40 large T antigen, and stably expressed STAT3-C by retroviral gene transfer methods. Surprisingly, the binding activity of STAT3-C to its target promoter regions, Socs3 and c-Fos, was abolished in the Crif1−/Δ MEFs, whereas the STAT3-C binding remained robust in the Crif1+/Δ MEFs (Figure 7B; Supplementary Figure 8B). In addition, EMSA experiments indicated that endogenous STAT3 associated with DNA upon OSM stimulation in Crif1+/Δ MEFs, whereas the DNA-binding ability of nuclear STAT3 was reduced in the Crif1−/Δ MEFs (Figure 7C). These results indicate that Crif1 enhances the transcriptional activity of STAT3 by modulating its DNA binding activity. To address whether Crif1 is also recruited to the Socs3 promoter, HA-Crif1 NIH3T3 cells were stimulated with OSM, and the chromatin was immunoprecipitated with an anti-HA antibody or an anti-STAT3 antibody. While Crif1 was not recruited to the Socs3 promoter without stimulation, we readily observed the accumulation of Crif1 in the STAT3 responsive region of the Socs3 promoter after OSM stimulation (Figure 7D; Supplementary Figure 8C). These data suggest that both Crif1 and STAT3 are recruited to the conserved response element in the promoter, which initiates the transcription of STAT3 target genes.
Crif1 is required for cellular transformation by STAT3-C
Immortalization of MEFs by SV40 large T antigen causes cellular transformation through a STAT3-dependent mechanism (Vultur et al, 2005). Fibroblasts immortalized with STAT3-C possess colony-forming potential in soft agar (Bromberg et al, 1999). To investigate the biological relevance of Crif1 in STAT3 signalling, we examined the transforming potential of STAT3-C in immortalized Crif1−/Δ MEFs. SV40 large T antigen alone resulted in anchorage-independent growth of Crif1+/Δ MEFs at 3 weeks. The combination of SV40 large T antigen and STAT3-C further increased the colony-forming efficiency (Figure 7E). In contrast, the colony formation activity was abolished in the Crif1−/Δ MEFs, despite the presence of both SV40 large T antigen and STAT3-C. These results show that Crif1 is indispensable for cellular transformation by STAT3 activation.
Discussion
STAT3 plays crucial roles in early embryogenesis as well as in a broad spectrum of biological activities, including cytokine-induced responses, differentiation, cell growth, and antiapoptosis (Levy and Darnell, 2002; Levy and Lee, 2002). Significant progress has been made in the elucidation of the regulation of STAT3 signalling. However, the transcriptional regulatory mechanism of STAT3 is poorly understood. In this report, the expression of STAT3 target genes was dramatically decreased in the Crif1−/− blastocyst culture, and the key activities of constitutively active STAT3-C, such as transcription, DNA binding, and cellular transformation, were abolished in the Crif1−/Δ MEFs. Thus, our study revealed that Crif1 is an essential transcriptional coactivator of STAT3.
Crif1 is an essential and specific transcriptional coactivator of STAT3 signalling
In Crif1-null MEFs, the receptor-proximal events, tyrosine phosphorylation and dimerization of STAT3 and its nuclear translocation, occurred properly, whereas the DNA binding and transcriptional activities of STAT3 were abolished, suggesting that Crif1 might be involved in the transcription mediated by STAT3. STAT3 requires several transcriptional cofactors for the proper expression of target genes (Giraud et al, 2002; Wang et al, 2005). Histone-modifying cofactors (CBP/p300 and NcoA/SRC1a) potentiate the STAT3-mediated transcription by linking STAT3 to the basal transcriptional machinery, and directly acetylate STAT3 to enhance the sequence-specific DNA binding ability (Giraud et al, 2002; Wang et al, 2005). However, these cofactors are not specific to STAT3. They are involved in the transcription by other STAT family members as well as oncoproteins and tumor-suppressor proteins (Shuai, 2000; Litterst and Pfitzner, 2002; Iyer et al, 2004). In this study, we found that Crif1 interacts with STAT3 in an OSM-stimulation-dependent manner and is recruited to the DNA-binding site of STAT3, while it does not interact with STAT1 or STAT5a. Furthermore, the transcription activity, DNA-binding ability, and transforming activity of STAT3-C, a constitutively active form of STAT3, were abolished in the Crif1-null MEFs. In contrast, the transcriptional activity of STAT1 was not impaired in Crif1−/Δ MEFs after IFNγ simulation. Thus, Crif1 is a specific coactivator of STAT3 among the STAT family members, and is crucial for the STAT3 transcriptional activity.
Crif1 interacts with the CCD of STAT3 (Figure 1E). The CCD of STAT3 is reportedly required for its recruitment to the cytokine receptor and for its ligand-induced nuclear translocation (Zhang et al, 2000; Ma et al, 2003). However, we showed that Crif1, as a nuclear protein, did not affect the phosphorylation, dimerization, and nuclear translocation of STAT3. Thus, Crif1 does not mediate the recruitment of STAT3 to the cytokine receptor and the nuclear translocation of STAT3 through the CCD. Moreover, the CCD of STAT proteins reportedly mediates associations with other transcriptional factors and coactivators, such as p48, IFN response factor (IRF) family members, c-Jun, and Nmi, an N-Myc interactor that regulates STAT transcriptional activity (Lufei et al, 2003). The C-terminal domain of Crif1 (C1), which binds to STAT3, did not rescue the transcription activity of STAT3-C in the Crif1−/Δ MEFs (Figure 6D), whereas the full-length Crif1 did. Therefore, the N-terminal non-STAT3-binding domain of Crif1 has an important role in STAT3 signalling, possibly through the recruitment of other transcriptional cofactors.
Crif1 and embryogenesis
STAT3 is essential for the self-renewal of embryonic stem cells, and it is the only STAT family member whose knockout leads to embryonic lethality. This lethality might be due to a functional defect in the visceral endoderm of the extraembryonic region, such as a nutritional insufficiency (Takeda et al, 1997). However, STAT3 also plays an important role in the ICM outgrowth of blastocysts (Takeda et al, 1997). Consistent with the indispensable function of Crif1 in the transcriptional activity of STAT3, the Crif1 deficiency in mice also resulted in early embryonic lethality around E6.5, as a result of defective proliferation and massive apoptosis. In addition, as in the Stat3−/− blastocysts, an in vitro culture of the Crif1−/− blastocysts exhibited impaired outgrowth of the ICM and decreased expression of STAT3 target genes, whereas the trophectodermal giant cells seemed to grow normally. Moreover, Crif1 mRNA was also expressed in the extra-embryonic visceral endoderm, suggesting that Crif1 might function as a transcriptional coactivator of STAT3 in the extra-embryonic tissues, although this needs to be delineated. Collectively, the STAT3–Crif1 interaction seems to be important for early embryonic development.
In the histological analysis of Crif1−/− embryos and in vitro cultured blastocysts, the Crif1−/− embryos showed more severe phenotypes than those of the Stat3−/− embryos. Besides STAT3, Crif1 has other binding partners, such as all three members, Gadd45α, Gadd45β, and Gadd45γ, of the Gadd45 protein family and Nur77 (Chung et al, 2003; Park et al, 2005). Although the early embryonic lethality of the Crif1−/− mice cannot be explained by the interactions with the Gadd45 family members and Nur77, because their mutant mice are viable and fertile, the more severe phenotypes of Crif1−/− embryos might be due to the additional dysfunctions of other binding partners, in the absence of STAT3 signalling (Lee et al, 1995; Hollander et al, 1999; Gupta et al, 2005).
Crif1- and STAT3-mediated transformation
STAT3 has been suspected of contributing to malignant transformation, since it is involved in cellular growth regulation and survival. A number of tumor cell lines and cells from primary tumours display constitutively activated STAT3 proteins (Bromberg, 2001). The constitutively active form of STAT3, STAT3-C, which substitutes cysteine residues for the phosphotyrosines of activated STAT3, causes cellular transformation of immortalized fibroblasts (Bromberg et al, 1999). However, in our study, this STAT3-C did not induce the cellular transformation of the immortalized Crif1−/Δ MEFs, indicating that Crif1 is required for the STAT3-C-mediated transformation. Moreover, in this study, the Crif1−/Δ MEFs immortalized using SV40 large T antigen, which requires STAT3 activity to induce neoplastic transformation, also failed to form colonies in soft-agar (Vultur et al, 2005). Thus, Crif1 inactivation completely blocked the transformation activity of STAT3.
Considerable effort is currently being focused on finding compounds capable of inhibiting various aspects of STAT3 function, through the inhibition of phosphorylation, dimerization, and DNA binding of STAT3 (Inghirami et al, 2005). SOCS1 reportedly suppressed STAT3 activation in a subset of hepatocellular carcinoma cells with STAT3 phosphorylated by JAKs (Yoshikawa et al, 2001). However, oncogenic STAT3, which is not dependent on phosphorylation by JAK, cannot be regulated by SOCS1. In contrast, Crif1 inactivation impaired the DNA-binding ability of activated STAT3. Therefore, the inhibition of the STAT3–Crif1 interaction could be a novel molecular target for the regulation of oncogenic STAT3 in various cancers.
Materials and methods
In vitro culture of blastocysts
Blastocysts collected at E3.5 were cultured in ES medium on a 0.1% gelatin-treated, multiwell slide chamber (Lab-Tek, Nalgene).
Yeast two-hybrid screening
Yeast two-hybrid screening was performed by Panbionet Corp. (www.panbionet.com), using the yeast strain PBN204 (MATα ura3-52 his3-200 ade2-101 trp-901 leu2-3,112 gal4Δ gal80Δ ura3∷kanMX6-pGAL1-URA3 pGAL1-lacZ ade2∷pGAL2-ADE2) and a human thymus cDNA library.
Plasmid construction
Full-length and mutant Crif1 were expressed with N-terminal HA-epitope tags, using the pcDNA3.0 vector (Invitrogen). The HA-Crif1 and STAT3-C genes were cloned into the retroviral vector pMSCV (Clontech). Flag-tagged STAT3 and V5-tagged STAT3Y705F plasmids were provided by Dr JY Yoo. All of the DNAs amplified by PCR were sequenced and tested for expression by western blotting.
Subcellular localization analysis
NIH3T3 cells were washed in PBS and fixed in 4% paraformaldehyde with 3% sucrose for 30 min at 4°C. The fixed cells were incubated in blocking solution (3% skim milk and 0.1% Triton X-100 in PBS) overnight at 4°C, and then were stained with mouse anti-STAT3 and anti-HA antibodies in 3% skimmed milk in PBS for 1 h at room temperature. Specific binding was detected with Alexa 488-labelled 594-labelled Ab (Molecular Probes), respectively.
Isolation of embryonic fibroblasts, immortalization, MSCV infection, and transfection
Embryonic fibroblasts were isolated from trypsin/EDTA-digested E13.5 embryos. The primary MEF cells were immortalized by transfection with SV40-large T antigen (gift from Dr J-Y Lee). For MSCV virus infection, a high-titre virus soup was produced with gp2-293 cells transfected with the pMSCV (Clontech) and VSV-G vectors. Embryonic fibroblasts were infected for 24 h and selected to eliminate the uninfected cells. Transient transfections of primary and immortalized MEFs were performed using Lipofectamine PLUS (Invitrogen) for the luciferase assay and a Microporator (Digital Biotechnology, South Korea) for the immunoprecipitation assay, ChIP, and EMSA, according to the manufacturers' instructions.
Luciferase assay
For the luciferase assay, NIH3T3 cells or primary MEF cells were transiently transfected using the Lipofectamine reagent (Life Technologies) with 0.5 μg of plasmid DNA (m67-Luc), the control pcDNA3, HA-Crif1, or STAT3-C in each well of 12-well plates. The Renilla reporter construct pRL-TK (Promega) was used to normalize the transfection efficiency. The cells were incubated for 36 h in DMEM containing 10% FBS and were harvested. Luciferase activity was determined using the Dual-Luciferase Reporter Assay System (Promega).
ChIP analysis
ChIP analyses of the MEF cells and NIH3T3 cells were performed using the EZ-Chip kit, according to the manufacturer's protocol (Upstate Biotechnology). Immunoprecipitation was performed with either the anti-STAT3 or the anti-HA antibody. The specific PCR primers were designed to contain putative STAT-binding sites, as determined by the MatInspector programme (Genomatix Software, Munchen, Germany), which spanned −166/−174 and −189/−197 in the SOCS3 promoter region (Zhang et al, 2006), −347/−355 in the c-fos promoter region (Yang et al, 2003), and −136/−146 in the c-myc promoter region (Barre et al, 2003). Real-time PCR was performed with a MyiQ single colour real-time PCR detection system (Bio-Rad) with iQ SYBR Green Supermix (Bio-Rad). Primer information is available in the Supplementary data.
Soft agar colony-forming assay
Colony-forming assays were performed in six-well dishes. Each well contained 3 ml of 0.6% agarose in DMEM as the bottom layer. The top layer consisted of 1 × 104 immortalized MEFs in 2 ml of 0.35% agarose in DMEM. After 20 days of incubation, colonies that were 0.2 mm or larger were counted.
Supplementary Material
Supplementary Information
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7
Supplementary Figure 8
Acknowledgments
We thank JK Han, SW Kim, and YJ Cho for helpful comments. This work was supported by grants from the 21C Frontier Functional Human Genome Project from the Ministry of Science and Technology of Korea (FG06-42-1), the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (0620020-1), and the Vascular System Research Center from KOSEF.
References
- Alexander WS, Hilton DJ (2004) The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol 22: 503–529 [DOI] [PubMed] [Google Scholar]
- Barre B, Avril S, Coqueret O (2003) Opposite regulation of myc and p21waf1 transcription by STAT3 proteins. J Biol Chem 278: 2990–2996 [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Schindler C (2003) Regulation of Stat3 nuclear export. J Clin Invest 111: 553–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE Jr (1999) Stat3 as an oncogene. Cell 98: 295–303 [DOI] [PubMed] [Google Scholar]
- Bromberg JF (2001) Activation of STAT proteins and growth control. Bioessays 23: 161–169 [DOI] [PubMed] [Google Scholar]
- Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K (1997) Specific inhibition of Stat3 signal transduction by PIAS3. Science 278: 1803–1805 [DOI] [PubMed] [Google Scholar]
- Chung HK, Yi YW, Jung NC, Kim D, Suh JM, Kim H, Park KC, Song JH, Kim DW, Hwang ES, Yoon SH, Bae YS, Kim JM, Bae I, Shong M (2003) CR6-interacting factor 1 interacts with Gadd45 family proteins and modulates the cell cycle. J Biol Chem 278: 28079–28088 [DOI] [PubMed] [Google Scholar]
- Fujita T, Kimura Y, Miyamoto M, Barsoumian EL, Taniguchi T (1989) Induction of endogenous IFN-alpha and IFN-beta genes by a regulatory transcription factor, IRF-1. Nature 337: 270–272 [DOI] [PubMed] [Google Scholar]
- Garcia R, Yu CL, Hudnall A, Catlett R, Nelson KL, Smithgall T, Fujita DJ, Ethier SP, Jove R (1997) Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ 8: 1267–1276 [PubMed] [Google Scholar]
- Giraud S, Bienvenu F, Avril S, Gascan H, Heery DM, Coqueret O (2002) Functional interaction of STAT3 transcription factor with the coactivator NcoA/SRC1a. J Biol Chem 277: 8004–8011 [DOI] [PubMed] [Google Scholar]
- Goenka S, Boothby M (2006) Selective potentiation of Stat-dependent gene expression by collaborator of Stat6 (CoaSt6), a transcriptional cofactor. Proc Natl Acad Sci USA 103: 4210–4215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornemann J, Hofmann TG, Will H, Muller M (2002) Interaction of human papillomavirus type 16 L2 with cellular proteins: identification of novel nuclear body-associated proteins. Virology 303: 69–78 [DOI] [PubMed] [Google Scholar]
- Gupta M, Gupta SK, Balliet AG, Hollander MC, Fornace AJ, Hoffman B, Liebermann DA (2005) Hematopoietic cells from Gadd45a- and Gadd45b-deficient mice are sensitized to genotoxic-stress-induced apoptosis. Oncogene 24: 7170–7179 [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F (2003) Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374: 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander MC, Sheikh MS, Bulavin DV, Lundgren K, Augeri-Henmueller L, Shehee R, Molinaro TA, Kim KE, Tolosa E, Ashwell JD, Rosenberg MP, Zhan Q, Fernandez-Salguero PM, Morgan WF, Deng CX, Fornace AJ Jr (1999) Genomic instability in Gadd45a-deficient mice. Nat Genet 23: 176–184 [DOI] [PubMed] [Google Scholar]
- Inghirami G, Chiarle R, Simmons WJ, Piva R, Schlessinger K, Levy DE (2005) New and old functions of STAT3: a pivotal target for individualized treatment of cancer. Cell Cycle 4: 1131–1133 [DOI] [PubMed] [Google Scholar]
- Iyer NG, Ozdag H, Caldas C (2004) p300/CBP and cancer. Oncogene 23: 4225–4231 [DOI] [PubMed] [Google Scholar]
- Kaptein A, Paillard V, Saunders M (1996) Dominant negative stat3 mutant inhibits interleukin-6-induced Jak-STAT signal transduction. J Biol Chem 271: 5961–5964 [DOI] [PubMed] [Google Scholar]
- Kuropatwinski KK, De Imus C, Gearing D, Baumann H, Mosley B (1997) Influence of subunit combinations on signaling by receptors for oncostatin M, leukemia inhibitory factor, and interleukin-6. J Biol Chem 272: 15135–15144 [DOI] [PubMed] [Google Scholar]
- Lee SL, Wesselschmidt RL, Linette GP, Kanagawa O, Russell JH, Milbrandt J (1995) Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nur77). Science 269: 532–535 [DOI] [PubMed] [Google Scholar]
- Levy DE, Darnell JE Jr (2002) Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 3: 651–662 [DOI] [PubMed] [Google Scholar]
- Levy DE, Lee CK (2002) What does Stat3 do? J Clin Invest 109: 1143–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litterst CM, Pfitzner E (2002) An LXXLL motif in the transactivation domain of STAT6 mediates recruitment of NCoA-1/SRC-1. J Biol Chem 277: 36052–36060 [DOI] [PubMed] [Google Scholar]
- Lufei C, Ma J, Huang G, Zhang T, Novotny-Diermayr V, Ong CT, Cao X (2003) GRIM-19, a death-regulatory gene product, suppresses Stat3 activity via functional interaction. EMBO J 22: 1325–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zhang T, Novotny-Diermayr V, Tan AL, Cao X (2003) A novel sequence in the coiled-coil domain of Stat3 essential for its nuclear translocation. J Biol Chem 278: 29252–29260 [DOI] [PubMed] [Google Scholar]
- Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, Miyazono K, Taga T (1999) Synergistic signaling in fetal brain by STAT3–Smad1 complex bridged by p300. Science 284: 479–482 [DOI] [PubMed] [Google Scholar]
- Nakayama K, Kim KW, Miyajima A (2002) A novel nuclear zinc finger protein EZI enhances nuclear retention and transactivation of STAT3. EMBO J 21: 6174–6184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimatsu M, Maeda H, Itoh S, Atsumi T, Ohtani T, Nishida K, Itoh M, Kamimura D, Park SJ, Mizuno K, Miyazaki J, Hibi M, Ishihara K, Nakajima K, Hirano T (2001) Tissue-specific autoregulation of the stat3 gene and its role in interleukin-6-induced survival signals in T cells. Mol Cell Biol 21: 6615–6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Burdon T, Chambers I, Smith A (1998) Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev 12: 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KC, Song KH, Chung HK, Kim H, Kim DW, Song JH, Hwang ES, Jung HS, Park SH, Bae I, Lee IK, Choi HS, Shong M (2005) CR6-interacting factor 1 interacts with orphan nuclear receptor Nur77 and inhibits its transactivation. Mol Endocrinol 19: 12–24 [DOI] [PubMed] [Google Scholar]
- Rodel B, Tavassoli K, Karsunky H, Schmidt T, Bachmann M, Schaper F, Heinrich P, Shuai K, Elsasser HP, Moroy T (2000) The zinc finger protein Gfi-1 can enhance STAT3 signaling by interacting with the STAT3 inhibitor PIAS3. EMBO J 19: 5845–5855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger K, Levy DE (2005) Malignant transformation but not normal cell growth depends on signal transducer and activator of transcription 3. Cancer Res 65: 5828–5834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Schlessinger K, Zhu X, Meffre E, Quimby F, Levy DE, Darnell JE Jr (2004) Essential role of STAT3 in postnatal survival and growth revealed by mice lacking STAT3 serine 727 phosphorylation. Mol Cell Biol 24: 407–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K (2000) Modulation of STAT signaling by STAT-interacting proteins. Oncogene 19: 2638–2644 [DOI] [PubMed] [Google Scholar]
- Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ, Sebti SM, Turkson J (2007) Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci USA 104: 7391–7396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi JJ, Ouchi T (2004) STAT1-induced apoptosis is mediated by caspases 2, 3, and 7. J Biol Chem 279: 4066–4074 [DOI] [PubMed] [Google Scholar]
- Snyder M, He W, Zhang JJ (2005) The DNA replication factor MCM5 is essential for Stat1-mediated transcriptional activation. Proc Natl Acad Sci USA 102: 14539–14544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ (1997) A family of cytokine-inducible inhibitors of signalling. Nature 387: 917–921 [DOI] [PubMed] [Google Scholar]
- Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S (1997) Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA 94: 3801–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vultur A, Arulanandam R, Turkson J, Niu G, Jove R, Raptis L (2005) Stat3 is required for full neoplastic transformation by the Simian Virus 40 large tumor antigen. Mol Biol Cell 16: 3832–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Cherukuri P, Luo J (2005) Activation of Stat3 sequence-specific DNA binding and transcription by p300/CREB-binding protein-mediated acetylation. J Biol Chem 280: 11528–11534 [DOI] [PubMed] [Google Scholar]
- Yang E, Lerner L, Besser D, Darnell JE Jr (2003) Independent and cooperative activation of chromosomal c-fos promoter by STAT3. J Biol Chem 278: 15794–15799 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, Fujiwara H, Hirata M, Yamagami T, Nakahata T, Hirabayashi T, Yoneda Y, Tanaka K, Wang WZ, Mori C, Shiota K, Yoshida N, Kishimoto T (1996) Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci USA 93: 407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H, Matsubara K, Qian GS, Jackson P, Groopman JD, Manning JE, Harris CC, Herman JG (2001) SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet 28: 29–35 [DOI] [PubMed] [Google Scholar]
- Yu H, Kortylewski M, Pardoll D (2007) Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 7: 41–51 [DOI] [PubMed] [Google Scholar]
- Zhang L, Badgwell DB, Bevers JJ III, Schlessinger K, Murray PJ, Levy DE, Watowich SS (2006) IL-6 signaling via the STAT3/SOCS3 pathway: functional analysis of the conserved STAT3 N-domain. Mol Cell Biochem 288: 179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Kee WH, Seow KT, Fung W, Cao X (2000) The coiled-coil domain of Stat3 is essential for its SH2 domain-mediated receptor binding and subsequent activation induced by epidermal growth factor and interleukin-6. Mol Cell Biol 20: 7132–7139 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7
Supplementary Figure 8