Abstract
Objective
To determine the impact of comorbidity on survival of bladder cancer patients.
Methods
The population included 675 patients with newly diagnosed bladder cancer whose medical information was abstracted from a hospital cancer registry. Adult Comorbidity Evaluation-27, a validated instrument, was used to prospectively categorize comorbidity. Independent variables assessed include comorbidity, American Joint Committee on Cancer (AJCC) stage, grade, age, gender, and race. Outcome measure was overall survival. We analyzed the entire cohort, patients with noninvasive disease, and patients requiring cystectomy. Cox proportional hazards analysis was used to assess impact of independent variables on survival.
Results
Median age at diagnosis for the entire cohort was 71 yr and median follow-up was 45 mo. Of 675 patients, 446 had at least one comorbid condition and 301 died during follow-up. On multivariable analysis for the entire cohort, comorbidity (p = 0.0001), AJCC stage (p = 0.0001), age (p = 0.0001), and race (p = 0.0045) significantly predicted overall survival. On subset analysis of noninvasive bladder cancer patients, comorbidity (p = 0.0001) and age (p = 0.0001) independently predicted overall survival, whereas stage, grade, race, and gender did not. On subset analysis of cystectomy patients, comorbidity (p = 0.0053), stage (p = 0.0001), and race (p = 0.0449) significantly predicted overall survival.
Conclusions
Comorbidity is an independent predictor of overall survival in the entire cohort of bladder cancer patients, the subset with noninvasive disease, and the subset treated with cystectomy.
Keywords: Bladder cancer, Comorbidity, Cystectomy, Epidemiology, Prognosis
1. Introduction
While prognostic factors for cancer in general and bladder cancer specifically traditionally focus on gross and microscopic tumor characteristics [1,2], overall health of patients often impacts survival. Comorbidity is defined as any coexisting disease or condition that may impact diagnosis, treatment, and prognosis for an index disease (in this study, bladder cancer). Many patients with cancer have comorbidities so severe that they prohibit use of preferred antineoplastic treatments and impact survival [3].
Cancers for which comorbidity is particularly important are those that are not rapidly fatal and affect people older than 50 yr. The prognostic impact of comorbidity has been determined in lung [4], oral cavity [5], and prostate cancer [6]. For some cancers, authors have found the prognostic impact of comorbidity to be independent of clinical aggressiveness [3,7]; therefore, assessment of comorbidity is warranted prior to aggressive intervention. In fact, because of the strong independent impact of comorbidity on survival, some authors have advocated inclusion of comorbidity information in cancer staging systems [5], insisting that such inclusion will improve accuracy in determining prognosis and in assessing treatment effectiveness.
Urothelial cancer is strongly associated with smoking and increased dietary fat [8,9]. Because these are factors that predispose to other medical conditions, including cardiovascular, cerebrovascular, and pulmonary disease, it is not surprising that patients with urothelial cancer often have significant comorbidities [10]. The goal of this study is to determine if comorbidity provides important independent prognostic information after controlling for morphological (stage and grade of tumor) and demographic (age, gender, and race) variables. Our end point is overall survival for the entire cohort. In addition, we investigated two subsets[em]patients with noninvasive disease and patients treated with cystectomy for muscle invasive or locally aggressive disease.
2. Methods
2.1. Study design
This was a retrospective analysis of data collected prospectively by certified tumor registrars at the time of cancer diagnosis.
2.2. Study population
After obtaining institutional review board approval, we reviewed clinical and pathological data of 794 patients diagnosed with bladder cancer between 1994 and 2006. The Barnes-Jewish Hospital Oncology Data Services is an American College of Surgeons Commission on Cancer (CoC)[en]approved cancer registry and actively maintains an electronic database of patients diagnosed or treated at our institution for malignancy. It does not mandate any practice pattern and all patients were managed at the discretion of the treating physician. We excluded 33 patients who did not have comorbidity information recorded, 31 patients who did not have pathological or clinical tumor stage recorded, and 55 patients who did not have transitional cell histology (specifically, patients with sarcomas, adenocarcinomas, and squamous cell carcinomas were excluded). The final study population consisted of 675 patients.
2.3. Data collection
Data collected include standard tumor registry data elements: date of diagnosis, demographic information, and tumor morphology and nodal spread at time of presentation or initial therapy. Follow-up was performed on all patients in the database on an annual basis by review of patient medical records, letters of inquiry to physicians and patients, and review of social security death index. Information collected at follow-up included disease status (including recurrence and progression), subsequent therapy, and outcome. The authors conducted verification of a random sample of the study population (5%) to ensure quality control.
2.4. Comorbidity data
Adult Comorbidity Evaluation-27 (ACE-27) is a 27-item comorbidity instrument validated on adult oncology patients [11,12] including 513 bladder cancer patients. Comorbidity was evaluated by review of medical records and other health information sources used by cancer registrars to obtain CoC-required information. Comorbidities were defined as preexisting medical conditions, or conditions present at time of cancer diagnosis, including previous or synchronous cancers. Examples of comorbid ailments included in ACE-27 are congestive heart failure (CHF), arrhythmias, hypertension, and diabetes. The ACE-27 can be viewed at: http://oto.wustl.edu/clinepi/Forms/com_form.doc. In ACE-27, specific diseases are graded into one of three levels of organ decompensation: Level 1 is mild decompensation, level 2 is moderate decompensation, and level 3 is severe decompensation. For instance, CHF would be rated as severe if ejection fraction was less than 20% or the patient was hospitalized within the previous 6 mo for CHF. It would be classified as moderate if the last hospitalization was more than 6 mo prior, or there was evidence of dyspnea limiting activity, but ejection fraction was more than 20%. Finally, CHF would be considered mild if there was evidence for dyspnea that responded to treatment.
Following classification of individual diseases, an overall comorbidity score (none, 0; mild, 1; moderate, 2; or severe, 3) is assigned on the basis of the highest-ranked single ailment or as “severe: in the case of two or more moderate organ decompensations in different organ systems.
2.5. Data classification
“Zero time” for each patient was defined as time of initial diagnosis of bladder cancer. Primary tumor histology was recorded from biopsy and surgical resections. Grading was performed at time of surgery with the use of the World Health Organization/International Society of Urological Pathology consensus classification [13] or the 1965 classification [14]. Given the different grading systems used during the span of our study, we standardized grade by classifying it into high and low grade for data analysis. Staging data included initial clinical TNM classification from physician reports and pathological TNM classification from surgical pathology reports. American Joint Committee on Cancer (AJCC) staging system was then derived from TNM classification: Stage 0 is TaN0M0 or TisN0M0; stage I is T1N0M0; stage II is T2aN0M0 or T2bN0M0; stage III is T3aN0M0, T3bN0M0 or T4aN0M0; stage IV is T4bNxMx, TxN+M0, or TxNxM1. For data analysis, pathological AJCC stage was used because since this parameter has been shown [15] to drive outcome. However, in some cases pathological stage incompletely assessed tumor extent (for example, neoadjuvant chemotherapy substantially alters pathological stage), so clinical AJCC stage was substituted.
2.6. Data analyses
Tabulation, sorting, and data analyses were performed with the use of the SAS® system (SAS Institute Inc, Cary, NC, USA). Bivariate and multivariate analyses were used to assess the prognostic impact of several factors. Variables with p < 0.1 on bivariate analysis were included in multivariate model. For each cohort studied, all predictor variables were entered into a single multivariate model (Cox proportional hazard analyses) and p < 0.05 was considered significant. Confidence intervals were calculated for each variable with the use of 1 degree of freedom. Interaction between comorbidity and age was assessed with the use of Spearman rank correlation coefficient.
We used bootstrap samples (with replacement) drawn from the development data set to estimate c-index from the logistic regression model predicting overall survival. The average of the performance measure was taken over 200 repetitions. To assess impact of comorbidity, discrimination was measured in the predictive model both with and without comorbidity assessment.
3. Results
The study population consisted of 519 (77%) men and 156 (23%) women, 568 (84%) European Americans, 100 (15%) African Americans, and 7 (1%) of other ethnicities. Median age was 71 yr. Of 675 patients, 446 (66%) had at least one comorbid condition.
3.1. Entire cohort
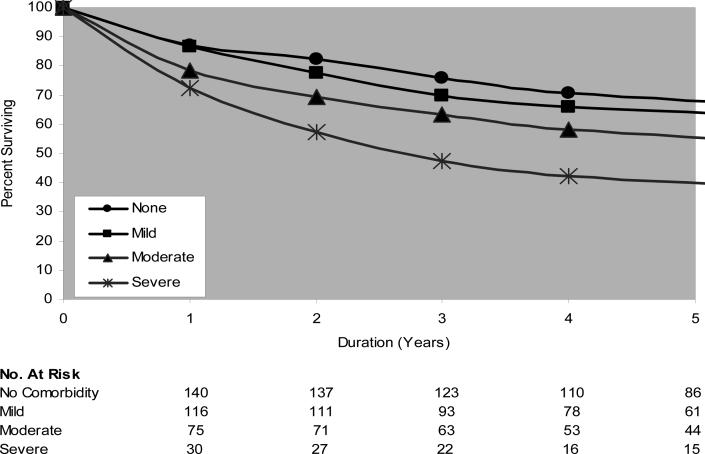
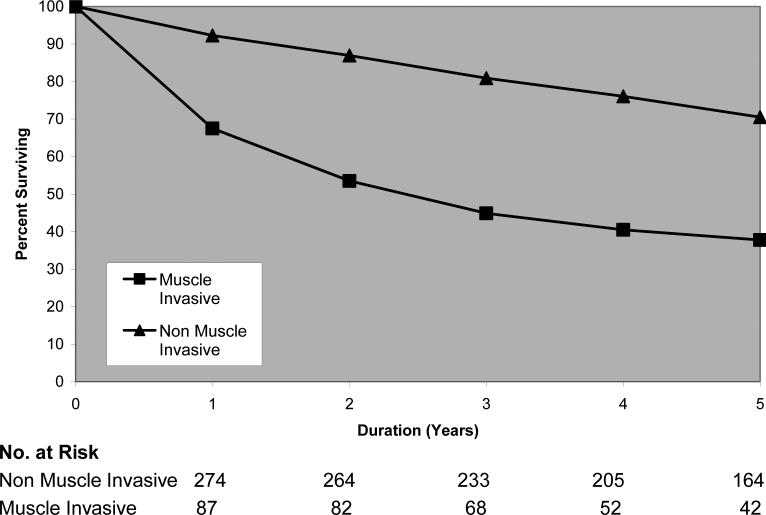
In the study population, 301 of 675 patients (44.6%) died during follow-up. Median follow-up was 45 mo. Demographic features and univariate analyses for the entire cohort are outlined in Table 1. The relationship between severity of comorbidity and overall survival was examined with the use of a Kaplan-Meier analysis (Fig. 1). At any point in time, patients with more severe comorbidity had worse overall survival. Stage is also associated with survival as demonstrated by Figure 2.
Table 1.
[en] Overall survival as a function of baseline demographic, clinical, and tumor features in the entire cohort
| Variable | Category | No. (%) of all patients | No. (%) of patients who died during follow-up | Unadjusted hazard ratio | 95% confidence interval | p value |
|---|---|---|---|---|---|---|
| Total | 675 (100) | 301 (44.6) | ||||
| Gender | Female | 156 (23.1) | 71 (45.5) | [em] | ||
| Male | 519 (76.9) | 230 (44.3) | 0.99 | 0.77[en]1.3 0 |
0.9952 | |
| Race* | White | 568 (84.1) | 247 (43.5) | [em] | ||
| Black | 100 (14.8) | 53 (53.0) | 1.51 | 1.12[en]2.0 3 |
0.0068 | |
| Age | ≤65 | 234 (34.7) | 77 (32.9) | [em] | ||
| 66[en]75 | 217 (32.1) | 93 (42.9) | 1.26 | 0.93[en]1.7 0 |
0.1348 | |
| ≥76 | 224 (33.2) | 131 (58.5) | 1.99 | 1.50[en]2.6 3 |
0.0001 | |
| Stage | 0 | 305 (45.2) | 98 (32.1) | [em] | ||
| I | 125 (18.5) | 50 (40.0) | 1.29 | 0.92[en]1.8 1 |
0.1473 | |
| II | 93 (13.8) | 44 (47.3) | 1.95 | 1.36[en]2.7 8 |
0.0003 | |
| III | 75 (11.1) | 45 (60.0) | 2.92 | 2.04[en]4.1 6 |
0.0001 | |
| IV | 77 (11.4) | 64 (83.1) | 5.81 | 4.22[en]8.0 0 |
0.0001 | |
| Comorbidity | 0 | 229 (33.9) | 83 (36.2) | [em] | ||
| 1 | 209 (31.0) | 89 (42.6) | 1.64 | 0.99[en]1.8 0 |
0.0585 | |
| 2 | 153 (22.7) | 77 (50.3) | 1.65 | 1.21[en]2.2 6 |
0.0015 | |
| 3 | 84 (12.4) | 52 (61.9) | 2.57 | 1.82[en]3.6 5 |
0.0001 | |
| Grade** | Low (I, II) | 297 (44.0) | 105 (35.4) | [em] | ||
| High (III, IV) |
327 (48.4) | 175 (53.5) | 1.95 | 1.55[en]2.4 5 |
0.0001 |
Seven patients were of other ethnicities and were censored owing to small sample size.
Fifty-one patients were not assigned a grade and were censored.
Fig. 1.
[en] Overall survival as a function of comorbidity severity. Log-rank chi-square = 31.74; p value = 0.0001; degrees of freedom = 3.
Fig. 2.
[en] Overall survival as a function of tumor aggressiveness. Log-rank chi-square = 89.53; p value = 0.0001; degrees of freedom = 1.
Because comorbidity, AJCC stage, age, race, and grade had p < 0.1 on bivariate analysis, they were included in multivariate analysis. In multivariate analysis, comorbidity (hazard ratio [HR], 1.39; 95% confidence interval [95%CI], 1.24[en]1.56; p = 0.0001), AJCC stage (HR, 1.62; 95%CI, 1.46[en]1.81; p = 0.0001), age (HR, 1.44; 95%CI, 1.24[en]1.68; p = 0.0001), and race (HR, 1.55; 95%CI, 1.15[en]2.10; p = 0.0045) were significant, whereas grade (HR, 0.91; 95%CI, 0.66[en]1.26; p = 0.5780) was not.
Bootstrapping demonstrated that inclusion of comorbidity improved the ability of the model to distinguish patients who did and did not survive after bladder cancer diagnosis. The c-index with inclusion of comorbidity was (c = 0.636; 95%CI, 0.633[en]0.639) compared with a predictive model without comorbidity as a covariate (c = 0.631; 95%CI, 0.629[en]0.634). This finding suggests that comorbidity significantly adds to the internal validity and fit of the model (t = 12.65, p = 0.0001).
Age was not a surrogate for comorbidity. Spearman rank correlation coefficient did not identify a meaningful relationship between age and comorbidity (r = 0.151; cut-off r >0.5 for interaction).
3.2. Patients with noninvasive bladder cancer
Demographic features and univariate analyses for patients with noninvasive bladder cancer are outlined in Table 2. On bivariate analysis, comorbidity, age, stage, and grade had p < 0.1, and were included in multivariate analysis. Comorbidity (HR, 1.52; 95%CI, 1.28[en]1.80; p = 0.0001) and age (HR, 1.63; 95%CI, 1.28[en]2.08; p = 0.0001) remained significant on multivariate analysis, whereas AJCC stage (HR, 1.39; 95%CI, 0.92[en]2.09; p = 0.1150) and grade (HR, 1.11; 95%CI, 0.72[en]1.70; p = 0.6510) did not.
Table 2.
[en] Overall survival as a function of baseline demographic, clinical, and tumor features in all patients with noninvasive disease
| Variable | Category | No. (%) of all patients | No. (%) of patients who died during follow-up | Unadjusted hazard ratio | 95% confidence interval | p value |
|---|---|---|---|---|---|---|
| Total | 390 (100) | 137 (35.1) | ||||
| Gender | Female | 91 (23.3) | 31 (34.1) | [em] | [em] | |
| Male | 299 (76.7) | 106 (35.5) | 1.13 | 0.76[en]1.6 9 |
0.5414 | |
| Race* | White | 325 (83.3) | 116 (35.7) | [em] | [em] | |
| Black | 59 (15.1) | 31 (52.5) | 1.25 | 0.78[en]1.9 9 |
0.3550 | |
| Age | ≤65 | 122 (31.3) | 27 (22.1) | [em] | [em] | |
| 66[en]75 | 128 (32.8) | 39 (30.5) | 1.26 | 0.77[en]2.0 6 |
0.3574 | |
| ≥76 | 140 (35.9) | 71 (50.7) | 2.64 | 1.69[en]4.1 7 |
0.0001 | |
| Stage | 0 | 287 (73.6) | 93 (32.4) | [em] | [em] | |
| I | 103 (26.4) | 44 (42.7) | 1.40 | 0.98[en]2.0 0 |
0.0685 | |
| Comorbidity | 0 | 137 (35.1) | 35 (25.5) | [em] | [em] | |
| 1 | 114 (29.2) | 34 (29.8) | 1.31 | 0.82[en]2.1 0 |
0.2614 | |
| 2 | 89 (22.8) | 40 (44.9) | 2.15 | 1.36[en]3.3 8 |
0.0010 | |
| 3 | 50 (12.8) | 28 (56.0) | 3.57 | 2.17[en]5.8 9 |
0.0001 | |
| Grade** | Low (I,II) | 266 (68.2) | 91 (34.2) | [em] | [em] | |
| High (III,IV) |
87 (22.3) | 34 (39.1) | 1.25 | 0.85[en]1.8 4 |
0.2633 |
Six patients were of another ethnicity and were censored owing to small sample size.
Thirty-seven patients were not assigned a grade and were censored.
3.3. Patients treated with cystectomy
Demographic features and univariate analyses for patients treated with cystectomy are outlined in Table 3. Because comorbidity, age, stage, grade, and race had p < 0.1 on bivariate analysis, they were included in multivariate analysis. In multivariate analysis, comorbidity (HR, 1.36; 95%CI, 1.10[en]1.69; p = 0.0053), AJCC stage (HR, 1.85; 95%CI, 1.47[en]2.32; p = 0.0001), and race (HR, 1.77; 95%CI, 1.01[en]3.08; p = 0.0449) were significant, whereas age (HR, 1.19; 95%CI, 0.92[en]1.54; p = 0.1810) and grade (HR, 1.05; 95%CI, 0.50[en]2.22; p = 0.9030) were not.
Table 3.
[en] Overall survival as a function of baseline demographic, clinical, and tumor features in all patients treated with cystectomy
| Variable | Category | No. (%) of all patients | No. (%) of patients who died during follow-up | Unadjusted hazard ratio | 95% confidence interval | p value |
|---|---|---|---|---|---|---|
| Total | 210 (100%) | 95 (45.2) | ||||
| Gender | Female | 44 (21.0) | 21 (47.7) | [em] | [em] | |
| Male | 166 (79.0) | 74 (44.6) | 0.87 | 0.54[en]1.4 2 |
0.5831 | |
| Race | White | 186 (88.6) | 79 (42.5) | [em] | [em] | |
| Black | 24 (11.4) | 16 (66.7) | 1.83 | 1.07[en]3.1 3 |
0.0281 | |
| Age | ≤65 | 93 (44.3) | 32 (34.4) | [em] | [em] | |
| 66[en]75 | 71 (33.8) | 39 (54.9) | 1.72 | 1.08[en]2.7 4 |
0.0237 | |
| ≥76 | 46 (21.9) | 24 (52.2) | 1.58 | 0.93[en]2.6 7 |
0.0928 | |
| Stage | 0 | 18 (8.6) | 5 (27.8) | [em] | [em] | |
| I | 22 (10.5) | 6 (27.3) | 0.99 | 0.30[en]3.2 5 |
0.9861 | |
| II | 61 (29.0) | 17 (27.9) | 1.14 | 0.42[en]3.0 9 |
0.7991 | |
| III | 66 (31.4) | 36 (54.5) | 2.95 | 1.15[en]7.5 2 |
0.0239 | |
| IV | 43 (20.5) | 31 (72.1) | 4.33 | 1.68[en]11. 16 |
0.0024 | |
| Comorbidity | 0 | 70 (33.3) | 28 (40.0) | [em] | [em] | |
| 1 | 72 (34.3) | 34 (47.2) | 1.40 | 0.85[en]2.3 2 |
0.1857 | |
| 2 | 46 (21.9) | 20 (43.5) | 1.15 | 0.65[en]2.0 4 |
0.6438 | |
| 3 | 22 (10.5) | 13 (59.1) | 2.33 | 1.20[en]4.5 1 |
0.0122 | |
| Grade** | Low (I, II) | 24 (11.4) | 8 (33.3) | [em] | [em] | |
| High (III, IV) |
178 (84.8) | 84 (47.2) | 1.74 | 0.93[en]3.2 6 |
0.0847 |
Eight patients were not assigned a grade and were censored.
Table 4 shows adjusted HRs for each level of comorbidity, with a side-by-side comparison of the entire cohort and the two subsets studied.
Table 4.
[en] Adjusted hazard ratios (HRs), p values (p), and 95% confidence interval (95%CI) for overall survival as a function of comorbidity severity (HRs were controlled for stage, grade, age, race, and gender)
| Comorbidity | Entire cohort | Subset with noninvasive disease | Subset treated with cystectomy |
|---|---|---|---|
| None | Reference | Reference | Reference |
| Mild | HR, 1.17 p = 0.3175 95%CI, 0.86[en]1.60 |
HR, 1.15 p = 0.6135 95%CI, 0.70[en]1.89 |
HR, 1.27 p = 0.3858 95%CI, 0.75[en]2.15 |
| Moderate | HR, 1.69 p = 0.0013 95%CI, 1.22[en]2.33 |
HR, 1.89 p = 0.0093 95%CI, 1.16[en]3.07 |
HR, 1.42 p = 0.2669 95%CI, 0.78[en]2.59 |
| Severe | HR, 2.54 p = 0.0001 95%CI, 1.78[en]3.63 |
HR, 3.68 p = 0.0001 95%CI, 2.19[en]6.19 |
HR, 2.65 p = 0.0047 95%CI, 1.33[en]5.28 |
4. Discussion
In evaluating patients for treatment, patients and physicians commonly focus on tumor characteristics such as grade and stage, which have proven to predict risk of recurrence, progression, metastasis, and death [1,2]. Efforts have been made to improve risk assessment by examining other pathological parameters such as number of lymph nodes [16], depth of tumor invasion [17], and biomarker status [18]. However, the primary cause of mortality is often not tumor progression, but comorbid disease [4[en]6]. Comorbidity can impact overall survival of bladder cancer patients either directly by causing the patient's demise or indirectly by limiting treatment options. Increased perioperative complications and less aggressive treatment have often been considered possible links between comorbidity and outcome [19].
In our study, overall survival for the entire cohort was related to comorbidity, age, stage, and race. This finding provides evidence that the most important end point, overall survival, is determined by a complex interaction of demographic, tumor, and patient factors. In noninvasive bladder cancer patients, comorbidity and age independently predicted overall survival, whereas grade, stage, race, and gender did not. This is consistent with the fact that noninvasive bladder cancer tends to recur locally but rarely progresses. As a result the primary risk of death is attributed to advancing age and comorbid disease, not bladder cancer. In contrast, analysis on patients undergoing cystectomy demonstrated that stage, comorbidity, and race significantly predicted overall survival. Thus, in the subset of patients requiring cystectomy, extent of tumor and comorbidity are important.
These findings have an impact for the practicing urologist. Although on the surface the relationship between comorbidity and overall survival seems intuitive, there are malignancies that are so aggressive (for instance pancreatic cancer) that the expected impact of comorbidity is minimal, whereas other malignancies can be indolent enough (eg, basal cell cancer) that survival is driven primarily by comorbidity. We found that for bladder carcinoma, both disease-specific and patient-specific variables impacted outcome. For patients with noninvasive disease, comorbidity drove survival. Clinicians can use these data to reassure patients that, whereas noninvasive tumor may recur frequently, if properly treated, it is unlikely to contribute to mortality. In contrast, for patients with invasive disease, even with severe comorbidity, tumor-specific variables impact survival. We believe that this finding provides important prognostic information for clinicians and patients following treatment.
The effect that small changes in comorbidity can have on survival is underappreciated. In our study, comorbidity strongly predicted outcome in patients healthy enough to be considered good surgical candidates. Although this group received gold standard treatment (cystectomy), relatively small changes in general health status led to significant differences in overall survival: 59.1% mortality for severe comorbidity versus 43.5% mortality for moderate comorbidity (Table 3). Therefore, comorbidity is a potentially valid adjunct along with tumor-specific variables, and patients' overall health should be optimized in addition to management of their bladder cancer.
Other patient-specific variables were also found to predict survival. The relationship between age and overall mortality should not be surprising. Clark et al [20] found that 5-year overall survival declined with increasing age for bladder cancer patients. Nielsen et al [21] found that advanced age was independently associated with poorer disease specific survival in cystectomy patients. The relationship between race and survival is striking, particularly in cystectomy patients (Tables 1 and 3). Although this relationship could be due to biological differences in tumor biology, a more plausible explanation is that access to care either before or after diagnosis is different. Finally, it is possible that competing causes of mortality unrelated to bladder cancer are higher in African Americans.
There have been several prior studies examining the effect of comorbidity on survival of patients with bladder cancer. Miller et al [22] examined 78 patients undergoing radical cystectomy and found that comorbidity was associated with surgical delay, adverse pathology, and decreased disease-free survival. They, however, failed to find a significant independent association between comorbidity and overall survival, and cited small sample size and weaknesses of the Charlson Comorbidity Index as possible explanations. Our study, with a larger sample size (subset analysis on 210 cystectomy patients) and a validated comorbidity instrument, demonstrated that comorbidity significantly influences overall survival in cystectomy patients.
Kock and Smith [23] examined 47 patients undergoing cystectomy and failed to demonstrate an association between comorbidity as assessed by American Society of Anesthesiologists (ASA) scores and survival. Lack of association may be secondary to relative lack of sensitivity of ASA and limited sample size. Chang et al [24] also used ASA scores and had relatively small samples sizes, which may have contributed to their negative results.
There are several limitations to our study. Mortality rates were higher than those reported in clinical trials, which likely reflects the fact that patients enrolled in clinical trials typically have good performance status compared with the average bladder cancer patient. Second, we did not examine the association between comorbidity and disease-specific survival because the data were not available. We believe that overall survival is a more critical end point[em]and is the end point of greatest interest to patients. Disease-specific survival is most important in assessing effect of treatment. Another limitation is that the relationship between treatment and outcome was not explored. However, the primary goal of this study was to examine the effect of comorbidity on all patients with newly diagnosed bladder cancer, not a subset of patients. Treatment for bladder cancer is dramatically different for patients with different stages of disease, ranging from local resection alone in patients with low-grade noninvasive disease to chemotherapy in patients with metastatic disease. Treatment is also influenced by patient and surgeon preference. Because of the complexity involved in treatment decisions and assessment of treatment effectiveness from observational data, we could not accurately model treatment in this study.
Despite these limitations, our study has certain strengths. To our knowledge, this is currently the largest study investigating the prognostic impact of comorbidity on survival for bladder cancer patients. The current study improves on previous studies by collecting comorbidity information prospectively with the use of ACE-27, an instrument validated on adult oncology patients. Lastly, our study population is diverse; it encompasses patients with all disease stages and does not limit our analysis to one specific stage or treatment group. This study, therefore, demonstrates the global effects of comorbidity on outcome for bladder cancer patients, while also addressing issues in specific subsets.
Because we have established the relationship between comorbidity and overall survival, future studies should investigate the means through which comorbidity influences outcome. The association between comorbidity and surgical delay and between comorbidity and type of surgery performed (including extent of lymph node dissection) should be further investigated because both are clear predictors of outcome for bladder cancer [16,25,26].
5. Conclusions
Comorbidity is a significant, independent predictor of overall survival in patients with bladder cancer. In addition, survival was associated with stage, age, and race. In patients with noninvasive disease, comorbidity and age predict survival, whereas tumor variables (stage and grade) do not. In contrast, for patients treated with cystectomy, comorbidity, stage, and race predict survival. Therefore, both tumor-specific variables and comorbidity should be evaluated in bladder cancer patients. Because we have shown that, even in patients with advanced bladder cancer who were considered good surgical candidates, small changes in severity of comorbidity can translate into significant differences in survival, attempts should be made to optimize the overall health of these patients, along with management of their bladder cancer.
Acknowledgments
Source of research support: I. Megwalu, Washington University Summer Research Grant; A. Vlahiotis, none; M. Radwan, none; J.F. Piccirillo, RO1 CA104797-01; A.S. Kibel, R01 CA112028-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None of the authors has an actual, potential, or apparent conflict of interest associated with this manuscript.
Take-home message
Outcome measures in bladder cancer traditionally focus on tumor characteristics. We demonstrate that comorbidity significantly predicts survival. In the subset with noninvasive disease, only comorbidity and age predict survival. In those treated with cystectomy, comorbidity, stage, and race predict survival.
References
- 1.Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Vicente-Rodriguez J. Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J Urol. 2000;163:73–8. doi: 10.1016/s0022-5347(05)67975-x. [DOI] [PubMed] [Google Scholar]
- 2.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 3.Piccirillo JF, Feinstein AR. Clinical symptoms and comorbidity: significance for the prognostic classification of cancer. Cancer. 1996;77:834–42. [PubMed] [Google Scholar]
- 4.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. In lung cancer patients, age, race-ethnicity, gender and smoking predict adverse comorbidity, which in turn predicts treatment and survival. J Clin Epidemiol. 2004;57:597–609. doi: 10.1016/j.jclinepi.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Pugliano FA, Piccirillo JF, Zequeira MR, Fredrickson JM, Perez CA, Simpson JR. Clinical-severity staging system for oral cavity cancer: five-year survival rates. Otolaryngol Head Neck Surg. 1999;120:38–45. doi: 10.1016/S0194-5998(99)70367-0. [DOI] [PubMed] [Google Scholar]
- 6.Post PN, Hansen BE, Kil PJ, Janssen-Heijnen ML, Coebergh JW. The independent prognostic value of comorbidity among men aged < 75 years with localized prostate cancer: a population-based study. BJU Int. 2001;87:821–6. doi: 10.1046/j.1464-410x.2001.02189.x. [DOI] [PubMed] [Google Scholar]
- 7.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120:104–10. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 8.Steineck G, Hagman U, Gerhardsson M, Norell SE. Vitamin A supplements, fried foods, fat and urothelial cancer. A case-referent study in Stockholm in 1985−87. Int J Cancer. 1990;45:1006–11. doi: 10.1002/ijc.2910450604. [DOI] [PubMed] [Google Scholar]
- 9.Wingo PA, Ries LA, Giovino GA, et al. Annual report to the nation on the status of cancer, 1973−1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999;91:675–90. doi: 10.1093/jnci/91.8.675. [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 11.Piccirillo JF, Creech CM, Zequeira R, Anderson S, Johnston AS. Inclusion of comorbidity into oncology data registries. J Reg Manag. 1999;26:66–70. [Google Scholar]
- 12.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–7. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 13.Epstein JI, Amin MB, Reuter VE, et al. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Am J Surg Pathol. 1998;12:1435–48. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Bergkvist A, Ljungqvist A, Moberger G. Classification of bladder tumors based on the cellular pattern. Acta Chir Scand. 1965;130:371–8. [PubMed] [Google Scholar]
- 15.Shariat SF, Palapattu GS, Karakiewicz PI, et al. Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. Eur Urol. 2007;51:137–49. doi: 10.1016/j.eururo.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Bochner BH, Cho D, Herr HW, Donat M, Kattan MW, Dalbagni G. Prospectively packaged lymph node dissections with radical cystectomy: evaluation of node count variability and node mapping. J Urol. 2004;172:1286–90. doi: 10.1097/01.ju.0000137817.56888.d1. [DOI] [PubMed] [Google Scholar]
- 17.Quek ML, Stein JP, Clark PE, et al. Microscopic and gross extravesical extension in pathological staging of bladder cancer. J Urol. 2004;171:640–5. doi: 10.1097/01.ju.0000108664.39035.51. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee SJ, Datar R, Youssefzadeh D, et al. Combined effects of p53, p21, and pRb expression in the progression of bladder transitional cell carcinoma. J Clin Oncol. 2004;22:1007–13. doi: 10.1200/JCO.2004.05.174. [DOI] [PubMed] [Google Scholar]
- 19.Derks W, de Leeuw RJ, Hordijk GJ. Elderly patients with head and neck cancer: the influence of comorbidity on choice of therapy, complication rate, and survival. Curr Opin Otolaryngol Head Neck Surg. 2005;13:92–6. doi: 10.1097/01.moo.0000156169.63204.39. [DOI] [PubMed] [Google Scholar]
- 20.Clark PE, Stein JP, Groshen SG, et al. Radical cystectomy in the elderly: comparison of survival between younger and older patients. Cancer. 2005;103:546–52. doi: 10.1002/cncr.20805. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen ME, Shariat SF, Karakiewicz PI, et al. Bladder Cancer Research Consortium (BCRC). Advanced age is associated with poorer bladder cancer-specific survival in patients treated with radical cystectomy. Eur Urol. 2007;51:699–706. doi: 10.1016/j.eururo.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Miller DC, Taub DA, Dunn RL, Montie JE, Wei JT. The impact of co-morbid disease on cancer control and survival following radical cystectomy. J Urol. 2003;169:105–9. doi: 10.1016/S0022-5347(05)64046-3. [DOI] [PubMed] [Google Scholar]
- 23.Koch MO, Smith JA., Jr Influence of patient age and co-morbidity on outcome of a collaborative care pathway after radical prostatectomy and cystoprostatectomy. J Urol. 1996;155:1681–4. [PubMed] [Google Scholar]
- 24.Chang SS, Alberts G, Cookson MS, Smith JA., Jr Radical cystectomy is safe in elderly patients at high risk. J Urol. 2001;166:938–41. [PubMed] [Google Scholar]
- 25.Sanchez-Ortiz RF, Huang WC, Mick R, Van Arsdalen KN, Wein AJ, Malkowicz SB. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol. 2003;169:110–5. doi: 10.1016/S0022-5347(05)64047-5. [DOI] [PubMed] [Google Scholar]
- 26.Fahmy NM, Mahmud S, Aprikian AG. Delay in the surgical treatment of bladder cancer and survival: systematic review of the literature. Eur Urol. 2006;50:1176–82. doi: 10.1016/j.eururo.2006.05.046. [DOI] [PubMed] [Google Scholar]