Abstract
Nasal trigeminal chemosensitivity in mice and rats is mediated in part by solitary chemoreceptor cells (SCCs) in the nasal epithelium (Finger et al., 2003). Many nasal SCCs express the G-protein α-gustducin as well as other elements of the bitter-taste signaling cascade including phospholipase Cβ2, TRPM5 and T2R bitter-taste receptors. While some populations of sensory cells are replaced throughout life (taste and olfaction), others are not (hair cells and carotid body chemoreceptors). These experiments were designed to test whether new SCCs are generated within the epithelium of adult mice. Wild type C57/B6 mice were injected with the thymidine analog 5-bromo-2'-deoxyuridine (BrdU) to label dividing cells. At various times after injection (1-40 days), the mice were perfused with 4% paraformaldehyde and prepared for dual-label immunocytochemistry. Double labeled cells were detected as early as 3 days post BrdU injection and remained for as long as 12 days post-injection suggesting that SCCs do undergo turnover like the surrounding nasal epithelium. No BrdU labeled cells were detected after 24 days suggesting relatively rapid replacement of the SCCs.
Keywords: Chemoreceptors, BrdU, nasal, trigeminal, immunocytochemistry
Introduction
Most epithelial cells are renewed throughout the life of an animal, most likely to replace damaged or dying cells. This process of cell replacement includes the sensory cells for the senses of taste and olfaction. The temporary nature of these cells complicates the development of specialized connections with the nervous system.
Recently, we described a population of epithelial chemoreceptor cells, called solitary chemoreceptor cells (SCCs), within the nasal epithelium of mammals (Finger et al., 2003). SCCs are morphologically similar to the individual cells in taste buds but unlike taste cells, form distinct synapses onto cutaneous nerve fibers of the trigeminal nerve (Finger et al., 2003). Also unlike taste buds, SCCs are not clustered into groups, but are scattered as isolated cells (Finger, 2000). SCCs are distributed throughout the nasal cavity, with the highest numbers anterior (Finger et al., 2003), and then extending down the airways through the larynx and trachea (Sbarbati et al., 2004; Sbarbati & Osculati, 2005).
While many trigeminal stimuli can diffuse across the nasal epithelium and interact with free nerve endings, lipophobic stimuli must find another way to activate the trigeminal nerve (Sekizawa & Tsubone, 1994). The apical process of SCCs extends to the surface of the epithelium and contain T2R “bitter-taste” receptors which presumably activate G-protein coupled intracellular signaling cascades (Finger et al., 2003). One transduction system that SCCs use is similar to that of type II (bitter-sensitive) taste cells since both cell types utilize similar transduction cascades including T2R bitter taste receptors, Phospholipase Cβ2 (PLCβ2), and the G protein α-gustducin (Clapp et al., 2001; Finger et al., 2003; Sbarbati & Osculati, 2005). Many bitter tasting substances are noxious and activate protective avoidance or rejection responses. The presence of bitter taste receptors and synapses with trigeminal nerve fibers suggests that SCCs are providing a detection system for noxious airborne substances which would not otherwise be able to reach nerve endings terminating below the level of the epithelial tight junctions (Finger et al., 2003).
Taking into account their exposure to noxious substances and similarities to some taste cells, which have a limited lifespan (Cho et al., 1998; Farbman, 1980), we hypothesized that nasal SCCs undergo continuous replacement. In taste buds, proliferative basal cells generate immature taste cells that require a 2-3 day period of maturation before they express elements of the transduction cascade like α-gustducin (Cho et al., 1998). We used the thymidine analog 5-bromo-2'-deoxyuridine (BrdU) to label dividing cells and by sacrificing animals at various times post injection, could follow the process of SCC generation and maturation (Miller & Nowakowski, 1988). The results suggest that SCCs undergo turnover and that the new cells mature at a rate similar to that of Type II taste cells.
Methods
Tissue preparation
All experiments referred to in this report were in accordance with approved UCDHSC IACUC animal protocols. Adult male wild type C57/B6 mice, maintained on a 12/12 light/dark cycle, were injected (80 mg/kg i.p.) with BrdU (Sigma-Aldrich, St Louis, MO). This concentration effectively labeled dividing cells in the nasal epithelium and was low enough to avoid obvious toxicity. The BrdU injections were made at the onset of the dark phase of the light cycle, when the greatest number of cells is expected to be in S-phase (Farbman, 1980). Mice were housed in Micro-Vent Environmental System racks (Allentown Caging, Allentown, N.J.). Airflow in the cages was 50-70 changes per hour and cage humidity was slightly higher than 38%. Mice were killed 24 hrs (n=1), 2 days (n=3), 3 days (n=3), 4 days (n=3), 6 days (n=3), 12 days (n=3), 24 days (n=1), 38 days (n=1), or 40 days (n=1) following injection with BrdU.
Mice were anesthetized with sodium pentobarbital (150 mg/kg) and perfused transcardially with 4 % paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB) (pH 7.2). The heads were then post-fixed in PFA for 2 hrs and transferred to 20% sucrose in PB overnight. The snout was decalcified with RDO rapid decalcifier (Apex, Plainsfield, IL) for 4 hrs and then rinsed for 2-4 hrs in 20% sucrose. The nasal cavities were then cut into 16 μm thick sections (4/slide spaced approximately 100 μm apart) with a cryostat. Sections were stored at −20° C until used for immunocytochemistry.
Antibodies and immunocytochemistry
After the sections were thawed and re-hydrated in PB, endogenous peroxidases were quenched with 3% H202 in methanol. Sections were then rinsed in phosphate-buffered saline (PBS) and lightly digested with trypsin for 5 min (Zymed BrdU kit, Zymed Laboratories, South San Francisco, CA). Slides were washed with water and then treated with 4N or 2N HCl for 20 min at 37°C to denature double-stranded DNA. For combined BrdU-gustducin immunocytochemistry, following PBS rinses and a 1 hr block with 1% normal goat serum (NGS), 1° antibodies (pre-diluted biotinylated mouse anti-BrdU [Zymed BrdU kit, Zymed Laboratories, South San Francisco, CA] and rabbit anti-α-gustducin 1:1,000 [Santa Cruz Biotechnology, Santa Cruz, CA]) were applied overnight at 4°C. The following day, sections were rinsed in PBS and then incubated with the 2° antibodies Alexa Fluor 568 goat anti-rabbit IgG (1:400) (Molecular Probes, Eugene, OR) and Alexa Fluor streptavidin 488 (1:400) (Molecular Probes, Eugene, OR) for 2hrs at room temperature in NGS block. After being rinsed in PBS, the slides were incubated with propidium iodide (Calbiochem, San Diego, CA) (1μg/ml in PBS) for 30 min to label all cell nuclei and then rinsed in 0.1M phosphate buffer. Slides were coverslipped with fluoromount-G (Southern Biotech, Birmingham, AL) and stored at 4°C until analysis.
Confocal microscopy
Immunofluorescence of labeled SCCs was imaged on an Olympus Fluoview laser scanning confocal microscope (Olympus America Inc., Melville, NY) using a 20X (0.80 n.a.) oil immersion lens. Optical sections (0.8 μm spacing) were acquired through each field of view and then compiled into a z-stack. Sequential scanning of the two separate fluorescence channels avoided “bleed through” of signal from the inappropriate channel.
Cell counting
Random sampling fields containing α-gustducin positive cells in the nasal respiratory epithelium were selected on sections at 20X and then imaged at 40X. The sampling area from which fields were selected contained the respiratory epithelium from the most anterior regions of the nasal cavity to the posterior end of the VNO. Five sampling fields were imaged per animal from this area. We used the z-stack 3D image acquired on the confocal to count all α-gustducin immunoreactive cells containing a nuclear profile and noted those that were double labeled. A summary of the cell counts is given in Table 1. α-gustducin cells were only counted when the non-reactive nucleus could be seen clearly. For a cell to be counted as double labeled, obvious BrdU-immunoreactivity had to be present in the nucleus surrounded by cytoplasmic α-gustducin staining.
Table 1.
Summary of cell counts in each mouse nose processed for α-gustducin and BrdU immunocytochemistry.
| animal | days post BrdU injection |
total # of SCCs counted |
# of counted SCCs co-labeled with BrdU |
frac lbl |
|---|---|---|---|---|
| 041203A | 2 day | 21 | 0 | 0 |
| 041203B | 2 day | 17 | 0 | 0 |
| 50721 | 2 day | 14 | 0 | 0 |
| 041206A | 3 day | 30 | 0 | 0 |
| 041206B | 3 day | 32 | 0 | 0 |
| 41111 | 3 day | 23 | 1 | 0.043 |
| 041206C | 4 day | 8 | 0 | 0 |
| 41112 | 4 day | 13 | 2 | 0.153 |
| 041206D | 4 day | 35 | 1 | 0.028 |
| 40830 | 6 day | 49 | 6 | 0.122 |
| 41207 | 6 day | 32 | 1 | 0.031 |
| 50725 | 6 day | 18 | 2 | 0.111 |
| 41007 | 12 day | 30 | 3 | 0.100 |
| 050801A | 12 day | 15 | 3 | 0.200 |
| 050801B | 12 day | 12 | 2 | 0.167 |
| 50425 | 24 day | 18 | 0 | 0 |
| 50509 | 38 day | 5 | 0 | 0 |
| 50511 | 40 day | 16 | 0 | 0 |
Propidium iodide staining revealed all cell nuclei in the epithelial sampling regions containing α-gustducin positive cells. The total number of epithelial cells, and the number labeled with BrdU, were counted. Basal cells were excluded from these counts and were identified by estimating that they rest on the basal lamina and are approximately 20-30 μm from the epithelial surface.
Results
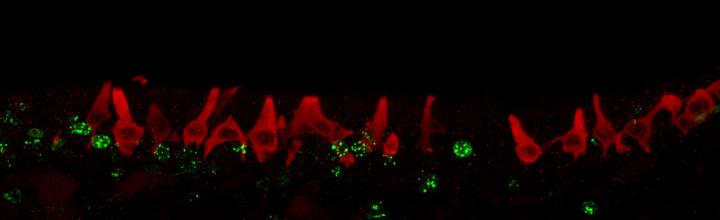
In BrdU injected mice, the anti-α-gustducin antibody reacted with the cytoplasm of SCCs and Type II taste cells (red staining in Fig.1). The positive SCCs were located throughout the nasal cavity with the highest numbers in the anterior portion as described previously (Finger et al., 2003). SCCs also occur on the septum and turbinates with a concentration of SCCs ventrolateral to the vomeronasal organ (VNO). Anteriorly, the most SCCs were located on the dorsal nasal walls and more posteriorly, ventrally beneath, or on the walls lateral to the VNO.
Figure 1.
Dual-label immunocytochemistry was used to identify SCCs (anti-α-gustducin = red) and cells which incorporated BrdU (anti-BrdU = green). At early survival times, most BrdU immunoreactive cells were located along the basal lamina. At later times, both basal cells and cells higher in the epithelium were labeled with BrdU (6 day survival shown above).
BrdU immunoreactivity occurred in the nucleus of basal cells as well as in cells higher in the epithelium (green staining in Fig.1). As expected, the number of cells labeled for BrdU in the epithelium increased with the intermediate survival periods. When the α-gustducin antibody was omitted, only BrdU immunoreactivity was observed and vice versa. In other words, neither secondary antibody bound to the inappropriate primary antibody.
Our first aim was to determine if newly generated nasal SCCs appear in the epithelium. Confocal microscopy revealed many cells that were immunoreactive for only α-gustducin, only BrdU, and a small percentage that were double labeled (Fig.2). The earliest that double labeled cells were observed was at 3 days post injection (Fig.2b). Thus, a cell requires 3 days between S-phase and the earliest expression of gustducin. Double labeled cells were observed more frequently in the 4, 6, and 12 day survival animals (Fig.2c, d, and e). This suggests that SCCs do undergo replacement. No double labeling was seen in longer survival times (24-40 days) and the BrdU labeling present in other cell types appeared fainter perhaps due to dilution of the BrdU label by repeated cell divisions.
Figure 2.
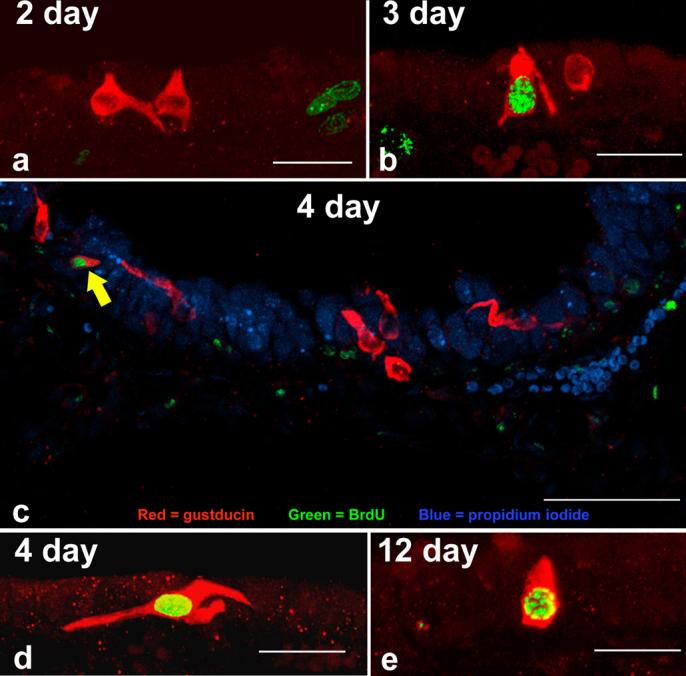
Triple label immunocytochemistry in the dorsal anterior nasal epithelium of a 4 day post-BrdU injection mouse (c). SCCs, identified with anti-α-gustducin, are shown in red. Nuclei which have incorporated BrdU are shown in green and all nuclei, stained with propidium iodide, are shown in blue. Scale bar = 50 μm. Note the double labeled SCC in the upper left (arrow). At the two day time point (a) no colocalization was seen. The first double labeled SCCs were observed at three days post-BrdU injection (b) and double labeled cells were still evident at 12 days post-BrdU (e). Scale bars in a, b, d, and e = 20 μm.
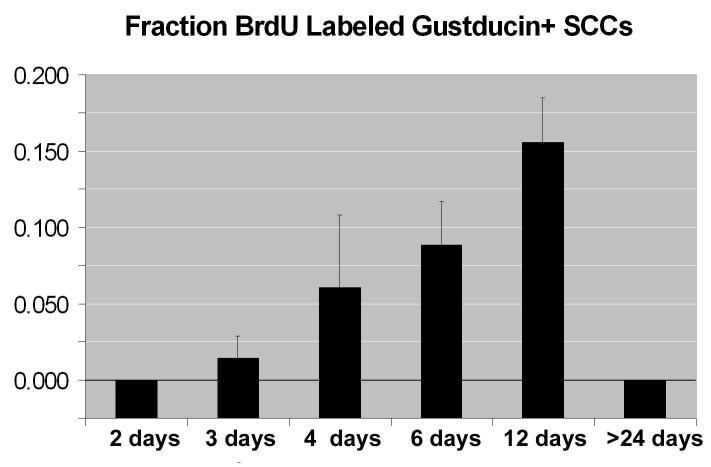
Next, we aimed to estimate a turnover rate for SCCs. A relatively low percentage of double labeled SCCs is present at three days (1.4%) and then increases to a maximum of about 16% by days 6-12 (Fig. 3). Counting all cells in the epithelium, the rate of generation is 1.84% at three days and 2.63% at four days post-BrdU injection. No significant differences (Chi-square test) were found between the total population of epithelial cells double labeled and the number of SCCs double labeled at these short survival times.
Figure 3.
Fraction of SCCs labeled with BrdU at different times after injection. n values indicate the number of animals used at each time point. N = 3 for each time.
In summary, these findings indicate that SCCs are generated continuously in the nasal cavity of an adult mouse at approximately the same rate as surrounding epithelial cells. Further, the SCCs have a limited lifespan in the epithelium rarely reaching 24 days post-mitosis.
Discussion
Nasal SCCs are a newly-discovered class of epithelial chemoreceptor cells which are located in the non-olfactory nasal epithelium (Finger et al., 2003). Like olfactory receptor neurons, the apical process of SCCs extends into the mucus layer overlying the nasal epithelium. Both cell populations then are vulnerable to toxic damage or pathogens carried on the respiratory air stream. Like olfactory receptor neurons, which are replaced throughout the life of the animal, SCCs undergo continuous proliferation in the adult animal.
Olfactory receptor neurons and SCCs both utilize signal transduction cascades involving different G-proteins. The time it takes for these signaling cascades to be established can be used to estimate maturation time for these cells. Olfactory neurons take several days after terminal mitosis to express odorant receptors (Iwema & Schwob, 2003). In fact, many sensory cells which signal with G-proteins mature at similar rates. Neurons in the opossum VNO have been estimated to mature within 3 days after exiting mitosis as evidenced by expression of olfactory marker protein (OMP) and the G-protein Gαo (Martinez-Marcos et al., 2000). Similarly, in Type II taste cells the G-protein α-gustducin is expressed in 2.5 days (Cho et al., 1998). Furthermore, rod photoreceptors express opsin, the transmembrane component of rhodopsin, 48-54 hr after the last S phase (Watanabe & Raff, 1990). We show that the timing of expression of α-gustducin in SCCs is within three days after completing mitosis. Timing of expression of this protein may indicate that SCCs mature at a rate similar to other sensory cells.
We observed a relatively low percentage of SCCs labeled with BrdU at this early stage (about 1.4% after 3 days). However, at later survival times, the number of double labeled cells we observed increased to a maximal fraction of about 16% by day 12. Assuming that the pulse of BrdU effectively labeled at maximum 25% of basal cells during the 2-4 hrs that it was bioavailable, we estimate that the entire population of SCCs is replaced on the order of 20 days. In support of this relatively short turnover time, we observed no double labeled cells at 24 days post-BrdU.
Our estimated turnover time for SCCs correlates well with other estimates of epithelial turnover in respiratory epithelium. The respiratory epithelium has a low steady-state level of cell division and turnover time decreases from the tracheobronchial tree toward the nasal epithelium (Basbaum & Jany, 1990). This may reflect the relative damage and thus need for replacement that the epithelium would sustain at varying locations in the respiratory tract. For example, adult mouse small bronchus turnover time is between 208 and 282 days while the tracheal epithelium has been estimated to turnover between 168 and 267 days (Basbaum & Jany, 1990). Nasal SCCs, being located in a more exposed and vulnerable position in the airway, could be subject to more frequent insult and thus be expected to have a shorter turnover time than more distal areas of the respiratory epithelium.
An alternative explanation for our results is that, during development, proliferation of SCCs serves to accommodate an expanding an epithelial surface area rather than for cell replacement. Weiler & Farbman (1997) observed an inverse relationship between the proliferation rate of cells in the olfactory and respiratory epithelium and age of the animal. In young animals, when the epithelium is growing rapidly, most cell proliferation is likely occurring to increase the total neuron population and surface area. In older animals where the epithelium is growing much more slowly, the main function of proliferation is replacement (Weiler & Farbman, 1997).
The proliferation we observed in SCCs is most likely due to cell replacement and not cell addition. The experiments done by Weiler & Farbman were conducted in rats, a species in which the total surface area of nasal epithelium increases with age (Gross et al., 1982; Weiler & Farbman, 1997). However, in the present study, we used mice to assess cell proliferation. Unlike rats, the total surface area of the nasal epithelium does not increase significantly after the animals enter adulthood (Gross et al., 1982). Since the mice we used were adults, we can assume that the majority of cell proliferation is due to cell replacement and not cell addition. Furthermore, the lack of double label at 24 days and longer suggests that the lifespan of a typical nasal SCC is shorter than 24 days.
Most epithelial cells are replaced actively (Vaigot et al., 1987). However, cells that form stable neuronal synapses such as neurons, muscle cells and many sensory cells are not normally replaced. Examples of non-proliferative sensory cell populations are common. Rods and cones are photoreceptor cells which are situated in the retina. In mammals, photoreceptors are not continually regenerated in the adult animal (Zeiss & Johnson, 2004). Hair cells, the mechanoreceptors found in the auditory epithelium, have a limited regenerative capacity and don't proliferate under normal conditions (Lopez-Schier, 2004). Merkel cells are non-keratinized epithelial cells which act as slowly adapting mechanoreceptors (Tachibana et al., 2000) and BrdU labeling indicates that Merkel cells are either unable to divide or do so very rarely (Vaigot et al., 1987).
Epithelial chemoreceptor cells are an apparent anomaly in terms of cell replacement. The sensory modalities of taste and olfaction possess chemoreceptor cells which form synapses and yet are still continuously replaced throughout adult life (Beidler & Smallman, 1965; Moulton, 1974). This does not seem to be a general property of all chemoreceptive cells though since chemoreceptor cells which reside in more protected environments in the body do not display this property. For example, the carotid body is located at the bifurcation of the common carotid artery, bilaterally in the neck, and within it are chemoreceptor cells which monitor alterations in blood PO2, PCO2, and pH (Gronblad, 1983). In pathological conditions, such as hypoxia, there is hypertrophy of the carotid body resulting from increased proliferation of the cells within it (Bee & Pallot, 1995). However, there is no evidence showing proliferation of cells under normal conditions. In fact, the number of sensory cells within the carotid body decreases with normal ageing (Hurst et al., 1985).
In contrast to protected chemoreceptor cell populations SCCs reside in a vulnerable position in the nasal cavity. They are directly exposed to the external environment. This is similar to olfactory receptor neurons and taste receptor cells, both of which are known to undergo turnover. Damage inflicted upon these cell populations necessitates that they be dynamic in order to remain responsive to stimuli. Turnover times should then be expected to reflect the relative amount of damage received by each population. Chemosensory cells in taste buds have been estimated to turnover in approximately 10.5 days (Moulton, 1974). Likewise, the chemosensory cells involved in olfaction (the olfactory neurons) are commonly thought to turnover in 30 days (Beidler & Smallman, 1965; Farbman, 1980). SCCs reside in an environment similar to olfactory receptor neurons so one might expect them to have comparable replacement requirements. No double labeled cells were detected at survival times greater than, or equal to, 24 days. This suggests that SCCs, like taste cells and olfactory receptor cells, have are relatively short lifespan in the epithelium.
In conclusion, our results indicate that nasal solitary chemoreceptor cells undergo continuous proliferation in adult mice. This property is shared by taste receptor cells and olfactory receptor neurons. Timing of α-gustducin expression in SCCs demonstrates that they mature at a rate comparable to taste receptor cells and other chemosensory cells utilizing G-protein signaling cascades. Taken together, our results demonstrate that chemosensory cells involved in perception of taste, smell, and trigeminal qualities all exhibit similar replacement requirements and maturation rates.
Acknowledgments
Supported by Grants from NIH RO1 DC 06070 (T.E.F.) and P30 DC 04657 (D. Restrepo)
Footnotes
1 halftone plate
2 color plate
Dedication: This work is dedicated to Al Farbman, a pioneering figure in studies of both the gustatory and olfactory epithelia. He is the rarest of individuals, an esteemed academic, an enthusiastic scientist, and a true gentleman.
References
- BASBAUM C, JANY B. American Journal of Physiology. 1990;259:L38–46. doi: 10.1152/ajplung.1990.259.2.L38. [DOI] [PubMed] [Google Scholar]
- BEE D, PALLOT DJ. Journal of Applied Physiolology. 1995;79:1504–1511. doi: 10.1152/jappl.1995.79.5.1504. [DOI] [PubMed] [Google Scholar]
- BEIDLER LM, SMALLMAN RL. Journal of Cell Biology. 1965;27:263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHO YK, FARBMAN AI, SMITH DV. Chemical Senses. 1998;23:735–742. doi: 10.1093/chemse/23.6.735. [DOI] [PubMed] [Google Scholar]
- CLAPP TR, STONE LM, MARGOLSKEE RF, KINNAMON SC. BMC Neuroscience. 2001;2:6. doi: 10.1186/1471-2202-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARBMAN AI. Cell and Tissue Kinetics. 1980;13:349–357. doi: 10.1111/j.1365-2184.1980.tb00474.x. [DOI] [PubMed] [Google Scholar]
- FINGER TE, BOTTGER B, HANSEN A, ANDERSON KT, ALIMOHAMMADI H, SILVER WL. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8981–8986. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINGER TE, SIMON SA. In: Cell Biology of Taste Epithelium. In The Neurobiology of Taste and Smell. FINGER TE, SILVER WL, RESTREPO D, editors. A John Wiley & Sons, Inc.; New York: 2000. pp. 287–314. [Google Scholar]
- GRONBLAD M. Medical Biology. 1983;61:229–248. [PubMed] [Google Scholar]
- GROSS EA, SWENBERG JA, FIELDS S, POPP JA. Journal of Anatomy. 1982;135:83–88. [PMC free article] [PubMed] [Google Scholar]
- HURST G, HEATH D, SMITH P. Journal of Pathology. 1985;147:181–187. doi: 10.1002/path.1711470306. [DOI] [PubMed] [Google Scholar]
- IWEMA CL, SCHWOB JE. The Journal of Comparative Neurology. 2003;459:209–222. doi: 10.1002/cne.10583. [DOI] [PubMed] [Google Scholar]
- LOPEZ-SCHIER H. Current Biology. 2004;14:R127–128. [PubMed] [Google Scholar]
- MARTINEZ-MARCOS A, UBEDA-BANON I, HALPERN M. Journal of Neurobiology. 2000;43:50–63. doi: 10.1002/(sici)1097-4695(200004)43:1<50::aid-neu5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- MILLER MW, NOWAKOWSKI RS. Brain Research. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- MOULTON DG. Annals of the New York Academy of Sciences. 1974;237:52–61. doi: 10.1111/j.1749-6632.1974.tb49843.x. [DOI] [PubMed] [Google Scholar]
- SBARBATI A, MERIGO F, BENATI D, TIZZANO M, BERNARDI P, OSCULATI F. Chemical Senses. 2004;29:683–692. doi: 10.1093/chemse/bjh071. [DOI] [PubMed] [Google Scholar]
- SBARBATI A, OSCULATI F. Progress in Neurobiology. 2005;75:295–307. doi: 10.1016/j.pneurobio.2005.03.001. [DOI] [PubMed] [Google Scholar]
- SEKIZAWA SI, TSUBONE H. Respiration Physiology. 1994;96:37–48. doi: 10.1016/0034-5687(94)90104-x. [DOI] [PubMed] [Google Scholar]
- TACHIBANA T, FUJIWARA N, NAWA T. Anatomy and Embryology. 2000;202:359–367. doi: 10.1007/s004290000124. [DOI] [PubMed] [Google Scholar]
- VAIGOT P, PISANI A, DARMON YM, ORTONNE JP. Acta DermatoVenereologica. 1987;67:517–520. [PubMed] [Google Scholar]
- WATANABE T, RAFF MC. Neuron. 1990;4:461–467. doi: 10.1016/0896-6273(90)90058-n. [DOI] [PubMed] [Google Scholar]
- WEILER E, FARBMAN AI. The Journal of Neuroscience. 1997;17:3610–3622. doi: 10.1523/JNEUROSCI.17-10-03610.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEISS CJ, JOHNSON EA. Investigative Opthalmology & Visual Science. 2004;45:971–976. doi: 10.1167/iovs.03-0301. [DOI] [PubMed] [Google Scholar]